Tuberculous meningitis (TBM), the most severe extrapulmonary manifestation of tuberculosis, is caused by the pathogen Mycobacterium tuberculosis. The M. tuberculosis complex includes seven lineages, all described to harbor a unique geographical dissemination pattern and clinical presentation. In this study, we set out to determine whether a certain M. tuberculosis lineage demonstrated tropism to cause TBM in patients from Cape Town, South Africa.
KEYWORDS: CNS tuberculosis, Mycobacterium tuberculosis lineage, childhood tuberculosis, genomic heterogeneity, genotyping, mixed infection, tuberculous meningitis
ABSTRACT
Tuberculous meningitis (TBM), the most severe extrapulmonary manifestation of tuberculosis, is caused by the pathogen Mycobacterium tuberculosis. The M. tuberculosis complex includes seven lineages, all described to harbor a unique geographical dissemination pattern and clinical presentation. In this study, we set out to determine whether a certain M. tuberculosis lineage demonstrated tropism to cause TBM in patients from Cape Town, South Africa. DNA was extracted from formalin-fixed paraffin-embedded central nervous system (CNS) tissue from a unique neuropathological cohort of 83 TBM patients, collected between 1975 and 2012. M. tuberculosis lineages 1, 2, 3, and 4 were determined using an allele-specific PCR and Sanger sequencing. Of the 83 patient specimens tested, bacterial characterization could be performed on 46 specimens (55%). M. tuberculosis lineage 4 was present in 26 patient specimens (56%), and non-lineage 4 was identified in 10 cases (22%). Moreover, genomic heterogeneity was detected in the CNS specimens of 7 adults and 3 children. We could show that infection of the CNS is not restricted to a single M. tuberculosis lineage and that even young children with rapid progression of disease can harbor more than one M. tuberculosis lineage in the CNS.
INTRODUCTION
The infectious disease tuberculosis (TB) is a major global health problem caused by infection with the pathogen Mycobacterium tuberculosis. Tuberculous meningitis (TBM), one of the most severe extrapulmonary manifestations of TB, can develop once M. tuberculosis disseminates from its primary pulmonary focus. TBM is generally accepted to be a consequence of preexisting granulomatous lesions in the central nervous system (CNS), so-called Rich foci. Once these foci rupture into the subarachnoid space, the subsequent inflammatory response induces meningitis (1, 2).
The M. tuberculosis complex includes seven large-sequence polymorphism (LSP)-based lineages, which are human adapted. Lineages 2, also known as the East Asian lineage, and 4, the Euro-American lineage, show the broadest distribution worldwide. In contrast, lineages 5 and 6 (Mycobacterium africanum) are restricted to West Africa and lineage 7 (Ethiopia lineage) is almost exclusively found in Ethiopia. Lineages 1 (Indo-Oceanic) and 3 (East African-Indian) display a more intermediate distribution (3, 4). The geographical distribution of lineages might suggest that lineages are adapted to particular human population groups (4). In addition, individual lineages are linked to distinct patterns of pathology and disease severity. Several studies suggest that infections with lineage 2 strains are more frequently associated with extrapulmonary TB and TBM (5–9). Other associations found with lineage 2 strains are a more acute presentation of disease and younger age, indicating that this lineage is the most pathogenic (10–12). In contrast, lineage 4 was associated with a higher prevalence of pulmonary TB than TBM, suggesting a protective association with this lineage (5, 11). In South Africa, a study among 285 children showed no difference in susceptibility to TBM between the prevalence of two dominant substrains belonging to lineages 2 and 4 (13), indicating that both strains might contribute to CNS involvement equally in this population.
Infection with M. tuberculosis is not restricted to infection with a single lineage. This so-called mixed infection, or genetic heterogeneity, is present in 10 to 20% of sputum cultures from individual TB patients and can have an enormous influence on treatment outcome and can select for drug resistance (14–16). The presence of genomic heterogeneity so far has not been described in cerebrospinal fluid (CSF) of patients with TBM. Since detection is restricted to our limited ability to culture bacteria from CSF and is dependent on the sensitivity of the genotyping method used (17–19), the actual number of patients with genomic heterogeneity in the CNS is most likely underestimated.
This study aimed to determine the M. tuberculosis lineage most commonly found in the granulomas and inflammatory foci in brain tissue from patients in the Western Cape of South Africa. To achieve this, we use a historical cohort of brain specimens from children and adults with advanced stages of TBM. Within this paucibacillary tissue, we demonstrate that lineage 4 is the most commonly detected lineage in our cohort. Furthermore, we could identify genomic heterogeneity within brain specimens and show for the first time that infection of the CNS is not restricted to a single M. tuberculosis lineage.
MATERIALS AND METHODS
Study cohort.
Material for this study was selected from a unique historical cohort study (S. D. Zaharie, D. J. Franken, M. van der Kuip, S. van Elsland, J. Hagoort, B. S. de Bakker, R. Solomons, R. van Toorn, M. Kruger, and A. M. van Furth, unpublished data), consisting of brain specimens from children (<14 years; n = 40) and adults (>14 years; n = 43) with definite or probable TBM registered at the Department of Anatomical Pathology between 1975 and 2012. Patients with medical records, radiographic imaging, and neuropathology brain specimens available were included (n = 83), and specimens were obtained postmortem (n = 76) or with diagnostic biopsy during life (n = 7). For this study, one or two blocks from each patient were selected based on positive Ziehl-Neelsen (ZN) staining. Boiled extracts from cultured M. tuberculosis isolates were included as technical controls for all tested bacterial lineages.
