Rapid and accurate differentiation of Salmonella spp. causing enteric fever from nontyphoidal Salmonella is essential for clinical management of cases, laboratory risk management, and implementation of public health measures. Current methods used for confirmation of identification, including biochemistry and serotyping as well as whole-genome sequencing analyses, take several days.
KEYWORDS: Nontyphoidal salmonellae, real-time PCR, Salmonella, whole-genome sequencing, typhoidal salmonellae
ABSTRACT
Rapid and accurate differentiation of Salmonella spp. causing enteric fever from nontyphoidal Salmonella is essential for clinical management of cases, laboratory risk management, and implementation of public health measures. Current methods used for confirmation of identification, including biochemistry and serotyping as well as whole-genome sequencing analyses, take several days. Here we report the development and evaluation of a real-time PCR assay that can be performed directly on crude DNA extracts from bacterial colonies for the rapid identification of typhoidal and nontyphoidal Salmonella.
INTRODUCTION
Our novel 2-h assay identifies the genus Salmonella by detecting the ttr gene, encoding tetrathionate reductase, and defines typhoidal Salmonella by the detection of Salmonella enterica serovar Typhi and Paratyphi-specific gene combinations. PCR assay performance was determined using 211 clinical cultures of Salmonella (114 nontyphoidal and 97 typhoidal strains) and 7 clinical non-Salmonella cultures. In addition, the specificity of the assay was evaluated in silico using a diverse in-house collection of 1,882 Salmonella whole-genome sequences. The real-time PCR results for 218 isolates and the genomic analysis of the 1,882 isolates produced 100% sensitivity and 100% specificity (based on a 7-gene profile) for identifying typhoidal Salmonella compared to the Salmonella whole-genome sequening identification methods currently used at Public Health England (PHE).
This paper describes a robust real-time PCR assay for the rapid, accurate identification of typhoidal and nontyphoidal Salmonella which will be invaluable for the urgent screening of isolates from symptomatic individuals, for the safe processing of isolates in laboratories, and for assisting the management of public health risks.
Salmonella is a diverse genus of gastrointestinal pathogens that cause a wide spectrum of diseases from self-limiting gastroenteritis (nontyphoidal salmonellae [NTS]) to systemic enteric fever (typhoidal salmonellae [TS]: Salmonella enterica serovar Typhi and serovars Paratyphi A, B, and C). Salmonellosis is global, but typhoidal salmonellae are found mainly in sub-Saharan Africa and South Asia, where enteric fever is endemic (1), although detailed local surveillance data from regions of endemicity remain poor (2). A current concern is the increase in bacteremia (and focal infections) associated with multidrug-resistant NTS infection in sub-Saharan Africa. In high-income countries such as the United Kingdom, invasive NTS infection is mainly confined to immunocompromised hosts, so the major risks are local outbreaks of NTS through poor food hygiene and typhoidal infections associated with travel to regions of endemicity.
Diagnostic hospital microbiology laboratories make only a presumptive identification of Salmonella spp.: they do not usually hold a sufficient range of specific antisera for full identification and rapid identification systems, such as matrix-assisted laser desorption/ionization–time of flight mass spectroscopy, are unable to fully identify Salmonella. In reference laboratories where definitive microbiological methods for the identification of Salmonella by serology and biochemistry (3) are available, the turnaround times are often lengthy because of weak expression of the somatic (O), flagellar (H), and Vi polysaccharide surface antigens leading to incomplete or incorrect identification of the serovars. Whole-genome sequencing (WGS) for Salmonella (4) has simplified the process for identifying Salmonella serovars substantially but still takes days rather than hours. Currently there are no rapid diagnostic tests for informing clinical and public health management of enteric fever or for ensuring that Salmonella isolates are processed appropriately with respect to laboratory safety.
In the United Kingdom, salmonellosis is a significant public health problem causing morbidity and financial loss due to sickness and absenteeism until clearance from infection for certain professions. The clinical management of salmonellosis patients depends on diagnosis. Enteric fever is treated with antibiotics, but nontyphoidal Salmonella gastroenteritis is usually self-limiting. Invasive disease needs to be treated with antibiotics specific to the strain causing infection. In addition, the processing of isolates or specimens in the laboratory from patients with suspected diarrheal infection depends on the identification of the causal agent. In the United Kingdom, microorganisms that pose a risk to human health are classified into one of four hazard groups based on the ability to infect healthy humans. The classification of these organisms allows the risk they pose to laboratory and health care workers to be controlled by implementing safety measures at the appropriate containment level (5). S. Typhi and S. Paratyphi A, B, and C are classified as hazard group 3 (HG3) pathogens in the United Kingdom and many regions globally (but not in all countries, such as those in North America), requiring processing in a specialized containment level 3 (CL3) laboratory (5). It is clear, therefore, that in order to treat patients effectively and protect health care and laboratory staff, the rapid identification of a patient as being infected with a typhoidal salmonella is critical.
