Abstract
This study aimed to shed light on the protective and therapeutic anti-osteoporotic effects and mechanisms of action of grapefruit juice (GFJ) on prednisolone-induced osteoporosis a rat femoral fracture model. We found that treating rats with GFJ before and/or after prednisolone-induced osteoporosis resulted in increased bone density, total mineral content, and calcium content to counteract the osteoporotic effects of prednisolone. In parallel, the histological and ultrastructural results of the GFJ-treated groups correlated well with enhanced breaking strength of femurs subjected to a constant load. Furthermore, GFJ treatment before and after prednisolone-induced osteoporosis decreased plasma alkaline phosphatase and tartrate-resistant acid phosphatase activities and increased the level of insulin-like growth factor 1. Mechanistically, our immunohistochemistry study showed that GFJ ameliorated prednisolone-induced osteocalcin depletion, decreased receptor activator of nuclear factor kappa-B ligand (RANKL) expression, and increased osteoprotegerin (OPG) expression. GFJ showed a beneficial anti-osteoporotic effect against prednisolone-induced osteoporosis in rats, possibly via the RANKL/OPG axis, suggesting that GFJ might be a good candidate for developing anti-osteoporotic drugs.
Keywords: Grapefruit juice, Bone density, Ultrastructure, Plasma alkaline phosphatase, Osteoprotegerin
Introduction
Prednisolone is a corticosterone medication with glucocorticoid properties, used for treating of a variety of diseases including asthma, autoimmune diseases, and other inflammatory conditions such as rheumatoid arthritis, and ulcerative colitis (Feldman 1992). Prednisolone is also considered one of the desired therapies in cancer treatment, especially malignant solid tumors (Umemoto et al. 1991; Davies et al. 2004). Formerly, many studies have considered that the daily use of low doses prednisolone (5 mg) cause plunge in the markers of bone formation while the daily use of high doses (prednisolone > 20 mg/day or equivalent) cause the loss of spinal bone density (Ton et al. 2005). In general, glucocorticoids, including prednisolone, affect bone in many ways. They adversely destroy the bone remodeling process. The bone resorption rate is determined by both osteoclasts number and activity. The number of osteoclasts is dependent on relative rates of cell differentiation and cell death (Hilton et al. 2008). Contrary to this, the bone formation rate is determined by the osteoblasts number, which reflects their longevity and the death rate via apoptosis (Manolagas 2000). Thus, the challenge is to try to disclose certain agents for protection or cure.
In this context, it has been noted that osteoporosis prevention is well correlated with diet components. For example, diet high in vegetables and fruits contents, is linked with diminished bone resorption, while processed foods diets are correlated with lower bone quality (Hardcastle et al. 2011a, b; McNaughton et al. 2011). In particular, phenolic compounds contained in fruits have great benefits on bone health (Welch and Hardcastle 2014). Flavonoids, and vitamin C have antioxidant and anti-inflammatory activities that inhibit osteoclastic differentiation and protect against bone loss (Lean et al. 2003; Wattel et al. 2004; Yu et al. 2005). As grapefruit belongs to citrus fruits rich with flavonoids and vitamin C, we wished to test its protective and curable effect against Osteoporosis model. Few studies have been showed the grapefruit juice effect (GFJ) on the bone quality (Riso et al. 2005; Jia et al. 2012; Ma et al. 2016; Wattel et al. 2004), nevertheless, osteoporotic suppressing effects and modes of action of GFJ are still elusive. Herein, we aimed to clarify the anti-osteoporotic effects and mechanisms of action of GFJ through studying its protective and curing role on a prednisolone-induced osteoporosis rat femoral fracture model.
Materials and methods
Drugs and chemicals
Prednisolone was purchased from Sigma-Aldrich (St. Louis, MO, USA). Grapefruit juice was freshly prepared as previously described by (Deyhim et al. 2008).
Total flavonoids content
Total flavonoids content was estimated with a solution composed by: 5% NaNO2, 0.1 mL of 10% aluminum chloride hexahydrate (AlCl3), and 1 M NaOH (Leontowicz et al. 2014). The mixture was diluted to 10 mL with water and the absorbance was read at 510 nm. The results were expressed as mg quercetin L−1 juice. Samples were analyzed in triplicate.
Total polyphenols content
The polyphenols content was measured by the Folin–Ciocalteu colorimetric method (Singleton et al. 1999). Briefly, 500 μL of juice was mixed with 1 mL of Folin–Ciocalteu’s reagent and 10 mL of a 20% in water solution of sodium carbonate solution and made up to 100 mL with ultrapure water. The reaction mixture was incubated at room temperature in the dark for 2 h and the absorbance was read at 760 nm. Results were expressed as mg L−1 of gallic acid equivalents. Samples were analyzed in triplicate.
Total anthocyanins content
Total anthocyanins were determined by a pH differential method (Nielsen et al. 2003). Briefly, grapefruit juice was diluted 1:5 with pH 1.0 buffer solution or pH 4.5 buffer solutions. After equilibration at room temperature for 10 min, the absorbance was measured at 520 nm (maximum absorbance of Cyd-3-Glu) and 700 nm (for turbidity corrections). Results were expressed as mg of cyanidin 3-glucoside L−1. All the measurements were made in triplicate.
Osteoporosis model and treatments
All animal experiments were carried out using protocols approved by the Committee on Animal Experimentation of Beni-Seuif University, Egypt. A total of thirty white albino rats (Rattus norvegicus), each weighing 100–150 g, were purchased from the National Research Center, Cairo and used for each experiment. The animals were brought for observation about 10 days before the start of the experiments to omit any intercurrent diseases. Then, the rats were housed in stainless steel cages in the animal facility at normal temperature and given food and water ad libitum. The animals were weighed weekly during the experimental period. The rats were then haphazardly divided into five groups (six rats each), given food and water ad libitum, and orally administered with the intended agent. Group 1 (control); The rats of this group were considered control where they were given the vehicle, group 2 (grapefruit); The rats of this group were non-osteoporotic and were given the grapefruit juice at a dose level of 250 mg/kg b. wt, three times/week for 3 months, group 3 (prednisolone); The rats of this group became osteoporotic by administration of the prednisolone daily for 2 months at dose level of 4.05 mg/kg b.wt, group 4 (protective); The rats of this group were protected by administration of the grapefruit juice three times/week for 1 month at a dose level of 250 mg/kg b. wt, before the induction of osteoporosis using the prednisolone daily for 2 months at a dose level of 4.05 mg/kg b. wt, and administered simultaneously the grapefruit juice for three times/week at a dose level of 250 mg/kg b. wt., half an hour before the prednisolone administration, and group 5 (treatable); The rats of this group became osteoporotic by administration of the prednisolone daily for 2 months at a dose level of 4.05 mg/kg b.wt and then cured with the grapefruit juice three times/week for 1 month at a dose level of 250 mg/kg b. wt.
At the end of the experimental period (3 months), all animals were sacrificed under mild diethyl ether anesthesia, and the femurs were collected, stripped off their soft tissues and blood samples were also collected.
Bone (femoral) density
The femoral bone density was calculated by Archimedes’ principle (Kalu 1991).
Determination of the mineral content in bone ash
The femurs were placed in a drying oven at 100 °C for 72 h. The weight of the samples was determined then bone samples were smashed in covered crucibles at 600 °C for 16 h. The samples were cooled and weighed to determine the percentage of mineral content (Shim et al. 2012).