Sample preparation.
The formalin-fixed paraffin-embedded (FFPE) brain tissues were cut using a microtome (block size, 8 to 54 cm2). To eliminate the possibility of cross-contamination, new microtome blades were used, and the microtome was cleaned with xylene, 100% ethanol, and 10% bleach between sectioning of each FFPE block. Blank, uninfected brain samples were tested as controls to exclude cross-contamination on the microtome. Three to five slices of 10 μm (depending on block size) were stored for processing in 1.5-ml Eppendorf safe-lock microcentrifuge tubes.
DNA extraction.
DNA extraction was performed using the NucleoSpin DNA FFPE XS kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instructions with the exception that the protocol was adjusted according to the amount of cut tissue.
PCR applications and Sanger sequencing.
Extracted DNA was used for PCR amplification of the lineage-specific alleles of lineages 1 through 4. Based on geographical distribution of M. tuberculosis lineages, we did not expect to find lineages 5 through 7 in the current cohort and focused on lineages 1 through 4. The Qiagen HotStarTaq Plus master mix kit (Qiagen, Hilden, Germany) was used with the following cycling conditions: initial denaturation for 10 min at 95°C; 40 cycles of 90 s denaturation at 94°C; 30 s at the optimal thermal denaturation (Tm) of the respective primers (see Table S1 in the supplemental material) and 1 min of extension at 72°C, with a final elongation at 72°C for 15 min.
Primers for the RD105 deletion were designed and adapted based on previously described work (20). Because of expected fragmentation of DNA, previously published primers could not be used. The primers were designed with Primer3 v. 0.4.0 and blasted on TubercuList (http://genolist.pasteur.fr/TubercuList/) using the flanking regions of RD239 (lineage 1), RD105 (lineage 2), RD181 (sublineage 2), RD150 (sublineage 2), RD142 (sublineage 2), and RD750 (lineage 3) (21). Details of all primer sequences and their regions are shown in Table S1. PCR products were visualized with GR Green DNA dye (Labgene Scientific SA, Châtel-Saint-Denis, Switzerland) after electrophoresis in 2% agarose. Samples with one or two visible bands of expected size were identified as samples with intact DNA in the amplified region. In contrast, samples with a smear or no band (empty lane) were described as “degraded DNA in the amplified region” or as “absent M. tuberculosis DNA,” respectively. Samples with degraded DNA in at least three different regions of the brain were not further tested. To identify lineage 4 strains, the presence or absence of the pks15/1 deletion was determined as previously described (22). PCR products with a clear band on agarose gel were sent for Sanger sequencing at Inqaba Biotec (South Africa).
The requirement for a reliable result was consistency between PCR result and sequencing. When a clear band on agarose gel is present and the sequencing is successful, results are called conclusive. If this is not the case, results are called inconclusive, meaning that a lineage could not be determined for this sample.
H&E, RS, and ZN staining.
Slides were stained according to conventional protocol with hematoxylin and eosin (H&E), reticulin silver (RS), and Ziehl-Neelsen (ZN) staining and analyzed by a skilled neuropathologist. Three categories were defined based on bacterial load per square millimeter identified by ZN staining with light microscopy using an Olympus BX43 (40× objective, 1 mm2 = 4.2 high-power field). Low bacterial load was defined as one or fewer bacteria per square millimeter, medium bacterial load was defined as 2 to 20 bacteria per square millimeter, and high bacterial load was defined as more than 20 bacteria per square millimeter.
Statistical analysis.
The database with patient information was managed with IBM SPSS Statistics, v. 23.0.0.0 (SPSS Inc., Chicago, IL, USA). Statistical analysis and creation of graphs were performed with the same program. Differences between children and adults were tested presenting P values for chi-square tests (Fisher’s exact test for a cell size less than five), Mann-Whitney U tests (median), and independent-samples t tests (mean). The correlations between sample age and PCR success rate and between ZN positivity and PCR success rate were tested with Spearman’s correlation. Significance was measured at a P value of 0.05. These analyses were done with IBM Statistics version 25 (IBM Corporation, South Africa).
Ethics.
The study was approved by the Human Research Ethics Committee of Stellenbosch University, Cape Town, Western Cape, South Africa (ethics approval number S12/11/298). Informed consent for this study was waived. The postmortem collection of neuropathology material was originally collected during patient admission at Tygerberg Hospital as part of standard care and is available because the patient or their family provided permission for initial autopsy at the time.
RESULTS
Baseline characteristics.
Bacterial characterization could be performed on FFPE neuropathology material of 46 out of 83 TBM patients (55%) with conclusive results. On pathological examination, the diagnosis TBM was probable when clear parenchymal inflammation and granulomas were found and definite when additional ZN-positive bacteria were found or when the CSF culture was positive for bacteria. Of the 46 patients included for analyses, 28 were male (61%) and 33 were of mixed ancestry (77%). The median age was 5.6 years (0.5 to 65.5). The majority of patients presented with stage 3 TBM (61%) on admission and had definite TBM (62%), and the duration between start of therapy until autopsy was 7 days (range, 0 to 153). Data on immunization was not available for most children (1/9 [11.1%] received BCG), and 11/21 (52%) had a registered TB contact in the household. Seventeen children were on the local standard 4-drug regimen of isoniazid, rifampin, pyrazinamide, and ethionamide (74%) (Table 1). Ethionamide is used instead of (WHO-recommended) ethambutol within a short intensified drug regimen because of better CSF penetration and good experience with respect to mortality and outcome (23). Definite TBM with a positive ZN staining was more frequently seen in children than in adults. Due to the historical time frame of this cohort, HIV coinfection and multidrug-resistant (MDR) or extensively drug-resistant (XDR) M. tuberculosis were not recorded.
