Tuberculous meningitis (TBM) is a devastating infection of the central nervous system lacking an adequate point-of-care diagnostic test. We conducted a prospective cohort study of 550 Zambian adults with suspected TBM to determine the diagnostic accuracy of cerebrospinal fluid (CSF) Xpert MTB/RIF, CSF lipoarabinomannan (LAM), urine LAM, CSF total protein, and CSF glucose compared with the gold standard of CSF culture.
KEYWORDS: Africa, LAM, tuberculous meningitis, Xpert MTB/RIF, Zambia, human immunodeficiency virus
ABSTRACT
Tuberculous meningitis (TBM) is a devastating infection of the central nervous system lacking an adequate point-of-care diagnostic test. We conducted a prospective cohort study of 550 Zambian adults with suspected TBM to determine the diagnostic accuracy of cerebrospinal fluid (CSF) Xpert MTB/RIF, CSF lipoarabinomannan (LAM), urine LAM, CSF total protein, and CSF glucose compared with the gold standard of CSF culture. We categorized patients with a positive CSF tuberculosis (TB) culture as definite TBM. We also assessed inpatient and 1-year mortality on definite TBM patients when CSF Xpert MTB/RIF results were available in real time to treating physicians relative to a historical comparison cohort in whom Xpert results were not available in real time. Of the 550 patients, 474 (86.2%) were HIV-infected and 105/550 (19.1%) had definite TBM based on a positive CSF culture. The sensitivity/specificity of the diagnostic tests were CSF Xpert MTB/RIF, 52.9%/94.2%; CSF LAM, 21.9%/94.2%; urine LAM, 24.1%/76.1%; and CSF glucose <40 mg/dl, and total protein, >100 mg/dl, 66.3%/90%. A model including CSF Xpert MTB/RIF, CSF LAM, CSF glucose, and CSF total protein demonstrated an area under the receiver operating curve of 0.90. The inpatient and 1-year mortality for definite TBM was 43% and 57%, respectively. There was low sensitivity for the diagnosis of TBM across all diagnostics tests. CSF Xpert MTB/RIF and CSF LAM are highly specific for the diagnosis of TBM. Despite the use of Xpert MTB/RIF for diagnostic purpose in real time, TBM was still associated with a high mortality in Zambian patients.
INTRODUCTION
TBM is a devastating infection of the central nervous system often leading to severe neurological impairment and death. Its effects are even more profound in sub-Saharan Africa where HIV is endemic. The diagnosis of TBM is problematic regardless of setting. CSF acid-fast bacterial staining has a sensitivity of 10% to 20% that varies greatly based on technical expertise (1). CSF Mycobacterium tuberculosis culture is widely considered to be an imperfect gold standard with a sensitivity from 50% to 70% (2, 3) and can take as long as 6 weeks to become positive. Neither of these techniques is practical in many low- and middle-income countries (LMIC) due to the complexity of widely instituting a specialized laboratory skill and the lack of effective systems to communicate results to patients in the community. As a result, treatment is often initiated empirically, with a lack of supportive diagnostic testing.
Xpert MTB/RIF is an automated real-time PCR platform that provides a diagnosis of TBM and rifampin resistance through analysis of CSF in under 2 hours. In 2013, the World Health Organization endorsed Xpert MTB/RIF as the initial test for the diagnosis of TBM after a review of multiple studies which demonstrated a sensitivity of 60% and specificity of 95% in comparison to CSF culture (4). Interestingly, no study to date has demonstrated that the use of Xpert MTB/RIF leads to improved survival in patients with TBM. Indeed, Xpert MTB/RIF on sputum has reduced the time to diagnosis of pulmonary TB but has not improved morbidity or mortality (5, 6). Additionally, Xpert MTB/RIF requires electricity, disposable cartridges, and servicing that prevents its scale-up to rural areas.
In contrast to TBM, cryptococcal meningitis diagnosis is facilitated by a simple low-cost lateral flow assay. One possible point-of-care candidate for the diagnosis of TBM is the LAM lateral flow assay (LFA) which requires <100 μl of urine applied to a small test strip at room temperature. It has been used to detect disseminated TB, with its greatest utility in HIV-infected patients with severe immunosuppression. A large multicenter randomized controlled trial of urine LAM-guided initiation of antituberculosis treatment in sub-Saharan on inpatients with suspected pulmonary TB showed a reduction in 8-week inpatient mortality (7). In a proof-of-concept study, LAM enzyme-linked immunosorbent assay (ELISA) testing of CSF on a cohort of South African adults with suspected TBM had a sensitivity of 64% and a specificity of 69% for the diagnosis of culture-positive TBM (8). An autopsy study of LAM LFA for the diagnosis of definite TBM showed a sensitivity of 75% and a specificity of 87% (9). There has been no report of LAM LFA on urine or CSF in living patients for the diagnosis of TBM.
