Abstract
A pivotal metabolic function of insulin is the stimulation of glucose uptake into muscle and adipose tissues. The discovery of the insulin-responsive glucose transporter type 4 (GLUT4) protein in 1988 inspired its molecular cloning in the following year. It also spurred numerous cellular mechanistic studies laying the foundations for how insulin regulates glucose uptake by muscle and fat cells. Here, we reflect on the importance of the GLUT4 discovery and chronicle additional key findings made in the past 30 years. That exocytosis of a multispanning membrane protein regulates cellular glucose transport illuminated a novel adaptation of the secretory pathway, which is to transiently modulate the protein composition of the cellular plasma membrane. GLUT4 controls glucose transport into fat and muscle tissues in response to insulin and also into muscle during exercise. Thus, investigation of regulated GLUT4 trafficking provides a major means by which to map the essential signaling components that transmit the effects of insulin and exercise. Manipulation of the expression of GLUT4 or GLUT4-regulating molecules in mice has revealed the impact of glucose uptake on whole-body metabolism. Remaining gaps in our understanding of GLUT4 function and regulation are highlighted here, along with opportunities for future discoveries and for the development of therapeutic approaches to manage metabolic disease.
Keywords: glucose transport, glucose metabolism, insulin, exercise, diabetes, signal transduction, GLUT4 storage vesicle (GSV), metabolic syndrome, solute carrier family 2 member 4 (SLC2A4), vesicle traffic
Introduction
Metabolic diseases like type 2 diabetes and nonalcoholic hepatosteatosis have been on a steady incline for the past 2 decades, and the economic burden to society is profound. Insulin resistance is a unifying feature of most if not all metabolic diseases. Hence, it is widely accepted that every effort must be made to understand the underlying cause of insulin resistance if we are to slow the progression of these diseases. Given that one of the most important actions of insulin in the body is to stimulate glucose transport into muscle and adipose tissue, defects in which contribute to whole-body insulin resistance in humans, there is much focus on delineating the regulatory features of this system. Here, we provide a perspective on key accomplishments that have been made over the past 30 years since the insulin responsive glucose transporter was first discovered.
Unlike most other cells in the body, where glucose transport is constitutively “on,” in muscle and fat cells, glucose transport is rapidly up-regulated severalfold in response to insulin, and in muscle also by exercise. The discovery of GLUT4 30 years ago was a paramount advance in metabolic research because it provided a molecular and cell biological explanation for how insulin and exercise regulate glucose transport into muscle and fat cells. GLUT4 is principally expressed in skeletal and cardiac muscles as well as in adipocytes, both brown and white, the same cells that exhibit a profoundly insulin-sensitive glucose transport system (1–5).
Here, we reflect on the importance of the GLUT4 discovery and chronicle several key findings made in the past 3 decades, highlight remaining open questions, and outline possible ways to capitalize on the knowledge to date for the treatment of metabolic disease.
A brief history
GLUT4 cloning, storage, and exocytosis
As early as 1939, Einar Lundsgaard showed that insulin stimulates glucose uptake into rodent muscle. However, our cellular understanding of this phenomenon was set in motion in 1980, with the pivotal report that insulin promotes the redistribution of a glucose transporter from inside the cell to the plasma membrane, giving rise to the translocation hypothesis, first in adipocytes (6–8) and a few years later in muscle (9, 10). These pioneering studies used subcellular fractionation and binding of cytochalasin B (a glucose-sensitive ligand) or glucose uptake into isolated vesicles to propose that glucose transporters “translocate” from intracellular membranes to the plasma membrane of adipocytes and muscle tissue prestimulated with insulin. At the time in the early 1980s, however, molecular cloning was relatively new, and the concept of gene families had not been widely considered, and therefore few had contemplated the possibility that there could be more than one member of the facilitated glucose transporter family. This concept became reality shortly after the cloning of the first mammalian glucose transporter in 1985, now known as GLUT1, one of the landmark discoveries in the field. At the time, it was assumed this was the same transporter that regulated glucose transport in muscle and fat cells. However, GLUT1 was expressed in all cells, yet insulin (and exercise) only activated glucose transport to any great extent in muscle and fat cells. In fact, one of the controversies at that time was that using antibodies against GLUT1, one could observe insulin-dependent movement of an immunoreactive band from intracellular membranes to the plasma membrane, but the magnitude of this change was far less than the observed change in cellular glucose transport. Was this due to some technical issue of subcellular fractionation, poor affinity of the antibodies used, or activation of the transporter? This controversy was largely laid to rest when in 1988 James et al. (4) produced a mAb against a glucose transporter species that had the cell biological properties expected for an insulin-responsive glucose transporter, another key finding in the ultimate resolution of insulin-regulated glucose transport. By immunizing mice with purified intracellular membranes thought to be enriched in what could have been insulin-responsive glucose transporters, they selected for antibodies that recognized highly insulin-responsive proteins. Fortuitously, one of these antibodies was highly selective for a protein later identified by molecular cloning as the insulin-regulated glucose transporter, eventually named GLUT4. This inspired five separate groups to isolate and sequence cDNA clones that encoded GLUT4 from diverse adipose and muscle systems (1–5).
The molecular cloning of GLUT4 was a crucial step in delineating the mechanism of regulated glucose transport in muscle and fat cells because it revealed that it was part of a larger highly homologous facilitated glucose transporter family, comprising at least five members. Each member was distinguished from the other by its unique tissue distribution, amino acid sequence, and kinetic transport properties (for a review, see Ref. 12).
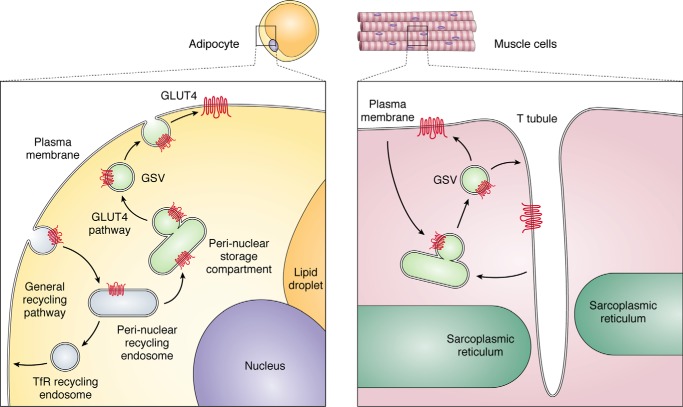
Second, it was crucial because it enabled the production of isoform-specific antibodies that provided unambiguous examination of the properties of GLUT4 independently of other isoforms. A series of cell biology studies verified the translocation hypothesis using GLUT4-specific antibodies and photoaffinity labeling reagents (13–17), followed by immunofluorescence microscopy (18–22) or high-resolution EM (23–26). Collectively, these studies showed that in basal adipocytes, GLUT4 was virtually absent from the cell surface and enriched in 70-nm-diameter tubulo-vesicular structures called GLUT4 storage vesicles (GSVs)5, clustered in the vicinity of the trans-Golgi network (TGN)/perinuclear endocytic recycling compartment and distributed in vesicles throughout the cytosol. With insulin stimulation, a new steady state is reached with ∼50% of GLUT4 redistributed from these intracellular structures to the plasma membrane. These studies provided clear evidence that insulin indeed stimulates the translocation of the glucose transporter from intracellular membranes to the cell surface, and based upon the characteristics and magnitude of this effect, this mechanism was sufficient to account for the respective change in glucose transport observed in response to insulin. General aspects of the GLUT4 itinerary are illustrated in Fig. 1. Attention next shifted to dissecting the cell biology of this process.
Figure 1.
General aspects of GLUT4 cycling that emerged over the years and have served as a template to understand the dynamic regulation of the transporter itinerary within adipose (left) and muscle (right) cells. TfR, transferrin receptor (recycling endosome marker); GSV, GLUT4 storage vesicles.
Dynamics of GLUT4 trafficking
From the early 1990s on, dynamic trafficking studies using tagged or fluorescent GLUT4 fusion proteins revealed that what distinguishes GLUT4 from other recycling proteins is its intrinsic slow exocytic rate in insulin's absence due to its unique partitioning into GSVs (28–34). Importantly, GSVs do not exist in nonspecialized cells, such as fibroblasts, explaining why GLUT4 trafficking in such cells is so different from that in bona fide insulin-sensitive cell types (adipocytes and muscle). Because of the need to perform spatiotemporal analysis of GLUT4 localization, most such mechanistic trafficking studies have been performed in cultured cells, predominantly 3T3-L1 adipocytes, as they possess a robust insulin-responsive GLUT4 system. L6 myotubes have been used as models to investigate GLUT4 trafficking in muscle cells. For the past 2 decades, studies in these cultured cell lines relied on tracking transfected, tagged versions of the transporter, using either static morphological approaches in fixed cells or more dynamic studies using live-cell microscopy aided by GFP-tagged versions of GLUT4.
Key aspects of GLUT4 traffic have been realized and are widely accepted; insulin increases GLUT4 at the plasma membrane principally by mobilizing GLUT4 from GSVs to the membrane and by accelerating the GLUT4 exocytosis rate. With insulin, GLUT4 is not statically maintained in the plasma membrane but continuously recycles. Upon insulin removal, cell surface GLUT4 rapidly returns to prestimulation levels as the kinetics of exocytosis and endocytosis reset back to basal rates. The re-sequestered GLUT4 can be recruited again to the plasma membrane with a second round of insulin stimulation. For the most part, these tenets have been verified in mature adipocytes for the native GLUT4 and, to some degree, by localizing endogenous or transfected GLUT4 in muscle fibers. Notably, through photoaffinity labeling of the endogenous GLUT4 in skeletal muscle, without the need for fractionation, it was verified that the gain in surface transporters matched the increase in glucose uptake in response to insulin (35).
The importance of fully understanding the elements and dynamics governing GLUT4 traffic is brought to light by the fact that plasma membrane levels of GLUT4 in rodent and human fat and muscle tissues are elevated in the postprandial state in vivo (36, 37) when glucose transport into muscle and fat cells is high, and conversely they are reduced in the fasted state when glucose transport into those cells is low.