TABLE 1.
Baseline characteristicsa
| Characteristic | Total no. of patients (%) | No. of patients aged <14 yrs (%) | No. of patients aged >14 yrs (%) | P valueb |
|---|---|---|---|---|
| Total no. of patients | 46 | 23 | 23 | |
| Age (years) | ||||
| Median [IQR]; range | 5.6 [2.3–32.6]; 0.5–65.5 | 2.6 [2.0–4.2]; 0.5–11.4 | 40.7 [23.8–48.5]; 14.3–65.5 | |
| Gender | ||||
| Male | 28 (60.9) | 15 (65.2) | 13 (56.5) | |
| Female | 18 (39.1) | 8 (34.8) | 10 (43.5) | 0.546 |
| Racec | ||||
| Caucasian | 1 (2.3) | 0 (0) | 1 (5.0) | |
| Mixed ancestry | 33 (76.7) | 21 (91.3) | 12 (60.0) | |
| African black | 9 (20.9) | 2 (8.7) | 7 (35.0) | 0.055 FE |
| BCG immunization | ||||
| Yes | 1 (11.1) | 8 (88.9) | ||
| No | 8 (88.9) | 1 (11.1) | ||
| Possible TB household contact | ||||
| No/unknown | 10 (47.6) | 10 (47.6) | ||
| Yes | 11 (52.4) | 11 (52.4) | ||
| TBM staged | ||||
| I | 2 (4.9) | 0 (0) | 2 (10.5) | |
| IIa | 8 (19.5) | 4 (18.2) | 4 (21.1) | |
| IIb | 6 (14.6) | 1 (4.5) | 5 (26.3) | 0.301 FE |
| III | 25 (61.0) | 17 (77.3) | 8 (42.1) | 0.420 FE |
| TBMe | ||||
| Probable | 17 (37.8) | 4 (17.4) | 13 (59.1) | |
| Proven | 28 (62.2) | 19 (82.6) | 9 (40.9) | 0.004 |
| Therapy received | ||||
| Isoniazid | 34 (87.2) | 21 (91.3) | 13 (81.3) | 0.631 FE |
| Rifampin | 34 (87.2) | 22 (95.7) | 12 (75.0) | 0.139 FE |
| Pyrazinamide | 29 (74.4) | 19 (82.6) | 10 (62.5) | 0.264 FE |
| Ethionamide | 18 (46.2) | 17 (73.9) | 1 (6.3) | <0.001 |
| Ethambutol | 20 (51.3) | 10 (43.5) | 10 (62.5) | 0.242 |
| Penicillin | 28 (71.8) | 19 (82.6) | 9 (56.3 | 0.146 FE |
| Streptomycin | 8 (20.5) | 3 (13.0) | 5 (31.3) | 0.235 FE |
| Therapy duration (days)f | ||||
| Median [IQR]; range | 7 [4–13]; 0–153 | 8 [4–25]; 0–153 | 7 [3–9]; 2–28 | 0.167 MW |
Baseline characteristics for patients <14 years old versus those >14 years old with probable or definite tuberculous meningitis whose samples are used in this study.
Chi-square used unless stated otherwise (FE, Fisher’s Exact test; MW, Mann-Whitney U test).
Significance level between mixed ancestry and African black.
Tuberculous meningitis stage at presentation is based on the “refined” British Medical Research Council scale (34). Stage I, Glasgow coma scale (GCS) of 15, without focal neurological deficits; Stage IIa, GCS of 15, with focal neurological deficits, or GCS of 13 to 14, with or without focal neurological deficits; Stage IIb, GCS of 10 to 12, with or without focal neurological deficits; Stage III, GCS of <10, with or without focal neurological deficits. P value represents difference between IIb or III and IIa.
Probable based on histopathology; proven based on histopathology and ZN positivity.
Time between treatment commenced until biopsy/death in days.
Paucibacillarity of tissue limits PCR-based genotyping.
In order to genotype M. tuberculosis, DNA was extracted from the available samples, and bacilli were genotyped using a targeted PCR-based method. Successful PCR amplification followed by sequencing was only observed in 46 patient specimens. This could be related to the fact that the tissue had been stored in either buffered or unbuffered formalin for an unknown time period before embedding in paraffin. Furthermore, the median sample age was 27.7 years (interquartile range [IQR], 23.2 to 32.6; range, 6.2 to 39.7); however, the age of the block was not associated with success of PCR-based genotyping (P > 0.05). The bacilli burden was not associated with positive PCR amplification; 19/46 (41%) patient specimens were ZN negative and 27/46 (59%) were ZN positive. Additionally, a low ZN positivity was found in the majority of these patient specimens (23/27 [85%]) while 3 and 1 patients had medium and high ZN positivity, respectively.
Lineage 4 is most prevalent in CNS granulomas between 1975 and 2012.
