Treatment of bacterial infections is increasingly challenged by resistance to currently available antibacterial agents. Not only are such agents less likely to be active today than they were in the past, but their very use has selected for and continues to select for further resistance. Additional strategies for the management of bacterial illnesses must be identified. In this review, bacteriophage-based therapies are presented as one promising approach.
KEYWORDS: bacteriophages
ABSTRACT
Treatment of bacterial infections is increasingly challenged by resistance to currently available antibacterial agents. Not only are such agents less likely to be active today than they were in the past, but their very use has selected for and continues to select for further resistance. Additional strategies for the management of bacterial illnesses must be identified. In this review, bacteriophage-based therapies are presented as one promising approach. In anticipation of their potential expansion into clinical medicine, clinical microbiologists may wish to acquaint themselves with bacteriophages and their antibacterial components and, specifically, with methods for testing them. Here, we reviewed the literature spanning January 2007 to March 2019 on bacteriophage and phage-encoded protein therapies of relevance to clinical microbiology.
INTRODUCTION
Following the early therapeutic application of bacteriophages (phages) in France in the 1930s, industrial investment in phage production took place across Europe, the former Soviet Union, and the United States (1). The popularity of human phage therapy waned in Western countries with the advent of antibiotics; as chemicals, antibiotics were easier to test in the clinical laboratory and administer than were bacteriophages. At the time, there was also an underdeveloped understanding of phage biology and a lack of standardized in vitro methods to assess bacteriophage activity, alongside a lack of clinical trial methodologies to evaluate them; what clinical data were available yielded inconsistent results (1). Today, phages continue to be used clinically in parts of Eastern Europe as well as the former Soviet Union (e.g., Poland, the Republic of Georgia) (1–4) and are also used in agronomy and food processing (5), as well as in veterinary medicine (6). With the current challenge of antibiotic resistance, the case for human phage therapy in Western medicine has been reopened. In addition, the potential use of phage components as antibacterial agents is being evaluated. While there are no U.S. Food and Drug Administration (FDA)-approved bacteriophages or phage component products available for human clinical application in the United States, some are being administered on an expanded-access basis, with others being used in clinical trials.
Clinical microbiologists have historically used phages for phage typing to differentiate between bacterial strains as part of outbreak investigations, though with the advent of molecular techniques, including, most recently, whole-genome sequencing, younger clinical microbiologists may be unfamiliar with phage-based testing. In anticipation of the potential expansion of phage therapy into clinical medicine, clinical microbiologists may wish to acquaint themselves with bacteriophages and their antibacterial components and specifically with methods for testing them. Here, we review the literature spanning January 2007 to March 2019 on bacteriophage and phage-encoded protein therapy of relevance to clinical microbiology.
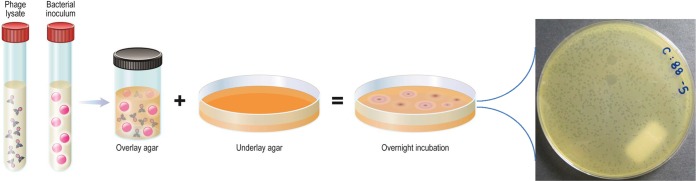
Bacteriophages are a clade of bacteriotropic viruses of individual sizes ranging from 20 to 200 nm that infect their hosts, typically manifesting strain- or species-level specificity (7, 8). More than 6,000 phages have been characterized to date, with genome sizes ranging from a few thousand to 480,000 nucleotides or more (7). Residing in close association with bacteria, phages are found throughout the biosphere, including in bodies of water and sewage, and in and on humans and animals, totaling an estimated 1 × 1031 virions and outnumbering bacterial cells by 10-fold (9). Given their abundance and diversity, it is not surprising that most are as yet undescribed. Phage bioprospecting efforts are ongoing; this typically involves adding target bacteria in a concentrated nutrient broth to a solid (e.g., soil) or liquid (e.g., water) sample, followed by incubation for several hours (10) and then assessment for phage using the double-overlay plaque assay described below (see Fig. 2), with isolated phages then being purified by serial plating. Thirteen (as many as 19, by some estimates) phage families are distinguished by morphology, the presence or absence of an envelope, as well as the size and composition of their genome (single-stranded or double-stranded DNA or RNA) (11).
FIG 2.
Double-overlay plaque assay. A combination of amplified phage and host bacterium in cooled, molten agar supplemented with divalent cations (e.g., CaCl2, MgSO4) is poured over solid agar and the medium is incubated overnight. Quantifiable clearings (plaques) in the bacterial lawn indicate the presence of infectious phage. At least two different plaque morphologies are observed in the example photograph, illustrating an experiment involving coculture of Salmonella enterica serotype Typhimurium and phages obtained from municipal sewage. (© Mayo Clinic).
That “phages are nature’s version of precision medicine” manifests advantages and disadvantages to their therapeutic application (12). While their specificity may minimize off-target effects on commensal bacteria compared to antibiotics, this same property may limit their empirical administration by requiring testing of the bacterium with which a particular patient is infected before selecting the specific phage to treat that patient, requiring the availability of laboratory testing and potentially engendering treatment delays. The narrow spectrum of individual phages may, however, be overcome by the concurrent use of combinations of phages in phage cocktails (12).
