Abstract
Objective
Obesity, defined by body mass index (BMI, kg/m2), is associated with lower mortality risk in numerous chronic disease states, a phenomenon termed the obesity paradox. Indices of obesity, including BMI and % body fat are confounded by muscle mass, while DXA-derived fat mass index (kg/m2) is not. We compared the associations between obesity and mortality in persons with chronic kidney disease (CKD) using several estimates of obesity, including fat mass index, and determined whether muscle mass, inflammation and weight loss modify these associations.
Design
Retrospective cohort study
Setting and Subjects
2,852 NHANES participants from 1999–2006, aged 20 years or more with body composition measures and CKD defined as estimated glomerular filtration rate <60 ml/min/1.73m2 or urine albumin:creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.
Intervention
Obesity defined using BMI and established DXA fat mass index and % body fat cut-offs
Main Outcome Measure
All-cause mortality
Results
In adjusted proportional hazards (Cox) regression models, obesity based on fat mass index was associated with lower mortality (HR 0.82, 95% CI, 0.70–0.97). As continuous variables, higher fat mass index, BMI and % body fat were associated with lower mortality. The apparent protective association of obesity was less pronounced among persons with higher lean (muscle) mass. The prevalence of >10% weight loss was 20% in participants that were obese by fat mass index, compared with 40% in the remainder. Prior weight loss was associated with mortality, and the protective association of obesity was no longer significant after adjustment for prior weight loss. Inflammation did not modify these associations.
Conclusions
An apparent protective association of high fat mass was seen in chronic kidney disease, particularly among persons with lower muscle mass. The prevalence of prior weight loss was two-fold lower among obese compared to non-obese persons, confounding these associations.
Keywords: Adiposity, kidney, mortality, inflammation, muscle
INTRODUCTION
Studies in end stage renal disease (ESRD)1–6, chronic kidney disease (CKD)7,8 and numerous other disease states9,10 demonstrated that obesity, defined by Quételet’s (body mass) index (BMI, kg/m2) conferred a survival advantage. This phenomenon has been termed the “obesity paradox.” ESRD11–13 and CKD14 are associated with sarcopenia. In this context, measures confounded by muscle mass (BMI and % Body Fat (%BF)) may fail to reveal the independent association between excess adiposity and mortality. Dual-energy X-ray absorptiometry (DXA)- derived fat mass index (FMI, kg/m2) overcomes the limitations of %BF and BMI.15–18
Further analyses of the obesity paradox in patients on hemodialysis demonstrated that inflammation modifies the association between BMI and mortality; the higher mortality rate among patients with lower BMI was only observed among patients with evidence of inflammation19. A potential explanation for this observation is that patients of excess weight have maintained or gained weight throughout their illness, while those with low weight and adiposity might have experienced illness-related weight loss due to the severity of their illness. Weight loss in excess of 5% of body weight over an average 5.7 years was seen in 20% of patients with CKD starting dialysis and was associated with mortality.20 It remains unclear to what degree inflammation and/or illness-related weight loss modify the association between obesity and mortality in CKD.
In the current study, we employed DXA measures of fat mass from the National Health and Nutrition Examination Survey (NHANES) 1999–2006 to determine the associations between obesity/adiposity and mortality in NHANES participants with CKD. We further assessed whether muscle mass, inflammation, and/or weight loss confounded or modified these associations. We hypothesized that higher FMI would be associated with lower mortality, but that adjustment for weight loss would attenuate the association. That is, the apparent protective association is due to the ability to maintain weight, not to adiposity itself. Further, persons with evidence of low muscle mass (sarcopenia) and systemic inflammation would be most protected by adiposity. BMI is higher with higher muscle mass, while %BF is lower with higher muscle mass; therefore, we expect higher BMI to be associated with lower mortality, and higher %BF with higher mortality.
METHODS
Study population
We examined data from NHANES, a nationally representative study of the non-institutionalized, U.S. civilian population using a complex, multistage probability sampling method including oversampling of Non-Hispanic Blacks and Hispanics to produce reliable race- and ethnicity-specific statistics. The current study was limited to years 1999–2006 as these years contained DXA derived body composition data. We included all 2,852 participants with CKD aged 20 years or more with body composition measures. CKD was defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 or urine albumin: creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.21
Measures of Fat and Muscle Mass
Whole body DXA scans were acquired using Hologic QDR 4500A fan-beam densitometers (Hologic, Inc, Bedford, MA) in participants 8 years of age and older. DXA exclusion criteria included pregnancy, weight >300 pounds (136 kg, due to the weight limit of the scanner), height >77 inches (195 cm), recent nuclear medicine scan, or exposure to radioactive contrast. To account for potential biases of non-random missing data, multiple imputation was performed by the National Center for Health Statistics for all participants with invalid or missing data (with the exception of pregnant women) using demographic, socioeconomic, and geographic variables, body measurements, health indicators, dietary and medication use variables, and blood test results.22,23
DXA-derived body composition measures included total body fat mass indexed to height2 (FMI, kg/m2) and skeletal muscle mass approximated using appendicular lean mass indexed to height2 (ALMI, kg/m2). We used previously published sex- and race/ethnicity-specific Lambda, Mu, Sigma (LMS) curves24 to convert ALMI and FMI results to sex- and race/ethnicity-specific Z-scores relative to age and T-scores based on LMS values in a 25 year old. The LMS method25,26 is the standard method for expressing body composition results as standard deviation score.