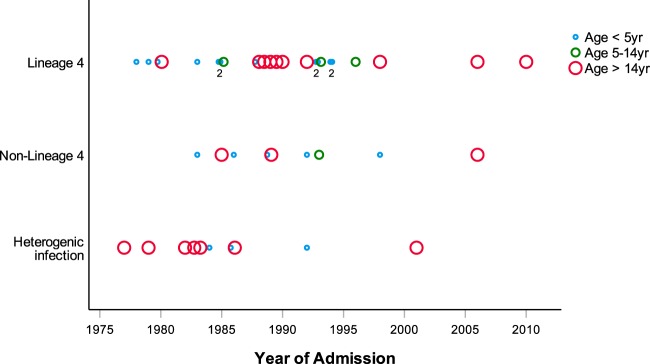
Lineage 4 was identified in 26/46 samples (56.5%), non-lineage 4 was found in 10 samples (22%), and a genomic heterogenic infection of lineage 4 and non-lineage 4 was found in another 10 samples (22%) (see Fig. S1 in the supplemental material). Age of the sample was not associated with detected lineage (P > 0.05) (Fig. 1). Genotyping for lineage 1 (RD239 deletion), lineage 2 (RD105 deletion and sublineages with deletion in RD181, RD150, and RD142), and lineage 3 (RD750 deletion) resulted in a large number of inconclusive results. Lineage distribution could, therefore, not be determined in the non-lineage 4 group or in the group with genetic heterogenic infection (Fig. S1).
FIG 1.
Distribution of lineages between 1975 and 2012. Identification of lineage 4, non-lineage 4, or heterogenic infection in patients <5 years of age (small blue circles), ages 5 to 14 years (medium green circles), and >14 years of age (large red circles) per year between 1975 and 2012. Number indicates multiple patients at a specific time point for the youngest group. No association was found between presence of a specific lineage and year of admission.
Genomic heterogenic infection in children.
Genomic heterogeneity was identified in 7 patients that were >14 years of age and 3 children that were <14 years of age (total of 10 patients [22%]). Since mixed infection has not previously been described in children, these three cases are described in more detail. The children were between 2 and 3 years of age, and their samples were collected in 1984, 1986, and 1992 (Table 2). Case 1 and 2 presented with TBM stage IIa, in which mild neurological symptoms were present. Case 3 presented with TBM stage III and showed improvement of staging during treatment. Both patients in cases 1 and 3 experienced a rapid disease progression with miliary TB. Both patients were deceased within 3 weeks of presentation, despite intensive treatment with at least four different tuberculous drugs. The patient in case 2 died without involvement of other organs and after a longer treatment duration of 52 days.
TABLE 2.
Patient characteristics of three children with heterogenic infectiona
| Characteristic | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Yr of administration | 1984 | 1986 | 1992 |
| General characteristic | |||
| Age (mo) | 32 | 27 | 26 |
| Gender | Male | Male | Female |
| Race | Black | Black | Mixed ancestry |
| BCG immunization | Unknown | Unknown | Yes |
| Possible TB household contact | Yes | Unknown | Unknown |
| TBM stageb | |||
| At presentation | IIa | IIa | III |
| During treatment | IIa | III | IIa |
| Before death | III | III | III |
| Outcome | Death | Death | Death |
| TBM diagnosis | Proven | Probable | Proven |
| Based on | Histology | Clinical symptoms | Histology |
| ZN positivity | Yes | No | Yes |
| Additional disease | |||
| Pulmonary TB | Yes | Yes | Yes |
| Miliary TB | Yes | Unknown | Yes |
| Organs involved | Thyroid, spleen, hilar lymph nodes | Unknown | Kidney, spleen, liver |
| Treatment | |||
| Tuberculostatics received | Rifampin, isoniazid, pyrazinamide, ethionamide | Rifampin, isoniazid, pyrazinamide, ethionamide, ethambutol | Rifampin, isoniazid, pyrazinamide, ethionamide |
| Duration until death (days) | 17 | 52 | 13 |
| Prednisone received | Yes | Yes | No |
Three children were found to have a heterogenic population of lineages in CNS granulomas. Characteristics of the individual patients are presented in this table.
Tuberculous meningitis stage at presentation is based on the “refined” British Medical Research Council scale (34).
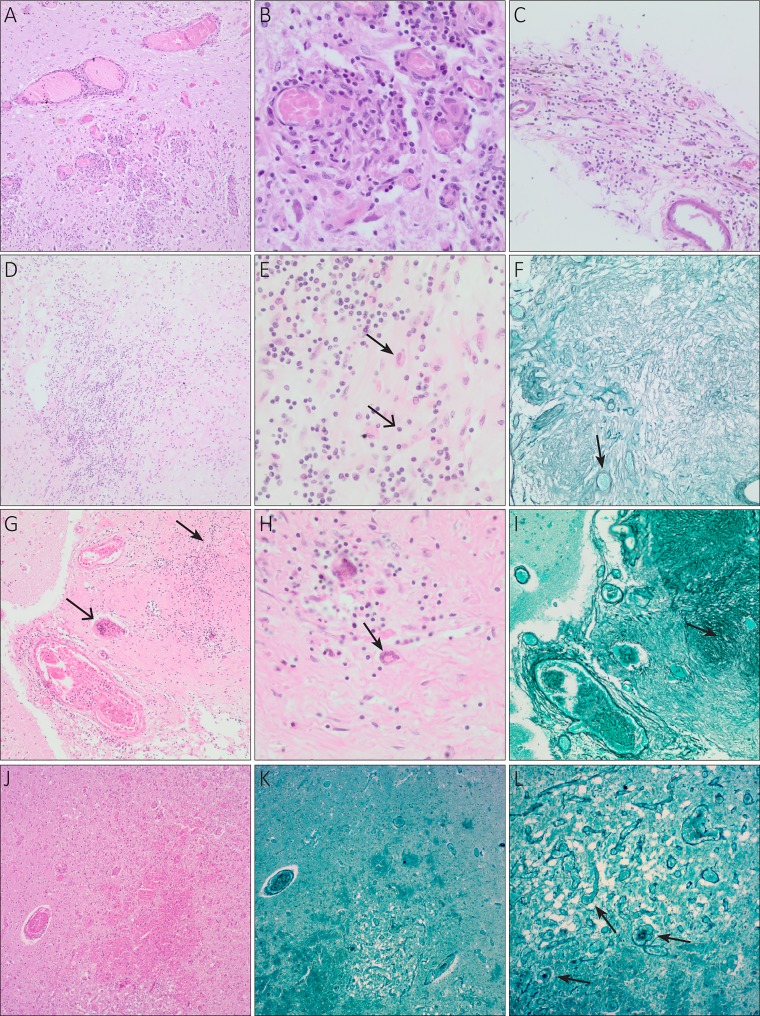
On neuropathological examination, all three children showed chronic inflammation in the leptomeninges, poorly formed granulomas, and fibrosis. Acute inflammation was present in the patient in case 2. All three cases were ZN negative (Fig. 2).
