Abstract
The vestibular (inner ear balance) system senses head movement and orientation in space. Vestibular sensory input plays a critical role in spatial cognitive abilities such as spatial memory and spatial navigation. Vestibular function declines with age, and recent studies have shown that age-related vestibular impairment is associated with poorer spatial cognitive skills in healthy older adults. Moreover, vestibular impairment is disproportionately prevalent among individuals with mild cognitive impairment and Alzheimer’s disease, and specifically in cognitively-impaired individuals who have spatial deficits such as disorientation and difficulty driving. Indeed, emerging evidence suggests that age-related vestibular impairment contributes to a “spatial” subtype of Alzheimer’s disease, characterized by highly morbid symptoms such as wandering and falls. Given that vestibular impairment can be treated through simple, physical-therapy based exercises, identifying and treating vestibular deficits in older adults with and without cognitive impairment may offer substantial benefit in preventing, mitigating and forestalling cognitive decline.
The vestibular system is an evolutionarily ancient and remarkably conserved sensory organ that plays a primal role in enabling stable locomotion. The vestibulo-ocular reflex, which is the fastest reflex in the human body, stabilizes the visual world during movement, while the vestibulo-spinal reflexes keep the body aligned with earth vertical during dynamic motion (Goldberg, Wilson, Angelaki, & Cullen, 2012). In addition to providing inputs to these critical brainstem reflexes, the vestibular system also sends projections to subcortical and cortical structures, where information from the vestibular system about head orientation and movement is used for higher-order cognitive processes such as spatial memory and navigation (Hitier, Besnard, & Smith, 2014). Vestibular loss occurs with age, and there is growing evidence in healthy older adults that reduced vestibular function is associated with poorer spatial cognitive skills including mental rotation, spatial memory and spatial navigation (Bigelow et al., 2015). Moreover, patients with Alzheimer’s disease (AD) have twice the level of vestibular impairment as healthy older adults, and AD patients with vestibular loss have disproportionate levels of spatial cognitive impairment relative to AD patients without vestibular loss, giving rise to the hypothesis that vestibular loss may contribute to a “spatial” subtype of AD (Wei, Oh, Harun, Ehrenburg, & Agrawal, 2017b). Impairment in spatial cognitive ability in both aging and neurodegeneration has profound clinical and functional impacts, specifically by increasing the risk of falls, institutionalization, and mortality. Evidence is mounting that loss of vestibular sensory function contributes to spatial cognitive impairment in cognitively-normal and impaired older adults. This is important given that effective therapies to treat vestibular loss exist, and deploying these therapies may have substantial clinical and public health impact. In this report, we briefly review the salient literature linking vestibular function and cognition, and outline the key research questions that need to be addressed to move the field forward.
Studies in animal models have long shown that effective spatial memory and navigation requires vestibular input. Bilateral vestibular lesion in rats resulted in aberrant activity of the rats’ place cells,(Stackman, Clark, & Taube, 2002) a population of neurons in the hippocampus that are thought to maintain the brain’s cognitive map of space (O’keefe & Nadel, 1978). Place cells are part of a complex neural network involved in spatial encoding that also includes grid cells in the entorhinal cortex (Cullen & Taube, 2017; Stackman et al., 2002). Theta rhythm, which is important for the coordination of place cell and grid cell firing, has also been demonstrated to be dysfunctional in both the hippocampus and the entorhinal cortex in rats with bilateral vestibular ablation (Aitken, Zheng, & Smith, 2017). Consistent with the electrophysiological data, behavioral studies in rats have shown that peripheral vestibular lesion results in impaired spatial memory and navigation as measured by performance on a food foraging task (Baek, Zheng, Darlington, & Smith, 2010). More recent studies in mice provide insight into which specific component of the vestibular system may be involved in spatial cognition. The vestibular organ consists of three orthogonally oriented semicircular canals that detect head rotations in the plane of the canals, and two otolith organs (the saccule and utricle) whose neuroepithelial membrane is covered with a layer of microscopic stones (otoconia) which sense the orientation of the head with respect to gravity (Goldberg et al., 2012). A recent study in otoconia-deficient tilted mice showed that the place cells in these mice lacked spatial specificity relative to wild-type mice,(Harvey et al., 2018) suggesting the particular importance of otolith input to the neural circuits involved in spatial cognition.
