Abstract
Aberrant activation of NLRP3 inflammasome has been implicated in the pathogenesis of diverse inflammation-related diseases, and pharmacological molecules targeting NLRP3 inflammasome are of considerable value to identifying potential therapeutic interventions. Cardamonin (CDN), the major active ingredient of the traditional Chinese medicinal herb Alpinia katsumadai, has exerted an excellent anti-inflammatory activity, but the mechanism underlying this role is not fully understood. Here, we show that CDN blocks canonical and noncanonical NLRP3 inflammasome activation triggered by multiple stimuli. Moreover, the suppression of CDN on inflammasome activation is specific to NLRP3, not to NLRC4 or AIM2 inflammasome. Besides, the inhibitory effect is not dependent on the expression of NF-κB-mediated inflammasome precursor proteins. We also demonstrate that CDN suppresses the NLRP3 inflammasome through blocking ASC oligomerization and speckle formation in a dose-dependent manner. Importantly, CDN improves the survival of mice suffering from lethal septic shock and attenuates IL-1β production induced by LPS in vivo, which is shown to be NLRP3 dependent. In conclusion, our results identify CDN as a broad-spectrum and specific inhibitor of NLRP3 inflammasome and a candidate therapeutic drug for treating NLRP3 inflammasome-driven diseases.
KEY WORDS: Cardamonin, NLRP3 inflammasome, IL-1β, Caspase-1, Septic shock
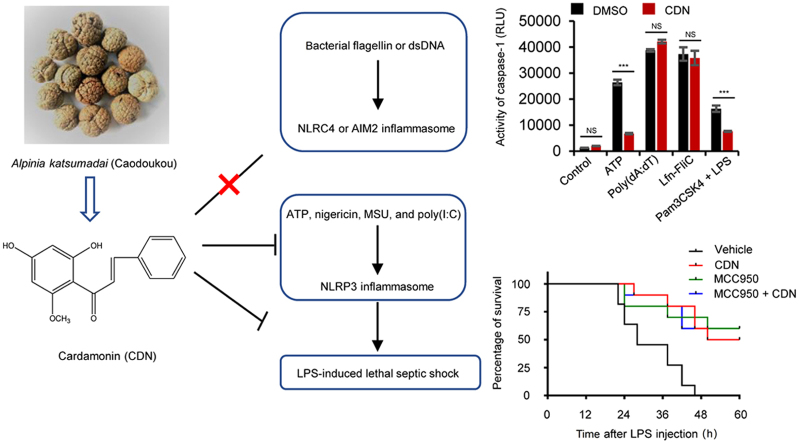
Graphical abstract
Cardamonin is a broad-spectrum inhibitor of NLRP3 inflammasome triggered by multiple stimuli. Moreover, the suppression of cardamonin on inflammasome activation is specific to NLRP3, not to NLRC4 or AIM2 inflammasome. Importantly, cardamonin improves the survival of mice suffering from LPS-induced lethal endotoxic shock, which is shown to be NLRP3 dependent.
1. Introduction
Inflammation is the body׳s defense responses to specific stimuli. A moderate inflammatory response contributes to alleviating infections, removing damaged cells, and initiating tissue recovery. Excessive inflammation, however, may exacerbate tissue damage or even induce serious diseases1., 2.. Recent studies have proved the crucial role of nod-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome in inflammatory responses3. The NLRP3 inflammasome is a multiprotein complex consisting of NLRP3, ASC [apoptosis-associated speck-like protein containing CARD (caspase activation and recruitment domain)], and cysteinyl aspartate-specific proteinase-1 (caspase-1)4. NLRP3 inflammasome activation requires double activation signal: priming and activation5. Pathogen-associated molecular patterns (PAMPs) are molecules associated with groups of pathogens, and most of which are recognized by Toll-like receptors (TLRs). Lipopolysaccharide (LPS) is the prototypical class of PAMPs, and the recognition of it by TLR4 will result in the activation of NF-κB and up-regulation of pro-IL-1β and NLRP3 protein expression, which is called the priming phase. During the activation step, the inflammasome complex is assembled and activated by various inducers, such as adenosine triphosphate (ATP), nigericin, monosodium urate crystals (MSU), and SiO2. Once activated, caspase-1 cleaves several protein substrates, including pro-IL-1β and pro-IL-18, to their mature forms, IL-1β and IL-18, respectively6.
The dysregulation of NLRP3 inflammasome activation leads to the occurrence and development of many inflammatory diseases7., 8.. Cryopyrin-associated periodic fever syndromes (CAPS), Muckle–Wells syndrome (MWS) and familial cold-induced autoinflammatory syndrome (FCAS) are associated with a predisposition for activation or genetic activation of the NLRP3 inflammasome9., 10.. In addition, NLRP3 inflammasome is involved in human complex diseases and chronic inflammation, including gout, type 2 diabetes (T2D), atherosclerosis, and nonalcoholic steatohepatitis (NASH)11., 12., 13., 14.. Therefore, targeting NLRP3 inflammasome is of great significance in the treatment of these diseases15. In recent years, a few active ingredients have been reported to have inhibitory effects on the NLRP3 inflammasome in animal models of human diseases. MCC950 treatment inhibits LPS-induced NLRP3 inflammasome activation and rescues NLRP3 dependent non-alcoholic fatty liver disease (NAFLD) pathology in obese diabetic mice13., 16.. Oridonin has both preventive and therapeutic effects on mouse models of NLRP3-mediated diseases like peritonitis, gouty arthritis, and T2D17. β-Hydroxybutyrate suppresses caspase-1 activation and IL-1β secretion in mouse models of MWS and FCAS18.
Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone, CDN) is a chalcone found mainly in the seeds of Alpinia katsumadai (Caodoukou in Chinese), a medicinal herb that has been widely used to treat digestive system-related diseases for thousands of years. CDN has shown considerable anti-inflammatory, anti-cancer, anti-oxidative, and vasorelaxant activities19., 20., 21., 22., 23.. Studies have been demonstrated that CDN exerts anti-inflammatory activity mediated by blocking NF-κB and MAPK signaling pathways24., 25., 26., 27.. The suppression of CDN on NLRP3 inflammasome and inflammatory colitis has been investigated in a recent preliminary research28. However, the broad-spectrum and specific inhibitory effect of CDN on NLRP3 inflammasome has not been investigated. In this study, we found that CDN could inhibit NLRP3 inflammasome activation specifically both in murine macrophages and human monocytes and prevent NLRP3-depedent septic shock in vivo.
2. Materials and methods
2.1. Mice
6–8-week-old female C57BL/6 mice were purchased from SPF Biotechnology Co., Ltd. (Beijing, China). All animals were maintained under 12-h light/dark conditions at 22–24 °C with unrestricted access to food and water for the duration of the experiment. All animal protocols in this study were performed according to the guidelines for care and use of laboratory animals and approved by the animal ethics committee of 302 Military Hospital (Beijing, China).