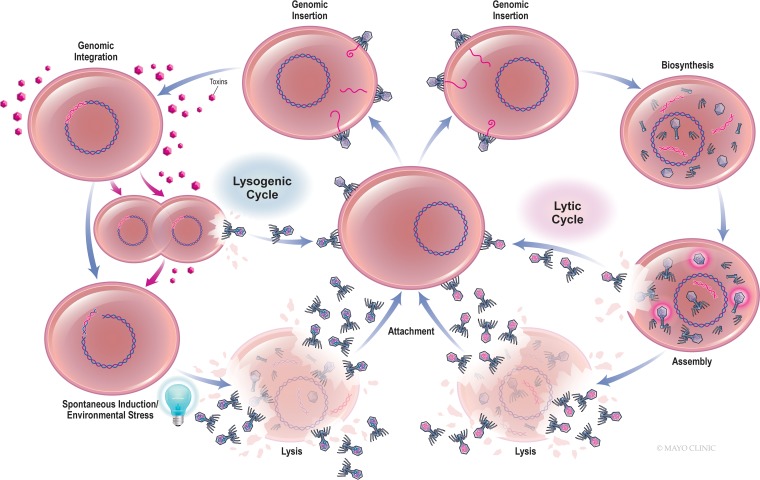
Although bacteriophages have traditionally been classified as lytic or lysogenic, a more precise terminology is strictly lytic and temperate (13) (Fig. 1). Temperate phages enter bacteria and become incorporated into the host genome, assuming a quiescent prophage form for a period of time, and may sometimes extrude progeny phages in the absence of lysis (7). Prophages may also be excised from the genome in a process known as spontaneous induction or following cellular stress (7). Phage excision may occur precisely or may result in the splicing and packaging of adjacent bacterial genes within newly synthesized virions, followed by host lysis. Many phages are capable of lytic and temperate cycles, with the switch between them being governed by such factors as the intracellular concentrations of second messenger molecules, extracellular phage density, and environmental resources (14). Temperate phages may encode virulence (including toxin) genes, preclude subsequent phage infection (including lytic phages), and promote bacterial fitness. Interestingly, Sweere et al. recently demonstrated that Pf4 phage infecting Pseudomonas aeruginosa sourced from human wounds contributed to chronic infection by binding Toll-like receptor 3, reducing phagocytosis and tumor necrosis factor secretion (15). Strictly lytic phages engage only the lytic cycle; that is, they enter their hosts, seize control of the bacterial replication machinery, and express proteins that destabilize the cell envelope, resulting in immediate bacteriolysis and the dissemination of new virions for subsequent infection. Because they effect temporally constrained cell death, are not frequent vectors for horizontal gene transfer, and are not known to interact with eukaryotic cells, strictly lytic phages are typically used for phage therapy. Still, generalized transduction of host genetic material may occur with strictly lytic phages as a consequence of the inadvertent encapsulation of host genes within newly produced viral capsids (7, 68).
FIG 1.
Bacteriophage life cycle.
The clinical use of phage protein components, including endolysins (described below), depolymerases (further categorized as hydrolases [or polysaccharases], endosialidases, endorhamnosidases, and polysaccharide lyases [16]), and holins (enzymes which insert holes into bacterial cell membranes), has recently been considered (17). The use of phage components eliminates the risk of genomic integration and gene transduction, while featuring ease of dosing, quality control, and, potentially, storage. In addition, phage protein components may have more predictable activity against strains of targeted species than phages. The drawbacks of lysin therapy include abbreviated circulation due to human host proteases and the absence of endogenous viral amplification associated with conventional phage therapy, in addition to immunogenicity, abrogating the future efficacy of the same phage protein component (18).
Endolysins, alternatively known as lysins or peptidoglycan hydrolases, hydrolyze bacterial cell walls, with those targeting Gram-positive bacteria generally being composed of an enzymatically active domain and a cell wall binding domain and with those targeting Gram-negative bacteria lacking the latter (19). Endolysins are classified on the basis of which murein bond that they cleave (20). There are two phage proteins in clinical trials at present, tonabacase (N-Rephasin SAL-200; iNtRON Biotechnology) (21) and exebacase (CF-301; ContraFect Corporation) (22), both of which are endolysin formulations specific to Staphylococcus aureus; tonabacase cleaves amide and peptide bonds, while exebacase cleaves amide bonds (23–25).
Adoption of phage therapy into human medicine would likely have implications for the clinical microbiology laboratory if the performance of phage susceptibility testing (PST) were to be required to select a phage or phage combinations for individual patients’ isolates and to assess whether resistance to particular phages or phage combinations has been selected for in cases of individual patient treatment failure. Testing the susceptibility of patient isolates to fixed-composition phage cocktails could be considered, with screening against individual phages from a phage library being performed only in cases that are not susceptible to generic cocktails (26).
If an individualized phage therapy approach were to be adopted, health care facilities would need not only to have access to PST but possibly also to engage in phage preparation. What role clinical laboratories versus pharmacies or other entities might play in the preparation of individualized phage formulations for patient administration, as is customary in some other countries and a paradigm that is discussed further below, remains to be determined (27). Once matched with the target bacteria, phages would need to be amplified to obtain viral titers sufficiently high for human administration. This entails incubation of the phage with its bacterial target, followed by purification for pyrogen removal. Care needs to be taken to avoid the use of lysogenized bacteria for phage amplification, lest genes carried by integrated temperate phages encoding bacterial resistance or toxins become inadvertently intermingled with lytic virions. Since physical phage isolation using centrifugation and filtration does not remove all bacterial components (e.g., endotoxin), methods for removal of these need to be applied, using monovalent cations (28) or elution filters and phase separation by 1-octanol, dialysis against ethanol and NaCl and speed vacuuming (29), for example.
