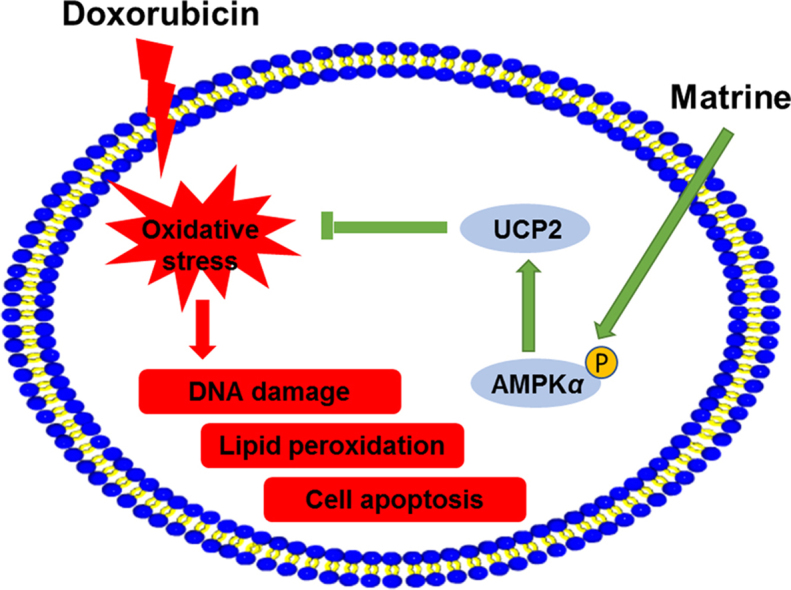
Abstract
Oxidative stress and cardiomyocyte apoptosis are involved in the pathogenesis of doxorubicin (DOX)-induced cardiotoxicity. Matrine is well-known for its powerful anti-oxidant and anti-apoptotic capacities. Our present study aimed to investigate the effect of matrine on DOX-induced cardiotoxicity and try to unearth the underlying mechanisms. Mice were exposed with DOX to generate DOX-induced cardiotoxicity or normal saline as control. H9C2 cells were used to verify the effect of matrine in vitro. DOX injection triggered increased generation of reactive oxygen species (ROS) and excessive cardiomyocyte apoptosis, which were significantly mitigated by matrine. Mechanistically, we found that matrine ameliorated DOX-induced uncoupling protein 2 (UCP2) downregulation, and UCP2 inhibition by genipin could blunt the protective effect of matrine on DOX-induced oxidative stress and cardiomyocyte apoptosis. Besides, 5′-AMP-activated protein kinase α2 (Ampkα2) deficiency inhibited matrine-mediated UCP2 preservation and abolished the beneficial effect of matrine in mice. Besides, we observed that matrine incubation alleviated DOX-induced H9C2 cells apoptosis and oxidative stress level via activating AMPKα/UCP2, which were blunted by either AMPKα or UCP2 inhibition with genetic or pharmacological methods. Matrine attenuated oxidative stress and cardiomyocyte apoptosis in DOX-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway, and it might be a promising therapeutic agent for the treatment of DOX-induced cardiotoxicity.
Abbreviations: 4-HNE, 4-hydroxynonenal; ACC, acetyl-CoA carboxylase; AMPKα, 5′-AMP-activated protein kinase α; ANOVA, analysis of variance; BAX, BCL-2-associated X protein; BCL-2, B-cell lymphoma 2; C-caspase 3, cleaved-caspase3; CCK-8, cell counting kit 8; CK-MB, creatine kinase isoenzymes; cTnT, cardiac isoform of Tropnin T; DCFH-DA, 2′,7′-dichlorodihydrofluorescein diacetate; DHE, dihydroethidium; DMEM, Dulbecco׳s modified Eagle׳s medium; DOX, doxorubicin; FBS, fetal bovine serum; FS, fractional shortening; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; HW, heart weight; LDH, lactate dehydrogenase; MDA, malondialdehyde; BCA, bicinchoninic acid; PPARs, peroxisomal proliferators-activated receptors; ROS, reactive oxygen species; SOD2, superoxide dismutase 2; T-caspase3, total-caspase3; TL, tibia length; Top2, topoisomerase-II; TUNEL, TdT-mediated dUTP nick end-labelling; UCP2, uncoupling protein 2
KEY WORDS: Matrine, Oxidative stress, Apoptosis, AMPKα, UCP2
Graphical abstract
Matrine attenuated oxidative stress and cardiomyocyte apoptosis in DOX-induced cardiotoxicity via maintaining AMPKα/UCP2 pathway, and it might be a promising therapeutic agent for the treatment of DOX-induced cardiotoxicity.