2.2. Reagents and antibodies
MSU, nigericin, ATP, poly(deoxyadenylic-thymidylic) acid sodium salt (poly (dA:dT)), polyinosinic:polycytidylic acid (poly (I:C)), phorbol-12-myristate-13-acetate (PMA), dimethyl sulfoxide (DMSO), and ultrapure LPS were purchased from Sigma–Aldrich (Munich, Germany). Pam3CSK4 was obtained from InvivoGen (Toulouse, France). MCC950 and CDN were from TargetMol (Boston, MA, USA). Lfn-Flic was kindly provided by Dr. Tao Li from National Center of Biomedical Analysis. Anti-mouse CASPASE-1 (1:1000, AG-20B-0042) was bought from Adipogen (San Diego, CA, USA). Anti-human cleaved IL-1β (1:2000, 12242), anti-human CASPASE-1 (1:2000, 4199 S), anti-mouse IL-1β (1:1000, 12507), anti-NLRP3 (1:2000, 15101S) were obtained from Cell Signaling Technology (Boston, MA, USA). Anti-ASC (1:1000, sc-22514-R) was from Santa Cruz Biotechnology (Dallas, TX, USA). Anti-GAPDH (1:2000, 60004-1-1g) was purchased from Proteintech (Chicago, IL, USA).
2.3. Cell culture
Bone-marrow-derived macrophages (BMDMs) were isolated from femoral bone marrow of 10-week-old female C57BL/6 mice and cultured in Dulbecco׳s modified Eagle׳s medium (DMEM) complemented with 10% fetal bovine serum (FBS), 1% penicillin/streptomycin (P/S) and 50 ng/mL murine macrophage colony-stimulating factor (M-CSF). Human THP-1 cells were grown in RPMI 1640 medium and stimulated by 100 nmol/L PMA overnight to differentiate into macrophages. Immortalized BMDMs (iBMDMs) were kindly provided by Dr. Tao Li from National Center of Biomedical Analysis (Beijing, China) and grown in DMEM containing 10% FBS, 1% P/S. Human peripheral blood mononuclear cells (PBMCs) were obtained from healthy donors and grown in RPMI 1640 medium and the experimental protocols were performed according to the approved guidelines established by the 302 Military Hospital (Beijing, China). All cell lines were cultured under a humidified 5% (v/v) CO2 atmosphere at 37 °C.
2.4. Inflammasome activation
We seeded BMDMs at 4×105 cells/well, THP-1 cells at 0.75×106 cells/well, PBMCs at 2.5×106 cells/well and iBMDMs at 2.5×105 cells/well in 24-well plates overnight. The following day, the medium was replaced, and cells were stimulated with 50 ng/mL LPS for 4 h. Then the medium was changed to Opti-MEM containing CDN for 1 h. For inducing NLRP3 inflammasome activation, cells were then stimulated with ATP (5 mmol/L), nigericin (7.5 μmol/L) for 1 h, or with MSU (200 μg/mL) for 6 h, poly(I:C) (2 μg/mL) transfected with Lipofectamine 2000 for 6 h according to the manufacturer׳s instructions. For absent in melanoma 2 (AIM2) inflammasome activation, poly (dA:dT) (2 μg/mL) was transfected using Lipofectamine 2000 for 6 h. For nod-like receptor containing a caspase activating and recruitment domain 4 (NLRC4) inflammasome activation, cells were stimulated with Lfn-Flic (200 ng/mL) for 6 h. For noncanonical inflammasome activation, BMDMs were primed with Pam3CSK4 (1 μg/mL) for 4 h, after which the medium was removed and replaced with Opti-MEM containing CDN for 1 h and 1 μg/mL LPS was transfected using Lipofectamine 2000 for 6 h.
2.5. Western blotting
Protein extraction method of cell culture supernatant has been described previously29. Immunoblot was used to test the expression of caspase-1 p20, IL-1β p17, pro-IL-1β, caspase-1 p45, NLRP3, and ASC. GAPDH served as a loading control in cell lysates. The samples were boiled at 105 °C for 15 min, then the protein samples were resolved in 12% or 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membrane via a wet-transfer system. Later, the membranes were incubated with 5% fat-free milk for 1 h at room temperature and incubated overnight with primary antibodies at 4 °C. Blots were washed three times with Tris-buffered saline Tween-20 (TBST) and incubated with corresponding horseradish peroxidase-conjugated secondary antibody (1:5000) for 1 h at room temperature, followed by another three times of washing, and the signals were analyzed using the enhanced chemiluminescent reagents (Promega, Beijing, China) detection system.
2.6. Cell viability assay
The cell counting kit-8 (CCK-8) assay was applied to detect the viability of cells. IBMDMs, PMA-primed THP-1 cells and BMDMs were seeded in 96-well growth-medium plate overnight at 1×105, 2×105 and 1×105 cells/well, respectively30., 31.. Then, iBMDMs and BMDMs were primed with LPS for 4 h. Next, LPS-primed iBMDMs, PMA-primed THP-1 cells and LPS-primed BMDMs were incubated at 37 °C followed by treatment with CDN for 24 h, then these cells were cultured with CCK-8 for 30 min. The optical density (O.D.) values at the wavelength of 450 nm were determined. The half-maximal inhibitory concentration (IC50) of CDN was performed using the software Prism 6 (GraphPad Software, San Diego, CA, US).
2.7. Caspase-1 activity assay
The Caspase-Glo® 1 Inflammasome Assay (Promega, Beijing, China), which is a homogeneous and bioluminescent method to measure the activity of caspase-1 selectively, was used to assess caspase-1 activity in cell culture supernatants according to the manufacturer׳s instructions.
2.8. Enzyme-linked immunosorbent assay (ELISA)
Supernatants from cell culture and mouse serum were assayed for mouse IL-1β, TNF-α, and human IL-1β according to manufacturer׳s instructions (Dakewei, Beijing, China).
2.9. Lactate dehydrogenase (LDH) assay
LPS-primed BMDMs were treated with inflammasome stimulants in the presence of CDN. The release of LDH into the culture supernatants was determined by LDH cytotoxicity assay kit (Beyotime, Shanghai, China) according to the manufacturer׳s instructions.
2.10. ASC oligomerization and speck staining detection
The assay for ASC oligomerization and speck staining detection has been described previously29.
2.11. LPS-induced septic shock in vivo
To induce septic shock, 6–8-week-old female C57BL/6 mice (n=10/group) were injected intraperitoneally (i.p.) with CDN (50 mg/kg), MCC950 (50 mg/kg) or MCC950 (50 mg/kg) plus CDN (50 mg/kg) 1 h before injection of LPS (20 mg/kg), and the mortality rate was monitored at regular intervals. In the second experiment, to induce inflammatory cytokines secretion, mice (n=6/group) were injected with LPS (20 mg/kg) after CDN or MCC950 (25 and 50 mg/kg) treatment 1 h. After 6 h, serum samples were collected, and the cytokines were measured by ELISA.
2.12. Statistical analyses
Statistical analysis was performed using the software Prism 6 (GraphPad Software, San Diego, CA, US). All experimental data were expressed as means±standard error of mean (SEM). The significant differences were statistically assessed using an unpaired Student׳s t-test and the difference was considered statistically significant when P<0.05.
