Abstract
Since metabolic process differs between humans and mice, studies were performed in hamsters, which are generally considered to be a more appropriate animal model for studies of obesity-related metabolic disorders. The modulation of gut microbiota, bile acids and the farnesoid X receptor (FXR) axis is correlated with obesity-induced insulin resistance and hepatic steatosis in mice. However, the interactions among the gut microbiota, bile acids and FXR in metabolic disorders remained largely unexplored in hamsters. In the current study, hamsters fed a 60% high-fat diet (HFD) were administered vehicle or an antibiotic cocktail by gavage twice a week for four weeks. Antibiotic treatment alleviated HFD-induced glucose intolerance, hepatic steatosis and inflammation accompanied with decreased hepatic lipogenesis and elevated thermogenesis in subcutaneous white adipose tissue (sWAT). In the livers of antibiotic-treated hamsters, cytochrome P450 family 7 subfamily B member 1 (CYP7B1) in the alternative bile acid synthesis pathway was upregulated, contributing to a more hydrophilic bile acid profile with increased tauro-β-muricholic acid (TβMCA). The intestinal FXR signaling was suppressed but remained unchanged in the liver. This study is of potential translational significance in determining the role of gut microbiota-mediated bile acid metabolism in modulating diet-induced glucose intolerance and hepatic steatosis in the hamster.
Abbreviations: ALT, alanine amino-transferase; ApoB, apolipoprotein B; AST, aspartate transaminase; AUC, area under curve; BAs, bile acids; BSH, bile acid hydrolase; CA, cholic acid; CAPE, caffeic acid phenethyl ester; CDCA, chenodeoxycholic acid; CETP, cholesterol ester transfer protein; CYP7A1, cytochrome P450 family 7 subfamily A member 1; CYP7B1, cytochrome P450 family 7 subfamily B member 1; CYP8B1, cytochrome P450 family 8 subfamily B member 1; CYP27A1, cytochrome P450 family 27 subfamily A member 1; DCA, deoxycholic acid; eWAT, epididymal white adipose tissue; FGF15/19, fibroblast growth factor 15/19; FXR, farnesoid X receptor; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GTT, glucose tolerance test; HFD, high fat diet; H&E, hematoxylin and eosin; ITT, insulin tolerance test; LCA, lithocholic acid; NASH, non-alcoholic steatohepatitis; NAFLD, non-alcoholic fatty liver disease; PBA/SBA, primary bile acids to secondary bile acids; sWAT, subcutaneous white adipose tissue; TC, total cholesterol; TCA, taurocholic acid; T2D, type 2 diabetes; TG, triglycerides; TβMCA, tauro-β-muricholic acid; UDCA, ursodeoxycholic acid; UPLC–MS/MS, ultra performance liquid chromatography–tandem mass spectrometry; VLDL, very low-density lipoprotein
KEY WORDS: Gut microbiota, CYP7B1, TβMCA, FXR, Metabolic disorders
Graphical abstract
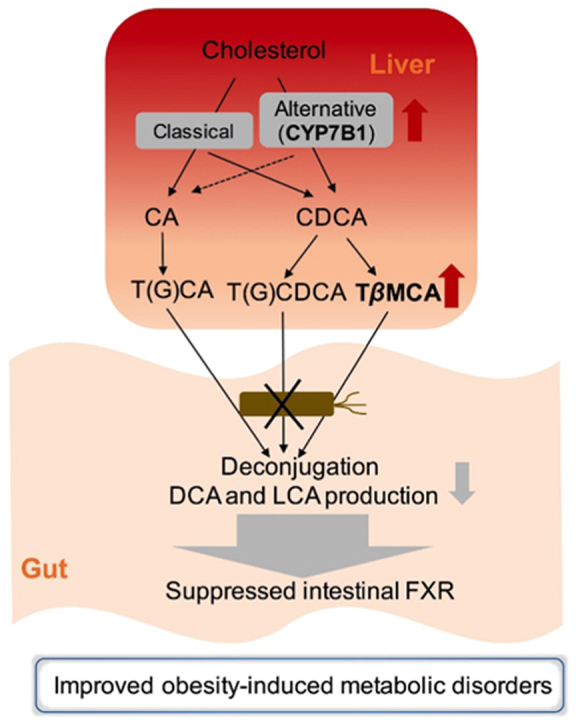
In the antibiotic-treated hamsters, hepatic CYP7B1-mediated alternative bile acid synthesis was activated. In hamsters, intestinal TβMCA accumulated and secondary bile acid levels were downregulated after gut microbiota depletion. Gut microbiota depletion-derived bile acid modulation results in intestinal FXR suppression and improvements of metabolic disorders in HFD-fed hamsters.
1. Introduction
The human gut is a complex ecosystem consisting of 10–100 trillion bacteria. Accumulating evidences suggest that the gut microbiota is associated with metabolic diseases, including obesity1, insulin resistance2., 3. and non-alcoholic fatty liver disease (NAFLD)4. The drugs for type 2 diabetes (T2D) also produce therapeutic effects by modulating microbial homeostasis5., 6., 7., 8.. The gut microbiota plays an essential role in the progress of metabolic diseases and is thus a potential drug target.
Dietary nutrients are the main drivers for remodeling the gut microbiota in the daily life of the host. In a cohort study, dietary fiber-induced improvement in glucose metabolism was due to the increased abundance of Prevotella9. Moreover, the content and diversity of gut microbiota in obese patients are lower than those in lean subjects10. Microbiota-derived metabolites, such as bile acids, short chain fatty acids and endotoxin, influence host metabolism by mediating the interaction between the gut and other organs11. Recent studies identified a link between the imbalance of intestinal flora and the pathological changes in the metabolism of bile acids in patients suffering from obesity and type 2 diabetes5., 12., 13.. In the liver, cholesterol is converted to primary bile acids via two pathways, with cytochrome P450 family 7 subfamily A member 1 (CYP7A1) as the rate-limiting enzyme in the classic pathway and cytochrome P450 family 7 subfamily B member 1 (CYP7B1) as an important enzyme in the alternative pathway14. It was reported that CYP7B1-mediated activation of the alternative pathway exerted metabolic benefits by shaping the gut microbiota and upregulating cold-induced thermogenesis and energy expenditure15. Primary bile acids synthesized in the liver are conjugated to taurine and glycine for secretion into the gut. In the gut, the conjugated primary bile acids are deconjugated by bile salt hydrolase (BSH) in gut bacteria flora and 7α/β-dehydroxylases, converting cholic acid (CA) and chenodeoxycholic acid (CDCA) to secondary bile acids, deoxycholic acid (DCA) and lithocholic acid (LCA), respectively16.