1. Introduction
Doxorubicin (DOX) is a widely used and highly potent chemotherapeutic agent to treat various types of cancers1. However, the clinical usage of DOX is greatly limited by its dose-dependent cardiotoxicity, which eventually leads to irreversible degenerative cardiomyopathy and congestive heart failure2. Despite the limited elucidation of pathogenesis of DOX-induced cardiotoxicity, accumulating evidence suggested that excessive production of ROS is the main factor involved in DOX-induced cardiotoxicity, which leads to oxidative damage and cardiomyocyte apoptosis, and eventually the occurrence of heart failure3., 4..
Mitochondria are the major source of intracellular ROS. The excessive production of ROS induces oxidative damage to biological macromolecules, including lipids, proteins and DNA, and disrupts cellular membrane structure and functions5., 6.. Mitochondrial uncoupling proteins (UCPs) are now well accepted as mitochondrial metabolite transporters, which mainly located in the inner membrane of mitochondria7., 8.. It has been reported that UCPs family could partially dissipate the proton concentration gradient of the inner mitochondria membrane, and play critical roles in controlling intracellular ROS homeostasis and preventing oxidative stress9. UCP2, a novel member of the UCPs family, sharing 60% sequence identity with the well-known thermogenic uncoupling protein 1 (UCP1) from brown adipose tissue, is the predominant type of UCPs in the heart10. Previous studies indicated that Ucp2 deficiency exacerbated oxidative stress and delayed liver regeneration in mice, whereas adenovirus-mediated UCP2 overexpression inhibited ROS production and protected endothelial cells from oxidative damage11., 12.. Besides, activation of UCP2 allowed for the prevention of ischemia-reperfusion induced oxidative stress and preservation of mitochondrial function13. Previously, Dhamrait et al.14 reported that gene mutation of UCP2 in man that led to a decreased UCP2 expression could reduce total antioxidant status. More importantly, a recent study demonstrated that knockdown of UCP2 could exacerbate DOX-induced cardiomyocyte apoptosis and myocardial oxidative stress15. 5′-AMP-activated protein kinase α (AMPKα) is a highly conserved eukaryotic serine/threonine protein kinase, and exerts beneficial effects in many physiological and pathological process, including energy homeostasis, endoplasmic reticulum stress, cardiac hypertrophy and fibrosis, etc.16., 17.. Current available studies showed that AMPKα also played essential roles in regulating oxidative stress and cell survival18., 19.. Kim et al.20 found that AMPKα activation decreased iron-induced ROS production and inhibited cell apoptosis. Ceolotto et al.21 further confirmed that activation of AMPKα prevented hyperactivity of NADPH oxidase and protected endothelial cells against glucose-induced oxidative damage. All these data defined indispensable roles of UCP2 and AMPKα in the regulation of oxidative stress and cell apoptosis. Therefore, targeting AMPKα and UCP2 may be of great therapeutic interest for the treatment of DOX-induced cardiotoxicity.
Matrine, a natural compound extracted from the root of Sophora flavescens Ait, has been shown several pharmacological activities, including anti-inflammatory, anti-fibrotic capacities, and served as a therapeutic agent clinically for the treatment of viral hepatitis and dementia22., 23., 24.. Previous studies showed that matrine and its derivatives reduced inflammatory response and attenuated sepsis-induced organ injury24. In addition, Liu et al.25 implied that matrine treatment increased the expression of NF-E2-related factor 2 and heme oxygenase-1, and suppressed ROS production in experimental autoimmune encephalomyelitis. Moreover, matrine administration resulted in an apparent decrease of ROS formation and prevented cardiomyocyte apoptosis in diabetic hearts26. Based on these data, we suppose that matrine might be a promising therapeutic agent against DOX-induced cardiotoxicity. Therefore, the present study aimed to evaluate the protective effect of matrine on DOX-induced cardiotoxicity and tried to elucidate the underlying mechanisms.
