Abstract
Drug uptake and efflux are two of the critical factors required in order to be able to define drug efficacy. This study aims to investigate cytotoxicity and uptake mechanisms of two FL118 analogues (7-Q20 and val-FL118) in parallel with FL118 in three-dimensional multi-cellular spheroids model. The influence of compound concentration, time, temperature, cell lines, and the inhibitors of P-gp, BCRP and LAT1 on drug uptake and efflux were investigated. In vitro cytotoxicity studies revealed that FL118, 7-Q20 and val-FL118 exhibited sensitive cytotoxicity to the HCT-116 cell line and the water-soluble compound 7-Q20 showed the lowest IC50. Cellular uptake and efflux of FL118 was independent of efflux pump proteins. Uptake and efflux of 7-Q20 were affected by P-gp, which was one of reasons that caused a lower uptake at 37 °C than at 4 °C. The carrier protein LAT1 played a role in the cellular intakes of val-FL118. These findings provided basic information for FL118 and the two novel FL118 derivatives for further development.
Keywords: 3D multi-cellular spheroids, FL118, FL118 derivatives, Uptake, Efflux
Introduction
FL118 (10,11-methylenedioxy-20(S)-camptothecin), Fig. 1a, is a camptothecin (CPT) derivative and appears to possess a novel mechanism of action (Ling et al. 2012; Li 2014); the antitumor activity of FL118 is irrelevant to the expression of topoisomerase I (Topo1) (Li et al. 2017).Specifically, FL118 differs from other CPT analogues (e.g. irinotecan, topotecan) that use Topo1 as their therapeutic target; FL118 selectively inhibits the expression of multiple antiapoptotic proteins including survivin, Mcl-1, XIAP and c-IAP2 (Ling et al. 2012). In fact, FL118’s distinguished antitumor activity of FL118 was recently recognized. FL118 has the potential to effectively eliminate human tumors that acquire irinotecan and/or topotecan resistance (Ling et al. 2015). FL118 is different from irinotecan and topotecan in that they are substrates of efflux pump proteins ATP-binding cassette transporter 2 (ABCG2)/breast cancer resistant protein (BCRP) and P-glycoprotein (P-gp); FL118 is not a substrate of ABCG2 (Westover et al. 2015) and P-gp substrates (Ling et al. 2015) and thus FL118 can effectively overcome resistance resulted from overexpression of ABCG2 and/or P-gp (Ling et al. 2015; Westover et al. 2015).
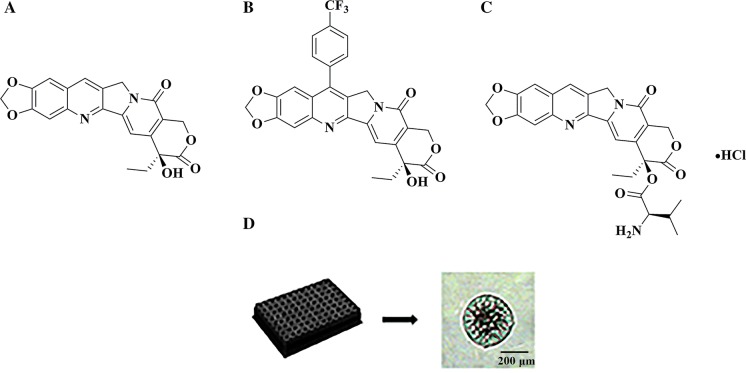
Fig. 1.
The structure of FL118 (a), 7-Q20 (b) and val-FL118 (c). The image of 3D Caco-2 cell spheroid (d) captured by OPTIKA, B-600TiFL (× 20)
High expression of ABCG2 is associated with decreased intracellular accumulation of irinotecan or topotecan and consequentially a decrease in drug potency. Over-expressing of adenosine triphosphate (ATP)-binding cassette (ABC) transporters is one of the common mechanisms that produce multidrug resistance in cancer cells. P-gp and BCRP are two typical proteins in the ABC transporter family. It appears that FL118 bypassed multiple efflux pump protein-induced resistance, which may have contributed to FL118 overcoming irinotecan and topotecan resistance in vivo (Ling et al. 2015). It was because of these findings, we decided to look into the transport substrate characteristics of two FL118 derivatives in parallel with FL118 as the potential treatment option for cancers with drug resistance.
Structural modification may affect the absorption and transport mechanism of compounds (Liu et al. 2015). Researchers (Zhao et al. 2014; Zhu et al. 2017) have reported that the camptothecin 7-substituents, especially the lipophilic group, possess unique properties that aid in preventing the incorporation of the ring-opened carboxylate produced by hydrolysis of the E-ring with human serum albumin in vivo. An introduction of glycinate at the 20-position of FL118 was highly active against human breast cancer xenografts by improving water solubility without sacrificing the stability of the E-ring ketone (critical for biological activity) (Wadkins et al. 1999). We synthesized 7-substituted and 20-substituted FL118 derivatives in our studies, we then focused on 7-Q20 (7-p-trifluoromethylphenyl-10,11-methylenedioxy-20(S)-camptothecin, Fig. 1b), and val-FL118 (20-valine-10,11-methylenedioxy-20(S)-camptothecin, Fig. 1c) in parallel with FL118 (Fig. 1a) to investigate their uptake kinetics and their possible transport mechanisms.