It is well established that bile acids bind to farnesoid X receptor (FXR), the nuclear receptor that is involved in the control of bile acid homeostasis and the enterohepatic circulation of bile acids and is known to influence multiple metabolic diseases16., 17.. Activation of intestinal FXR induces fibroblast growth factor 15 (FGF15) to activate hepatic FGF receptor 4/β-Klotho signaling to inhibit bile acid synthesis. Notably, recent studies in mice demonstrated that tauro-β-muricholic acid (T-βMCA) antagonizes intestinal FXR, inhibiting the FXR-FGF15 axis to influence hepatic bile acid and cholesterol metabolism18., 19., 20., and that inhibition or ablation of intestinal FXR prevented obesity-related metabolic dysfunction in high-fat diet (HFD)-fed and genetically obese mice21., 22., 23.. However, there are many differences in the bile acid composition between humans and mice, because humans only produce CA and CDCA in the liver whereas mice also produce ursodeoxycholic acid (UDCA) which therefore should be considered as primary bile acid in mice. Intestinal FXR signaling induces FGF15 in mice but FGF19 in humans, with a different regulation of CYP7A1 and cytochrome P450 family 8 subfamily B member 1 (CYP8B1)21, thus revealing the limitation of translational applications of studies performed in mouse models. Compared with mice, the hamster is considered to be a more appropriate model for studies of metabolic diseases24. Notably, lipid metabolism in hamsters is more similar to that in humans, including the production of HFD-induced high plasma triglycerides, the levels of high cholesterol ester transfer protein (CETP) and very low-density lipoprotein (VLDL), and the specific apolipoprotein B (ApoB) expression in the intestine25., 26.. The HFD-fed golden Syrian hamster model has been used as an animal model of insulin resistance, hepatic steatosis and dyslipidemia, as hamsters have similar lipid profiles to human and are predisposed to HFD-induced hyperlipidemia27., 28., 29.. Collectively, the hamster is a more ideal animal model to study the pathogenesis and pharmacological interventions of human diet-related metabolic diseases. In the current study, the metabolic phenotypes, bile acid metabolism and FXR signaling were examined after the ablation of the gut microbiota in hamsters.
2. Material and methods
2.1. Animals
Golden Syrian hamsters were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. Six- to eight-week-old male hamsters were housed in a standard SPF environment (12 h daylight cycle, lights off at 18:00). The hamsters were divided into two groups and given 0.5% CMC-Na (Sigma, St. Louis, MO, USA) or an antibiotic cocktail suspended in 0.5% CMC-Na by gavage twice a week under a 60% high-fat diet (D12492, Research Diet, New Brunswick, NJ, USA) treatment for 4 weeks. The combined antibiotics were consisted of vancomycin (50 mg/kg, Sigma), metronidazole (100 mg/kg, Sigma), kanamycin (100 mg/kg, Sigma) and ampicillin (100 mg/kg, Sigma). The antibacterial spectrum of each antibiotic is as follows (data from Antimicrobe.org):
Vancomycin: coagulase negative Staphylococci, Streptococcus spp., Streptococcus pneumoniae, Staphylococcus aureus (vancomycin susceptible), Enterococcus spp. (vancomycin-susceptible), Clostridium spp., Corynebacterium jeikeium, Listeria monocytogenes, and Actinomyces.
Metronidazole: anaerobic Gram-negative bacilli including Bacteroides species, Prevotella spp.; Fusobacterium spp., Porphyromonas spp.; anaerobic Gram-positive bacilli including Clostridium spp.; Protozoa including Blastocystis hominis, Entamoeba histolytica, Giardia lamblia, and Trichomonas vaginalis; Anaerobic cocci including Peptostreptococcus species, Veillonella species.
Kanamycin: Proteus species (both indole-positive and indole-negative), E. coli, Enterobacter aerogenes, Serratia marcescens, Klebsiella pneumoniae, and Acinetobacter species.
Ampicillin: Gram-positive including Enterococcus spp., Streptococcus spp., and Listeria monocytogenes; Gram-negative including Haemophilus influenzae, Salmonella spp., E. coli, Proteus mirabilis, and Shigella spp.
Body weights and food intakes were recorded once a week. Glucose tolerance tests and insulin tolerance tests were conducted during 4th week. The hamsters were sacrificed after 4-h fasting at the end of the 4th week. All animal experiment protocols were approved by the Animal Care and Use Committee of Peking University (Beijing, China).
2.2. Metabolic assays
For the glucose tolerance test, the hamsters were fasted for 16 h overnight. The blood glucose at different time points (0, 15, 30, 60, 90 and 120 min) was measured with an Accu-Check glucometer (Bayer, Leverkusen, Germany) before and after glucose (2.5 g/kg) challenge. The insulin tolerance test was carried out after fasting for 6 h. Blood glucose was measured before (0 min) and after (15, 30, 60 and 90 min) intraperitoneal injections of insulin (1.5 UI/kg). Blood glucose levels during glucose and insulin tolerance tests were detected using blood from the tails. Blood samples were collected after fasting for 4 h to measure fasting blood glucose and insulin (Insulin ELISA kit, Alpco, Salem, OR, USA) levels. HOMA-IR was calculated according to Eq. (1) to assess glucose homeostasis in hamsters.
| (1) |
2.3. Histological analysis
Liver tissues were fixed in 4% paraformaldehyde for 4–6 h, and paraffin sections were cut and stained by hematoxylin and eosin (H&E). Tissues embedded in OCT were cut to obtain liver frozen sections, which were stained by oil red O. A minimum of three discontinuous sections were evaluated for each sample, with more than three samples per group.
2.4. Lipid measurements
Total triglycerides (TG) and cholesterol (TC) in the liver and plasma were measured (TGKIT and TCKIT, BioSINO, Beijing, China) to evaluate hepatic steatosis and dyslipidemia. The degree of liver injury was estimated based on plasma alanine amino-transferase (ALT) and aspartate transaminase (AST) (ALT/GPTKIT and AST/GOTKIT, BioSINO, Beijing, China).