3. Results
3.1. CDN suppresses caspase-1 activation and IL-1β secretion
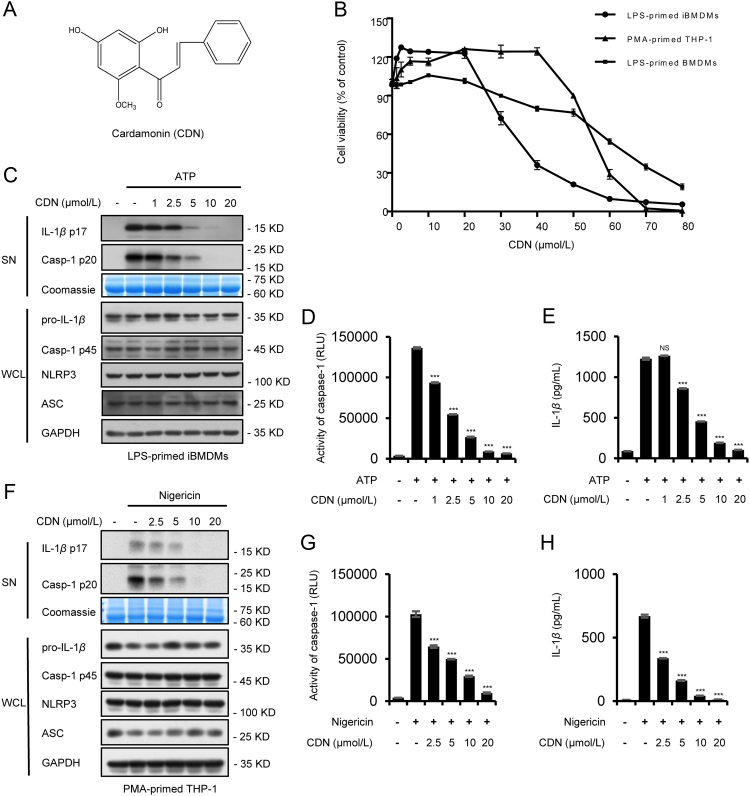
NLRP3 inflammasome activation leads to caspase-1 activation, which processes inactive cytoplasmic precursors (pro-IL-1β, pro-IL-18) into the mature active form (IL-1β, IL-18). Before investigating the role of CDN (Fig. 1A) on inflammasome, we first tested the toxicity of CDN by CCK-8 cytotoxicity assay in LPS-primed immortalized bone-marrow-derived macrophages (iBMDMs), PMA-primed THP-1 cells and LPS-primed bone-marrow-derived macrophages (BMDMs). After being exposed to CDN for 24 h, the half-maximal inhibitory concentration (IC50) of CDN were 32.16, 55.05, and 61.03 μmol/L, respectively. CDN promoted cell viability at low concentrations, and no toxic effect was observed at the concentration below 20 μmol/L (Fig. 1B). Therefore, 0–20 μmol/L was chosen to test the inhibitory effect of CDN on the caspase-1 cleavage and IL-1β secretion. IBMDMs were primed with LPS, then treated with CDN, and finally stimulated with ATP. Results showed that CDN treatment markedly reduced the caspase-1 cleavage and IL-1β maturation in a dose-dependent manner (Fig. 1C–E). NLRP3, ASC, pro-IL-1β, and caspase-1 (p45) expression in whole cell lysates were not affected by CDN (Fig. 1C). Similarly, PMA-primed THP-1 cells were pretreated with CDN and then stimulated with nigericin. Results also showed that caspase-1 cleavage and mature IL-1β secretion were dose-dependently inhibited by CDN (Fig. 1F–H). Taken together, these results demonstrate that CDN could inhibit caspase-1 activation and IL-1β secretion in both mouse and human macrophages.
Figure 1.
CDN suppresses caspase-1 activation and IL-1β secretion. (A) CDN structure. (B) Cytotoxicity of CDN in LPS-primed iBMDMs, PMA-primed THP-1 cells, and LPS-primed BMDMs. (C) LPS-primed iBMDMs were treated with various doses of CDN and then stimulated with ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in culture supernatants (SN) and pro- IL-1β, caspase-1 (p45), NLRP3, ASC in whole cell lysates (WCL). (D and E) Activity of caspase-1 (D), secretion of IL-1β (E) in SN from iBMDMs described in (C). (F) PMA-primed THP-1 cells were stimulated with nigericin after treated with CDN. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (G and H) Activity of caspase-1 (G), secretion of IL-1β (H) in SN from THP-1 cells described in (F). RLU, recombinant luciferase, which is proportional to caspase-1 activity. Coomassie blue staining was provided as the loading control for the SN (C and F). GAPDH served as a loading control in WCL (C) and (F). Data are expressed as mean±SEM (n=3) from three independent experiments with biological duplicates in (B, D, E, G, and H). Statistics differences were analyzed using an unpaired Student׳s t-test: ***P<0.001 vs. the control. NS, not significant.
3.2. CDN inhibits NLRP3 inflammasome activation in primary mouse bone-marrow-derived macrophages (BMDMs) and human peripheral blood mononuclear cells (PBMCs)
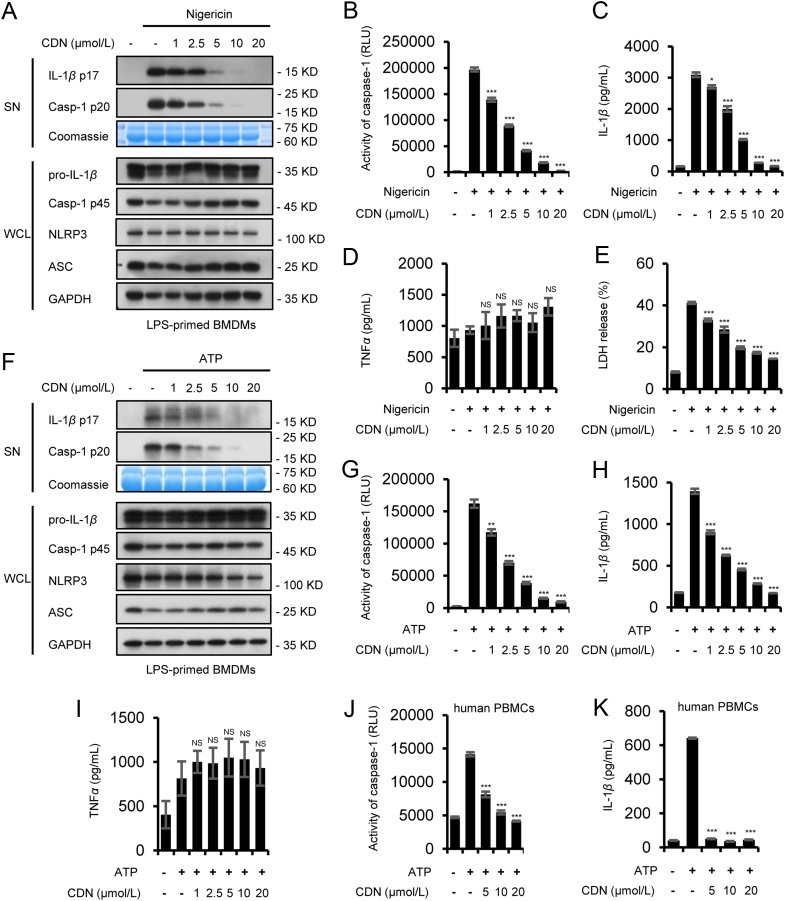
We used primary mouse bone-marrow-derived macrophages (BMDMs) from femoral bone marrow of 10-week-old female C57BL/6 mice to illuminate the inhibitory effect of CDN on NLRP3 inflammasome. We pretreated LPS-primed BMDMs with CDN and then stimulated them with nigericin. CDN exhibited dose-dependently inhibitory effects on caspase-1 cleavage, IL-1β secretion, and LDH release, but no significant effect was found on inflammasome-independent cytokine TNF-α production (Fig. 2A–E and Supporting Information Fig. S1A and B). Besides, CDN treatment did not affect the expression of NLRP3, ASC, pro-IL-1β, and caspase-1 p45 in whole cell lysates (Fig. 2A and Supporting Information Fig. S1C–F).