At present there is no single rapid method to identify all TS, even though genomic data on the presence or absence of genes in both typhoidal and nontyphoidal Salmonella are in abundance. The ttr gene, encoding tetrathionate reductase, has been used as a PCR gene target to detect and identify Salmonella since it is present in all Salmonella spp. (6). However, it is not intended to distinguish typhoid and nontyphoidal subspecies. A few potential candidate genes for identifying TS have been described previously. For example, the tviB gene, encoding a Vi polysaccharide capsule, which is present in S. Typhi and S. Paratyphi C (7) but not in S. Paratyphi A or S. Paratyphi B, can identify a subset of TS but does not distinguish S. Typhi or S. Paratyphi C (8). In order to differentiate Salmonella serovars causing enteric fever, additional genes are required. Nga et al. proposed using SPA2308, encoding a hypothetical protein, for the detection of S. Paratyphi A and STY0201 (also known as the staG gene), encoding a putative fimbrial protein, for the detection of S. Typhi in clinical blood samples via PCR (9). Connor et al. suggested that S. Paratyphi B (TS) could be distinguished from S. Java (NTS) using two genes encoding type III secretion system (TTSS) effector proteins, sseJ and srfJ (10), with S. Paratyphi B possessing only srfJ but S. Java possessing both sseJ and srfJ. However, as sseJ is also absent in S. Typhi and S. Paratyphi A, this gene cannot be used to differentiate all TS serovars or used alone as an NTS marker. A potential gene target for S. Paratyphi C identification is the SPC0869 target, a gene encoding a hypothetical protein, shown to be present only in S. Paratyphi C (8). The use of this gene requires further assessment to ensure that it is a unique target among the S. Paratyphi C population, as only five serovars were investigated in the study by Liu et al. (8).
The design of a PCR assay to identify Salmonella and differentiate NTS and TS requires a multitargeted approach with defined gene profiles and rigorous validation. The aim of this study was to develop and validate a real-time PCR assay to distinguish TS (HG3) and NTS (HG2) and identify specific serovars of TS using traditional molecular and bioinformatic techniques.
MATERIALS AND METHODS
Bacterial strains.
A total of 211 Salmonella enterica subspecies I isolates, received at the Gastrointestinal Bacterial Reference Unit (GBRU), Public Health England (PHE) between 2008 and 2017 (Table 1), were used in this PCR study. Representative NTS isolates from the two most common serovars, S. Enteritidis and S. Typhimurium, as well as serovars that can be difficult to distinguish from TS isolates by traditional methods, including S. Dublin, S. Java, and S. Choleraesuis, were selected (Table 1). Assay specificity was further investigated by the inclusion of four Shigella isolates (Shigella flexneri, S. sonnei, S. dysenteriae, and S. boydii) and three Escherichia coli isolates (containing either eae or stx genes) as representatives to test the specificity against other Enterobacteriaceae that are occasionally misidentified by referring clinical laboratories using automated identification platforms (see Table S1 in the supplemental material).
TABLE 1.
Number and type of Salmonella serovars tested via molecular PCR
| Sequence type(s) | eBURST group(s) | Serovar or species | Serotype | Hazard groupb | No. (n = 218) |
|---|---|---|---|---|---|
| Typhoidal Salmonella | |||||
| 1, 2, 2173 | 13 | Salmonella Typhi | 9,12[Vi]:d:– | 3 | 61 |
| 85, 129 | 11 | Salmonella Paratyphi A | 1,2,12:a:[1,5] | 3 | 15 |
| 86 | 5 | Salmonella Paratyphi B | 1,4,[5],12:b:1,2 | 3 | 15 |
| 146 | 20 | Salmonella Paratyphi C | 6,7,[Vi]:c:1,5 | 3 | 6 |
| Total | 97 | ||||
| Nontyphoidal Salmonella isolates | |||||
| 11, 183 | 11, 183 | Salmonella Enteritidis | 1,9,12:g,m:– | 2 | 14 |
| 19, 34, 36 | 19, 34, 36 | Salmonella Typhimurium | 1,4,[5],12:i:1,2 | 2 | 14 |
| 10 | 10 | Salmonella Dublin | 1,9,12[Vi]:g,p:– | 2 | 14 |
| 43, 88, 2545 | 43, 88, 0 | Salmonella Java | 1,4,[5],12:b:1,2 | 2 | 13 |
| 2902, 3226, 139, 145 | 0, 0, 6,6 | Salmonella Choleraesuis | 6,7,:c:1,5 | 2 | 6 |
| Variablea | Variablea | Selection of Salmonella spp. from GeneFinder analysisa | Variable; see Table S1 | 2 | 53 |
| Total | 114 | ||||
| Non-Salmonella isolates | |||||
| 245, 152, 252, 7375 | CC245, 152, 145, 0 | Shigella flexneri, S. sonnei, S. boydii, S. dysenteriae | 3a, NA, O6, O1 | 2 | 4 |
| 11, 29, 40 | CC11, 21, 40 | EPEC (eae), STEC (stx1a or eae stx2a)c | O55:H12, O77:H1, O157:H7 | 2 | 3 |
| Total | 7 | ||||
A random selection of hazard group 2 ST containing sporadic gene targets was chosen.