In this study, we used 3D multi-cellular spheroids models to study the uptake and efflux of FL118 and its two derivatives. The 96-well ultra-low attachment plates (Howes et al. 2014) were used to form 3D spheroids (Fig. 1d). It is well established that 3D multi-cellular spheroid models can overcome the limitations of 2D cell cultures because they more closely resemble the mechanical and physiological micro-environments of cells in solid tumors (Bonnier et al. 2015; Casey et al. 2016; Nath and Devi 2016). Cell spheroids of 200–300 μm are 3D microscale tissues which exhibit an inherent gradient of nutrients, oxygen and metabolites within themselves; this is consistent with the in vivo effectiveness of drug and carrier system (Mehta et al. 2012). Additionally, intestinal uptake and efflux are two major factors that determine the bioavailability of orally administrated compounds (Fang et al. 2018). Caco-2 cell line mimics intestinal absorption and is usually regarded as a model for studying permeability and uptake characteristics of compounds (Pullakhandam et al. 2008). Given these characteristics, Caco-2 cell line was used in this study.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Gibco BRL Life Technology (Carlsbad, CA). Dimethyl sulfoxide (DMSO) was obtained from Sigma-Aldrich (St. Louis, MO). Fetal bovine serum (FBS) was purchased from Hyclone (Thermo Fisher Scientific). Hanks buffered salt solution (Hanks), 0.25% trypsin with ethylenediaminetetraacetic acid (Trypsin–EDTA) and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT), 100 μg/mL of penicillin and 100 μg/mL of streptomycin were purchased from Genom. Verapamil (CAS: 152-11-4), Iressa (CAS: 184475-35-2) were purchased from Aladdin, BCH (CAS: 20448-79-7) was purchased from Efebio. All reagents for high performance liquid chromatography (HPLC) were of analytical grade. 7-Q20, val-FL118 and FL118 were synthesized by our laboratory, all the purity are > 95%. 7-Q20, val-FL118, FL118, verapamil, Iressa and BCH were dissolved in DMSO at 1 mM as stock solutions (the content of DMSO in final solution was limited < 0.1% (v/v) in this study). Blood counting chamber was purchased from QIU JING® (Shanghai). 96-well and 6-well ultra low attachment plates were purchased from Costar® (Corning Incorporated, USA).
Cell lines
We used five cell lines (HCT-116, HepG-2, MCF-7, A549, HeLa) in our previous experiments to test the cytotoxicity of FL118, 7-Q20 and val-FL118, and the results showed that the HCT-116 cell line had the highest cytotoxicity and HepG-2 had the lowest. We used these two cell lines to determine whether differences in uptake exist for these compounds. The human colorectal cancer cell lines Caco-2 (CCTCC, No.GDC153) were purchased from the China Center for Type Culture Collection (CCTCC, Wuhan, China), the human liver cancer cell lines HepG2 (Crisprbio, No.CE10193) and the human colon cancer cell lines HCT116 (Crisprbio, No.CE10187) were purchased from the Crisprbio (Beijing, China).
Cell culture
Caco-2, HepG-2 and HCT-116 were cultured with DMEM containing 10% heat-inactivated FBS, 100 μg/ml penicillin and 100 μg/ml streptomycin in a humidified incubator (5% CO2, 37 °C). 48 h after seeding, the medium was replaced with 1% DMEM containing various concentrations of compounds. All cells used in this study were between passages 10–20.