Figure 2.
CDN inhibits NLRP3 inflammasome activation in primary BMDMs and PBMCs. (A) LPS-primed BMDMs were treated with various doses of CDN and then stimulated with nigericin. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (B–E) Activity of caspase-1 (B), production of IL-1β (C), TNF-α (D) and LDH (E) in SN from BMDMs described in (A). (F) LPS-primed BMDMs were treated with various doses of CDN and then stimulated with ATP. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, ASC in WCL. (G–I) Activity of caspase-1 (G), secretion of IL-1β (H), TNF-α (I) in SN from BMDMs described in (F). (J and K) Activity of caspase-1 (J), secretion of IL-1β (K) in SN from LPS-primed PBMCs treated with various doses of CDN and then stimulated with ATP. Coomassie blue staining was provided as the loading control for the SN (A and F). GAPDH served as a loading control in WCL (A and F). Data are expressed as mean±SEM (n = 3) from three independent experiments with biological duplicates in (B–E, G–K). Statistics differences were analyzed using an unpaired Student׳s t-test: *P<0.05, **P<0.01, ***P<0.001 vs. the control. NS, not significant.
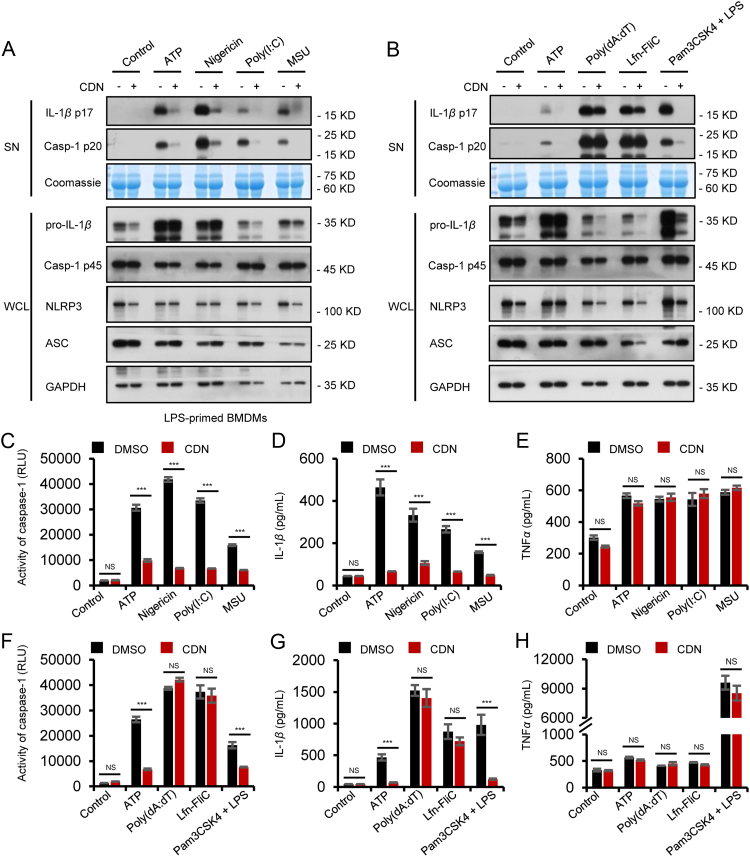
To further clear the inhibitory effect of CDN on NLRP3 inflammasome activation, we tested other NLRP3 agonists in BMDMs. The results showed that CDN also blocked caspase-1 cleavage and IL-1β secretion triggered by ATP, MSU and poly(I:C) but had no effects on TNF-α production (Fig. 2F–I, Fig. 3A, C–E and Supporting Information Fig. S2A–F), suggesting that CDN is a potent and broad inhibitor for NLRP3 inflammasome activation. Intracellular LPS is sensed by a noncanonical NLRP3 inflammasomes pathway, which produces caspase-11-dependent IL-1β maturation and secretion4., 32.. We then activated noncanonical NLRP3 inflammasome in BMDMs pretreated with Pam3CSK4 and transfected with LPS16., 33., and the results showed that CDN significantly blocked caspase-11-dependent caspase-1 cleavage and IL-1β secretion (Fig. 3B, F–H and Supporting Information Fig. S3A–F). These results suggest that CDN could inhibit canonical and noncanonical NLRP3 inflammasome activation in BMDMs. We next detected whether CDN inhibits NLRP3 inflammasome activation in response to human cells. LPS-primed PBMCs were stimulated with ATP after pretreated with CDN. The results showed that caspase-1 activity and IL-1β production were also suppressed by CDN (Fig. 2J and K).
Figure 3.
CDN inhibits canonical and noncanonical NLRP3 activation, but does not inhibit NLRC4, AIM2 inflammasome. (A) LPS-primed BMDMs were treated with CDN (10 μmol/L) and then stimulated with ATP, nigericin, poly(I:C), and MSU. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, and ASC in WCL. (B) LPS-primed BMDMs were treated with CDN (10 μmol/L) and then stimulated with ATP, poly (dA:dT), Lfn-Flic, or Pam3CSK4-primed BMDMs were treated with CDN (10 μmol/L) and then stimulated with LPS. Western blot analysis of IL-1β (p17), caspase-1 (p20) in SN and pro-IL-1β, caspase-1 (p45), NLRP3, and ASC in WCL. (C–E) Activity of caspase-1 (C), secretion of IL-1β (D), and TNF-α (E) in SN from BMDMs described in (A). (F–H) Activity of caspase-1 (F), secretion of IL-1β (G), and TNF-α (H) in SN from BMDMs described in (B). Coomassie blue staining was provided as the loading control for the SN (A and B). GAPDH served as a loading control in WCL (A and B). Data are expressed as mean±SEM (n = 3) from three independent experiments with biological duplicates in (C–H). Statistics differences were analyzed using an unpaired Student׳s t-test: ***P<0.001 vs. the control. NS, not significant.