The GLUT4 itinerary and its many unknowns
A major challenge to the full dissection of how GLUT4 navigates throughout the cell is that its normal itinerary includes transit through both “specialized” and general intracellular compartments. GLUT4 is internalized from the plasma membrane in great part via clathrin-mediated endocytosis together with other recycling cargo, such as the transferrin receptor, albeit the recycling rate of GLUT4 is much slower than that of other recycling proteins, under both basal and insulin-stimulated conditions (18, 38). GLUT4 diverges from other recycling cargo and moves from endosomes to a subdomain of the TGN demarcated by the SNARE proteins Syntaxin6 and Syntaxin16, and from here it is packaged into GSVs (33, 39–41), from which insulin causes its discharge (31). Hence, GLUT4 transits through endosomal and TGN compartments as it cycles through the cell, and therefore it is also cargo of those pathways. Consequently, the membrane transport machineries dedicated to those pathways, which are not specific to the GSV exocytosis pathway, are also required for proper GLUT4 traffic. Importantly, whereas the net amount of GLUT4 in the endosomal and TGN compartments is quite high in the presence of insulin, under basal conditions, the majority of GLUT4 is kinetically sequestered in GSVs with only a small amount of GLUT4 localized to endosomes, TGN, and the plasma membrane at any given time (42). The continued relay of GLUT4 away from the constitutive recycling pathway is also responsible for the long half-life of the protein, and this becomes a hallmark of the existence of a sequestration mechanism represented by redirection toward GSVs.
It is not surprising that, because a portion of GLUT4 is found in endosomes and the TGN even under basal conditions, proteomic analysis of immunopurified GLUT4-containing membranes from adipocytes revealed not just GSV proteins like GLUT4, the insulin-regulated aminopeptidase (IRAP), and the low-density lipoprotein receptor-related protein-1 (LRP1), but also constituents of the generic endosomal and TGN systems, including all of the Rab GTPases found in these compartments (Rabs 1–8, 10, 11, 14, 18, and 35) (43, 44). Corresponding to its itinerary, the GLUT4 molecule contains a number of amino acid motifs that choreograph its journey throughout this complex endomembrane system. These include an FQQI motif in the amino cytoplasmic domain and LL and TELEY motifs in the carboxyl cytoplasmic domain (18, 23, 45–48). These motifs regulate GLUT4 endocytosis and its sorting into GSVs (28, 33, 49–51). The large intracellular loop has also been implicated in intracellular sorting (51). The trafficking machinery that interacts with these different motifs is poorly defined, although roles for the GGA, retromer, and AP1 adaptor complexes have been proposed (52–55). The core molecular machinery required for GSV formation remains to be identified. In this regard, a handful of molecules like sortilin, retromer, and clathrin heavy chain-22 (56, 57) have been proposed to participate in retrograde GLUT4 trafficking and GSV biogenesis. Clathrin heavy chain-22 is particularly interesting because it is expressed in humans but not in rodents. Additionally, TUG and TC10 have been implicated in GLUT4 exocytosis (58, 59). Detailed analysis of in vivo models is required to fully understand the role of all these proteins in regulating metabolism. There are a number of review articles describing the various roles ascribed to these and additional proteins in fine-tuning GLUT4 traffic, so they will not be described in detail here (60–67).
Reflecting on the components established for the GLUT4 traffic model, the next goal should be to analyze GLUT4 traffic spatiotemporally in mature adipocytes and muscle fibers, whether by transfecting tagged GLUT4 or detecting the endogenous transporter. To some degree, this should become possible through the use of new antibodies that recognize extracellularly facing GLUT4 epitopes (68) and mini-antibodies. Of particular interest will be to identify the machinery that accelerates the rate constant of GLUT4 exocytosis in addition to the increase in the GLUT4 available for discharge. Another feature of GLUT4 trafficking that has confounded the analysis of insulin-regulated GSV exocytosis is that most cells, including generic fibroblasts, exhibit some kind of regulated traffic between endosomes and the plasma membrane. For example, several different growth factors increase surface levels of a range of nutrient receptors and transporters, such as the transferrin receptor, amino acid transporters, and other glucose transporters like GLUT1. What has recently become clear is that adipocytes and muscle cells also possess this generic regulated recycling system in addition to the more specialized GSV exocytosis system (69). However, it has proven challenging to molecularly distinguish these systems from each other. Importantly, whereas there is a 2-fold increase in the level of generic recycling proteins at the cell surface with insulin, the increase in GLUT4 and other GSV proteins like IRAP is >10-fold. This has given rise to the concept that GSVs are distinct from endosomes.
In the years 2004–2012, employing live-cell total internal reflection fluorescence microscopy (TIRFM) to examine GSVs approaching the cell surface in response to insulin, it was shown that these vesicles are not only enriched in Rab10, a GTPase involved in insulin-dependent GLUT4 exocytosis, see later, but they move to and fuse with the plasma membrane independently of endosomes (70). Several other studies have used TIRFM to analyze the effect of insulin on docking versus fusion of GSVs at the plasma membrane (71–75). However, these studies are limited by the inability to distinguish between GSVs and GLUT4-positive endosomes. Hence, it may be fruitful to combine expression of tagged Rabs with high-resolution TIRFM to compare docking and fusion of GSVs versus endosomes in adipocytes in the presence and absence of insulin.
Another question that arises is whether GLUT4 that is discharged into endosomes in the presence of insulin requires repackaging into GSVs to recycle back to the cell surface, or would it simply recycle via the generic pathway until insulin is withdrawn? Analysis of GLUT4 recycling in fibroblasts and adipocytes favors the former (31, 76); however, such observations are complicated by the aforementioned challenge that to get to GSVs, GLUT4 and other cargo first must transit through the endosomal/TGN system.
Relaying the information of insulin signaling to vesicle traffic
Whereas the studies above provided essential information about the complexity of GLUT4 trafficking in cells, particularly adipocytes, they did not address the specific components that actually orchestrated the redistribution of the transporters to the cell surface. Fortuitously, the details of the insulin-signaling pathway were also being defined in parallel, and in the 1990s, through elegant studies by a number of groups, it was shown that the insulin receptor was a tyrosine kinase, and upon activation it phosphorylates insulin receptor substrate proteins like IRS1 that acted to recruit adaptors to the plasma membrane. One of these, phosphatidylinositol 3-kinase, led to increased phosphatidylinositol 3,4,5-trisphosphate at the plasma membrane, leading to the activation of Akt. Using small-molecule inhibitors of the phosphatidylinositol 3-kinase/Akt pathway, it was shown that this pathway plays an essential role in insulin-regulated glucose transport in both muscle and fat cells. The discovery in 2003 that a RabGAP, TBC1D4 (also known as AS160), is a highly insulin-responsive Akt target (77) provided one of the first links between insulin signaling and GLUT4 translocation, given that a major function of Rab GTPases is to regulate vesicle traffic. TBC1D4 emerged as a negative regulator in the insulin transduction relay, since overexpressing a phosphorylation-defective mutant reduced insulin-dependent GLUT4 translocation and, conversely deletion of TBC1D4 elevated GLUT4 levels in the plasma membrane in the absence of insulin stimulation. Active research is still uncovering the various mechanisms for how TBC1D4 exerts its regulation, and hence we next expand on these to expose current questions (77).
TBC1D4 possesses four separate functions relevant to regulated GLUT4 trafficking: 1) GAP activity, 2) Akt substrate and 14-3-3 binding, 3) lipid binding, and 4) GSV binding
GAP activity
The active site of TBC1D4 displays GAP activity in vitro against a variety of Rab GTPases, including Rab8, Rab10, and Rab14, thereby maintaining them inactive, and functionally, these Rab GTPases along with the closely related Rab13 play a role in GLUT4 trafficking in adipose and/or muscle cells (78, 79). Rab10 and Rab14 have been identified on purified GSVs by MS (80), and TIRF microscopy identified Rab10 as the main Rab protein associated with GSVs arriving at the plasma membrane of insulin-stimulated adipocytes (70).
Akt substrate and 14-3-3 binding
Initially, six insulin-regulated phosphorylation sites were described for TBC1D4, and proteomic analysis has identified up to 26, with three proven to be Akt substrates (77). Akt-dependent phosphorylation catalyzes binding of 14-3-3 to TBC1D4 (81) and the TBC1D4 phosphorylation-defective mutant re-engineered to constitutively bind to 14-3-3 protein can no longer prevent GLUT4 translocation (81). Accordingly, it has been suggested, although not experimentally proven, that phosphorylation and/or 14-3-3 binding directly inhibits TBC1D4 GAP activity. A recent study has shown that the TBC1D4 ortholog TBC1D1 that is also phosphorylated by Akt and AMPK does not undergo any significant change in GAP activity following its phosphorylation (82). However, the stoichiometry of TBC1D1 phosphorylation was not carefully assessed in this study, so it is unclear what proportion of the total TBC1D1 pool that was under investigation was actually phosphorylated.
Lipid binding
TBC1D4 possesses an N-terminal phosphotyrosine-binding domain that regulates its association with the plasma membrane (83), suggesting that it may impart regulation at this location.
GSV binding
TBC1D4 binds to GSV cargo, including the cytosolic tails of IRAP and LRP1, and this interaction is reduced in response to insulin (43, 84). In fact, phosphorylation of TBC1D1, determines its dissociation from IRAP rather than its inactivation (82). Assuming that, as with TBC1D1, phosphorylation of TBC1D4 does not regulate its GAP activity, then phosphorylation-dependent release of TBC1D1/TBC1D4 from GSVs may be the major mechanism for regulating the activity of this protein, enabling GTP loading of Rabs on GSVs. However, it has also been shown that a GLUT4-TBC1D4 fusion protein translocates to the cell surface in adipocytes in response to insulin, whereas a fusion protein comprising mutations in the major Akt phosphorylation sites in TBC1D4 was blocked (84). These studies tend to exclude a central role for TBC1D4 release from membranes as a major regulatory mechanism. Thus, further work is required to resolve the role of phosphorylation in the activity of these proteins.
The above observations have led to a model where, in the absence of insulin, TBC1D4 binds to GSVs and represses GLUT4 exocytosis by blocking activation (GTP loading) of its Rab targets. Akt-dependent phosphorylation of TBC1D4 triggers 14-3-3 binding, inhibiting the interaction between TBC1D4 and GSV components like IRAP, releasing TBC1D4 into the cytosol, and enhancing GTP loading of its Rab GTPases on GSVs, which in turn must engage effectors to enact mobilization to and/or fusion with the plasma membrane. The N-terminal lipid-binding phosphotyrosine-binding domain in TBC1D4 may contribute to GSV docking at the cell surface, raising the possibility that TBC1D4 phosphorylation by Akt may also occur after GSVs have docked at the cell surface. Aligned with this, an in vitro reconstitution assay showed that most of the insulin regulation required for GSV fusion with the plasma membrane tracks with the purified plasma membrane (85), and phosphorylated TBC1D4 was found to be enriched at the plasma membrane (86). Hence, in this model, TBC1D4 may actually contribute to docking GSVs at the plasma membrane prior to their fusion. Based upon the similarities between TBC1D1 and TBC1D4 function, it is predicted that the same model applies to exercise-mediated GLUT4 translocation in muscle via TBC1D1 and other kinases like AMPK.