Femoral Ca+2 content
Bone (femur) ash was prepared in a muffle furnace (7000 °C for 6 h) and dissolved in 0.1 mol/l HCl solution. Bone mineral calcium was measured by a UV–visible spectrophotometer (RMS-BCA 201) (Cheng et al. 2004).
Time to pressure-induced fracture by the three-points bending test
The femurs were immersed in normal saline for 48 h at 4 °C. Femoral biomechanical properties were measured by a three-points bending test (Stable Micro System, Canton, MA, USA). Bone samples were placed in a similar direction on two opposite bars spaced 20 mm apart and a crosshead force moving at a constant speed of 1.0 mm/min was delivered to the mid-shaft. A force against distortion curve was recorded. Time to pressure-induced fracture was recorded upon the breakage of the intact femur (Kiebzak et al. 1988).
Light microscopic examination
Bone samples were submerged in PLP fixative (2% paraformaldehyde containing 0.075 M lysine and 0.01 M sodium periodate solution, pH 7.4) at 4 °C. Then demineralization with 10% EDTA solution and dehydration with ascending concentration series of ethanol before being embedded in paraffin. A 5-μm transverse sections were obtained using a microtome. After deparaffinization, the sections were stained in filtered hematoxylin for 3 min and then rinsed in running water until the water became clear. The sections were then immersed in eosin stain for 1–2 min and rinsed in running water until the water became clear. Thereafter, the sections were dehydrated in ascending series of alcohol solutions of 50, 70, 80, 95% (2 times), and 100% (2 times) and cleared with xylene (3 times) and then examined under a light microscope (Humanson 1961).
Scanning electron microscopic (SEM) examination
The fresh femur specimens were trimmed down into 5-mm transverse sections and fixed in 2% glutaraldehyde solution. Before SEM characterization, drying the bone samples were performed on mounted stubs with adhesive. Carbon coatings were applied at a thickness of about 20 nm with the help of sputter coater. SEM images were obtained on a low vacuum SEM in the Faculty of Science, Beni Suef University, Egypt.
Biochemical variables
The plasma was separated from the collected blood samples, then the plasma alkaline phosphatase (ALP) and tartrate resistant-acid phosphatase (TRAP) activities were measured using commercially convenient kits as an indication of bone resorption (Thermo Electron, Louisville, CO). Plasma insulin-like growth factor I (IGF-I) was evaluated using a commercially available kit (R&D Systems, Minneapolis, MN) as a quantitative measure of osteoblast proliferation and consequently as an indicator for bone formation.
Immunohistochemical studies
Two immunohistochemical techniques were used: an Avidin–Biotin-Horseradish Peroxidase Complex System (ABC) was used for detecting osteoprotegerin (OPG, a marker for bone formation) and receptor activator of nuclear factor kappa-B ligand (RANKL, a marker for bone resorption) and an Envision+ System (Dako, K4006) for detecting osteocalcin (OCC, a marker for bone formation) (Graham and Karnovsky 1966). Immunohistochemistry staining intensity score was calculated using immunohistochemistry profiler plugin in ImageJ software.
Statistical analysis
Data are represented as mean ± SD. Student’s t test was performed to define the statistical significance compared to the prednisolone group. Statistical significance was defined as * p < 0.05 and ** p < 0.005. The data shown in the figures are representative data for three independent experimental results.
Results
Total contents of flavonoids, polyphenols, and anthocyanins
As an initial step in the measurement of the anti-osteoporotic activity of grapefruit juice (GFJ), the total flavonoids, total polyphenols, and total anthocyanins contents of the GFJ were quantified, because these values correlate in most cases with the anti-osteoporotic activities. Results in Fig. 1 showed that flavonoids content (304.17 ± 8.71 mg L−1) is the highest content among the measured phytochemicals in GFJ. Meanwhile, the total polyphenols content (124.57 ± 3.23 mg L−1) and the total anthocyanins content (2.5 ± 0.68 mg L−1) were less than that of flavonoids content. These results showed that flavonoids are the major phytochemicals content in GFJ.
Fig. 1.
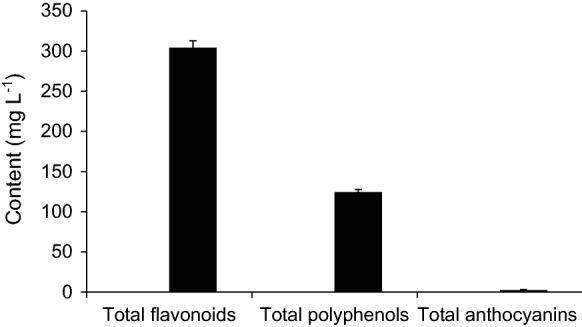
Contents of total flavonoids, total polyphenols and total anthocyanins in Grapefruit juice (GFJ). Total flavonoids, total polyphenols and total anthocyanins in grapefruit juice were determined and expressed in mg L−1. Results are the averages of three replicates
GFJ enhanced the morphology, bone density, total mineral content, and calcium content in prednisolone-induced osteoporosis rat femoral bone
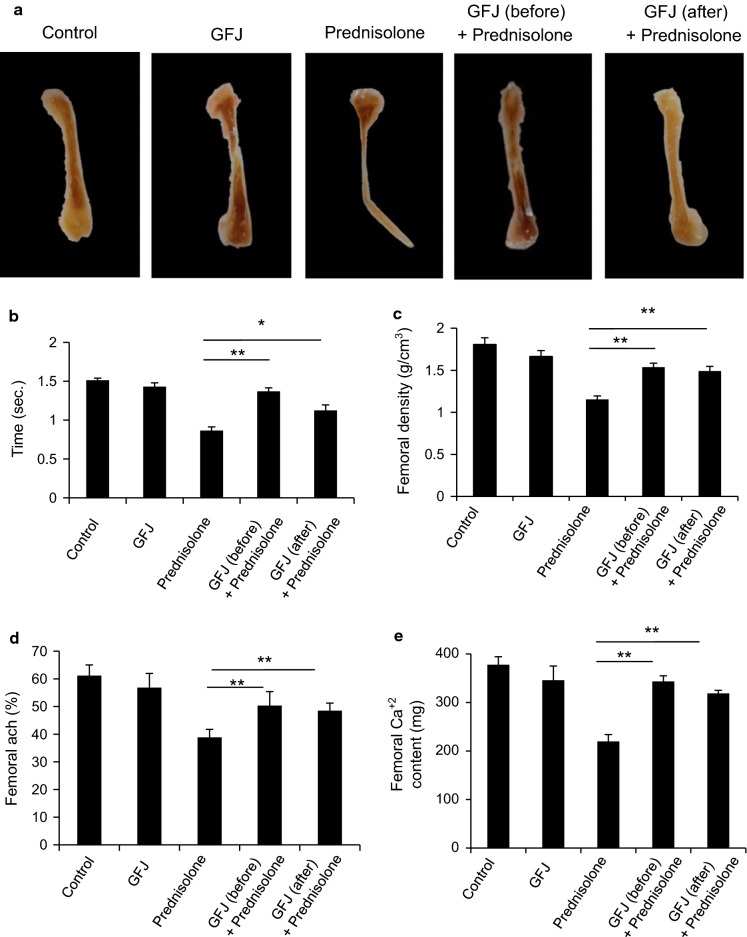