To define obesity based on FMI (obeseFMI) we used sex- and race/ethnicity specific FMI cut point values developed by Kelly, et al. that correlate to the same prevalence of obesity as a BMI cut point of 30 kg/m2 in 25year-old NHANES participants.24 The values range from an FMI of 8.1 kg/m2 in black men to 12.9 kg/m2 in white women. For comparative purposes, we also defined obesity as BMI ≥ 30 kg/m2 (ObeseBMI) and %BF > 42.1% in women and > 29.6% in men (Obese%BF).27,28
The BMI was initially developed in the 19th century; the indexing of weight to height squared. However, the correlation of BMI with height could bias its validity when comparing populations of variable stature. Therefore, we also calculated Benn’s Index, weight/height p, where the exponent p is calculated within sex and race groups, such that index no longer correlated with height, and conducted analyses in parallel with those using BMI.29
Kidney Disease and Co-morbidities
Laboratory assays for serum creatinine, C-reactive protein (CRP), and albumin, and urine albumin and creatinine were standardized and calibrated in accordance with established methods.22 Serum creatinine values from 1999 to 2000 were calibrated to the Cleveland Clinic laboratory standard by multiplying by 1.013 and then adding 0.147.22 Estimated GFR was calculated using the age-, sex- and race- (Black versus non-Black) specific Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.30,31
We defined a state of heightened Inflammation by serum C-reactive protein (CRP) ≥3.0 mg/L or albumin < 3.5 g/dL. Percent weight loss was defined as percent difference in weight between the maximum self-reported weight and current measured weight.32 The elapsed time period of weight loss is not accounted for in this variable.
Smoking status was defined by self-report of smoking >100 cigarettes/lifetime. Diabetes was defined by an affirmative response to whether the participant had been told by a doctor that he or she had “diabetes or borderline diabetes” while not pregnant, the current use of insulin or oral hypoglycemic medications, or a glycohemoglobin >6.5%. Cardiovascular disease was defined by self-report of a physician diagnosis of congestive heart failure, coronary heart disease, angina, myocardial infarction, or stroke. Cancer was defined by an affirmative response to the question has a doctor or medical professional “ever told you had cancer or malignancy?” Liver disease was defined by an affirmative response to “Have you ever been told that you had any liver condition?”
Leisure time physical activity information was obtained and given a MET score according to the Compendium of Physical Activities33. Participants were categorized as meeting the minimum goal (<450 MET/min/week), more than meeting the minimum goal (450 to <750 MET/min/week) and exceeding the recommended goal (≥750 MET/min/week).34
Mortality
The National Center for Health Statistics has linked mortality data from NHANES to death certificate data in the National Death Index (NDI) with follow-up through December 31, 2011. The NDI matches individuals on personal and demographic criteria, such as social security number and date of birth, and provides mortality status and months of follow-up.35
Statistical Analysis
The study population was characterized using descriptive weighted statistics with categorical characteristics summarized as counts/percentages and continuous characteristics summarized as mean with standard error or median with 25th, 75th percentile range.
We tested the associations between different measures of obesity (FMI, BMI, %BF, Benn’s index, obeseFMI, obeseBMI and obese%BF) using unadjusted and adjusted multivariable proportional hazards (Cox) regression analyses. Adjusted models included demographics (Model 1), demographics, diabetes and cardiovascular disease (Model 2) and all variables determined to be significantly associated with mortality on univariate screen (Model 3). We then tested whether weight loss was a confounder in these associations by adding percent weight loss as a categorical variable (none or minimal weight loss, 5–10% weight loss, or >10% weight loss) to Model 3 to generate Model 4.36 We tested for non-linearity of these associations by adding a quadratic term to all adjusted models with continuous exposures (i.e., adjusted FMI models additionally for FMI2 term). Finally, we repeated Model 3 with additional adjustment for eGFR.
To determine whether the association of FMI with mortality was different among persons with lower muscle mass, we evaluated for effect modification by separately testing the significance of an FMI x ALMI multiplicative interaction term and stratifying by ALMI T-score ≥ or < 0 in the multivariable model.
To determine whether the association of FMI with mortality differed by the presence of systemic inflammation, we tested the significance of the FMI x inflammatory status (as defined above), FMI x continuous serum albumin, and FMI x continuous serum CRP interaction terms in the multivariable model. We also stratified by serum albumin ≥ or < 3.5 g/dL in the multivariable model.
We considered 2-tailed p-values <0.05 statistically significant. We performed all analyses using survey procedures with SAS version 9.4 for Unix (SAS Institute, Cary, North Carolina) to account for the complex sampling design of NHANES and appropriately weighted participants in statistical models.
RESULTS
Participant Characteristics
Participant characteristics organized by obeseFMI, obeseBMI and obese%BF status are shown in Table 1. Regardless of the definition, obese patients were older, less physically active, had lower serum albumin, and higher serum CRP concentrations, and were more likely to have diabetes and cardiovascular disease. Obese%BF participants had the lowest mean FMI and ALMI compared to persons with obeseFMI and obeseBMI. Overall, persons with obeseBMI had the highest mean ALMI and FMI.