FIG 2.
Neuropathological findings in three children with tuberculous meningitis and heterogenic infection. Characteristics of the three cases presented are specified in Table 2. (A) Case 1, medulla oblongata; multiple intraparenchymal granulomas. H&E, ×40 magnification. (B) Case 1, medulla oblongata; granulomas with lymphocytes (chronic inflammation). H&E, ×100 magnification. (C) Case 1, leptomeninges; lymphocytes (chronic inflammation), no granulomas present. H&E, ×100 magnification. (D) Case 2, leptomeninges; poorly formed granulomatous inflammation. H&E, ×100 magnification. (E) Case 2, leptomeninges; lymphocytes (open arrow, chronic inflammation) and macrophages (closed arrow). H&E, ×400 magnification. (F) Case 2, leptomeninges; fibrosis and vascular proliferation (arrow). Reticulin silver stain, ×100 magnification. (G) Case 3, brain parenchyma and leptomeninges; poorly formed granuloma (closed arrow) and foreign body giant cell (open arrow). H&E, ×100 magnification. (H) Case 3, brain parenchyma and leptomeninges; Langhans giant cell (arrow). H&E, ×400 magnification. (I) Case 3, brain parenchyma and leptomeninges; fibrosis (arrow) and vascular proliferation. Reticulin silver stain, ×100 magnification. (J) Case 3, brain parenchyma; infarct. H&E, ×40 magnification. (K) Case 3, brain parenchyma; infarct with vascular proliferation. Reticulin silver stain, ×40 magnification. (L) Case 3, brain parenchyma; infarct with vascular proliferation (arrows). Reticulin silver stain, ×100 magnification.
DISCUSSION
In this study, we successfully identified lineage 4 as the predominant M. tuberculosis strain in CNS granulomas. At Tygerberg Hospital, the hospital where this study was performed, 15% of all culture-confirmed TB cases in patients under the age of 14 years are TBM (24). However, little information was available about M. tuberculosis lineage specificity until recently. Based on previous studies performed in the same geographical region, lineage 4 was expected to be most prevalent in pulmonary TB (25), and lineage 2 was expected to be most prevalent in children with TBM (6). However, additional studies show an equal distribution of lineages 2 and 4 for both pulmonary and extrapulmonary TB (3, 13).
Lineage 4, also known as the Euro-American lineage, is geographically the most widespread lineage and shows substantial variation among different ethnic groups and in different clinical settings (4). This diversity may be explained by the fact that lineage 4 consists of at least ten different sublineages, which have evolved independently and thereby may have adapted to specific host populations. The overrepresentation of lineage 4 in our TBM samples may be unexpected and is in contrast with previous results. However, most of these studies were performed in Vietnam, China, and Thailand, thereby representing patients with a different ethnic background. The widespread geographical variation of strains suggests the possibility of a different M. tuberculosis genotype distribution among the South African population as described here (5–8, 10–12, 26). The predominance of lineage 4 is a logical consequence of it being the most dominant lineage in Cape Town. In addition, it can be explained by the hypothesis that lineage distribution is subject to time in addition to geography. A study performed in Cape Town with archived postmortem samples reported an increase in infection with the Beijing strain over the past decades. This strain was never found before 1966, but prevalence significantly increased after 1995. Simultaneously, the proportion of Euro-American strains declined (25). However, Beijing prevalence never exceeded lineage 4. Although a correlation between time and prevalence was not found in the current cohort, the effect of altering prevalence of specific strains cannot be completely excluded.
The second finding in this study is the presence of more than one M. tuberculosis lineage within the CNS of a single patient, even when patients were ZN negative during initial histopathological examination. Genetic heterogeneity in lung tissue, in which multiple lineages can be found in a single individual at the same time, is in fact not new. Numerous reports, including a South African cohort in the same region as the current study, report 10 to 20% of sputum cultures containing more than one M. tuberculosis lineage (15–18, 20). However, this is likely an underestimation of the actual amount of genetic heterogenic infections due to the difficulty of isolating these strains and differences in genotyping methods (17, 18, 27). In addition to the presence of multiple strains in sputum samples, heterogenic populations of strains were also found in spleen, blood, and lymph node specimens (17, 28–30), indicating that genetic heterogeneity is not restricted to the lung. The current study is the first to identify genetic heterogeneity in the CNS in both adults and children in 10 of 46 patients, which is similar to the 22% occurrence described in literature (15–18, 20). Laboratory contamination during sample processing cannot be ruled out completely, but it can be assumed to only contribute to a small proportion of genetic heterogeneity. This is based on a previous study that showed a maximum of 4% of contamination in cultures (16) and the negatively tested blank controls in the current study.