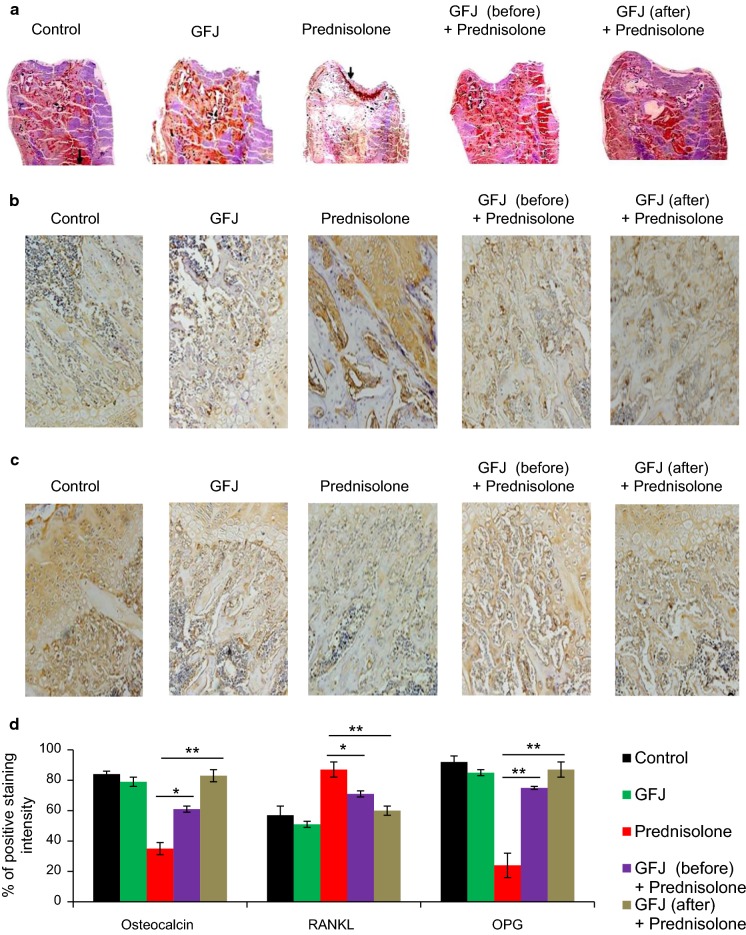
To test the anti-osteoporotic effect of GFJ on femur bone tissue, we first examined femur bone morphology in all groups. Our results in Fig. 2a show normal bone morphology in control and GFJ groups. The osteoporotic group treated with prednisolone showed many morphological changes including distortion and destruction of the femur bone head, shaft, and condyles. Treatment with GFJ before prednisolone treatment inhibited the development of osteoporosis. Additionally, applying GFJ treatment after inducing osteoporosis markedly rescued these morphological changes. Then, we observed that prednisolone shortened the time taken to develop pressure-induced fracture. The rate of femoral fracture was delayed significantly in rats treated with GFJ before or after prednisolone treatment in comparison with the rate in their corresponding control (Fig. 2b). To understand how GFJ maintained the normal morphology of the bone and delayed its fracture, we measured femoral bone density as well as the mineral content, particularly calcium content. We found that the mean femoral density of prednisolone-treated rats significantly diminished comparing to the corresponding control group (Fig. 2c). GFJ administration before prednisolone treatment inhibited the development of osteoporosis, and applying this treatment after induction of osteoporosis ameliorated this decrease in osteoporotic rats (Fig. 2c). As decreases in bone mineral content, particularly the calcium percentage, are a specific indicator for osteoporosis, we tested the effect of GFJ on the mineral and calcium contents of bone. We found that the mineral and calcium contents in the ash of the bone of the prednisolone-treated group were less than that of the corresponding control group (Fig. 2d, e). Treating these osteoporotic rats with GFJ before prednisolone treatment inhibited the progression of osteoporosis. In addition, applying this treatment after inducing osteoporosis increased mineral content and calcium content in the ash of femur bones (Fig. 2d, e).
Fig. 2.
Grapefruit juice (GFJ) administration enhanced the morphology, bone density, total mineral content, and calcium content in prednisolone-induced osteoporosis rat femoral bone. a Administration of GFJ prevented and repaired the prednisolone-induced femur morphological changes. b Administration of GFJ before or after the osteoporosis induction increased the time consumed until pressure-induced fracture occurred. c GFJ prevented and ameliorated the decrease in the mean femur density of osteoporotic rats. d GFJ prevented and ameliorated the decrease in the total mineral content of osteoporotic rats. e GFJ prevented and ameliorated the decrease in the Ca2+ content of osteoporotic rats. Data are expressed as the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.005 indicate significant differences, compared to the prednisolone group as analyzed by Student’s t test
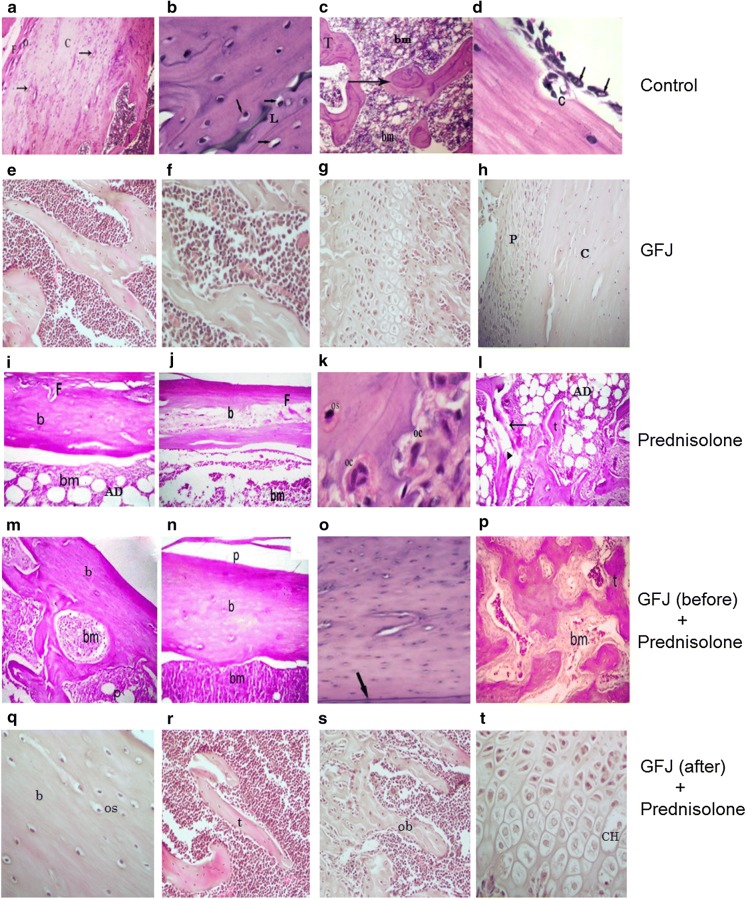
GFJ prevented and reversed the prednisolone-induced femur bone histological changes
After staining thin sections of the femoral heads, histological analysis showed the following:
Control and GFJ groups:
We found a similar histological appearance between control and GFJ groups. Hematoxylin- and eosin-stained sections of the proximal femur diaphysis and epiphysis of the control rats revealed that an external shell of cortical bone formed to which the periosteum was attached to its external surface (Fig. 3a, h), and the endosteum was attached to its internal surface. The cortical bone appeared as a layer of dense bone containing osteocytes inside their lacunae with densely-stained oval nuclei (Fig. 3b). The internal cancellous bone of the secondary spongiosa of the proximal femur metaphysis of the control rats consisted of a network of bony trabeculae separated by interconnecting spaces containing bone marrow. The bone marrow is formed of hematopoietic tissue, scattered adipocytes, and blood sinusoids. The bone trabeculae consist of irregular bone lamellae and osteocytes within their lacunae in between bone lamellae (Fig. 3a, e, f). The cortical bone showed subperiosteal bone deposition appearing as a distinct basophilic cement line demarcating the border between newly-added bone matrix and the older bone (Fig. 3b). The endosteal surface of the cortical bone had a smooth appearance; this surface was lined with many osteoprogenitor cells and osteoblasts. The osteoclasts were also present in their Howship’s lacunae (Fig. 3c). The proximal epiphyseal plate of the femur showed the four distinct zones of the epiphyseal growth plate with regular chondrocyte column arrangement. These zones are: columns of resting zone, proliferative zone, the prominent hypertrophic zone, and calcification zone (Fig. 3g).