TBC1D4-regulated Rab GTPases directing GLUT4 vesicle trafficking
The implication of a RabGAP connecting the Akt pathway to GLUT4 trafficking brought into full focus the identity of the Rab GTPase itself that serves as the substrate of TBC1D4. Using a biochemical approach, Lienhard and colleagues (87) showed that the TBC domain of TBC1D4 had reasonable GAP activity toward a subset of the 60 Rabs expressed in mammals, namely Rabs 2A, 8A, 10, and 14. It is notable that in this assay, the GAP activity of the TBC1D4 fragment used was relatively low compared with that observed for other GAPs. In a recent study examining TBC1D1 expressed in Sf9 cells, it was shown that the GAP activity of the full-length protein was markedly higher than that of the GAP domain alone (82). Hence, it would be fruitful to re-examine the Rab GTPase specificity of TBC1D4 using the full-length protein. Rab10 is the only known TBC1D4 substrate in adipocytes that is clearly functionally linked to GLUT4 translocation to the plasma membrane for the following reasons: 1) Rab10 knockdown blunts insulin-stimulated GLUT4 translocation to the plasma membrane, similar in phenotype to overexpression of the TBC1D4 dominant inhibitory mutant; 2) Rab10 knockdown reverses the effects of TBC1D4 knockdown in adipocytes, providing strong evidence that Rab10 is downstream of TBC1D4 (79); 3) adipose-specific Rab10 knockout blunts insulin-stimulated glucose uptake into adipose tissue in vivo and in vitro and blunts GLUT4 insulin-stimulated GLUT4 translocation in in vitro differentiated adipocytes from Rab10 knockout mice (88); and 4) adipose-specific Rab10 knockout is accompanied by dysregulation of whole-body glucose homeostasis similar to adipose-specific GLUT4 knockout (88). Rab14 has also been implicated to some degree in GLUT4 trafficking in adipocytes (89, 90). Conversely, in L6 myocytes, Rab8a and Rab13 act downstream of TBC1D4 and have dominating effects on GLUT4 translocation (91, 92).
The above work on TBC1D4 and the identification of the Rab substrates has pinpointed three major putative sites of action of insulin required to implement increased GLUT4 at the cell surface: release of GSVs from their intracellular retention, movement of GSVs to the plasma membrane, and docking and fusion of GSVs with the plasma membrane. The current controversy around which of these steps is regulated by TBC1D4/Rab10 represents a challenge to our full understanding of this process. One view proposes that the main regulated step is the docking/fusion with the plasma membrane (93), possibly through myosin Va (70), thereby raising the possibility that additional Akt substrates are required to execute insulin-dependent GSV traffic to this site. On the other hand, the identification of Sec16A as a major effector of Rab10 in adipocytes supports its input at the level of GSV formation (94). In myoblasts, myosin Va is a Rab8a effector regulating GLUT4 at perinuclear regions (91), and it is the Rab13 effectors MICAL-L2 and actinin-4 that act at the cell periphery (92). Future studies are required to come to consensus as to the precise GLUT4-trafficking steps that are regulated by these effectors and to establish whether they are cell type–specific.
Beyond TBC1D4 and its Rab GTPases
Although highly relevant, only about half of the effect of insulin-stimulated GLUT4 translocation can be accounted for by the TBC1D4-Rab10 signaling module in adipocytes (79, 88), establishing that additional Akt targets are required for insulin control of GLUT4. The small GTPase RalA, acting via the exocyst, contributes to GLUT4 translocation, and recent evidence supports its activation downstream of Rab10. Whether this accounts for the remaining insulin effect on GLUT4 not explained by TBC1D4 remains to be determined. In addition, especially in muscle, other signals downstream of phosphatidylinositol 3-kinase, such as activation of Rho-family GTPases, in particular Rac1, exert regulatory inputs in parallel to the Akt axis (95). A major outcome of Rac1 activation by insulin is the reorganization of actin filaments beneath the membrane, required for GLUT4 translocation (96). Cortical actin filaments interact with the molecular motor Myo1c that aids in GSV tethering beneath the membrane (97, 98) and contribute to GLUT4 vesicle positioning for subsequent fusion (99). The next years should focus on elucidating these collective mechanisms and how they exert the overall displacement of GSVs and their positioning for optimal fusion with the plasma membrane.
Contrasting with the wealth of knowledge gained to date on the mechanism and regulation of GLUT4 intracellular traffic, the catalytic activity of the transporter remains far less explored, although it has been considered in studies over the 30 years since GLUT4 cloning (100–105). Given that the activity of most other nutrient transporters is subject to regulation, a compelling future goal will be to solve the structure of GLUT4 and to gain understanding of how metabolic and hormonal signals potentially control glucose transport activity via alterations in GLUT4 architecture.
Regulation of GLUT4 by exercise
The discovery of GLUT4 also contributed significantly to our understanding of the metabolic actions of exercise. A substantial increase in muscle glucose uptake is pivotal to sustain the energetic cost of muscle activity. A rise in glucose uptake into muscle during exercise was formally documented by Holloszy and Narahara in 1965 (106). When GLUT4 was cloned and its abundance in skeletal muscle was determined, it became pressing to determine its participation in the exercise response. This was evinced by the reduction in glucose uptake into contracting muscles of GLUT4-null mice (107, 108). Before that, however, studies in the 1990s using subcellular fractionation showed that exercise and muscle contraction promote a gain in GLUT4 at both the sarcolemma and transverse tubule membranes (13, 109, 110). Importantly, the gain in surface GLUT4 upon simultaneous muscle contraction and insulin stimulation was additive (111). These findings were nicely reinforced using immunofluorescence and immunoelectron microscopy, whereby contraction appeared to deplete GLUT4 from transferrin receptor–positive endosomes that were not affected by insulin (24, 112), visually supporting the concept that the intracellular depots mobilized by exercise differ from those accessed by insulin (13, 24). However, like insulin, exercise also reduces GLUT4 in the TGN area, in the subsarcolemmal cytoplasm, and along the sarcomeric bands, consistent with the concept that there is overall mobilization of the continuum of GLUT4-containing compartments. This suggests that, as in adipocytes, the diverse GLUT4 pools are in constant communication with each other.
Tracing the signals that promote increased surface GLUT4 in contracting muscle has proven challenging, due in part to the lack of suitable cell culture models that faithfully reproduce muscle contraction and the limitations of performing live analysis in a contracting muscle. The signals involved include an increase in cytosolic Ca2+, activation of the enzyme AMPK (although this has been contested in some studies (113)), and several other and possibly additive pathways (24–27) that may converge downstream to mobilize GLUT4 and thus regulate glucose uptake. Indeed, beyond promoting GLUT4 increases in the plasma membrane, a single bout of exercise can also sensitize the muscle to insulin, causing a left shift in the glucose uptake response to insulin (114). How GLUT4 traffic is sensitized under these conditions is still being investigated.
Notably, both TBC1D4 and particularly TBC1D1 are phosphorylated in response to muscle contraction/exercise, illustrating that, although different vesicular pools and signals are engaged by contraction and insulin, there is convergence in some downstream signals. Whereas TBC1D4 regulates aspects of GLUT4 exocytosis in response to insulin, the steps that either TBC1D1 or TBC1D4 regulate during exercise remain to be mapped, and their relative contributions remain to be discerned (115). Depletion of these RabGAPs is associated with reduced GLUT4 protein levels, raising the possibility that under these conditions, the transporter is mis-sorted and rerouted to the degradative pathway (11). If so, these RabGAPs are not only involved in GSV translocation to the plasma membrane in response to stimuli but may also play a crucial role in GLUT4 sequestration in GSVs, protecting it from degradation (116, 117).
Importantly, insulin resistance does not affect the acute exercise-mediated increase in glucose uptake or its underlying elevation in muscle surface GLUT4. This, along with the near additivity in the GLUT4 response caused by insulin plus exercise, supports the concept that the two stimuli access distinct intracellular pools of the transporter. It is tempting to consider that the distinct pools of GSVs might be demarcated by TBC1D4 or TBC1D1 regulation, respectively. The available and future evidence supporting the difference in pools and signals will present the opportunity to bypass insulin resistance of GLUT4 translocation by promoting GLUT4 traffic through the exercise/contraction pathway. Further work is required to achieve a full definition of the mechanistic similarities and differences in GLUT4 regulation by insulin and muscle contraction.
GLUT4 in human metabolic disease
A hallmark clinical feature of insulin-resistant individuals and those suffering from type 2 diabetes (T2D) is reduced clearance of glucose from the blood, and this is mainly due to impaired insulin-stimulated glucose transport into muscle and adipocytes. Compelling evidence using 31P NMR measurements shows that glucose transport into muscle is rate-limiting for insulin-stimulated glycogen synthesis and nonoxidative glucose metabolism in individuals with T2D (118). Earlier studies had shown reduced uptake of the nonmetabolizable sugar 3-O-methylglucose into perfused or isolated muscles of diabetic rats that notably could be improved, although not restored, by muscle contraction (119, 120). These collective studies suggested that defective GLUT4 translocation to the cell surface is a major lesion contributing to insulin resistance in muscle and possibly adipocytes, and evidence to this effect soon emerged.
The total level of GLUT4 is reduced by 50% in adipose tissue from humans with T2D but unchanged in skeletal muscle (121). Is the glucose transport defect in adipose tissue purely a function of reduced GLUT4 levels? This is likely not the case, at least in gestational diabetes, because not all individuals display reduced GLUT4 levels in their fat cells, yet all present a similar reduction in insulin-stimulated glucose transport (122). Direct investigation of this question had started already in the late 1980s, when a defect in insulin-dependent GLUT4 translocation to the muscle plasma membrane was documented in rodent models with diverse origins of insulin resistance (123–126). In the late 1990s, impaired insulin-dependent redistribution of GLUT4 was shown in muscle from humans with T2D (127–129). Subcellular fractionation studies also showed a defect in insulin-dependent GLUT4 translocation in gestational diabetes, whereas insulin-regulated movement of the constitutively recycling GLUT1 transporter was unaffected.
Several of the studies mentioned above also revealed aberrant intracellular distribution of GLUT4 already in basal conditions, raising the possibility that the defect may lie in the intracellular sorting of the transporter (125, 127). Notably, the defect in muscle GLUT4 traffic in T2D is relatively specific to insulin action because exercise-modulated GLUT4 translocation to the cell surface is unaffected (109). This is consistent with the observations that insulin and exercise draw on GLUT4 from distinct intracellular compartments. Hence, one of the beneficial effects of exercise in relieving metabolic disease may lie in the differential action of exercise and insulin on GLUT4 traffic. Thus, it is tempting to speculate that insulin resistance involves defective trafficking of GLUT4 selectively into insulin-responsive GSVs. Moreover, the defect is restricted to insulin regulation of GLUT4/glucose uptake, consistent with observations in model adipocyte systems and fat tissue from mice with diet-induced obesity that insulin resistance is selective and specific to GLUT4 traffic relative to other metabolic outcomes (130, 131).
