Abstract
Monoclonal antibody (McAb) has been established as one of the most successful therapeutic strategies for the treatment of cancer. M1A2 (McAb) as a new monoclonal antibody was designed to recognize heat shock protein (HSP60), but its optimum production condition has not been studied. In this study, the cell culture conditions for both Roswell Park Memorial Institute Medium (RPMI 1640) and Dulbecco’s Modified Eagle Medium (DMEM) were optimized using artificial neural network (ANN) analysis to obtain maximum production of IgM McAb by hybridoma M1A2 cells. By using a central composite design, an experimental matrix with cultivation parameters of incubation time, temperature and fetal bovine serum (FBS) concentration on IgM McAb production was designed. The results was analysed by ANN network with different learning algorithms. From the analysis, batch back propagation (BBP) trained ANN composed of eight hidden nodes using a hyperbolic tangent sigmoid transfer function was capable to provide the highest McAb production for both RPMI and DMEM media. Under optimum conditions of 12.5% of FBS, at 33 °C after 3(1/2) days of incubation, maximum McAb production (1132.69 μg/ml) in DMEM was achieved. With PRMI 1640 medium, maximum McAb production (1105.12 μg/ml) was achieved at optimum conditions of 11% of FBS, at 33 °C after 4 days of incubation. The results of this study will provide information for optimum culture conditions of M1A2 McAb production in both DMEM and RPMI 1640 media and also give some clues for the other hybridoma excreting antibodies in the development of in vitro cell culture.
Keywords: Monoclonal antibody, Hybridoma, Response surface methodology, Artificial neural network, Backpropagation, Optimization
Introduction
Despite major advances in our understanding of cancer biology and technological advances in cancer diagnosis and therapy over past three decades, cancer is a global health problem and a major cause of death worldwide. The advent of hybridoma technology makes monoclonal antibodies McAb become essential tools in the discovery of novel and overexpressed cell surface antigens and in the diagnosis and therapy of many diseases including human cancers. This characteristic feature of the McAb makes them an ideal tool for many applications including cancer diagnosis and therapy, leading to huge demand of McAb (Harris 2004).
To develop a suitable in vitro cell culture, many studies have been conducted to discover the cultivation parameters involved in optimizing hybridoma growth and McAb concentration (Heilmann et al. 2005; Terada et al. 2005). A study reported by Chong et al. (2008) showed that the culture viability of hybridoma C2E7 cells was substantially improved and the specific antibody productivity was enhanced under mild hypothermic growth conditions. For media optimization, the study by Maizirwan et al. (2008) showed that the cell viability was greatly enhanced by the optimum concentrations of glutamine (1.68%), serum (13.5%) and NaCO3 (0.87%). This indicated that media optimization provides a way to increase the hybridoma cell viability. Dulbecco’s Modified Eagle Medium (DMEM) and Roswell Park Memorial Institute Medium (RPMI 1640) are generally used to grow different types of cell lines and modification of their compositions could potentially affect the cell proliferation, viability and differentiation (Wu et al. 2009).
A neural network-based approach has become remarkably successful in the last few years, as this system persist high predicting capabilities of nonlinear functions. Artificial neural networks (ANN) had been widely used by researchers as the mathematical or statistical modeling tool in biotechnology applications (Nor et al. 2017; Prabhu et al. 2017a, b; Tan et al. 2010). ANN is biologically inspired by the information processing strategies of the human brain. ANN is trained by experience, when applied a new input to the network it can generalize from past experiences and produce a new result (Unni et al. 2018). The simple structure of ANN normally consists of an input layer, a hidden layer and an output layer (Abbasiliasi et al. 2016). Hence, ANN can predict an optimum values in maximizing the production McAb by applying algorithms that mimic the processes of real neurons.
The information on the factors affecting the production of IgM McAb is scarce and the optimization of cultivation parameters for improved production remains elusive. Thus, improvement of IgM McAb production requires a comprehensive understanding of the factors affecting its production. The objective of this study was to optimize the cultivation parameters particularly incubation time, temperature and FBS concentration in both DMEM and RPMI 1640 media for enhancement of production of IgM McAb by hybridoma M1A2.
Materials and methods
Cell line, media preparation and optimization
The M1A2 hybridoma clone which was established by the fusion of lymphocytes from BALB/c mice sensitized by MCF7 breast carcinoma cell line and Sp20/0-Ag 14 myeloma cells was used in this study (Bakar et al. 2009; Bashokouh 2012). MCF-7 is human breast cancer cell line obtained from ATCC (American Type Culture Collection, Rockville, MD). M1A2 hybridoma cells were inoculated into 10 ml of RPMI 1640 and DMEM in 25 cm2 T-flasks. The culture was incubated at 37 °C with supply of 5% CO2 until the cell number achieved 1 × 105 cells/ml and used as inoculum at 10%.
A total of 39 experimental runs were designed by central composite design (CCD) in response surface methodology (RSM) using Design-Expert software (version 6.0.6, Stat-Ease, Inc., MN). FBC concentration (%) and cultivation time (day) were selected as numerical variables, while types of medium (RPMI 1640 and DMEM) and temperature levels [low (33 °C) and high (37 °C)] were chosen as categorical variables with M1A2 McAb concentration (µg/ml) as response (Table 1). The range of the selected parameters was determined by preliminary experiments (data not shown). The samples were taken from supernatant to measure IgM concentration. All of the experiments were performed in triplicates. The results were analyzed using an ANN software (Neural Power version 2.5, CPC-X). A total of 2 ml supernatant was taken for antibody quantification and replaced with 2 ml of fresh medium.