Fig. 3.
Grapefruit juice (GFJ) reversed and prevented the prednisolone-induced femur bone histological changes. a–d The histological pattern of the control group. a Periosteum with fibrous layer (F) and osteogenic layer (O) surrounding the compact bone (C) which consists of many osteons (arrows). b Osteocytes and basophilic cement line (L). c Spongy bone with trabeculae (T) surrounding the bone marrow (bm). d The endosteal surface lined with many osteoprogenitor cells (arrows) and osteoclasts (C) residing in their Howship’s lacunae. e–h Effects of GFJ alone on the histological pattern of the femur. e Inner cancellous bone consists of a network of bony trabeculae separated by interconnecting spaces containing bone marrow. f Bone marrow with its hematopoietic tissue. g The epiphyseal plate with its four distinct zones and regular chondrocyte column arrangement. h The periosteum layer (P) surrounding the compact bone (C). i–l Effects of prednisolone alone on the histological pattern of the femur. i, j show thickening of the fibrous layer (f) and erosion of the bone matrix (b) with many adipocytes (AD) within the bone marrow (bm). k the increased number of osteoclasts with multiple nuclei (OC) and less number of osteocytes (OS). l the eroded spongy bone trabeculae (arrow) compared to an unaffected one (t), in addition to many adipocytes (AD) located in the bone marrow. m–p Effects of applying of GFJ treatment before osteoporosis induction on the histological pattern in rat femur. m The normalized structure of the periosteum (p) and the compact bone matrix (b) and (bm). n The normalized periosteum (p), bone matrix (b), and Bone marrow (bm), remove (sh). o The cement line (arrow). p showing the normalized structure of the spongy bone with its trabeculae (t) enclosing the bone marrow (bm). q–t Effects of applying of GFJ treatment after osteoporosis induction on the histological patterns in rat femurs. q The bone matrix (b) with its osteocytes residing in their lacunae (os). r The histological structure of the spongy bone with its trabeculae (t) embracing the bone marrow (bm). s The endosteal surface of bone trabeculae lined with osteoblasts (ob). t The almost normalized structure of the epiphyseal growth plate with its chondrocytes residing in their lacunae (CH)
Osteoporotic group: Examination of the sections of the proximal femur diaphysis of rats treated with prednisolone revealed no signs of novel subperiosteal bone deposition and indistinct basophilic cement lines in the lamellae of the cortical bone compared with the control. In addition, resorption cavities were observed in the cortical bone. Moreover, the endosteal surface of the cortical bone appeared irregularly eroded with faintly stained matrix having less distinct cement lines compared with that of the normal and GFJ groups (Fig. 3i, j). Moreover, the bone resorbing cells, osteoclasts, with their highly acidophilic cytoplasm and numerous nuclei were located in the corroded bone surface (resorption areas) (Fig. 3k). The bone marrow adipocytes depicted an obvious increase compared with the level observed in the controls. Some trabeculae were observed as islands of widely separated spicules, whereas other trabeculae appeared thinned out compared with the controls, with faintly stained bone matrix areas, in addition to the resorption areas presence (Fig. 3l).
Osteoporotic-GFJ treated groups: On the other hand, the GFJ treatment before giving rats prednisolone precluded the development of the aforementioned osteoporotic signs (Fig. 3m–o). Additionally, the growth plate was almost identical with that of the control and GFJ-treated rats in thickness, and the chondrocytes exhibited an almost preserved structure (Fig. 3p). Moreover, applying these treatments after inducing osteoporosis restored the architecture of the cortical and trabecular structure with a structurally-organized bone matrix showing the disappearance of osteoporotic cavities, thinning of the fibrous layer and diminishing in the number of adipocytes in the bone marrow. In addition, within the marrow cavity, many branching and anastomosing bony trabeculae that contain osteocytes enclosed in their lacunae were observed arising from the cortical bone (Fig. 3q–t).
The histological results correlated well with enhanced breaking strength of femur bones subjected to a constant moving load.
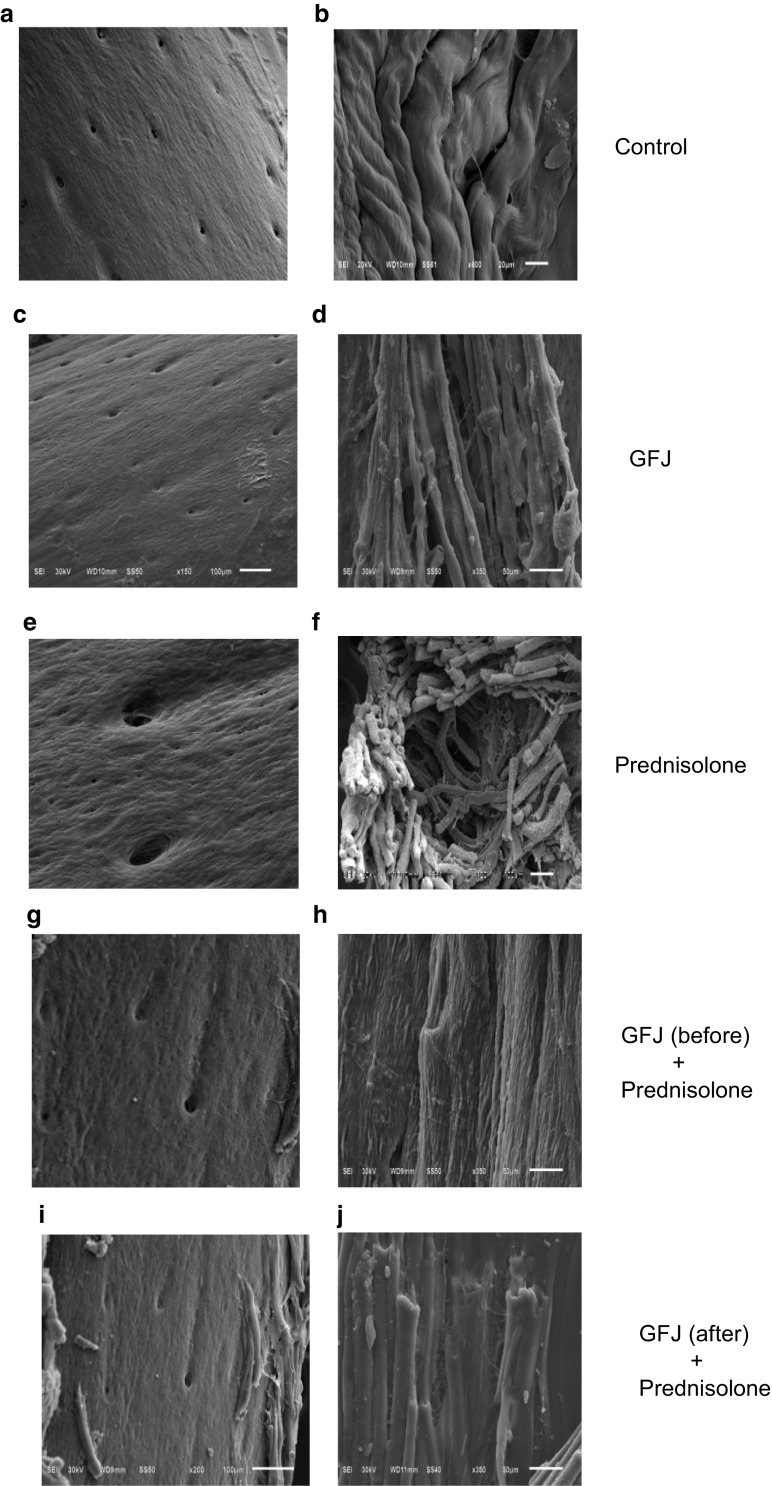
Scanning electron microscopic examination
We found similar ultrastructural appearance between control and GFJ groups. In control and GFJ groups, the compact bone consists of a repeated structure known as an osteon or Haversian system, and in each bone lamella, the protein (collagen) fibers are mainly oriented parallelly to each other (Fig. 4a–d).