The fact that insulin resistance is relatively specific to GLUT4 trafficking in muscle and fat cells is inconsistent with the disease phenotype arising from a generic defect in one of the signaling components proximal to the insulin receptor, as this would result in defects in all of the hormone's actions (132, 133). Whereas numerous studies report a reduction in Akt phosphorylation in fat and muscle tissue of diabetic and obese prediabetic animals and humans, cellular studies show that, as a single defect, Akt must be reduced by over 80% or specifically at the plasma membrane to significantly impact on GLUT4 translocation (reviewed in 67, 134). In fact, in muscle from humans with insulin resistance and/or T2D, despite a 40–50% defect in insulin-stimulated Akt phosphorylation at its two major regulatory sites (Thr-308 and Ser-473), there is little disruption of insulin-dependent phosphorylation of a range of Akt substrates (135). Thus, it is unlikely that defects in proximal insulin signaling are major contributors to insulin resistance in human muscle and adipose tissue. On the other hand, mutations in the Akt substrate TBC1D4 have been associated with insulin resistance and diabetes in Greenlandic Inuit people (134). Despite the fact that this mutation is not common to other ethnic groups, it highlights that lesions that are specific to the GLUT4 regulatory arm of insulin action are associated with metabolic disease in humans.
A more refined description of the nature of the defect(s) in GLUT4 traffic in both muscle and adipose tissue awaits, especially defining whether the defect(s) involves impaired sorting of GLUT4 into GSVs or defective GSV mobilization and/or fusion with the cell surface. Resolving this could benefit from new advances in serial block-face scanning EM combined with automated three-dimensional reconstruction and quantification, as well as from detection with high-affinity, conformation-specific antibodies that recognize exofacial domains of the transporter (68). The information gained could provide essential clues toward understanding the molecular underpinnings of insulin resistance.
The analysis of the role of GLUT4 in metabolism and disease would be incomplete without pointing out its localization in discrete loci outside of muscle and adipose tissues, specifically within areas of the brain (136–138), cerebellum (139), and kidney (140, 141). Undoubtedly, the future will disclose more about the function of the transporter in these areas and its impact on whole-body metabolism as well as its potential failure leading to disease.
GLUT4 or GLUT4 pathway as a therapeutic target?
In addition to providing considerable insight into vesicle transport and the insulin-signaling pathway, the discovery of GLUT4 has advanced our understanding of whole-body metabolism and in particular of tissue cross-talk. Most notably, as shown in 2005, deletion of GLUT4 specifically in adipocytes leads to major metabolic defects throughout the body, including in liver and muscle (142). This supports the concept that defects in insulin regulation of GLUT4 trafficking could contribute to whole-body insulin resistance in humans. The impact of tissue-specific deletion of GLUT4 on metabolic defects in other tissues has given rise to the notion that glucose metabolism in one tissue regulates the release of circulating factors that influence metabolism elsewhere. Several such factors like the secretory hormone RBP4 (143) or a novel class of adipocyte lipids (branched fatty acid esters of hydroxy fatty acids) have been identified (144), and substantiation of their participation is being vigorously pursued (145). Alternatively, this tissue cross-talk may reflect the metabolic switching first proposed by Randle and colleagues in 1963 (146), describing a reciprocity in the use of either fatty acids or glucose by muscle and fat cells. Whether this is mediated by allosteric control, as originally proposed (i.e. glucose uptake responding to a “pull” signal), or by fatty acids directly blocking insulin-mediated GLUT4 translocation (a “push” mechanism) (147–149) requires further investigation.
Attempts to positively manipulate glucose uptake have also been made by overexpressing GLUT4 in various tissues of transgenic mice. Whereas these studies have in general yielded very positive outcomes on glucose metabolism (150), they have also shown how important it is to maintain GLUT4 expression within its physiological range. Indeed, supraphysiological expression of GLUT4 caused hypoglycemia, hypoinsulinemia, and lactacidemia. Other strategies, more directly focused on the process that is actually defective in insulin resistance or on the pathways utilized during exercise to evoke GLUT4 translocation, are likely to be more effective.
Despite the caveats with the GLUT4 overexpression studies, they have provided important clues of future clinical utility. First, as observed in GLUT4-overexpressing mice, improving insulin-mediated GLUT4 translocation in muscle will likely lower blood glucose levels and consequently insulin as well, both outcomes being metabolically desirable in insulin-resistant states. Such an approach might be more efficacious than alternate strategies, such as insulin administration, which has other undesirable consequences. Second, increasing GLUT4 levels may alleviate lipotoxicity particularly in liver, a common feature of metabolic disease, by enhancing the capacity of fat cells to store lipid. Importantly, as shown a decade after GLUT4 cloning from muscle, a boost in its expression is a physiological consequence of exercise training (151, 152), demonstrating that there are physiological pathways that could be accessed to improve glucose redirection to this tissue.
The future
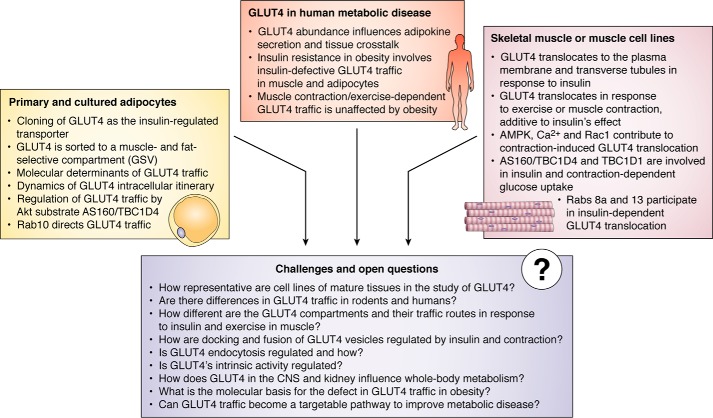
Notwithstanding the intensive efforts to understand GLUT4 biology over the past 30 years, briefly reviewed here and highlighted in Figs. 1 and 2, a number of gaps in our understanding of transporter function and regulation remain. These include the regulation of GLUT4 transport activity in addition to regulation of its translocation to the cell surface; the molecular differences in the GLUT4 pools and traffic pathways regulated by insulin and exercise; the signals beyond the RabGAPs that contribute to GLUT4 mobilization and how these are integrated; the Rab effectors and how these directly enact vesicle budding, mobilization, and fusion; whether GLUT4 vesicle fusion is part of the regulated process; the identification of other GLUT4-regulatory Akt substrates; and ultimately which of these components are altered in the most common forms of human insulin resistance.
Figure 2.
Salient findings in the history of GLUT4 pertaining to its intracellular cycling in adipose and muscle cells or cell models, key aspects of GLUT4's contribution to metabolic dysregulation in obesity/diabetes, and outstanding questions on GLUT regulation and its potential as a therapeutic target to improve health
Beyond mechanistic enquiry, the next 30 years will certainly build on the knowledge gained in the past 30 to investigate how GLUT4 and the GLUT4 pathway can be sourced to treat metabolic disease. It will be paramount to elucidate in molecular detail how the regulation of GLUT4 is altered in insulin-resistant states, whether it is a cause or a consequence, and importantly whether it is a sole or major cause of this condition. As alluded to here, this defect probably does not involve alterations in individual, proximal insulin signaling components such as Akt. However, the nature of the actual defect remains unknown, and identifying it will have a profound impact on our understanding of insulin resistance. On the shoulders of the past 30 years of knowledge, and with rapid developments in a range of technologies, we can expect considerable progress in these and related areas to bring closer the advent of novel classes of insulin sensitizing agents suitable to treat metabolic disease (Fig. 2).
Acknowledgments
We recognize the vast scientific contribution by numerous scientists over 30 years of GLUT4 and that only representative studies are cited in this short retrospective. We sincerely appreciate the help and useful input by Victoria Tokarz as well as the insight and comments by Victoria Tikarz, Daniel Fazakerley, James Krycer, Phil Bilan, Erik Richter, Morrie Birnbaum, and Michael Czech. We thank Yingke Xu for an inspiring conversation toward the writing of this piece.
The authors declare that they have no conflicts of interest with the contents of this article.
- GSV
- GLUT4 storage vesicle
- TGN
- trans-Golgi network
- IRAP
- insulin-regulated aminopeptidase
- LRP1
- low-density lipoprotein receptor-related protein-1
- TIRFM
- total internal reflection fluorescence microscopy
- AMPK
- AMP-activated protein kinase
- T2D
- type 2 diabetes.