We evaluated the diagnostic accuracy of Xpert MTB/RIF on CSF, LAM LFA on fresh urine and/or CSF, and current standard of care for TBM diagnosis in Zambia which combines clinical presentation with CSF glucose and total protein values. CSF M. tuberculosis culture served as the gold standard. We also studied whether the use of Xpert MTB/RIF improved survival of TBM patients in a tertiary care facility in Zambia.
MATERIALS AND METHODS
Study design and patient population.
We conducted a prospective cohort study of TBM at the University Teaching Hospital (UTH) in Lusaka, Zambia between 4 April 2014 and 31 August 2017. We enrolled adults (age, ≥18 years) who presented with signs and symptoms concerning for TBM and already received a lumbar puncture as part of routine care. Patients with unknown HIV status were offered HIV testing as part of routine clinical care and not as part of the study. All potential study subjects were examined by a study neurologist. After a lumbar puncture was completed, study staff identified patients from the UTH microbiology laboratories who had ≥3 ml of excess CSF remaining after routine testing composed of Gram stain, India ink stain, cryptococcoal antigen testing, and bacterial culture on a blood agar plate. Mycobacterial growth indicator tube (MGIT) culture is currently not part of routine testing on all CSF. Patients with a positive CSF bacterial Gram stain, India ink stain, cryptococcal antigen test, or positive bacterial culture were excluded from enrollment. A study nurse obtained written informed consent from the patient or health care proxy for study enrollment to use excess CSF from the lumbar puncture for additional M. tuberculosis testing. No lumbar puncture was performed solely for study purposes or at the recommendation of study staff. The nurse also documented demographic data, presenting symptoms, past medical history, and medications through a formal interview with the patient or health care proxy and review of the medical record as explicitly requested in the consent form. Study subjects then provided an additional sample of whole blood and urine. A study neurologist conducted a structured neurologic assessment with particular attention to focal abnormalities. We recorded Medical Research Council severity using the established grading system, namely, grade I (GCS score, 15; no focal neurological signs), grade II (GCS score, 11 to 14 or 15 with focal neurological signs), or grade III (GCS score, ≤10) (10). Study staff recorded inpatient mortality. A study coordinator called patients or members of the household quarterly for 1 year to ask only about patient survival. The study was approved by the University of Zambia School of Medicine Biomedical Research Ethics Committee and Beth Israel Deaconess Medical Center Institutional Review Board.
Laboratory testing.
Routine CSF testing at the UTH microbiology and biochemistry laboratories includes cell count, Gram stain, India ink stain, bacterial culture, fungal culture, and total protein and glucose concentration. When the UTH biochemistry laboratory did not have the necessary reagents to run CSF total protein, samples were processed at a private medical laboratory in Lusaka. Both the UTH laboratory and private laboratory are locally accredited. CSF Xpert MTB/RIF, CSF LAM, urine LAM, and CSF TB culture were performed in the Zambart research laboratories.
Study staff delivered 3 ml of CSF and 3 ml of urine in plain sterile tubes in a cooler filled with ice packs to the Zambart research laboratories. In the laboratory, staff would bring the samples to room temperature 1 hour prior to use. Using a sterile filtered tip pipette, a lab technician removed 60 μl of CSF and urine and transferred the specimens to a LAM LFA. When possible, two investigators trained in LAM LFA interpretation read the samples in a blind manner after 25 to 35 minutes in comparison to a kit reference card according to the manufacturer’s instructions, and they scored the test as positive, negative, or indeterminate. If there was disagreement, the lowest reading was taken as the final result. For 20% of the samples, only one LAM LFA reader was available. Indeterminate samples were treated as negative for purposes of the analysis.
The remaining CSF was centrifuged at 3,500 × g for 20 minutes. Supernatant was removed, leaving a pellet and 500 μl of residual fluid. The samples were then vortexed. One hundred microliters each was used to inoculate two separate MGIT tubes that were placed in a Bactec960 instrument for culture. Samples positive on MGIT culture were confirmed as M. tuberculosis with the Capilia TB MPT64 Ag assay (Tauns Laboratories, Japan). Phosphate-buffered saline was added to the remaining CSF to bring it to a volume of 500 μl. Laboratory staff then added 1.5 ml of Xpert sample reagent. The specimen was incubated for 10 minutes at room temperature. The specimen was then either shaken vigorously for 20 seconds or vortexed for 10 seconds and was left to incubate for an additional 5 minutes. A laboratory technician then transferred 2 ml of specimen/sample reagent mixture to an Xpert cartridge for testing. Results were rated as negative or positive for M. tuberculosis with or without rifampin resistance according to the manufacturer’s instructions. Samples found to be rifampin resistant by Xpert MTB/RIF had confirmatory drug susceptibility testing (DST) for rifampin and isoniazid conducted separately. Xpert MTB/RIF results were placed in the patient’s file by study staff within 24 hours of the test being completed.