Genetic heterogeneity is thought to be a consequence of reinfection or superinfection after an (incomplete) recovery of a previous M. tuberculosis infection, and it has been hypothesized that this second strain may reactivate the primary infection (17, 20, 31). In young children, the amount of genomic heterogenic infection is thought to be reduced due to the rapid progression of disease and the small time frame in which reinfection can occur (18, 19). Therefore, the genomic heterogeneity identified in the CNS of three young children was a major surprise. Hypothetically, a high rate of reinfection can exist in areas with high M. tuberculosis burden. Subsequently, once the first strain causes a latent CNS infection, a reinfection can easily trigger progression of disease in the vulnerable brain of a child with a relatively immature immune system. Another possibility is exposure of the child to two different strains at once. Children are in close contact with adults, in which mixed infection in sputum has been shown before. Infection with more than one strain might than promote extrapulmonary dissemination.
Heterogenic infection was concentrated in the first half of the study period, and it would be likely that a higher TBM prevalence increases the probability for finding genomic heterogeneity. Nevertheless, exact incidence rates for childhood TB and TBM are scarce, due to diagnostic challenges and lack of disease reports in rural areas. One study performed between 1985 and 1994 in two rural areas of Cape Town reported no increase or decrease in incidence of childhood TB over this time period (32). Furthermore, clinicians at Tygerberg Hospital report an increasing incidence of TBM over the last 5 to 10 years (R. van Toorn, personal communication). Therefore, the concentration of genomic heterogeneity in the first half of the study is rather an effect of a larger amount of samples collected during that time than a consequence of a higher TBM prevalence.
This study has certain limitations. Although genomic heterogeneity was identified, the combination of the historical sample collection with partly degraded DNA, the paucibacillary nature of the samples, and the chosen genotyping methods did not allow for a further characterization of sublineages or strain classification at the sublineage level. Ideally, the genotyping performed in this study would be repeated to confirm the findings, but the quality of the samples did not allow for additional testing. Also, CSF of patients with active TBM has an extremely low bacterial load (19). Therefore, it is questionable if it was possible to identify a large number of bacteria at a single location in an individual patient’s brain specimen, which might lead to an underestimation of the amount of mixed infections. Another limitation was the archived FFPE samples, which were stored in buffered or unbuffered formalin for an unknown period of time under unknown circumstances with most likely damaging effects on the samples (33). Given the paucibacillary nature of the specimens and previous genotyping studies describing inconclusive results on FFPE material with spoligotyping (21), PCR-based genotyping seemed to be the best option to distinguish the different lineages. However, this method resulted in a large proportion of inconclusive results as well, perhaps explained by the expected high rate of fragmentation in the DNA in combination with the size of the PCR products. This is supported by the observation that the primers generating the smallest products were the most reliable. Nevertheless, results concerning lineage 4 were conclusive. The sequences of the PCR products made it possible to clearly distinguish between lineage 4, non-lineage 4, and a mixed infection with lineage 4 and another lineage. However, since these primers group many different sublineages of lineage 4, it is likely that this method results in an underestimation of mixed infections of more than one sublineage of lineage 4.
Taken together, this unique cohort reveals valuable insight in the overrepresentation of lineage 4 and the presence of genomic heterogenic infection in the CNS of young children in TBM samples in the Western Cape of South Africa over the past several decades. This might explain the rapid progression of disease and insufficient response to standard therapy seen in this population. Not only the advanced stage of TBM can explain the outcomes of this patient group, and the genomic heterogeneity may contribute to treatment unresponsiveness leading to unfavorable outcomes.
Supplementary Material
ACKNOWLEDGMENTS
We thank Willy Pieterse and Daniel Franken for technical assistance.
This study was supported by a travel grant of the Catharine van Tussenbroek foundation (awarded to L.M.V.L.).
P.V., E.M.S., and M.V.D.K. conceived and designed the experiments with input from L.M.V.L., R.M.W., and A.M.V.F. L.M.V.L., P.V., A.J., E.M.S., and S.D.Z. collected and analyzed neuropathology material. S.L.V.E. and M.V.D.K. managed the project. L.M.V.L. and P.V. analyzed the data. L.M.V.L. and S.L.V.E. performed statistical tests. L.M.V.L. and P.V. wrote the paper with input from S.L.V.E., R.M.W., M.V.D.K., and A.M.V.F.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JCM.00415-19.