As defined in the Advisory Committee on Dangerous Pathogens (ACDP) guidelines (5).
EPEC, enteropathogenic E. coli; STEC, Shiga toxin-producing E. coli.
Salmonella whole-genome sequence data.
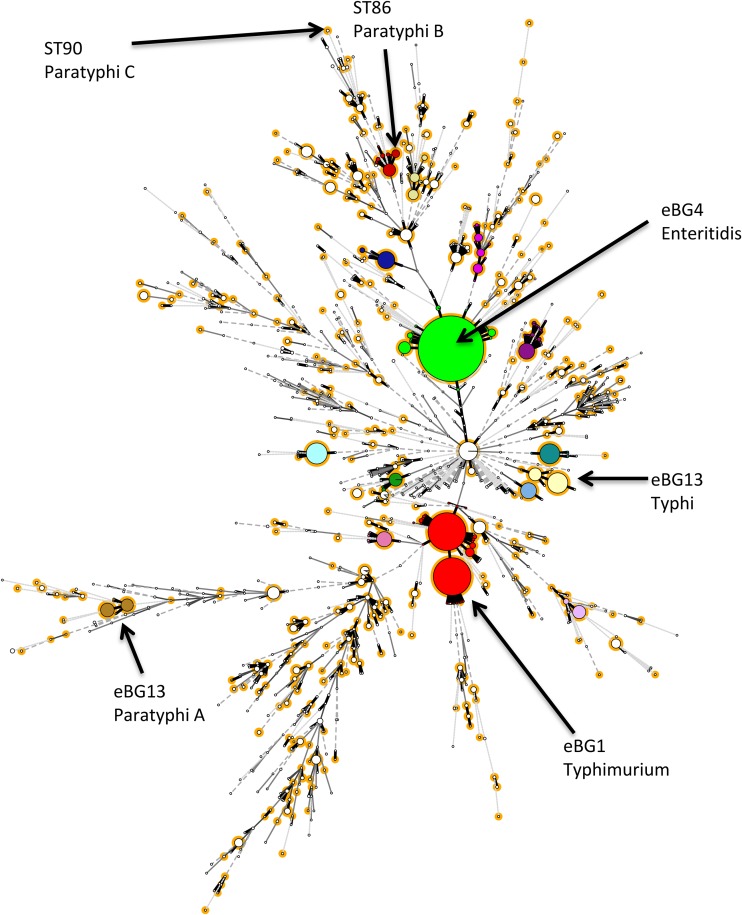
A total of 1,882 Salmonella whole-genome sequences (including the 211 Salmonella isolates), representing the diversity of salmonellae tested by the GBRU, were included in an in silico validation of the specificity of the selected target genes (Fig. 1). This data set included representative sequence types (ST) of the 19,221 strains validated and reported at the GBRU between 2016 and 2017. The strains selected included all subspecies of Salmonella and the common (3 or more isolates received between 2016 and 2017 at PHE) Salmonella serovars, representing in total 477 different sequence types (Table 2 and Table S1).
FIG 1.
Selection of representative strains to test in silico. Shown is a population structure of Salmonella received at PHE between 2016 and 2017 and strains tested by PCR in this study, totaling 19,221 strains. Strains are color coded by main eBURST groups (eBG). Representative strains (highlighted in orange) from each sequence type within an eBG containing 3 or more isolates were selected for in silico detection of the seven genes (ttr, sseJ, srfJ, tviB, SPC0869, SPA2308, and staG).
TABLE 2.
Number and type of Salmonella serovars tested via in silico (GeneFinder) analysis
| Type of organism | No. |
|---|---|
| Salmonella Typhi | 556 |
| Salmonella Paratyphi A | 315 |
| Salmonella Paratyphi B | 53 |
| Salmonella Paratyphi C | 6 |
| HG2 serovars | 952 |
| Nonsalmonellae | 7 |
| Total | 1,889 |
| Sequence types | 480 |
| Subspecies I | 1,821/1,889 |
| Subspecies II | 14/1,889 |
| Subspecies IIIa | 14/1,889 |
| Subspecies IIIb | 29/1,889 |
| Subspecies IV | 3/1,889 |
| Subspecies V | 1/1,889 |
DNA extraction and real-time PCR assays.
DNA from 218 isolates was extracted via a crude extraction method in which a single colony from MacConkey agar (Thermo Fisher Scientific, Waltham, MA) was inoculated into 490 μl of sterile distilled water in a screw-cap microtube (Eppendorf, Hamburg, Germany) and placed in a boiling water bath for 20 min. Primers and probes for ttr (detection of all Salmonella), tviB (detection of S. Typhi and S. Paratyphi C), SPA2308 (detection of S. Paratyphi A), and staG (detection of S. Typhi) were based on previously published studies (Table 3). Primers and probes for SPC0869 (detection of S. Paratyphi C) and sseJ and srfJ (detection of S. Paratyphi B) were designed using the PrimerQuest Tool V8 (https://www.idtdna.com/PrimerQuest/Home/Index) using sequences obtained from the NCBI nucleotide database (https://www.ncbi.nlm.nih.gov/nucleotide/) (Table 3).