Diagnostic categorization.
We classified patients as having definite or probable TBM to evaluate the performance of the Xpert MTB/RIF, CSF LAM, and Urine LAM. Definite TBM was defined as a CSF sample that was MGIT culture positive. Probable TBM was defined as patients with a CSF white blood cell count between 10 and 500, CSF total protein of >100 mg/dl, and CSF glucose of <40 mg/dl. These values were adapted from a uniform case definition of probable TBM for use in clinical research (11).
Statistical analyses.
Descriptive measures (such as median, interquartile range, frequencies, and percentages) were used to summarize the data. Wilcoxon rank-sum and Fisher exact tests were used to compare continuous and categorical study variables, respectively, between groups.
The primary outcome of interest was the performance of Xpert MTB/RIF, CSF LAM, and urine LAM for the diagnosis of TBM relative to CSF MGIT culture as the gold standard. Sensitivity and specificity together with their corresponding 95% confidence intervals were calculated to assess the predictive accuracy of the diagnostic tests (CSF Xpert MTB/RIF, CSF LAM, urine LAM, and CSF total protein and glucose) at specific thresholds. Exact binomial confidence intervals were used to estimate confidence intervals for sensitivities and specificities. Receiver operating characteristic (ROC) curves and the area under the ROC curves were used to evaluate and compare the overall predictive accuracy of the diagnostic tests. Secondary outcomes of interest were factors associated with inpatient and 1-year mortality.
Univariate and multivariate logistic regression models were utilized to assess the clinical predictors of inpatient and 1-year mortality. A significance level of <0.10 was used to select variables for the multivariate analyses. The inpatient mortality rate for the HIV-infected TBM patients were compared to the inpatient mortality rate of HIV-infected TBM controls from a prior study at the same facility where PCR results were not readily available (12) to assess the impact of real-time use of CSF Xpert MTB/RIF. All P values were 2-sided and considered statistically significant if <0.05.
RESULTS
Patients characteristics.
Five hundred and fifty patients were enrolled into the study as shown in Fig. 1. Of those patients, 474 (86.2%) were HIV infected. Eleven patients (2%) had an unknown HIV status because they either declined testing or died prior to testing. The incidence of TB culture-positive CSF was higher among HIV-infected patients than uninfected patients (20.5% versus 12.3%), although this difference was not statistically significant.
FIG 1.
Flow sheet of patient recruitment and percentages with positive CSF culture, CSF Xpert, CSF LAM, and urine LAM testing based on HIV status. TBM, tuberculous meningitis; MTB, Mycobacterium tuberculosis; Cx, culture; CSF, cerebrospinal fluid; Xpert, GeneXpert MTB/RIF; RIF, rifampin; LAM, lipoarabinomannan.
Table 1 shows the patient demographics stratified by HIV status. The median age of patients who were TBM culture positive was significantly higher in HIV-positive patients than in HIV-negative patients (P = 0.005). There were significantly more HIV-infected men 64/190 (33.7%) than women 33/188 (17.6%) diagnosed with TBM (P = 0.006). There was no significant difference in CD4+ T-cell counts or age between these two groups. In HIV-negative patients with TBM, the median CD4+ T-cell count was <200 cells/μl and significantly less than HIV-negative patients without TBM. Overall, more patients with a negative CSF culture had received a prior diagnosis of TB, although this difference was only significant in the HIV-infected population. For those patients taking antiretroviral therapy (ART) at the time of enrollment, TBM patients were on ART for a significantly shorter period than those who did not have TBM. Fifty-one percent of HIV-infected men with TBM received their HIV diagnosis within 1 week of enrollment compared with 27% of females (P = 0.03). Among HIV-infected patients with TBM, there was no significant difference between men and women for a prior diagnosis of TB, taking ART, or inpatient mortality.
TABLE 1.
