Abstract
Microglial activation followed by neuroinflammation is a defense mechanism of the brain to eliminate harmful endogenous and exogenous materials including pathogens and damaged tissues, while excessive or chronic neuroinflammation may cause or exacerbate neurodegeneration observed in brain injuries and neurodegenerative diseases. Depending on conditions/environments during activation, microglia acquire distinct phenotypes, such as pro-inflammatory, anti-inflammatory, and disease-associated phenotypes, and show their ability to phagocytose various objects and produce pro-and anti-inflammatory mediators. Prevention of excessive inflammation by regulating the microglia’s pro/anti-inflammatory balance is important for alleviating progression of brain injuries and diseases. Among many factors involved in the regulation of microglial phenotypes, cellular energy status plays an important role. Adenosine monophosphate-activated protein kinase (AMPK), which serves as a master sensor and regulator of energy balance, is considered a candidate molecule. Accumulating evidence from adult rodent studies indicates that AMPK activation promotes anti-inflammatory responses in microglia exposed to danger signals or various stressors mainly through inhibition of the nuclear factor κB (NF-κB) signaling and activation of the nuclear factor erythroid-2-related factor-2 (Nrf2) pathway. However, AMPK activation in neurons exposed to stressors/insults may exacerbate neuronal damage if AMPK activation is excessive or prolonged. While AMPK affects microglial activation states and neuronal cell survival rates in both the adult and the developing brain, studies in the developing brain are still scarce, even though activated AMPK is highly expressed especially in the neonatal brain. More in depth studies in the developing brain are important, because neuroinflammation/neurodegeneration occurred during development can result in long-lasting brain damage.
Keywords: AMPK, microglia, inflammation, NF-kB, neuroprotection, developing brain
Introduction
Microglial activation and neuroinflammation in the brain are the innate immune processes to defend the brain against harmful agents, such as bacterial and viral pathogens and dead cells and injured tissues (Rivest, 2009). In the healthy state, microglia, the primary innate immune cells of the brain, survey danger signals derived from those harmful agents (Lenz and Nelson, 2018). Depending on the conditions under which microglia are exposed to the danger signals/stressors, activated microglia acquire different phenotypes: some are more phagocytic and some secrete higher amounts of pro- or anti-inflammatory mediators than the others. These microglia with diverse characteristics are often classified using nomenclatures of macrophages: classical proinflammatory (M1) and alternate anti-inflammatory (M2) phenotypes (Orihuela et al., 2016;Cherry et al., 2014b;Hu et al., 2015;Jha et al., 2016), although this classification is only one of the designation systems for characterizing functions of activated microglia (Cherry et al., 2014b) and is not precisely grouped by distinct molecular patterns (Ransohoff, 2016).
Pro- and anti-inflammatory states of microglia affect the progression of brain injuries, neuroimmune disorders, neurodegenerative diseases, and other inflammation-related disorders. Therefore, factors which promote pro- or anti-inflammatory microglia may serve as therapeutic targets for treatment of such disorders (Song and Suk, 2017;Block et al., 2007;Colonna and Butovsky, 2017;Norden et al., 2015). While many factors are involved in microglial activation, recent literature highlights the importance of cellular energy status of microglia. It is indicated that mitochondrial function, glucose availability, and glycolytic rate in microglia influence pro-inflammatory gene expression at both transcriptional and post translational levels (Ghosh et al., 2018). Some molecules which regulate energy metabolism seem to influence microglial inflammatory status. For instance, silent information regulator 1 (SIRT1), a major regulator of energy metabolism, is a key regulator of NF-κB, a transcription factor inducing various pro-inflammatory factors (Chen et al., 2005;Cho et al., 2015;Ge et al., 2015), and peroxisome proliferator-activated receptors γ (PPARγ), that is also highly involved in energy metabolism, is suggested to be a key regulator of anti-inflammatory functions of microglia (Flores et al., 2016;Savage et al., 2015;Yamanaka et al., 2012;Zhao et al., 2015b). Nuclear factor erythroid-2-related factor-2 (Nrf2), which regulates expression of antioxidant proteins, and triggering receptor expressed on myeloid cells 2 (TREM2), which is necessary for phagocytic activity of microglia, are also involved in both energy metabolism and inflammation in microglia (Zhang et al., 2017;Vilhardt et al., 2017;Ulland et al., 2017). All these molecules show tight relationship with adenosine monophosphate-activated protein kinase (AMPK). AMPK, a highly conserved serine/threonine protein kinase, serves as a master sensor and regulator of energy balance and controls many cellular processes, such as autophagy, cell death, and inflammation to maintain cellular homeostasis (Peixoto et al., 2017). Once activated, it acts to restore energy homeostasis by activating catabolic pathways that generate ATP and by inhibiting anabolic pathways that consume ATP (Hardie et al., 2012;Hardie et al., 2016). While AMPK in the hypothalamus is known to be a central integrator of energy status and a master controller of feeding behavior through circulating orexigenic and anorexigenic hormones, AMPK also plays an important role in cellular energy homeostasis in the brain especially after insults, such as ischemia and hypoxia (Ramamurthy and Ronnett, 2006), and may affect neuroinflammation. In this review, roles of AMPK in microglial inflammation will be discussed based on studies using in vitro microglial culture and in vivo rodent models. Since microglial inflammation is tightly linked to neurodegeneration in brain injuries, neuroimmune disorders, neurodegenerative diseases, involvement of AMPK in neurodegeneration is also discussed to explore the possible anti-inflammatory/neuroprotective effects of AMPK activation in the adult and the developing brain.
1. Pro- and anti-inflammatory states of microglia
Microglia, which are developed from myeloid precursor cells in the embryonic yolk sac, are the primary innate immune cells in the brain, and respond to danger signals such as damage-associated molecular patterns (DAMPS), pathogen-associated molecular patterns (PAMPS), and dead cells/debris, although they have many other functions including synaptic organization and control of neuronal excitability. Once activated by danger signals and other stressors, microglia acquire diverse phenotypes. Traditionally, activated microglia are distinguished from homeostatic microglia (M0) and classified into pro-inflammatory (M1-like), anti-inflammatory (M2-like), or the mixture of both, primarily based on whether pro- or anti-inflammatory mediators and other cellular markers are differentially expressed, or how microglia are activated [e.g. via lipopolysaccharides (LPS), IL4, or IL13] (Lenz and Nelson, 2018;Kabba et al., 2018;Mosser et al., 2017;Hamzei et al., 2018). A typical pro-inflammatory state of microglia can be seen in animals and in cultured cells treated with LPS, a well-known PAMP derived from Gram-negative bacteria. These LPS-induced neuroinflammation models have been extensively studied (Lehnardt et al., 2003;Lehnardt et al., 2002;Orihuela et al., 2016). LPS trigger Toll-like receptor (TLR) 4 on microglia, activate a transcription factor NF-ĸB, and enhance expression of proinflammatory cytokines, chemokines, and cytotoxic factors, such as tumor necrosis factor α (TNF-α), interleukin-1 β (IL-1β), TLRs, cytokine receptors, nitric oxide, and reactive oxygen species (ROS) (Lehnardt et al., 2003;Lehnardt et al., 2002;Akira and Takeda, 2004;Orihuela et al., 2016). In contrast, IL-4 and IL-13, which are T-helper type 2 (Th2) cytokines, are often used to induce anti-inflammatory functions of microglia (Mori et al., 2016;Hamzei et al., 2018). These cytokines promote expression of Arg1 (Arginase-1), Ym-1 (chitinase-like protein), CD200R, IL-10, TGF-β (transforming growth factor), and Fizz-1 (found in inflammatory zone 1), which are considered specific markers for anti-inflammatory (M2-like) microglia (Song and Suk, 2017). M2-like microglial states may be subclassified into M2a, M2b, and M2c, as shown in macrophages (Subramaniam and Federoff, 2017). Furthermore, recent studies using single-cell RNA sequencing have led to findings of more diversified microglial populations during development or in response to injury (Mosser et al., 2017;Hammond et al., 2018). It has been reported that the profile of LPS-induced activated microglia (M1-like) is different from that of a microglial population uniquely associated with neurodegenerative diseases (Sousa et al., 2018) and called disease associated microglia (DAM) or microglial neurodegenerative (MGnD) phenotypes (Keren-Shaul et al., 2017;Krasemann et al., 2017;Shi and Holtzman, 2018). Also, when microglia are activated by multiple signals in the brain injury or neurodegenerative diseases, microglia may show more complex phenotypes: pro-inflammatory, anti-inflammatory, disease-associated, or mixture of these phenotypes, depending on the severity of triggers and cellular and environmental conditions of microglia including the effects from other cell types (e.g., neurons and astrocytes). Such inflammatory states of microglia may show dynamic changes during the process of inflammation. For instance, after ischemic stroke, microglia are activated by receiving danger signals from damaged neurons through CX3CL1 (a chemokine, also called fractalkine)/CX3CR1 (a receptor of CX3CL1) signaling, release pro-inflammatory factors, and aggravate ischemia-mediated brain damage (Tang et al., 2014). However, in response to sub lethal ischemia, neurons may express IL-4, which polarizes microglia to an anti-inflammatory phenotype. Such microglia initiate phagocytosis for clearance of debris and produce trophic factors that may promote brain repair (Zhao et al., 2015a;Wang et al., 2018a). Thus, pro- and anti-inflammatory states of microglia are involved in the progression of neuroimmune disorders, neurodegenerative diseases, and other inflammation-related disorders. Therefore, factors which regulate microglial activation states, especially, factors which shift microglial polarization from pro- to anti-inflammatory (repair-promoting) phenotypes, may serve as therapeutic agents for treatment of such disorders (Song et al., 2015).
2. Factors affecting neuroinflammation and energy metabolism
While many factors are involved in pro- and anti-inflammatory pathways in microglia, recent literature indicates the importance of cellular energy status of microglia. Microglia has a capacity to generate ATP by both glycolytic and oxidative pathways, and while quiescent microglia primarily rely on oxidative phosphorylation for ATP production, activated microglia seem to increase reliance on glycolysis (Cherry et al., 2014a;Ghosh et al., 2018;Orihuela et al., 2016;Gimeno-Bayon et al., 2014;Voloboueva et al., 2013). In macrophages, activated pro-inflammatory cells mainly generate energy through glycolysis, whereas anti-inflammatory cells predominantly generate energy through oxidative phosphorylation (O’Neill and Hardie, 2013). In microglia, it has been reported that mitochondrial function, glucose availability, and glycolytic rate influence pro-inflammatory gene expression at both transcriptional and post translational levels (Ghosh et al., 2018). When glucose utilization is suppressed by caloric restriction, ketogenic diet, and a glycolytic inhibitor [e.g., 2-deoxyglucose (2DG)], brain injury-induced neuroinflammation (e.g., microglial activation, induction of TNF-α), neurodegeneration, tissue loss, and functional impairment are reduced, while hypoxia and hyperglycemia, which promote glucose utilization, exacerbate inflammation and worsen brain injury (Shen et al., 2017;Robbins and Swanson, 2014;Loncarevic-Vasiljkovic et al., 2012). , It has also been reported that hyperglycolysis and induction of the key glycolytic enzyme hexokinase 2 (HK2) are essential for microglia-mediated neuroinflammation under hypoxia (Li et al., 2018b).