Table 1.
Characteristics of 2,852 chronic kidney disease participants of the National Health and Nutrition Examination Survey 1999–2006
| Obese FMI |
Non-Obese FMI |
Obese BMI |
Non-Obese BMI |
Obese %BF |
Non-Obese %BF |
|
|---|---|---|---|---|---|---|
| N (Unweighted) | 1326 | 1526 | 1134 | 1712 | 1526 | 1320 |
| Age (median, 25th, 75th percentile) | 59 (47–70) | 57 (40–72) | 57 (45–68) | 61 (42–73) | 63 (49–72) | 53 (37–69) |
| Female | 47.8% | 54.4% | 50.2% | 52.3% | 47.5% | 55.7% |
| Mexican American | 7.3% | 8.0% | 8.2% | 7.3% | 7.3% | 8.1% |
| >750 | 10.8% | 17.8% | 11.3% | 17.0% | 10.7% | 19.0% |
| Smoker | 53.0% | 56.6% | 50.9% | 57.5% | 53.7% | 56.1% |
| Diabetes | 35.5% | 15.6% | 36.0% | 16.8% | 31.7% | 16.7% |
| Cancer | 12.2% | 14.1% | 11.1% | 14.7% | 14.2% | 12.1% |
| CVD | 22.7% | 19.1% | 21.4% | 20.2% | 24.1% | 17.0% |
| Liver Disease | 7.3% | 5.9% | 6.8% | 6.4% | 7.7% | 5.2% |
| 12+ years | 69.4% | 68.9% | 69.8% | 68.6% | 68.8% | 69.5% |
| $75,000 + | 17.2% | 15.7% | 17.8% | 15.5% | 14.8% | 18.1% |
|
Vitamin D Deficiency (<50 nmol/L) |
57.5% | 45.7% | 57.5% | 46.6% | 54.3% | 47.3% |
| >10% | 20.2% | 40.4% | 17.7% | 40.6% | 22.8% | 40.8% |
| Laboratory Values | ||||||
|
Serum Albumin (g/dL) |
4.16 ± 0.01 | 4.31 ± 0.01 | 4.17 ± 0.01 | 4.30 ± 0.01 | 4.18 ± 0.01 | 4.32 ± 0.01 |
|
Low Serum Albumin (<3.5 g/dl) |
2.6% | 1.9% | 2.6% | 1.9% | 2.5% | 1.8% |
|
Serum CRP (mg/dL) |
0.77 ± 0.04 | 0.49 ± 0.04 | 0.79 ± 0.04 | 0.50 ± 0.04 | 0.71 ± 0.03 | 0.51 ± 0.05 |
|
Elevated Serum CRP (≥3.0 mg/L) |
2.6% | 2.6% | 2.7% | 2.5% | 2.9% | 2.3% |
| Cystatin C eGFR | 61 ± 1.1 | 67 ± 1.3 | 62 ± 1.2 | 66 ± 1.2 | 61 ± 1.0 | 69 ± 1.3 |
| Creatinine eGFR | 76 ± 0.9 | 78 ± 0.9 | 78 ± 1.0 | 77 ± 0.8 | 74 ± 0.9 | 80 ± 0.9 |
|
Serum Bicarbonate (mEq/L) |
24.10 ± 0.12 | 24.35 ± 0.14 | 24.03 ± 0.12 | 24.38 ± 1.14 | 24.19 ± 0.12 | 24.29 ± 0.14 |
| Body Composition | ||||||
| Female | 36.36 ± 0.34 | 24.20 ± 0.20 | 36.90 ± 0.32 | 24.27 ± 0.19 | 34.48 ± 0.33 | 24.39 ± 0.25 |
| Female | 17.19 ± 0.22 | 9.08 ± 0.13 | 17.33 ± 0.23 | 9.27 ± 0.14 | 16.28 ± 0.20 | 8.90 ± 0.15 |
| FMI T-score | 1.35 ± 0.02 | −0.07 ± 0.03 | 1.37 ± 0.02 | 0.02 ± 0.03 | 1.22 ± 0.02 | −0.15 ± 0.03 |
| FMI Z-score | 1.08 ± 0.03 | −0.63 ± 0.03 | 1.12 ± 0.03 | −0.54 ± 0.02 | 0.88 ± 0.03 | −0.69 0.03 |
| Female | 47.00 ± 0.21 | 36.91 ± 0.31 | 46.59 ± 0.25 | 37.52 ± 0.32 | 46.79 ± 0.16 | 35.81 ± 0.31 |
| Benn’s Index | 39.05 ± 0.32 | 27.59 ± 0.15 | 40.21 ± 0.33 | 27.64 ± 0.14 | 37.30 ± 0.33 | 27.72 ± 0.20 |
| Female | 7.86 ± 0.09 | 6.00 ± 0.04 | 8.06 ± 0.07 | 5.94 ± 0.04 | 7.40 ± 0.08 | 6.19 ± 0.06 |
| ALMI T-score | 0.59 ± 0.04 | −0.65 ± 0.03 | 0.87 ± 0.04 | −0.75 ± 0.03 | 0.26 ± 0.05 | −0.48 ± 0.04 |
| ALMI Z-score | 0.80 ± 0.04 | −0.45 ± 0.04 | 1.04 ± 0.04 | −0.52 ± 0.03 | 0.51 ± 0.05 | −0.32 ± 0.04 |
Data is presented as weighted mean +/− standard error or weighted % unless otherwise stated.