TABLE 3.
Primer and probe sequences used for each gene target with the fluorescent dye coloreda
| Gene | Name | Sequence (5′–3′) |
GenBank accession no. |
Reference |
|---|---|---|---|---|
| ttr | ttr_F | CTCACCAGGAGATTACAACATGG | AF282268 | 6 |
| ttr_R | AGCTCAGACCAAAAGTGACCATC | |||
| ttr_P | FAM-CACCGACGGCGAGACCGACTTT-BHQ1 | |||
| sseJ | sseJ_F | CGAGACTGCCGATGCATTTA | AF294582 | This study |
| sseJ_R | GTACATAGCCGTGGTGAGTATAAG | |||
| sseJ_P | CY3-TGGAGGCGGCCAGTAATATTGGTT-BHQ1 | |||
| srfJ | srfJ_F | CTGTCTGTATAGCGTGGAAGAG | AF231759 | This study |
| srfJ_R | GTCCACCAGGCCATCTTTAT | |||
| srfJ_P | JOE-CGGCAGGGTATGGATGAGATGGAG-BHQ1 | |||
| tviB | tviB_F | TGTGGTAAAGGAACTCGGTAAA | NC_003198 | 7 (modified) |
| tviB_R | GACTTCCGATACCGGGATAATG | |||
| tviB_P | JOE-TGGATGCCGAAGAGGTAAGACGAGA-BHQ1 | |||
| SPC0869 | SPC0869_F | CTGGCTGACACATGAACAAATC | NC_012125 | This study |
| SPC0869_R | CCTGAGAACGAGTCAGGTTTAC | |||
| SPC0869_P | CY5-TGTACGACTGCAAACGCCAAAGTC-BHQ2 | |||
| SPA2308 | SPA2308_F | ACGATGATGACTGATTTATCGAAC | FM200053 | 9 |
| SPA2308_R | TGAAAAGATATCTCTCAGAGCTGG | |||
| SPA2308_P | CY5-CCCATACAATTTCATTCTTATTGAGAATGCGC-BHQ2 | |||
| staG | staG_F | CGCGAAGTCAGAGTCGACATAG | AL513382 | 9 |
| staG_R | AAGACCTCAACGCCGATCAC | |||
| staG_P | FAM-CATTTGTTCTGGAGCAGGCTGACGG-BHQ1 |
The color of the reporter is related to spectrum of detection; quenchers are in bold. FAM, 6-carboxyfluorescein; BHQ, black hole quencher; F, forward primer; R, reverse primer; P, probe.
The real-time PCR reported here was carried out as seven individual monoplex reactions but also worked as duplex and triplex PCR assays with interchangeable primer and probe targets (and probe dyes) depending on the target gene combination required (data not shown, but master mix details are in Data Set S1). The master mix for the monoplex assay consisted of 12.5 μl of Takyon Low Rox probe master mix (Eurogentec, Liège, Belgium), 8 μl of nuclease-free water, 0.5 μl each of 20 μM forward and reverse primers, 1 μl of 5 μM probe, and 2.5 μl of DNA in a final reaction volume of 25 μl. A negative control was run with each PCR using 2.5 μl of nuclease-free water for the template (Severn Biotech, Kidderminster, UK), and the following positive controls were used: NCTC 8385 for S. Typhi (ttr, tviB, and staG), NCTC 11803 for S. Paratyphi A (ttr and SPA2308), NCTC 8299 for S. Paratyphi B (ttr and srfJ), NCTC 96 for S. Paratyphi C (ttr, sseJ, tviB, and SPC0869), NCTC 6676 for S. Enteritidis (ttr and sseJ), and NCTC 14013 for S. Typhimurium (ttr, sseJ, and srfJ). The PCR was run on an ABI Prism 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The conditions for the PCR were an initial activation of 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 10 s. A positive result was assigned when a threshold cycle (CT) value was achieved between 12 and 30 with a threshold set at a maximal fluorescence (ΔR) of 0.03.
Identification of TS and differentiation from NTS were based on a profile of seven genes (Table 4). The molecular and/or in silico PCR identification was compared with the original identification of the serovar obtained via a combination of WGS identification, phenotype, and serology carried out by the Salmonella laboratory as described previously (4) (Table S1).
TABLE 4.
Gene profiles for the identification of S. Typhi and S. Paratyphi from other serovarsa
| Profile | Salmonella identification | ttr | sseJ | tviBb | srfJ | SPC0869 | SPA2308 | staG |
|---|---|---|---|---|---|---|---|---|
| 1 | S. Typhi | + | − | +/− | − | − | − | + |
| 2 | S. Paratyphi A | + | − | − | − | − | + | − |
| 3 | S. Paratyphi B | + | − | − | + | − | − | − |
| 4 | S. Paratyphi C | + | + | + | − | + | − | − |
| 5 | NTS serovarc | + | + | − | +/− | − | − | − |
| 6 | Non-Salmonella spp. | − | − | − | − | − | − | − |
+, present; −, absent.
tviB+ means that the strain is genotypically Vi positive.