It is indicated that such relationship between inflammatory reactions and energy metabolism are related to the coordinated actions of NF-κB, which drives pro-inflammatory phenotype with glycolytic metabolism, and SIRT1, a major regulator of energy metabolism and tissue survival (Kauppinen et al., 2013). SIRT1, a member of the sirtuins (a family of NAD+-dependent lysine deacetylase), activates or inhibits several protein targets via deacetylation of histones and non-histone proteins, and SIRT1 deacetylates and inactivates the p65 component of the NF-κB pathway, thus limiting the expression of NF-κB-dependent genes and suppressing inflammatory responses (Yeung et al., 2004). Although these studies are mainly performed in peripheral tissues, studies in the brain indicate similar functions of SIRT1 on NF-κB. Resveratrol, a potent SIRT1 activator, ameliorates LPS-induced NF-κB activation in mouse hippocampus (Ge et al., 2015), and activation of SIRT1 markedly reduces NF-κB signaling and shows strong neuroprotection against amyloid β toxicity in primary cortical cultures (Chen et al., 2005). On the other hand, SIRT1 deficiency in microglia leads to enhanced expression of IL-1β and causes cognitive decline (Cho et al., 2015). These studies suggest that SIRT1 is a key regulator of NF-κB signaling. PPARs are members of a type II nuclear hormone receptor superfamily of ligand-activated nuclear transcription factors. Among them PPARγ regulates the expression of genes involved in inflammation, redox equilibrium, trophic factor production, insulin sensitivity, and the metabolism of lipids and glucose (Cai et al., 2018), thus representing another key molecule to connect inflammation and energy metabolism. PPARγ is abundant in immune-related cell types, particularly in adipocytes, macrophages, dendritic cells, and microglia (Yuan et al., 2015;Villapol, 2018), and is indicated as a key regulator of anti-inflammatory functions of microglia, because PPARγ activation increases anti-inflammatory-related gene expression and down-regulates pro-inflammatory mediators in activated microglia/macrophages and promotes phagocytosis of apoptotic cells, contributing to the resolution of inflammation (Flores et al., 2016;Savage et al., 2015;Villapol, 2018;Yamanaka et al., 2012;Zhao et al., 2015b), although there is a report showing that an antagonist of PPARγ promotes the pro-inflammatory to anti-inflammatory phenotypic shift in LPS-treated microglia (Ji et al., 2018). The Nrf2 transcription factor is a master regulator of antioxidant responses. Target genes of Nrf2 include NADPH quinone oxidoreductase-1 (NQO-1), heme oxygenase-1 (HO-1) and glutathione S-transferase (GST), and Nrf2 is also considered one of the anti-inflammatory molecules (Vilhardt et al., 2017). TREM2 is another molecule involved in energy metabolism and inflammation. TREM2 has been postulated to be a phagocytic receptor on myeloid cells including microglia, and the expression is associated with suppression of inflammatory gene expression by attenuating PI3K/NF-κB signaling. TREM2 overexpression inhibits LPS-induced microglial activation and pro-inflammatory reaction (Zhang et al., 2017). TREM2 sustains cell energetic and biosynthetic metabolism through mTOR signaling in microglia, and its deficiency disturbs the mTOR pathway, anabolic and energetic metabolism, leading to compensatory autophagy (Ulland et al., 2017).
Thus, SIRT1, PPARγ, Nrf2, and TREM2 are related to energy metabolism and enhance pro- to anti-inflammatory polarization of microglia, and these molecules are tightly linked to AMPK, which is also a key molecule for cellular energy balance. It has been shown that SIRT1 activates AMPK via deacetylation of the tumor suppressor liver kinase B1 (LKB1), an upstream kinase of AMPK, and AMPK promotes the synthesis of cellular NAD+, which is required for SIRT activity (Kauppinen et al., 2013). Many studies have supported the notion that the Nrf2 antioxidant pathway is downstream of AMPK (Yaku et al., 2013;Iwasaki et al., 2013). PPARγ phosphorylation by AMPK represses both the ligand-dependent and -independent trans activating function of the receptor (Burns and Vanden Heuvel, 2007). Also, TREM2 deficiency causes AMPK phosphorylation (activation), impaired mTOR signaling, and enhancement of compensatory autophagy (Ulland et al., 2017). As described below, AMPK regulate microglial inflammation.
3. AMPK as a regulator of inflammation
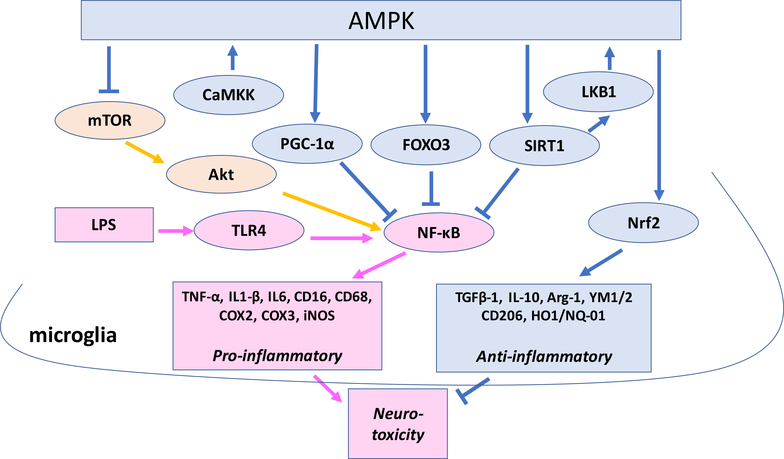
AMPK seems to play an important role in promoting microglial polarization from the pro- to anti-inflammatory phenotype. AMPK, a highly conserved serine/threonine protein kinase, serves as a master sensor and regulator of energy balance and controls various cellular processes, such as autophagy, cell death, and inflammation to maintain cellular homeostasis (Peixoto et al., 2017) and can be activated by many natural and synthesized agonists and activators (Fig.1). AMPK is a heterotrimer consisting of a catalytic subunit (α1 or α2) coupled to two regulatory subunits (β1 or β2; γ1, γ2, or γ3), which is ubiquitously expressed in mammalian cells (Hardie et al., 2016;Stapleton et al., 1996). The activity is controlled by: 1) phosphorylation at threonine 172 of the α subunit via up-stream protein kinases; LKB1 that is constitutively active (Hawley et al., 2003;Woods et al., 2003;Shaw et al., 2004), calmodulin kinase kinase β (CaMKKβ) (Hawley et al., 2005;Oakhill et al., 2012), and the transforming growth factor-β activated kinase (TAK1) (Momcilovic et al., 2006), 2) allosteric activation by AMP binding to γ subunit, and 3) activation of LKB1-induced Thr172 phosphorylation by AMP binding to γ subunit and inhibition of protein phosphatase-2Cα (PP2C-α)-induced Thr172 dephosphorylation by AMP or ADP binding to γ subunit (Hardie et al., 2016;O’Neill and Hardie, 2013;Xiao et al., 2007;Xiao et al., 2011;Gowans et al., 2013). Since ATP antagonizes these effects of AMP and ADP, AMPK acts as a sensor of cellular AMP/ATP and ADP/ATP ratios, which increase during cellular energy stress, although AMPK can also be activated by phosphorylation at threonine 172 by CaMKKβ activation through an increase in cellular Ca2+ levels, which is independent of cellular ADP/ATP ratios (Hawley et al., 2005). Activated AMPK acts to restore energy homeostasis by activating catabolic pathways that generate ATP, such as fatty acid oxidation, mitochondrial oxidative phosphorylation, glucose uptake through the phosphorylation of Akt and other enzymes necessary to the translocation of glucose transporter type 4 (GLUT4), and by inhibiting anabolic pathways that consume ATP, such as protein and lipid synthesis (Hardie et al., 2016;Hardie et al., 2012). AMPK also upregulates several antioxidant genes by activating Nrf2, which regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation (Iwasaki et al., 2013;Yaku et al., 2013). AMPK also reduces protein synthesis and stimulates autophagic pathways via inhibition of the mechanistic target of rapamycin (mTOR) (Peixoto et al., 2017). In the brain, AMPK is predominantly expressed in neurons but also found in astrocytes, oligodendrocytes, and microglia (Culmsee et al., 2001;Lu et al., 2010;Ramamurthy and Ronnett, 2006;Turnley et al., 1999), and AMPK affects microglial pro- and anti-inflammatory phenotypes. Accumulating data from experiments using LPS as a trigger for pro-inflammatory reactions indicate that AMPK activation induces a microglial phenotypic shift from pro-inflammatory to anti-inflammatory in AMPK activation-dependent manner. In primary cultured microglia or BV2, LPS-induced pro-inflammatory reaction is associated with reduction in phospho (p)-AMPK (activated AMP) (Li et al., 2018b;Park et al., 2018;Xu et al., 2015;Zhou et al., 2014), and AMPK activators including metformin and 5-aminoimidazole-4-carboxamide 1-β-D-ribofuranoside (AICAR) suppress pro-inflammatory and enhance anti-inflammatory reactions (Giri et al., 2004;Lee et al., 2015;Lu et al., 2010;Li et al., 2018a;Xu et al., 2015;Park et al., 2018;Wang et al., 2018d;Zhou et al., 2014;Velagapudi et al., 2017;Chen et al., 2014). For example, AICAR (an analogue of AMP) attenuates LPS-induced activation of NF-κB and inhibits expression of proinflammatory cytokines (TNF-α, IL1β, IL-6) and iNOS, and these effects of AICAR are inhibited by the dominant negative form of AMPK and anti-sense AMPK treatment in primary rat astrocytes, microglia, and peritoneal macrophages (Giri et al., 2004). Betulinic acid (BA), a naturally occurring pentacyclic triterpenoid, attenuates LPS-induced pro-inflammation reaction in BV-2 microglial cells via CaMKKβ-dependent AMPK activation, and compound C (an AMPK inhibitor) and AMPK siRNA abolish the effects, although BA does not affect phagocytic activation induced by LPS (Li et al., 2018a). ENERGI-F704, an AMPK agonist, decreases the protein level and nuclear translocation of NF-κB, leading to reduction of pro-inflammatory mediators, such as IL-6, TNF-α, iNOs, and COX-3 in LPS-stimulated microglia (BV2) (Chen et al., 2014). Also, AMPK/Nrf2 signaling is involved in anti-inflammatory action of Petatewalide B against LPS in microglia (Park et al., 2018). Similar to the results shown in microglial culture, LPS decreases p-AMPK levels (Zhou et al., 2014), and AMPK activation by various activators suppresses pro-inflammatory reactions and enhances anti-inflammatory reactions in LPS-induced rodent neuroinflammation models (Lu et al., 2010;Wang et al., 2018d;Xu et al., 2015;Zhou et al., 2014;Li et al., 2018a). For instance, while LPS [intracerebroventricular (icv) injection] decreases p-AMPK levels and induces expression of M1 signature genes (iNOS, IL-1β, TNFα, and IL-6) in the lateral septal complex area, administration of hydrogen sulfide (H2S) donors enhances AMPK activation, attenuates M1 signature gene expression, and enhances expression of M2 signature genes (Arg-1, YM1/2, and IL-10) (Zhou et al., 2014). Treatment with BA significantly increases p-CaMKKβ and p-AMPK in the cerebral cortex and suppresses LPS [intraperitoneal (i.p.) injection]-induced mRNA expression of TNF-α and iNOS and promotes M2 gene CD206 and Arg-1 mRNA expression (Li et al., 2018a). Balasubramide derivative 3C ameliorates depressive behaviors of LPS (i.p.)-induced neuroinflammatory mice by promoting the anti-inflammatory activation of microglia through the CaMKKβ-dependent AMPK/peroxisome proliferator-activated receptor-γ coactivator (PGC-1α) signaling pathway (Wang et al., 2018d). Salvanoic acid C inhibits LPS-induced inflammatory response and NF-ĸB activation through the activation of AMPK/Nrf2 signaling in vivo and in vitro (Song et al., 2018). Thus, AMPK activation seems to attenuate proinflammatory reaction induced by LPS and enhance anti-inflammatory reaction both in in vivo and in vitro. Although metformin and other AMPK activators used in these experiments may not be specific to AMPK, experiments using AMPK siRNAs or other inhibitors and genetic modifications of AMPK indicate the importance of AMPK activation in the microglial shift from pro- to anti-inflammatory phenotypes (Giri et al., 2004;Li et al., 2018a;Park et al., 2018;Song et al., 2018;Wang et al., 2018d;Zhou et al., 2014). These experiments also show that LPS treatment decreases microglial p-AMPK levels, which are restored by AMPK activators.