Table 1.
Levels of numerical and categorical factors on response used in CCD
| Factors | Level | |
|---|---|---|
| Numerical | ||
| FBC concentration (%) | 7 | 17 |
| Cultivation time (day) | 3 | 5 |
| Categorical | ||
| Temperature levels | Low (33 °C) | High (37 °C) |
| Types of medium | DMEM | RMPI 1640 |
| Response | ||
| M1A2 McAb concentration (µg/ml) | ||
Artificial neural network analysis
The experimental data obtained from the factorial design were analyzed using ANN with a multilayer feed-forward structure to establish the predictive model for optimizing the production of IgM McAb. The network developed consisted of three layers—an input layer with three neurons (FBC concentration, cultivation time and temperature levels), one hidden layer with eight neurons and an output layer with one neuron (IgM McAb concentration). The categorical variable (type of medium) was run separately as ANN could not perform modeling on categorical variable. After generating ANN, it was trained to accurately model the test system of the interest. The test system was modelled by training using learning algorithms [incremental back propagation (IBP), batch back propagation (BBP), quick propagation (QP), genetic algorithm (GA) and Levenberg–Marquardt (LM)]. A cut-and-try approach was used to adjust the number of neurons from 3 to 12 in hidden layer. The …… experiments were performed for the selection group, ….. for the training group and …. For testing group. The aim of adjusting the structure was to obtain the fastest network convergence speed and the lowest mean-square-error (MSE).
The ANN model was trained with the back-propagation function, using the learning rate at 0.01. The optimum topology was determined based on the lowest root mean squared error (RMSE) and the coefficient of determination (R2) close to one. Details of training an optimal ANN model with outstanding generalization capacity have been described in our previous reports (Tan et al. 2010). The ideal network was used to predict and optimize of McAb production.
Antibody determination
IgM concentration was determined quantitatively as suggested by Ishida et al. (2006) using an ELISA kit (Bethyl Laboratories, Montgomery, TX, USA). At the same time IgM standards with defined concentration was added to wells and preceded the same as samples. IgM standard carve was plotted and sample IgM content calculated according achieved equilibrium.
Results
Effect of FBS concentration on IgM McAb production
In Table 2, the highest (1220 µg/ml) IgM McAb production was obtained in DMEM medium containing 17% FBS, cultivated for 5 days at 33 °C. However, the production of IgM McAb was reduced to 719.24 µg/ml when DMEM medium contains 7% FBS, cultivated for 5 days at 33 °C. The reduction of IgM McAb production was observed when the FBS concentration decreased. This could be seen in Fig. 1a where the IgM McAb production increased with increasing FBS concentration. On the other hand, the McAb production was the highest (1100 µg/ml) in RPMI 1640 medium containing 10% FBS, cultivated for 4 days at 33 °C. The IgM McAb production reduced to 125.29 µg/ml when FBS was supplemented at 20% in RPMI 1640. In Fig. 1b, the one factor plot shows the increase of FBS concentration increased the IgM McAb production to an extent and gradually decreased after that.
Table 2.
Experimental data of training and testing of artificial neural network designed using RSM
| Type of medium | FBS (%) | Cultivation time (day) | Temperature (°C) | Experimental McAb (µg/ml) | Predicted McAb (µg/ml) |
|---|---|---|---|---|---|
| Training data | |||||
| DMEM | 5 | 3 | 33 | 673.97 | 673.88 |
| 7 | 5 | 33 | 719.24 | 719.34 | |
| 7 | 2 | 33 | 638.02 | 638.08 | |
| 20 | 3 | 33 | 821.39 | 821.37 | |
| 17 | 2 | 33 | 509.83 | 509.88 | |
| 17 | 5 | 33 | 1220 | 1219.8 | |
| 5 | 3 | 37 | 529.14 | 529.14 | |
| 10 | 4 | 37 | 603.78 | 607.98 | |
| 10 | 4 | 37 | 610 | 605.77 | |
| 10 | 4 | 33 | 1066.7 | 1033.8 | |
| 10 | 4 | 33 | 1006 | 1039.5 | |
| 17 | 5 | 37 | 600 | 600.04 | |
| 10 | 6 | 37 | 600 | 599.98 | |
| 10 | 1 | 33 | 411.87 | 411.78 | |
| 20 | 3 | 37 | 529.14 | 529.15 | |
| 10 | 6 | 33 | 1097.2 | 1097.1 | |
| Testing data | |||||
| DMEM | 10 | 4 | 37 | 610 | 608 |
| 10 | 4 | 33 | 1050 | 1034 | |
| 7 | 2 | 37 | 504.31 | 545.62 | |
| 7 | 5 | 37 | 569.24 | 585.93 | |
| 10 | 1 | 33 | 400 | 411.72 | |
| Training data | |||||
| RPMI 1640 | 5 | 3 | 33 | 354.06 | 354.06 |
| 7 | 5 | 33 | 600 | 600 | |
| 7 | 2 | 33 | 446.9 | 446.90 | |
| 20 | 3 | 33 | 125.29 | 125.28 | |
| 17 | 2 | 33 | 194.82 | 194.81 | |
| 17 | 5 | 33 | 568.18 | 568.17 | |
| 5 | 3 | 37 | 289.52 | 289.52 | |
| 10 | 4 | 37 | 547.45 | 547.45 | |
| 10 | 4 | 37 | 520 | 520 | |
| 10 | 4 | 33 | 1100 | 1100 | |
| 17 | 5 | 37 | 120 | 120 | |
| 10 | 6 | 37 | 100 | 100 | |
| 10 | 1 | 37 | 332.01 | 332.00 | |
| Testing data | |||||
| RPMI 1640 | 10 | 4 | 37 | 520 | 533.73 |
| 10 | 4 | 33 | 1002 | 1100 | |
| 7 | 2 | 37 | 451.424 | 504.14 | |
| 7 | 5 | 37 | 110 | 213.54 | |
| 10 | 1 | 33 | 153.76 | 243.23 | |
Fig. 1.