2. Materials and methods
2.1. Reagents and antibodies
Matrine (M5319), DOX (D1515) and genipin (G4796) were purchased from Sigma–Aldrich (St. Louis, MO, USA). Primary antibodies for the following proteins were purchased from Cell Signaling Technology (Danvers, MA, USA): UCP2 (1:1000), total-caspase3 (T-caspase3, 1:1000), cleaved-caspase3 (C-caspase3, 1:1000), BCL-2-associated X protein (BAX, 1:1000), T-AMPKα (1:1000), phosphorylated-AMPKα (P-AMPKα, 1:1000), T-acetyl-CoA carboxylase (T-ACC, 1:1000), p-ACC (1:1000) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:1000). Anti-B-cell lymphoma 2 (BCL-2, 1:1000), superoxide dismutase 2 (SOD2, 1:1000) and 4-hydroxynonenal (4-HNE, 1:200 for staining) were obtained from Abcam (Cambridge, UK). Dihydroethidium (DHE) was obtained from Keygen Biotech (Nanjing, China), and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) was purchased from Beyotime Biotechnology (Shanghai, China). Malondialdehyde (MDA) assay kit, total SOD assay kit and NADPH oxidase assay kit were all purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The cell counting kit-8 (CCK-8) was obtained from Dōjindo Laboratories (Kumamoto, Japan). ApopTag® Plus In Situ Apoptosis Fluorescein Detection Kit was purchased from Millipore (Billerica, MA, USA). Small interfering RNA against UCP2 (siUcp2) and its negative control (siRNA) were synthesized by RiboBio (RiboBio Co. Ltd, Guangzhou, China). Replication-defective adeno-vectors carrying small hairpin RNA against Ampkα2 (shAmpkα2) were obtained from Vigene Bioscience (Rockville, MD, USA), and the efficiency was confirmed by western blot as previously described27., 28.. Anti-rabbit/mouse EnVisionTM+/HRP reagent was purchased from Gene Technology (Shanghai, China). The bicinchoninic acid (BCA) protein assay kit was obtained from Pierce (Rockford, IL, USA).
2.2. Animals and treatment
All animal care and experimental procedures were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University (Wuhan, China) and performed in accordance with the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication, revised 2011). Adult male C57/B6 mice (weight: 23.5–27.5 g, age: 8–10 weeks) were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Sciences (Beijing, China). Mice were bred in the specific pathogen free conditions with a controlled temperature (20–25 °C) and suitable humidity (50 ± 5%). The animals were allowed free access to food or water, and were housed with regular 12/12 h light/dark cycles. After an adaptive feeding for 1 week, the mice were randomly exposed to intraperitoneal injections of DOX (4 mg/kg) weekly for consecutive 4 weeks to generate DOX-induced cardiotoxicity or equal volume of normal saline (NS) as a control. To investigate the effect of matrine, mice were intragastrically administered with matrine (200 mg/kg/day) simultaneously from the first injection of DOX referring to previous studies26., 29.. All the mice were monitored and weighed weekly, and were sacrificed after 4-week matrine treatment, with hearts collected for further experiment. For UCP2 inhibition, genipin (30 mg/kg) was administered biweekly by intraperitoneal injection for two times referring to previous data30. To verify the involvement of AMPKα, Ampkα2 knockout (KO) mice were used, and the source has been described in our previous studies27.
2.3. Echocardiography and hemodynamics
Echocardiography and invasive hemodynamic monitoring were performed referring to our previous studies27. Briefly, mice were lightly anaesthetized with isoflurane (1.5%) and subjected to a MyLab 30CV ultrasound (Esaote SpA, Genoa, Italy) to detect the morphological and functional parameters of the heart, averaged from three to five cardiac cycles. Hemodynamic variables were collected by the PowerLab system (AD Instruments Ltd., Oxford, UK) using a 1.4-French Millar pressure-volume catheter (SPR-839; Millar Instruments, Houston, TX) and the data were analyzed using the PVAN data analysis software.
2.4. Immunohistochemistry staining
The paraffin-embedded heart sections were dewaxed, rehydrated and incubated with citric acid buffer for antigen retrieval, and then they were treated with 3% hydrogen peroxide and 10% goat serum to block endogenous peroxidase and the nonspecific binding of the antibody. After incubated with primary antibodies against 4-HNE (1:100) at 4 °C overnight, sections were incubated with anti-rabbit/mouse EnVisionTM+/HRP reagent for 1 h at 37 °C. After being visualized with diaminobenzidin for 2 min at room temperature, these sections were observed and analyzed by two investigators in a blinded manner.