Fig. 1.
AMPK-signaling and its functions regulated by agonists and activators. Arrows indicate activation or elevation of AMPK.
In addition to LPS-induced inflammatory models, the microglial shift from pro- to anti-inflammatory phenotypes by AMPK activation has been also reported in several animal models of inflammation-related diseases. In a MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) plus probenecid (MPTPp) mouse model of Parkinson’s, metformin enhances AMPK phosphorylation and autophagy and inhibits NF-ĸBp65 nuclear translocation and activation of NLRP3 (NOD-, LRR-and pyrin domain-containing 3) inflammasome in the middle brain. Metformin also reduces the transcription of pro-inflammatory cytokines (TNF-α and IL6) and restores the transcription of anti-inflammatory cytokines (Lu et al., 2016). In the mouse model of diabetic encephalopathy induced by streptozotocin (STZ), elevation of AMPK by metformin reduces expression of astrocytic and microglial markers and inflammation mediators (Oliveira et al., 2016). It has also been reported that resveratrol reduces TNF-α and IL-6 transcripts, the expression of NF-ĸB, p38, and p-ERK1/2, as well as astrocytic activation in STZ-induced diabetic rats (Jing et al., 2013), which show lower hippocampal p-AMPK levels compared to the control rats. In a rat model of global cerebral ischemia, activation of AMPK by metformin suppresses pro-inflammatory responses (such as production of TNF-α, IL-6, iNOS, and ROS) and confers anti-inflammatory effects (such as production of IL-10) in the AMPK dependent manner (Ashabi et al., 2015). Metformin treatment for 3 weeks before permanent middle cerebral artery occulsion suppresses inflammation reaction (Zhu et al., 2015). Metformin treatment for 2 weeks before subjecting to 9 min asphyxia cardiac arrest (CA) ameliorates CA-induced glial activation in the hippocampal CA1 region and cortex, which is accompanied by augmented AMPK phosphorylation and autophagy activation (Zhu et al., 2018). Not only metformin, but also other compounds which activate AMPK supress inflammation. The compound 3C (Balasubramide derivative 3C)-mediated activation of CaMKKβ, AMPK, and PGC-1α is involved in the anti-inflammatory effects of 3C in the brain of LPS-treated mice and ischemic rats (Wang et al., 2018d). A dual AMPK/Nrf2 activator (HP-1c) reduces brain inflammation after focal ischemic stroke (Wang et al., 2018c).
Thus, in both cultured microglia and the brain, AMPK activators inhibit inflammation caused by LPS and other stressors, and the inhibition is attenuated by blocking AMPK activity genetically or pharmacologically, indicating that AMPK is involved in the pro- to anti-inflammatory shift of microglia. While multiple mechanisms behind the action of AMPK have been suggested, the aforementioned studies indicate that inhibition of NF-ĸB and activation of Nrf2 may be important (Fig. 2). Activated AMPK, which acts to restore energy homeostasis by activating catabolic pathways that generate ATP and by inhibiting anabolic pathways that consume ATP (Hardie et al., 2016;Hardie et al., 2012), indirectly inhibits NF-κB activation through multiple downstream pathways including SIRT1, Folkhead box O (FOXO), and PGC1α (Peixoto et al., 2017;Jeon, 2016). AMPK activates SIRT1, which directly inhibits NF-κB, or AMPK phosphorylates p53 and FOXO, which inhibit NF-κB signaling (Kauppinen et al., 2013;Peixoto et al., 2017). PGC-1α also promotes microglial anti-inflammatory activation by inhibiting NF-κB activity (Yang et al., 2017). AMPK can also modulate the NF-κB pathway by phosphorylating eNOS at Ser177 (Peixoto et al., 2017). In addition, AMPK may induce anti-inflammatory microglia by Nrf2 activation (Wang et al., 2018b; Song et al., 2018; Park et al., 2018).
Fig.2.
Possible mechanisms behind the pro- to anti-inflammatory phenotypic shift by AMPK activation in microglia activated by LPS and other cellular stressors.
Such anti-inflammatory polarization of microglia induced by AMPK activation is often associated with neuroprotection in animal models of brain disorders, such as ischemic, Parkinson’s, and streptozotocin-induced diabetic models (Jing et al., 2013;Lu et al., 2016;Oliveira et al., 2016;Wang et al., 2018d;Wang et al., 2018c). However, in Parkinson’s (MPTP) model, while metformin reduces microglial morphological changes as well as TNF-α, IL-1β and iNOS mRNAs levels, it exacerbates dopaminergic neuron damage (Ismaiel et al., 2016). It seems that AMPK activation may or may not exert neuroprotective effects depending on degrees and length of AMPK activation as described below.
4. AMPK and neurodegeneration
Dysregulated inflammation of the CNS may induce neurodegeneration. Once microglia are excessively activated, they can damage neurons by secreting neurotoxic molecules, such as proinflammatory cytokines, metalloproteinases, nitric oxide (NO), and ROS (Block et al., 2007;Norden et al., 2015;Colonna and Butovsky, 2017). As described above, the anti-inflammatory effects of AMPK activation are consistent across different models and different AMPK activators (Curry et al., 2018). Whether this anti-inflammatory action of AMPK via regulation of microglia results in the survival of neurons and other cell types is an important question when we consider the AMPK pathways as a therapeutic target for brain disorders related to neuroinflammation. If the neurodegeneration is induced or enhanced by neuroinflammation as predicted in some neurodegenerative diseases and brain injuries (Block et al., 2007;Norden et al., 2015;Colonna and Butovsky, 2017), AMPK activation may exert neuroprotection by attenuating neuroinflammation. However, AMPK, a master regulator of cellular energy balance in various cell types, may provide protective or harmful effects directly on neurons, which show high metabolic demands. In the brain, AMPK is activated in response to a number of pathologically relevant stresses, such as hypoxia (Gusarova et al., 2011;Mungai et al., 2011) and excitotoxicity (Weisova et al., 2013;Concannon et al., 2010), when AMP/ATP or ADP/ATP ratios increase. Therefore, AMPK activation is recognized as the development of neurological disorders, although it is unclear whether this activation is beneficial or deleterious (McCullough et al., 2005). Such AMPK activation may be necessary to restore AMP/ATP balance, but excessive or prolonged AMPK activation may be harmful. In cultured hippocampal neurons, AICAR, an AMPK activator, attenuates neuronal death induced by glucose deprivation, chemical hypoxia, glutamate, and amyloid β peptide (Culmsee et al., 2001). Also, metformin reduces neuronal apoptosis in cultured cortical neurons exposed to ethanol (Ullah et al., 2012). However, prolonged AMPK activation by kainic acid seems to induce apoptosis in cultured hippocampal neurons (Ullah et al., 2014). Also, zinc-induced neuronal death, which is thought to be a key mechanism of ischemic neuronal death, is mediated by enhanced AMPK via up-regulation of Bim (Bcl-2 homology domain 2 (BH3)-only protein) and activation of caspase-3 in cultured neurons (Eom et al., 2016).
In vivo, a number of studies show that metformin provides neuroprotection against cerebral ischemia induced by middle cerebral artery occlusion (Arbelaez-Quintero and Palacios, 2017). For example, metformin treatment for 2 weeks before global cerebral ischemia inhibits inflammatory responses and attenuates cell death in the rat hippocampus in AMPK-dependent manner (Ashabi et al., 2014), and metformin treatment for 3 weeks before permanent middle cerebral artery occulsion suppresses inflammation, reduces infarct volume, and improves neurological deficits in rats (Zhu et al., 2015). Further, acute metformin preconditioning activates AMPK and autophagy in the rat brain and reduces infarct bolume, neurological deficits and cell apoptosis during the focal cerebral ischaemia (Jiang et al., 2014). Also, Metformin treatment for 2 weeks before subjecting to asphyxia cardiac arrest (CA) ameliorates CA-induced neuronal degeneration as well as glial activation in the rat hippocampal CA1 and cortex, which is accompanied by augmented AMPK phosphorylation and autophagy activation (Zhu et al., 2018). These metformin’s effects are attenuated by an AMPK inhibitor (compound C) or an autophagy inhibitor (3-methladenine) (Jiang et al., 2014). Not only metformin, but also other compounds which activate AMPK show neuroprotection. The compound 3C (Balasubramide derivative 3C)-mediated activation of CaMKKβ, AMPK, and PGC-1α is involved in the anti-neuroinflammatory and neuroprotective effects of 3C in the brain of LPS-treated mice and ischemic rats (Wang et al., 2018d). A dual AMPK/Nrf2 activator (HP-1c) reduces rat brain inflammation and provides neuroprotection after focal ischemic stroke by enhacing anti-inflammatory microglia population and by anti-oxidative effects (Wang et al., 2018c).
However, AMPK activation induced by focal stroke in adult mice enhances metabolic dysfunction, and AMPK inhibition or AMPKα−2 deletion is neuroprotective and induces smaller infarct volumes compared with wild-type littermates (Li et al., 2007). Similarly, pharmacological inhibition of AMPK by C75 or compound C provides neuroprotection in stroke (middle cerebral artery occlusion) in mice (McCullough et al., 2005). Since acute metformin treatment exacerbates stroke injury in mice, while chronic metformin given pre-stroke is neuroprotective, the timing, duration and amount of AMPK activation may be factors which determine the effects of AMPK on the ischemic brain (Li et al., 2010).
Neuroprotection by AMPK activation is also reported in inflammatioin-related diseases other than stroke/ischemia. In mouse models of Parkinson’s, ghrelin, a gut peptide hormone, mediates the neuroprotective effects of calorie restriction by elevating p-AMPK levels and reducing MPTP-induced loss of substantia nigra dopamine neurons (Bayliss et al., 2016). Also, metformin reduces pro-inflammatory cytokines and dopaminergic neurodegeneration via AMPK-mediated autophagy and mitochondrial ROS clearance in a MPTP+probenecid (MPTPp) mouse model (Lu et al., 2016). In these studies, MPTP treatment increases p-AMPK levels as shown in ischemia models. However, not all stress-inducers activate cellular AMPK. As described above, LPS treatment, which induces inflammation, decreases p-AMPK levels in cultured microglia (Li et al., 2018a;Xu et al., 2015;Park et al., 2018;Zhou et al., 2014) and also reduces p-AMPK in a mouse neuroinflammation model (Zhou et al., 2014), although it is not clear which cell types are responsible for the reduction in vivo. In relevant to this, in the mouse model of experimental autoimmune encephalomyelitis (EAE), AMPK levels are low in immune cells including microglia (Nath et al., 2009) and treatment of mice with AICAR attenuates EAE by reducing the inflammatory response and protecting neurons (Nath et al., 2005). In STZ-induced diabetic rats, which show lower hippocampal p-AMPK levels compared to the control rats, resveratrol treatment increases hippocampal p-AMPK, diminishes neurodegeneration and astrocytic activation, and reduces TNF-α and IL-6 transcripts as well as the expression of NF-κB, p38, and ERK1/2 phosphorylation (Jing et al., 2013). In the mouse model of diabetic encephalopathy produced by STZ, metformin reduces neuroinflammation and decreases the loss of neurons in the hippocampus (Oliveira et al., 2016). In this study, STZ does not increase p-AMPK levels. Traumatic adult brain injury decreases AMPK activity, and AICAR/metformin improves cognitive outcome (Hill et al., 2016). In Alzheimer disease (AD) mouse models, resveratrol improves proteostasis and enhances cognitive functions, and these effects are mainly mediated by increased activation of the SIRT-1/AMPK pathway (Porquet et al., 2014;Corpas et al., 2018). In these studies, activation levels of AMPK seem to be no difference between AD and wild-type mice. Also, in a AD mouse model, metformin atenuates spatial memory deficit and neuron loss, and decreases amyloid-β plaque and chronic inflammation through AMPK activation (Ou et al., 2018). In SOD(G93A) amyotrophic lateral sclerosis (ALS) mice, which do not show the elevation of AMPK, resveratrol exerts neuroprotective effects on motoneurons by activating the SIRT1/AMPK pathway (Mancuso et al., 2014).