Patient demographics
| Demographic | Values of HIV-positive patients |
Values of HIV-negative patients |
||||
|---|---|---|---|---|---|---|
| TBM culture positive (n = 97) | TBM culture negative (n = 378) | P value | TBM culture positive (n = 8) | TBM culture negative (n = 57) | P value | |
| Median age (yr) (IQR) | 35 (30–41) | 36 (30–43) | 0.36 | 25 (24–27) | 41 (28–55) | 0.006 |
| Sex, male (n [%]) | 64 (66) | 190 (50) | 0.006 | 3 (38) | 27 (47) | 0.88 |
| Median CD4+ T cells/μl (IQR) | 104 (45–167) | 129 (42–346) | 0.06 | 165 (142–264) | 593 (498–842) | 0.003 |
| Prior TB diagnosis (n [%]) | 20 (21) | 163 (43) | <0.0001 | 0 (0) | 5 (9) | 0.38 |
| Median days since HIV diagnosis (IQR) | 14 (3–152) | 270 (30–1825) | <0.0001 | |||
| Taking ART (n [%]) | 33 (34) | 231 (61) | < 0.0001 | |||
| Duration of ART, (median days [IQR]) | 105 (19–723) | 730 (60–1825) | < 0.0001 | |||
| MRCb TBM severity grade (n [%]) | ||||||
| Grade 1 | 13 (13) | 0 (0) | ||||
| Grade 2 | 44 (46) | 3 (37.5) | ||||
| Grade 3 | 32 (33) | 3 (37.5) | ||||
| Unknowna | 8 (8) | 2 (25) | ||||
| Inpatient mortality (n [%]) | 42 (43) | 83 (22) | 0.0002 | 2 (25) | 14 (25) | 0.68 |
| 1-yr morality (n [%]) | 59 (61) | 165 (44) | 0.003 | 2 (25) | 26 (46) | 0.47 |
Incomplete neurological examination due to patient’s inability to cooperate or death prior to examination.
MRC, Medical Research Council.
Accuracy of diagnostic tests for TBM.
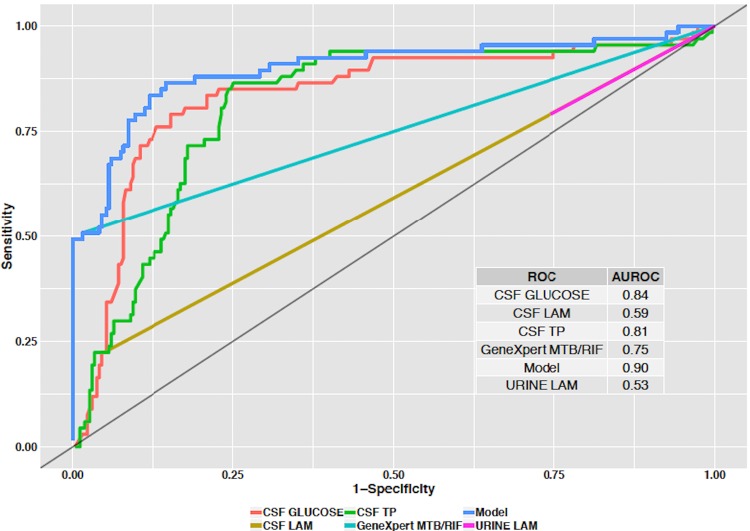
The sensitivity/specificity for the diagnostic tests were CSF Xpert MTB/RIF, 52.9%/94.2%; CSF LAM, 21.9%/94.2%; and urine LAM, 24.1%/76.1%. The combination of CSF glucose of <40 mg/dl and total protein of >100 mg/dl had sensitivity/specificity of 66.3%/90%. Figure 2 shows the receiver operating characteristic (ROC) curves with area under the curve (AUC) for CSF Xpert MTB/RIF, CSF LAM, Urine LAM, CSF glucose, and CSF total protein in comparison to the gold standard of CSF TB culture. The AUCs were as follows: CSF Xpert MTB/RIF, 0.75 (95% confidence interval [CI], 0.69 to 0.81; P < 0.0001); CSF LAM, 0.59 (95% CI, 0.54 to 0.64; P = 0.001); Urine LAM, 0.53 (95% CI, 0.47 to 0.58; P = 0.94); CSF total protein, 0.81 (95% CI, 0.76 to 0.87; P = 0.001), and CSF glucose, 0.84 (95% CI, 0.78 to 0.90; P < 0.0001). Based on the Youden Index, the optimal cutoff values when equally weighting for sensitivity and specificity for the diagnostic tests with continuous variables were as follows: CSF glucose, <36 mg/dl (sensitivity, 75%; specificity, 87%); and CSF total protein, >110 mg/dl (sensitivity, 82%; specificity, 75%). Figure 2 also shows the performance of a diagnostic model that includes CSF Xpert MTB/RIF, CSF LAM, CSF total protein, and CSF glucose with an AUC of 0.90 (95% CI, 0.84 to 0.94; P < 0.0001).