HPLC conditions
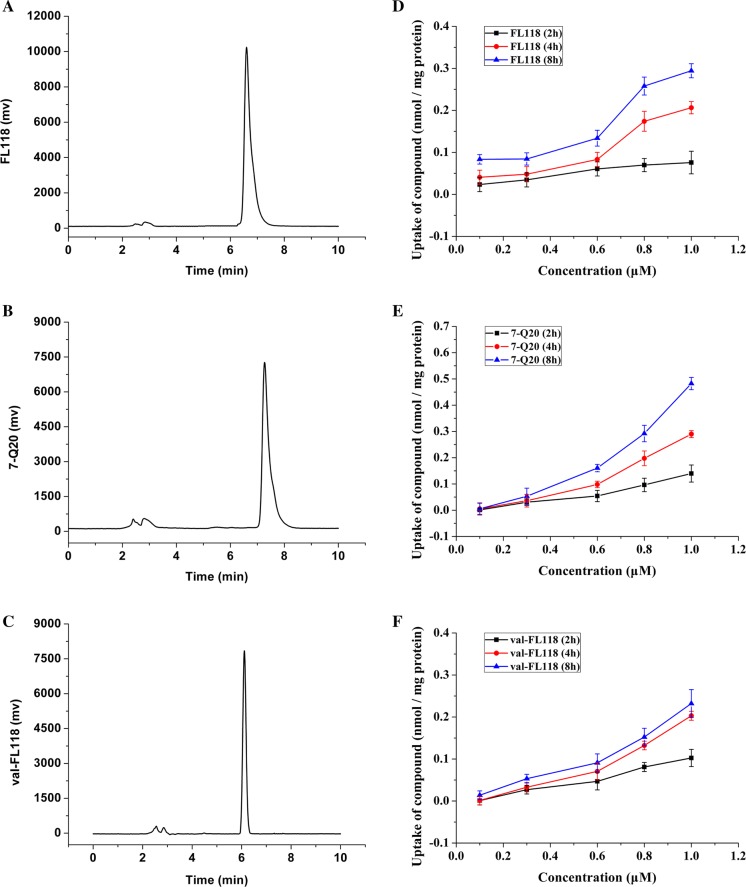
Shimadzu HPLC system equipped with LC solution software, a LC-20AT binary gradient pump, a CTO-10AS column oven, and a SPD-20A UV/VIS detector (Shimadzu, Kyoto, Japan). Column: Sinochrom C18 (250 mm × 4.6 mm, 5 μm, Elite, Dalian); Mobile phase: acetonitrile: 0.1% formic acid (V/V) = 65:45 (7-Q20), 40:60 (FL118), 30:70 (val-FL118); Flow rate: 1 mL/min; Column temperature: 40 °C; Sample volume: 20 μL; Detection wavelength: 360 nm. The retention times for the three compounds were 6.59 min (FL118, Fig. 2a), 7.26 min (7-Q20, Fig. 2b) and 6.22 min (val-FL118, Fig. 2c). To quantify the concentration of FL118, 7-Q20 and val-FL118 in cells, a calibration curve was established over a concentration range of 0.03–3.2 μM. Linear regression analysis of the peak area and concentration revealed a typical equation y = 10506x − 41.154 with a mean correlation coefficient R2 = 0.9995 for FL118, a typical equation y = 8746.5x + 111.96 with a mean correlation coefficient R2 = 0.9995 for 7-Q20, a typical equation y = 3841.7x + 59.86 with a mean correlation coefficient R2 = 0.9986 for val-FL118. All of these equations indicate good linearity in this calibration curve and that the equation could be used to determine the concentration of FL118, 7-Q20 and val-FL118 in this work.
Fig. 2.
Representative chromatograph, FL118 with retention time of 6.59 min (a), 7-Q20 with retention time of 7.26 min (b), and val-FL118 with retention time of 6.22 min (c). Compound concentration and time courses on the uptake of FL118 (d), 7-Q20 (e) and val-FL118 (f) in the 3D Caco-2 cell model are shown. Results are the mean ± SD (n = 3 replicates)
The content changes of 7-Q20, val-FL118 and FL118 were detected before and after keeping them in Hanks solution at 37 °C for 4 h. The stability of 7-Q20 was the highest with 98.46% remaining after 4 h, the stability of FL118 and val-FL118 were 98.11% and 96.39% remaining.
Cytotoxicity assay
The cytotoxicity of 7-Q20, val-FL118 and FL118 were evaluated by MTT assay. The Caco-2, HepG-2 and HCT-116 cell lines were cultured at a density of 2.5 × 105 cells/well in 96-well microplates. After incubation with 0.0064, 0.064, 0.16, 0.4, 0.8, 1 μM 7-Q20, val-FL118 and FL118 for 72 h for cytotoxicity, and 0.01, 0.05, 0.1, 0.5, 1, 5 μM 7-Q20, val-FL118 and FL118 for 8 h for optimizing experimental conditions, MTT solution (0.5 mg/mL) 20 μL/well was added for 4 h. Then, MTT solution was removed and DMSO (150 μL/well) was added to dissolve crystallized MTT. A multiskan spectrum microplate reader was used for measuring absorbance at 570 nm with a reference at 630 nm. Three technical repeats were done. The percentage of cell viability relative to that of the control cells was used as the cytotoxicity measure.
Uptake of 7-Q20, val-FL118 and FL118
Caco-2 cell lines (2.5 × 105 cells/well) were seeded in 6-well ultra low attachment plates (Corning, 7007) for 3D multi-cellular spheroids cultivation. Cells were allowed to grow 48 h to reach confluence and get 200–300 μm spheroid (Howes et al. 2014). Before pre-incubating with Hanks buffer for 20 min, each well was washed with Hanks buffer thrice. The medium in each well was then removed and we added 2 mL of Hanks containing 0.1, 0.3, 0.6, 0.8, 1 μM compounds of 7-Q20, val-FL118 or FL118. After incubating 2, 4, 8 h at 37 °C, the uptake was stopped by rinsing the cells twice with ice-cold Hanks buffer. The cells were then collected and centrifuged at 5000 rpm for 10 min. Adding 250 μL of new Hanks buffer to re-suspend the deposition, the new cell suspension was thawed and frozen at 40 °C versus − 20 °C thrice, then placed into an ultrasonic oscillator for 10 min to break the cells totally. After that, the suspension were centrifuged at 10,000 rpm for 10 min and then divided 250 μL supernatants into two parts, 40 μL supernatants for protein concentration detection, the rest 200 μL for HPLC analysis.