Fig. 4.
Grapefruit juice (GFJ) reversed and prevented the prednisolone-induced femur bone ultrastructure changes. a–b The ultrastructure pattern of the control group. c–d Effects of GFJ alone on the ultrastructure pattern of the femur. e–f Effects of prednisolone alone on the ultrastructure pattern of the femur. g–h Effects of applying of GFJ treatment before osteoporosis induction on the ultrastructure pattern in rat femur. i–j Effects of applying of GFJ treatment after osteoporosis induction on the ultrastructure pattern in rat femur
In the osteoporotic groups, the cortical bones showed resorption of the collagen fibrils on the trabecular surface. Under high magnification, the collagen fibrils were loosely scattered and randomly distributed (Fig. 4e, f). Treatment with GFJ either before or after induction of osteoporosis resulted in reduced resorption of the collagen fibrils on the trabecular surface and they returned to their intact normal folded structure (Fig. 4g–j).
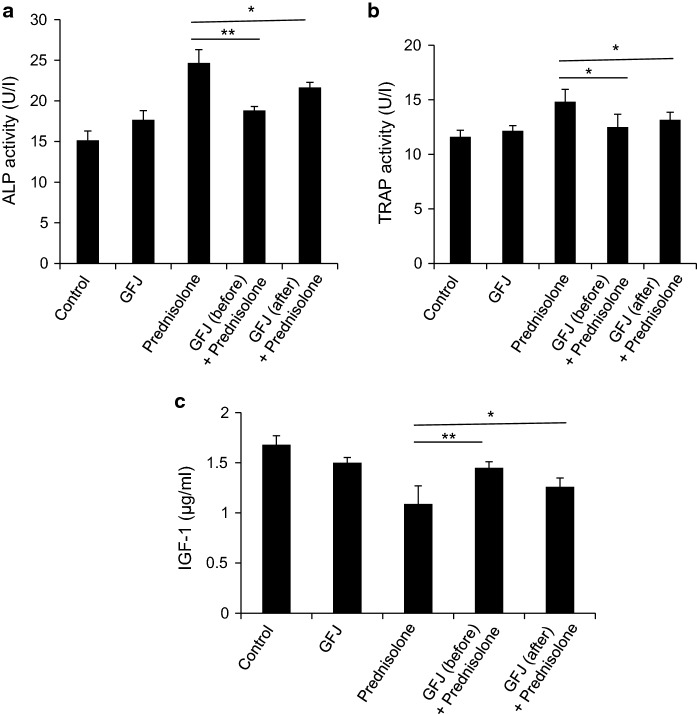
Biochemical variables
The plasma ALP and TRAP activities increased with the development of osteoporosis. Similarly, our results showed that ALP and TRAP activities increased in the prednisolone-treated group (Figs. 5a, 4b). On the other hand, administering GFJ before treating with prednisolone precluded the increase in the activities of these enzymes. Moreover, ALP and TRAP activities decreased in the osteoporotic rats treated with GFJ after the osteoporosis induction. Treatment with GFJ returned the value of TRAP to the normal value but failed to do so with ALP (Fig. 5a, b). In contrast, the plasma IGF-1 concentration decreased in the osteoporotic group (Fig. 5c). Administering GFJ to the osteoporotic rats before giving prednisolone precluded the decrease in the concentration of IGF-1. Additionally, applying this treatment after inducing osteoporosis ameliorated the decreased level of IGF-1 (Fig. 5c).
Fig. 5.
Grapefruit juice (GFJ) administration decreased ALP and TRAP activities and increased the IGF-1 level in osteoporotic rats. Applying GFJ treatment before or after osteoporosis induction significantly decreased the alkaline phosphatase (ALP) activity (a) and tartrate-resistant acid phosphatase (TRAP) activity (b) in osteoporotic rats. c. Applying GFJ treatment before or after the osteoporosis induction significantly increased IGF-1 level in osteoporotic rats. Data are expressed as the mean ± SD of three independent experiments. *p < 0.05 and **p < 0.005 indicate significant differences, compared to the prednisolone group as analyzed by a Student’s t test
Immunohistochemical study
Osteocalcin (OCC)
The osteoporotic group showed a decrease in OCC labeling compared with that of the control group (Fig. 6a, d). Administering GFJ before prednisolone treatment precluded the decline in OCC level. Additionally, applying this treatment after inducing osteoporosis ameliorated the depletion in OCC level. The GFJ group showed similar OCC activity to that of the control group (Fig. 6a, d).
Receptor activator of nuclear factor kappa-B ligand (RANKL) and osteoprotegerin (OPG)
Fig. 6.