References
- 1. Charron M. J., Brosius F. C. 3rd, Alper S. L., and Lodish H. F. (1989) A glucose transport protein expressed predominately in insulin-responsive tissues. Proc. Natl. Acad. Sci. U.S.A. 86, 2535–2539 10.1073/pnas.86.8.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., and Seino S. (1989) Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J. Biol. Chem. 264, 7776–7779 [PubMed] [Google Scholar]
- 3. Garcia de Herreros A., and Birnbaum M. J. (1989) The acquisition of increased insulin-responsive hexose transport in 3T3-L1 adipocytes correlates with expression of a novel transporter gene. J. Biol. Chem. 264, 19994–19999 [PubMed] [Google Scholar]
- 4. James D. E., Brown R., Navarro J., and Pilch P. F. (1988) Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature 333, 183–185 10.1038/333183a0 [DOI] [PubMed] [Google Scholar]
- 5. Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., and Lane M. D. (1989) Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc. Natl. Acad. Sci. U.S.A. 86, 3150–3154 10.1073/pnas.86.9.3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cushman S. W., and Wardzala L. J. (1980) Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell: apparent translocation of intracellular transport systems to the plasma membrane. J. Biol. Chem. 255, 4758–4762 [PubMed] [Google Scholar]
- 7. Suzuki K., and Kono T. (1980) Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc. Natl. Acad. Sci. U.S.A. 77, 2542–2545 10.1073/pnas.77.5.2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wardzala L. J., Cushman S. W., and Salans L. B. (1978) Mechanism of insulin action on glucose transport in the isolated rat adipose cell. Enhancement of the number of functional transport systems. J. Biol. Chem. 253, 8002–8005 [PubMed] [Google Scholar]
- 9. Klip A., Ramlal T., Young D. A., and Holloszy J. O. (1987) Insulin-induced translocation of glucose transporters in rat hindlimb muscles. FEBS Lett. 224, 224–230 10.1016/0014-5793(87)80452-0 [DOI] [PubMed] [Google Scholar]
- 10. Wardzala L. J., and Jeanrenaud B. (1981) Potential mechanism of insulin action on glucose transport in the isolated rat diaphragm: apparent translocation of intracellular transport units to the plasma membrane. J. Biol. Chem. 256, 7090–7093 [PubMed] [Google Scholar]
- 11. Chadt A., Immisch A., de Wendt C., Springer C., Zhou Z., Stermann T., Holman G. D., Loffing-Cueni D., Loffing J., Joost H.-G., and Al-Hasani H. (2015) Deletion of both Rab-GTPase-activating proteins TBC1D1 and TBC1D4 in mice eliminates insulin- and AICAR-stimulated glucose transport. Diabetes 64, 746–759 10.2337/db14-0368 [DOI] [PubMed] [Google Scholar]
- 12. Mueckler M., and Thorens B. (2013) The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 34, 121–138 10.1016/j.mam.2012.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Douen A. G., Ramlal T., Rastogi S., Bilan P. J., Cartee G. D., Vranic M., Holloszy J. O., and Klip A. (1990) Exercise induces recruitment of the “insulin-responsive glucose transporter”: evidence for distinct intracellular insulin- and exercise-recruitable transporter pools in skeletal muscle. J. Biol. Chem. 265, 13427–13430 [PubMed] [Google Scholar]
- 14. Holman G. D., Kozka I. J., Clark A. E., Flower C. J., Saltis J., Habberfield A. D., Simpson I. A., and Cushman S. W. (1990) Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel: correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J. Biol. Chem. 265, 18172–18179 [PubMed] [Google Scholar]
- 15. Holman G. D., Lo Leggio L., and Cushman S. W. (1994) Insulin-stimulated GLUT4 glucose transporter recycling: a problem in membrane protein subcellular trafficking through multiple pools. J. Biol. Chem. 269, 17516–17524 [PubMed] [Google Scholar]
- 16. Satoh S., Nishimura H., Clark A. E., Kozka I. J., Vannucci S. J., Simpson I. A., Quon M. J., Cushman S. W., and Holman G. D. (1993) Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells: evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 268, 17820–17829 [PubMed] [Google Scholar]
- 17. Zorzano A., Wilkinson W., Kotliar N., Thoidis G., Wadzinkski B. E., Ruoho A. E., and Pilch P. F. (1989) Insulin-regulated glucose uptake in rat adipocytes is mediated by two transporter isoforms present in at least two vesicle populations. J. Biol. Chem. 264, 12358–12363 [PubMed] [Google Scholar]
- 18. Garippa R. J., Judge T. W., James D. E., and McGraw T. E. (1994) The amino terminus of GLUT4 functions as an internalization motif but not an intracellular retention signal when substituted for the transferrin receptor cytoplasmic domain. J. Cell Biol. 124, 705–715 10.1083/jcb.124.5.705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haney P. M., Slot J. W., Piper R. C., James D. E., and Mueckler M. (1991) Intracellular targeting of the insulin-regulatable glucose transporter (GLUT4) is isoform specific and independent of cell type. J. Cell Biol. 114, 689–699 10.1083/jcb.114.4.689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malide D., Dwyer N. K., Blanchette-Mackie E. J., and Cushman S. W. (1997) Immunocytochemical evidence that GLUT4 resides in a specialized translocation post-endosomal VAMP2-positive compartment in rat adipose cells in the absence of insulin. J. Histochem. Cytochem. 45, 1083–1096 10.1177/002215549704500806 [DOI] [PubMed] [Google Scholar]
- 21. Marette A., Richardson J. M., Ramlal T., Balon T. W., Vranic M., Pessin J. E., and Klip A. (1992) Abundance, localization, and insulin-induced translocation of glucose transporters in red and white muscle. Am. J. Physiol. 263, C443–C452 10.1152/ajpcell.1992.263.2.C443 [DOI] [PubMed] [Google Scholar]
- 22. Piper R. C., Hess L. J., and James D. E. (1991) Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am. J. Physiol. 260, C570–C580 10.1152/ajpcell.1991.260.3.C570 [DOI] [PubMed] [Google Scholar]
- 23. Piper R. C., Tai C., Slot J. W., Hahn C. S., Rice C. M., Huang H., and James D. E. (1992) The efficient intracellular sequestration of the insulin-regulatable glucose transporter (GLUT-4) is conferred by the NH2 terminus. J. Cell Biol. 117, 729–743 10.1083/jcb.117.4.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ploug T., van Deurs B., Ai H., Cushman S. W., and Ralston E. (1998) Analysis of GLUT4 distribution in whole skeletal muscle fibers: identification of distinct storage compartments that are recruited by insulin and muscle contractions. J. Cell Biol. 142, 1429–1446 10.1083/jcb.142.6.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slot J. W., Geuze H. J., Gigengack S., James D. E., and Lienhard G. E. (1991) Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc. Natl. Acad. Sci. U.S.A. 88, 7815–7819 10.1073/pnas.88.17.7815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., and James D. E. (1991) Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113, 123–135 10.1083/jcb.113.1.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sylow L., Kleinert M., Richter E. A., and Jensen T. E. (2017) Exercise-stimulated glucose uptake: regulation and implications for glycaemic control. Nat. Rev. Endocrinol. 13, 133–148 10.1038/nrendo.2016.162 [DOI] [PubMed] [Google Scholar]
- 28. Govers R., Coster A. C. F., and James D. E. (2004) Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol. Cell Biol. 24, 6456–6466 10.1128/MCB.24.14.6456-6466.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kanai F., Nishioka Y., Hayashi H., Kamohara S., Todaka M., and Ebina Y. (1993) Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J. Biol. Chem. 268, 14523–14526 [PubMed] [Google Scholar]
- 30. Lampson M. A., Racz A., Cushman S. W., and McGraw T. E. (2000) Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. J. Cell Sci. 113, 4065–4076 [DOI] [PubMed] [Google Scholar]
- 31. Lampson M. A., Schmoranzer J., Zeigerer A., Simon S. M., and McGraw T. E. (2001) Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell 12, 3489–3501 10.1091/mbc.12.11.3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li D., Randhawa V. K., Patel N., Hayashi M., and Klip A. (2001) Hyperosmolarity reduces GLUT4 endocytosis and increases its exocytosis from a VAMP2-independent pool in l6 muscle cells. J. Biol. Chem. 276, 22883–22891 10.1074/jbc.M010143200 [DOI] [PubMed] [Google Scholar]
- 33. Shewan A. M., van Dam E. M., Martin S., Luen T. B., Hong W., Bryant N. J., and James D. E. (2003) GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973–986 10.1091/mbc.e02-06-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang Q., Khayat Z., Kishi K., Ebina Y., and Klip A. (1998) GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett. 427, 193–197 10.1016/S0014-5793(98)00423-2 [DOI] [PubMed] [Google Scholar]
- 35. Lund S., Holman G. D., Zierath J. R., Rincon J., Nolte L. A., Clark A. E., Schmitz O., Pedersen O., and Wallberg-Henriksson H. (1997) Effect of insulin on GLUT4 cell surface content and turnover rate in human skeletal muscle as measured by the exofacial bis-mannose photolabeling technique. Diabetes 46, 1965–1969 10.2337/diab.46.12.1965 [DOI] [PubMed] [Google Scholar]
- 36. Goodyear L. J., Hirshman M. F., Napoli R., Calles J., Markuns J. F., Ljungqvist O., and Horton E. S. (1996) Glucose ingestion causes GLUT4 translocation in human skeletal muscle. Diabetes 45, 1051–1056 10.2337/diab.45.8.1051 [DOI] [PubMed] [Google Scholar]
- 37. Kahn B. B. (1994) Dietary regulation of glucose transporter gene expression: tissue specific effects in adipose cells and muscle. J. Nutr. 124, 1289S–1295S 10.1093/jn/124.suppl_8.1289S [DOI] [PubMed] [Google Scholar]
- 38. Habtemichael E. N., Brewer P. D., Romenskaia I., and Mastick C. C. (2011) Kinetic evidence that Glut4 follows different endocytic pathways than the receptors for transferrin and α2-macroglobulin. J. Biol. Chem. 286, 10115–10125 10.1074/jbc.M111.217935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Proctor K. M., Miller S. C. M., Bryant N. J., and Gould G. W. (2006) Syntaxin 16 controls the intracellular sequestration of GLUT4 in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 347, 433–438 10.1016/j.bbrc.2006.06.135 [DOI] [PubMed] [Google Scholar]