2.5. Real-time PCR analysis
The ileal epithelium and liver tissues were frozen in liquid nitrogen and stored at –80 °C The standard chloroform—isopropanol extraction was applied to isolate total RNA from lytic tissues in Trizol reagent. cDNA was synthesized from 2 μg total RNA with a Reverse Transcription Kit (ABM, Richmond, Canada). The relative expression of genes was normalized to 18 S rRNA. All of the primer sequences used in the real-time PCR are listed in Supporting Information Table S1.
2.6. Sample collection
The ileal epithelium was scraped from the lower 1/3 of the small intestine after washing with PBS. Fresh fecal samples were collected from the cecum.
2.7. Bile acid analysis
Ten milligrams each of feces from the cecum, liver and ileal epithelium tissues was dissolved in 100 µL double-distilled water and pulverized. The protein was precipitated with 300 µL methanol containing 0.1% ammonium hydroxide. The supernatants were collected for further analysis, and chlorpropamide (Sigma) was added as an internal standard to quantify bile acid levels. The bile acid concentrations in the supernatants were measured by a UPLC/Synapt G2-Si QTQF MS system (Water Corp, Milford, MA, USA) with an ESI source. An Acquity BEH C18 column (100 mm × 2.1 mm i.d., 1.7 μm, Waters Crop, Milford, MA, USA) was used for chromatographic separation. The column temperature was 45 °C, and the flow rate was 0.4 mL/min. The mobile phase consisted of a mixture of 0.1% formic acid in water and 0.1% formic acid in acetonitrile. A gradient elution was conducted, and MS detection proceeded in the negative mode. A mass range of m/z 50 to 850 was acquired.
2.8. Data analysis
GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis. The sample sizes were determined by power analysis using StatMate version 2.0 (GraphPad Software, San Diego, CA, USA). Experimental data were shown as the mean±SEM. The investigators involved in this study were not completely blinded in the animal experiments during sample collection and analysis. No data were excluded from the data analysis. A two-tailed unpaired Student׳s t-test was used for all comparisons of the hamster-related experiments. P values of less than 0.05 were considered significant.
3. Results
3.1. Antibiotic treatment alleviated hepatic steatosis, inflammation and glucose intolerance in HFD-fed hamsters
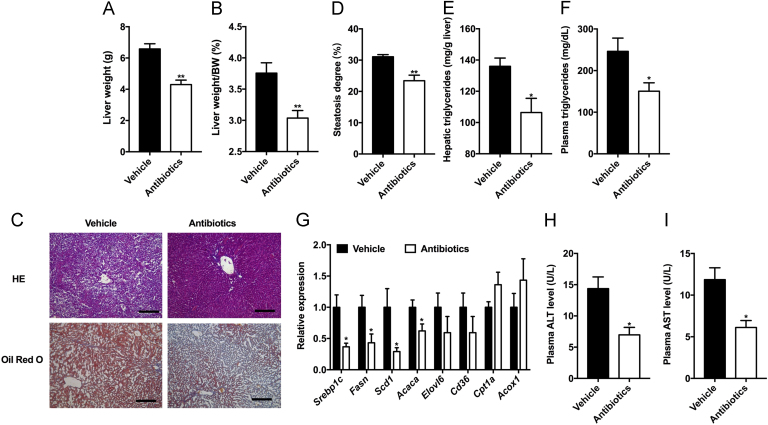
To explore the role of gut microbiota in influencing obesity-related diseases, hamsters under HFD treatment were treated with vehicle or antibiotic cocktail by gavage for four weeks. In antibiotic-treated hamsters, the liver weights and the liver weight to body weight ratios were both reduced (Fig. 1A and B). H&E and oil red O staining revealed a reduced accumulation of lipids in the liver of antibiotic-treated hamsters compared with the vehicle (Fig. 1C and D). Moreover, antibiotic treatment significantly decreased hepatic and plasma triglyceride levels (Fig. 1E and F). Consistent with that, qPCR analysis indicated that lipid synthesis in the liver was downregulated in antibiotic-treated hamsters, while lipid uptake and oxidation had no changes (Fig. 1G). The reduced levels of plasma alanine aminotransferase (ALT) and aspartate transaminase (AST) further demonstrated the improvement in the liver injury after antibiotic treatment (Fig. 1H and I). Above results suggested that antibiotic treatment had beneficial effects in reducing hepatic lipid accumulation and liver injury under 60% HFD treatment in hamsters.
Figure 1.
Ablation of gut microbiota protects from obesity-induced hepatic steatosis and liver injury in hamsters. The hamsters were fed a 60% high-fat diet and given vehicle or antibiotics for 4 weeks (n=5 hamsters/group). (A) The liver weight. (B) The ratio of liver weight to body weight. (C) Representative H&E (upper panel) and oil red O (lower panel) staining of liver sections of the two groups. Scale bars: 200 μm (3 images/hamster). (D) Steatosis degree (the ratio of vacuolar area to whole area in one image, 3 images/hamster). (E) Hepatic triglycerides. (F) Plasma triglycerides. (G) The relative expression of genes involved in fatty acid synthesis, uptake and oxidation in the liver. (H) Plasma ALT levels. (I) Plasma AST levels. Data are presented as the mean±SEM. *P<0.05, **P<0.01 versus vehicle by two-tailed Student׳s t-test.