3.3. CDN does not inhibit NLRC4 and AIM2 inflammasome activation
In addition to NLRP3 inflammasome, other inflammasomes, such as NLRC4 and AIM2 which can activate caspase-1 in response to endogenous and exogenous danger signals have been characterized34., 35.. We further investigated the specificity inhibition effect of CDN on NLRP3 inflammasome as compared with other inflammasomes. LPS-primed BMDMs were infected with Lfn-Flic to activate NLRC4 inflammasomes, and results indicated that CDN did not inhibit caspase-1 activation, IL-1β or TNF-α production in response to Lfn-Flic treatment (Fig. 3B, F–H and Supporting Information Fig. S3A and B). The expression of NLRP3, ASC, caspase-1 (p45) in whole cell lysates was not substantially affected by CDN either (Fig. 3B, Supporting Information Fig. S3C–F). The effect of CDN on AIM2 inflammasome was examined by transfecting BMDMs with poly (dA:dT), and CDN did not attenuate the caspase-1 activation, IL-1β or TNF-α production in response to the AIM2 inflammasome activator poly (dA:dT) (Fig. 3B, F–H and Supporting Information Fig. S3A and B). Taken together, these results demonstrate that CDN could specifically inhibit NLRP3 but not NLRC4 or AIM2 inflammasome activation.
3.4. CDN does not inhibit LPS-induced NLRP3 priming but blocks ASC oligomerization
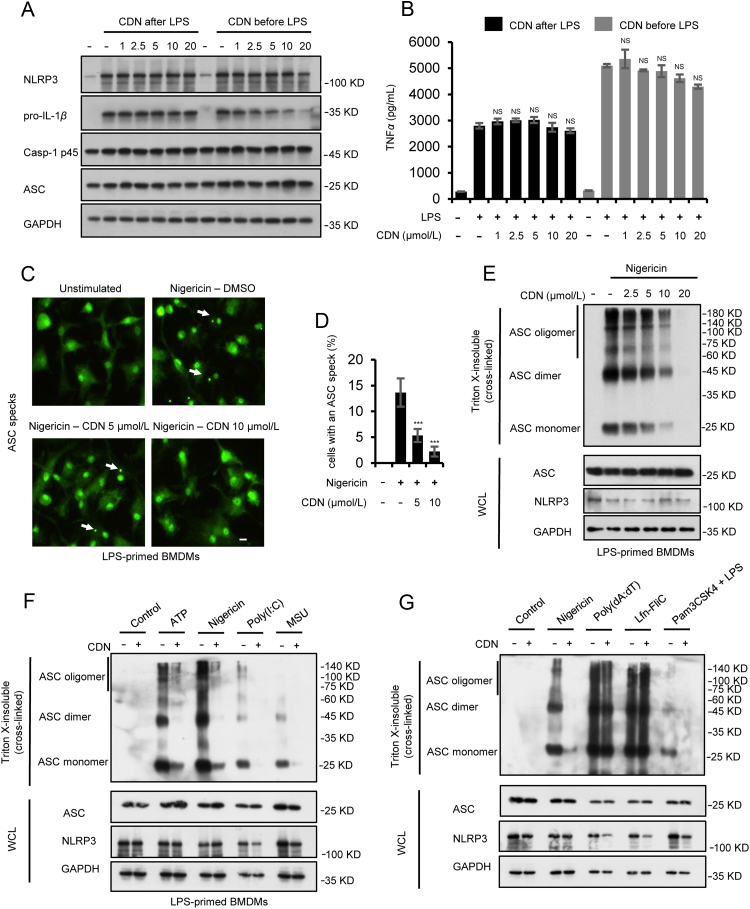
Previous studies have demonstrated that CDN could inhibit NF-κB activation24., 25., 26.. We then examined whether CDN could affect the priming phase of NLRP3 inflammasome activation via blocking NF-κB-dependent NLRP3 or pro-IL-1β expression according to the described method previously36., 37.. When BMDMs were stimulated with CDN after LPS treatment, NLRP3 and pro-IL-1β expression were not affected by CDN, as well as TNF-α production (Fig. 4A and B), suggesting that CDN does not affect LPS-induced priming phase of NLRP3 inflammasome activation in this condition. In contrast, when BMDMs were stimulated with CDN before LPS treatment, CDN inhibited pro-IL-1β expression while little effect was observed on NLRP3 expression and TNF-α production (Fig. 4A and B). These results suggest that CDN could suppress NLRP3 inflammasome activation without affecting the expression of NF-κB-mediated inflammasome precursor proteins.
Figure 4.
CDN does not inhibit LPS-induced NLRP3 priming, but blocks NLRP3-dependent ASC oligomerization. (A) Western blot analysis of the indicated proteins in WCL from BMDMs treated with LPS for 4 h and left stimulated with different doses of CDN for 1 h (CDN after LPS) or treated with different doses of CDN for 1 h and then stimulated with LPS for 4 h (CDN before LPS). (B) secretion of TNF-α in SN from BMDMs described in (A). (C) Live-cell imaging of ASC-green cells treated with CDN and stimulated with nigericin. At least ten different images were taken of each condition at an original magnification of 40×. Results are representative of three independent experiments. Scale bar: 5 μm. (D) The percentage of ASC green cells containing an ASC speck after treatment with CDN (5 and 10 μmol/L) and stimulation with nigericin. (E) LPS-primed BMDMs were treated with various doses of CDN and then stimulated with nigericin. Western blot analysis of WCL and cross-linked cytosolic pellets. (F) Western blot analysis of WCL and cross-linked cytosolic pellets of LPS-primed BMDMs treated with CDN (10 μmol/L) and then stimulated with ATP, nigericin, poly(I:C), MSU. (G) Western blot analysis of WCL and cross-linked cytosolic pellets of LPS-primed BMDMs treated with CDN (10 μmol/L) and then stimulated with ATP, poly (dA:dT), Lfn-Flic, or Pam3CSK4-primed BMDMs treated with CDN (10 μmol/L) and then stimulated with LPS. GAPDH served as a loading control in WCL (A, E–G). Data are expressed as mean±SEM (n=3) from three independent experiments with biological duplicates in (B and D). Statistics differences were analyzed using an unpaired Student׳s t-test: ***P<0.001 vs. the control. NS, not significant.
During NLRP3 inflammasome activation, ASC oligomerization is a critical step for the subsequent caspase-1 activation38. We further examined whether the inhibitory effect of CDN on NLRP3 inflammasome activation was mediated by inhibition of ASC oligomerization. Immunoblot analysis was used to test ASC oligomerization in BMDMs treated or untreated with CDN and then BMDMs were stimulated with different stimuli. Cytosolic fractions from cell lysates were cross-linked, and ASC monomers and higher order complexes were observed after co-stimulation with LPS and nigericin, but ASC-complex formation was attenuated by CDN in a dose dependent manner (Fig. 4E and Supporting Information Fig. S4A and B). Upon NLRP3 inflammasome activation by nigericin, ASC condenses into a large speck in cells. Therefore, ASC oligomerization was also observed when we used BMDMs stably to express ASC tagged with green fluorescent protein, but pretreatment with CDN dose-dependently inhibited speck formation (Fig. 4C and D). Further results showed that CDN had a favorable inhibitory effect on ASC oligomerization of NLRP3 inflammasome activators, such as ATP, nigericin, poly(I:C), MSU and intracellular LPS (Fig. 4F, G and Supporting Information Fig. S4C and D). Whereas, ASC oligomerization of NLRC4 and AIM2 inflammasome, stimulated by Lfn-Flic and poly (dA:dT), were not affected by CDN (Fig. 4G, Supporting Information Fig. S4E and F). These results indicate that CDN inhibits canonical and noncanonical NLRP3 inflammasome activation by attenuating NLRP3-dependent ASC oligomerization.