In recent years, studies in humans using structural and functional neuro-imaging and behavioral assays are providing compelling evidence that vestibular sensory inputs are critical for human spatial cognitive abilities as well. A landmark study in patients with Neurofibromatosis 2 (NF2) who underwent bilateral vestibular nerve section observed that the NF2 patients had significantly poorer spatial navigation skills as well as reduced hippocampal volumes compared to matched controls (Brandt et al., 2005). Subsequent studies have documented impaired spatial memory and navigation as well as hippocampal atrophy in patients with vestibular disorders such as Menière’s disease (Kremmyda et al., 2016; Popp et al., 2017). Progressive loss of vestibular function occurs with age, and several large epidemiologic studies of US adults have shown that age-related vestibular impairment is associated with an increased odds specifically of poorer spatial cognitive ability (and not an increased odds of poorer language or verbal memory skills) as measured by neurocognitive tests (see Box 1 for summary of studies) (Bigelow, Semenov, du Lac, Hoffman, & Agrawal, 2015; Bigelow et al., 2015; Semenov, Bigelow, Xue, Lac, & Agrawal, 2015). A further study in over 100 healthy adults found that poorer vestibular function was associated with significantly reduced hippocampal volume (Kamil, Jacob, Ratnanather, Resnick, & Agrawal, 2018). Hippocampal atrophy may represent the neuroanatomic correlate of poorer spatial cognitive ability associated with vestibular impairment, and may also underlie the link between vestibular loss and AD, given the primacy of hippocampal atrophy to the pathology of AD.
Box 1.
Summary of 3 US population-based studies reporting a significant association between reduced vestibular function and poorer cognitive performance.
| Study population | Year | N | Finding |
|---|---|---|---|
| Baltimore Longitudinal Study of Aging (BLSA) | 2013–2014 | 183 | Reduced saccular function associated with significantly poorer scores on neurocognitive tests of visuospatial ability in cross-sectional analyses; no significant relationship between saccular function and tests of language, executive function, and verbal memory |
| National Health and Nutrition Examination Survey (NHANES) | 1999–2002 | 1303 | Vestibular impairment (based on modified Romberg test) associated with significantly poorer Digit Symbol Subsitution Score in cross-sectional analyses |
| National Health Interview Survey (NHIS) | 2008 | 20,950 | Vestibular vertigo (based on questionnaire responses) significantly associated with 4-fold increased odds of “difficulty remembering and confusion” in cross-sectional analyses |
Given growing evidence that vestibular function contributes to cognitive function, further studies have investigated whether vestibular loss increases the odds of clinical syndromes of cognitive impairment, notably mild cognitive impairment (MCI) and AD. The peripheral vestibular system provides major inputs to cholinergic neurons in the medial temporal region, which are specifically degraded in AD, giving rise to the hypothesis that loss of vestibular input contributes to the degradation of these neurons and thus plays a causal role in the pathogenesis of AD (Previc, 2013). A recent study evaluated the prevalence of vestibular impairment in ~50 patients with AD and found that vestibular impairment was two-fold more common in AD patients (~50% prevalence) relative to cognitively-normal age-matched controls (~25%) (Harun, Oh, Bigelow, Studenski, & Agrawal, 2016). The reason for the higher prevalence of vestibular loss in AD patients is unclear. It is possible that AD pathology (e.g. β-amyloid (Aβ)) accumulates in central vestibular pathways, thereby impairing vestibular physiologic function. However, a recent study of ~100 participants from the Baltimore Longitudinal Study of Aging did not find a significant association between vestibular function and Aβ deposition (Kamil, Bilgel, Resnick, & Agrawal, 2018). Alternatively, vestibular impairment may directly contribute to medial temporal neurodegeneration and AD, possibly due to reduced vestibular input and consequent degeneration of central pathways, as is known to occur in the auditory system (Cui et al., 2012).