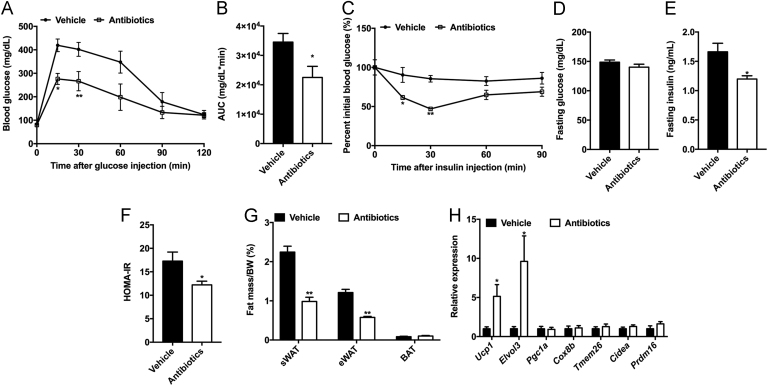
The glucose tolerance test (GTT) and insulin tolerance test (ITT) revealed that glucose intolerance and insulin resistance were substantially improved after antibiotic treatment (Fig. 2A–C). Antibiotic-treated hamsters displayed lower fasting serum insulin level and HOMA-IR than the vehicle (Fig. 2D–F). Antibiotic-treated hamsters were resistant to HFD-induced body weight gain (Supporting Information Fig. S1A and B). The fat mass and fat weight/body weight ratios of subcutaneous white adipose tissue (sWAT) and epididymal white adipose tissue (eWAT) were markedly decreased after antibiotic treatment, while there were no differences in brown adipose tissue (Fig. 2G and Fig. S1C). It was reported that cold-induced microbial alteration was beneficial for beiging in the sWAT, resulting in an ameliorative metabolic phenotype in mice30. Indeed, the mRNA levels of thermogenic genes, such as Ucp1 and Elovl3, were significantly elevated in the sWAT of antibiotic-treated hamsters (Fig. 2H). Food intake remained unchanged between vehicle- and antibiotic-treated hamsters (Fig. S1D). The above results highlighted the effects of gut microbiota on the progress of HFD-induced metabolic disorders in hamsters.
Figure 2.
Ablation of gut microbiota improves obesity-induced insulin resistance in hamsters. The hamsters were fed a 60% high-fat diet and given vehicle or antibiotics for 4 weeks (n=5 hamsters/group). On the fourth week, glucose tolerance tests (GTT) and insulin tolerance tests (ITT) were performed. (A) GTT; (B) Area under the curve (AUC); (C) ITT. (D) Fasting glucose levels; (E) Fasting insulin levels; (F) HOMA-IR; (G) The ratios of fat mass to body weight in subcutaneous adipose tissue (sWAT), epididymal adipose tissue (eWAT) and brown adipose tissue (BAT); (H) The relative expression of thermogenic gene mRNAs in the sWAT. Data are presented as the mean±SEM, n=5 hamsters/group. *P<0.05, **P<0.01 versus vehicle by two-tailed Student׳s t-test.
3.2. Modulation of bile acid synthesis in the liver by antibiotics
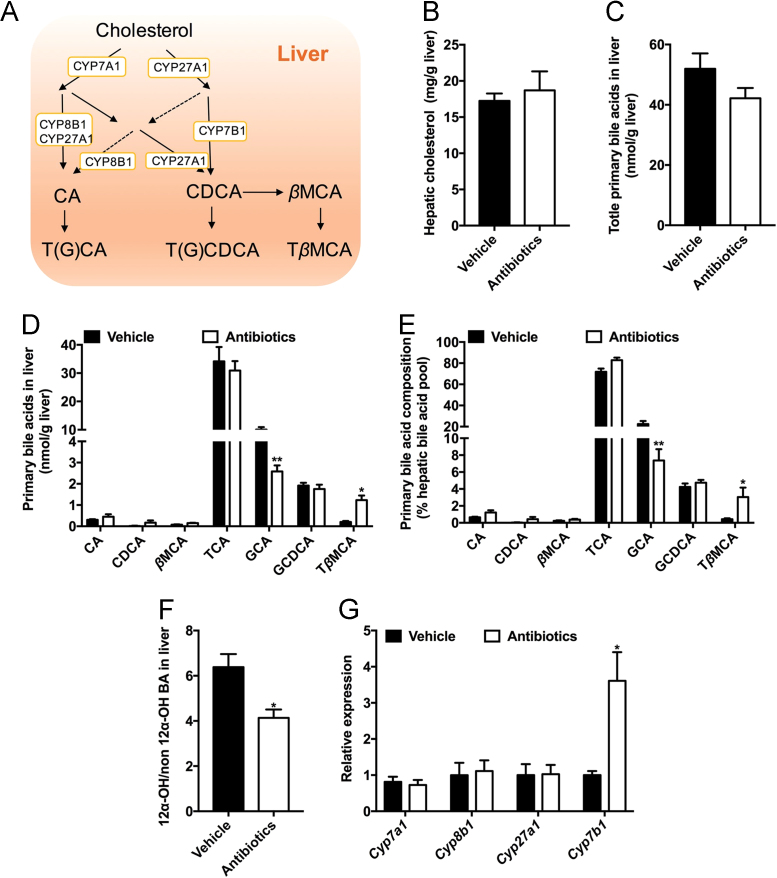
Bile acids are mainly synthesized in the liver via both classical and alternative pathways. The classical pathway is initiated by the rate-limiting enzyme CYP7A1, which accounts for approximately 75% of bile acid production under physiological conditions. CYP7B1 is mainly involved in the alternative pathway31. Moreover, the formation of bile acids involves multiple enzymes, including cytochrome P450 family 27 subfamily A member 1 (CYP27A1) and CYP8B1 (Fig. 3A). Recent studies revealed that the expression of CYP7A1, CYP27A1 and CYP7B1 was influenced by the gut microbiota in mice20. However, it remains unknown whether bile acid synthesis is under microbial regulation in hamsters. There was no significant difference in hepatic content of cholesterol and total primary bile acids in the liver between the two groups (Fig. 3B and C). Antibiotic treatment significantly decreased glycocholic acid (GCA) levels and significantly increased TβMCA levels in liver (Fig. 3D and E). Alterations in the bile acid composition were associated with metabolic disorders in the clinic. It was reported that the ratio of 12α-OH to non-12α-OH bile acids (12α-OH to non-12α-OH BAs) was positively associated with insulin resistance and hyperlipidemia32 and that the ratio of primary bile acids to secondary bile acids (PBA/SBA) declined in non-alcoholic steatohepatitis (NASH) patients33. In antibiotic-treated hamsters, the PBA/SBA ratio was markedly elevated, while there was a decrease in the ratio of 12α-OH to non-12α-OH BAs (Fig. 3F and Supporting Information Fig. S2A). The conjugation of bile acids was not different between the two groups (Fig. S2B). Furthermore, Cyp7b1 mRNA was upregulated, while there were no significant changes in the relative expression of Cyp7a1, Cyp27a1 and Cyp8b1 (Fig. 3G). According to the hydrophobicity indices of bile acids, DCA, LCA, CA and their conjugates are hydrophobic, while β-muricholic acid (βMCA) and TβMCA are hydrophilic34. In hamsters, gut microbiota depletion led to a more hydrophilic bile acid profile, with an elevation of TβMCA in the liver.