Effect of grapefruit juice (GFJ) administration on the osteocalcin, receptor activator of nuclear factor kappa-B ligand (RANKL), and Osteoprotegerin (OPG) levels in the osteoporotic rats. a GFJ administration before or after osteoporosis induction prevented and ameliorated the depletion in osteocalcin level. b Applying GFJ treatment before or after the osteoporosis induction decreased the RANKL expression. c Applying GFJ treatment before or after the osteoporosis induction increased the OPG expression d Graphical representation of percentage of positive staining intensity of osteocalcin, RANKL, and osteoprotegerin (OPG). *p < 0.05 and **p <0.005 indicate significant differences, compared to the prednisolone group as analyzed by a Student’s t test
RANKL and OPG expression was observed via immunohistochemical techniques (Graham and Karnovsky 1966) as yellow–brown staining in the cytoplasm. RANKL labeling was significantly raised in the osteoporotic group compared with that of the control one (Fig. 6b, d). The GFJ group showed similar staining to that of the control group. Applying GFJ before prednisolone treatment precluded the increase in RANKL expression. Moreover, the RANKL expression of the osteoporotic group treated with GFJ after the induction of osteoporosis was lower than that of the osteoporotic group without GFJ treatment (Fig. 6b, d). A plunge in OPG labeling was noticed in the osteoporotic groups compared with that of the control, whereas the expression of OPG of the osteoporotic groups treated with GFJ before or after the induction of osteoporosis was higher than that of the osteoporotic group (Fig. 6c, d). The GFJ group showed similar OPG labeling to that of the control (Fig. 6c, d).
Discussion
Prednisolone is a glucocorticoid anti-inflammatory drug. Prolonged prednisolone consumption can result in osteoporosis development of which makes bones more brittle and susceptible to fractures (Hara et al. 1993; Gudbjornsson et al. 2002). The present study evaluated the potential protective and therapeutic anti-osteoporotic effects and mechanisms of action of GFJ in a prednisolone-induced osteoporosis rat femoral fracture model. BMD is used in the clinic as an indirect indicator of osteoporosis and fracture risk. Our data confirm earlier findings that prednisolone administration reduces BMD (Kasonde et al. 2014). Certainly, this study showed the bone-protective and therapeutic properties of GFJ as observed by increased femoral densities and ash percentage, and from delayed femoral fracture despite the numerical improvement in femoral strength in comparison with the prednisolone-treated group.
In terms of chemical composition, we found that flavonoids are the major phytochemicals in GFJ. Researchers showed naringin, a major flavonoid found in GFJ, enhances the proliferation of bone marrow stromal cells (BMSCs) and promotes their osteogenic differentiation (Yu et al. 2016). The enhanced bone mineral density and ash percentage could be attributed to the flavonoids content particularly naringin in GFJ. Moreover, glucocorticoids lower the absorption of intestinal calcium and increase the loss of urinary calcium, thereby decreasing femoral calcium content; these are in accordance with our results (Gennari 1993). Our finding that administering GFJ increased calcium concentration significantly in femur bone is in agreement with results of a previous study (Plotkin 2014). In addition, treatment with GFJ before osteoporosis induction restored Ca2+ values to a normal level. One possible explanation is the interaction between GFJ and calcium metabolism and/or execration that could be the main mechanism to increase the calcium content in the femur; although, more studies are needed to confirm our hypothesis. Moreover, the current study suggested that GFJ may have a favorable effect on Ca2+ absorption and its deposition in bone, which may explain the observed increase in BMD. Many studies have been shown the valuable anti-osteoporotic effects of some citrus fruits components; however these studies didn’t clarify the mechanistic pathways (Zhang et al. 2014; Cancalon 2016; Singh et al. 2018).
In parallel, we found that the histological results of the osteoporotic animals were in agreement with the results shown by many previous studies (Devogelaer 2006; Hatakeyama et al. 2012); a progressive increase in bone resorption and a plunge in bone formation were observed. However, after induction of osteoporosis, the trabeculae of the inner spongy bone lost their normal architecture and appeared as separated bony ossicles surrounding widened bone marrow spaces that were extensively filled with adipose tissue; the tissue exhibited a substantial decrease in cancellous bone. Moreover, Haney and Warden (2008) reported that the trabecular bones were very thin and the inter-trabecular spaces were wide. Most trabecular bones acquired rod-like shapes with less trabecular connectivity. These findings strongly suggest that there was a seriously advanced osteoporosis with trabecular disconnectivity caused by prednisolone administration. In accordance, the histological results of the rats in the osteoporotic-treated groups in the present study showed that GFJ partially healed erosion cavities in the outer cortical bone in which deep basophilic areas were observed around erosion cavities. It is possible that flavonoids content of GFJ may activate the BMP-2 promoter and increase bone formation, and the result is that the histological sections showed a healing process after the fracture as observed by many osteoblasts, newly formed bone, and increased trabecular bone and connectivity (Hatakeyama et al. 2012). Furthermore, the antiresorptive effect of GFJ was confirmed through ultrastructural studies. When osteoporotic groups were treated with GFJ either before or after prednisolone administration, the results revealed almost normal structure of the rat femur. This suggestion evidenced by the bone lamellae of the femur was mostly composed of collagen fibers arranged in parallel with a normalized structure of the Haversian systems. It seems that the histological and ultrastructural results of the GFJ-treated groups correlated well with enhanced breaking strength of femur bones subjected to a constant burden.
On the other hand, ALP and TRAP are key enzymes for bone calcification and provide an index of cell differentiation for bone formation (Elabd et al. 2008; Hrvačić et al. 2015). It is well known that prednisolone increases serum levels of these markers. In the present study, prednisolone also resulted in high levels of bone remodeling as seen by a surge in the markers of bone turnover as ALP and TRAP; this effect was reduced by GFJ supplementation. In agreement with results of previous reports (Bonucci and Nanci 2001; Sontakke and Tare 2002), the present decrease in the activity of ALP and TRAP may be due to a disturbance in the function of osteoblasts and/or an incoordination between the activities of osteoclasts and osteoblasts, in which the preceding replaces the latter. The ability of GFJ to reduce these markers may be due to their flavonoid contents. Similarly, researchers mentioned that citrus flavonoids elicited improved osteoblast development by modulating ALP and TRAP activities (Chiba et al. 2003; Hu et al. 2008). Another critical bone marker, IGF-1, that corresponding to the bone mass and can act as foreteller for the osteoporosis (Amin et al. 2007; Ohlsson et al. 2009). In this study, we found that IGF-1 level decreased in osteoporotic rats in accordance with previous results (Agnusdei and Gentilella 2005), while IGF-1 levels were restored to normal upon GFJ administration either before or after osteoporosis induction as reported by Ohlsson et al. (2009) and Rizzoli et al. (2010). However, our statistical analyses showed that treatment with GFJ before the induction of osteoporosis increased IGF-1 values more than when GFJ was applied after osteoporosis induction. This can be explained as IGF-1 released during bone resorption leading to new bone formation (Xian et al. (2012). From a mechanistic point of view, as GFJ decreased ALP and TRAP and increased IGF-1, this may suggest the ability of GFJ to slow down the bone resorption process.
To further explore the mechanisms that could be implicated in GFJ-induced anti-osteoporotic effects, we tested the effect of GFJ on osteocalcin, RANKL, and OPG through immunohistochemistry. Osteocalcin is implicated in bone mineralization and calcium ion homeostasis (Kim et al. 2010). GFJ treatment increased the osteocalcin levels. This increase could be explained by the following possible mechanisms: decreased elimination of osteocalcin by the kidney, decreased osteocalcin degradation, increased production, osteocalcin displacement from hydroxyapatite by EHDP, or the stimulated production of the calciotropic hormones 1,25-dihydroxyvitamin D and PTH (Jagetia et al. 2003; Marie 2007). On the other side, the prime regulator of bone remodeling is the RANK/RANKL/OPG system. There was an increased OPG/RANKL ratio as revealed by the immunohistochemical findings in the treated groups. These observations suggest that when GFJ administration stimulates the osteoblasts, two types of signals are produced simultaneously, thereby activating the anabolic pathways in the pre-osteoblasts and osteoblasts, and an anti-catabolic pathway in the pre-osteoclasts and osteoclasts (Marie 2007). Abnormalities of the RANKL/RANK/OPG system have been implicated in the resorption of bone during osteoporosis. RANKL stimulates osteoclast precursors to differentiate by binding to its receptor, RANK (Tay et al. 2004). Therefore, it seems logical to postulate that GFJ reduced bone resorption by acting on the RANKL/OPG system.