3.5. CDN ameliorates LPS-induced septic shock and IL-1β production in vivo
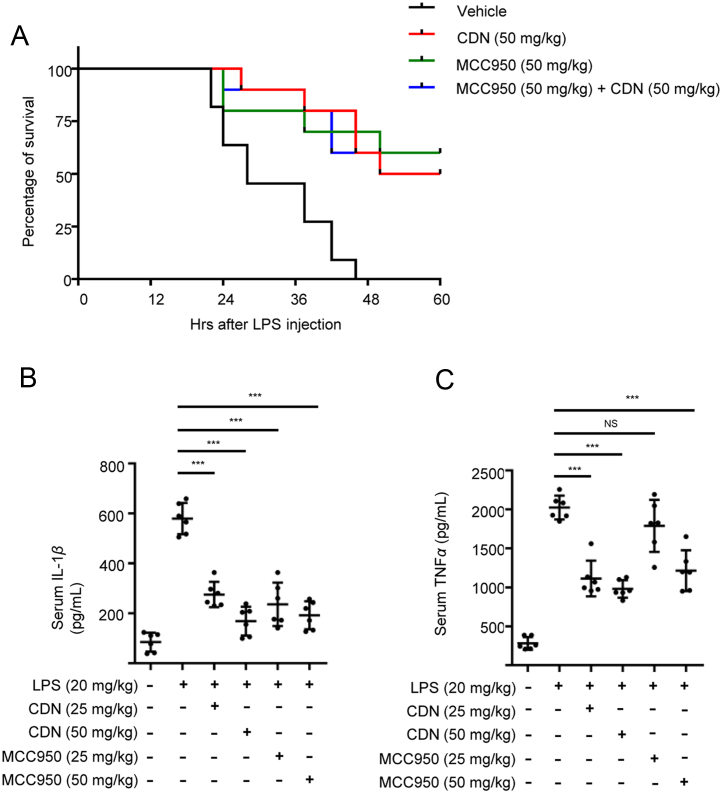
The septic shock and production of IL-1β induced by intraperitoneal (i.p.) injection of LPS is shown to be NLRP3 inflammasome-dependent16., 39., 40., 41., 42.. Therefore, LPS-induced lethality was chosen for the evaluation of the effect of CDN in vivo. Meanwhile, administration of MCC950, which is the most selective tool inhibitor available for investigation of NLRP3-related effects in vitro and in vivo15., 16., was used to mimic the consequences of NLRP3 knock-down in our study. Results showed that pretreatment with MCC950 greatly improved the survival of mice suffering from septic shock (Fig. 5A), which indicated that NLRP3 inflammasome plays an important role in septic shock. And there was no significant difference between CDN- and MCC950-treated group (Fig. 5A). Furthermore, the survival rate of LPS-induced septic shock after CDN treatment combined with MCC950 was not significantly better than the group of MCC950 or CDN alone (Fig. 5A), suggesting that CDN ameliorates LPS-induced septic shock mainly through suppressing NLRP3 inflammasome. Owing to the inflammatory disorder of septic shock, we also examined whether CDN could suppress the inflammatory cytokines through the inhibitory effect of NLRP3 inflammasome in septic shock. We pretreated mice with CDN for 1 h before intraperitoneal injection of LPS for 6 h, and the result indicated that CDN treatment resulted in significant reduction of serum IL-1β and TNF-α production, which was equivalent to the effect of MCC950 (Fig. 5B–C). Taken together, these results demonstrate that CDN could inhibit LPS-induced NLRP3 inflammasome activation in vivo and improve the survival of mice suffering from septic shock in mice model.
Figure 5.
CDN ameliorates LPS-induced septic shock and IL-1β production. (A) Survival of 8-week-old C57BL/6 female mice intraperitoneally injected with LPS (20 mg/kg) in the presence or absence of CDN, MCC950 (50 mg/kg, n=10). Lethality was monitored for 60 h. (B and C) Serum levels of IL-1β (B), TNF-α (C) from C57BL/6 mice pretreated with CDN, MCC950 or vehicle control (n=6) as measured by ELISA 6 h after intraperitoneally LPS (20 mg/kg) injection. Data are shown mean±SEM. Statistics differences were analyzed using an unpaired Student׳s t-test: ***P<0.001 vs. the control. NS, not significant.
4. Discussion
The inflammatory cytokine IL-1β is produced in very low amounts under normal circumstances. However, in terms of tissue damage, environmental stress, infection or chronic inflammation, the production of IL-1β is highly increased43., 44.. The production and secretion of IL-1β is regulated by diverse inflammasomes, including NLRP3, NLRC4, and AIM2 inflammasomes. NLRP3 inflammasome is involved in many inflammatory, metabolic, degenerative and aging-related diseases45. Therefore, developing some small molecules that target NLRP3 inflammasome specifically without inhibiting other inflammasomes is very useful for treating various inflammatory diseases as described above. Traditional Chinese medicines (TCMs) have been used in China for thousands of years, and many TCMs have obvious anti-inflammatory activity, but the active ingredients and mechanisms remain to be studied. It has been reported that Oridonin is the major active ingredient of Rabdosia rubescens (Donglingcao in Chinese) and inhibits NLRP3 inflammasome assembly and activation17. Curcumin is a common NLRP3 inflammasome activation inhibitor found in the herb Curcuma Longa (Jianghuang in Chinese)46. Isoliquiritigenin from the roots and rhizomes of Glycyrrhiza plants also inhibits NLRP3 inflammasome activation and subsequent IL-1β production as well47.
Alpinia katsumadai (Caodoukou in Chinese) is a medicinal herb which is supposed to have potent anti-inflammatory and immunomodulatory properties for the treatment of multiple inflammatory disorders, including rheumatoid arthritis, colitis, ulcerative colitis, and inflammatory edema24., 26., 48., 49.. Previous study has demonstrated that CDN could inhibit ATP-induced NLRP3 inflammasome activation in THP-1 cells and BMDMs28. However, since NLRP3 inflammasome can be induced by various endogenous and exogenous stimuli and the activation mechanism is also different, the specific inhibition of CDN on NLRP3 inflammasome is not explained. In the current study, we found that CDN, the major active constituent of Alpinia katsumadai, could inhibit canonical and noncanonical NLRP3 inflammasome activation and the subsequent release of mature IL-1β both in murine macrophages and human monocytes triggered by multiple stimuli. Our results show that CDN does not affect the activation of the NLRC4 or AIM2 inflammasomes, suggesting that CDN inhibits the NLRP3 inflammasome activation specifically. Though previous studies demonstrate that CDN is an inhibitor of NF-κB pathway, which reduces the expression of IL-1β, TNF-α and IL-625., 26., our results indicate that the doses needed for NLRP3 inflammasome inhibition does not affect NF-κB -dependent pro-IL-1β, NLRP3 or TNF-α production. ASC-complex formation is a key event in NLRP3 inflammasome activation38, which was attenuated by CDN treatment, demonstrating that ablation of ASC oligomerization is the specific inhibition effect of CDN for the NLRP3 inflammasome.