Group categorization is not exclusive; i.e. there is some overlap between groups. Chronic kidney disease was defined as eGFR <60 ml/min/1.73m2 or urine albumin: creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.
Participants in non-obese groups were more likely to have experienced significant weight loss compared to obese groups. For example, > 10% weight loss was observed in 40.4% of patient who were not obese by FMI, compared with 20.2% of those who were obese by FMI.
Association Between Obesity and Mortality
Table 2 shows adjusted and unadjusted associations between obesity measures (obeseFMI, obeseBMI, obese%BF) and mortality. In unadjusted analyses, relationships between definitions of obesity and mortality varied. ObeseFMI and obeseBMI, were associated with lower mortality risk, although only obeseBMI reached statistical significance. Obese%BF was associated with higher mortality.
Table 2.
Hazard Ratios and 95% confidence intervals for the associations of mortality with estimates of adiposity in 2,852 chronic kidney disease participants of the National Health and Nutrition Examination Survey 1999–2006
| Categorical Indices of Obesity | Continuous Measures | ||||||
|---|---|---|---|---|---|---|---|
| ObeseFMI | ObeseBMI | Obeseo%BF | FMI | BMI | %BF | Benn’s Index |
|
| Unadjusted | 0.93 (0.77–1.12) p=0.41 |
0.76 (0.60–0.95) p=0.02 |
1.22 (0.99–1.49) p=0.05 |
0.98 (0.96–1.00) p=0.09 |
0.98 (0.97–0.99) p=0.02 |
0.99 (0.99–1.01) p=0.69 |
0.99 (0.97–0.99) p=0.01 |
| Model 1 | 0.91 (0.77–1.08) p=0.28 |
0.91 (0.73–1.14) p=0.40 |
0.92 (0.77–1.10) p=0.38 |
0.99 (0.96–1.02) p=0.39 |
0.98 (0.97–1.01) p=0.36 |
0.99 (0.97–1.00) p=0.15 |
0.99 (0.98–1.01) p=0.26 |
| Model 2 | 0.85 (0.72–0.99) p=0.04 |
0.83 (0.66–1.03) p=0.09 |
0.90 (0.75–1.08) p=0.24 |
0.97 (0.95–0.99) p=0.04 |
0.9 (0.96–0.99) p=0.03 |
0.98 (0.97–0.99) p=0.03 |
0.98 (0.97–0.99) p=0.02 |
| Model 3 | 0.82 (0.70–0.97) p=0.02 |
0.81 (0.65–1.01) p=0.06 |
0.89 (0.74–1.08) p=0.23 |
0.97 (0.95–0.99) p=0.03 |
0.98 (0.97–0.99) p=0.03 |
0.98 (0.96–0.99) p=0.03 |
0.98 (0.97–0.99) p=0.01 |
| Model 4 | 1.01 (0.84–1.22) p=0.92 |
1.01 (0.79–1.29) p=0.95 |
1.09 (0.90–1.33) p=0.37 |
1.00 (0.97–1.03) p=0.87 |
1.00 (0.98–1.02) p=0.94 |
1.00 (0.98–1.02) p=0.99 |
0.99 (0.98–1.01) p=0.75 |
Chronic kidney disease was defined as eGFR <60 ml/min/1.73m2 or urine albumin: creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.
Model 1: Adjusted for age, sex and race/ethnicity. Model 2: Covariates included in Model 1 with further adjustment for diabetes and cardiovascular disease. Model 3: Covariates included in Model 2 with further adjustment for cancer, physical activity, income, and education. Model 4: Covariates included in Model 3 with further adjustment for weight loss.
Table 2 shows results of multivariable models. Age, sex, race/ethnicity, diabetes, cardiovascular disease, liver disease, cancer, physical activity, income, and education were all significantly associated with mortality in univariate analyses and included in models 3 and 4. After adjustment for demographics, diabetes and cardiovascular disease (Model 2) higher FMI, BMI, %BF, Benn’s Index and obeseFMI were significantly associated with lower mortality and remained “protective” after additional adjustment for liver disease, cancer, physical activity, income, and education (Model 3). ObeseBMI and obese%BF were associated with numerically but not significantly lower mortality. Additional testing for non-linearity of the associations of continuous exposures with mortality by adding a quadratic term to model 3 was significant only for BMI suggesting the protective association of higher BMI attenuates at higher BMI. The association between obesity based on FMI and mortality among individuals with CKD was similar after adding eGFR to Model 3 (HR 0.81, 95% CI, 0.69–0.95).
Confounding by Weight Loss
Table 2 (Model 4) shows the associations of mortality with obeseFMI, obeseBMI, obese%BF and respective continuous variables with additional adjustment for percent weight loss categories. Once percent weight loss was accounted for, none of the obesity measures were significantly associated with mortality.