Figure 3.
Alternative bile acid synthesis in the liver is elevated after antibiotic treatment. The hamsters were fed a 60% high-fat diet and given vehicle or antibiotics for 4 weeks (n=5 hamsters/group). (A) Bile acid synthesis in the liver; (B) Hepatic cholesterol; (C) Total primary bile acids in the liver; (D) Primary bile acid profiles in the liver; (E) Primary bile acid composition in the liver; (F) The ratio of 12α-OH bile acids to non-12α-OH bile acids in the liver [12α-OH/non12α-OH BA=(GCA+CA+TCA+GDCA+DCA+TDCA)/(LCA+HDCA+CDCA+UDCA+βMCA+GCDCA+GUDCA+TLCA+TUDCA+THDCA+TβMCA)]; (G) The relative expression of genes involved in bile acid synthesis. Data are presented as the mean±SEM, n=5 hamsters/group. *P<0.05, **P<0.01 versus vehicle by two-tailed Student׳s t-test.
3.3. Antibiotic treatment remodeled the bile acid profiles in the gut
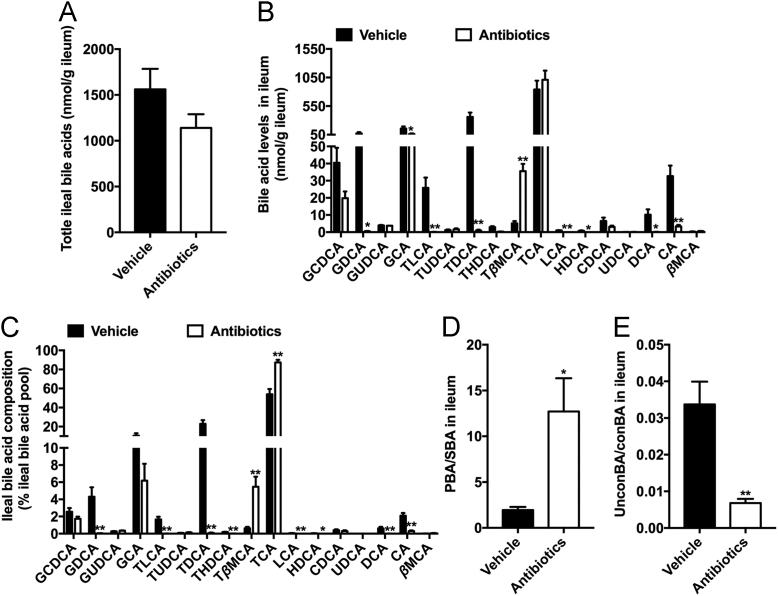
Multiple gut microbiota-derived enzymes are involved in bile acid modification, including deconjugation, transport and 7α/β-dehydroxylation5. CA, CDCA and βMCA are produced from conjugated bile acids via BSH and are further converted to secondary bile acids after 7α/β-dehydroxylation (Supporting Information Fig. S3A). The total ileal bile acid levels did not change after antibiotic treatment (Fig. 4A). Further metabolomics analysis showed that the levels of ileal and fecal secondary bile acids in the antibiotic-treated group were much lower than those in the vehicle-treated group, which was likely due to the absence of 7α/β-dehydroxylase activity (Fig. 4B, C and Fig. S3C). Consistently, the ratio of PBA/SBA was significantly upregulated by antibiotic treatment (Fig. 4D and Fig. S3D). The reduction of total fecal bile acids was probably due to the deficient transformation from primary unconjugated bile acids to secondary bile acids (Fig. S3B). In the ileum, there was a reduced ratio of unconBA/conBA, with a remarkable accumulation of TβMCA (Fig. 4B, C and E), which was previously identified as an intestinal FXR antagonist in mice20. The conjugated bile acids, such as glycochenodeoxycholic acid (GCDCA), GCA and taurocholic acid (TCA), were more abundant in the feces of antibiotic-treated hamsters but there was no increase in the ileum, indicating the excretion of GCDCA, GCA and TCA was elevated after antibiotic treatment (Fig. S3C). The elevation in ileal TβMCA levels might have resulted from the increased CYP7B1-mediated synthesis and the decreased microbial deconjugation.
Figure 4.
Microbial modulation of bile acid metabolism in the gut. (A) Total ileal bile acids; (B) Bile acid levels in the ileum; (C) Bile acid composition in the ileum; (D) The ratio of primary bile acids to secondary bile acids in the ileum; (E) The ratio of unconjugated bile acids to conjugated bile acids in the ileum. Data are presented as the mean±SEM, n=5 hamsters/group. *P<0.05, **P<0.01 versus vehicle by two-tailed Student׳s t-test.
3.4. Antibiotic treatment suppressed intestinal FXR signaling
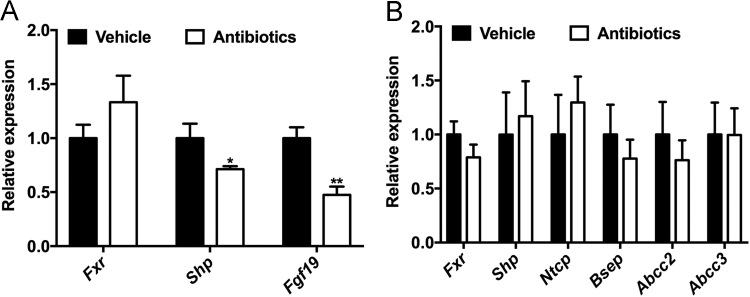
Inhibition of intestinal FXR exerted a protective role in insulin resistance and hepatic steatosis22., 35.. TβMCA, the antagonist of intestinal FXR in mice, had been found to be markedly increased in the ileum of antibiotic-treated hamsters (Fig. 3D). In antibiotic-treated hamsters, FXR signaling was selectively suppressed in the gut but not in the liver (Fig. 5A and B). These results suggested that intestinal FXR inhibition was the potential target of gut microbiota-derived bile acid signaling in the metabolic diseases.
Figure 5.
Intestinal FXR is suppressed in the absence of gut microbiota. (A) The relative abundance of intestinal FXR and its target genes; (B) The mRNA levels of genes in the hepatic FXR signaling. Data are presented as the mean±SEM, n=5 hamsters/group. *P<0.05, **P<0.01 versus vehicle by two-tailed Student׳s t-test.