The production of IL-1β and septic shock induced by intraperitoneal injection of LPS is associated with the NLRP3 inflammasome activation16., 39., 40., 41., 42.. Our results demonstrated that CDN improved the survival of LPS-induced septic shock in a mouse model (P<0.05). Studies have demonstrated that CDN could inhibit NF-κB activation24., 25., 26.. The effect of CDN on LPS-induced septic shock via NF-κB or NLRP3 inflammasome signal pathway was not well investigated. Therefore, MCC950, a specific inhibitor of the NLRP3 inflammasome16, was used to mimic the consequences of NLRP3 knock-down. Results showed that the survival rate after CDN treatment combined with MCC950 (in the condition of NLRP3 inflammasome knock-down) was not significantly better than the group of MCC950 or CDN alone (P>0.05), suggesting that CDN ameliorates LPS-induced endotoxic shock mainly through suppressing NLRP3 inflammasome. In addition, survival rate continued to decrease after 24 h when the mice were injected intraperitoneally with CDN only once, which may be related to rapid metabolism in vivo. After injection of CDN, the content of CDN in serum reached a peak value of 1.5 μmol/L at 1 h. With the extension of time, the content of CDN rapidly decreased (1–4 h) and became stable (4–6 h) (Supporting Information Fig. S5). Therefore, in order to assess the therapeutic potential of CDN, the pharmacokinetic profile in a number of assays about in vivo absorption, distribution, metabolism and excretion (ADME) should be tested50. In the second experiment, both CDN and MCC950 reduced serum IL-1β and TNF-α production induced by LPS stimulation. It has been reported that MCC950 does not affect the NF-κB signaling pathway, as well as TNF-α production16. However, the reason why TNF-α has a decreasing trend after 6 h of treatment with CDN and MCC950 may be related to the subsequent immune inflammatory response mediated by inhibition of IL-1β production51., 52., but the underlying mechanism needs further study. In short, CDN may be a suitable drug candidate for the treatment of NLRP3-driven diseases.
In summary, our study shows that CDN is a broad-spectrum and specific inhibitor of NLRP3 inflammasome. Importantly, CDN significantly attenuates IL-1β secretion and TNF-α production induced by LPS and improves the survival of mice suffering from LPS-induced lethal endotoxic shock. These findings offer a mechanistic basis for the therapeutic potential of CDN in septic shock and other NLRP3 inflammasome-driven diseases, such as CAPS, T2D, NASH and gout.
Acknowledgments
This work was supported by the National Key Technology R&D Program (No. 2017ZX09301022), the National Key Technology R&D Program (No. 2015ZX09501-004-001-008), the Beijing Nova program (No. Z181100006218001), the National Natural Science Foundation of China (No. 81630100).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2019.02.003.
Contributor Information
Youping Liu, Email: liuyouping@163.com.
Xiaohe Xiao, Email: pharmacy_302@126.com.
Zhaofang Bai, Email: baizf2008@hotmail.com.
Appendix A. Supplementary material
Supplementary material.
.
References
- 1.Hanke T., Merk D., Steinhilber D., Geisslinger G., Schubert-Zsilavecz M. Small molecules with anti-inflammatory properties in clinical development. Pharmacol Ther. 2016;157:163–187. doi: 10.1016/j.pharmthera.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 2.Ribeiro D., Freitas M., Lima J.L., Fernandes E. Proinflammatory pathways: the modulation by flavonoids. Med Res Rev. 2015;35:877–936. doi: 10.1002/med.21347. [DOI] [PubMed] [Google Scholar]
- 3.Baroja-Mazo A., Martín-Sánchez F., Gomez A.I., Martínez C.M., Amores-Iniesta J., Compan V. The NLRP3 inflammasome is released as a particulate danger signal that amplifies the inflammatory response. Nat Immunol. 2014;15:738–748. doi: 10.1038/ni.2919. [DOI] [PubMed] [Google Scholar]
- 4.Lamkanfi M., Dixit V. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Afonina I.S., Zhong Z., Karin M., Beyaert R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat Immunol. 2017;18:861–869. doi: 10.1038/ni.3772. [DOI] [PubMed] [Google Scholar]
- 6.Abderrazak A., Syrovets T., Couchie D., Hadri K.E., Friguet B., Simmet T. NLRP3 inflammasome: from a danger signal sensor to a regulatory node of oxidative stress and inflammatory diseases. Redox Biol. 2015;4:296–307. doi: 10.1016/j.redox.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elliott E.I., Sutterwala F.S. Initiation and perpetuation of NLRP3 inflammasome activation and assembly. Immunol Rev. 2015;265:35–52. doi: 10.1111/imr.12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S., Sun B. Negative regulation of NLRP3 inflammasome signaling. Protein Cell. 2013;4:251–258. doi: 10.1007/s13238-013-2128-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis B.K., Wen H., Ting J.P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol. 2011;29:707–735. doi: 10.1146/annurev-immunol-031210-101405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortimer L., Moreau F., Macdonald J.A., Chadee K. NLRP3 inflammasome inhibition is disrupted in a group of auto-inflammatory disease CAPS mutations. Nat Immunol. 2016;17:1176–1186. doi: 10.1038/ni.3538. [DOI] [PubMed] [Google Scholar]
- 11.Martinon F., Pétrilli V., Mayor A., Tardivel A., Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 12.Masters S.L., Dunne A., Subramanian S.L., Hull R.L., Tannahill G.M., Sharp F.A. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mridha A.R., Wree A., Robertson A.A., Yeh M.M., Johnson C.D., van Rooyen D.M. NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol. 2017;66:1037–1046. doi: 10.1016/j.jhep.2017.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wree A., Eguchi A., Mcgeough M.D., Pena C.A., Johnson C.D., Canbay A. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation and fibrosis. Hepatology. 2014;59:898–910. doi: 10.1002/hep.26592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 16.Coll R.C., Robertson A.A., Chae J.J., Higgins S.C., Inserra M.C., Vetter I. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21:248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He H., Jiang H., Chen Y., Ye J., Wang A., Wang C. Oridonin is a covalent NLRP3 inhibitor with strong anti-inflammasome activity. Nat Commun. 2018;9:2550. doi: 10.1038/s41467-018-04947-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Youm Y.H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D. Ketone body β-hydroxybutyrate blocks the NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Shi D., Niu P., Zhu Y., Zhou J. Anti-inflammatory effects of cardamonin in ovarian cancer cells are mediated via mTOR suppression. Planta Med. 2018;84:1183–1190. doi: 10.1055/a-0626-7426. [DOI] [PubMed] [Google Scholar]