A proportion of NTS serovars will be positive for the ttr gene and a combination of targets that do not match any of the TS profiles (Table S1).
PCR assay evaluation.
The sensitivity and specificity of the ttr, sseJ, srfJ, tviB, staG, SPA2308, and SPC0869 primers and probes (Table 4) used in the real-time PCR assays were calculated according to the method of Martin (11).
In addition, PCR assay specificity was assessed by in silico genomic analysis using a diverse in-house WGS data set covering the population structure of Salmonella (Fig. 1). A total of 1,882 Salmonella sequences (Fig. 1 and Table S1), including the 211 Salmonella isolates tested by PCR, were screened for the presence of seven target genes (ttr, sseJ, srfJ, tviB, staG, SPA2308, and SPC0869) using a PHE in-house bioinformatics tool called GeneFinder (developed by M. Doumith [unpublished data]). This tool takes paired-end Illumina FASTQ reads and aligns them to a reference sequence of the target genes, as a multi-FASTA file (accession numbers in Table 3), using Bowtie2 v2.1.0 (12) and Samtools v1.0.18 (13) and determines metrics such as coverage, presence of indels (insertions or deletions), amino acid alterations, the presence of single nucleotide polymorphisms, and overall similarity of the test sequence to the reference gene sequence. Target genes were designated present when sequences achieved a detection threshold of 80% sequence similarity to the reference gene, apart from ttr, for which the threshold was set at 70% sequence similarity, due to the size and variability of this particular gene (in-house validation). Any discrepant results between GeneFinder and the PCR were investigated further by assembling the sequence data using Spades v3.1.1 to default parameters and examining the variability of primer and probe binding sites.
Assay reproducibility was determined by testing 20 of the 211 Salmonella isolates in triplicate. Precision was evaluated by the standard deviation of CT values of 10 replicates of each of the positive controls for each target. Each target was assessed individually and as a multiplex in separate assay runs by different individuals and had the threshold set at 25% of the maximal fluorescence (ΔR) of each respective target.
RESULTS
Comparison of real-time PCR and current PHE methods for distinguishing NTS and TS.
Of the 211 Salmonella isolates subjected to PCR identification, all gave the expected gene profile identification (Table 5 and Table S1), matching the original identification, except for 3 S. Typhi isolates in which the tviB gene was not detected. This was confirmed by in silico analysis (see below). Previously described TS gene targets SPA0869, staG, and SPA2308 were found sporadically (41/114 [35%] of the NTS tested; 2 isolates had two TS gene targets present), confirming that use of single targets to differentiate TS from NTS is not appropriate (Table 6 and Table S1). None of the 7 target genes were detected in the four shigellae or three E. coli isolates that were tested.
TABLE 5.
Summary of gene profile results
| Profile | Salmonella serovar (no. tested) | Expected genes present | Result | Matches (%) |
|---|---|---|---|---|
| 1 | Salmonella Typhi (556) | ttr, (tviB+/−), staG | 556 | 100 |
| 2 | Salmonella Paratyphi A (315) | ttr, SPA2308 | 315 | 100 |
| 3 | Salmonella Paratyphi B (53) | ttr, srfJ | 53 | 100 |
| 4 | Salmonella Paratyphi C (6) | ttr, sseJ, tviB, SPC0869 | 6 | 100 |
| 5 | NTS serovar (952) | ttr (plus combination of any of the following not fitting the above profiles: sseJ, srfJ, SPC0869, SPA2308, and staG) | 952 | 100 |
| 6 | Non-Salmonella spp. (7) | Negative for all genes | 7 | 100 |
TABLE 6.
Summary of individual gene target results
| Salmonella strain (no. tested) | No. with indicated gene |
||||||
|---|---|---|---|---|---|---|---|
| ttr | sseJ | tviB | srfJ | SPC0869 | SPA2308 | staG | |
| Salmonella Typhi (556) | 556 | 0 | 553 | 0 | 0 | 0 | 556 |
| Atypical Salmonella Typhi (3) | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| Salmonella Paratyphi A (315) | 315 | 0 | 0 | 0 | 0 | 315 | 0 |
| Salmonella Paratyphi B (53) | 53 | 0 | 0 | 53 | 0 | 0 | 0 |
| Salmonella Paratyphi C (6) | 6 | 6 | 6 | 0 | 6 | 0 | 0 |
| NTS serovara (952) | 952 | 938 | 0 | 380 | 19 | 50 | 41 |
| Non-Salmonella spp. (7) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The combination of genes present was heterogeneous; please see Table S1 for details.
Whole-genome sequencing in silico analysis.