- 40. Koumanov F., Pereira V. J., Whitley P. R., and Holman G. D. (2012) GLUT4 traffic through an ESCRT-III-dependent sorting compartment in adipocytes. PLoS One 7, e44141 10.1371/journal.pone.0044141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Foley K. P., and Klip A. (2014) Dynamic GLUT4 sorting through a syntaxin-6 compartment in muscle cells is derailed by insulin resistancecausing ceramide. Biol. Open. 3, 314–325 10.1242/bio.20147898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeigerer A., Lampson M. A., Karylowski O., Sabatini D. D., Adesnik M., Ren M., and McGraw T. E. (2002) GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13, 2421–2435 10.1091/mbc.e02-02-0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jedrychowski M. P., Gartner C. A., Gygi S. P., Zhou L., Herz J., Kandror K. V., and Pilch P. F. (2010) Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling. J. Biol. Chem. 285, 104–114 10.1074/jbc.M109.040428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Larance M., Ramm G., Stöckli J., van Dam E. M., Winata S., Wasinger V., Simpson F., Graham M., Junutula J. R., Guilhaus M., and James D. E. (2005) Characterization of the role of the Rab GTPase-activating protein AS160 in insulin-regulated GLUT4 trafficking. J. Biol. Chem. 280, 37803–37813 10.1074/jbc.M503897200 [DOI] [PubMed] [Google Scholar]
- 45. Garippa R. J., Johnson A., Park J., Petrush R. L., and McGraw T. E. (1996) The carboxyl terminus of GLUT4 contains a serine-leucine-leucine sequence that functions as a potent internalization motif in Chinese hamster ovary cells. J. Biol. Chem. 271, 20660–20668 10.1074/jbc.271.34.20660 [DOI] [PubMed] [Google Scholar]
- 46. Haney P. M., Levy M. A., Strube M. S., and Mueckler M. (1995) Insulin-sensitive targeting of the GLUT4 glucose transporter in L6 myoblasts is conferred by its COOH-terminal cytoplasmic tail. J. Cell Biol. 129, 641–658 10.1083/jcb.129.3.641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shewan A. M., Marsh B. J., Melvin D. R., Martin S., Gould G. W., and James D. E. (2000) The cytosolic C-terminus of the glucose transporter GLUT4 contains an acidic cluster endosomal targeting motif distal to the dileucine signal. Biochem. J. 350, 99–107 10.1042/0264-6021:3500099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verhey K. J., Yeh J. I., and Birnbaum M. J. (1995) Distinct signals in the GLUT4 glucose transporter for internalization and for targeting to an insulin-responsive compartment. J. Cell Biol. 130, 1071–1079 10.1083/jcb.130.5.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blot V., and McGraw T. E. (2006) GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J. 25, 5648–5658 10.1038/sj.emboj.7601462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Blot V., and McGraw T. E. (2008) Molecular mechanisms controlling GLUT4 intracellular retention. Mol. Biol. Cell 19, 3477–3487 10.1091/mbc.e08-03-0236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Capilla E., Suzuki N., Pessin J. E., and Hou J. C. (2007) The glucose transporter 4 FQQI motif is necessary for Akt substrate of 160-kilodalton-dependent plasma membrane translocation but not Golgi-localized γ-ear-containing Arf-binding protein-dependent entry into the insulin-responsive storage compartment. Mol. Endocrinol. 21, 3087–3099 10.1210/me.2006-0476 [DOI] [PubMed] [Google Scholar]
- 52. Gillingham A. K., Koumanov F., Pryor P. R., Reaves B. J., and Holman G. D. (1999) Association of AP1 adaptor complexes with GLUT4 vesicles. J. Cell Sci. 112, 4793–4800 [DOI] [PubMed] [Google Scholar]
- 53. Li L. V., and Kandror K. V. (2005) Golgi-localized, γ-ear-containing, Arf-binding protein adaptors mediate insulin-responsive trafficking of glucose transporter 4 in 3T3-L1 adipocytes. Mol. Endocrinol. 19, 2145–2153 10.1210/me.2005-0032 [DOI] [PubMed] [Google Scholar]
- 54. Watson R. T., Khan A. H., Furukawa M., Hou J. C., Li L., Kanzaki M., Okada S., Kandror K. V., and Pessin J. E. (2004) Entry of newly synthesized GLUT4 into the insulin-responsive storage compartment is GGA dependent. EMBO J. 23, 2059–2070 10.1038/sj.emboj.7600159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yang Z., Hong L. K., Follett J., Wabitsch M., Hamilton N. A., Collins B. M., Bugarcic A., and Teasdale R. D. (2016) Functional characterization of retromer in GLUT4 storage vesicle formation and adipocyte differentiation. FASEB J. 30, 1037–1050 10.1096/fj.15-274704 [DOI] [PubMed] [Google Scholar]
- 56. Pan X., Zaarur N., Singh M., Morin P., and Kandror K. V. (2017) Sortilin and retromer mediate retrograde transport of Glut4 in 3T3-L1 adipocytes. Mol. Biol. Cell 28, 1667–1675 10.1091/mbc.e16-11-0777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vassilopoulos S., Esk C., Hoshino S., Funke B. H., Chen C.-Y., Plocik A. M., Wright W. E., Kucherlapati R., and Brodsky F. M. (2009) A role for the CHC22 clathrin heavy-chain isoform in human glucose metabolism. Science 324, 1192–1196 10.1126/science.1171529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Habtemichael E. N., Li D. T., Alcázar-Román A., Westergaard X. O., Li M., Petersen M. C., Li H., DeVries S. G., Li E., Julca-Zevallos O., Wolenski J. S., and Bogan J. S. (2018) Usp25m protease regulates ubiquitin-like processing of TUG proteins to control GLUT4 glucose transporter translocation in adipocytes. J. Biol. Chem. 293, 10466–10486 10.1074/jbc.RA118.003021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Okada S., Yamada E., Saito T., Ohshima K., Hashimoto K., Yamada M., Uehara Y., Tsuchiya T., Shimizu H., Tatei K., Izumi T., Yamauchi K., Hisanaga S., Pessin J. E., and Mori M. (2008) CDK5-dependent phosphorylation of the Rho family GTPase TC10α regulates insulin-stimulated GLUT4 translocation. J. Biol. Chem. 283, 35455–35463 10.1074/jbc.M806531200 [DOI] [PubMed] [Google Scholar]
- 60. Huang S., and Czech M. P. (2007) The GLUT4 glucose transporter. Cell Metab. 5, 237–252 10.1016/j.cmet.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 61. Rowland A. F., Fazakerley D. J., and James D. E. (2011) Mapping insulin/GLUT4 circuitry. Traffic 12, 672–681 10.1111/j.1600-0854.2011.01178.x [DOI] [PubMed] [Google Scholar]
- 62. Stöckli J., Fazakerley D. J., and James D. E. (2011) GLUT4 exocytosis. J. Cell Sci. 124, 4147–4159 10.1242/jcs.097063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kandror K. V., and Pilch P. F. (2011) The sugar is sIRVed: sorting Glut4 and its fellow travelers. Traffic 12, 665–671 10.1111/j.1600-0854.2011.01175.x [DOI] [PubMed] [Google Scholar]
- 64. Bogan J. S. (2012) Regulation of glucose transporter translocation in health and diabetes. Annu. Rev. Biochem. 81, 507–532 10.1146/annurev-biochem-060109-094246 [DOI] [PubMed] [Google Scholar]
- 65. Leto D., and Saltiel A. R. (2012) Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13, 383–396 10.1038/nrm3351 [DOI] [PubMed] [Google Scholar]
- 66. Jaldin-Fincati J. R., Pavarotti M., Frendo-Cumbo S., Bilan P. J., and Klip A. (2017) Update on GLUT4 vesicle traffic: a cornerstone of insulin action. Trends Endocrinol. Metab. 28, 597–611 10.1016/j.tem.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 67. Tokarz V. L., MacDonald P. E., and Klip A. (2018) The cell biology of systemic insulin function. J. Cell Biol. 217, 2273–2289 10.1083/jcb.201802095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Tucker D. F., Sullivan J. T., Mattia K.-A., Fisher C. R., Barnes T., Mabila M. N., Wilf R., Sulli C., Pitts M., Payne R. J., Hall M., Huston-Paterson D., Deng X., Davidson E., Willis S. H., et al. (2018) Isolation of state-dependent monoclonal antibodies against the 12-transmembrane domain glucose transporter 4 using virus-like particles. Proc. Natl. Acad. Sci. U.S.A. 115, E4990–E4999 10.1073/pnas.1716788115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beg M., Abdullah N., Thowfeik F. S., Altorki N. K., and McGraw T. E. (2017) Distinct Akt phosphorylation states are required for insulin regulated Glut4 and Glut1-mediated glucose uptake. ELife 6, e26896 10.7554/eLife.26896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen Y., Wang Y., Zhang J., Deng Y., Jiang L., Song E., Wu X. S., Hammer J. A., Xu T., and Lippincott-Schwartz J. (2012) Rab10 and myosin-Va mediate insulin-stimulated GLUT4 storage vesicle translocation in adipocytes. J. Cell Biol. 198, 545–560 10.1083/jcb.201111091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bai L., Wang Y., Fan J., Chen Y., Ji W., Qu A., Xu P., James D. E., and Xu T. (2007) Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 5, 47–57 10.1016/j.cmet.2006.11.013 [DOI] [PubMed] [Google Scholar]
- 72. Huang S., Lifshitz L. M., Jones C., Bellve K. D., Standley C., Fonseca S., Corvera S., Fogarty K. E., and Czech M. P. (2007) Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol. Cell Biol. 27, 3456–3469 10.1128/MCB.01719-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li C. H., Bai L., Li D. D., Xia S., and Xu T. (2004) Dynamic tracking and mobility analysis of single GLUT4 storage vesicle in live 3T3-L1 cells. Cell Res. 14, 480–486 10.1038/sj.cr.7290251 [DOI] [PubMed] [Google Scholar]
- 74. Lizunov V. A., Matsumoto H., Zimmerberg J., Cushman S. W., and Frolov V. A. (2005) Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J. Cell Biol. 169, 481–489 10.1083/jcb.200412069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stenkula K. G., Lizunov V. A., Cushman S. W., and Zimmerberg J. (2010) Insulin controls the spatial distribution of GLUT4 on the cell surface through regulation of its postfusion dispersal. Cell Metab. 12, 250–259 10.1016/j.cmet.2010.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Karylowski O., Zeigerer A., Cohen A., and McGraw T. E. (2004) GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell 15, 870–882 10.1091/mbc.e03-07-0517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sano H., Kane S., Sano E., Mîinea C. P., Asara J. M., Lane W. S., Garner C. W., and Lienhard G. E. (2003) Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278, 14599–14602 10.1074/jbc.C300063200 [DOI] [PubMed] [Google Scholar]
- 78. Ishikura S., Bilan P. J., and Klip A. (2007) Rabs 8A and 14 are targets of the insulin-regulated Rab-GAP AS160 regulating GLUT4 traffic in muscle cells. Biochem. Biophys. Res. Commun. 353, 1074–1079 10.1016/j.bbrc.2006.12.140 [DOI] [PubMed] [Google Scholar]
- 79. Sano H., Eguez L., Teruel M. N., Fukuda M., Chuang T. D., Chavez J. A., Lienhard G. E., and McGraw T. E. (2007) Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 5, 293–303 10.1016/j.cmet.2007.03.001 [DOI] [PubMed] [Google Scholar]