Thus, AMPK activation not only induces anti-inflammatory reactions in microglia but also exerts neuroprotection, especially when initial AMPK activation levels by insults are not excessive. AMPK activation may provide neuroprotection through anti-inflammatory action, enhanced autophagy, anti-oxidant activation through the Nrf2 pathway, and restoration of energy levels. It is also suggested that activation of pro-survival Akt is mediated by AMPK-induced insulin-like growth factor1 receptor (IGF-1R) activation via phosphorylation of the insulin receptor substrate-1 (IRS-1), and inhibition of AMPK by compound-C results in decreased p-Akt , suggesting a central role for AMPK in Akt activation (Leclerc et al., 2010). However, when energy levels/metabolic states are deeply disturbed by stressors, such as severe excitotoxicity or oxygen/glucose deprivation-induced injury, and AMPK is highly activated, further AMPK activation may be neurotoxic. It is suggested that within a short period of energy deficiency, AMPK activation enhances astrocytic glycolysis and ketosis to compensate energy deficiency in ischemic neurons. However, prolonged glycolysis in astrocytes leads to progressive acidosis and inhibits the ability of neurons to use lactate as energy sources, leading to neuronal death (Li and McCullough, 2010). Also, activation of AMPK may enhance the level of oxidative stress (Ju et al., 2014) leading to neurodegeneration. It is also indicated that activation of AMPK reduces Akt activation through inhibition of mTOR and leads to dephosphorylation and nuclear translocation of FOXO3, and FOXO3 phosphorylation by AMPK in the nucleus induces the pro-apoptotic Bcl-2 family protein Bim elevation during excitotoxic injury (Davila et al., 2012). It is shown that rotenone activates AMPK and inactivates Akt, causing neuronal cell death in culture (Xu et al., 2014), while metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress, and neuroinflammation via Akt activation (Tang et al., 2017). Thus, AMPK activation may lead to Akt activation or inhibition depending on the conditions of cells under stress and may regulate neurodegeneration/neuroprotection.
These studies imply that cellular AMPK levels modified by both stressors (e.g., ischemia, excitotoxicity) and AMPK activators (e.g., AICAR, metformin) may determine subsequent cell fates. AMPK activators may exert more efficient anti-inflammatory and neuroprotective actions against stressors which lower cellular AMPK levels, such as LPS, while AMPK activators induce or enhance neurotoxicity against stressors which exceedingly elevate cellular AMPK levels, such as severe ischemia. Also, cellular levels of p-Akt may affect the AMPK activation-induced cell fate, because activated Akt in microglia may enhance inflammation while activated Akt is generally neuroprotective.
Neuroprotection by AMPK observed in some of the in vivo disease/injury models may block microglial proinflammatory activation, which can be triggered by receiving danger signals from damaged neurons (Roh and Sohn, 2018;Venegas and Heneka, 2017;Schindler et al., 2018). Therefore, the AMPK-induced microglial shift to the proinflammatory phenotype may be caused not only by the direct effects of AMPK on microglia described in the previous section but also by the neuroprotective effects of AMPK.
5. Microglia in the developing brain
Microglia is originated from myeloid precursors born in the yolk sac around E7 in mice. Then immature microglia invade the CNS from E8.5 and follow tangential and radial migration pathways to colonize all CNS regions. In the CNS, proliferation and differentiation of immature microglia continue during early postnatal period (Mosser et al., 2017). It is found that microglia express distinct sets of genes that can divide microglia into three distinct groups: early (E10.5–14), pre-microglia (E14-P9], and adult microglia (P28 and on) (Matcovitch-Natan et al., 2016). Morphology of some pre-microglia are amoeboid and similar to activated phagocytic microglia in the adult brain although gene expression profiles are different (Lenz and Nelson, 2018). In the developing brain, microglia play a critical role both in normal brain development and in response to infection and other early life stresses. During the development of healthy CNS, microglia support a variety of processes, such as neurogenesis, synaptogenesis, synaptic elimination, myelination, cell death, cell survival, and angiogenesis/vascularization via functions such as phagocytosis and release of diffusible factors (Pierre et al., 2017;Lenz and Nelson, 2018). For example, at P5, microglia start engulfing inappropriate pre-synaptic elements via interactions between microglia and unwanted synapses through classical complement cascade with complement proteins C1q and C3 as identification signals (Stephan et al., 2012;Schafer et al., 2012;Bialas and Stevens, 2013). Also, TREM2 is indicated to be essential for microglia-mediated synaptic refinement during the early stages of brain development (Filipello et al., 2018). A subset of microglia in the early postnatal subventricular zone seems to enhance neurogenesis and oligodendrogenesis through production of several cytokines (Shigemoto-Mogami et al., 2014), and the survival of layer V cortical neurons requires microglia, which are accumulated close to the subcerebral region and secrete IGF1 (Ueno et al., 2013). It has been also indicated that a CD11c-positive microglial subset, which is the major source of IGF-1, displays myelinogenic and neurogenic phenotypes (Wlodarczyk et al., 2017). Mice lacking the colony stimulating factor 1 receptor (CSF1R) for CSF1 or IL34, which is critical for microglial proliferation, differentiation and survival, show abnormal brain development including behavioral defects (Erblich et al., 2011;Rosin et al., 2018), while microglial loss in adulthood seems to have little impact on behavioral outcomes (Lenz and Nelson, 2018). Thus, microglia play critical roles in normal neurodevelopment. Therefore, if microglia are activated and disturbed by early life stress including infection, normal neurodevelopmental trajectories can be disrupted, and neurodevelopmental disorders, neurodegenerative diseases, and brain injuries may be triggered or worsened. Microglia-involved neuroinflammation is also important in the pathogenesis of injury to the immature brain (Pierre et al., 2017;Mottahedin et al., 2017). Especially, complications during preterm birth, such as neonatal infection, meningitis, neonatal hypoxia-ischemia, and neonatal stroke, in which inflammation is a common factor, impose a risk of injury to the developing brain (Mottahedin et al., 2017). While the peripheral immune response is indicated to be suppressed in neonates, it is unclear if immune response is less active in the developing CNS (Christensen et al., 2014). In rodent infection models, it has been shown that LPS and CpG oligodeoxynucleotides (CpG ODN, PAMP present in B cells) trigger to produce higher levels of cytokines in postnatal day 2 (P2) mouse neonates compared to weanlings (Christensen et al., 2014). The hippocampus of P4 rats, which has a high number of microglia with activated/amoeboid morphology, produces pro-inflammatory cytokines such as IL-1β, IL6, and TNF-α upon LPS exposure (Schwarz and Bilbo, 2011), and stimulation of TLRs by LPS or Poly(I:C) during the prenatal or neonatal period results in robust inflammatory responses in the fetal and newborn brain, including marked microglial proliferation and increased cytokine expression (Hagberg et al., 2015). Systemic injection of LPS in P5 mouse pups induces an acute and transient increase in reactive microglia with both M1 and M2 phenotypes and dysregulation of the ongoing process of neurogenesis in the developing hippocampal germinal niche (Smith et al., 2014). In neonatal rodent hypoxia-ischemia/stroke models, microglial activation and widespread neuronal apoptosis are observed, while necrosis is a dominant type of neuronal cell death in adult brain (Vexler and Yenari, 2009). In a mouse model of neonatal excitotoxic brain damage, injection of ibotenate induces excitotoxic lesions along with microglial activation, and the inhibition of microglial and/or blood-derived monocytes activation is accompanied by a significant reduction in severity of ibotenate-induced brain lesions (Dommergues et al., 2003). Prenatal alcohol exposure at E14/15 triggers higher expression of proinflammatory cytokines in embryonic brain than maternal brains, which may be due to less developed anti-oxidant systems in the developing brain (Akhtar et al., 2017). Also, alcohol injection into P4 mice triggers MCP-1 (monocyte chemoattractant protein-1)/CCR2 (C-C Motif Chemokine Receptor 2) signaling-mediated microglial activation and neuroinflammation (Zhang et al., 2018a). Cultured microglia isolated from neonatal brains show higher production of proinflammatory cytokines after being activated by ATP compared to other ages (Lai et al., 2013). Also, it has been shown that the acute phase of neonatal stroke, excitotoxic injury, or systemic infection, microglia are major cells for inflammation responses, and monocyte infiltration is low compared to adult brain injury (Denker et al., 2007;Smith et al., 2014;Dommergues et al., 2003).
Thus, embryonic and neonatal microglia in the brain seem to show prominent pro-inflammatory phenotypes after activation and may be prone to activation following an immune challenge (Pierre et al., 2017). Furthermore, the inflammatory activation of microglia during embryonic and neonatal periods seems to induce neurodevelopmental abnormalities: Prenatal exposure of rodents to influenza, Poly(I:C), or LPS increases cytokines such as IL-1β, TNF-α, IL6 and induces neurodevelopmental abnormalities in the offspring (Meyer, 2014). Acute exposure to LPS in prenatal mice induces inflammation and alters glial cytoarchitecture in the P40 brain (O’Loughlin et al., 2017). Alcohol intake during gestation and lactation increases levels of several cytokines/chemokines in the maternal sera, amniotic fluid, and brains of fetuses and offspring through the TLR4 response, leading to long-term behavioral abnormalities (Pascual et al., 2017). Neonatal hyperglycemia induces CXCL (C-X-C Motif Chemokine Ligand) 10/CXCR3 (C-X-C Motif Chemokine Receptor) signaling, microglial activation, and astrocytosis in the rat hippocampus and alters long-term synaptogenesis and behavior (Shi et al., 2017;Satrom et al., 2018).
Anti-inflammatory functions of microglia have been also reported in the developing brain. Pharmacological depletion of microglia prior to neonatal stroke exacerbates the release of inflammatory cytokines, suggesting that at least a subpopulation of microglia shows anti-inflammatory responses (Hagberg et al., 2015;Fernandez-Lopez et al., 2016;Faustino et al., 2011). During the acute phase of neonatal ischemia, genetic deletion of the scavenger receptor CD36 reduces phagocytosis of neuronal debris by microglia and enhances neuroinflammation (Li et al., 2015;Woo et al., 2012). Our studies indicate that alcohol injection into P7 mice induces acute microglial activation along with apoptotic neurodegeneration (Saito et al., 2010;Saito et al., 2012;Saito et al., 2015). These activated microglia are localized in the same area where apoptotic neurodegeneration is detected by Fluoro-Jade stain, and the Fluoro-Jade stain is frequently observed in Iba1-positive microglia (Saito et al., 2015). In addition, the rod- or amoeba-shaped microglia are strongly labeled with antibody against CD68, a marker for phagocytes (Saito et al., 2015), and labeled with antibody against cleaved tau (tau cleaved by activated caspase-3) present in degenerating neurons (Saito et al., 2010), suggesting that these microglia are phagocytes engulfing degenerating neurons. Ahlers and his colleagues (Ahlers et al., 2015) also show that P7 alcohol activates microglia which contain markers of late-stage apoptotic neurons, and apoptotic bodies and are deactivated within 1–2 days. The authors show further that alcohol-induced microglial activation and transient elevation of TNF-α and IL-1β are largely abolished in BAX (Bcl-2-associated X protein) null mice lacking apoptotic neurodegeneration. Thus, neonatal microglia have high capacity of phagocytosis and eliminate apoptotic neurons without prolonged inflammation, probably because one of the physiological roles of microglia in the neonatal brain is to eliminate neurons dying by programmed cell death. However, it has been also shown that minocycline, which inhibits microglial activation and alleviates neuroinflammation, protects neurons from ethanol-induced apoptosis in the P4 mouse brain (Wang et al., 2018b), suggesting that the initial phase of pro-inflammatory microglia may contribute to ethanol-induced neurodegeneration.