Conclusion
In conclusion, grapefruit juice (GFJ) improved the bone quality and exerted anti-osteoporotic effect in prednisolone-induced osteoporosis as seen by increased bone density, total mineral content, and calcium content, and decreased plasma alkaline phosphatase (ALP) and tartrate-resistant acid phosphatase (TRAP) activities. GFJ possibly exerts its effect against prednisolone-induced osteoporosis in rats via the RANKL/OPG pathway. Furthermore, our results also showed that there was a great improvement in the bone parameters when GFJ was given both before and after osteoporosis induction. We conclude that GFJ may serve as a good protective agent for treating prednisolone-induced osteoporosis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
The authors declare that there are no studies conducted with human participants. Animals were treated in accordance with the guidelines of the Committee on Animal Experimentation of Beni-Seuif University, Egypt.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Eslam Muhammad Bastawy, Phone: +8170-7565-7087, Email: eslam-bastawy@hiroshima-u.ac.jp, Email: eslam_bastawy@sci.asu.edu.eg.
Amer Ali Abd El-Hafeez, Phone: +1-216-269-4145, Email: amer.ali@nci.cu.edu.eg, Email: aam002@ucsd.edu.
References
- Agnusdei D, Gentilella R. GH and IGF-I as therapeutic agents for osteoporosis. J Endocrinol Investig. 2005;28:32–36. [PubMed] [Google Scholar]
- Amin S, Riggs BL, Melton LJ, Achenbach SJ, Atkinson EJ, Khosla S. High serum IGFBP-2 is predictive of increased bone turnover in aging men and women. J Bone Miner Res. 2007;22:799–807. doi: 10.1359/jbmr.070306. [DOI] [PubMed] [Google Scholar]
- Bonucci E, Nanci A. Alkaline phosphatase and tartrate-resistant acid phosphatase in osteoblasts of normal and pathologic bone. Italian Journal of Anatomy and Embryology Archivio italiano di anatomia ed embriologia. 2001;106:129–133. [PubMed] [Google Scholar]
- Cancalon PF (2016) Citrus juices health benefits. In: Beverage impacts on health and nutrition (pp 115–127). Humana Press, Cham
- Cheng X, Filiaggi M, Roscoe SG. Electrochemically assisted co-precipitation of protein with calcium phosphate coatings on titanium alloy. Biomaterials. 2004;25:5395–5403. doi: 10.1016/j.biomaterials.2003.12.045. [DOI] [PubMed] [Google Scholar]
- Chiba H, et al. Hesperidin, a citrus flavonoid, inhibits bone loss and decreases serum and hepatic lipids in ovariectomized mice. J Nutr. 2003;133:1892–1897. doi: 10.1093/jn/133.6.1892. [DOI] [PubMed] [Google Scholar]
- Davies DR, Wyatt KM, Jardine JE, Robertson ID, Irwin PJ. Vinblastine and prednisolone as adjunctive therapy for canine cutaneous mast cell tumors. J Am Anim Hosp Assoc. 2004;40:124–130. doi: 10.5326/0400124. [DOI] [PubMed] [Google Scholar]
- Devogelaer J-P. Glucocorticoid-induced osteoporosis: mechanisms and therapeutic approach. Rheum Dis Clin. 2006;32:733–757. doi: 10.1016/j.rdc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Deyhim F, Mandadi K, Faraji B, Patil BS. Grapefruit juice modulates bone quality in rats. J Med Food. 2008;11:99–104. doi: 10.1089/jmf.2007.537. [DOI] [PubMed] [Google Scholar]
- Elabd C, et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells. 2008;26:2399–2407. doi: 10.1634/stemcells.2008-0127. [DOI] [PubMed] [Google Scholar]
- Feldman SR. The biology and clinical application of systemic glucocorticoids. Curr Probl Dermatol. 1992;4:211–235. doi: 10.1016/1040-0486(92)90007-5. [DOI] [Google Scholar]
- Gennari C. Differential effect of glucocorticoids on calcium absorption and bone mass. Rheumatology. 1993;32:11–14. doi: 10.1093/rheumatology/32.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- Graham RC, Karnovsky MJ. The early stages of absorption of injected horseradish peroxidase in the peroximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Gudbjornsson B, Juliusson U, Gudjonsson F. Prevalence of long term steroid treatment and the frequency of decision making to prevent steroid induced osteoporosis in daily clinical practice. Ann Rheum Dis. 2002;61:32–36. doi: 10.1136/ard.61.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney EM, Warden S. Skeletal effects of serotonin (5-hydroxytryptamine) transporter inhibition: evidence from clinical studies. J Musculoskelet Neuronal Interact. 2008;8:133–145. [PubMed] [Google Scholar]
- Hara K, Akiyama Y, Ohkawa I, Tajima T. Effects of menatetrenone on prednisolone-induced bone loss in rats. Bone. 1993;14:813–818. doi: 10.1016/8756-3282(93)90309-X. [DOI] [PubMed] [Google Scholar]
- Hardcastle A, Aucott L, Fraser W, Reid D, Macdonald H. Dietary patterns, bone resorption and bone mineral density in early post-menopausal Scottish women. Eur J Clin Nutr. 2011;65:378. doi: 10.1038/ejcn.2010.264. [DOI] [PubMed] [Google Scholar]
- Hardcastle AC, Aucott L, Reid DM, Macdonald HM. Associations between dietary flavonoid intakes and bone health in a Scottish population. J Bone Miner Res. 2011;26:941–947. doi: 10.1002/jbmr.285. [DOI] [PubMed] [Google Scholar]
- Hatakeyama Y, et al. Vertebral histomorphometry in a child with glucocorticoid-induced osteoporosis. Tohoku J Exp Med. 2012;227:263–267. doi: 10.1620/tjem.227.263. [DOI] [PubMed] [Google Scholar]
- Hilton MJ, et al. Notch signaling maintains bone marrow mesenchymal progenitors by suppressing osteoblast differentiation. Nat Med. 2008;14:306. doi: 10.1038/nm1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrvačić B, et al. Relative potencies of three glucocorticoids to induce hypoplasia of the physis and concomitant biochemical alterations in the rat. Drug Chem Toxicol. 2015;38:272–277. doi: 10.3109/01480545.2014.947502. [DOI] [PubMed] [Google Scholar]
- Hu J-P, Nishishita K, Sakai E, Yoshida H, Kato Y, Tsukuba T, Okamoto K. Berberine inhibits RANKL-induced osteoclast formation and survival through suppressing the NF-κB and Akt pathways. Eur J Pharmacol. 2008;580:70–79. doi: 10.1016/j.ejphar.2007.11.013. [DOI] [PubMed] [Google Scholar]
- Humanson G. Basic procedure—animal tissue technique Part I. San Francisco: WH Freeman and Company; 1961. pp. 130–132. [Google Scholar]
- Jagetia GC, Venkatesha V, Reddy TK. Naringin, a citrus flavonone, protects against radiation-induced chromosome damage in mouse bone marrow. Mutagenesis. 2003;18:337–343. doi: 10.1093/mutage/geg001. [DOI] [PubMed] [Google Scholar]