FIG 2.
Receiver operating curves (ROCs) for diagnostic tests to distinguish TBM from non-TBM cases. The model (blue line) demonstrates the area under curve incorporating all variables except for urine LAM. TP, total protein.
CSF LAM detected 5 cases missed by Xpert MTB/RIF. When both tests were used in combination in HIV-infected patients, the sensitivity was 59.1% with a specificity of 93.2%. We identified 14 cases of probable TBM. The inclusion of these cases did not significantly change the performance of Xpert MTB/RIF, CSF LAM, or urine LAM for the diagnosis of TBM. Of the CSF LAM indeterminate samples, 8% (1/13) were CSF TB culture positive. Of the urine LAM indeterminate samples 19% (3/16) were CSF TB culture positive. A sensitivity analysis treating these 4 indeterminate samples as positive did not significantly change the overall test performance for CSF or urine LAM.
Inpatient and 1-year mortality.
Table 2 demonstrates the risk factors for inpatient and 1-year mortality. No patients were lost during the period they were hospitalized, but 113/550 (20.5%) were lost to follow-up after 1 year postdischarge. The 1-year mortality outcomes among those who could be tracked was 58.9%. A sensitivity analysis treating all lost to follow-ups as having survived showed a 1-year mortality rate of 46.7%. A sensitivity analysis treating all lost to follow-ups as having died showed a 1-year mortality rate of 67.3%. Longer time period since HIV diagnosis, longer duration of treatment with ART, and a higher CD4+ T-cell count at the time of hospitalization were associated with surviving to discharge. Positive CSF TB culture and a higher Medical Research Council (MRC) TBM severity grade (10) were associated with inpatient mortality. Higher CD4+ T-cell count was associated with lower odds of 1-year mortality. TB positive CSF culture and higher MRC TBM severity grade were associated with higher odds of 1-year mortality. In the multivariate analysis in Table 2, only the MRC grade remained significant for both inpatient mortality and 1-year mortality. A higher CD4 count was associated with 1-year survival on multivariate regression analysis.
TABLE 2.
Variables associated with inpatient and 1-year mortality in univariate and multivariate analysis
| Variable by type (n = 550) | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CIa ) | P value | Odds ratio (95% CI) | P value | |
| Inpatient mortality | ||||
| Categorical (n) | ||||
| Female (261) | 0.81 (0.56–1.19) | 0.29 | ||
| HIV (475) | 1.02 (0.57–1.85) | 0.93 | ||
| Taking ART (190) | 0.85 (0.57–1.26) | 0.42 | ||
| History of TB (189) | 0.75 (0.50–1.12) | 0.17 | ||
| TB-positive CSF culture (107) | 2.51 (1.61–3.90) | <0.0001 | 2.38 (0.92–6.14) | 0.07 |
| Continuous | ||||
| Age | 0.99 (0.97–1.0005) | 0.19 | ||
| Duration of HIV diagnosis (days) | 0.9995 (0.9993–0.9998) | 0.001 | 0.999 (0.998–1.000) | 0.20 |
| Duration of ART (days) | 0.9996 (0.9994–0.9999) | 0.02 | 1.0002 (0.9994–1.000) | 0.60 |
| CD4 | 0.9990 (0.9982–0.9998) | 0.02 | 0.9996 (0.9981–1.0012) | 0.70 |
| MRC severity | 2.933 (2.103–4.091) | <0.001 | 2.40 (1.34–4.27) | 0.003 |
| 1-yr mortality | ||||
| Categorical (n) | ||||
| Female (261) | 0.82 (0.56–1.21) | 0.32 | ||
| HIV (475) | 1.37 (0.77–2.42) | 0.28 | ||
| Taking ART (190) | 1.25 (0.83–1.86) | 0.28 | ||
| History of TB (189) | 1.47 (0.97–2.24) | 0.07 | 0.62 (0.35–1.10) | 0.10 |
| TB-positive CSF culture (107) | 1.68 (1.03–2.74) | 0.04 | 1.28 (0.67–2.43) | 0.45 |
| Continuous | ||||
| Age | 1.01 (0.99–1.03) | 0.33 | ||
| Duration of HIV diagnosis (days) | 0.9998 (0.9996–0.9999) | 0.05 | 0.9999 (0.9997–1.0001) | 0.68 |
| Duration of ART (days) | 0.9999 (0.9996–1.0001) | 0.45 | ||
| CD4 | 0.9985 (0.9978–0.9993) | 0.0001 | 0.998 (0.997–0.999) | 0.04 |
| MRC severity | 2.08 (1.52–2.86) | <0.0001 | 1.72 (1.16–2.55) | 0.006 |
CI, confidence interval.