REFERENCES
- 1.Donald PR, Schaaf HS, Schoeman JF. 2005. Tuberculous meningitis and miliary tuberculosis: the Rich focus revisited. J Infect 50:193–195. doi: 10.1016/j.jinf.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Rich AR, McCordock HA. 1933. The pathogenesis of tuberculous meningitis. Bull Johns Hopkins Hosp 52:2–33. [Google Scholar]
- 3.Brites D, Gagneux S. 2017. The nature and evolution of genomic diversity in the Mycobacterium tuberculosis complex. Adv Exp Med Biol 1019:1–26. doi: 10.1007/978-3-319-64371-7_1. [DOI] [PubMed] [Google Scholar]
- 4.Stucki D, Brites D, Jeljeli L, Coscolla M, Liu Q, Trauner A, Fenner L, Rutaihwa L, Borrell S, Luo T, Gao Q, Kato-Maeda M, Ballif M, Egger M, Macedo R, Mardassi H, Moreno M, Vilanova GT, Fyfe J, Globan M, Thomas J, Jamieson F, Guthrie JL, Asante-Poku A, Yeboah-Manu D, Wampande E, Ssengooba W, Joloba M, Boom WH, Basu I, Bower J, Saraiva M, Vasconcellos SEG, Suffys P, Koch A, Wilkinson R, Gail-Bekker L, Malla B, Ley SD, Beck H-P, de Jong BC, Toit K, Sanchez-Padilla E, Bonnet M, Gil-Brusola A, Frank M, Penlap Beng VN, Eisenach K, Alani I, et al. 2016. Mycobacterium tuberculosis lineage 4 comprises globally distributed and geographically restricted sublineages. Nat Genet 48:1535–1543. doi: 10.1038/ng.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caws M, Thwaites G, Dunstan S, Hawn TR, Lan NTN, Thuong NTT, Stepniewska K, Huyen MNT, Nguyen DB, Tran HL, Gagneux S, Van Soolingen D, Kremer K, Van Der Sande M, Small P, Anh PTH, Nguyen TC, Hoang TQ, Duyen NTH, Dau QT, Hieu NT, Torok E, Tran TH, Nguyen HD, Nhu NTQ, Phan MD, Chau NVV, Farrar J. 2008. The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 4:e100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maree F, Hesseling AC, Schaaf HS, Marais BJ, Beyers N, van Helden P, Warren RM, Schoeman JF. 2007. Absence of an association between Mycobacterium tuberculosis genotype and clinical features in children with tuberculous meningitis. Pediatr Infect Dis J 26:13–18. doi: 10.1097/01.inf.0000247044.05140.c7. [DOI] [PubMed] [Google Scholar]
- 7.Pan Y, Yang Z, Liu R, Xing L, Peng Z, Zhu C. 2015. Host and microbial predictors of childhood extrathoracic tuberculosis and tuberculosis meningitis. Pediatr Infect Dis J 34:1289–1295. doi: 10.1097/INF.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 8.Xing L, Liu R, Li Q, Peng Z, Zhu C. 2012. Clinical and genotypic characteristics of childhood tuberculosis in Chongqing, China. Eur J Clin Microbiol Infect Dis 31:1735–1739. doi: 10.1007/s10096-011-1494-5. [DOI] [PubMed] [Google Scholar]
- 9.Hesseling AC, Marais BJ, Kirchner HL, Mandalakas AM, Brittle W, Victor TC, Warren RM, Schaaf HS. 2010. Mycobacterial genotype is associated with disease phenotype in children. Int J Tuberc Lung Dis 14:1252–1258. [PubMed] [Google Scholar]
- 10.Yorsangsukkamol J, Chaiprasert A, Prammananan T, Palittapongarnpim P, Limsoontarakul S, Prayoonwiwat N. 2009. Molecular analysis of Mycobacterium tuberculosis from tuberculous meningitis patients in Thailand. Tuberculosis (Edinb) 89:304–309. doi: 10.1016/j.tube.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Faksri K, Drobniewski F, Nikolayevskyy V, Brown T, Prammananan T, Palittapongarnpim P, Prayoonwiwat N, Chaiprasert A. 2011. Epidemiological trends and clinical comparisons of Mycobacterium tuberculosis lineages in Thai TB meningitis. Tuberculosis (Edinb) 91:594–600. doi: 10.1016/j.tube.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 12.Thwaites G, Caws M, Chau TTH, D'Sa A, Lan NTN, Huyen MNT, Gagneux S, Anh PTH, Tho DQ, Torok E, Nhu NTQ, Duyen NTH, Duy PM, Richenberg J, Simmons C, Hien TT, Farrar J. 2008. Relationship between Mycobacterium tuberculosis genotype and the clinical phenotype of pulmonary and meningeal tuberculosis. J Clin Microbiol 46:1363–1368. doi: 10.1128/JCM.02180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicol MP, Sola C, February B, Steyn L, Wilkinson RJ, Rastogi N. 2005. Distribution of strain families of Mycobacterium tuberculosis causing pulmonary and extrapulmonary disease in hospitalized children in Cape Town, South Africa. J Clin Microbiol 43:5779–5781. doi: 10.1128/JCM.43.11.5779-5781.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Rie A, Victor TC, Richardson M, Johnson R, Van Der Spuy GD, Murray EJ, Beyers N, Van Pittius NCG, Van Helden PD, Warren RM. 2005. Reinfection and mixed infection cause changing Mycobacterium tuberculosis drug-resistance patterns. Am J Respir Crit Care Med 172:636–642. doi: 10.1164/rccm.200503-449OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen T, Chindelevitch L, Misra R, Kempner ME, Galea J, Moodley P, Wilson D. 2016. Within-host heterogeneity of mycobacterium tuberculosis infection is associated with poor early treatment response: a prospective cohort study. J Infect Dis 213:1796–1799. doi: 10.1093/infdis/jiw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Warren RM, Victor TC, Streicher EM, Richardson M, Beyers N, van Pittius NCG, van Helden PD. 2004. Patients with active tuberculosis often have different strains in the same sputum specimen. Am J Respir Crit Care Med 169:610–614. doi: 10.1164/rccm.200305-714OC. [DOI] [PubMed] [Google Scholar]