These studies indicate that although there are differences in the process of microglial activation/neuroinflammation between the developing and the adult brain, microglial polarization from the pro-to anti-inflammatory phenotypes seems to be also beneficial in the developing brain for alleviating acute neurodegeneration as well as the long-lasing adverse effects on brain development.
6. AMPK and neuroinflammation/neurodegeneration in the developing brain
AMPK is present in the developing brain. It has been shown that catalytic (α1 and α2) and noncatalytic (β2 and γ1) subunits of AMPK are abundant in the embryonic rat brain (Culmsee et al., 2001). Mouse brain mRNA levels for α2 and β2 subunits of AMPK are higher between E14 and P8 compared to adults, whereas expression of α1, β1, and γ1 subunits is consistent at all ages examined, and immunostaining shows a mainly neuronal distribution of all isoforms (Turnley et al., 1999). Our observation also indicates that AMPK is highly expressed in neurons during the early neonatal period in the developing mouse brain. Specifically, the content of p-AMPK (activated form) and p-acetyl-CoA carboxylase (p-ACC) is high at P4 and P7 and decreases to 20% of the amount of P4 by P19, indicating that AMPK in the neonatal brain is more activated and phosphorylates its substrate ACC more strongly than AMPK in the P19 brain (Saito et al., 2009). It has been established that AMPK regulates lipogenesis and β-oxidation through modifying ACC and SREBP-1 transcriptionally and post-transcriptionally in the peripheral organs (Hardie and Pan, 2002). The regulation of ACC by AMPK has been observed not only in the peripheral tissues but also in hypothalamic neurons, cortical neurons, and Neuro2a cells (Dasgupta and Milbrandt, 2007;Landree et al., 2004). Our previous studies (Saito et al., 2007) have shown that ethanol decreases the content of p-AMPK and p-ACC, and increases triglyceride (TG) synthesis, which is considered a marker for lipogenesis, in P7 mouse brains. Conversely, nutrient deprivation increases p-AMPK and decreases TG levels in these brains. However, neither ethanol exposure nor nutrient deprivation affects levels of p-AMPK and TG in P19 brains. These observations, combined with the neuronal localization of AMPK, ACC, and fatty acid synthase (FAS) and the high content of pAMPK and pACC in early neonatal brains, support the notion that AMPK regulates lipogenesis in neurons during the early neonatal period (Saito et al., 2009). Higher AMPK activity or higher phosphorylated levels of AMPK during the mouse neonatal period is also observed in other studies (Rousset et al., 2015;Williams et al., 2011).
Functions of AMPK during brain development have not been well clarified. It has been reported that knockout of AMPK β1 (AMPK β−/−) subunit in mice leads to severe loss of neurons and oligodendrocytes as well as abnormal astrocyte proliferation (Dasgupta and Milbrandt, 2009). However, it has also been shown that AMPK β−/− mice display no visible brain developmental deficits (Dzamko et al., 2010), and using transgenic mice that are ubiquitously inactivated for the AMPKα1 gene and conditionally inactivated for the AMPKα2 gene, it has been indicated that AMPK is not required for neurogenesis, neuronal differentiation, or neuronal survival in vivo under normal conditions, while overactivation of AMPK during metabolic stress impairs neuronal polarization in a mTOR-dependent manner (Williams et al., 2011). Similarly, in differentiating hippocampal neurons, activation of AMPK by energetic stress suppresses both mTOR and Akt signaling pathways and inhibits neuronal development during axon outgrowth, dendrite growth and arborization (Ramamurthy et al., 2014).
Althlough studies on the relatioship between AMPK activation and microglial activation/neurodegeneration in the developing brain are still scarce, some studies show that AMPK activation is associated with anti-inflammatory action of microglia in the developing brain. Chemerin (a chemokine, also classified as a adipokine) suppresses inflammation and improves neurological recovery via ChemR23 (the receptor for chemerin)/CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage (GMH) in neonatal rats (Zhang et al., 2018b). Experiments using Lipo-Alpha-NETA (ChemR23 inhibitor) and Lipo-Dorsomorphin (AMPK inhibitor) indicate that alleviation of GMH-induced inflammation by chemerin may be primarily mediated through AMPK/Nrf2 signaling in microglia. In a model of neonatal hypoxic-ischemic brain injury, metformin treatment attenuates brain infarct volumes and brain edema 24 h after hypoxic-ischemic injury, and the neuroprotection by metformin is associated with suppression of the neuroinflammation assessed by decreases in expression levels of proinflammatory mediators, such as TNF-α, IL-6, IL-Iβ, and iNOS, and also decreases in levels of TLR4 and NF-B, although the direct involvement of AMPK has not been proved in these studies (Fang et al., 2017). Also, it has been shown that pioglitazone, a PPARγ agonist, effectively blocks ethanol-induced neuroinflammation manifested by microglial morphological changes and production of cytokines and chemokines in neonatal mice (Drew et al., 2015). Although the effects of AMPK are not examined in this study, previous studies indicating that AMPK activation is linked to increased expression of PPARγ (Li et al., 2017a;Chen et al., 2018;Zhong et al., 2018;Sun et al., 2017) suggest the involvement of AMPK.
Neuroprotective effects of AMPK in the developing brain are also indicated in several studies. Intranasal administration of adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia in P10 rats at least in part by activating adipoR1/APPL1 (an adaptor protein mediating adiponectin signaling)/LKB1/AMPK signaling pathway (Xu et al., 2018). Similarly, osmotin (a homolog of adiponectin) and adiponectin attenuate alcohol-induced apoptosis in P7 rats partially via AMPK activation (Naseer et al., 2014). Sestrin 2, a highly conserved stress-inducible protein, provides neuroprotection against rat neonatal hypoxic-ischemic encephalopathy by AMPK signaling activation (Shi et al., 2017). It has been also indicated that up-regulation of nicotinamide mononucleotide adenylyltransferase 1 (NMNAT1) induces neuroprotection against ischemic injury through AMPK activation (Liang et al., 2015). However, AMPK activation also induces apoptosis under some conditions as observed in the adult brain. It has been shown that AMPK activates FOXO3a and promotes neuronal apoptosis in the P7 rat brain during the early phase after hypoxia-ischemia. Activation of AMPK is accompanied by the decrease of p-mTOR, p-Akt and p-FOXO3a, which induces FOXO3a translocation into the nucleus and up-regulates the expression of Bim and cleaved caspase 3 (Li et al., 2017b). High dose of glutamate significantly increases brain glutamate levels and activates AMPK, leading to cell death in the hippocampus of P7 rats, SH-SY5Y, and BV2 cells probably through the enhanced level of oxidative stress (Shah et al., 2016), as observed in kainic acid-induced hippocampal neurons (Ullah et al., 2014).
Thus, although the effects of AMPK on neuroinflammation and neurodegeneration have not been extensively studied in the developing brain, available data indicate that AMPK affects brain development especially after the metabolic stress. As observed in the adult brain, AMPK activation in the neonatal brain appears to promote anti-inflammatory responses in microglia and may reduce or enhance neuroapoptosis, depending on the AMPK activation conditions.
Highlights.
AMPK activation shifts microglia from a pro- to anti-inflammatory phenotype.
NF-κB and Nrf2 are involved in anti-inflammatory functions of AMPK.
AMPK activation, if not excessive or prolonged, exerts neuroprotection.
High levels of activated AMPK are present in the neonatal mouse brain.
Anti-inflammatory/neuroprotective effects of AMPK are seen in adults and neonates.
Summary.
Accumulating evidence indicates that regulation of metabolic homeostasis by the AMPK signaling pathway plays important roles in determining phenotypes of microglia activated by stressors such as pathogens, environmental toxins, and ischemia in the adult brain. Activation of AMPK has been shown to polarize microglia to anti-inflammatory phenotypes in various rodent models. It is suggested that Inhibition of the NF-ĸB pathway and activation of the Nrf2 pathway are the major mechanisms behind the AMPK-induced anti-inflammatory reactions in microglia. Also, AMPK activation often exerts neuroprotection, while excess or prolonged elevation of AMPK induces neuronal cell death. The degree of AMPK activation as well as the status of Akt activation seem to be important factors which determine the fates of neurons under stress. Similarly, AMPK activation appears to promote anti-inflammatory microglial polarization in the developing brain and may attenuate or enhance neuroapoptosis depending on the AMPK activation conditions. However, studies in the developing brain are still scarce and need to be explored further, because neuroinflammation /neurodegeneration in the developing brain may cause the long-term adverse effects, which may lead to neurodevelopmental or neurodegenerative diseases.
Acknowledgement
This work was partly supported by NIH/NIAAA R01 AA023181 (to M.S.) and NIH/NIAID R01AI132614 (to B.C.D.).