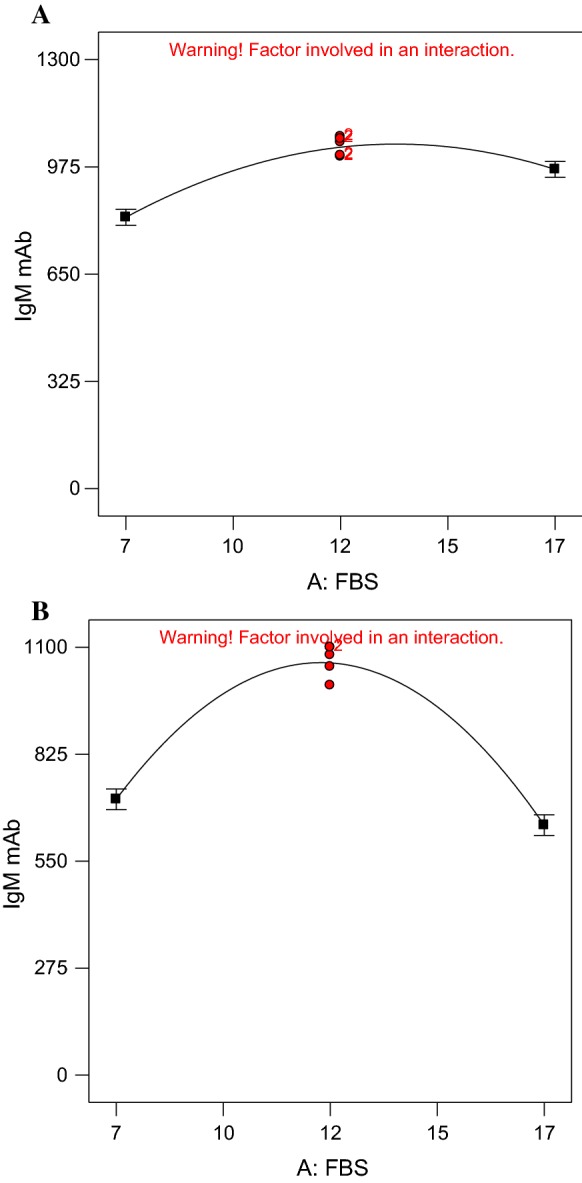
One factor plot for main effect of FBS concentration on the production of McAb (y-axis) in DMEM medium (a) and RPMI 1640 medium (b)
Effect of temperature level on IgM McAb production
In Table 2, the IgM McAb was obtained at 1066.7 µg/ml when the cultivation temperature was at 33 °C in DMEM medium containing 10% FBS, cultivated for 4 days while the production of IgM McAb (610 µg/ml) reduced about 74% in the similar medium and cultivation time but incubated at temperature of 37 °C. Similar production trend could be observed in RPMI 1640 media where the McAb concentration had twofold increment from 520 to 1100 µg/ml when the temperature increased from 33 to 37 °C. The main effect of temperature on the IgM McAb production in both media could be seen in Fig. 2 which the IgM McAb production increased with decrease in temperature level.
Fig. 2.