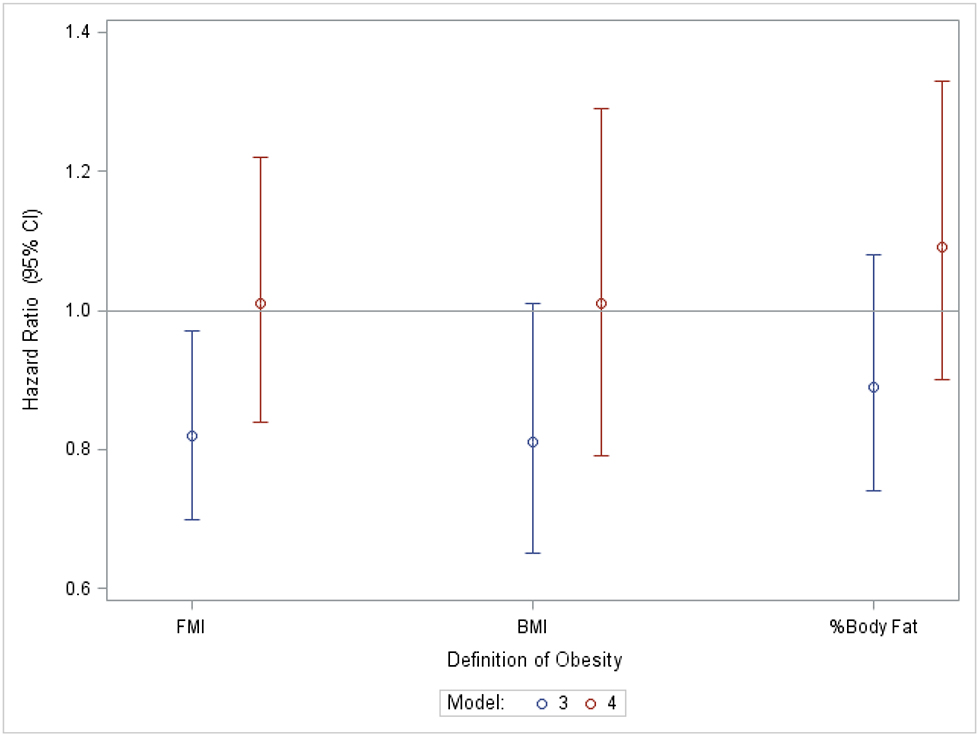
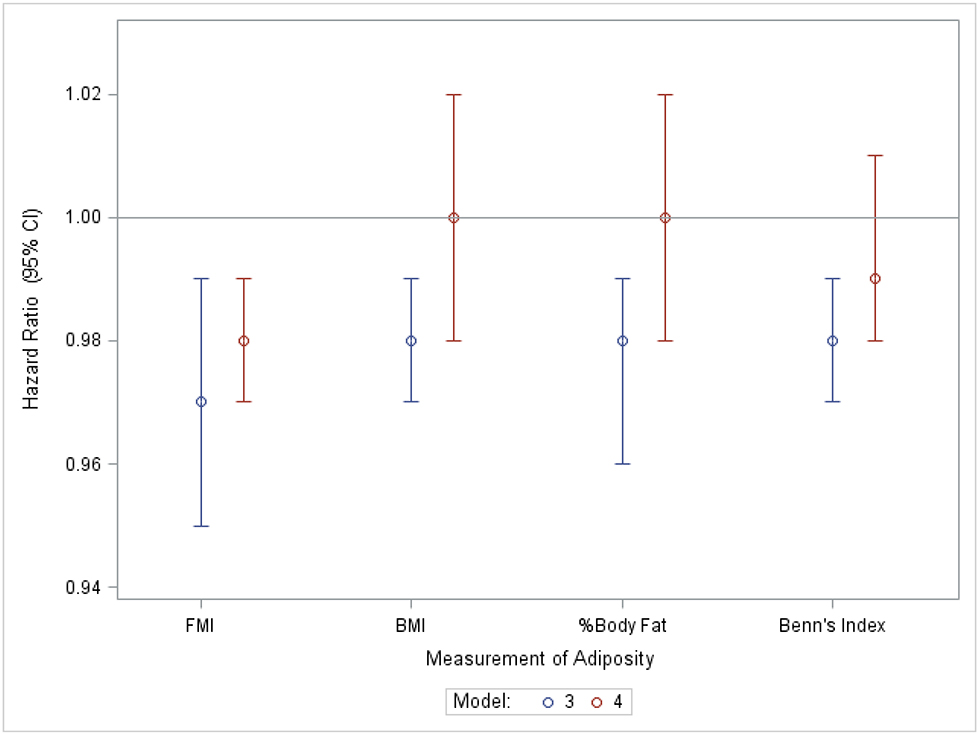
Figure 1 demonstrates the associations of the categorical (Figure 1A) and continuous (Figure 1B) measures of adiposity with mortality in Model 3, and the impact of adjustment for percent weight loss categories (Model 4).
Figure 1A.