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahlers KE, Karacay B, Fuller L, Bonthius DJ, Dailey ME (2015) Transient activation of microglia following acute alcohol exposure in developing mouse neocortex is primarily driven by BAX-dependent neurodegeneration. Glia 63:1694–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar F, Rouse CA, Catano G, Montalvo M, Ullevig SL, Asmis R, Kharbanda K, Maffi SK (2017) Acute maternal oxidant exposure causes susceptibility of the fetal brain to inflammation and oxidative stress. J Neuroinflammation 14:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511. [DOI] [PubMed] [Google Scholar]
- Arbelaez-Quintero I, Palacios M (2017) To Use or Not to Use Metformin in Cerebral Ischemia: A Review of the Application of Metformin in Stroke Rodents. Stroke Res Treat 2017:9756429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashabi G, Khalaj L, Khodagholi F, Goudarzvand M, Sarkaki A (2015) Pre-treatment with metformin activates Nrf2 antioxidant pathways and inhibits inflammatory responses through induction of AMPK after transient global cerebral ischemia. Metab Brain Dis 30:747–754. [DOI] [PubMed] [Google Scholar]
- Ashabi G, Khodagholi F, Khalaj L, Goudarzvand M, Nasiri M (2014) Activation of AMP-activated protein kinase by metformin protects against global cerebral ischemia in male rats: interference of AMPK/PGC-1alpha pathway. Metab Brain Dis 29:47–58. [DOI] [PubMed] [Google Scholar]
- Bayliss JA, Lemus MB, Stark R, Santos VV, Thompson A, Rees DJ, Galic S, Elsworth JD, Kemp BE, Davies JS, Andrews ZB (2016) Ghrelin-AMPK Signaling Mediates the Neuroprotective Effects of Calorie Restriction in Parkinson’s Disease. J Neurosci 36:3049–3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas AR, Stevens B (2013) TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci 16:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69. [DOI] [PubMed] [Google Scholar]
- Burns KA, Vanden Heuvel JP (2007) Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta 1771:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W, Yang T, Liu H, Han L, Zhang K, Hu X, Zhang X, Yin KJ, Gao Y, Bennett MVL, Leak RK, Chen J (2018) Peroxisome proliferator-activated receptor gamma (PPARgamma): A master gatekeeper in CNS injury and repair. Prog Neurobiol 163–164:27–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Lin JT, Cheng YF, Kuo CY, Huang CF, Kao SH, Liang YJ, Cheng CY, Chen HM (2014) Amelioration of LPS-induced inflammation response in microglia by AMPK activation. Biomed Res Int 2014:692061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280:40364–40374. [DOI] [PubMed] [Google Scholar]
- Chen W, Xi X, Zhang S, Zou C, Kuang R, Ye Z, Huang Y, Hu H (2018) Pioglitazone Protects Against Renal Ischemia-Reperfusion Injury via the AMP-Activated Protein Kinase-Regulated Autophagy Pathway. Front Pharmacol 9:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK (2014a) Are “resting” microglia more “m2”? Front Immunol 5:594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK (2014b) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SH, Chen JA, Sayed F, Ward ME, Gao F, Nguyen TA, Krabbe G, Sohn PD, Lo I, Minami S, Devidze N, Zhou Y, Coppola G, Gan L (2015) SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci 35:807–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen LB, Woods TA, Carmody AB, Caughey B, Peterson KE (2014) Age-related differences in neuroinflammatory responses associated with a distinct profile of regulatory markers on neonatal microglia. J Neuroinflammation 11:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, Butovsky O (2017) Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu Rev Immunol 35:441–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concannon CG, Tuffy LP, Weisova P, Bonner HP, Davila D, Bonner C, Devocelle MC, Strasser A, Ward MW, Prehn JH (2010) AMP kinase-mediated activation of the BH3-only protein Bim couples energy depletion to stress-induced apoptosis. J Cell Biol 189:83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corpas R, Grinan-Ferre C, Rodriguez-Farre E, Pallas M, Sanfeliu C (2018) Resveratrol Induces Brain Resilience Against Alzheimer Neurodegeneration Through Proteostasis Enhancement. Mol Neurobiol. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Monnig J, Kemp BE, Mattson MP (2001) AMP-activated protein kinase is highly expressed in neurons in the developing rat brain and promotes neuronal survival following glucose deprivation. J Mol Neurosci 17:45–58. [DOI] [PubMed] [Google Scholar]
- Curry DW, Stutz B, Andrews ZB, Elsworth JD (2018) Targeting AMPK Signaling as a Neuroprotective Strategy in Parkinson’s Disease. J Parkinsons Dis 8:161–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J (2007) Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A 104:7217–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J (2009) AMP-activated protein kinase phosphorylates retinoblastoma protein to control mammalian brain development. Dev Cell 16:256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila D, Connolly NM, Bonner H, Weisova P, Dussmann H, Concannon CG, Huber HJ, Prehn JH (2012) Two-step activation of FOXO3 by AMPK generates a coherent feed-forward loop determining excitotoxic cell fate. Cell Death Differ 19:1677–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, Ji S, Dingman A, Lee SY, Derugin N, Wendland MF, Vexler ZS (2007) Macrophages are comprised of resident brain microglia not infiltrating peripheral monocytes acutely after neonatal stroke. J Neurochem 100:893–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dommergues MA, Plaisant F, Verney C, Gressens P (2003) Early microglial activation following neonatal excitotoxic brain damage in mice: a potential target for neuroprotection. Neuroscience 121:619–628. [DOI] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJ (2015) Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzamko N, van Denderen BJ, Hevener AL, Jorgensen SB, Honeyman J, Galic S, Chen ZP, Watt MJ, Campbell DJ, Steinberg GR, Kemp BE (2010) AMPK beta1 deletion reduces appetite, preventing obesity and hepatic insulin resistance. J Biol Chem 285:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom JW, Lee JM, Koh JY, Kim YH (2016) AMP-activated protein kinase contributes to zinc-induced neuronal death via activation by LKB1 and induction of Bim in mouse cortical cultures. Mol Brain 9:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW (2011) Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One 6:e26317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Jiang H, Ye L, Cai C, Hu Y, Pan S, Li P, Xiao J, Lin Z (2017) Metformin treatment after the hypoxia-ischemia attenuates brain injury in newborn rats. Oncotarget 8:75308–75325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustino JV, Wang X, Johnson CE, Klibanov A, Derugin N, Wendland MF, Vexler ZS (2011) Microglial cells contribute to endogenous brain defenses after acute neonatal focal stroke. J Neurosci 31:12992–13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lopez D, Faustino J, Klibanov AL, Derugin N, Blanchard E, Simon F, Leib SL, Vexler ZS (2016) Microglial Cells Prevent Hemorrhage in Neonatal Focal Arterial Stroke. J Neurosci 36:2881–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello F, et al. (2018) The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 48:979–991. [DOI] [PubMed] [Google Scholar]
- Flores JJ, Klebe D, Rolland WB, Lekic T, Krafft PR, Zhang JH (2016) PPARgamma-induced upregulation of CD36 enhances hematoma resolution and attenuates long-term neurological deficits after germinal matrix hemorrhage in neonatal rats. Neurobiol Dis 87:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge L, Liu L, Liu H, Liu S, Xue H, Wang X, Yuan L, Wang Z, Liu D (2015) Resveratrol abrogates lipopolysaccharide-induced depressive-like behavior, neuroinflammatory response, and CREB/BDNF signaling in mice. Eur J Pharmacol 768:49–57. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Castillo E, Frias ES, Swanson RA (2018) Bioenergetic regulation of microglia. Glia 66:1200–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, Mahy N (2014) Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res 92:723–731. [DOI] [PubMed] [Google Scholar]
- Giri S, Nath N, Smith B, Viollet B, Singh AK, Singh I (2004) 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside inhibits proinflammatory response in glial cells: a possible role of AMP-activated protein kinase. J Neurosci 24:479–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowans GJ, Hawley SA, Ross FA, Hardie DG (2013) AMP is a true physiological regulator of AMP-activated protein kinase by both allosteric activation and enhancing net phosphorylation. Cell Metab 18:556–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusarova GA, Trejo HE, Dada LA, Briva A, Welch LC, Hamanaka RB, Mutlu GM, Chandel NS, Prakriya M, Sznajder JI (2011) Hypoxia leads to Na,K-ATPase downregulation via Ca(2+) release-activated Ca(2+) channels and AMPK activation. Mol Cell Biol 31:3546–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Ferriero DM, Vannucci SJ, Levison SW, Vexler ZS, Gressens P (2015) The role of inflammation in perinatal brain injury. Nat Rev Neurol 11:192–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, Walker AJ, Gergits F, Segel M, Nemesh J, Marsh SE, Saunders A, Macosko E, Ginhoux F, Chen J, Franklin RJM, Piao X, McCarroll SA, Stevens B (2018) Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei TS, Le BD, Hoornaert C, Daans J, Quarta A, Praet J, Van der Linden A, Ponsaerts P, Hoehn M (2018) Targeted intracerebral delivery of the anti-inflammatory cytokine IL13 promotes alternative activation of both microglia and macrophages after stroke. J Neuroinflammation 15:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Pan DA (2002) Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans 30:1064–1070. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13:251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Schaffer BE, Brunet A (2016) AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol 26:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG (2003) Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG (2005) Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2:9–19. [DOI] [PubMed] [Google Scholar]
- Hill JL, Kobori N, Zhao J, Rozas NS, Hylin MJ, Moore AN, Dash PK (2016) Traumatic brain injury decreases AMP-activated protein kinase activity and pharmacological enhancement of its activity improves cognitive outcome. J Neurochem 139:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J (2015) Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol 11:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismaiel AA, Espinosa-Oliva AM, Santiago M, Garcia-Quintanilla A, Oliva-Martin MJ, Herrera AJ, Venero JL, de Pablos RM (2016) Metformin, besides exhibiting strong in vivo anti-inflammatory properties, increases mptp-induced damage to the nigrostriatal dopaminergic system. Toxicol Appl Pharmacol 298:19–30. [DOI] [PubMed] [Google Scholar]
- Iwasaki K, Ray PD, Huang BW, Sakamoto K, Kobayashi T, Tsuji Y (2013) Role of AMP-activated protein kinase in ferritin H gene expression by resveratrol in human T cells. Biochemistry 52:5075–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon SM (2016) Regulation and function of AMPK in physiology and diseases. Exp Mol Med 48:e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha MK, Lee WH, Suk K (2016) Functional polarization of neuroglia: Implications in neuroinflammation and neurological disorders. Biochem Pharmacol 103:1–16. [DOI] [PubMed] [Google Scholar]
- Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, Huang JY, Zhao XJ, Sun XL (2018) Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Celle 12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L (2014) Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol 171:3146–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing YH, Chen KH, Kuo PC, Pao CC, Chen JK (2013) Neurodegeneration in streptozotocin-induced diabetic rats is attenuated by treatment with resveratrol. Neuroendocrinology 98:116–127. [DOI] [PubMed] [Google Scholar]
- Ju TC, Chen HM, Chen YC, Chang CP, Chang C, Chern Y (2014) AMPK-alpha1 functions downstream of oxidative stress to mediate neuronal atrophy in Huntington’s disease. Biochim Biophys Acta 1842:1668–1680. [DOI] [PubMed] [Google Scholar]
- Kabba JA, Xu Y, Christian H, Ruan W, Chenai K, Xiang Y, Zhang L, Saavedra JM, Pang T (2018) Microglia: Housekeeper of the Central Nervous System. Cell Mol Neurobiol 38:53–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A (2013) Antagonistic crosstalk between NF-kappaB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal 25:1939–1948. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, David E, Baruch K, Lara-Astaiso D, Toth B, Itzkovitz S, Colonna M, Schwartz M, Amit I (2017) A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169:1276–1290. [DOI] [PubMed] [Google Scholar]