No standard method for PST exists. A common approach is the double-overlay plaque assay or a modification thereof (including the spot plate method, used for screening high numbers of phage-bacterium combinations), in which known concentrations of phage and bacteria are combined with semisoft agar and incubated, with the presence or absence of plaques denoting bacterial susceptibility or a lack thereof, respectively (Fig. 2). Another method is the cross-streak method, in which phage lysate is plated across an agar plate and bacteria are plated in perpendicular fashion, with susceptibility being inferred based on the zone of inhibition size (30). Merabishvili et al. described the use of large square petri dishes with 2% Luria broth agar inoculated with the target bacteria in horizontal strips, air dried, and then spotted with 5 μl of 107-PFU/ml suspensions of the individual bacteriophages (65). Following incubation for 16 to 18 h at 37°C, the degree of phage activity is classified as confluent lysis, opaque lysis, semiconfluent lysis, several plaques, or negative. These approaches can be time- and labor-intensive and may not be user-friendly in clinical laboratories. The OmniLog system (Biolog, Haywood, CA) has been adapted for PST by assaying various phage concentrations and bacteria in the presence of a tetrazolium dye in microtiter plates. If bacterial growth occurs, cells respire and reduce the tetrazolium dye to induce a color change; conversely, cell lysis secondary to phage infection abrogates host respiration which is colorimetrically apparent. This method also serves as the basis of the Host Range Quick Test, which is capable of screening a single bacterial sample against as many as 5,000 bacteriophages in less than 18 h (Adaptive Phage Therapeutics, Gaithersburg, MD). While the technology is not commercially available, it may portend the phage screening capacity of clinical laboratories in the future.
An unanswered question pertaining to PST regards the need for the establishment of positive, intermediate, and negative phage infectivity thresholds, as is standard for interpretation of the MICs of traditional antibiotics. The convention in phage research is to measure the multiplicity of infection (MOI), that is, the ratio of phages to bacteria. This metric may be challenging to apply in phage therapy because one would not necessarily know how many bacteria are being treated, that number could change over time, and some bacteria may be more or less accessible to phage than others (31). Additionally, unlike traditional antibiotics, phage can amplify in the host, such that the dose increases in vivo, the very attractiveness of phage therapy. In the end, phage will likely be delivered as a specific number of phages; how that delivery will take place is the subject of ongoing studies and may vary depending on the site and type of infection. This, too, could influence the interpretation of results of laboratory studies on phage activity. Further, it is likely that phage will be applied in challenging-to-treat infections, which, in some cases, will involve biofilms. As such, methods for determining phage antibiofilm activity, which is not even standardized for traditional antibiotics, may be needed.
In contrast to PST, testing for susceptibility to phage-encoded proteins is more similar to conventional antibacterial susceptibility testing. For example, a standard or modified broth microdilution method is used for susceptibility testing of tonabacase (21) and exebacase (32), respectively, with the modification comprised of the addition of 0.5 mM dl-dithiothreitol and 25% horse serum to cation-adjusted Mueller-Hinton broth (33). Quality control ranges for exebacase are being developed by the Clinical and Laboratory Standards Institute (34). As a result of the use of broth microdilution, lysin susceptibility testing should be amenable to automation on commercial susceptibility testing instruments (35).
Exercising proper storage and maintenance is as crucial as selecting a phage(s) active against the target bacteria toward successful phage therapy. Given the proteinaceous composition of viruses, shear forces or variations in the temperature and pH to which phages are subjected during handling may impact titers as a result of protein misfolding or denaturation. Their shelf life may vary by bacteriophage species, as a result of phage size and morphology (29, 36–39). The storage methods utilized in the Republic of Georgia, where phage therapy is used clinically, have included freeze-drying, freezing, refrigeration, and short-term ambient storage (Marina Tediashvili, personal communication), though some studies suggest that phase changes, including freezing or drying, may result in drops in titer, at least among certain phages (37). These effects may be abrogated by the addition of excipients, such as skim milk (37, 40). Phage viability over long-term storage appears to be maximized at 4°C, −80°C, or −196°C (41). It is likely that conditions for ideal stability will need to be evaluated for each phage considered for clinical use. Lysins, in contrast to whole phages, may exhibit stability at a wider range of temperatures and pHs (22).
Whether or not phages will ultimately be adopted into the therapeutic armamentarium in Western medicine is unknown. Case studies of phage therapy continue to be added to the published literature (Table 1). Patey et al. note that the generally positive results reported from isolated applications stand in contrast to the negative results of some clinical trials (Table 2) (42). The routes of phage administration and dosing intervals, as well as the diversity of target bacteria and infection types, portend that the investigational use of phage therapy will likely continue for some time in the United States. PST methods ought to be standardized in order to guide studies and enable results between studies to be compared and observations generalized and extrapolated.
TABLE 1.