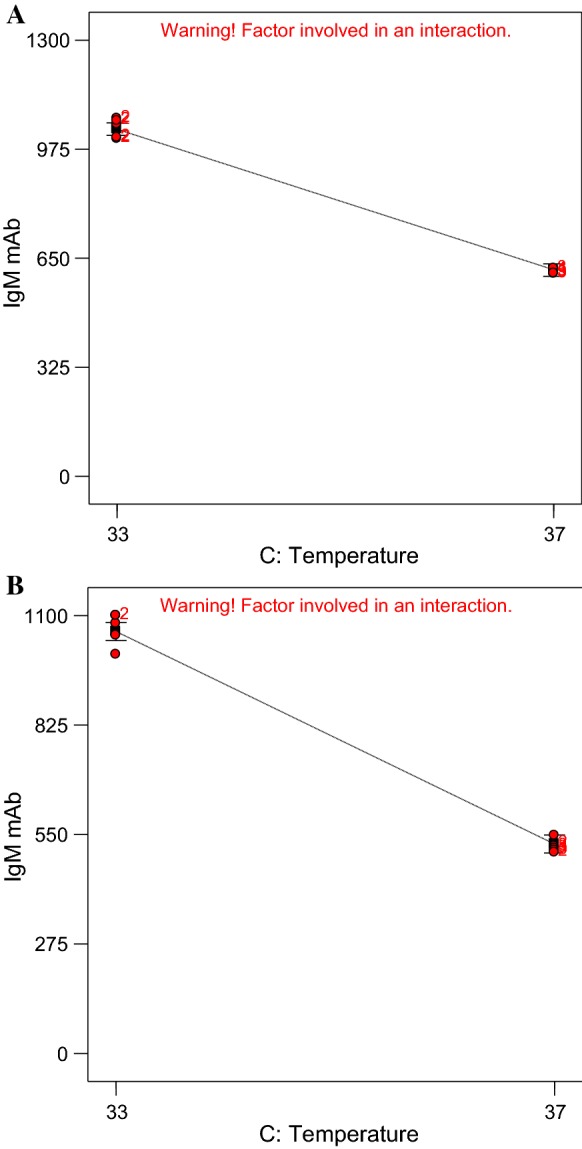
One factor plot for main effect of temperature on the production of McAb (y-axis) in DMEM medium (a) and RPMI 1640 medium (b)
Effect of cultivation time on IgM McAb production
As shown in Table 2, the cultivation time of 1, 4 and 6 days gave production of IgM McAb of 411.87 µg/ml, 1006 µg/ml and 1097.2 µg/ml, respectively in DMEM medium containing 10% FBS at 33 °C. The McAb production had only slight increase from day 4 to day 6. Figure 3a also shows the production rate of IgM McAb slowed down with increasing cultivation time. Similar trend was observed in RPMI 1640 media where the IgM McAb production increased from 332.01 to 520 µg/ml when the cultivation time increased from day 1 to day 4. However, the IgM McAb production decreased to 100 µg/ml when the cultivation time prolonged to day 6. Figure 3b also shows that the decrease of McAb production when the cultivation time reached day 6.
Fig. 3.
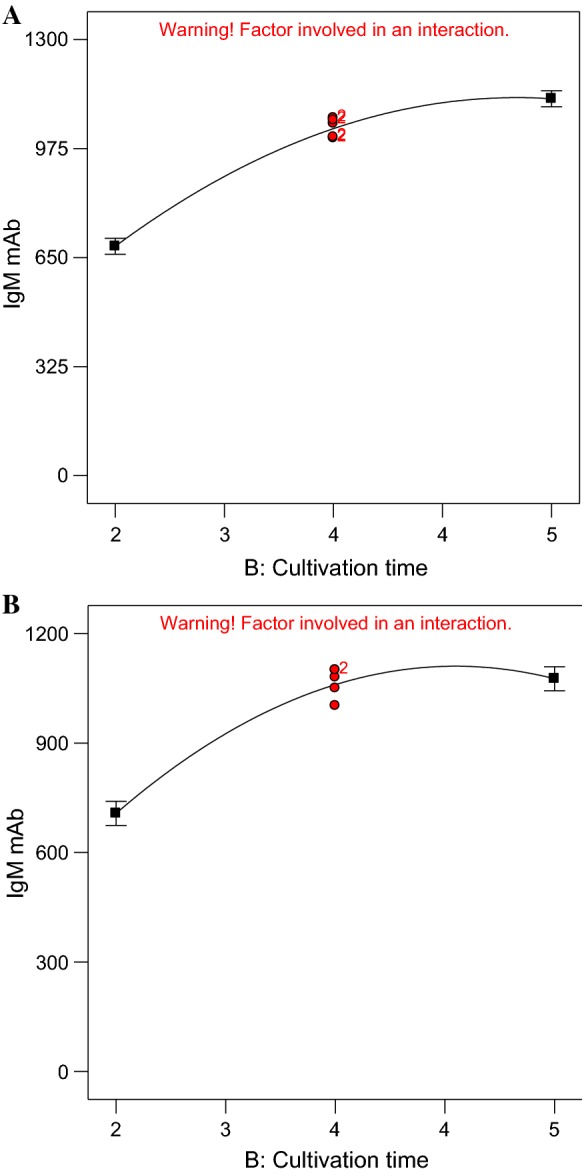
One factor plot for main effect of cultivation time on the production of McAb (y-axis) in DMEM medium (a) and RPMI 1640 medium (b)
Learning and training process of ANN
For minimization of the learning error during the neural network training it is necessary to iteratively update the network weights. Different learning algorithms namely IBP, BBP, QP, GA, and LM were used in this work for training multilayer normal feed forward for obtaining best result of the McAb production from hybridoma. Table 3 shows the results of ANN performance on the basis of R2 values obtained for the different training algorithms used in the present study. It is also notable to see the results presented where the decreasing order of the RMSE character is as follows: GA > QP > IBP > LM > BBP. From the results, BBP was the selected learning algorithm as it had lowest value of RMSE (0.046 for DMEM and 0.066 for RPMI 1640) and highest R2 value (0.989 for DMEM and 0.987 for RPMI 1640) as compared to other learning algorithms. The optimal number of neurons in hidden layer is usually determined through trial and error process and in the current study the eight optimum neurons were selected based on the average R2 values. Table 4 shows the dependency of performance quality of the ANN system on the neuron numbers in the hidden layer. The average R2 values with normalized data were 0.997 and 0.999 for DMEM and RPMI 1640, respectively using BBP at 3-8-1 model as training method.
Table 3.