The cumulative inpatient and 1-year mortality for TBM patients that were diagnosed as CSF Xpert MTB/RIF positive was 49% and 76%, respectively. This is in comparison to an inpatient and 1-year mortality of 37% and 62%, respectively, for patients that were Xpert MTB/RIF negative but CSF TB culture positive. These differences were not statistically significant. There was also no significant difference in MRC grade between CSF Xpert MTB/RIF positive and negative TBM patients. Xpert MTB/RIF detected rifampin resistance on 6/55 (11%) positive samples. The cumulative inpatient and 1-year mortality for these patients was 67% and 83%, respectively. The inpatient mortality of 49% in this study was not significantly different from the inpatient mortality of 46% from a prior study at the same institution where PCR results from TBM patients were not shared with the treating team in real time.(12)
DISCUSSION
Xpert MTB/RIF performance and impact on mortality.
Xpert MTB/RIF performed similarly compared with what has been previously reported for the diagnosis of TBM (13, 14). It misses almost half of definite TBM cases, which results in frequent empirical treatment based on diagnostic algorithms or provider suspicion. There was no difference in inpatient mortality among HIV-positive patients in this study when Xpert MTB/RIF results were turned over to the treating team within 24 hours compared with a prior PCR study in the same institution when results were not immediately available for clinical care purposes (12). This may reflect the high rate of empirical treatment in cases that were Xpert negative.
Utility of LAM and CSF biochemistry.
CSF LAM testing was highly specific and detected 5 cases that were missed by Xpert MTB/RIF. The additional detection of cases by CSF LAM comes with little additional resources given LAM’s cost and ease of use. These findings highlight that there are novel CSF biomarkers for TBM that can be used to enhance the diagnosis.
Based on the performance of urine LAM, it does not appear to be of value as a stand-alone test for the diagnosis of TBM. However, urine LAM was positive in 51 HIV-infected patients with CD4+ T-cell counts of <200 who had a negative CSF culture. In a prior study, urine LAM had a sensitivity of 46% and a specificity of 93% for the diagnosis of disseminated TB in HIV patients with advanced immunosuppression (15). Thus, it is likely that the majority of these 51 patients had disseminated TB with unclear central nervous system (CNS) involvement. In a recently developed uniform case definition of TBM, clinical research patients have been assigned to categories of probable or possible TBM based on a numerical score derived from a combination of clinical presentation, CSF findings, neuroimaging, and evidence of TB elsewhere (11). Urine LAM is currently not factored into this scoring system as evidence of TB elsewhere. Based on our findings, we feel it deserves strong consideration for inclusion.
CSF glucose and total protein demonstrated the highest AUC compared with all of the other diagnostic tests. The optimal cutoff values of a CSF glucose of <36 mg/dl and CSF total protein of >110 mg/dl established by the Youden index when providing equal weight to sensitivity and specificity are consistent with the cutoff values established in the consensus paper for the diagnosis of TBM for clinical research (11). It is important to note that cryptococcal meningitis and bacterial meningitis patients were screened out in our population.
High mortality.
The inpatient and 1-year mortality among TBM patients was extremely high. MRC grade was the only significant factor associated with both inpatient and 1-year mortality in the multivariate model, suggesting that the major driver of mortality is the advanced stage of illness at the time of initial medical evaluation. Additionally, there were significantly more men than women diagnosed with TBM in this study. These findings support numerous research studies that document poor health-seeking behavior among HIV-infected males, resulting in presentation during advanced stages of illness (16).
HIV-negative TBM patients.
There was a relatively small number of HIV-negative patients diagnosed with TBM. These patients were younger than the HIV-positive population and had similarly low CD4+ T-cell counts. The etiology for the CD4+ lymphocytopenia in this population was likely TB infection itself rather than another source of immunodeficiency (17).
Limitations.
This study has a number of limitations. There is stigma around lumbar punctures in Zambia that leads to a high refusal rate of nearly 25% (18). As a result, this cohort may not be representative of all patients with TBM but rather only those who provided CSF. This study used approximately 3 ml of centrifuged CSF for Xpert MTB/RIF testing. It has been shown that larger volumes of centrifuged CSF can increase Xpert MTB/RIF sensitivity (19). Additionally, CSF TB culture is widely accepted to be an imperfect gold standard for the diagnosis of TBM, especially in patients with paucibacillary disease in whom false-negative cultures are likely problematic. As a result, the true performance of Xpert MTB/RIF, CSF LAM, and urine LAM are likely better than reported here. A consensus definition of probable and possible TBM was developed for clinical research in 2010 (11). Data collection in this study did not provide sufficient information to use the recommended definitions. An adapted definition of probable TBM was used, and we did not classify patients with possible TBM.