Human case reports and case series involving phage and phage lysin therapy published between January 2007 and March 2019
| Bacterium | Clinical presentation | Phage(s) administered | Method of phage administration | Outcome | Reference(s) |
|---|---|---|---|---|---|
| Acinetobacter baumannii | 68-yr-old man, necrotizing pancreatitis | ΦPC cocktail (AC4, C1P12, C2P21, C2P24), ΦIV cocktail (AB-Navy1, AB-Navy4, AB-Navy71, AB-Navy97), ΦIVB cocktail (AB-Navy71, AbTP3Φ1) | Intracavitary and intravenous administration of approximately 109 PFU/dose alongside multiple antibiotics | Recovery | 50 |
| 77-yr-old man, craniectomy site infection | 1 proprietary phage | Intravenous administration of 2.14 × 107 PFU/ml in 4 ml lactated Ringer solution every 2 h for 8 days | Withdrawal of treatment and death | 51 | |
| Enterococcus faecalis | 3 men (32–45 yr old), chronic bacterial prostatitis | Assorted lytic phages (from the Institute of Immunology and Experimental Therapy of the Polish Academy of Sciences) screened against prostatic secretion isolates | Twice daily intrarectal administration of 10 ml of 4.5 × 107 to 2.8 × 108 PFU/ml for 28–33 days | Recovery | 52, 53 |
| Multiple-antibiotic-resistant bacteria | 62 patients, genitourinary tract infections, prostatitis, bone infections, upper or lower respiratory tract infections, or skin or soft tissue infections | MS-1 cocktail, OPMS-1 cocktail, monovalent Staphylococcus aureus phages (unspecified), monovalent Enterococcus faecalis phages (unspecified), monovalent Gram-negative bacterial phages (unspecified) | Oral, oral and local, intrarectal, or local bacteriophage (106–109 PFU/ml) twice or three times daily for 12 wk or more at dosing volumes of 10 ml for oral and intrarectal administration (volume unspecified for local administration) | Favorable response in 40–55% of patients | 54 |
| Pseudomonas aeruginosa | 76-yr-old man, infected, fistulated aortic graft | OMKO1 | 10 ml of 1 × 107 PFU/ml of intrafistular phage and ceftazidime | Recovery | 55 |
| 61-yr-old man, pressure sores and septicemia | BFC1 cocktail (PNM, 14/1, ISP) | Topical (50 ml 109 PFU/ml every 8 h) and intravenous (50 μl 109 PFU/ml/6 h) for 10 days | Blood cultures negative, but local infection persisted and patient died 4 mo later due to unrelated infection | 56 | |
| 67-yr-old woman, urinary tract infection | Pyophage 051007 (proprietary 6-phage cocktail) | Bladder irrigation of 20 ml containing 2 × 107 PFU twice daily for 10 days with concurrent colistin and meropenem and subsequent meropenem | Recovery | 57 | |
| 2-yr-old boy, bacteremia | Proprietary 2-phage cocktail | Intravenous administration of 3.5 × 105 PFU every 6 h for 3 days alongside meropenem, tobramycin, aztreonam, colistin, and polymyxin B | Recovery | 58 | |
| Staphylococcus aureus | 65-yr-old woman, bacterial keratitis | SATA-8505 | Intravenous, intranasal, and intraocular administration over 4 wk (dose unspecified) | Recovery | 59 |
| 6 men (44–92 years old), diabetic foot ulcers | Sb-1 | Weekly tissue debridement and topical phage treatment (0.1–0.5 ml of 107–108 PFU/ml) | Recovery | 60 |
TABLE 2.
Phage and phage lysin therapy in human clinical trials from January 2007 to March 2019
| Yr of trial start | Trial registration no. | Reference | Phage/lysin formulation (investigator/sponsor) | Bacteria or bacterium targeted | Treatment indication | Trial phase | Outcome |
|---|---|---|---|---|---|---|---|
| 2006 | NCT00663091 | 61 | WPP-201 cocktail (Southwest Regional Wound Care Center) | Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus | Infected venous leg ulcers | I | No adverse effects observed |
| 2009 | NCT00937274 | 62, 66 | T4 coliphage cocktail: AB2, 4, 6, 11, 46, 50, 55, JS34, 37, 98, D1.4 (Nestlé) | E. coli | Dysentery | I/II | No adverse effects or benefit noted; study discontinued prematurely |
| 2009 | EudraCT 2004-001691-39 | 63 | Biophage-PA (Biocontrol Ltd.) | P. aeruginosa | Otitis externa | I/II | No adverse effects noted; at 42 days posttreatment, the phage-treated cohort exhibited reduced median bacterial abundance compared to placebo group |
| 2015 | NCT02116010 | 67 | PhagoBurn (Pherecydes Pharma) | P. aeruginosa, E. coli | Infected burn wounds | I/II | Fewer adverse effects noted in phage versus antibiotic treatment arms; median time to sustained bacterial reduction comparable between treatment groups by day 7; study prematurely discontinued |
| 2017 | NCT03089697 | NAa | SAL200 (Tonabacase; iNtRON Biotechnology) | S. aureus | Bacteremia | II | Ongoing |
| 2017 | NCT03163446 | 64 | Exebacase (CF-301; ContraFect Corp.) | S. aureus | Bacteremia, including endocarditis (single intravenous dose added to standard-of-care antibiotics) | II | Well tolerated; higher clinical response rate compared to antibiotics alone in methicillin-resistant S. aureus- but not methicillin-susceptible S. aureus-infected subgroups |
| 2017 | NCT03140085 | NA | Pyophage (Tzulakidze National Center of Urology) | Enterococcus species, E. coli, Proteus mirabilis, staphylococci, streptococci, P. aeruginosa | Urinary tract infections | II/III | Results pending (Thomas Kessler, personal communication) |
| 2019 | NCT03808103 | NA | EcoActive (Intralytix, Inc.) | Adherent invasive E. coli (AIEC) | Exacerbation of inflammation in Crohn disease secondary to AIEC | I/II | Recruitment ongoing |
| 2019 | NCT02664740 | NA | PhagoPied (Pherecydes Pharma) | S. aureus | Diabetic wounds | I/II | Not yet recruiting |
NA, not applicable.
Phages may be used as a complementary approach to currently available antimicrobial strategies rather than a replacement (42). As viruses, they and their components may be recognized by the immune system and eliminated via innate and/or adaptive defenses before reaching sites of infection, and their use may also compromise the future use of similar species in the same patient. Phages are also 6-fold larger than small-molecule antibiotics, limiting their distribution and ability to diffuse across some physiological membranes, such as the blood-brain barrier, and, subsequently, their utility to treat certain types of infection (3, 43). Finally, their host specificity requires identification of the pathogen prior to treatment, such that empirical therapy may be challenging (43, 44).