For the 1,882 Salmonella isolates subjected to in silico analysis, identification based on gene profiles (Table 4) matched the original identification but did highlight that individual gene targets could be found sporadically across the Salmonella population. In silico analysis identified 952/1,882 nontyphoidal Salmonella isolates that were positive for ttr and a combination of other TS gene targets (Table 6 and Table S1), designated profile 5 (Table 5). None of the gene profiles of these isolates matched the designated TS profiles (profiles 1 to 4), and thus, our interpretation is that ttr-positive strains with a profile not matching the TS profiles should be classified as NTS (Tables 4 and 5 and Table S1).
As with the real-time PCR assay, the Salmonella processed via in silico analysis identified the three SPI-7-negative S. Typhi isolates. The real-time PCR and GeneFinder correctly identified the deletion of this gene.
In this study, 8 of the 1,882 sequences were positive by PCR and yet negative for the same gene by GeneFinder. Further in silico analysis revealed that the genes concerned had an intact primer and probe binding site, thus confirming the PCR result, but variation outside these regions resulted in average similarity values below the GeneFinder threshold value (Table S1).
Reproducibility and precision of PCR assay.
Reproducibility was assessed by performing the PCR 3 times on 20 isolates. The results indicated that the PCR was reproducible for differentiating between NTS and TS and for the identification of serovars within TS (Table S1). The precision analysis demonstrated that five out of the seven gene targets were considered precise (i.e., standard deviation < 0.167). The following average CTs (with standard deviations in parentheses) were obtained for the indicated genes: ttr, 25.12 (0.154); sseJ, 23.59 (0.127); srfJ, 24.51 (0.179); tviB, 25.01 (0.115); staG, 24.97 (0.121); SPC0869, 25.68 (0.142); and SPA2308, 20.59 (0.248). Both srfJ and SPA2308 have standard deviations above the value of 0.167, which is considered not precise. The explanation for this is that these two primer/probe sets are more susceptible to variation due to the srfJ reverse primer having no G/C’s in the GC clamp, therefore increasing the possibility of variable binding to the target gene. The SPA2308 forward primer has less than 40% GC content, making it more thermally variable, and both reverse primer and probes do self-anneal and form hairpins. This is the case as the SPA2308 gene has a very low GC content of 32.25% and as a result will lead to more variable results. Another important note is that this validation process occurred using boiled cells as the DNA extraction method (as this is the intended use for rapidity) and there is always the possibility of slight levels of PCR inhibition, in comparison to using purified DNA, which will also affect the precision results. The lower precision level did not affect the molecular PCR in practice and was deemed suitable for use.
Sensitivity and specificity.
Sensitivity and specificity were based on the 7 gene profiles (and not individual gene markers) detected by real-time PCR and GeneFinder (Table 5). It showed 100% sensitivity and specificity for the detection of TS compared to the routine reference identification by WGS and serotyping.
DISCUSSION
This study describes for the first time a robust real-time PCR assay for the specific identification of each of the four TS serovars, S. Typhi and S. Paratyphi A, B, and C; this assay is 100% reliable (Fig. 1, Tables 5 and 6, and Table S1). This assay was validated as a monoplex PCR providing the flexibility to use individual targets of interest and is now in use routinely at PHE. The rapid (2-h) turnaround time of this PCR assay has potential for expediting reporting of results to customers (urgent TS confirmatory requests from hospitals, coupled with antimicrobial susceptibility testing) and the management of suspected cases of typhoid fever. Many laboratories are moving away from serology (due to the cost of maintaining sera and results being subjective, especially for the Vi polysaccharide sera used to identify S. Typhi) and are depending on whole-genome sequencing identification (sequence type), which can take up to 7 to 10 days. Public Health England stopped routine serology in 2015 (4).
With additional optimization, the application of this assay could be extended to direct testing of clinical specimens (blood and stool) as well as food, water, and environmental specimens. This would further increase the value of the assay, although such use may risk the possibility of fewer isolates being referred to reference laboratories for further characterization, leading to loss of typing for surveillance purposes (including antimicrobial resistance monitoring) as well as outbreak detection and investigation. Thus, it is essential that isolates continue to be isolated and referred to reference laboratories.
Recently, there has been an emphasis on using rapid and accurate molecular tools (e.g., real-time PCR) for the detection of TS in low- and middle-income countries where the disease is endemic for epidemiological surveillance purposes. Data from these surveillance studies will help countries make evidence-based decisions to facilitate control and prevention (vaccine) measures and allows rapid outbreak detection (14–18).
Many assays for identifying TS have been described previously, but these usually involved single gene targets with much lower specificity and sensitivity, as the assays were not tested against a large set of diverse Salmonella serovars or were aimed at just one or two of the TS serovars and some of the assays were carried out by conventional PCR (9, 19–21). However, these important studies have provided input for the selection of candidate gene targets in designing a gene profile-based PCR assay; the validation of this PCR assay was strengthened by the use of WGS sequence data for high-through put testing on a more diverse collection of Salmonella isolates.