Comparison of the RMSE and R2 values for training data in different learning algorithms
| Learning algorithm | RMSE | R2 value | ||
|---|---|---|---|---|
| DMEM | RPMI 1640 | DMEM | RPMI 1640 | |
| BBP | 0.046 | 0.066 | 0.989 | 0.987 |
| IBP | 0.094 | 0.104 | 0.865 | 0.883 |
| QP | 0.098 | 0.112 | 0.824 | 0.816 |
| LM | 0.083 | 0.097 | 0.904 | 0.901 |
| GA | 0.195 | 0.267 | 0.756 | 0.698 |
Table 4.
R2 values of the ANN models with respect to data obtained according to BBP learning algorithms after training and testing
| Model | Connection type | Transfer function | Average R2 value | |
|---|---|---|---|---|
| DMEM | RPMI | |||
| 3-2-1 | Linear | Tanh | 0.73225 | 0.72567 |
| 3-3-1 | Linear | Tanh | 0.69856 | 0.79178 |
| 3-4-1 | Linear | Tanh | 0.91255 | 0.92546 |
| 3-5-1 | Linear | Tanh | 0.68914 | 0.78621 |
| 3-6-1 | Linear | Tanh | 0.75699 | 0.83110 |
| 3-7-1 | Linear | Tanh | 0.96421 | 0.94682 |
| 3-8-1 | Linear | Tanh | 0.99732 | 0.99923 |
| 3-9-1 | Linear | Tanh | 0.89662 | 0.90478 |
| 3-10-1 | Linear | Tanh | 0.90498 | 0.99846 |
Optimization of IgM McAb production using BBP trained ANN
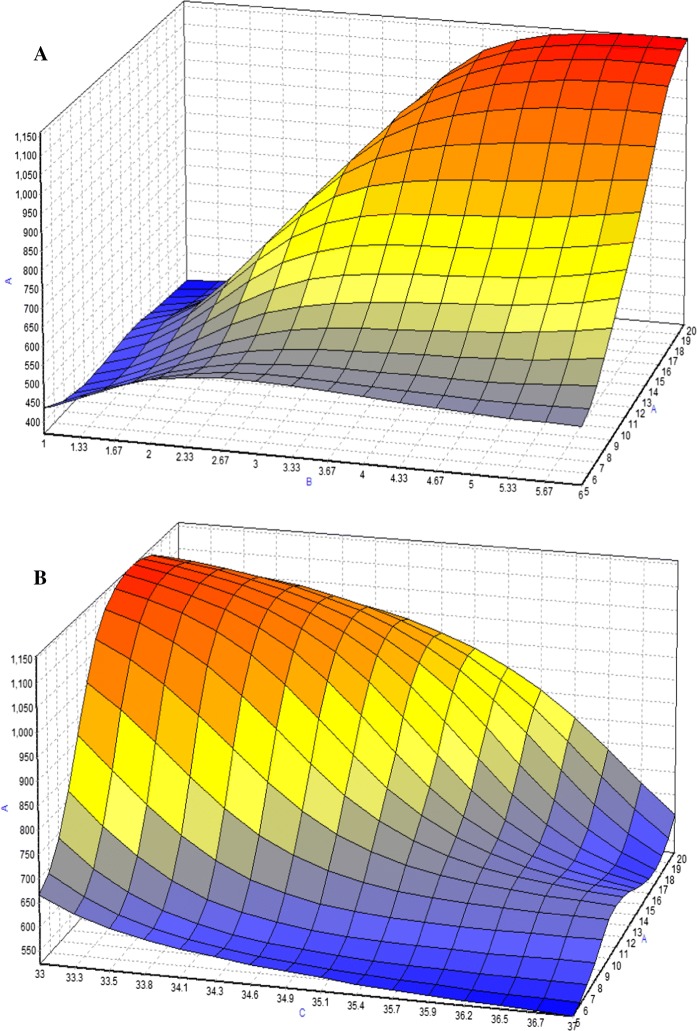
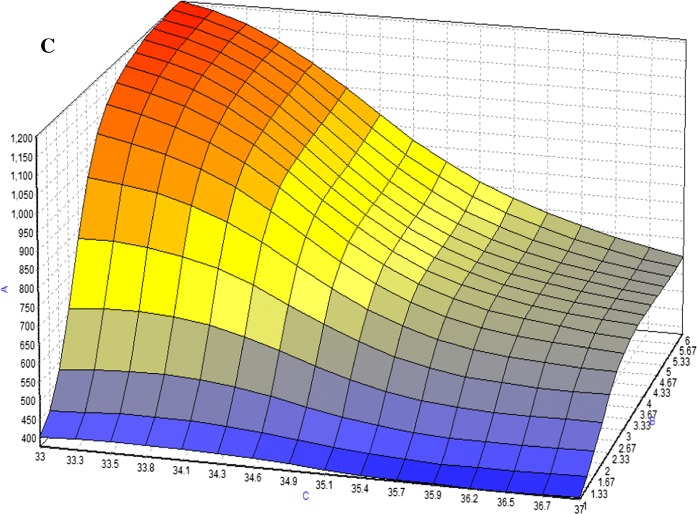
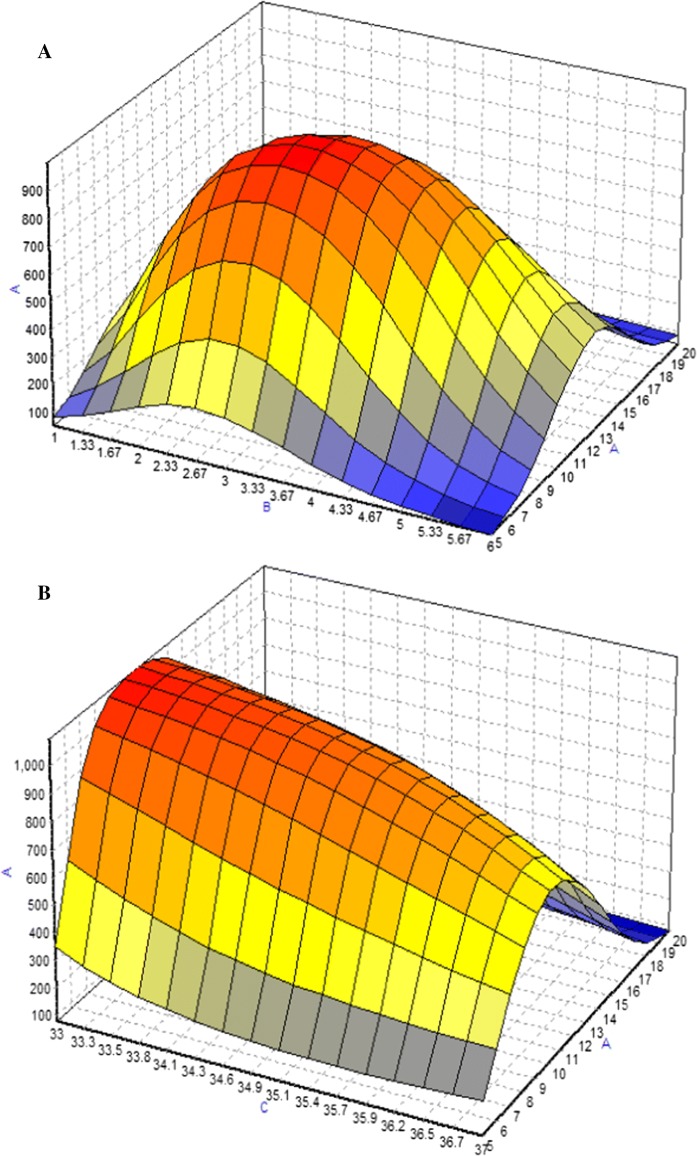
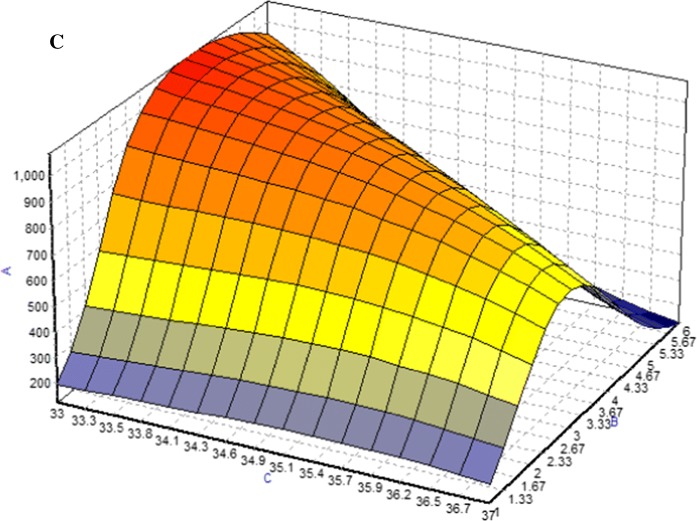
Based on experimental data, maximum McAb, was obtained using/with BBP, where the optimization method offered by the NeuralPower software was Rotation Inherit Optimization (RIO). In Fig. 4, response surface curves revealed that the studied parameters have a combinatory effect on McAb production. For DMEM, it shows the McAb increased with the cultivation time and FBS concentration. For the variable such as temperature, it had negative effect on McAb production at high level (37 °C). Figure 5 showed a dome shape