For the temperature effect, the compounds exposures were done at 4 °C for 4 h with Caco-2 cell lines only.
For the cell line effect, the exposures of compounds were done at 37 °C for 4 h with HCT-116 and HepG-2 cell lines.
For the effect of transporter protein, the LAT1 inhibitor BCH (2 mL of 1 mM solution) was added to each well for 30 min.
Efflux of 7-Q20, val-FL118 and FL118
The 3D Caco-2 multi-cellular spheroids in 6-well plates were incubated to meet the experimental requirements. After 72 h, each well was washed with Hanks buffer thrice, and then balanced with 1 mL Hanks buffer per well for 20 min. The P-gp inhibitor verapamil (2 mL of 1 mM solution) was added to each well for 30 min. It was then replaced by 2 mL of Hanks containing 0.1, 0.3, 0.6, 0.8, 1 μM compounds of 7-Q20, val-FL118 and FL118 respectively. The BCRP inhibitor Iressa solution (1 mL of 1 mM solution) was added to 1 mL Hanks containing 0.1, 0.3, 0.6, 0.8, 1 μM compounds of 7-Q20, val-FL118 and FL118, respectively. Then the mixture was added to each well for and incubating 4 h in 37 °C, the uptake was stopped by rinsing the cells twice with ice-cold Hanks buffer. The following steps were the same as 2.7.
Protein concentration detection
Coomassie brilliant blue (CBB) was used to determine the protein concentration in the supernatants as was done in Liang and Li’s (Liang and Li 2013). The corresponding protein concentration was calculated based on the protein content standard curve and then multiplied by the dilution factor to obtain the total intracellular protein content in the original supernatant.
HPLC analysis
The 1 mM stock solutions of FL118, 7-Q20, val-FL118 were added to the Hanks buffer to prepare standard solutions at 0.03, 0.05, 0.10, 0.20, 0.40, 0.80, 1.60 μM. Before detection by HPLC, these solutions were filtered with 0.45 μm filters to remove insoluble salts. The compound concentration was plotted on the abscissa and the peak area was plotted on the ordinate to obtain the standard curve of each compound.
The 200 μL cell supernatant from “Uptake of 7-Q20, val-FL118 and FL118” and “Efflux of 7-Q20, val-FL118 and FL118” sections was added to 200 μL acetonitrile to precipitate the protein, the protein was then removed by centrifugation at 10,000 rpm for 10 min. The supernatant was filtered with a 0.45 μm filter and the contents of intracellular FL118, 7-Q20 and val-FL118 were determined by HPLC. According to the compound standard curve, the corresponding compound concentration was calculated and then multiplied by the dilution factor to obtain the compound concentration in the original supernatant. The intracellular compound uptake was expressed as the ratio of the intracellular compound content to the total intracellular protein content in nmol/mg protein.
Statistical analysis
The process data were analyzed by SPSS 10.0 software and displayed as mean ± standard deviation (SD). Statistical significance was determined by one-way ANOVA. P values ≤ 0.05 were considered significant.
Results
A FL118 analogue with a water-soluble group on Position 7 shows better cytotoxicity
Based on the previously known cytotoxicity of FL118 in cancer cells, we first compared the relative cytotoxicity of FL118 with two FL118 analogues 7-Q20 and val-FL118. The studies indicated that 7-Q20 showed better anticancer activity at a nanomolar concentration range against HCT-116 and HepG2 cells than FL118 and val-FL118 presented in Table 1.
Table 1.
IC50 values for the compounds studied in the HCT-116 and HepG-2 cell lines, determined by MTT assay
| Cell line | Compounds | IC50 (nM) |
|---|---|---|
| HCT-116 | FL118 | 46.31 ± 1.69 |
| 7-Q20 | 40.89 ± 2.91 | |
| val-FL118 | 99.40 ± 2.73 | |
| HepG-2 | FL118 | 134.2 ± 2.94 |
| 7-Q20 | 86.60 ± 1.23 | |
| val-FL118 | 200.1 ± 2.56 |
Values were expressed as mean ± SD (n = 3 replicates)
Table 2 showed the 3D cellular viability after 7-Q20, FL118 and val-FL118 exposure for 8 h, and all cells in drug exposure were at low-cytotoxicity in 3D model (cell viability > 80%), so we considered 0.1, 0.3, 0.6, 0.8, 1 μM of these compounds in our subsequent uptake experiments.
Table 2.