How phage therapy would be regulated in the United States is presently unclear, with some questioning whether this can be done within the existing drug and device approval infrastructure of the U.S. Food and Drug Administration, founded on the concept of drugs as small molecules of fixed composition (42, 44, 45). Subjecting the production and oversight of therapeutic phage to current good manufacturing practice and evaluation within conventional randomized clinical trials will be associated with significant cost and incite process development inefficiencies (e.g., in a phage cocktail, each phage as well as the final cocktail may require FDA approval; the composition of off-the-shelf phage products might require updating at regular intervals to avoid resistance and to maintain clinical relevance to current pathogens), culminating in approval delays (42, 44, 46, 47). Some countries in which phages are used therapeutically operate under different regulatory guidelines; for example, Belgium uses magistral preparations, the adoption of which some favor on a broader scale (27, 46). By this course, phage is prescribed by a clinician, and the corresponding formulation is produced by an associated pharmacy using master phage stocks that are quality controlled by an accredited laboratory (27). Regulation will also depend upon the level at which oversight of phage treatment is controlled: individual patients, hospitals, local or national governments, or some combination thereof (48, 49). While offering a critique or suggestions for the regulation of phage therapy is beyond the scope of this review, clinical microbiologists should be aware of the possibilities receiving consideration as well as their predicted impact on laboratory practice.
Although more translational research must be completed before the clinical implementation of bacteriophage therapy is feasible, it is not too early to consider how this antibacterial strategy may shift current treatment paradigms. Such a shift will likely impact the clinical microbiology laboratory, upon which the burden of PST, reporting parameters, and possibly phage storage (at least for in vitro testing) will rest.
ACKNOWLEDGMENTS
We gratefully acknowledge Larry J. Prokop for his medical library expertise and assistance in curating the collection of articles which inform this review. We thank Raymond Schuch (ContraFect) for his thoughtful review of the manuscript.
R.P. reports grants from ContraFect, CD Diagnostics, Merck, Hutchison Biofilm Medical Solutions, Accelerate Diagnostics, and Shionogi (monies are paid to Mayo Clinic), serving as a consultant to Curetis, Specific Technologies, Next Gen Diagnostics, Selux Dx, GenMark Diagnostics, PathoQuest, and Qvella (monies are paid to Mayo Clinic), patents on a Bordetella pertussis/B. parapertussis PCR and an antibiofilm substance issued, a patent on a device/method for sonication with royalties paid by Samsung to Mayo Clinic, receiving editor’s stipends from ASM and IDSA, and receiving honoraria from the NBME, Up-to-Date, and the Infectious Diseases Board Review Course. K.M.C. has no conflicts to report.
R.P. is supported by UM1 AI104681-01, R01 AR056647, and R21 AI125870. K.M.C. is supported by T32 AR056950.
REFERENCES
- 1.Summers W. 2005. History of phage research and phage therapy, p 3–17. In Waldor MK, Friedman DI, Adhya SL (ed), Phages: their role in bacterial pathogenesis and biotechnology. ASM Press, Washington, DC. [Google Scholar]
- 2.Górski A, Międzybrodzki R, Łobocka M, Głowacka-Rutkowska A, Bednarek A, Borysowski J, Jończyk-Matysiak E, Łusiak-Szelachowska M, Weber-Dąbrowska B, Bagińska N, Letkiewicz S, Dąbrowska K, Scheres J. 2018. Phage therapy: what have we learned? Viruses 10:288. doi: 10.3390/v10060288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kortright KE, Chan BK, Koff JL, Turner PE. 2019. Phage therapy: a renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 25:219–232. doi: 10.1016/j.chom.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Brussow H. 2012. What is needed for phage therapy to become a reality in Western medicine? Virology 434:138–142. doi: 10.1016/j.virol.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Harada LK, Silva EC, Campos WF, Del Fiol FS, Vila M, Dabrowska K, Krylov VN, Balcao VM. 2018. Biotechnological applications of bacteriophages: state of the art. Microbiol Res 212-213:38–58. doi: 10.1016/j.micres.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Hawkins C, Harper D, Burch D, Anggard E, Soothill J. 2010. Topical treatment of Pseudomonas aeruginosa otitis of dogs with a bacteriophage mixture: a before/after clinical trial. Vet Microbiol 146:309–313. doi: 10.1016/j.vetmic.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 7.Guttman B, Raya R, Kutter E. 2005. Basic phage biology, p 29–66. In Kutter E, Sulakvelidze A (ed), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL. doi: 10.1201/9780203491751.ch3. [DOI] [Google Scholar]
- 8.Pantůcek R, Rosypalová A, Doskar J, Kailerová J, Růzicková V, Borecká P, Snopková S, Horváth R, Götz F, Rosypal S. 1998. The polyvalent staphylococcal phage phi 812: its host-range mutants and related phages. Virology 246:241–252. doi: 10.1006/viro.1998.9203. [DOI] [PubMed] [Google Scholar]