- 80. Fazakerley D. J., Naghiloo S., Chaudhuri R., Koumanov F., Burchfield J. G., Thomas K. C., Krycer J. R., Prior M. J., Parker B. L., Murrow B. A., Stöckli J., Meoli C. C., Holman G. D., and James D. E. (2015) Proteomic analysis of GLUT4 storage vesicles reveals tumor suppressor candidate 5 (TUSC5) as a novel regulator of insulin action in adipocytes. J. Biol. Chem. 290, 23528–23542 10.1074/jbc.M115.657361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ramm G., Larance M., Guilhaus M., and James D. E. (2006) A role for 14-3-3 in insulin-stimulated GLUT4 translocation through its interaction with the RabGAP AS160. J. Biol. Chem. 281, 29174–29180 10.1074/jbc.M603274200 [DOI] [PubMed] [Google Scholar]
- 82. Mafakheri S., Flörke R. R., Kanngiesser S., Hartwig S., Espelage L., De Wendt C., Schönberger T., Hamker N., Lehr S., Chadt A., and Al-Hasani H. (2018) AKT and AMP-activated protein kinase regulate TBC1D1 through phosphorylation and its interaction with the cytosolic tail of insulin-regulated aminopeptidase IRAP. J. Biol. Chem. 293, 17853–17862 10.1074/jbc.RA118.005040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tan S.-X., Ng Y., Burchfield J. G., Ramm G., Lambright D. G., Stöckli J., and James D. E. (2012) The Rab GTPase-activating protein TBC1D4/AS160 contains an atypical phosphotyrosine-binding domain that interacts with plasma membrane phospholipids to facilitate GLUT4 trafficking in adipocytes. Mol. Cell Biol. 32, 4946–4959 10.1128/MCB.00761-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Stöckli J., Davey J. R., Hohnen-Behrens C., Xu A., James D. E., and Ramm G. (2008) Regulation of glucose transporter 4 translocation by the Rab guanosine triphosphatase-activating protein AS160/TBC1D4: role of phosphorylation and membrane association. Mol. Endocrinol. 22, 2703–2715 10.1210/me.2008-0111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Koumanov F., Jin B., Yang J., and Holman G. D. (2005) Insulin signaling meets vesicle traffic of GLUT4 at a plasma-membrane-activated fusion step. Cell Metab. 2, 179–189 10.1016/j.cmet.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 86. Ng Y., Ramm G., Burchfield J. G., Coster A. C. F., Stöckli J., and James D. E. (2010) Cluster analysis of insulin action in adipocytes reveals a key role for Akt at the plasma membrane. J. Biol. Chem. 285, 2245–2257 10.1074/jbc.M109.060236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Mîinea C. P., Sano H., Kane S., Sano E., Fukuda M., Peränen J., Lane W. S., and Lienhard G. E. (2005) AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 391, 87–93 10.1042/BJ20050887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Vazirani R. P., Verma A., Sadacca L. A., Buckman M. S., Picatoste B., Beg M., Torsitano C., Bruno J. H., Patel R. T., Simonyte K., Camporez J. P., Moreira G., Falcone D. J., Accili D., Elemento O., et al. (2016) Disruption of adipose Rab10-dependent insulin signaling causes hepatic insulin resistance. Diabetes 65, 1577–1589 10.2337/db15-1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Reed S. E., Hodgson L. R., Song S., May M. T., Kelly E. E., McCaffrey M. W., Mastick C. C., Verkade P., and Tavaré J. M. (2013) A role for Rab14 in the endocytic trafficking of GLUT4 in 3T3-L1 adipocytes. J. Cell Sci. 126, 1931–1941 10.1242/jcs.104307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sadacca L. A., Bruno J., Wen J., Xiong W., and McGraw T. E. (2013) Specialized sorting of GLUT4 and its recruitment to the cell surface are independently regulated by distinct Rabs. Mol. Biol. Cell 24, 2544–2557 10.1091/mbc.e13-02-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sun Y., Chiu T. T., Foley K. P., Bilan P. J., and Klip A. (2014) Myosin Va mediates Rab8A-regulated GLUT4 vesicle exocytosis in insulin-stimulated muscle cells. Mol. Biol. Cell 25, 1159–1170 10.1091/mbc.e13-08-0493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sun Y., Jaldin-Fincati J., Liu Z., Bilan P. J., and Klip A. (2016) A complex of Rab13 with MICAL-L2 and α-actinin-4 is essential for insulin-dependent GLUT4 exocytosis. Mol. Biol. Cell 27, 75–89 10.1091/mbc.E15-05-0319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Brewer P. D., Romenskaia I., Kanow M. A., and Mastick C. C. (2011) Loss of AS160 Akt substrate causes Glut4 protein to accumulate in compartments that are primed for fusion in basal adipocytes. J. Biol. Chem. 286, 26287–26297 10.1074/jbc.M111.253880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bruno J., Brumfield A., Chaudhary N., Iaea D., and McGraw T. E. (2016) SEC16A is a RAB10 effector required for insulin-stimulated GLUT4 trafficking in adipocytes. J. Cell Biol. 214, 61–76 10.1083/jcb.201509052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sylow L., Kleinert M., Pehmøller C., Prats C., Chiu T. T., Klip A., Richter E. A., and Jensen T. E. (2014) Akt and Rac1 signaling are jointly required for insulin-stimulated glucose uptake in skeletal muscle and downregulated in insulin resistance. Cell. Signal. 26, 323–331 10.1016/j.cellsig.2013.11.007 [DOI] [PubMed] [Google Scholar]
- 96. Chiu T. T., Patel N., Shaw A. E., Bamburg J. R., and Klip A. (2010) Arp2/3- and cofilin-coordinated actin dynamics is required for insulin-mediated GLUT4 translocation to the surface of muscle cells. Mol. Biol. Cell 21, 3529–3539 10.1091/mbc.e10-04-0316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Boguslavsky S., Chiu T., Foley K. P., Osorio-Fuentealba C., Antonescu C. N., Bayer K. U., Bilan P. J., and Klip A. (2012) Myo1c binding to submembrane actin mediates insulin-induced tethering of GLUT4 vesicles. Mol. Biol. Cell 23, 4065–4078 10.1091/mbc.e12-04-0263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chen X.-W., Leto D., Chiang S.-H., Wang Q., and Saltiel A. R. (2007) Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391–404 10.1016/j.devcel.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 99. Lopez J. A., Burchfield J. G., Blair D. H., Mele K., Ng Y., Vallotton P., James D. E., and Hughes W. E. (2009) Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol. Biol. Cell 20, 3918–3929 10.1091/mbc.e09-03-0187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hansen P. A., Wang W., Marshall B. A., Holloszy J. O., and Mueckler M. (1998) Dissociation of GLUT4 translocation and insulin-stimulated glucose transport in transgenic mice overexpressing GLUT1 in skeletal muscle. J. Biol. Chem. 273, 18173–18179 10.1074/jbc.273.29.18173 [DOI] [PubMed] [Google Scholar]
- 101. Kahn B. B., and Cushman S. W. (1985) Subcellular translocation of glucose transporters: role in insulin action and its perturbation in altered metabolic states. Diabetes Metab. Rev. 1, 203–227 10.1002/dmr.5610010301 [DOI] [PubMed] [Google Scholar]
- 102. Kristiansen S., Youn J., and Richter E. A. (1996) Effect of vanadate on glucose transporter (GLUT4) intrinsic activity in skeletal muscle plasma membrane giant vesicles. Biochim. Biophys. Acta. 1282, 71–75 10.1016/0005-2736(96)00041-7 [DOI] [PubMed] [Google Scholar]
- 103. Shamni O., Cohen G., Gruzman A., Zaid H., Klip A., Cerasi E., and Sasson S. (2017) Regulation of GLUT4 activity in myotubes by 3-O-methyl-d-glucose. Biochim. Biophys. Acta Biomembr. 1859, 1900–1910 10.1016/j.bbamem.2017.06.013 [DOI] [PubMed] [Google Scholar]
- 104. Wang W., Hansen P. A., Marshall B. A., Holloszy J. O., and Mueckler M. (1996) Insulin unmasks a COOH-terminal Glut4 epitope and increases glucose transport across T-tubules in skeletal muscle. J. Cell Biol. 135, 415–430 10.1083/jcb.135.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zaid H., Talior-Volodarsky I., Antonescu C., Liu Z., and Klip A. (2009) GAPDH binds GLUT4 reciprocally to hexokinase-II and regulates glucose transport activity. Biochem. J. 419, 475–484 10.1042/BJ20081319 [DOI] [PubMed] [Google Scholar]
- 106. Holloszy J. O., and Narahara H. T. (1965) Studies of tissue permeability. X. Changes in permeability to 3-methylglucose associated with contraction of isolated frog muscle. J. Biol. Chem. 240, 3493–3500 [PubMed] [Google Scholar]
- 107. Howlett K. F., Andrikopoulos S., Proietto J., and Hargreaves M. (2013) Exercise-induced muscle glucose uptake in mice with graded, muscle-specific GLUT-4 deletion. Physiol. Rep. 1, e00065 10.1002/phy2.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zisman A., Peroni O. D., Abel E. D., Michael M. D., Mauvais-Jarvis F., Lowell B. B., Wojtaszewski J. F., Hirshman M. F., Virkamaki A., Goodyear L. J., Kahn C. R., and Kahn B. B. (2000) Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nat. Med. 6, 924–928 10.1038/78693 [DOI] [PubMed] [Google Scholar]
- 109. Kennedy J. W., Hirshman M. F., Gervino E. V., Ocel J. V., Forse R. A., Hoenig S. J., Aronson D., Goodyear L. J., and Horton E. S. (1999) Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48, 1192–1197 10.2337/diabetes.48.5.1192 [DOI] [PubMed] [Google Scholar]
- 110. Kristiansen S., Hargreaves M., and Richter E. A. (1996) Exercise-induced increase in glucose transport, GLUT-4, and VAMP-2 in plasma membrane from human muscle. Am. J. Physiol. 270, E197–E201 10.1152/ajpendo.1996.270.1.E197 [DOI] [PubMed] [Google Scholar]
- 111. Ploug T., Wojtaszewski J., Kristiansen S., Hespel P., Galbo H., and Richter E. A. (1993) Glucose transport and transporters in muscle giant vesicles: differential effects of insulin and contractions. Am. J. Physiol. 264, E270–E278 10.1152/ajpendo.1993.264.2.E270 [DOI] [PubMed] [Google Scholar]
- 112. Fazakerley D. J., Lawrence S. P., Lizunov V. A., Cushman S. W., and Holman G. D. (2009) A common trafficking route for GLUT4 in cardiomyocytes in response to insulin, contraction and energy-status signalling. J. Cell Sci. 122, 727–734 10.1242/jcs.041178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sylow L., Møller L. L. V., Kleinert M., D'Hulst G., De Groote E., Schjerling P., Steinberg G. R., Jensen T. E., and Richter E. A. (2017) Rac1 and AMPK account for the majority of muscle glucose uptake stimulated by ex vivo contraction but not in vivo exercise. Diabetes 66, 1548–1559 10.2337/db16-1138 [DOI] [PubMed] [Google Scholar]
- 114. Richter E. A., and Hargreaves M. (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 93, 993–1017 10.1152/physrev.00038.2012 [DOI] [PubMed] [Google Scholar]
- 115. Whitfield J., Paglialunga S., Smith B. K., Miotto P. M., Simnett G., Robson H. L., Jain S. S., Herbst E. A. F., Desjardins E. M., Dyck D. J., Spriet L. L., Steinberg G. R., and Holloway G. P. (2017) Ablating the protein TBC1D1 impairs contraction-induced sarcolemmal glucose transporter 4 redistribution but not insulin-mediated responses in rats. J. Biol. Chem. 292, 16653–16664 10.1074/jbc.M117.806786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hargett S. R., Walker N. N., and Keller S. R. (2016) Rab GAPs AS160 and Tbc1d1 play nonredundant roles in the regulation of glucose and energy homeostasis in mice. Am. J. Physiol. 310, E276–E288 10.1152/ajpendo.00342.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mafakheri S., Chadt A., and Al-Hasani H. (2018) Regulation of RabGAPs involved in insulin action. Biochem. Soc. Trans. 46, 683–690 10.1042/BST20170479 [DOI] [PubMed] [Google Scholar]