- Krasemann S, et al. (2017) The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47:566–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai AY, Dibal CD, Armitage GA, Winship IR, Todd KG (2013) Distinct activation profiles in microglia of different ages: a systematic study in isolated embryonic to aged microglial cultures. Neuroscience 254:185–195. [DOI] [PubMed] [Google Scholar]
- Landree LE, Hanlon AL, Strong DW, Rumbaugh G, Miller IM, Thupari JN, Connolly EC, Huganir RL, Richardson C, Witters LA, Kuhajda FP, Ronnett GV (2004) C75, a fatty acid synthase inhibitor, modulates AMP-activated protein kinase to alter neuronal energy metabolism. J Biol Chem 279:3817–3827. [DOI] [PubMed] [Google Scholar]
- Leclerc GM, Leclerc GJ, Fu G, Barredo JC (2010) AMPK-induced activation of Akt by AICAR is mediated by IGF-1R dependent and independent mechanisms in acute lymphoblastic leukemia. J Mol Signal 5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YY, Park JS, Lee EJ, Lee SY, Kim DH, Kang JL, Kim HS (2015) Anti-inflammatory mechanism of ginseng saponin metabolite Rh3 in lipopolysaccharide-stimulated microglia: critical role of 5’-adenosine monophosphate-activated protein kinase signaling pathway. J Agric Food Chem 63:3472–3480. [DOI] [PubMed] [Google Scholar]
- Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T (2002) The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci 22:2478–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnardt S, Massillon L, Follett P, Jensen FE, Ratan R, Rosenberg PA, Volpe JJ, Vartanian T (2003) Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A 100:8514–8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz KM, Nelson LH (2018) Microglia and Beyond: Innate Immune Cells As Regulators of Brain Development and Behavioral Function. Front Immunol 9:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhang C, Zhou H, Feng Y, Tang F, Hoi MPM, He C, Ma D, Zhao C, Lee SMY (2018a) Inhibitory Effects of Betulinic Acid on LPS-Induced Neuroinflammation Involve M2 Microglial Polarization via CaMKKbeta-Dependent AMPK Activation. Front Mol Neurosci 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Gong D, Chen LY, Zhang M, Xia XD, Cheng HP, Huang C, Zhao ZW, Zheng XL, Tang XE, Tang CK (2017a) Puerarin promotes ABCA1-mediated cholesterol efflux and decreases cellular lipid accumulation in THP-1 macrophages. Eur J Pharmacol 811:74–86. [DOI] [PubMed] [Google Scholar]
- Li D, Luo L, Xu M, Wu J, Chen L, Li J, Liu Z, Lu G, Wang Y, Qiao L (2017b) AMPK activates FOXO3a and promotes neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia. Brain Res Bull 132:1–9. [DOI] [PubMed] [Google Scholar]
- Li F, Faustino J, Woo MS, Derugin N, Vexler ZS (2015) Lack of the scavenger receptor CD36 alters microglial phenotypes after neonatal stroke. J Neurochem 135:445–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Benashski SE, Venna VR, McCullough LD (2010) Effects of metformin in experimental stroke. Stroke 41:2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McCullough LD (2010) Effects of AMP-activated protein kinase in cerebral ischemia. J Cereb Blood Flow Metab 30:480–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD (2007) Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke 38:2992–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, et al. (2018b) Hexokinase 2-dependent hyperglycolysis driving microglial activation contributes to ischemic brain injury. J Neurochem 144:186–200. [DOI] [PubMed] [Google Scholar]
- Liang J, Wang P, Wei J, Bao C, Han D (2015) Nicotinamide Mononucleotide Adenylyltransferase 1 Protects Neural Cells Against Ischemic Injury in Primary Cultured Neuronal Cells and Mouse Brain with Ischemic Stroke Through AMP-Activated Protein Kinase Activation. Neurochem Res 40:1102–1110. [DOI] [PubMed] [Google Scholar]
- Loncarevic-Vasiljkovic N, Pesic V, Todorovic S, Popic J, Smiljanic K, Milanovic D, Ruzdijic S, Kanazir S (2012) Caloric restriction suppresses microglial activation and prevents neuroapoptosis following cortical injury in rats. PLoS One 7:e37215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu DY, Tang CH, Chen YH, Wei IH (2010) Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem 110:697–705. [DOI] [PubMed] [Google Scholar]
- Lu M, Su C, Qiao C, Bian Y, Ding J, Hu G (2016) Metformin Prevents Dopaminergic Neuron Death in MPTP/P-Induced Mouse Model of Parkinson’s Disease via Autophagy and Mitochondrial ROS Clearance. Int J Neuropsychopharmacol 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R, del VJ, Modol L, Martinez A, Granado-Serrano AB, Ramirez-Nunez O, Pallas M, Portero-Otin M, Osta R, Navarro X (2014) Resveratrol improves motoneuron function and extends survival in SOD1(G93A) ALS mice. Neurotherapeutics 11:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matcovitch-Natan O, et al. (2016) Microglia development follows a stepwise program to regulate brain homeostasis. Science 353:aad8670. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV (2005) Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem 280:20493–20502. [DOI] [PubMed] [Google Scholar]
- Meyer U (2014) Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biol Psychiatry 75:307–315. [DOI] [PubMed] [Google Scholar]
- Momcilovic M, Hong SP, Carlson M (2006) Mammalian TAK1 activates Snf1 protein kinase in yeast and phosphorylates AMP-activated protein kinase in vitro. J Biol Chem 281:25336–25343. [DOI] [PubMed] [Google Scholar]
- Mori S, Maher P, Conti B (2016) Neuroimmunology of the Interleukins 13 and 4. Brain Sci 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser CA, Baptista S, Arnoux I, Audinat E (2017) Microglia in CNS development: Shaping the brain for the future. Prog Neurobiol 149–150:1–20. [DOI] [PubMed] [Google Scholar]
- Mottahedin A, Ardalan M, Chumak T, Riebe I, Ek J, Mallard C (2017) Effect of Neuroinflammation on Synaptic Organization and Function in the Developing Brain: Implications for Neurodevelopmental and Neurodegenerative Disorders. Front Cell Neurosci 11:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, Schumacker PT (2011) Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 31:3531–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseer MI, Ullah I, Narasimhan ML, Lee HY, Bressan RA, Yoon GH, Yun DJ, Kim MO (2014) Neuroprotective effect of osmotin against ethanol-induced apoptotic neurodegeneration in the developing rat brain. Cell Death Dis 5:e1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath N, Giri S, Prasad R, Salem ML, Singh AK, Singh I (2005) 5-aminoimidazole-4-carboxamide ribonucleoside: a novel immunomodulator with therapeutic efficacy in experimental autoimmune encephalomyelitis. J Immunol 175:566–574. [DOI] [PubMed] [Google Scholar]
- Nath N, Khan M, Rattan R, Mangalam A, Makkar RS, de MC, Bertrand L, Singh I, Chen Y, Viollet B, Giri S (2009) Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem Biophys Res Commun 386:16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, Godbout JP (2015) Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology 96:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Loughlin E, Pakan JMP, Yilmazer-Hanke D, McDermott KW (2017) Acute in utero exposure to lipopolysaccharide induces inflammation in the pre- and postnatal brain and alters the glial cytoarchitecture in the developing amygdala. J Neuroinflammation 14:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill LA, Hardie DG (2013) Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 493:346–355. [DOI] [PubMed] [Google Scholar]
- Oakhill JS, Scott JW, Kemp BE (2012) AMPK functions as an adenylate charge-regulated protein kinase. Trends Endocrinol Metab 23:125–132. [DOI] [PubMed] [Google Scholar]
- Oliveira WH, Nunes AK, Franca ME, Santos LA, Los DB, Rocha SW, Barbosa KP, Rodrigues GB, Peixoto CA (2016) Effects of metformin on inflammation and short-term memory in streptozotocin-induced diabetic mice. Brain Res 1644:149–160. [DOI] [PubMed] [Google Scholar]
- Orihuela R, McPherson CA, Harry GJ (2016) Microglial M1/M2 polarization and metabolic states. Br J Pharmacol 173:649–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Kong X, Sun X, He X, Zhang L, Gong Z, Huang J, Xu B, Long D, Li J, Li Q, Xu L, Xuan A (2018) Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 69:351–363. [DOI] [PubMed] [Google Scholar]
- Park SY, Choi MH, Li M, Li K, Park G, Choi YW (2018) AMPK/Nrf2 signaling is involved in the anti-neuroinflammatory action of Petatewalide B from Petasites japonicus against lipopolysaccharides in microglia. Immunopharmacol Immunotoxicol 40:232–241. [DOI] [PubMed] [Google Scholar]
- Pascual M, Montesinos J, Montagud-Romero S, Forteza J, Rodriguez-Arias M, Minarro J, Guerri C (2017) TLR4 response mediates ethanol-induced neurodevelopment alterations in a model of fetal alcohol spectrum disorders. J Neuroinflammation 14:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peixoto CA, Oliveira WH, Araujo SMDR, Nunes AKS (2017) AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol 298:31–41. [DOI] [PubMed] [Google Scholar]
- Pierre WC, Smith PLP, Londono I, Chemtob S, Mallard C, Lodygensky GA (2017) Neonatal microglia: The cornerstone of brain fate. Brain Behav Immun 59:333–345. [DOI] [PubMed] [Google Scholar]
- Porquet D, Grinan-Ferre C, Ferrer I, Camins A, Sanfeliu C, del VJ, Pallas M (2014) Neuroprotective role of trans-resveratrol in a murine model of familial Alzheimer’s disease. J Alzheimers Dis 42:1209–1220. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Chang E, Cao Y, Zhu J, Ronnett GV (2014) AMPK activation regulates neuronal structure in developing hippocampal neurons. Neuroscience 259:13–24. [DOI] [PubMed] [Google Scholar]
- Ramamurthy S, Ronnett GV (2006) Developing a head for energy sensing: AMP-activated protein kinase as a multifunctional metabolic sensor in the brain. J Physiol 574:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM (2016) A polarizing question: do M1 and M2 microglia exist? Nat Neurosci 19:987–991. [DOI] [PubMed] [Google Scholar]
- Rivest S (2009) Regulation of innate immune responses in the brain. Nat Rev Immunol 9:429–439. [DOI] [PubMed] [Google Scholar]
- Robbins NM, Swanson RA (2014) Opposing effects of glucose on stroke and reperfusion injury: acidosis, oxidative stress, and energy metabolism. Stroke 45:1881–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JS, Sohn DH (2018) Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw 18:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin JM, Vora SR, Kurrasch DM (2018) Depletion of embryonic microglia using the CSF1R inhibitor PLX5622 has adverse sex-specific effects on mice, including accelerated weight gain, hyperactivity and anxiolytic-like behaviour. Brain Behav Immun 73:682–697. [DOI] [PubMed] [Google Scholar]
- Rousset CI, Leiper FC, Kichev A, Gressens P, Carling D, Hagberg H, Thornton C (2015) A dual role for AMP-activated protein kinase (AMPK) during neonatal hypoxic-ischaemic brain injury in mice. J Neurochem 133:242–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Mao RF, Paik SM, Vadasz C, Saito M (2010) Tau phosphorylation and cleavage in ethanol-induced neurodegeneration in the developing mouse brain. Neurochem Res 35:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Mao RF, Vadasz C, Saito M (2009) Developmental profiles of lipogenic enzymes and their regulators in the neonatal mouse brain. Neurochem Res 34:1945–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Mao RF, Wang R, Cooper TB, Vadasz C, Saito M (2007) Ethanol alters lipid profiles and phosphorylation status of AMP-activated protein kinase in the neonatal mouse brain. J Neurochem 103:1208–1218. [DOI] [PubMed] [Google Scholar]
- Saito M, Chakraborty G, Shah R, Mao RF, Kumar A, Yang DS, Dobrenis K, Saito M (2012) Elevation of GM2 ganglioside during ethanol-induced apoptotic neurodegeneration in the developing mouse brain. J Neurochem 121:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Wu G, Hui M, Masiello K, Dobrenis K, Ledeen RW, Saito M (2015) Ganglioside accumulation in activated glia in the developing brain: comparison between WT and GalNAcT KO mice. J Lipid Res 56:1434–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satrom KM, Ennis K, Sweis BM, Matveeva TM, Chen J, Hanson L, Maheshwari A, Rao R (2018) Neonatal hyperglycemia induces CXCL10/CXCR3 signaling and microglial activation and impairs long-term synaptogenesis in the hippocampus and alters behavior in rats. J Neuroinflammation 15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage JC, Jay T, Goduni E, Quigley C, Mariani MM, Malm T, Ransohoff RM, Lamb BT, Landreth GE (2015) Nuclear receptors license phagocytosis by trem2+ myeloid cells in mouse models of Alzheimer’s disease. J Neurosci 35:6532–6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B (2012) Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74:691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler SM, Frank MG, Annis JL, Maier SF, Klegeris A (2018) Pattern recognition receptors mediate pro-inflammatory effects of extracellular mitochondrial transcription factor A (TFAM). Mol Cell Neurosci 89:71–79. [DOI] [PubMed] [Google Scholar]