Further studies have explored whether vestibular impairment is associated with specific phenotypes of cognitive deficits – notably spatial cognitive deficits – in patients with AD. There is increasing recognition that AD may be a heterogeneous condition with multiple phenotypes, some characterized by predominantly amnestic features while others are characterized by greater motoric and spatial impairment (Mapstone, Steffenella, & Duffy, 2003). One study of 50 patients with MCI or AD found that patients with vestibular impairment were significantly more likely to have deficits in neurocognitive tests of spatial skills such as the Money Road Map test. Moreover, when patients were stratified into “spatially normal” vs. “spatially impaired” groups based on their performance on the Money Road Map test, 25% of spatially normal patients had vestibular impairment compared to 96% of spatially impaired patients (Wei et al., 2017b). Another study of 60 patients with MCI or AD observed that patients with vestibular impairment were significantly more likely to have difficulty driving, which is an activity closely linked to spatial ability (Wei, Oh, Harun, Ehrenburg, & Agrawal, 2017a). Vestibular impairment may contribute to a “spatial” subtype of AD, and may increase the risk of highly morbid symptoms in AD such as spatial disorientation, wandering, and increased fall risk that occur in a subset of patients.
As noted previously, the vestibular system consists of five discrete end-organs: three semicircular canals involved in detecting head rotation and two otolith organs (the saccule and the utricle) which detect head orientation with respect to gravity. Interestingly, most of the studies reviewed above in both cognitively-normal and impaired adults specifically demonstrated a link between saccular function and cognition. Reduced saccular function was found to have the strongest association with spatial ability in healthy older adults. Moreover, patients with AD were noted to have specifically poorer saccular and utricular function relative to age-matched controls (Harun et al., 2016). The saccule is the vestibular end-organ involved in detecting the orientation of the head with respect to gravity. As noted previously, otolith deficient tilted mice were shown to have aberrant hippocampal place cell activity (Harvey et al., 2018). In human fMRI and EEG studies, saccular stimulation has been shown to activate the multisensory vestibular cortex involved in spatial processing (Kammermeier, Singh, Noachtar, Krotofil, & Bötzel, 2015; Miyamoto, Fukushima, Takada, WAELE, & VIDAL, 2005; Schlindwein et al., 2008). The saccule is thought to play a pre-eminent role in the orientation and encoding of space and appears to have particular relevance for spatial cognitive function.
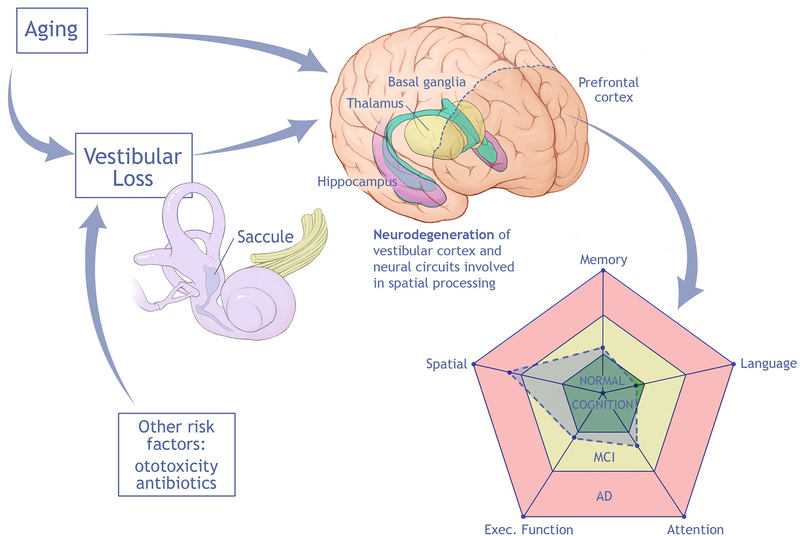
This review highlights recent evidence of a link between vestibular impairment, cognitive decline, and AD, and specifically a “spatial” subtype of AD characterized by spatial disorientation and impaired spatial navigation (e.g. driving difficulty). Loss of vestibular (specifically saccular) input to spatial processing networks centered on the hippocampus appears to be a mechanism behind this link. A conceptual model summarizing these data is depicted in Figure 1. Further establishing and understanding the contribution of vestibular impairment to cognitive decline has the potential for substantial impact to the health of older adults, given that effective therapies are available to treat vestibular loss (Hillier & McDonnell, 2016). The mainstay of treatment for vestibular impairment is vestibular rehabilitation, which is a set of physical therapy-based exercises involving head movements that stimulate the vestibular system and over time foster adaptation and compensation for vestibular loss (Herdman & Clendaniel, 2014). Given that vestibular impairment may represent a modifiable risk factor for cognitive decline and AD, the impact of vestibular loss on cognition should be considered a critical new area of investigation. Currently, key knowledge gaps include:
Figure 1.