4. Discussion
Obesity, type 2 diabetes and non-alcohol fatty liver disease are aspects of metabolic syndrome, which is a major public health problem worldwide36., 37., 38., 39., 40.. Thermogenesis is considered a therapeutic target for obesity41. The progress of metabolic syndrome results from interactions between the gut microbiota and the host42. Some of these phenotypes could be transferred to germ-free mice by fecal transplantation from mice or patients43., 44.. It was also found that the therapeutic effects in clinical treatment were determined by the composition of the gut microbiota in patients5., 45., 46.. Thus, a more complete understanding of how the gut microbiota influences host metabolism and the development of novel therapeutic approaches are important research priorities. Previous studies demonstrated that mice on an HFD that were treated with antibiotics or tempol displayed reduced hepatic triglyceride accumulation and increased beiging of white adipose tissue. In the present study, another animal model, the golden Syrian hamster, was used to more accurately reflect metabolic dysfunction found in humans. There was an obvious hepatic lipid accumulation after only four weeks of HFD treatment. To evaluate the role of the gut microbiota in the progress of HFD-induced metabolic disorders, the HFD-treated hamsters were given vehicle or antibiotics by gavage twice a week for four weeks. After antibiotic treatment, the body weights and the percent of fat mass showed a significant reduction, accompanied by elevated expression of genes involved in thermogenesis in the sWAT. Furthermore, insulin sensitivity and hepatic steatosis were both ameliorated in the absence of the gut microbiota for hamsters on an HFD as a result of the inhibition of lipid synthesis in the liver.
Bile acids are metabolized in the gut, and the bile acid compositions vary widely among mice and humans. It is interesting to note that glycine-conjugated bile acids are much higher in hamsters than mice, which is more like humans. To further determine the microbial metabolism of bile acids in hamsters, a metabolomics analysis of liver, ileum and feces samples was performed. Due to the upregulated excretion in the feces, there was no significant change in the levels of hepatic and ileal GCDCA. In mice, but not in humans, CDCA is converted into α,βMCA through the activity of a sterol-6β-hydroxylase, cytochrome P450, family 2, subfamily c, polypeptide 70 (CYP2C70), which is expressed in the mouse liver47. In hamsters, antibiotic treatment markedly upregulated CYP7B1 expression, leading to a more hydrophilic bile acid composition with an increase in TβMCA and a reduction in GCA in the liver. CA feeding was shown to elevate fat absorption and aggravate glucose intolerance48. A recent study showed that whole-body Cyp7a1-null (Cyp7a1–/–) mice displayed an improvement of glucose tolerance and insulin sensitivity when fed an HFD49. In Cyp7a1–/– mice, the TβMCA content in bile was observed to increase as a result of CYP7B1 upregulation. TβMCA is the most efficacious endogenous FXR antagonist, and inhibition of intestinal FXR signaling was found to improve hepatic steatosis and insulin resistance8., 22.. According to reported clinical data, hepatic CYP7B1 expression was down-regulated, while CYP7A1 and CYP8B1 expression remained similar in the obese individuals with type 2 diabetes (T2D) compared to health controls15. Moreover, cold-induced upregulation of CYP7B1 promoted thermogenesis and energy expenditure by increasing brown and beige adipocytes15. Above findings suggested that hepatic CYP7B1 potentially plays a vital role in obesity-related T2D in humans. In germ-free mice, hepatic CYP7A1 enzyme activity was mainly up-regulate20. Gut microbiota-induced bile acid synthesis enzymes remodeling in liver differed between mice and humans. Consistent with humans, antibiotic-treated hamsters displayed a higher relative expression level of hepatic Cyp7b1, but Cyp7a1, Cyp8b1 and Cyp27a1 relative mRNA levels remain unchanged. Moreover, hamsters displayed more similarities in bile acids metabolism and composition with human. Collectively, hamsters are more proper animals used for confirming whether modulation of gut microbiota-CYP7B1-bile acids axis is a potential therapeutic strategy for clinical T2D treatment.
In the gut, microbiota-derived enzymes mediated the complex biotransformations of bile acids produced in the liver, including deconjugation, 7-dehydroxylation and oxidation of hydroxyl groups50. Bile acid deconjugation is carried out by BSH, expressed in Lactobacilli, Bifidobacteria, Clostridium and Bacteroides51., 52., 53.. Both the preferential reduction of the abundance of Lactobacilli and its BSH by the antioxidant tempol and the direct suppression of BSH activity by caffeic acid phenethyl ester (CAPE) resulted in the accumulation of TβMCA in the ileum to ameliorate obesity and insulin resistance35. Consistently, the levels of TβMCA in the ileum and feces were significantly elevated due to the absence of microbial BSH activity after antibiotic treatment in hamsters. Due to the markedly increased excretion in the feces of conjugated bile acids (Fig. S3C), such as TCA, GCA and GCDCA, the levels of these bile acids were not increased or even slightly decreased in the ileum (Fig. 4B). Collectively, the accumulation of TβMCA was due to both the upregulation of the alternative bile acid synthesis pathway and the inhibition of deconjugation. Secondary bile acids are generated by 7-dehydroxylation with microbial bile acid-inducible (bai) gene products. The antibiotic-treated hamsters had an overall reduction in secondary bile acids in the enterohepatic cycle, resulting from the lack of multiple 7-dehydroxylation steps.
FXR is a ligand-activated nuclear receptor that regulates bile acid homeostasis and is primarily expressed in the liver and gut54. FXR can be bound by a variety of endogenous bile acids with various affinities, with the potency of FXR activation in reporter gene assays estimated at CDCA>LCA = DCA55., 56.. The activated FXR signaling resulted in downregulated bile acid synthesis via the inhibition of the expression of CYP7A157. In contrast, the murine conjugated bile acid, TβMCA, was proven to be an endogenous FXR antagonist20., 21.. It was verified that inhibition and knockout of intestinal FXR prevented obesity-related metabolic diseases in mice21., 22., 23.. In hamsters treated with antibiotics, intestinal FXR signaling (in humans and hamsters) was inhibited, associated with the increased TβMCA and the reduced DCA and LCA. However, there was no obvious difference in hepatic FXR signaling between vehicle- and antibiotic-treated hamsters. Thus, this revealed that modulation of bile acids by ablation of the gut microbiota mainly influenced intestinal FXR signaling in hamsters.