- Schwarz JM, Bilbo SD (2011) LPS elicits a much larger and broader inflammatory response than Escherichia coli infection within the hippocampus of neonatal rats. Neurosci Lett 497:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah SA, Amin FU, Khan M, Abid MN, Rehman SU, Kim TH, Kim MW, Kim MO (2016) Anthocyanins abrogate glutamate-induced AMPK activation, oxidative stress, neuroinflammation, and neurodegeneration in postnatal rat brain. J Neuroinflammation 13:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC (2004) The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 101:3329–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Kapfhamer D, Minnella AM, Kim JE, Won SJ, Chen Y, Huang Y, Low LH, Massa SM, Swanson RA (2017) Bioenergetic state regulates innate inflammatory responses through the transcriptional co-repressor CtBP. Nat Commun 8:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Xu L, Doycheva DM, Tang J, Yan M, Zhang JH (2017) Sestrin2, as a negative feedback regulator of mTOR, provides neuroprotection by activation AMPK phosphorylation in neonatal hypoxic-ischemic encephalopathy in rat pups. J Cereb Blood Flow Metab 37:1447–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Holtzman DM (2018) Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol 18:759–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K (2014) Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci 34:2231–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PL, Hagberg H, Naylor AS, Mallard C (2014) Neonatal peripheral immune challenge activates microglia and inhibits neurogenesis in the developing murine hippocampus. Dev Neurosci 36:119–131. [DOI] [PubMed] [Google Scholar]
- Song GJ, Suk K (2017) Pharmacological Modulation of Functional Phenotypes of Microglia in Neurodegenerative Diseases. Front Aging Neurosci 9:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Han Y, Pan C, Deng X, Dai W, Hu L, Jiang C, Yang Y, Cheng Z, Li F, Zhang G, Wu X, Liu W (2015) Activation of Adenosine Monophosphate-activated Protein Kinase Suppresses Neuroinflammation and Ameliorates Bone Cancer Pain: Involvement of Inhibition on Mitogen-activated Protein Kinase. Anesthesiology 123:1170–1185. [DOI] [PubMed] [Google Scholar]
- Song J, Zhang W, Wang J, Yang H, Zhao X, Zhou Q, Wang H, Li L, Du G (2018) Activation of Nrf2 signaling by salvianolic acid C attenuates NFkappaB mediated inflammatory response both in vivo and in vitro. Int Immunopharmacol 63:299–310. [DOI] [PubMed] [Google Scholar]
- Sousa C, Golebiewska A, Poovathingal SK, Kaoma T, Pires-Afonso Y, Martina S, Coowar D, Azuaje F, Skupin A, Balling R, Biber K, Niclou SP, Michelucci A (2018) Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, House CM, Fernandez CS, Cox T, Witters LA, Kemp BE (1996) Mammalian AMP-activated protein kinase subfamily. J Biol Chem 271:611–614. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Federoff HJ (2017) Targeting Microglial Activation States as a Therapeutic Avenue in Parkinson’s Disease. Front Aging Neurosci 9:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Zhu X, Lin W, Zhou Y, Cai W, Qiu L (2017) Interactions of TLR4 and PPARgamma, Dependent on AMPK Signalling Pathway Contribute to Anti-Inflammatory Effects of Vaccariae Hypaphorine in Endothelial Cells. Cell Physiol Biochem 42:1227–1239. [DOI] [PubMed] [Google Scholar]
- Tang G, Yang H, Chen J, Shi M, Ge L, Ge X, Zhu G (2017) Metformin ameliorates sepsis-induced brain injury by inhibiting apoptosis, oxidative stress and neuroinflammation via the PI3K/Akt signaling pathway. Oncotarget 8:97977–97989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Gan Y, Liu Q, Yin JX, Liu Q, Shi J, Shi FD (2014) CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J Neuroinflammation 11:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF (1999) Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 72:1707–1716. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T (2013) Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci 16:543–551. [DOI] [PubMed] [Google Scholar]
- Ullah I, Park HY, Kim MO (2014) Anthocyanins protect against kainic acid-induced excitotoxicity and apoptosis via ROS-activated AMPK pathway in hippocampal neurons. CNS Neurosci Ther 20:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah I, Ullah N, Naseer MI, Lee HY, Kim MO (2012) Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci 13:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulland TK, Song WM, Huang SC, Ulrich JD, Sergushichev A, Beatty WL, Loboda AA, Zhou Y, Cairns NJ, Kambal A, Loginicheva E, Gilfillan S, Cella M, Virgin HW, Unanue ER, Wang Y, Artyomov MN, Holtzman DM, Colonna M (2017) TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell 170:649–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velagapudi R, El-Bakoush A, Lepiarz I, Ogunrinade F, Olajide OA (2017) AMPK and SIRT1 activation contribute to inhibition of neuroinflammation by thymoquinone in BV2 microglia. Mol Cell Biochem 435:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas C, Heneka MT (2017) Danger-associated molecular patterns in Alzheimer’s disease. J Leukoc Biol 101:87–98. [DOI] [PubMed] [Google Scholar]
- Vexler ZS, Yenari MA (2009) Does inflammation after stroke affect the developing brain differently than adult brain? Dev Neurosci 31:378–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilhardt F, Haslund-Vinding J, Jaquet V, McBean G (2017) Microglia antioxidant systems and redox signalling. Br J Pharmacol 174:1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villapol S (2018) Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol Neurobiol 38:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva LA, Emery JF, Sun X, Giffard RG (2013) Inflammatory response of microglial BV-2 cells includes a glycolytic shift and is modulated by mitochondrial glucose-regulated protein 75/mortalin. FEBS Lett 587:756–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jamnik J, Garcia-Bailo B, Nielsen DE, Jenkins DJA, El-Sohemy A (2018a) ABO Genotype Does Not Modify the Association between the “Blood-Type” Diet and Biomarkers of Cardiometabolic Disease in Overweight Adults. J Nutr 148:518–525. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhang K, Yang F, Ren Z, Xu M, Frank JA, Ke ZJ, Luo J (2018b) Minocycline protects developing brain against ethanol-induced damage. Neuropharmacology 129:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, Saavedra JM, Zhang L, Huang Z, Pang T (2018c) A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation After Stroke by Enhancing Microglia M2 Polarization. Antioxid Redox Signal 28:141–163. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ruan W, Mi J, Xu J, Wang H, Cao Z, Saavedra JM, Zhang L, Lin H, Pang T (2018d) Balasubramide derivative 3C modulates microglia activation via CaMKKbeta-dependent AMPK/PGC-1alpha pathway in neuroinflammatory conditions. Brain Behav Immun 67:101–117. [DOI] [PubMed] [Google Scholar]
- Weisova P, Alvarez SP, Kilbride SM, Anilkumar U, Baumann B, Jordan J, Bernas T, Huber HJ, Dussmann H, Prehn JH (2013) Latrepirdine is a potent activator of AMP-activated protein kinase and reduces neuronal excitability. Transl Psychiatry 3:e317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T, Courchet J, Viollet B, Brenman JE, Polleux F (2011) AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc Natl Acad Sci U S A 108:5849–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wlodarczyk A, Holtman IR, Krueger M, Yogev N, Bruttger J, Khorooshi R, Benmamar-Badel A, de Boer-Bergsma JJ, Martin NA, Karram K, Kramer I, Boddeke EW, Waisman A, Eggen BJ, Owens T (2017) A novel microglial subset plays a key role in myelinogenesis in developing brain. EMBO J 36:3292–3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MS, Wang X, Faustino JV, Derugin N, Wendland MF, Zhou P, Iadecola C, Vexler ZS (2012) Genetic deletion of CD36 enhances injury after acute neonatal stroke. Ann Neurol 72:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, Schlattner U, Wallimann T, Carlson M, Carling D (2003) LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 13:2004–2008. [DOI] [PubMed] [Google Scholar]
- Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, Walker PA, Haire L, Eccleston JF, Davis CT, Martin SR, Carling D, Gamblin SJ (2007) Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 449:496–500. [DOI] [PubMed] [Google Scholar]
- Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, Carmena D, Jing C, Walker PA, Eccleston JF, Haire LF, Saiu P, Howell SA, Aasland R, Martin SR, Carling D, Gamblin SJ (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472:230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Zhang Y, Doycheva DM, Ding Y, Zhang Y, Tang J, Guo H, Zhang JH (2018) Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology 133:415–428. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu C, Chen S, Ye Y, Guo M, Ren Q, Liu L, Zhang H, Xu C, Zhou Q, Huang S, Chen L (2014) Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal 26:1680–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Xu Y, Wang Y, Wang Y, He L, Jiang Z, Huang Z, Liao H, Li J, Saavedra JM, Zhang L, Pang T (2015) Telmisartan prevention of LPS-induced microglia activation involves M2 microglia polarization via CaMKKbeta-dependent AMPK activation. Brain Behav Immun 50:298–313. [DOI] [PubMed] [Google Scholar]
- Yaku K, Matsui-Yuasa I, Konishi Y, Kojima-Yuasa A (2013) AMPK synergizes with the combined treatment of 1’-acetoxychavicol acetate and sodium butyrate to upregulate phase II detoxifying enzyme activities. Mol Nutr Food Res 57:1198–1208. [DOI] [PubMed] [Google Scholar]
- Yamanaka M, Ishikawa T, Griep A, Axt D, Kummer MP, Heneka MT (2012) PPARgamma/RXRalpha-induced and CD36-mediated microglial amyloid-beta phagocytosis results in cognitive improvement in amyloid precursor protein/presenilin 1 mice. J Neurosci 32:17321–17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Xu S, Qian Y, Xiao Q (2017) Resveratrol regulates microglia M1/M2 polarization via PGC-1alpha in conditions of neuroinflammatory injury. Brain Behav Immun 64:162–172. [DOI] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23:2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan G, Chen X, Li D (2015) Modulation of peroxisome proliferator-activated receptor gamma (PPAR gamma) by conjugated fatty acid in obesity and inflammatory bowel disease. J Agric Food Chem 63:1883–1895. [DOI] [PubMed] [Google Scholar]
- Zhang K, Wang H, Xu M, Frank JA, Luo J (2018a) Role of MCP-1 and CCR2 in ethanol-induced neuroinflammation and neurodegeneration in the developing brain. J Neuroinflammation 15:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yan F, Cui J, Wu Y, Luan H, Yin M, Zhao Z, Feng J, Zhang J (2017) Triggering Receptor Expressed on Myeloid Cells 2 Overexpression Inhibits Proinflammatory Cytokines in Lipopolysaccharide-Stimulated Microglia. Mediators Inflamm 2017:9340610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu N, Ding Y, Zhang Y, Li Q, Flores J, Haghighiabyaneh M, Doycheva D, Tang J, Zhang JH (2018b) Chemerin suppresses neuroinflammation and improves neurological recovery via CaMKK2/AMPK/Nrf2 pathway after germinal matrix hemorrhage in neonatal rats. Brain Behav Immun 70:179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang H, Sun G, Zhang J, Edwards NJ, Aronowski J (2015a) Neuronal Interleukin-4 as a Modulator of Microglial Pathways and Ischemic Brain Damage. J Neurosci 35:11281–11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XR, Gonzales N, Aronowski J (2015b) Pleiotropic role of PPARgamma in intracerebral hemorrhage: an intricate system involving Nrf2, RXR, and NF-kappaB. CNS Neurosci Ther 21:357–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J, Gong W, Chen J, Qing Y, Wu S, Li H, Huang C, Chen Y, Wang Y, Xu Z, Liu W, Li H, Long H (2018) Micheliolide alleviates hepatic steatosis in db/db mice by inhibiting inflammation and promoting autophagy via PPAR-gamma-mediated NF-small ka, CyrillicB and AMPK/mTOR signaling. Int Immunopharmacol 59:197–208. [DOI] [PubMed] [Google Scholar]
- Zhou X, Cao Y, Ao G, Hu L, Liu H, Wu J, Wang X, Jin M, Zheng S, Zhen X, Alkayed NJ, Jia J, Cheng J (2014) CaMKKbeta-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid Redox Signal 21:1741–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Liu K, Huang K, Gu Y, Hu Y, Pan S, Ji Z (2018) Metformin Improves Neurologic Outcome Via AMP-Activated Protein Kinase-Mediated Autophagy Activation in a Rat Model of Cardiac Arrest and Resuscitation. J Am Heart Assoc 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XC, Jiang T, Zhang QQ, Cao L, Tan MS, Wang HF, Ding ZZ, Tan L, Yu JT (2015) Chronic Metformin Preconditioning Provides Neuroprotection via Suppression of NF-kappaB-Mediated Inflammatory Pathway in Rats with Permanent Cerebral Ischemia. Mol Neurobiol 52:375–385. [DOI] [PubMed] [Google Scholar]