Conceptual model of impact of aging on vestibular function (notably saccular function), which contributes to neurodegeneration of neural circuits involved in vestibular processing and deterioration specifically in spatial cognitive ability.
Does vestibular sensory loss, which commonly occurs with age but also from exposure to other risk factors (e.g. ototoxic medications, infections), cause cognitive decline and AD? Careful prospective studies of vestibular function and cognitive outcomes, with rigorous consideration of potential confounding factors such as hearing and vision loss, will be needed to answer this fundamental question.
What are the mechanisms by which vestibular loss may result in cognitive impairment? Studies in animal models will be required to further elucidate the multiple central nervous system targets of vestibular input involved in cognitive (notably spatial cognitive) processes, including the thalamic head direction system, hippocampus and entorhinal cortex, and also the striatum and prefrontal cortex. Peripheral vestibular lesion studies in animal models will be critical to determine the timing and occurrence of central effects across these structures. Moreover, structural and functional neuroimaging studies in humans will be needed to define the homologous CNS networks involved in human vestibular processing, and how these networks might overlap with known sites of AD neuropathology.
Are current vestibular therapies or novel variants effective as symptomatic or even disease-modifying interventions against cognitive (specifically spatial cognitive) impairment? Controlled clinical trials of vestibular therapy in cognitively-normal and cognitively-impaired populations will be needed to determine whether vestibular impairment is a modifiable risk factor for cognitive decline, for which very few effective treatments currently exist.
Footnotes
The authors report no conflict of interest.
References
- Aitken P, Zheng Y, & Smith PF (2017). The modulation of hippocampal theta rhythm by the vestibular system. Journal of Neurophysiology, 119(2), 548–562. [DOI] [PubMed] [Google Scholar]
- Baek JH, Zheng Y, Darlington CL, & Smith PF (2010). Evidence that spatial memory deficits following bilateral vestibular deafferentation in rats are probably permanent. Neurobiology of Learning and Memory, 94(3), 402–413. [DOI] [PubMed] [Google Scholar]
- Bigelow RT, Semenov YR, du Lac S, Hoffman HJ, & Agrawal Y (2015). Vestibular vertigo and comorbid cognitive and psychiatric impairment: The 2008 national health interview survey. Journal of Neurology, Neurosurgery & Psychiatry, , 310319. [DOI] [PubMed] [Google Scholar]
- Bigelow RT, Semenov YR, Trevino C, Ferrucci L, Resnick SM, Simonsick EM, … Agrawal Y (2015). Association between visuospatial ability and vestibular function in the baltimore longitudinal study of aging. Journal of the American Geriatrics Society, 63(9), 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt T, Schautzer F, Hamilton DA, Brüning R, Markowitsch HJ, Kalla R, … Strupp M (2005). Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain, 128(11), 2732–2741. [DOI] [PubMed] [Google Scholar]
- Cui B, Zhu L, She X, Wu M, Ma Q, Wang T, … An G (2012). Chronic noise exposure causes persistence of tau hyperphosphorylation and formation of NFT tau in the rat hippocampus and prefrontal cortex. Experimental Neurology, 238(2), 122–129. [DOI] [PubMed] [Google Scholar]
- Cullen KE, & Taube JS (2017). Our sense of direction: Progress, controversies and challenges. Nature Neuroscience, 20(11), 1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM, Wilson VJ, Angelaki DE, & Cullen KE (2012). The vestibular system: A sixth sense Oxford University Press. [Google Scholar]