Hazard ratios and 95% confidence intervals for mortality in obese vs. non-obese persons with chronic kidney disease before and after adjustment for % weight loss.
Chronic kidney disease was defined as eGFR <60 ml/min/1.73m2 or urine albumin: creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.
Model 3: Adjusted for age, sex, race, physical activity level, smoking status, diabetes, cancer, cardiovascular disease, liver disease, income and education. Model 4: Adjusted for percent weight loss category, age, sex, race, physical activity level, smoking status, diabetes, cancer, cardiovascular disease, and liver disease.
Figure 1B.
Hazard ratios and 95% confidence intervals for mortality by continuous measures of adiposity in persons with chronic kidney disease before and after adjustment for % weight loss.
Chronic kidney disease was defined as eGFR <60 ml/min/1.73m2 or urine albumin: creatinine ratio ≥ 25 mg/g for women and ≥ 17 mg/g for men.
Model 3: Adjusted for age, sex, race, physical activity level, smoking status, diabetes, cancer, cardiovascular disease, liver disease, income and education. Model 4: Adjusted for percent weight loss category, age, sex, race, physical activity level, smoking status, diabetes, cancer, cardiovascular disease, and liver disease.
Effect Modification by Muscle Mass and Inflammation Status
Muscle mass (ALMI) modified the association between each measure of adiposity and mortality (β=0.28, 0.28, 0.21 for obeseFMI, obeseBMI, and obese%BF, respectively; p for obese%BF x ALMI= 0.04; p for all other interactions <0.01). That is, the apparent protective association of obesity was more pronounced among persons with lower muscle mass. This phenomenon was demonstrated in analyses stratified by ALMI T score < 0 or ≥ 0, suggesting a beneficial association of obeseFMI, in the lower ALMI group (HR ALMI T-score <0= 0.70, 95% CI 0.54 to 0.89; HR ALMI T-score ≥ 0= 1.35, 95% CI 0.96 to 1.90).
Tests for the modification of the FMI – mortality association by inflammation status were not significant.
DISCUSSION
Using a direct measure of adiposity independent of muscle mass, higher FMI was associated with lower mortality in NHANES participants with CKD, independent of demographics, comorbidities, and lifestyle covariates. Participants with the lowest muscle mass demonstrated the most pronounced benefit of adiposity. Weight loss, a known poor prognostic indicator, was two-fold more prevalent among non-obese compared to obese NHANES participants, and was associated with mortality. Accordingly, weight loss confounded the association between obesity and mortality, such that the apparent protective association between FMI and mortality was eliminated after adjustment for weight loss.
In the general population, obesity is associated with cardiovascular events and mortality.37 These associations are attributed to the direct effects of obesity, such as increased cholesterol synthesis and insulin resistance as well as the indirect effects of hypertension and progression of diabetes.38 However, obesity has been associated with lower mortality risk among persons with CKD and other chronic diseases.2,10 Numerous potential explanations for the obesity paradox have been proposed. Obesity may represent nutritional reserve and adipose tissue may help sequester uremic toxins in persons with CKD.7 However, the obesity paradox can be alternatively explained by reverse causality; namely, more severe illness results in weight loss, and loss of fat mass confounds these epidemiologic relationships.
Previous studies on the association between higher BMI and mortality in CKD have shown either no association39 or lower mortality risk7,40. These studies are limited by their use of BMI, a measure confounded by muscle mass. Lower muscle mass is associated with mortality in CKD and may explain the observed relation between BMI and mortality. Few studies have used more direct estimates of adiposity to determine the relation between obesity and mortality in CKD. Using NHANES data, two studies explored the relationship of %BF with mortality in persons with CKD. Navaneethan et al. assessed mortality risk per 10% higher%BF, while Androga et al. used sex-specific %BF cutpoints. Neither found an association between %BF and mortality in CKD.41,42 Similarly, these studies were limited by their use of %BF to assess adiposity, a measure that increases with lower muscle mass. Our study similarly found no association of obesity defined by %BF with mortality using sex-specific cutpoints. When assessed as a continuous variable, higher %BF was associated with a slight reduction in mortality risk.
To our knowledge, our study is the first to use a direct estimate of adiposity to assess the association of obesity with mortality in CKD and to assess the impact of weight loss on these findings. Weight loss is associated with mortality in the general population43 and among persons with ESRD44 and CKD20 and was an important confounder in our analyses. Adjustment for weight loss eliminated any apparent protective effect of obesity across all measures.
Our study had several limitations including the measurement of body composition at a single point in time. As such, we cannot infer a causal relation between mortality and altered body composition. Further, percent weight loss was determined using self-reported highest weight and current measured weight. Therefore, exact weight lost and over what timeframe could not be determined. The NHANES study population had a very low prevalence of inflammation based on surrogates of elevated CRP or low albumin limiting the power of our inflammation analyses. Finally, variation in soft tissue hydration seen in chronic diseases states such as CKD may cause errors in DXA fat estimates.45
Strengths of our analysis included the use of FMI and a novel definition to define obesity. We also used multiple measures of obesity including the Benn’s Index that relies on a measured weight and provides a closer approximation of adiposity relative to BMI, because of the correlation between height and BMI which is eliminated when calculating Benn’s index.46
CONCLUSION
In conclusion, patients with CKD and higher fat mass have a better prognosis with regard to mortality, particularly among those with lower muscle mass. Weight loss, a known poor prognostic indicator in CKD, was two-fold more prevalent among non-obese participants compared to obese participants and confounded these associations, suggesting that these epidemiologic associations suffer from reverse-causality as opposed to a biologically protective effect.