- 17.Cohen T, van Helden PD, Wilson D, Colijn C, McLaughlin MM, Abubakar I, Warren RM. 2012. Mixed-strain Mycobacterium tuberculosis infections and the implications for tuberculosis treatment and control. Clin Microbiol Rev 25:708–719. doi: 10.1128/CMR.00021-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plazzotta G, Cohen T, Colijn C. 2015. Magnitude and sources of bias in the detection of mixed strain M. tuberculosis infection. J Theor Biol 368:67–73. doi: 10.1016/j.jtbi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson RJ, Rohlwink U, Misra UK, Van Crevel R, Mai NTH, Dooley KE, Caws M, Figaji A, Savic R, Solomons R, Thwaites GE. 2017. Tuberculous meningitis. Nat Rev Neurol 13:581–598. doi: 10.1038/nrneurol.2017.120. [DOI] [PubMed] [Google Scholar]
- 20.Hanekom M, Streicher EM, Van de Berg D, Cox H, McDermid C, Bosman M, Gey van Pittius NC, Victor TC, Kidd M, van Soolingen D, van Helden PD, Warren RM. 2013. Population structure of mixed Mycobacterium tuberculosis infection is strain genotype and culture medium dependent. PLoS One 8:5–10. doi: 10.1371/journal.pone.0070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsolaki AG, Hirsh AE, DeRiemer K, Enciso JA, Wong MZ, Hannan M, Goguet de la Salmoniere Y-O, Aman K, Kato-Maeda M, Small PM. 2004. Functional and evolutionary genomics of Mycobacterium tuberculosis: insights from genomic deletions in 100 strains. Proc Natl Acad Sci U S A 101:4865–4870. doi: 10.1073/pnas.0305634101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagneux S, DeRiemer K, Van T, Kato-Maeda M, de Jong B, Narayanan S, Nicol M, Niemann S, Kremer K, Gutierrez MC, Hilty M, Hopewell PC, Small PM. 2006. Variable host-pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103:2869–2873. doi: 10.1073/pnas.0511240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Toorn R, Schaaf HS, Laubscher JA, van Elsland SL, Donald PR, Schoeman JF. 2014. Short intensified treatment in children with drug-susceptible tuberculous meningitis. Pediatr Infect Dis J 33:248–252. doi: 10.1097/INF.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 24.Schaaf HS, Marais BJ, Whitelaw A, Hesseling AC, Eley B, Hussey GD, Donald PR. 2007. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: a review of 596 cases. BMC Infect Dis 7:140. doi: 10.1186/1471-2334-7-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cowley D, Govender D, February B, Wolfe M, Steyn L, Evans J, Wilkinson RJ, Nicol MP. 2008. Recent and rapid emergence of W-Beijing strains of Mycobacterium tuberculosis in Cape Town, South Africa. Clin Infect Dis 47:1252–1259. doi: 10.1086/592575. [DOI] [PubMed] [Google Scholar]
- 26.Wang T, Feng GD, Pang Y, Liu JY, Zhou Y, Yang YN, Dai W, Zhang L, Li Q, Gao Y, Chen P, Zhan LP, Marais BJ, Zhao YL, Zhao G. 2016. High rate of drug resistance among tuberculous meningitis cases in Shaanxi province, China. Sci Rep 6:25251. doi: 10.1038/srep25251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng C, Li S, Luo Z, Pi R, Sun H, He Q, Tang K, Luo M, Li Y, Couvin D, Rastogi N, Sun Q. 2015. Mixed infections and rifampin heteroresistance among Mycobacterium tuberculosis clinical isolates. J Clin Microbiol 53:2138–2147. doi: 10.1128/JCM.03507-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Navarro Y, Herranz M, Pérez-Lago L, Lirola MM, Ruiz-Serrano MJ, Bouza E, De Viedma DG. 2011. Systematic survey of clonal complexity in tuberculosis at a populational level and detailed characterization of the isolates involved. J Clin Microbiol 49:4131–4137. doi: 10.1128/JCM.05203-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bates JH, Stead WW, Rado TA. 1976. Phage type of tubercle bacilli isolated from patients with two or more sites of organ involvement. Am Rev Respir Dis 114:353–358. [DOI] [PubMed] [Google Scholar]
- 30.Chaves F, Dronda F, Alonso-Sanz M, Noriega AR. 1999. Evidence of exogenous reinfection and mixed infection with more than one strain of Mycobacterium tuberculosis among Spanish HIV-infected inmates. AIDS 13:615–620. doi: 10.1097/00002030-199904010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Du Plessis DG, Warren R, Richardson M, Joubert JJ, van Helden PD. 2001. Demonstration of reinfection and reactivation in HIV-negative autopsied cases of secondary tuberculosis: multilesional genotyping of Mycobacterium tuberculosis utilizing IS 6110 and other repetitive element-based DNA fingerprinting. Tuberculosis (Edinb) 81:211–220. doi: 10.1054/tube.2000.0278. [DOI] [PubMed] [Google Scholar]
- 32.van Rie A, Beyers N, Gie RP, Kunneke M, Zietsman L, Donald PR. 1999. Childhood tuberculosis in an urban population in South Africa: burden and risk factor. Arch Dis Child 80:433–437. doi: 10.1136/adc.80.5.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miething F, Hering S, Hanschke B, Dressler J. 2006. Effect of fixation to the degradation of nuclear and mitochondrial DNA in different tissues. J Histochem Cytochem 54:371–374. doi: 10.1369/jhc.5B6726.2005. [DOI] [PubMed] [Google Scholar]
- 34.van Toorn R, Springer P, Laubscher JA, Schoeman JF. 2012. Value of different staging systems for predicting neurological outcome in childhood tuberculous meningitis. Int J Tuberc Lung Dis 16:628–632. doi: 10.5588/ijtld.11.0648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.