- Harun A, Oh ES, Bigelow RT, Studenski S, & Agrawal Y (2016). Vestibular impairment in dementia. Otology & Neurotology, 37(8), 1137–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey RE, Rutan SA, Willey GR, Siegel JJ, Clark BJ, & Yoder RM (2018). Linear self-motion cues support the spatial distribution and stability of hippocampal place cells. Current Biology, 28(11), 1810. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman SJ, & Clendaniel R (2014). Vestibular rehabilitation FA Davis. [Google Scholar]
- Hillier S, & McDonnell M (2016). Is vestibular rehabilitation effective in improving dizziness and function after unilateral peripheral vestibular hypofunction? an abridged version of a cochrane review. Eur J Phys Rehabil Med, 52(4), 541–556. [PubMed] [Google Scholar]
- Hitier M, Besnard S, & Smith PF (2014). Vestibular pathways involved in cognition. Front Integr Neurosci, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil RJ, Bilgel M, Resnick SM, & Agrawal Y (2018). Vestibular function and brain beta-amyloid deposition in the baltimore longitudinal study of aging. In Review [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamil RJ, Jacob AJ, Ratnanather JT, Resnick SM, & Agrawal Y (2018). Vestibular function and hippocampal volume in the baltimore longitudinal study of aging (BLSA). Otol Neurotol, In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammermeier S, Singh A, Noachtar S, Krotofil I, & Bötzel K (2015). Intermediate latency evoked potentials of cortical multimodal vestibular areas: Acoustic stimulation. Clinical Neurophysiology, 126(3), 614–625. [DOI] [PubMed] [Google Scholar]
- Kremmyda O, Hüfner K, Flanagin VL, Hamilton DA, Linn J, Strupp M, … Brandt T (2016). Beyond dizziness: Virtual navigation, spatial anxiety and hippocampal volume in bilateral vestibulopathy. Frontiers in Human Neuroscience, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Steffenella TM, & Duffy CJ (2003). A visuospatial variant of mild cognitive impairment getting lost between aging and AD. Neurology, 60(5), 802–808. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Fukushima K, Takada T, WAELE C, & VIDAL P (2005). Saccular projections in the human cerebral cortex. Annals of the New York Academy of Sciences, 1039(1), 124–131. [DOI] [PubMed] [Google Scholar]
- O’keefe J, & Nadel L (1978). The hippocampus as a cognitive map Clarendon Press Oxford. [Google Scholar]
- Popp P, Wulff M, Finke K, Rühl M, Brandt T, & Dieterich M (2017). Cognitive deficits in patients with a chronic vestibular failure. Journal of Neurology, 264(3), 554–563. [DOI] [PubMed] [Google Scholar]
- Previc FH (2013). Vestibular loss as a contributor to alzheimerā€™ s disease. Medical Hypotheses, 80(4), 360–367. [DOI] [PubMed] [Google Scholar]
- Schlindwein P, Mueller M, Bauermann T, Brandt T, Stoeter P, & Dieterich M (2008). Cortical representation of saccular vestibular stimulation: VEMPs in fMRI. NeuroImage, 39(1), 19–31. [DOI] [PubMed] [Google Scholar]
- Semenov YR, Bigelow RT, Xue Q, Lac S. d., & Agrawal Y (2015). Association between vestibular and cognitive function in US adults: Data from the national health and nutrition examination survey. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences, 71(2), 243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, & Taube JS (2002). Hippocampal spatial representations require vestibular input. Hippocampus, 12(3), 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EX, Oh ES, Harun A, Ehrenburg M, & Agrawal Y (2017a). Saccular impairment in alzheimer’s disease is associated with driving difficulty. Dementia and Geriatric Cognitive Disorders, 44(5–6), 294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei EX, Oh ES, Harun A, Ehrenburg M, & Agrawal Y (2017b). Vestibular loss predicts poorer spatial cognition in patients with alzheimer’s disease. Journal of Alzheimer’s Disease, , 1–9. [DOI] [PubMed] [Google Scholar]