In silico analysis has its limitations if relied on as the sole method. Although PHE utilizes a multilocus sequence type (MLST)-based approach with genomic data for Salmonella identification (4), other organizations may use a gene-based approach for Salmonella identification, and the use of set thresholds with in silico testing in the current study on target genes (i.e., at what threshold is the test positive) may need to be flexible depending on the gene. Unlike detection via PCR, the entire target gene is evaluated using in silico analysis, and therefore, we can draw conclusions on the presence or absence of the target gene. However, selecting a threshold value (and therefore a percent identity of a match) for which a gene is considered present or absent can be difficult. Discrepancies between real-time PCR and genomic detection of target genes occur when a gene has less than the set threshold of sequence similarity. There were initially eight negative gene results using GeneFinder that were positive by PCR. These were due to a lower percentage of gene similarity and below the 80% set threshold (Table S1) and were positive for the presence of the gene (matching the PCR result). When mismatches between PCR and in silico methods occur, explicit consideration is required to ascertain if the PCR primer/probe binding region is intact and how much of the gene is present. Specifically, in our targets, ttr showed a large range of variability among isolates in terms of sequence similarity to the reference gene, with five of eight of these samples having <80% ttr sequence similarity. After assessing the primer/probe binding sites of the genes, there were no discrepancies between GeneFinder and the PCR assay.
This current study showed that 17 of the 952 NTS isolates were positive for only a single gene target (ttr gene) (Table S1) and belonged to Salmonella subspecies III, IV, and V. Therefore, most NTS contain one or more of the other genes markers normally associated with TS (Table 6). This highlights that a single gene target method is not appropriate for distinguishing between typhoidal and nontyphoidal Salmonella, with a gene profile-based method being more accurate for identification and differentiation of TS. The reassuring finding, however, is that not one of the 935 NTS had the same gene profile as the TS (Tables 4 and 5 and Table S1).
Another notable observation is that three S. Typhi isolates from Pakistan lacked the 134-kb SPI-7 pathogenicity island harboring the viaB operon (tvi genes, associated with the production of the Vi capsule). Although rare, the absence of the SPI-7 pathogenicity island, including the tvi region, in S. Typhi has previously been described (7). This is potentially an important public health finding, as the current typhoid Vi polysaccharide vaccine stimulates immunity against the Vi capsule. It is known that SPI-7-negative (Vi-negative) S. Typhi can cause typhoid fever (22), so there is a need to monitor the loss of the SPI-7 island in regions of endemicity where S. Typhi vaccination programs are being conducted (22). The assay described here could be used to monitor the emergence of Vi-negative S. Typhi through the emergence of ttr- and staG-positive tviB-negative strains.
Conclusion.
In conclusion, ours is the first real-time PCR assay that can rapidly distinguish between typhoidal, i.e., S. Typhi, Paratyphi A, Paratyphi B, and Paratyphi C, and nontyphoidal Salmonella serovars. The assay has the ability to be implemented in diagnostic and reference laboratories globally as a safe and cost-effective way of differentiating salmonellae as well as to be used for epidemiological surveillance purposes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sarah Alexandra and Julie Russell from the National Collection of Type Cultures for providing positive-control strains. We also thank Lailanie Aqunino from GBRU for support in undertaking PCR.
We have no conflict of interest to declare.
This study was funded by PHE.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00167-19.
REFERENCES
- 1.Crump JA, Sjolund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wain J, Hendriksen RS, Mikoleit ML, Keddy KH, Ochiai RL. 2015. Typhoid fever. Lancet 385:1136–1145. doi: 10.1016/S0140-6736(13)62708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bale J, Meunier D, Weill FX, dePinna E, Peters T, Nair S. 2016. Characterization of new Salmonella serovars by whole-genome sequencing and traditional typing techniques. J Med Microbiol 65:1074–1078. doi: 10.1099/jmm.0.000325. [DOI] [PubMed] [Google Scholar]
- 4.Ashton PM, Nair S, Peters TM, Bale JA, Powell DG, Painset A, Tewolde R, Schaefer U, Jenkins C, Dallman TJ, de Pinna EM, Grant KA, Salmonella Whole Genome Sequencing Implementation Group. 2016. Identification of Salmonella for public health surveillance using whole genome sequencing. PeerJ 4:e1752. doi: 10.7717/peerj.1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Health and Safety Executive. 2013. The approved list of biological agents. Health and Safety Executive, Bootle, United Kingdom. [Google Scholar]