- 20.Lu S., Lin C., Cheng X., Hua H., Xiang T., Huang Y. Cardamonin reduces chemotherapy resistance of colon cancer cells via the TSP50/NF-κB pathway in vitro. Oncol Lett. 2018;15:9641–9646. doi: 10.3892/ol.2018.8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mkb B., Hossan M.S., Khoo Y., Qazzaz M.E., Mzk A.H., Chow S.C. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the mTOR pathway. Fitoterapia. 2018;125:161–173. doi: 10.1016/j.fitote.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Peng S., Hou Y., Yao J., Fang J. Activation of Nrf2-driven antioxidant enzymes by cardamonin confers neuroprotection of PC12 cells against oxidative damage. Food Funct. 2017;8:997–1007. doi: 10.1039/c7fo00054e. [DOI] [PubMed] [Google Scholar]
- 23.Je H.D., Jeong J.H. Cardamonin inhibits agonist-induced vascular contractility via Rho-kinase and MEK inhibition. Korean J Physiol Pharmacol. 2016;20:69–74. doi: 10.4196/kjpp.2016.20.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y.Y., Huang S.S., Lee M.M., Deng J.S., Huang G.J. Anti-inflammatory activities of cardamonin from Alpinia katsumadai through heme oxygenase-1 induction and inhibition of NF-κB and MAPK signaling pathway in the carrageenan-induced paw edema. Int Immunopharmacol. 2015;25:332–339. doi: 10.1016/j.intimp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Chow Y.L., Lee K.H., Vidyadaran S., Lajis N.H., Akhtar M.N., Israf D.A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-κB signalling pathway. Int Immunopharmacol. 2012;12:657–665. doi: 10.1016/j.intimp.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Ren G., Sun A., Deng C., Zhang J., Wu X., Wei X. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G517–G527. doi: 10.1152/ajpgi.00133.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatziieremia S., Gray A.I., Ferro V.A., Paul A., Plevin R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NF-κB signaling pathways in monocytes/macrophages. Br J Pharmacol. 2006;149:188–198. doi: 10.1038/sj.bjp.0706856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang K., Lv Q., Miao Y.M., Qiao S.M., Dai Y., Wei Z.F. Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol. 2018;155:494–509. doi: 10.1016/j.bcp.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 29.Song N., Liu Z.S., Xue W., Bai Z.F., Wang Q.Y., Dai J. NLRP3 phosphorylation is an essential priming event for inflammasome activation. Mol Cell. 2017;68:185–197. doi: 10.1016/j.molcel.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Brandelli J.R., Griener T.P., Laing A., Mulvey G., Armstrong G.D. The effects of Shiga toxin 1, 2 and their subunits on cytokine and chemokine expression by human macrophage-like THP-1 cells. Toxins. 2015;7:4054–4066. doi: 10.3390/toxins7104054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honda H., Nagai Y., Matsunaga T., Okamoto N., Watanabe Y., Tsuneyama K. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. 2014;96:1087–1100. doi: 10.1189/jlb.3A0114-005RR. [DOI] [PubMed] [Google Scholar]
- 32.Kayagaki N., Warming S., Lamkanfi M., Vande W.L., Louie S., Dong J. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–122. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 33.Kayagaki N., Dixit V.M. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science. 2013;341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 34.Zhao Y., Yang J., Shi J., Gong Y.N., Lu Q., Xu H. The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature. 2011;477:596–600. doi: 10.1038/nature10510. [DOI] [PubMed] [Google Scholar]
- 35.Ming M.S., Thirumala-Devi K. Regulation of inflammasome activation. Immunol Rev. 2015;265:6–21. doi: 10.1111/imr.12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., Jiang H., Chen Y., Wang X., Yang Y., Tao J. Tranilast directly targets NLRP3 to treat inflammasome-driven diseases. Embo Mol Med. 2018;10:e8689. doi: 10.15252/emmm.201708689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang H., He H., Chen Y., Huang W., Cheng J., Ye J. Identification of a selective and direct NLRP3 inhibitor to treat inflammatory disorders. J Exp Med. 2017;214:3219–3238. doi: 10.1084/jem.20171419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu A., Magupalli V.G., Ruan J., Yin Q., Atianand M.K., Vos M.R. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell. 2014;156:1193–1206. doi: 10.1016/j.cell.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He Y., Franchi L., Núñez G. Toll-like receptor agonists stimulate NLRP3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190:334–339. doi: 10.4049/jimmunol.1202737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamkanfi M., Mueller J.L., Vitari A.C., Misaghi S., Fedorova A., Deshayes K. Glyburide inhibits the cryopyrin/NALP3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanjeev M., David S.W., Kim N., Jacqueline M., Karen O., Meron R. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 42.Sutterwala F.S., Ogura Y., Szczepanik M., Lara-Tejero M., Lichtenberger G.S., Grant E.P. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Somakala K., Amir M. Synthesis, characterization and pharmacological evaluation of pyrazolyl urea derivatives as potential anti-inflammatory agents. Acta Pharm Sin B. 2017;7:230–240. doi: 10.1016/j.apsb.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C., Zhao W., Zhang X., Chen X. Neocryptotanshinone inhibits lipopolysaccharide-induced inflammation in RAW264.7 macrophages by suppression of NF-κB and iNOS signaling pathways. Acta Pharm Sin B. 2015;5:323–329. doi: 10.1016/j.apsb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guo H., Callaway J.B., Ting J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yin H., Guo Q., Li X., Tang T., Li C., Wang H. Curcumin suppresses IL-1β secretion and prevents inflammation through inhibition of the NLRP3 inflammasome. J Immunol. 2018;200:2835–2846. doi: 10.4049/jimmunol.1701495. [DOI] [PubMed] [Google Scholar]
- 47.Honda H., Nagai Y., Matsunaga T., Okamoto N., Watanabe Y., Tsuneyama K. Isoliquiritigenin is a potent inhibitor of NLRP3 inflammasome activation and diet-induced adipose tissue inflammation. J Leukoc Biol. 2014;96:1087–1100. doi: 10.1189/jlb.3A0114-005RR. [DOI] [PubMed] [Google Scholar]
- 48.Voon F.L., Sulaiman M.R., Akhtar M.N., Idris M.F., Akira A., Perimal E.K. Cardamonin (2′,4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur J Pharmacol. 2017;794:127–134. doi: 10.1016/j.ejphar.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Ali A.A., En A.A.H., Khaleel S.A., Sallam A.S. Protective effect of cardamonin against acetic acid-induced ulcerative colitis in rats. Pharmacol Rep. 2017;69:268–275. doi: 10.1016/j.pharep.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Yu A.M., Zhong X.B. Advanced knowledge in drug metabolism and pharmacokinetics. Acta Pharm Sin B. 2016;6:361–362. doi: 10.1016/j.apsb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Granowitz E.V., Clark B.D., Vannier E., Callahan M.V., Dinarello C.A. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: ii. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood. 1992;79:2364–2369. [PubMed] [Google Scholar]
- 52.Dinarello C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.