Future research.
GeneXpert MTB/RIF Ultra (Xpert Ultra) is the next generation Xpert MTB/RIF test that has shown increased sensitivity for the diagnosis of TBM. It has been endorsed by the WHO as the test of choice for the diagnosis of TBM (20). A larger study that incorporates Xpert Ultra would be beneficial to see if it results in a decrease in TBM-associated mortality. In settings where TB is endemic, many TBM patients are empirically commenced on TB medication based on clinical presentation and limited laboratory data. It is possible that Xpert Ultra will make no difference on mortality in these settings. This is an important question given the significant financial resources Xpert Ultra requires for scale-up in LMIC.
Supplementary Material
ACKNOWLEDGMENTS
We thank the UTH microbiology lab for technical assistance as well as the patients and their families.
Igor J. Koralnik has served on scientific advisory boards for Hoffman La Roche, Glaxo Smith Klyne, and Merck Serono and received consulting fees from Bristol Myers Sqibb, Ono Pharmaceuticals, Merck Serono, Hoffman La Roche, GlaxoSmithKline, Perseid Therapeutics, Vertex Pharmaceutical, and Johnson & Johnson. He is an editorial board member for the Journal of NeuroVirology and receives royalties from UpToDate for topics on the management of HIV, CNS mass lesions, and progressive multifocal leukoencephalopathy. Gretchen L. Birbeck has served as a consultant for GlaxoSmithKline. She is on the advisory board for the US NIH Fogarty International Center, the board of directors for the American Neurological Association, and editorial board for BMC Medicine and Neurology. Keertan Dheda has received speaker fees from Alere. Omar K. Siddiqi, Musie Ghebremichael, Eugene Mubanga, Shawn Love, Clayton Buback, Barry Kosloff, Helen Ayles, and Masharip Atadzhanov report no conflict of interest.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00652-19.
REFERENCES
- 1.Clarridge JE III, Shawar RM, Shinnick TM, Plikaytis BB. 1993. Large-scale use of polymerase chain reaction for detection of Mycobacterium tuberculosis in a routine mycobacteriology laboratory. J Clin Microbiol 31:2049–2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garg RK. 1999. Tuberculosis of the central nervous system. Postgrad Med J 75:133–140. doi: 10.1136/pgmj.75.881.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahr NC, Boulware DR. 2014. Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med 8:1085–1103. doi: 10.2217/bmm.14.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. 2013. Automated real-time nucleic acid amplification technology for rapid and simultaneous detection of tuberculosis and rifampicin resistance: Xpert MTB/RIF assay for the diagnosis of pulmonary and extrapulmonary tuberculous in adults and children Policy update. World Health Organization Press, Geneva, Switzerland. [PubMed] [Google Scholar]
- 5.Churchyard GJ, Stevens WS, Mametja LD, McCarthy KM, Chihota V, Nicol MP, Erasmus LK, Ndjeka NO, Mvusi L, Vassall A, Sinanovic E, Cox HS, Dye C, Grant AD, Fielding KL. 2015. Xpert MTB/RIF versus sputum microscopy as the initial diagnostic test for tuberculosis: a cluster-randomised trial embedded in South African roll-out of Xpert MTB/RIF. Lancet Glob Health 3:e450–e457. doi: 10.1016/S2214-109X(15)00100-X. [DOI] [PubMed] [Google Scholar]
- 6.Theron G, Zijenah L, Chanda D, Clowes P, Rachow A, Lesosky M, Bara W, Mungofa S, Pai M, Hoelscher M, Dowdy D, Pym A, Mwaba P, Mason P, Peter J, Dheda K, Team T-N. 2014. Feasibility, accuracy, and clinical effect of point-of-care Xpert MTB/RIF testing for tuberculosis in primary-care settings in Africa: a multicentre, randomised, controlled trial. Lancet 383:424–435. doi: 10.1016/S0140-6736(13)62073-5. [DOI] [PubMed] [Google Scholar]
- 7.Peter JG, Zijenah LS, Chanda D, Clowes P, Lesosky M, Gina P, Mehta N, Calligaro G, Lombard CJ, Kadzirange G, Bandason T, Chansa A, Liusha N, Mangu C, Mtafya B, Msila H, Rachow A, Hoelscher M, Mwaba P, Theron G, Dheda K. 2016. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 387:1187–1197. doi: 10.1016/S0140-6736(15)01092-2. [DOI] [PubMed] [Google Scholar]