The 8 h cell viability exposed to compounds in 3D Caco-2 cells
| Concentration (μM) | Cell viability (%) | ||
|---|---|---|---|
| FL118 | 7-Q20 | val-FL118 | |
| 0.01 | 102.12 ± 2.76 | 102.25 ± 1.52 | 104.58 ± 1.82 |
| 0.05 | 102.22 ± 3.91 | 101.34 ± 1.97 | 102.28 ± 2.77 |
| 0.1 | 100.32 ± 1.62 | 100.53 ± 2.93 | 102.02 ± 1.73 |
| 0.5 | 99.90 ± 2.91 | 99.61 ± 1.05 | 101.81 ± 1.81 |
| 1 | 96.68 ± 2.82 | 96.16 ± 2.60 | 99.86 ± 1.92 |
Cell viability was expressed as mean ± SD (n = 3 replicates)
Results of uptake of FL118, 7-Q20, val-FL118 in cancer cell model
Effect of compound concentrations and time on the uptake of drug in 3D cell model
The uptake of the three tested compounds showed dose- and time-dependence. The intake of FL118 (Fig. 2d) had a rapid increment at longer compound exposure periods (at 4 and 8 h), indicating that FL118 needs a period of time to accumulate in this Caco-2 cancer cell model. The water-soluble compound 7-Q20 (Fig. 2e) showed higher cellular uptake compared to FL118 (Fig. 2d) and val-FL118 (Fig. 2f).
Uptake of drugs in HCT-116 and HepG-2 cells in 3D cell model
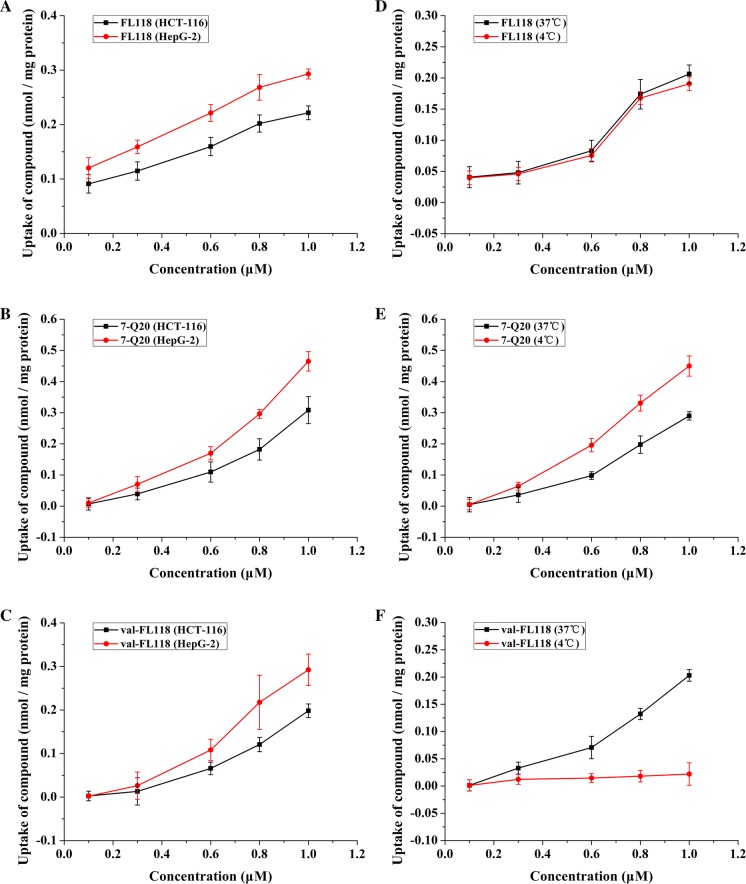
Next, we looked into the effect of different cancer cell types on drug uptake. We found that the absorption quantities of FL118 (Fig. 3a), 7-Q20 (Fig. 3b) and val-FL118 (Fig. 3c) varied in different cell types. We observed a better uptake of all three compounds in HepG-2 cells than in HCT-116 in our 3D multi-cellular spheroids. Distinctly, the sequence of the uptake quantities of these compounds was 7-Q20 > val-FL118 > FL118.
Fig. 3.
Cell line affect on the uptake of FL118 (a), 7-Q20 (b) and val-FL118 (c) in 3D HCT-116 and HepG-2 cell models. Temperature affects on the uptake of FL118 (d), 7-Q20 (e) and val-FL118 (f) at 4 °C and 37 °C in 3D Caco-2 cell model; Results are the mean ± SD (n = 3 replicates)
Effect of temperature on the uptake of drug in 3D cell model
The uptake of 7-Q20, FL118, and val-FL118 were measured at 4 °C and 37 °C to identify their transport paths. Figure 3d shows that FL118’s uptake was affected minimally by temperature, indicating that FL118 may not be affected by the efflux pump activity of the efflux pump proteins. In contrast, the uptake of 7-Q20 (Fig. 3e) was richer at 4 °C than the uptake at 37 °C, suggesting that the uptake of these two compounds by cancer cells may be affected by efflux pump protein efflux pump activity, since these proteins will have a lower efflux pump activity at 4 °C than at 37 °C. Next we explored the effect of several efflux pumps on the uptake of these compounds, such as P-gp, BCRP. In contrast to the above finding for FL118 and 7-Q20, val-FL118’s intake (Fig. 3f) showed significant dependence on temperature in our model, and near zero absorption of val-FL118 at 4 °C suggesting a carrier-mediated transport mechanism related to amino acid transport; we next explored the effect of LAT1 on the uptake of these compounds.