PRACTICAL APPLICATION
Widely used definitions of obesity, %BF and BMI, are confounded by muscle mass, which must be considered when interpreting studies using these measures. In clinical practice, providers should consider prior weight loss even in obese patients as this may be a sign of higher mortality risk.
Acknowledgments
Funding
This research was supported by National Institutes of Health grants F32 DK111083 (SZ) and K24 DK076808 (MBL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
REFERENCES
- 1.Johansen KL, Kutner NG, Young B, Chertow GM. Association of body size with health status in patients beginning dialysis. The American journal of clinical nutrition. 2006;83(3):543–549. [DOI] [PubMed] [Google Scholar]
- 2.Vashistha T, Mehrotra R, Park J, et al. Effect of age and dialysis vintage on obesity paradox in long-term hemodialysis patients. Am J Kidney Dis. 2014;63(4):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chazot C, Gassia JP, Di Benedetto A, Cesare S, Ponce P, Marcelli D. Is there any survival advantage of obesity in Southern European haemodialysis patients? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2009;24(9):2871–2876. [DOI] [PubMed] [Google Scholar]
- 4.Park J, Jin DC, Molnar MZ, et al. Mortality predictability of body size and muscle mass surrogates in Asian vs white and African American hemodialysis patients. Mayo Clinic proceedings. 2013;88(5):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kopple JD, Kilpatrick RD, et al. Association of morbid obesity and weight change over time with cardiovascular survival in hemodialysis population. Am J Kidney Dis. 2005;46(3):489–500. [DOI] [PubMed] [Google Scholar]
- 6.Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney international. 1999;55(4):1560–1567. [DOI] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Paradoxical association between body mass index and mortality in men with CKD not yet on dialysis. Am J Kidney Dis. 2007;49(5):581–591. [DOI] [PubMed] [Google Scholar]
- 8.Lu JL, Kalantar-Zadeh K, Ma JZ, Quarles LD, Kovesdy CP. Association of body mass index with outcomes in patients with CKD. Journal of the American Society of Nephrology : JASN. 2014;25(9):2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Costanzo P, Cleland JG, Pellicori P, et al. The obesity paradox in type 2 diabetes mellitus: relationship of body mass index to prognosis: a cohort study. Annals of internal medicine. 2015;162(9):610–618. [DOI] [PubMed] [Google Scholar]
- 10.Oreopoulos A, Padwal R, Kalantar-Zadeh K, Fonarow GC, Norris CM, McAlister FA. Body mass index and mortality in heart failure: a meta-analysis. American heart journal. 2008;156(1):13–22. [DOI] [PubMed] [Google Scholar]
- 11.Kittiskulnam P, Carrero JJ, Chertow GM, Kaysen GA, Delgado C, Johansen KL. Sarcopenia among patients receiving hemodialysis: weighing the evidence. Journal of Cachexia, Sarcopenia and Muscle. 2017;8(1):57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gracia-Iguacel C, Gonzalez-Parra E, Perez-Gomez MV, et al. Prevalence of protein-energy wasting syndrome and its association with mortality in haemodialysis patients in a centre in Spain. Nefrologia : publicacion oficial de la Sociedad Espanola Nefrologia. 2013;33(4):495–505. [DOI] [PubMed] [Google Scholar]
- 13.Lamarca F, Carrero JJ, Rodrigues JC, Bigogno FG, Fetter RL, Avesani CM. Prevalence of sarcopenia in elderly maintenance hemodialysis patients: the impact of different diagnostic criteria. The journal of nutrition, health & aging. 2014;18(7):710–717. [DOI] [PubMed] [Google Scholar]
- 14.Ziolkowski SL J; Baker J; Simard J; Chertow G; Leonard MB. Sarcopenia, Relative Sarcopenia and Excess Adiposity in Chronic Kidney Disease. Journal of Cachexia, Sarcopenia and Muscle- Clinical Reports. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molfino A, Don BR, Kaysen GA. Comparison of bioimpedance and dual-energy x-ray absorptiometry for measurement of fat mass in hemodialysis patients. Nephron Clinical practice. 2012;122(3–4):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Formica C, Atkinson MG, Nyulasi I, McKay J, Heale W, Seeman E. Body composition following hemodialysis: studies using dual-energy X-ray absorptiometry and bioelectrical impedance analysis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 1993;3(4):192–197. [DOI] [PubMed] [Google Scholar]
- 17.Abrahamsen B, Hansen TB, Hogsberg IM, Pedersen FB, Beck-Nielsen H. Impact of hemodialysis on dual X-ray absorptiometry, bioelectrical impedance measurements, and anthropometry. The American journal of clinical nutrition. 1996;63(1):80–86. [DOI] [PubMed] [Google Scholar]
- 18.Stenver DI, Gotfredsen A, Hilsted J, Nielsen B. Body composition in hemodialysis patients measured by dual-energy X-ray absorptiometry. American journal of nephrology. 1995;15(2):105–110. [DOI] [PubMed] [Google Scholar]