in the plots which introduced the optimum condition of McAb production in T-flask environment with the specified factors. Increasing FBS to 11% increased the concentration of McAb but further increase did not increase McAb concentration in the same increasing slope. Similar trend was observed in cultivation time. Results of the optimization study are shown in Table 5. The final predicted conditions for DMEM supplemented with 12.5% of FBS and cultivated for 3(1/2) days at 33 °C which was estimated to produce 1132.69 µg/ml of McAb. On the other hand, the final predicted conditions for RMPI 1640 which supplemented with 11% of FBS and cultivated for 4 days at 33 °C was predicted to produce 1105.12 µg/ml of McAb. The model validation was carried out by running the predicted conditions, where, as a result of successive runs, only slight variation (< 10%) in the value of McAb production was observed, indicating ANN successful predict the optimum condition for both media on maximum production of McAb.
Fig. 4.
3D plots for combined effect of a FBS concentration and cultivation time b FBS concentration and temperature c cultivation time and temperature on the production of mAb (y-axis) in DMEM medium. The symbols indicated in the graph were a is FBS concentration; b is cultivation time and c is temperature. Color from red to blue indicated maximum to minimum production of McAb
Fig. 5.
3D plots for combined effect of a FBS concentration and cultivation time b FBS concentration and temperature c cultivation time and temperature on the production of mAb (y-axis) in RPMI 1640 medium. The symbols indicated in the graph were a is FBS concentration; b is cultivation time and c is temperature. Color from red to blue indicated maximum to minimum production of McAb
Table 5.