- 9.Comeau AM, Hatfull GF, Krisch HM, Lindell D, Mann NH, Prangishvili D. 2008. Exploring the prokaryotic virosphere. Res Microbiol 159:306–313. doi: 10.1016/j.resmic.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Van Twest R, Kropinski AM. 2009. Bacteriophage enrichment from water and soil In Clokie MRJ, Kropinski AM (ed), Bacteriophages: isolation, characterization, and interactions, vol 1 Humana Press, Totowa, NJ. [Google Scholar]
- 11.Ackermann H-W. 2004. Bacteriophage classification, p 67–89. In Kutter E, Sulakvelidze A (ed), Bacteriophages: biology and applications. CRC Press, Boca Raton, FL. [Google Scholar]
- 12.Lyon J. 2017. Phage therapy's role in combating antibiotic-resistant pathogens. JAMA 318:1746–1748. doi: 10.1001/jama.2017.12938. [DOI] [PubMed] [Google Scholar]
- 13.Hobbs Z, Abedon ST. 2016. Diversity of phage infection types and associated terminology: the problem with 'lytic or lysogenic'. FEMS Microbiol Lett 363:fnw047. doi: 10.1093/femsle/fnw047. [DOI] [PubMed] [Google Scholar]
- 14.Howard-Varona C, Hargreaves KR, Abedon ST, Sullivan MB. 2017. Lysogeny in nature: mechanisms, impact and ecology of temperate phages. ISME J 11:1511–1520. doi: 10.1038/ismej.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sweere JM, Van Belleghem JD, Ishak H, Bach MS, Popescu M, Sunkari V, Kaber G, Manasherob R, Suh GA, Cao X, de Vries CR, Lam DN, Marshall PL, Birukova M, Katznelson E, Lazzareschi DV, Balaji S, Keswani SG, Hawn TR, Secor PR, Bollyky PL. 2019. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 363:eaat9691. doi: 10.1126/science.aat9691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B, Delattre AS, Lavigne R. 2012. Learning from bacteriophages—advantages and limitations of phage and phage-encoded protein applications. Curr Protein Pept Sci 13:699–722. doi: 10.2174/138920312804871193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan X, Li W, Zheng F, Xie J. 2013. Bacteriophage inspired antibiotics discovery against infection involved biofilm. Crit Rev Eukaryot Gene Expr 23:317–326. doi: 10.1615/CritRevEukaryotGeneExpr.2013007717. [DOI] [PubMed] [Google Scholar]
- 18.Maciejewska B, Olszak T, Drulis-Kawa Z. 2018. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: an ambitious and also a realistic application? Appl Microbiol Biotechnol 102:2563–2581. doi: 10.1007/s00253-018-8811-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Parisien A, Allain B, Zhang J, Mandeville R, Lan CQ. 2008. Novel alternatives to antibiotics: bacteriophages, bacterial cell wall hydrolases, and antimicrobial peptides. J Appl Microbiol 104:1–13. doi: 10.1111/j.1365-2672.2007.03498.x. [DOI] [PubMed] [Google Scholar]
- 20.Vollmer W, Joris B, Charlier P, Foster S. 2008. Bacterial peptidoglycan (murein) hydrolases. FEMS Microbiol Rev 32:259–286. doi: 10.1111/j.1574-6976.2007.00099.x. [DOI] [PubMed] [Google Scholar]
- 21.Kim N-H, Park WB, Cho JE, Choi YJ, Choi SJ, Jun SY, Kang CK, Song K-H, Choe PG, Bang J-H, Kim ES, Park SW, Kim N-J, Oh M-D, Kim HB. 2018. Effects of phage endolysin SAL200 combined with antibiotics on Staphylococcus aureus infection. Antimicrob Agents Chemother 62:e00731-18. doi: 10.1128/AAC.00731-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuch R, Khan BK, Raz A, Rotolo JA, Wittekind M. 2017. Bacteriophage lysin CF-301, a potent antistaphylococcal biofilm agent. Antimicrob Agents Chemother 61:e02666-16. doi: 10.1128/AAC.02666-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother 57:2743–2750. doi: 10.1128/AAC.02526-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun SY, Jung GM, Son J-S, Yoon SJ, Choi Y-J, Kang SH. 2011. Comparison of the antibacterial properties of phage endolysins SAL-1 and LysK. Antimicrob Agents Chemother 55:1764–1767. doi: 10.1128/AAC.01097-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lood R, Molina H, Fischetti VA. 2017. Determining bacteriophage endopeptidase activity using either fluorophore-quencher labeled peptides combined with liquid chromatography-mass spectrometry (LC-MS) or Forster resonance energy transfer (FRET) assays. PLoS One 12:e0173919. doi: 10.1371/journal.pone.0173919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kvachadze L, Balarjishvili N, Meskhi T, Tevdoradze E, Skhirtladze N, Pataridze T, Adamia R, Topuria T, Kutter E, Rohde C, Kutateladze M. 2011. Evaluation of lytic activity of staphylococcal bacteriophage Sb-1 against freshly isolated clinical pathogens. Microb Biotechnol 4:643–650. doi: 10.1111/j.1751-7915.2011.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirnay JP, Verbeken G, Ceyssens PJ, Huys I, De Vos D, Ameloot C, Fauconnier A. 2018. The magistral phage. Viruses 10:E64. doi: 10.3390/v10020064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drab M. 2018. Phage aggregation-dispersion by ions: striving beyond antibacterial therapy. Trends Biotechnol 36:875–881. doi: 10.1016/j.tibtech.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Bonilla N, Rojas MI, Netto Flores Cruz G, Hung SH, Rohwer F, Barr JJ. 2016. Phage on tap—a quick and efficient protocol for the preparation of bacteriophage laboratory stocks. PeerJ 4:e2261. doi: 10.7717/peerj.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balouiri M, Sadiki M, Ibnsouda SK. 2016. Methods for in vitro evaluating antimicrobial activity: a review. J Pharm Anal 6:71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abedon ST. 2016. Phage therapy dosing: the problem(s) with multiplicity of infection (MOI). Bacteriophage 6:e1220348. doi: 10.1080/21597081.2016.1220348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh J, Sauve KL, Wittekind M, Schuch R. 2017. Development of an antimicrobial susceptibility test (AST) for the antistaphylococcal lysin CF-301. ECCMID, Vienna, Austria. [Google Scholar]