2.5. Western blot and quantitative real-time PCR
Western blot and quantitative real-time PCR were performed according to our previous studies31. Total proteins were extracted from murine hearts or cell lysates, and the protein concentration was determined by BCA protein assay kit. Then, the proteins were separated on 10% SDS-PAGE and transferred to PVDF membranes (EMD Millipore, Billerica, MA, USA; No. IPFL00010). Membranes were blocked with 5% skim milk for 1 h at room temperature, and incubated with the primary antibodies at 4 °C overnight followed with the secondary antibodies for an additional 1 h. The images were detected and quantified by Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA). Total RNA was extracted using TRIzol reagent and reverse transcribed with Maxima First Strand cDNA Synthesis Kit (Roche, Basel, Switzerland; 04896866001). The expression level of each individual transcript was normalized to Gapdh.
2.6. Cell culture and treatment
H9C2 cells were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China) and were cultured in Dulbecco׳s modified Eagle׳s medium (DMEM, GIBCO, USA) with 10% fetal bovine serum (FBS, GIBCO, USA). After being starved for 16 h to synchronize, H9C2 cells were assigned randomly to incubate with matrine (200 μmol/L) or equal volume of vehicle in the presence or absence of DOX (1 μmol/L) for 24 h32. To knock down the expression of UCP2 in vitro, cells were transfected with siUcp2 (50 nmol/L) for 24 h using Lipo6000TM transfection reagent (Beyotime, Shanghai, China) according to the manufacturer׳s protocol as our previous described33. The efficiency was confirmed by western blot, and we chose the most effective sequence for further study. To evaluate the role of AMPKα in matrine-mediated protective effects, we knocked down AMPKα with shAmpkα2 as previously described34. The efficiency was determined with western blot. Cell viability was detected by CCK-8 according to the protocol.
2.7. Oxidative stress detection in vivo and in vitro
ROS generation was detected by DHE staining in vivo and DCFH-DA staining in vitro. Briefly, frozen heart sections or H9C2 cells were incubated with DHE (5 μmol/L) or DCFH-DA (5 μmol/L) at 37 °C for 30 min in a dark chamber. Fluorescent images were observed with a fluorescence microscope (Olympus IX53, Tokyo, Japan) in a blinded manner. To further assess oxidative stress level, the content of MDA, total SOD activity and NADPH oxidase activity in the myocardium or H9C2 cells were measured according to our previous study by commercially available kits35.
2.8. TdT-mediated dUTP nick end-labelling (TUNEL) staining
A commercially available kit was used to detect apoptosis levels in vivo and in vitro as we previously described36, and the images were captured via the OLYMPUS DX51 fluorescence microscope (Tokyo, Japan).
2.9. Biochemical analysis
Serum levels of cardiac isoform of Tropnin T (cTnT), lactate dehydrogenase (LDH) and creatine kinase isoenzymes (CK-MB) were measured by an automatic biochemical analyzer (ADVIA® 2400, Siemens Ltd., China) as previously described37.
2.10. Statistical analysis
All data in this research were presented as mean ± standard error of the mean (SEM) and analyzed using SPSS 22.0 software. One-way analysis of variance (ANOVA) followed by Tukey post hoc test was performed when comparing multiple groups, whereas differences in two groups were evaluated using unpaired Student׳s t-test. A value of P<0.05 was considered statistically significant.