Optimum conditions for McAb production by BBP RIO approach in ANN
| Input parameter | Responses | ||||
|---|---|---|---|---|---|
| Type of medium | FBS (%) | Cultivation time (day) | Temperature (°C) | Predicted maximized McAb Production (µg/ml) |
Experimental maximized McAb Production (µg/ml) |
| DMEM | 12.5 | 3(1/2) | 33 | 1132.69 | 1224.36 |
| RMPI 1640 | 11 | 4 | 33 | 1105.12 | 1184.55 |
Discussion
The results revealed the FBS affected IgM McAb production. Increase in FBS concentration increased the IgM McAb production in this study. This could be due to FBS provides nutrient and energy for cell growth which leads to higher McAb production. However, in higher concentration e.g. 20%, the effect diminished. This is in agreement to the study by Maizirwan et al. (2008) who investigated the effect of FBS at the range of 5% to 15% on the viability of RC1 hybridoma cell, a McAb (IgG)-secreting cell line. They reported that the concentration of 13.5% serum was required to get high cell viability in RPMI 1640 medium. This showed that further increment of FBS was not favour to the cell growth. This could be due to the low availability of nutrient in cells microenvironment when the cell number increase in confluent T-flask which leads to cell starvation and death. Besides, increasing number of dead cell could inhibit the viable cells in culture media (Gregory and Pound 2010; Sakagami et al. 2009).
Higher McAb production obtained with lower level of temperature
This study showed that the lower temperature had higher McAb production in both DMEM and RPMI 1640 media. This is in agreement to with the study by of Chong et al. (2008) who reported that the growth of hybridoma cells was slower during the mild hypothermic condition as compared to that at 37 °C. However, under those hypothermic conditions, the cell viability was improved and the antibody production was increased as compared to that at 37 °C.
The cultivation time also greatly affected the McAb production where prolonged cultivation time to day 6 decreased the McAb production. The cells grow and McAb production was carried out through an interconnected network of reactions known as cell metabolism by consuming FBS as energy source. However, the nutrient deprivation and accumulation of mass toxicity of compounds in the cell metabolism can induce cell death. When the cultivation time was prolonged, the number of viable cells in a culture would be reduced due to nutrient deprivation and accumulation of toxic compounds, which indirectly reduced the overall McAb productivity (Kontoravdi et al. 2007).
In vitro production of McAb in conventional culture methods can produce McAb at concentration between 1 and 100 μg/ml (Trebak et al. 1999). Our results show a higher amount of IgM production (10 times higher) compared to other studies. Nevertheless, McAb secreted by mammalian cells may not be fully functional (Kontoravdi et al. 2007). For ANN, the convergence rate and complexity of a model is highly depended on the type of transfer function used. By performing the trial an error process, it is possible to find the transfer function with the best performance. In the present study transfer functions for the hidden and output layer both were hyperbolic tangent (Tanh). The key element for obtaining an appropriate accuracy of the suggested model in ANN system is to find the optimal number of neurons in the hidden layer. Too few neurons in the hidden layer limit the modeling ability of the ANN system while excessive number of neurons resulted in over-fitting and decreasing the predictability of the system (Tan et al. 2010). It was reported that the modelling ability of the ANN system could be limited by too few neuron.
It should be noted that the current study is the media development in first stage of hybridoma development and the fitness in applying ANN in a small volume to find out the best condition for a new hybridoma. Thus, further study has to be performed in a laboratory bioreactor using the optimum medium formulation and culture conditions for the up-scaling preparation. It is also suggested for the increase in antibody concentration through lyophilization and protein concentration through filtration to provide enough antibodies for primary research. ANN could be a tool in optimizing the purification of the antibody for further study as it has proven to improve the efficiency of extraction (Prabhu et al. 2017a, b).
Conclusion
Results form this study revealed that ANN provided a confident estimation capability through the range of variables in the optimization of cultivation condition for production of monoclonal IgM antibody production by M1A2 hybridoma. The ability of ANN to predict the process characteristics with little prior knowledge is desirable which simplify its implementation and increase its modelling potential. This property makes ANN a powerful and flexible tool well-suited to modelling of complex bioprocesses. Under optimized conditions, addition of 12.5% of FBS and incubation at 33 °C for 3(1/2) days yielded maximum McAb production (1132.69 μg/ml) in DMEM medium. In RPMI medium, maximum McAb production (1105.12 μg/ml) was obtained at optimum conditions of 11% of FBS when incubated at 33 °C for 4 days. Monoclonal IgM antibody production by M1A2 hybridoma in optimized cultivation conditions was about 2 times higher than that obtained in non-optimized condition (603.78 μg/ml for DMEM and 547.45 μg/ml for RMPI 1640). However, chemically defined cell culture media to replaced fetal bovine serum in mammalian in vitro models could be studied for its utilization for future cost-effective use for in vivo application. Furthermore, the growth inhibitory substance during the cell metabolism in optimization study is suggested to increase the productivity of the cells.
Acknowledgements
This work was funded by the short-term Grant of Universiti Sains Malaysia (PO4865, 304/PTENKIND/6315138).