- 118. Rothman D. L., Shulman R. G., and Shulman G. I. (1992) 31P nuclear magnetic resonance measurements of muscle glucose-6-phosphate: evidence for reduced insulin-dependent muscle glucose transport or phosphorylation activity in non-insulin-dependent diabetes mellitus. J. Clin. Invest. 89, 1069–1075 10.1172/JCI115686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wallberg-Henriksson H., and Holloszy J. O. (1985) Activation of glucose transport in diabetic muscle: responses to contraction and insulin. Am. J. Physiol. 249, C233–C237 10.1152/ajpcell.1985.249.3.C233 [DOI] [PubMed] [Google Scholar]
- 120. Wallberg-Henriksson H., and Holloszy J. O. (1984) Contractile activity increases glucose uptake by muscle in severely diabetic rats. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 57, 1045–1049 10.1152/jappl.1984.57.4.1045 [DOI] [PubMed] [Google Scholar]
- 121. Shepherd P. R., and Kahn B. B. (1999) Glucose transporters and insulin action—implications for insulin resistance and diabetes mellitus. N. Engl. J. Med. 341, 248–257 10.1056/NEJM199907223410406 [DOI] [PubMed] [Google Scholar]
- 122. Garvey W. T., Maianu L., Zhu J. H., Hancock J. A., and Golichowski A. M. (1993) Multiple defects in the adipocyte glucose transport system cause cellular insulin resistance in gestational diabetes: heterogeneity in the number and a novel abnormality in subcellular localization of GLUT4 glucose transporters. Diabetes 42, 1773–1785 10.2337/diab.42.12.1773 [DOI] [PubMed] [Google Scholar]
- 123. Etgen G. J. Jr., Jensen J., Wilson C. M., Hunt D. G., Cushman S. W., and Ivy J. L. (1997) Exercise training reverses insulin resistance in muscle by enhanced recruitment of GLUT-4 to the cell surface. Am. J. Physiol. 272, E864–E869 10.1152/ajpendo.1997.272.5.E864 [DOI] [PubMed] [Google Scholar]
- 124. Etgen G. J. Jr., Wilson C. M., Jensen J., Cushman S. W., and Ivy J. L. (1996) Glucose transport and cell surface GLUT-4 protein in skeletal muscle of the obese Zucker rat. Am. J. Physiol. 271, E294–E301 [DOI] [PubMed] [Google Scholar]
- 125. Klip A., Ramlal T., Bilan P. J., Cartee G. D., Gulve E. A., and Holloszy J. O. (1990) Recruitment of GLUT-4 glucose transporters by insulin in diabetic rat skeletal muscle. Biochem. Biophys. Res. Commun. 172, 728–736 10.1016/0006-291X(90)90735-6 [DOI] [PubMed] [Google Scholar]
- 126. Ramlal T., Rastogi S., Vranic M., and Klip A. (1989) Decrease in glucose transporter number in skeletal muscle of mildly diabetic (streptozotocin-treated) rats. Endocrinology 125, 890–897 10.1210/endo-125-2-890 [DOI] [PubMed] [Google Scholar]
- 127. Garvey W. T., Maianu L., Zhu J. H., Brechtel-Hook G., Wallace P., and Baron A. D. (1998) Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. J. Clin. Invest. 101, 2377–2386 10.1172/JCI1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ryder J. W., Yang J., Galuska D., Rincón J., Björnholm M., Krook A., Lund S., Pedersen O., Wallberg-Henriksson H., Zierath J. R., and Holman G. D. (2000) Use of a novel impermeable biotinylated photolabeling reagent to assess insulin- and hypoxia-stimulated cell surface GLUT4 content in skeletal muscle from type 2 diabetic patients. Diabetes 49, 647–654 10.2337/diabetes.49.4.647 [DOI] [PubMed] [Google Scholar]
- 129. Zierath J. R., He L., Gumà A., Odegoard Wahlström E., Klip A., and Wallberg-Henriksson H. (1996) Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia 39, 1180–1189 10.1007/BF02658504 [DOI] [PubMed] [Google Scholar]
- 130. Gonzalez E., Flier E., Molle D., Accili D., and McGraw T. E. (2011) Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc. Natl. Acad. Sci. U.S.A. 108, 10162–10167 10.1073/pnas.1019268108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Tan S.-X., Fisher-Wellman K. H., Fazakerley D. J., Ng Y., Pant H., Li J., Meoli C. C., Coster A. C. F., Stöckli J., and James D. E. (2015) Selective insulin resistance in adipocytes. J. Biol. Chem. 290, 11337–11348 10.1074/jbc.M114.623686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Czech M. P. (2017) Insulin action and resistance in obesity and type 2 diabetes. Nat. Med. 23, 804–814 10.1038/nm.4350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Fazakerley D. J., Krycer J. R., Kearney A. L., Hocking S. L., and James D. E. (2018) Muscle and adipose tissue insulin resistance: malady without mechanism? J. Lipid Res. pii: jlr.R087510 10.1194/jlr.R087510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Manousaki D., Kent J. W. Jr., Haack K., Zhou S., Xie P., Greenwood C. M., Brassard P., Newman D. E., Cole S., Umans J. G., Rouleau G., Comuzzie A. G., and Richards J. B. (2016) Toward precision medicine: TBC1D4 disruption is common among the Inuit and leads to underdiagnosis of type 2 diabetes. Diabetes Care 39, 1889–1895 10.2337/dc16-0769 [DOI] [PubMed] [Google Scholar]
- 135. Tonks K. T., Ng Y., Miller S., Coster A. C. F., Samocha-Bonet D., Iseli T. J., Xu A., Patrick E., Yang J. Y. H., Junutula J. R., Modrusan Z., Kolumam G., Stöckli J., Chisholm D. J., James D. E., and Greenfield J. R. (2013) Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia 56, 875–885 10.1007/s00125-012-2811-y [DOI] [PubMed] [Google Scholar]
- 136. Ashrafi G., Wu Z., Farrell R. J., and Ryan T. A. (2017) GLUT4 mobilization supports energetic demands of active synapses. Neuron 93, 606–615.e3 10.1016/j.neuron.2016.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ren H., Lu T. Y., McGraw T. E., and Accili D. (2015) Anorexia and impaired glucose metabolism in mice with hypothalamic ablation of Glut4 neurons. Diabetes 64, 405–417 10.2337/db14-0752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Reno C. M., Puente E. C., Sheng Z., Daphna-Iken D., Bree A. J., Routh V. H., Kahn B. B., and Fisher S. J. (2017) Brain GLUT4 knockout mice have impaired glucose tolerance, decreased insulin sensitivity, and impaired hypoglycemic counterregulation. Diabetes 66, 587–597 10.2337/db16-0917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bakirtzi K., Belfort G., Lopez-Coviella I., Kuruppu D., Cao L., Abel E. D., Brownell A.-L., and Kandror K. V. (2009) Cerebellar neurons possess a vesicular compartment structurally and functionally similar to Glut4-storage vesicles from peripheral insulin-sensitive tissues. J. Neurosci. 29, 5193–5201 10.1523/JNEUROSCI.0858-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Di Chiara M., Glaudemans B., Loffing-Cueni D., Odermatt A., Al-Hasani H., Devuyst O., Faresse N., and Loffing J. (2015) Rab-GAP TBC1D4 (AS160) is dispensable for the renal control of sodium and water homeostasis but regulates GLUT4 in mouse kidney. Am. J. Physiol. 309, F779–F790 10.1152/ajprenal.00139.2015 [DOI] [PubMed] [Google Scholar]
- 141. Wasik A. A., and Lehtonen S. (2018) Glucose transporters in diabetic kidney disease—friends or foes? Front. Endocrinol. 9, 155 10.3389/fendo.2018.00155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yang Q., Graham T. E., Mody N., Preitner F., Peroni O. D., Zabolotny J. M., Kotani K., Quadro L., and Kahn B. B. (2005) Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature 436, 356–362 10.1038/nature03711 [DOI] [PubMed] [Google Scholar]
- 143. Norseen J., Hosooka T., Hammarstedt A., Yore M. M., Kant S., Aryal P., Kiernan U. A., Phillips D. A., Maruyama H., Kraus B. J., Usheva A., Davis R. J., Smith U., and Kahn B. B. (2012) Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory cytokines in macrophages through a c-Jun N-terminal kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol. Cell Biol. 32, 2010–2019 10.1128/MCB.06193-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Moraes-Vieira P. M., Saghatelian A., and Kahn B. B. (2016) GLUT4 expression in adipocytes regulates de novo lipogenesis and levels of a novel class of lipids with antidiabetic and anti-inflammatory effects. Diabetes 65, 1808–1815 10.2337/db16-0221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Hammarstedt A., Syed I., Vijayakumar A., Eliasson B., Gogg S., Kahn B. B., and Smith U. (2018) Adipose tissue dysfunction is associated with low levels of the novel palmitic acid hydroxystearic acids. Sci. Rep. 8, 15757 10.1038/s41598-018-34113-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Randle P. J. (1998) Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes Metab. Rev. 14, 263–283 [DOI] [PubMed] [Google Scholar]
- 147. Dresner A., Laurent D., Marcucci M., Griffin M. E., Dufour S., Cline G. W., Slezak L. A., Andersen D. K., Hundal R. S., Rothman D. L., Petersen K. F., and Shulman G. I. (1999) Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J. Clin. Invest. 103, 253–259 10.1172/JCI5001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hoehn K. L., Hohnen-Behrens C., Cederberg A., Wu L. E., Turner N., Yuasa T., Ebina Y., and James D. E. (2008) IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 7, 421–433 10.1016/j.cmet.2008.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Pillon N. J., Frendo-Cumbo S., Jacobson M. R., Liu Z., Milligan P. L., Hoang Bui H., Zierath J. R., Bilan P. J., Brozinick J. T., and Klip A. (2018) Sphingolipid changes do not underlie fatty acid-evoked GLUT4 insulin resistance nor inflammation signals in muscle cells. J. Lipid Res. 59, 1148–1163 10.1194/jlr.M080788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Gibbs E. M., Stock J. L., McCoid S. C., Stukenbrok H. A., Pessin J. E., Stevenson R. W., Milici A. J., and McNeish J. D. (1995) Glycemic improvement in diabetic db/db mice by overexpression of the human insulin-regulatable glucose transporter (GLUT4). J. Clin. Invest. 95, 1512–1518 10.1172/JCI117823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Daugaard J. R., Nielsen J. N., Kristiansen S., Andersen J. L., Hargreaves M., and Richter E. A. (2000) Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes 49, 1092–1095 10.2337/diabetes.49.7.1092 [DOI] [PubMed] [Google Scholar]
- 152. McGee S. L., and Hargreaves M. (2004) Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes 53, 1208–1214 10.2337/diabetes.53.5.1208 [DOI] [PubMed] [Google Scholar]