- 8.Patel VB, Singh R, Connolly C, Kasprowicz V, Zumla A, Ndungu T, Dheda K. 2010. Comparison of a clinical prediction rule and a LAM antigen-detection assay for the rapid diagnosis of TBM in a high HIV prevalence setting. PLoS One 5:e15664. doi: 10.1371/journal.pone.0015664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox JA, Lukande RL, Kalungi S, Van Marck E, Lammens M, Van de Vijver K, Kambugu A, Nelson AM, Colebunders R, Manabe YC. 2015. Accuracy of lipoarabinomannan and Xpert MTB/RIF testing in cerebrospinal fluid to diagnose tuberculous meningitis in an autopsy cohort of HIV-infected adults. J Clin Microbiol 53:2667–2673. doi: 10.1128/JCM.00624-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilkinson RJ, Rohlwink U, Misra UK, van Crevel R, Mai NTH, Dooley KE, Caws M, Figaji A, Savic R, Solomons R, Thwaites GE, Tuberculous Meningitis International Research Consortium. 2017. Tuberculous meningitis. Nat Rev Neurol 13:581–598. doi: 10.1038/nrneurol.2017.120. [DOI] [PubMed] [Google Scholar]
- 11.Marais S, Thwaites G, Schoeman JF, Torok ME, Misra UK, Prasad K, Donald PR, Wilkinson RJ, Marais BJ. 2010. Tuberculous meningitis: a uniform case definition for use in clinical research. Lancet Infect Dis 10:803–812. doi: 10.1016/S1473-3099(10)70138-9. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqi OK, Ghebremichael M, Dang X, Atadzhanov M, Kaonga P, Khoury MN, Koralnik IJ. 2014. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin Infect Dis 58:1771–1777. doi: 10.1093/cid/ciu191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel VB, Theron G, Lenders L, Matinyena B, Connolly C, Singh R, Coovadia Y, Ndung'u T, Dheda K. 2013. Diagnostic accuracy of quantitative PCR (Xpert MTB/RIF) for tuberculous meningitis in a high burden setting: a prospective study. PLoS Med 10:e1001536. doi: 10.1371/journal.pmed.1001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nhu NT, Heemskerk D, Thu d. D, Chau TT, Mai NT, Nghia HD, Loc PP, Ha DT, Merson L, Thinh TT, Day J, Chau N, Wolbers M, Farrar J, Caws M. 2014. Evaluation of GeneXpert MTB/RIF for diagnosis of tuberculous meningitis. J Clin Microbiol 52:226–233. doi: 10.1128/JCM.01834-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shah M, Hanrahan C, Wang ZY, Dendukuri N, Lawn SD, Denkinger CM, Steingart KR. 2016. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in HIV-positive adults. Cochrane Database Syst Rev 10:CD011420. doi: 10.1002/14651858.CD011420.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mills EJ, Beyrer C, Birungi J, Dybul MR. 2012. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med 9:e1001167. doi: 10.1371/journal.pmed.1001167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skogmar S, Schon T, Balcha TT, Jemal ZH, Tibesso G, Bjork J, Bjorkman P. 2013. CD4 cell levels during treatment for tuberculosis (TB) in Ethiopian adults and clinical markers associated with CD4 lymphocytopenia. PLoS One 8:e83270. doi: 10.1371/journal.pone.0083270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elafros MA, Belessiotis C, Birbeck GL, Bond V, Sikazwe I, Kvalsund MP. 2017. Barriers to lumbar puncture in Zambia: insights into the “Tap Gap,” abstr 350332. American Neurological Association Annual Meeting, San Diego, CA. [Google Scholar]
- 19.Bahr NC, Marais S, Caws M, van Crevel R, Wilkinson RJ, Tyagi JS, Thwaites GE, Boulware DR, Tuberculous Meningitis International Research Consortium. 2016. GeneXpert MTB/Rif to diagnose tuberculous meningitis: perhaps the first test but not the last. Clin Infect Dis 62:1133–1135. doi: 10.1093/cid/ciw083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahr NC, Nuwagira E, Evans EE, Cresswell FV, Bystrom PV, Byamukama A, Bridge SC, Bangdiwala AS, Meya DB, Denkinger CM, Muzoora C, Boulware DR, Williams DA, Taseera K, Nyehangane D, Ivan M, Orikiriza P, Rhein J, Hullsiek KH, Musubire A, Pastick K, Nabeta P, Mwesigye J, Rajasingham R. 2018. Diagnostic accuracy of Xpert MTB/RIF Ultra for tuberculous meningitis in HIV-infected adults: a prospective cohort study. Lancet Infect Dis 18:68–75. doi: 10.1016/S1473-3099(17)30474-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.