Compliance with ethical standards
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbasiliasi S, Tan JS, Kadkhodaei S, Nelofer R, Ibrahim TAT, Mustafa S, Ariff AB. Enhancement of BLIS production by Pediococcus acidilactici kp10 in optimized fermentation conditions using an artificial neural network. RSC Adv. 2016;6:6342–6349. doi: 10.1039/C5RA22879D. [DOI] [Google Scholar]
- Bakar AFA, Alitheen NB, Keong YS, Hamid M, Ali SAM, Ali AM. A mouse igM monoclonal antibody recognizes breast and colon cancer. Hybridoma. 2009;28:199–203. doi: 10.1089/hyb.2007.0531. [DOI] [PubMed] [Google Scholar]
- Bashokouh F. Characterization of M1A2 monoclonal antibody and in vitro cytotoxicity assessment. Serdang: Universiti Putra Malaysia; 2012. [Google Scholar]
- Chong SL, Mou DG, Ali AM, Lim SH, Tey BT. Cell growth, cell-cycle progress, and antibody production in hybridoma cells cultivated under mild hypothermic conditions. Hybridoma. 2008;27:107–111. doi: 10.1089/hyb.2007.0548. [DOI] [PubMed] [Google Scholar]
- Gregory CD, Pound JD. Microenvironmental influences of apoptosis in vivo and in vitro. Apoptosis. 2010;15:1029–1049. doi: 10.1007/s10495-010-0485-9. [DOI] [PubMed] [Google Scholar]
- Harris M. Monoclonal antibodies as therapeutic agents for cancer. Lancet Oncol. 2004;5:292–302. doi: 10.1016/S1470-2045(04)01467-6. [DOI] [PubMed] [Google Scholar]
- Heilmann K, Groth T, Behrsing O, Albrecht W, Schossig M, Lendlein A, Micheel B. The influence of the chemical composition of cell culture material on the growth and antibody production of hybridoma cells. J Biotechnol. 2005;115:291–301. doi: 10.1016/j.jbiotec.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Ishida T, Ichihara M, Wang X, Yamamoto K, Kimura J, Majima E, Kiwada H. Injection of PEGylated liposomes in rats elicits PEG specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112(1):15–25. doi: 10.1016/j.jconrel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kontoravdi C, Asprey SP, Pistikopoulos EN, Mantalaris A. Development of a dynamic model of monoclonal antibody production and glycosylation for product quality monitoring. Comput Chem Eng. 2007;31:392–400. doi: 10.1016/j.compchemeng.2006.04.009. [DOI] [Google Scholar]
- Maizirwan M, Mat Saad M, Yumi Zuhanis HH, Mohamed Salleh MR. Monoclonal antibody production: media optimization for enhancement the cell viability of hybridoma cell. Asian J Sci Res. 2008;1:525–531. doi: 10.3923/ajsr.2008.525.531. [DOI] [Google Scholar]
- Nor NM, Mohamed MS, Loh TC, Foo HL, Rahim RA, Tan JS, Mohamad R. Comparative analyses on medium optimization using one-factor-at-a-time, response surface methodology, and artificial neural network for lysine–methionine biosynthesis by Pediococcus pentosaceus RF-1. Biotechnol Biotechnol Equip. 2017;31:935–947. doi: 10.1080/13102818.2017.1335177. [DOI] [Google Scholar]
- Prabhu AA, Chityala S, Garg Y, Venkata Dasu V. Reverse micellar extraction of papain with cationic detergent based system: an optimization approach. Prep Biochem Biotechnol. 2017;47:236–244. doi: 10.1080/10826068.2016.1201685. [DOI] [PubMed] [Google Scholar]
- Prabhu AA, Mandal B, Dasu VV. Medium optimization for high yield production of extracellular human interferon-γ from Pichia pastoris: a statistical optimization and neural network-based approach. Korean J Chem Eng. 2017;34:1109–1121. doi: 10.1007/s11814-016-0358-1. [DOI] [Google Scholar]
- Sakagami H, Kishino K, Amano O, Kanda Y, Kunii S, Yokote Y, Oizumi H, Oizumi T. Cell death induced by nutritional starvation in mouse macrophage-like RAW264.7 cells. Anticancer Res. 2009;29:343–347. [PubMed] [Google Scholar]
- Tan J, Ramanan R, Ling T, Shuhaimi M, Ariff A. Comparison of predictive capabilities of response surface methodology and artificial neural network for optimization of periplasmic interferon-alpha 2b production by recombinant Escherichia coli. Minerva Biotecnologica. 2010;22:63–73. [Google Scholar]
- Terada S, Sasaki M, Yanagihara K, Yamada H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J Biosci Bioeng. 2005;100:667–671. doi: 10.1263/jbb.100.667. [DOI] [PubMed] [Google Scholar]
- Trebak M, Chong JM, Herlyn D, Speicher DW. Efficient laboratory-scale production of monoclonal antibodies using membrane-based high-density cell culture technology. J Immunol Methods. 1999;230:59–70. doi: 10.1016/S0022-1759(99)00122-2. [DOI] [PubMed] [Google Scholar]
- Unni S, Prabhu AA, Pandey R, Hande R, Veeranki VD. Artificial neural network-genetic algorithm (ANN-GA) based medium optimization for the production of human interferon gamma (hIFN-γ) in Kluyveromyces lactis cell factory. Can J Chem Eng. 2018;4:843–858. [Google Scholar]
- Wu X, Lin M, Li Y, Zhao X, Yan F. Effects of DMEM and RPMI 1640 on the biological behavior of dog periosteum-derived cells. Cytotechnology. 2009;59:103–111. doi: 10.1007/s10616-009-9200-5. [DOI] [PMC free article] [PubMed] [Google Scholar]