5. Conclusions
The present results highlighted that microbial modulation of bile acids was pivotal to regulating obesity-induced metabolic disorders in hamsters. It was suggested that removal of the gut microbiota mainly elevated alternative bile acid synthesis in the liver and suppressed the microbial deconjugation and de-hydroxylation in the gut, leading to increased TβMCA and decreased secondary bile acids in the ileum. Remodeling of bile acid profiles due to antibiotic treatment had beneficial effects in improving glucose intolerance and hepatic steatosis. Furthermore, we found that intestinal FXR, but not hepatic FXR, was suppressed after antibiotic treatment in hamsters. These data support the view that intestinal FXR inhibition is a potential target for the clinical treatment of obesity-induced metabolic diseases.
Acknowledgments
We thank John J. Chiang for editing the manuscript. This work was supported by the National Key Research and Development Program of China (grant No. SQ2018YFC100236); the National Natural Science Foundation of China (grant Nos. 91857115, 81522007, 81470554, 31401011, and 81700010); the Fundamental Research Funds for the Central Universities: Clinical Medicine Plus X - Young Scholars Project of Peking University (grant No. PKU2018LCXQ013, China); and Beijing Nova Program (grant No. Z161100004916056, China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2019.02.004.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 2.Anhe F.F., Roy D., Pilon G., Dudonne S., Matamoros S., Varin T.V. A polyphenol-rich cranberry extract protects from diet-induced obesity, insulin resistance and intestinal inflammation in association with increased Akkermansia spp. population in the gut microbiota of mice. Gut. 2015;64:872–883. doi: 10.1136/gutjnl-2014-307142. [DOI] [PubMed] [Google Scholar]
- 3.Forslund K., Hildebrand F., Nielsen T., Falony G., Le Chatelier E., Sunagawa S. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leung C., Rivera L., Furness J.B., Angus P.W. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol. 2016;13:412–425. doi: 10.1038/nrgastro.2016.85. [DOI] [PubMed] [Google Scholar]
- 5.Gu Y., Wang X., Li J., Zhang Y., Zhong H., Liu R. Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat Commun. 2017;8:1785. doi: 10.1038/s41467-017-01682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu H., Esteve E., Tremaroli V., Khan M.T., Caesar R., Manneras-Holm L. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat Med. 2017;23:850–858. doi: 10.1038/nm.4345. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X., Zhao Y., Xu J., Xue Z., Zhang M., Pang X. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L., Xie C., Wang G., Wu Y., Wu Q., Wang X. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med. 2018;24:1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kovatcheva-Datchary P., Nilsson A., Akrami R., Lee Y.S., De Vadder F., Arora T. Dietary fiber-induced improvement in glucose metabolism is associated with increased abundance of Prevotella. Cell Metab. 2015;22:971–982. doi: 10.1016/j.cmet.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 10.Le Chatelier E., Nielsen T., Qin J., Prifti E., Hildebrand F., Falony G. Richness of human gut microbiome correlates with metabolic markers. Nature. 2013;500:541–546. doi: 10.1038/nature12506. [DOI] [PubMed] [Google Scholar]
- 11.Schroeder B.O., Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–1089. doi: 10.1038/nm.4185. [DOI] [PubMed] [Google Scholar]
- 12.Albaugh V.L., Flynn C.R., Cai S., Xiao Y., Tamboli R.A., Abumrad N.N. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab. 2015;100:E1225–E1233. doi: 10.1210/jc.2015-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kars M., Yang L., Gregor M.F., Mohammed B.S., Pietka T.A., Finck B.N. Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59:1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu Y., Liu H., Zhang M., Guo G.L. Fatty liver diseases, bile acids, and FXR. Acta Pharm Sin B. 2016;6:409–412. doi: 10.1016/j.apsb.2016.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worthmann A., John C., Ruhlemann M.C., Baguhl M., Heinsen F.A., Schaltenberg N. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med. 2017;23:839–849. doi: 10.1038/nm.4357. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara T., Li F., Gonzalez F.J. FXR signaling in the enterohepatic system. Mol Cell Endocrinol. 2013;368:17–29. doi: 10.1016/j.mce.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng L., Piekos S., Guo G.L., Zhong X.B. Role of farnesoid X receptor in establishment of ontogeny of phase-I drug metabolizing enzyme genes in mouse liver. Acta Pharm Sin B. 2016;6:453–459. doi: 10.1016/j.apsb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Degirolamo C., Rainaldi S., Bovenga F., Murzilli S., Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the FXR-FGF15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 19.Wahlstrom A., Sayin S.I., Marschall H.U., Backhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-β-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez F.J., Jiang C., Patterson A.D. An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology. 2016;151:845–859. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Investig. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang C., Xie C., Lv Y., Li J., Krausz K.W., Shi J. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gardes C., Chaput E., Staempfli A., Blum D., Richter H., Benson G.M. Differential regulation of bile acid and cholesterol metabolism by the farnesoid X receptor in Ldlr–/– mice versus hamsters. J Lipid Res. 2013;54:1283–1299. doi: 10.1194/jlr.M033423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haidari M., Leung N., Mahbub F., Uffelman K.D., Kohen-Avramoglu R., Lewis G.F. Fasting and postprandial overproduction of intestinally derived lipoproteins in an animal model of insulin resistance. Evidence that chronic fructose feeding in the hamster is accompanied by enhanced intestinal de novo lipogenesis and ApoB48-containing lipoprotein overproduction. J Biol Chem. 2002;277:31646–31655. doi: 10.1074/jbc.M200544200. [DOI] [PubMed] [Google Scholar]
- 26.Gao S., He L., Ding Y., Liu G. Mechanisms underlying different responses of plasma triglyceride to high-fat diets in hamsters and mice: roles of hepatic MTP and triglyceride secretion. Biochem Biophys Res Commun. 2010;398:619–626. doi: 10.1016/j.bbrc.2010.05.114. [DOI] [PubMed] [Google Scholar]