- 19.Stenvinkel P, Gillespie IA, Tunks J, et al. Inflammation Modifies the Paradoxical Association between Body Mass Index and Mortality in Hemodialysis Patients. Journal of the American Society of Nephrology : JASN. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ku E, Kopple JD, Johansen KL, et al. Longitudinal Weight Change During CKD Progression and Its Association With Subsequent Mortality. Am J Kidney Dis. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer IH, Astor BC, Kramer H, et al. Mild elevations of urine albumin excretion are associated with atherogenic lipoprotein abnormalities in the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis. 2008;197(1):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper R, Hardy R, Bann D, et al. Body mass index from age 15 years onwards and muscle mass, strength, and quality in early old age: findings from the MRC National Survey of Health and Development. The journals of gerontology Series A, Biological sciences and medical sciences. 2014;69(10):1253–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schenker N, Borrud LG, Burt VL, et al. Multiple imputation of missing dual-energy X-ray absorptiometry data in the National Health and Nutrition Examination Survey. Statistics in medicine. 2011;30(3):260–276. [DOI] [PubMed] [Google Scholar]
- 24.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flegal KM, Cole TJ. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. National health statistics reports. 2013(63):1–3. [PubMed] [Google Scholar]
- 26.Cole TJ. The LMS method for constructing normalized growth standards. European journal of clinical nutrition. 1990;44(1):45–60. [PubMed] [Google Scholar]
- 27.Sharma D, Hawkins M, Abramowitz MK. Association of sarcopenia with eGFR and misclassification of obesity in adults with CKD in the United States. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(12):2079–2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baumgartner RN, Wayne SJ, Waters DL, Janssen I, Gallagher D, Morley JE. Sarcopenic obesity predicts instrumental activities of daily living disability in the elderly. Obesity research. 2004;12(12):1995–2004. [DOI] [PubMed] [Google Scholar]
- 29.Benn RT. Some mathematical properties of weight-for-height indices used as measures of adiposity. British journal of preventive & social medicine. 1971;25(1):42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens LA, Coresh J, Schmid CH, et al. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51(3):395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levey AS, Stevens LA, Schmid CH, et al. A New Equation to Estimate Glomerular Filtration Rate. Annals of internal medicine. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stokes A Using maximum weight to redefine body mass index categories in studies of the mortality risks of obesity. Population health metrics. 2014;12(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and science in sports and exercise. 2000;32(9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 34.Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and science in sports and exercise. 2007;39(8):1423–1434. [DOI] [PubMed] [Google Scholar]
- 35.Fillenbaum GG, Burchett BM, Blazer DG. Identifying a national death index match. American journal of epidemiology. 2009;170(4):515–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hatoum IJ, Kaplan LM. Advantages of percent weight loss as a method of reporting weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring, Md). 2013;21(8):1519–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW, Jr. Body-mass index and mortality in a prospective cohort of U.S. adults. The New England journal of medicine. 1999;341(15):1097–1105. [DOI] [PubMed] [Google Scholar]
- 38.Haslam DW, James WP. Obesity. Lancet (London, England). 2005;366(9492):1197–1209. [DOI] [PubMed] [Google Scholar]
- 39.Madero M, Sarnak MJ, Wang X, et al. Body mass index and mortality in CKD. Am J Kidney Dis. 2007;50(3):404–411. [DOI] [PubMed] [Google Scholar]
- 40.Kwan BC, Murtaugh MA, Beddhu S. Associations of body size with metabolic syndrome and mortality in moderate chronic kidney disease. Clinical journal of the American Society of Nephrology : CJASN. 2007;2(5):992–998. [DOI] [PubMed] [Google Scholar]
- 41.Androga L, Sharma D, Amodu A, Abramowitz MK. Sarcopenia, obesity, and mortality in US adults with and without chronic kidney disease. Kidney international reports. 2017;2(2):201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navaneethan SD, Kirwan JP, Arrigain S, Schold JD. Adiposity measures, lean body mass, physical activity and mortality: NHANES 1999–2004. BMC nephrology. 2014;15:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cornoni-Huntley JC, Harris TB, Everett DF, et al. An overview of body weight of older persons, including the impact on mortality. The National Health and Nutrition Examination Survey I--Epidemiologic Follow-up Study. Journal of clinical epidemiology. 1991;44(8):743–753. [DOI] [PubMed] [Google Scholar]
- 44.Kalantar-Zadeh K, Streja E, Kovesdy CP, et al. The Obesity Paradox and Mortality Associated With Surrogates of Body Size and Muscle Mass in Patients Receiving Hemodialysis. Mayo Clinic proceedings. 2010;85(11):9911001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. The American journal of physiology. 1998;274(5 Pt 1):E808–816. [DOI] [PubMed] [Google Scholar]
- 46.Garn SM, Pesick SD. Comparison of the Benn index and other body mass indices in nutritional assessment. The American journal of clinical nutrition. 1982;36(4):573–575. [DOI] [PubMed] [Google Scholar]