- 33.Oh JT, Cassino C, Schuch R. 2019. Postantibiotic and sub-MIC effects of Exebacase (lysin CF-301) enhance antimicrobial activity against Staphylococcus aureus. Antimicrob Agents Chemother 63:e02616-18. doi: 10.1128/AAC.02616-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuch R. 2017. Update on CF-301 AST development: effect of variation in horse serum sources on BMD MIC values, p 37–45. In Methods development and standardization January 2017. CLSI Antimicrobial Susceptibility Testing Meeting, January 2017. Clinical and Laboratory Standards Institute, Wayne, PA: https://clsi.org/meetings/ast/ast-meeting-files-resources/. [Google Scholar]
- 35.Xie Y, Wahab L, Gill JJ. 2018. Development and validation of a microtiter plate-based assay for determination of bacteriophage host range and virulence. Viruses 10:E189. doi: 10.3390/v10040189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leung SSY, Parumasivam T, Nguyen A, Gengenbach T, Carter EA, Carrigy NB, Wang H, Vehring R, Finlay WH, Morales S, Britton WJ, Kutter E, Chan HK. 2018. Effect of storage temperature on the stability of spray dried bacteriophage powders. Eur J Pharm Biopharm 127:213–222. doi: 10.1016/j.ejpb.2018.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clark WA. 1962. Comparison of several methods for preserving bacteriophages. Appl Microbiol 10:466–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark WA, Geary D. 1973. Proceedings: preservation of bacteriophages by freezing and freeze-drying. Cryobiology 10:351–360. doi: 10.1016/0011-2240(73)90057-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y, Peng X, Zhang H, Watts AB, Ghosh D. 2018. Manufacturing and ambient stability of shelf freeze dried bacteriophage powder formulations. Int J Pharm 542:1–7. doi: 10.1016/j.ijpharm.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Menendez E, Fernandez L, Gutierrez D, Rodriguez A, Martinez B, Garcia P. 2018. Comparative analysis of different preservation techniques for the storage of Staphylococcus phages aimed for the industrial development of phage-based antimicrobial products. PLoS One 13:e0205728. doi: 10.1371/journal.pone.0205728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fortier LC, Moineau S. 2009. Phage production and maintenance of stocks, including expected stock lifetimes In Clokie MRJ, Kropinski AM (ed), Bacteriophages: methods and protocols, isolation, characterization, and interactions. vol 1:Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- 42.Patey O, McCallin S, Mazure H, Liddle M, Smithyman A, Dublanchet A. 2018. Clinical indications and compassionate use of phage therapy: personal experience and literature review with a focus on osteoarticular infections. Viruses 11:E18. doi: 10.3390/v11010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harper DR. 2018. Criteria for selecting suitable infectious diseases for phage therapy. Viruses 10:E177. doi: 10.3390/v10040177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cooper CJ, Khan Mirzaei M, Nilsson AS. 2016. Adapting drug approval pathways for bacteriophage-based therapeutics. Front Microbiol 7:1209. doi: 10.3389/fmicb.2016.01209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration. 29 JuneE 2018. The history of FDA's fight for consumer protection and public health. https://www.fda.gov/AboutFDA/History/default.htm. Accessed 19 April 2019.
- 46.Nagel TE. 2018. Delivering phage products to combat antibiotic resistance in developing countries: lessons learned from the HIV/AIDS epidemic in Africa. Viruses 10:E345. doi: 10.3390/v10070345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Górski A, Międzybrodzki R, Weber-Dąbrowska B, Fortuna W, Letkiewicz S, Rogóż P, Jończyk-Matysiak E, Dąbrowska K, Majewska J, Borysowski J. 2016. Phage therapy: combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front Microbiol 7:1515. doi: 10.3389/fmicb.2016.01515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huys I, Pirnay JP, Lavigne R, Jennes S, De Vos D, Casteels M, Verbeken G. 2013. Paving a regulatory pathway for phage therapy: Europe should muster the resources to financially, technically and legally support the introduction of phage therapy. EMBO Rep 14:951–954. doi: 10.1038/embor.2013.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Verbeken G, De Vos D, Vaneechoutte M, Merabishvili M, Zizi M, Pirnay J-P. 2007. European regulatory conundrum of phage therapy. Future Microbiol 2:485–491. doi: 10.2217/17460913.2.5.485. [DOI] [PubMed] [Google Scholar]