- 27.Briand F., Thieblemont Q., Muzotte E., Sulpice T. High-fat and fructose intake induces insulin resistance, dyslipidemia, and liver steatosis and alters in vivo macrophage-to-feces reverse cholesterol transport in hamsters. J Nutr. 2012;142:704–709. doi: 10.3945/jn.111.153197. [DOI] [PubMed] [Google Scholar]
- 28.Basciano H., Miller A.E., Naples M., Baker C., Kohen R., Xu E. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am J Physiol Endocrinol Metab. 2009;297:E462–E473. doi: 10.1152/ajpendo.90764.2008. [DOI] [PubMed] [Google Scholar]
- 29.Dong B., Kan C.F., Singh A.B., Liu J. High-fructose diet downregulates long-chain acyl-CoA synthetase 3 expression in liver of hamsters via impairing LXR/RXR signaling pathway. J Lipid Res. 2013;54:1241–1254. doi: 10.1194/jlr.M032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chevalier C., Stojanovic O., Colin D.J., Suarez-Zamorano N., Tarallo V., Veyrat-Durebex C. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163:1360–1374. doi: 10.1016/j.cell.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Thomas C., Pellicciari R., Pruzanski M., Auwerx J., Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 32.Haeusler R.A., Astiarraga B., Camastra S., Accili D., Ferrannini E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes. 2013;62:4184–4191. doi: 10.2337/db13-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiao N., Baker S.S., Chapa-Rodriguez A., Liu W., Nugent C.A., Tsompana M. Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut. 2018;67:1881–1891. doi: 10.1136/gutjnl-2017-314307. [DOI] [PubMed] [Google Scholar]
- 34.Heuman D.M. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 35.Xie C., Jiang C., Shi J., Gao X., Sun D., Sun L. An intestinal farnesoid X receptor—ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes. 2017;66:613–626. doi: 10.2337/db16-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 37.Jin W., Cui B., Li P., Hua F., Lv X., Zhou J. 1,25-Dihydroxyvitamin D3 protects obese rats from metabolic syndrome via promoting regulatory T cell-mediated resolution of inflammation. Acta Pharm Sin B. 2018;8:178–187. doi: 10.1016/j.apsb.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu Q., Liu S., Gao L., Sun S., Huan Y., Li C. Anti-diabetic effects and mechanisms of action of a Chinese herbal medicine preparation JQ-R in vitro and in diabetic KKAy mice. Acta Pharm Sin B. 2017;7:461–469. doi: 10.1016/j.apsb.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang Y., Zhang X., Chen Z., Yin W., Nan G., Tian J. Novel benzamido derivatives as PTP1B inhibitors with anti-hyperglycemic and lipid-lowering efficacy. Acta Pharm Sin B. 2018;8:919–932. doi: 10.1016/j.apsb.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang S., Wu C., Li X., Zhou Y., Zhang Q., Ma F. Syringaresinol-4-O-β-d-glucoside alters lipid and glucose metabolism in HepG2 cells and C2C12 myotubes. Acta Pharm Sin B. 2017;7:453–460. doi: 10.1016/j.apsb.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J., Wang Y., Lin L. Small molecules for fat combustion: targeting obesity. Acta Pharm Sin B. 2018 doi: 10.1016/j.apsb.2018.09.007. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonnenburg J.L., Backhed F. Diet-microbiota interactions as moderators of human metabolism. Nature. 2016;535:56–64. doi: 10.1038/nature18846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khoruts A., Sadowsky M.J. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13:508–516. doi: 10.1038/nrgastro.2016.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khoruts A. Faecal microbiota transplantation in 2013: developing human gut microbiota as a class of therapeutics. Nat Rev Gastroenterol Hepatol. 2014;11:79–80. doi: 10.1038/nrgastro.2013.231. [DOI] [PubMed] [Google Scholar]
- 45.Hegazy A.N., West N.R., Stubbington M.J.T., Wendt E., Suijker K.I.M., Datsi A. Circulating and tissue-resident CD4+ T cells with reactivity to intestinal microbiota are abundant in healthy individuals and function is altered during inflammation. Gastroenterology. 2017;153:1320–1337. doi: 10.1053/j.gastro.2017.07.047. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu A.M., Ingelman-Sundberg M., Cherrington N.J., Aleksunes L.M., Zanger U.M., Xie W. Regulation of drug metabolism and toxicity by multiple factors of genetics, epigenetics, lncRNAs, gut microbiota, and diseases: a meeting report of the 21st International Symposium on Microsomes and Drug Oxidations (MDO) Acta Pharm Sin B. 2017;7:241–248. doi: 10.1016/j.apsb.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi S., Fukami T., Masuo Y., Brocker C.N., Xie C., Krausz K.W. CYP2C70 is responsible for the species difference in bile acid metabolism between mice and humans. J Lipid Res. 2016;57:2130–2137. doi: 10.1194/jlr.M071183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang D.Q., Tazuma S., Cohen D.E., Carey M.C. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285:G494–G502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 49.Ferrell J.M., Boehme S., Li F., Chiang J.Y. Cholesterol 7α-hydroxylase-deficient mice are protected from high-fat/high-cholesterol diet-induced metabolic disorders. J Lipid Res. 2016;57:1144–1154. doi: 10.1194/jlr.M064709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ussar S., Griffin N.W., Bezy O., Fujisaka S., Vienberg S., Softic S. Interactions between gut microbiota, host genetics and diet modulate the predisposition to obesity and metabolic syndrome. Cell Metab. 2015;22:516–530. doi: 10.1016/j.cmet.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 52.Jones B.V., Begley M., Hill C., Gahan C.G., Marchesi J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci U S A. 2008;105:13580–13585. doi: 10.1073/pnas.0804437105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyce S.A., MacSharry J., Casey P.G., Kinsella M., Murphy E.F., Shanahan F. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A. 2014;111:7421–7426. doi: 10.1073/pnas.1323599111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 55.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 56.Parks D.J., Blanchard S.G., Bledsoe R.K., Chandra G., Consler T.G., Kliewer S.A. Bile acids: natural ligands for an orphan nuclear receptor. Science. 1999;284:1365–1368. doi: 10.1126/science.284.5418.1365. [DOI] [PubMed] [Google Scholar]
- 57.Gonzalez F.J., Jiang C., Bisson W.H., Patterson A.D. Inhibition of farnesoid X receptor signaling shows beneficial effects in human obesity. J Hepatol. 2015;62:1234–1236. doi: 10.1016/j.jhep.2015.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material