Effect of LAT 1 on drug uptake in 3D cell model
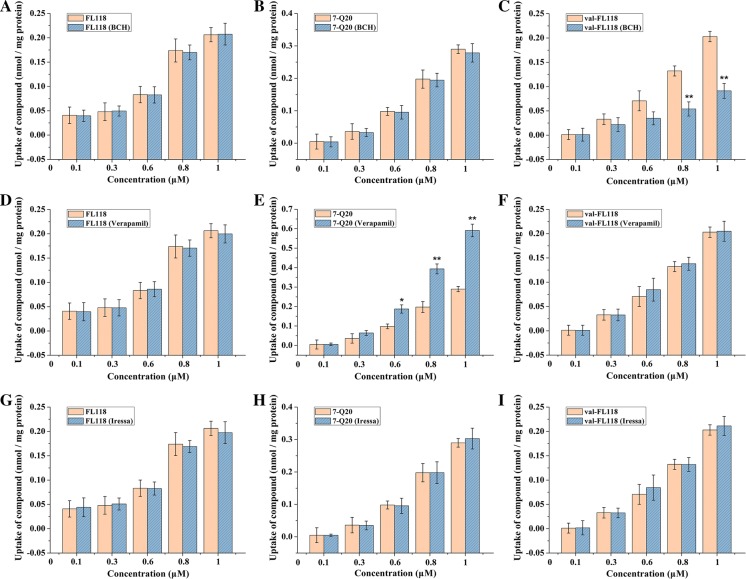
The amino acid analogue BCH was added to investigate the possible involvement of uptake transporters of LAT1. The intake of val-FL118 (Fig. 4c) was reduced significantly at higher compound concentrations in the presence of BCH. The intakes of FL118 (Fig. 4a) and 7-Q20 (Fig. 4b) were basically unchanged.
Fig. 4.
The uptake of FL118, 7-Q20 and val-FL118 in 3D Caco-2 cell model in the presence or absence of LAT1 inhibitor-BCH (a–c), P-gp inhibitor-Verapamil (d–f), BCRP inhibitor-Iressa (g–i). Results are shown as the mean ± SD (n = 3 replicates); p < 0.05 when compared to controls, *p ≤ 0.05; **p ≤ 0.01
Results of efflux of FL118, 7-Q20, val-FL118 in cancer cell model
Effect of P-gp on drug uptake in 3D cell model
Verapamil, a substrate of P-gp, was used to determine the status and functionality of P-gp in the process of uptake. From the data shown in Fig. 4, the uptake of 7-Q20 (Fig. 4e) was promoted significantly with Verapamil, indicating that P-gp was related to the uptake of 7-Q20. However, the uptake of FL118 (Fig. 4d) or val-FL118 (Fig. 4f) was not, suggesting that FL118 and val-FL118 are not substrates of P-gp or other Verapamil-inhibited efflux pump proteins (if any).
Effect of BCRP on drug uptake in 3D cell model
Iressa was expected to inhibit the efflux of compounds occurring via BCRP. In Fig. 4g, FL118’s cellular uptake had little increment in the presence of Iressa. This result agreed with previous studies (Westover et al. 2015), that FL118 was not a substrate of ABCG2. The intracellular uptake of 7-Q20 (Fig. 4h) and val-FL118 (Fig. 4i) were also immune to the effect of Iressa, suggesting that all three of these compounds are potentially not ABCG2 substrates.
Discussion
The aim of this work was to study FL118 and its water-soluble derivatives’ uptake kinetics in parallel with FL118 in the condition to change multiple parameters including compound concentrations, time, temperature, cell lines, the inhibitors of P-gp, BCRP and LAT1, and to understand their absorption mechanisms by 3D multi-cellular spheroids model.
Combined with our previous research in 2D monolayer cell uptake model, drug diffusion in 2D models occurred at an increased, faster rate when compared to 3D spheroids. In 3D spheroids, it took time for the drugs to be taken up by the spheroids, similar to the absorption process in the human body (Chitrangi et al. 2017).
The water-soluble compound 7-Q20 appeared to be accessible to permeating cytomembrane and exerting its cytotoxicity. The presence of amino acid ligand enhanced water solubility but weakened lipid bounding membrane permeability and as a result, val-FL118 was difficult to get through cells and had a lower cytotoxicity.
Surprisingly, FL118, 7-Q20 and val-FL118 had better intakes in HepG-2 cells and poor absorption in HCT-116 (Fig. 3a–c). However, though FL118, 7-Q20 and val-FL118 were more accumulated in HepG-2 cells; HepG-2 cells showed lower sensitivity to these compounds than HCT-116 (Table 1). The high sensitivity of these compounds to colon cancer cells suggests that these compounds should be further investigated.