- 6.Hopkins KL, Peters TM, Lawson AJ, Owen RJ. 2009. Rapid identification of Salmonella enterica subsp. arizonae and S. enterica subsp. diarizonae by real-time polymerase chain reaction. Diagn Microbiol Infect Dis 64:452–454. doi: 10.1016/j.diagmicrobio.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Nair S, Alokam S, Kothapalli S, Porwollik S, Proctor E, Choy C, McClelland M, Liu SL, Sanderson KE. 2004. Salmonella enterica serovar Typhi strains from which SPI7, a 134-kilobase island with genes for Vi exopolysaccharide and other functions, has been deleted. J Bacteriol 186:3214–3223. doi: 10.1128/jb.186.10.3214-3223.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu WQ, Feng Y, Wang Y, Zou QH, Chen F, Guo JT, Peng YH, Jin Y, Li YG, Hu SN, Johnston RN, Liu GR, Liu SL. 2009. Salmonella paratyphi C: genetic divergence from Salmonella choleraesuis and pathogenic convergence with Salmonella typhi. PLoS One 4:e4510. doi: 10.1371/journal.pone.0004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nga TV, Karkey A, Dongol S, Thuy HN, Dunstan S, Holt K, Tu Le TP, Campbell JI, Chau TT, Chau NV, Arjyal A, Koirala S, Basnyat B, Dolecek C, Farrar J, Baker S. 2010. The sensitivity of real-time PCR amplification targeting invasive Salmonella serovars in biological specimens. BMC Infect Dis 10:125. doi: 10.1186/1471-2334-10-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor TR, Owen SV, Langridge G, Connell S, Nair S, Reuter S, Dallman TJ, Corander J, Tabing KC, Le Hello S, Fookes M, Doublet B, Zhou Z, Feltwell T, Ellington MJ, Herrera S, Gilmour M, Cloeckaert A, Achtman M, Parkhill J, Wain J, De Pinna E, Weill FX, Peters T, Thomson N. 2016. What’s in a name? Species-wide whole-genome sequencing resolves invasive and noninvasive lineages of Salmonella enterica serotype Paratyphi B. mBio 7:e00527-16. doi: 10.1128/mBio.00527-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin SW. 1984. Estimating disease prevalence and the interpretation of screening. Prev Vet Med 2:463–472. doi: 10.1016/0167-5877(84)90091-6. [DOI] [Google Scholar]
- 12.Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R, 1000 Genome Project Data Processing Subgroup. 2009. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong VK, Dallman TJ, Nair S, Baker S, Shaheen G, Qureshi S, Yousafzai MT, Saleem MK, Hasan Z, Dougan G, Hasan R. 2018. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 9:e00105-18. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godbole GS, Day MR, Murthy S, Chattaway MA, Nair S. 2018. First report of CTX-M-15 Salmonella Typhi from England. Clin Infect Dis 66:1976–1977. doi: 10.1093/cid/ciy032. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay S, Garrett D, Steele D. 2019. Evidence to action: the 10th International Conference on Typhoid and Other Invasive Salmonelloses. Clin Infect Dis 68:S1–S3. doi: 10.1093/cid/ciy962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha S, Tanmoy AM, Andrews JR, Sajib MSI, Yu AT, Baker S, Luby SP, Saha SK. 2019. Evaluating PCR-based detection of Salmonella Typhi and Paratyphi A in the environment as an enteric fever surveillance tool. Am J Trop Med Hyg 100:43–46. doi: 10.4269/ajtmh.18-0428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carey ME, Diaz ZI, Zaidi AKM, Steele AD. 2019. A global agenda for typhoid control—a perspective from the Bill & Melinda Gates Foundation. Clin Infect Dis 68:S42–s45. doi: 10.1093/cid/ciy928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirose K, Itoh K, Nakajima H, Kurazono T, Yamaguchi M, Moriya K, Ezaki T, Kawamura Y, Tamura K, Watanabe H. 2002. Selective amplification of tyv (rfbE), prt (rfbS), viaB, and fliC genes by multiplex PCR for identification of Salmonella enterica serovars Typhi and Paratyphi A. J Clin Microbiol 40:633–636. doi: 10.1128/JCM.40.02.633-636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy H, Diallo S, Tennant SM, Livio S, Sow SO, Tapia M, Fields PI, Mikoleit M, Tamboura B, Kotloff KL, Lagos R, Nataro JP, Galen JE, Levine MM. 2008. PCR method to identify Salmonella enterica serovars Typhi, Paratyphi A, and Paratyphi B among Salmonella isolates from the blood of patients with clinical enteric fever. J Clin Microbiol 46:1861–1866. doi: 10.1128/JCM.00109-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tennant SM, Toema D, Qamar F, Iqbal N, Boyd MA, Marshall JM, Blackwelder WC, Wu Y, Quadri F, Khan A, Aziz F, Ahmad K, Kalam A, Asif E, Qureshi S, Khan E, Zaidi AK, Levine MM. 2015. Detection of typhoidal and paratyphoidal Salmonella in blood by real-time polymerase chain reaction. Clin Infect Dis 61(Suppl 4):S241–S250. doi: 10.1093/cid/civ726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker S, Sarwar Y, Aziz H, Haque A, Ali A, Dougan G, Wain J, Haque A. 2005. Detection of Vi-negative Salmonella enterica serovar typhi in the peripheral blood of patients with typhoid fever in the Faisalabad region of Pakistan. J Clin Microbiol 43:4418–4425. doi: 10.1128/JCM.43.9.4418-4425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.