- Jia M, Nie Y, Cao DP, Xue YY, Wang JS, Zhao L, Rahman K, Zhang QY, Qin LP. Potential antiosteoporotic agents from plants: a comprehensive review. Evid Based Complement Altern Med. 2012 doi: 10.1155/2012/364604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalu DN. The ovariectomized rat model of postmenopausal bone loss. Bone Miner. 1991;15:175–191. doi: 10.1016/0169-6009(91)90124-I. [DOI] [PubMed] [Google Scholar]
- Kasonde M, et al. Bone mineral density changes among HIV-uninfected young adults in a randomised trial of pre-exposure prophylaxis with tenofovir-emtricitabine or placebo in Botswana. PLoS ONE. 2014;9:e90111. doi: 10.1371/journal.pone.0090111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiebzak GM, Smith R, Howe JC, Gundberg CM, Sacktor B. Bone status of senescent female rats: chemical, morphometric, and biomechanical analyses. J Bone Miner Res. 1988;3:439–446. doi: 10.1002/jbmr.5650030411. [DOI] [PubMed] [Google Scholar]
- Kim Y-S, Paik I-Y, Rhie Y-J, Suh S-H. Integrative physiology: defined novel metabolic roles of osteocalcin. J Korean Med Sci. 2010;25:985–991. doi: 10.3346/jkms.2010.25.7.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lean JM, et al. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Investig. 2003;112:915–923. doi: 10.1172/JCI200318859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontowicz M, Jesion I, Leontowicz H, Park YS, Namiesnik J, Jastrzebski Z, Katrich E, Tashma Z, Gorinstein S. Bioactivity and bioavailability of minerals in rats loaded with cholesterol and kiwi fruit. Microchem J. 2014;114:148–154. doi: 10.1016/j.microc.2013.12.015. [DOI] [Google Scholar]
- Ma X, Lv J, Sun X, Ma J, Xing G, Wang Y, Sun L, Wang J, Li F, Li Y, Zhao Z. Naringin ameliorates bone loss induced by sciatic neurectomy and increases Semaphorin 3A expression in denervated bone. Sci Rep. 2016;6:24562. doi: 10.1038/srep24562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Marie PJ. Strontium ranelate: new insights into its dual mode of action. Bone. 2007;40:S5–S8. doi: 10.1016/j.bone.2007.02.003. [DOI] [Google Scholar]
- McNaughton SA, Wattanapenpaiboon N, Wark JD, Nowson CA. An energy-dense, nutrient-poor dietary pattern is inversely associated with bone health in women. J Nutr. 2011;141:1516–1523. doi: 10.3945/jn.111.138271. [DOI] [PubMed] [Google Scholar]
- Nielsen IL, Haren GR, Magnussen EL, Dragsted LO, Rasmussen SE. Quantifcation of anthocyanins in commercial black currant juices by simple high-performance liquid chromatography. Investigation of their pH stability and antioxidative potency. J Agric Food Chem. 2003;51:5861–5866. doi: 10.1021/jf034004+. [DOI] [PubMed] [Google Scholar]
- Ohlsson C, et al. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494–535. doi: 10.1210/er.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin LI. Apoptotic osteocytes and the control of targeted bone resorption. Curr Osteoporos Rep. 2014;12:121–126. doi: 10.1007/s11914-014-0194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riso P, et al. Effects of blood orange juice intake on antioxidant bioavailability and on different markers related to oxidative stress. J Agric Food Chem. 2005;53:941–947. doi: 10.1021/jf0485234. [DOI] [PubMed] [Google Scholar]
- Rizzoli R, Bianchi ML, Garabédian M, McKay HA, Moreno LA. Maximizing bone mineral mass gain during growth for the prevention of fractures in the adolescents and the elderly. Bone. 2010;46:294–305. doi: 10.1016/j.bone.2009.10.005. [DOI] [PubMed] [Google Scholar]
- Shim M, Karnuah A, Mitchell A, Anthony N, Pesti G, Aggrey S. The effects of growth rate on leg morphology and tibia breaking strength, mineral density, mineral content, and bone ash in broilers. Poult Sci. 2012;91:1790–1795. doi: 10.3382/ps.2011-01968. [DOI] [PubMed] [Google Scholar]
- Singh Z, Sharma S, Kaur A. Antitoxic effects of naringin: a flavonoid with diverse biological activities. World J Pharm Res. 2018;7:484–489. [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Sontakke A, Tare RS. A duality in the roles of reactive oxygen species with respect to bone metabolism. Clin Chim Acta. 2002;318:145–148. doi: 10.1016/S0009-8981(01)00766-5. [DOI] [PubMed] [Google Scholar]
- Tay J, Bay B, Yeo J, Harris M, Meghji S, Dheen S. Identification of RANKL in osteolytic lesions of the facial skeleton. J Dent Res. 2004;83:349–353. doi: 10.1177/154405910408300415. [DOI] [PubMed] [Google Scholar]
- Ton FN, Gunawardene SC, Lee H, Neer RM. Effects of low-dose prednisone on bone metabolism. J Bone Miner Res. 2005;20:464–470. doi: 10.1359/JBMR.041125. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Makuuchi H, Amemiya T, Yamaguchi H, Oka S, Owada T, Koizumi K. Intra-abdominal desmoid tumors in familial polyposis coli: a case report of tumor regression by prednisolone therapy. Dis Colon Rectum. 1991;34:89–93. doi: 10.1007/BF02050216. [DOI] [PubMed] [Google Scholar]
- Wattel A, Kamel S, Prouillet C, Petit JP, Lorget F, Offord E, Brazier M. Flavonoid quercetin decreases osteoclastic differentiation induced by RANKL via a mechanism involving NFκB and AP-1. J Cell Biochem. 2004;92:285–295. doi: 10.1002/jcb.20071. [DOI] [PubMed] [Google Scholar]
- Welch AA, Hardcastle AC. The effects of flavonoids on bone. Curr Osteoporos Rep. 2014;12:205–210. doi: 10.1007/s11914-014-0212-5. [DOI] [PubMed] [Google Scholar]
- Xian L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wang L, Walzem RL, Miller EG, Pike LM, Patil BS. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. 2005;53:2009–2014. doi: 10.1021/jf0484632. [DOI] [PubMed] [Google Scholar]
- Yu GY, Zheng GZ, Chang B, Hu QX, Lin FX, Liu DZ, Wu CC, Du SX, Li XD. Naringin stimulates osteogenic differentiation of rat bone marrow stromal cells via activation of the notch signaling pathway. Stem Cells Int. 2016 doi: 10.1155/2016/7130653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zheng L, Zhao Z, Shi J, Wang X, Huang J. Grape seed proanthocyanidins inhibit H2O2-induced osteoblastic MC3T3-E1 cell apoptosis via ameliorating H2O2-induced mitochondrial dysfunction. J Toxicol Sci. 2014;39:803–813. doi: 10.2131/jts.39.803. [DOI] [PubMed] [Google Scholar]