Changes of temperature are used to confirm the mode of uptake, as a temperature-dependent uptake appears to be evidence of a carrier-mediated transport process (Wang et al. 2000) The variations of val-FL118 in different temperatures (Fig. 3f) demonstrated that the uptake process was almost carrier-mediated transport, which supports the fact that the entry of amino acid complexes into cells required the assistance of vectors (Fan et al. 1998). The nearly equivalent uptake of FL118 in 4 °C and 37 °C (Fig. 3d) suggests that efflux pump proteins are not involved in the FL118 efflux process. The difference of uptakes of 7-Q20 at 4 °C versus 37 °C (Fig. 3e) suggests an efflux of 7-Q20 increase at 37 °C, and this is likely due to an increase of efflux pump protein activity at 37 °C. This is consistent with the finding that 7-Q20 is a substrate of P-gp (Fig. 4e), in response to this we chose inhibitors to verify.
Our results showed that the uptake of FL118 was not related to P-gp (Fig. 4d), BCRP (Fig. 4g) or LAT1 (Fig. 4a) in this study, which was in line with the previous findings that FL118 could overcome P-gp and BCRP induced resistance (Westover et al. 2015; Ling et al. 2015, Yang et al. 2018). The efflux of 7-Q20 was competitively inhibited by Verapamil (Fig. 4e), suggesting the involvement of efflux P-gp transporters and other possible efflux-pump proteins, as Verapamil inhibits proteins other than P-gp. Previous studies showed that both P-gp and MRP2 proteins were involved in the uptake of irinotecan and topotecan (Luo et al. 2002). Our results indicated that BCRP (Fig. 4h) and LAT-1 (Fig. 4b) do not play a role in the uptake or efflux of 7-Q20. Val-FL118 was partially transported by LAT1 (Fig. 4c), and other transporters might exist, as Kinne et al. (2011) reported that amino acid derivatives binding to LAT1 may also be substrates for other amino acid transporters. We assumed that the hydrophilic group of val-FL118 made it harder to get through the phospholipid bilayer but the help of amino acid transport proteins, such as LAT1, increased its intake.
In conclusion, the water-soluble derivative 7-Q20 or val-FL118 did change some transport characters of FL118: (a) the water-soluble derivative 7-Q20 which modified FL118 at 7-position significantly improved the uptake into all the tested cells; however, this group affected 7-Q20 uptake by efflux pump protein; (b) the FL118 derivative val-FL118 which introduced valine at 20-position showed an intake near to FL118 and maintained FL118’s insensitivity to BCRP or P-gp. The results reported in this study provide possible uptake characteristics of FL118 and its two derivatives in 3D cancer cell models.
Acknowledgements
We thank Ms. Amanda Hess for editorial proofreading of the manuscript before submission and final publication.
Abbreviations
- FL118
10,11-Methylenedioxy-20(S)-camptothecin
- 7-Q20
7-p-Trifluoromethylphenyl-10,11-methylenedioxy-20(S)-camptothecin
- val-FL118
10,11-Methylenedioxy-20 (S)-valine-camptothecin
- MTT
3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide
- 3D
Three dimension
- P-gp
P-glycoprotein
- BCRP
Breast cancer resistant protein
- LAT1
L-type amino acid transporter 1
Author contributions
All authors contributed to the study conception and design. Material preparation was performed by YZ and YZ, theoretical advice was provided by QL and FL, data collection and analysis were performed by LZ and QW. The first draft of the manuscript was written by LZ. All authors read and approved the final manuscript.
Funding
This work was financially supported by Qianjiang Talents Project in Zhejiang Province.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bonnier F, et al. Cell viability assessment using the Alamar blue assay: a comparison of 2D and 3D cell culture models. Toxicol In Vitro. 2015;29:124–131. doi: 10.1016/j.tiv.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Casey A, Gargotti M, Bonnier F, Byrne HJ. Chemotherapeutic efficiency of drugs in vitro: comparison of doxorubicin exposure in 3D and 2D culture matrices. Toxicol In Vitro. 2016;33:99–104. doi: 10.1016/j.tiv.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Chitrangi S, Nair P, Khanna A. 3D engineered In vitro hepatospheroids for studying drug toxicity and metabolism. Toxicol In Vitro. 2017;38:8–18. doi: 10.1016/j.tiv.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Fan MZ, Adeola O, Mcburney MI, Cheeseman CI. Kinetic analysis of l-glutamine transport into porcine jejunal enterocyte brush-border membrane vesicles. Comp Biochem Physiol Part A Mol Integr Physiol. 1998;121:411–422. doi: 10.1016/S1095-6433(98)10152-6. [DOI] [PubMed] [Google Scholar]