- 50.Schooley RT, Biswas B, Gill JJ, Hernandez-Morales A, Lancaster J, Lessor L, Barr JJ, Reed SL, Rohwer F, Benler S, Segall AM, Taplitz R, Smith DM, Kerr K, Kumaraswamy M, Nizet V, Lin L, McCauley MD, Strathdee SA, Benson CA, Pope RK, Leroux BM, Picel AC, Mateczun AJ, Cilwa KE, Regeimbal JM, Estrella LA, Wolfe DM, Henry MS, Quinones J, Salka S, Bishop-Lilly KA, Young R, Hamilton T. 2017. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob Agents Chemother 61:e00954-17. doi: 10.1128/AAC.00954-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaVergne S, Hamilton T, Biswas B, Kumaraswamy M, Schooley RT, Wooten D. 2018. Phage therapy for a multidrug-resistant Acinetobacter baumannii craniectomy site infection. Open Forum Infect Dis 5:ofy064. doi: 10.1093/ofid/ofy064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Letkiewicz S, Miedzybrodzki R, Fortuna W, Weber-Dabrowska B, Górski A. 2009. Eradication of Enterococcus faecalis by phage therapy in chronic bacterial prostatitis—case report. Folia Microbiol (Praha) 54:457–461. doi: 10.1007/s12223-009-0064-z. [DOI] [PubMed] [Google Scholar]
- 53.Letkiewicz S, Międzybrodzki R, Kłak M, Jończyk E, Weber-Dąbrowska B, Górski A. 2010. The perspectives of the application of phage therapy in chronic bacterial prostatitis. FEMS Immunol Med Microbiol 60:99–112. doi: 10.1111/j.1574-695X.2010.00723.x. [DOI] [PubMed] [Google Scholar]
- 54.Łusiak-Szelachowska M, Żaczek M, Weber-Dąbrowska B, Międzybrodzki R, Letkiewicz S, Fortuna W, Rogóż P, Szufnarowski K, Jończyk-Matysiak E, Olchawa E, Walaszek KM, Górski A. 2017. Antiphage activity of sera during phage therapy in relation to its outcome. Future Microbiol 12:109–117. doi: 10.2217/fmb-2016-0156. [DOI] [PubMed] [Google Scholar]
- 55.Chan BK, Turner PE, Narayan D, Kim S, Mojibian HR, Elefteriades JA. 2018. Phage treatment of an aortic graft infected with Pseudomonas aeruginosa. Evol Med Public Health 2018:60–66. doi: 10.1093/emph/eoy005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jennes S, Merabishvili M, Soentjens P, Pang KW, Rose T, Keersebilck E, Soete O, François P-M, Teodorescu S, Verween G, Verbeken G, De Vos D, Pirnay J-P. 2017. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury—a case report. Crit Care 21:129. doi: 10.1186/s13054-017-1709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khawaldeh A, Morales S, Dillon B, Alavidze Z, Ginn AN, Thomas L, Chapman SJ, Dublanchet A, Smithyman A, Iredell JR. 2011. Bacteriophage therapy for refractory Pseudomonas aeruginosa urinary tract infection. J Med Microbiol 60:1697–1700. doi: 10.1099/jmm.0.029744-0. [DOI] [PubMed] [Google Scholar]
- 58.Duplessis C, Wolfe D, Quinones J, Estrella L, Henry M, Hamilton T, Biswas B, Hanisch B, Perkins M. 2018. Refractory Pseudomonas bacteremia in a 2-year-old sterilized by bacteriophage therapy. J Pediatr Infect Dis Soc 7:253–256. doi: 10.1093/jpids/pix056. [DOI] [PubMed] [Google Scholar]
- 59.Fadlallah A, Chelala E, Legeais JM. 2015. Corneal infection therapy with topical bacteriophage administration. Open Ophthalmol J 9:167–168. doi: 10.2174/1874364101509010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fish R, Kutter E, Wheat G, Blasdel B, Kutateladze M, Kuhl S. 2016. Bacteriophage treatment of intransigent diabetic toe ulcers: a case series. J Wound Care 25:S27–S33. doi: 10.12968/jowc.2016.25.Sup7.S27.26949862 [DOI] [Google Scholar]
- 61.Rhoads DD, Wolcott RD, Kuskowski MA, Wolcott BM, Ward LS, Sulakvelidze A. 2009. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J Wound Care 18:237. doi: 10.12968/jowc.2009.18.6.42801. [DOI] [PubMed] [Google Scholar]
- 62.Vandenheuvel D, Lavigne R, Brussow H. 2015. Bacteriophage therapy: advances in formulation strategies and human clinical trials. Annu Rev Virol 2:599–618. doi: 10.1146/annurev-virology-100114-054915. [DOI] [PubMed] [Google Scholar]
- 63.Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin Otolaryngol 34:349–357. doi: 10.1111/j.1749-4486.2009.01973.x. [DOI] [PubMed] [Google Scholar]
- 64.Fowler VG, Das A, Lipka J, Schuch R, Cassino C. 2019. Exebacase (lysin CF-301) improved clinical responder rates in methicillin resistant Staphylococcus aureus (MRSA) bacteremia including endocarditis compared to standard of care antibiotics (SOC) alone in a first-in-patient phase II study. https://d1io3yog0oux5.cloudfront.net/_53ec10a3e327f1e291fe5c77b7504a7a/contrafect/db/226/1306/pdf/ECCMID+ORAL+Ph2_FINAL.pdf. Accessed 30 April 2019.
- 65.Merabishvili M, Pirnay JP, Verbeken G, Chanishvili N, Tediashvili M, Lashkhi N, Glonti T, Krylov V, Mast J, Van Parys L, Lavigne R, Volckaert G, Mattheus W, Verween G, De Corte P, Rose T, Jennes S, Zizi M, De Vos D, Vaneechouette M. 2009. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4(3):e4944. doi: 10.1371/journal.pone.0004944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarker SA, Sultana S, Reuteler G, Moine D, Descombes P, Charlton F, Bourdin G, McCallin S, Ngom-Bru C, Neville T, Akter M, Huq S, Qadri F, Talukdar K, Kassam M, Delley M, Loiseau C, Deng Y, El Aidy S, Berger B, Brussow H. 2016. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: a randomized trial in children from Bangladesh. EBioMedicine 4:124–137. doi: 10.1016/j.ebiom.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jault P, Leclerc T, Jennes S, Pirnay JP, Que Y-A, Resch G, Rosseau AF, Ravat F, Carsin H, Le Floch R, Schaal JV, Soler C, Fevre C, Arnaud I, Bretaudeau L, Gabard J. 2019. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): a randomised, controlled, double-blind phase 1/2 trial. Lancet Infect Dis 19:35–45. doi: 10.1016/S1473-3099(18)30482-1. [DOI] [PubMed] [Google Scholar]
- 68.Calendar R, Inman R. 2005. Phage biology, p 18–36. In Waldor MK, Friedman DI, Adhya SL (ed), Phages: their role in bacterial pathogenesis and biotechnology. ASM Press, Washington, DC. [Google Scholar]