- Fang Y, Liang F, Liu K, Qaiser S, Pan S, Xu X. Structure characteristics for intestinal uptake of flavonoids in Caco-2 cells. Food Res Int. 2018;105:353–360. doi: 10.1016/j.foodres.2017.11.045. [DOI] [PubMed] [Google Scholar]
- Howes AL, Richardson RD, Finlay D, Vuori K. 3-Dimensional culture systems for anti-cancer compound profiling and high-throughput screening reveal increases in EGFR inhibitor-mediated cytotoxicity compared to monolayer culture systems. PLoS ONE. 2014;9:e108283. doi: 10.1371/journal.pone.0108283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne A, Schülein R, Krause G. Primary and secondary thyroid hormone transporters. Thyroid Res. 2011;4:S7. doi: 10.1186/1756-6614-4-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Anticancer drug FL118 is more than a survivin inhibitor: where is the Achilles’ heel of cancer? Am J Cancer Res. 2014;4:304–311. [PMC free article] [PubMed] [Google Scholar]
- Li F, et al. Topoisomerase I (Top1): a major target of FL118 for its antitumor efficacy or mainly involved in its side effects of hematopoietic toxicity? Am J Cancer Res. 2017;7:370–382. [PMC free article] [PubMed] [Google Scholar]
- Liang QQ, Li YS. A rapid and accurate method for determining protein content in dairy products based on asynchronous-injection alternating merging zone flow-injection spectrophotometry. Food Chem. 2013;141:2479–2485. doi: 10.1016/j.foodchem.2013.05.075. [DOI] [PubMed] [Google Scholar]
- Ling X, Cao S, Cheng Q, Keefe JT, Rustum YM, Li F. A novel small molecule FL118 that selectively inhibits survivin, Mcl-1, XIAP and cIAP2 in a p53-independent manner, shows superior antitumor activity. PLoS ONE. 2012;7:e45571. doi: 10.1371/journal.pone.0045571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X, Liu X, Zhong K, Smith N, Prey J, Li F. FL118, a novel camptothecin analogue, overcomes irinotecan and topotecan resistance in human tumor xenograft models. Am J Transl Res. 2015;7:1765–1781. [PMC free article] [PubMed] [Google Scholar]
- Liu J-A, Guo X-P, Liang S, An F, Shen H-Y, Xu Y-J. Regioselective synthesis of 5′-amino acid esters of some nucleosides via orthogonal protecting protocol. Tetrahedron. 2015;71:1409–1412. doi: 10.1016/j.tet.2015.01.023. [DOI] [Google Scholar]
- Luo FR, Paranjpe Guo A, Rubin E, Sinko P. Intestianl transport of irinotecon in Caco-2 cells and MDCK11 cells overexpressing efflux transporters PGP, cMOAT, and MRP1. Drug Metab Distrib. 2002;30:763–770. doi: 10.1124/dmd.30.7.763. [DOI] [PubMed] [Google Scholar]
- Mehta G, Hsiao AY, Ingram M, Luker GD, Takayama S. Opportunities and challenges for use of tumor spheroids as models to test drug delivery and efficacy. J Control Release. 2012;164:192–204. doi: 10.1016/j.jconrel.2012.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath S, Devi GR. Three-dimensional culture systems in cancer research: focus on tumor spheroid model. Pharmacol Ther. 2016;163:94–108. doi: 10.1016/j.pharmthera.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullakhandam R, Nair MK, Kasula S, Kilari S, Thippande TG. Ferric reductase activity of low molecular weight human milk fraction is associated with enhanced iron solubility and uptake in Caco-2 cells. Biochem Biophys Res Commun. 2008;374:369–372. doi: 10.1016/j.bbrc.2008.07.029. [DOI] [PubMed] [Google Scholar]
- Wadkins RM, et al. Water soluble 20(S)-glycinate esters of 10,11-methylenedioxycamptothecins are highly active against human breast cancer xenografts. Cancer Res. 1999;59:3424–3428. [PubMed] [Google Scholar]
- Wang W, Clarkson TW, Ballatori N. Gamma-glutamyl transpeptidase and l-cysteine regulate methylmercury uptake by HepG2 cells, a human hepatoma cell line. Toxicol Appl Pharmacol. 2000;168:72–78. doi: 10.1006/taap.2000.9018. [DOI] [PubMed] [Google Scholar]
- Westover D, et al. FL118, a novel camptothecin derivative, is insensitive to ABCG2 expression and shows improved efficacy in comparison with irinotecan in colon and lung cancer models with ABCG2-induced resistance. Mol Cancer. 2015;14:92–103. doi: 10.1186/s12943-015-0362-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, et al. FL118, a novel camptothecin analogue, suppressed migration and invasion of human breast cancer cells by inhibiting epithelial-mesenchymal transition via the Wnt/beta-catenin signaling pathway. Biosci Trends. 2018;12:40–46. doi: 10.5582/bst.2017.01288. [DOI] [PubMed] [Google Scholar]
- Zhao X, et al. Design and synthesis of new 7-(N-substituted-methyl)-camptothecin derivatives as potent cytotoxic agents. Bioorg Med Chem Lett. 2014;24:3850–3853. doi: 10.1016/j.bmcl.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G, et al. Design, synthesis and potent cytotoxic activity of novel 7-(N-[(substitutedsulfonyl)piperazinyl]-methyl)-camptothecin derivatives. Bioorg Med Chem Lett. 2017;27:1750–1753. doi: 10.1016/j.bmcl.2017.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]