3. Results
3.1. Matrine attenuated DOX-induced cardiotoxicity in mice
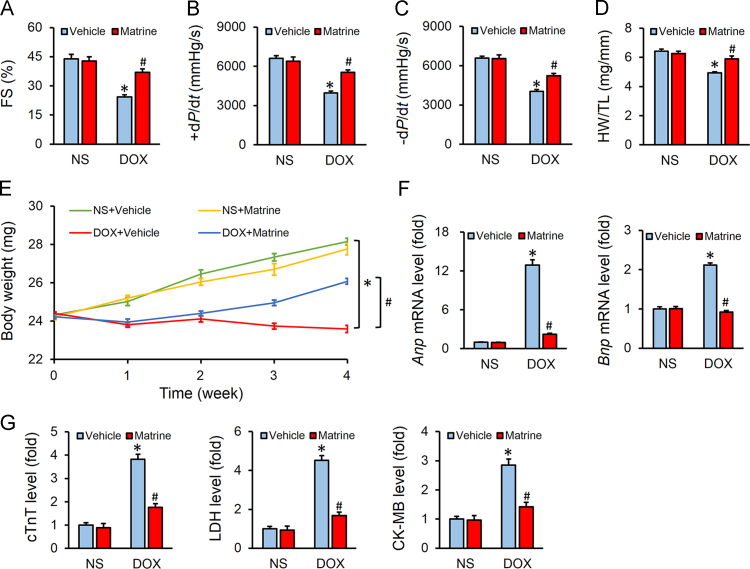
Mice were challenged with DOX (4 mg/kg, i.p.) weekly for consecutive 4 weeks to generate DOX-induced cardiotoxicity. As shown in Fig. 1A–C, DOX injection significantly impaired the left ventricular function as indicated by the decreased fractional shortening (FS) and ±dP/dt, which were significantly attenuated by matrine administration. However, matrine treatment alone showed no beneficial effects on heart function under basal conditions (Fig. 1A–C). Consistent with the fact that DOX injection results in massive loss of cardiac cells, we observed that heart weight (HW)/tibia length (TL) was markedly decreased in DOX-treated mice, which was mitigated with matrine protection (Fig. 1D). Previous studies indicated that chemotherapeutic agents-induced body weight loss predicted bad prognosis in cancer patients and DOX clinical usage is usually accompanied with decreased body weight38., 39.. Our present data suggested that systematic administration of matrine prevented DOX-induced body weight loss in mice (Fig. 1E). Matrine also overtly decreased the mRNA levels of Anp and Bnp in DOX-treated murine hearts (Fig. 1F). Myocardial injury was also assessed by the serum levels of cTnT, LDH and CK-MB, and we observed that matrine alleviated DOX-induced myocardial injury (Fig. 1G). Collectively, we concluded that matrine attenuated DOX-induced cardiotoxicity in mice.
Figure 1.
Matrine attenuated doxorubicin (DOX)-induced cardiotoxicity in mice. (A) Quantitative analysis of fraction shortening (FS) obtained from echocardiography (n = 8). (B) and (C) Hemodynamic analysis of mice with or without matrine treatment (n = 8). (D) Statistical results of the heart weight (HW)/tibia length (TL) (n = 12). (E) Body weight of four groups (n = 12). (F) Relative mRNA levels of Anp, Bnp (n = 6). (G) Plasma levels of cTnT, LDH and CK-MB in mice (n = 6). Values represent mean ± SEM. *P< 0.05 versus NS+Vehicle; #P< 0.05 versus DOX+Vehicle.
3.2. Matrine prevented oxidative damage in response to DOX insult
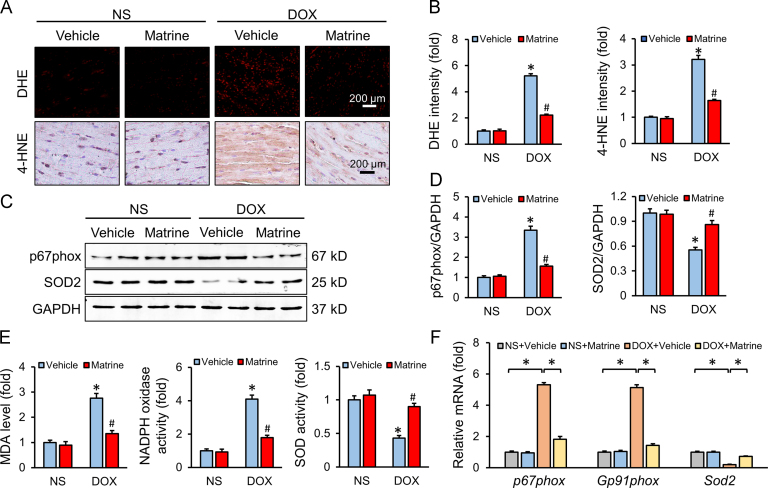
Oxidative stress has been proved to be the primary mechanism in DOX-induced cardiotoxicity, which eventually resulted in massive cardiomyocyte apoptosis4. As shown in Fig. 2A and B, matrine administration decreased lipid peroxidation and ROS generation in DOX-treated murine hearts. Western blot results also verified the protective effect of matrine on DOX-induced oxidative stress, as confirmed by the decreased p67phox and increased SOD2 protein levels (Fig. 2C and D). We also observed that matrine administration reduced the abnormal MDA level and NADPH oxidase activity, and preserved total SOD activity (Fig. 2E). Such results were further confirmed by the mRNA levels of p67phox, Gp91phox and Sod2 (Fig. 2F). All these data defined a beneficial effect of matrine on DOX-induced oxidative damage.
Figure 2.
Matrine prevented oxidative damage in response to DOX insult. (A) and (B) Representative DHE and 4-HNE staining images and the statistical results (n = 6). (C) and (D) Representative Western blot and quantitative data (n = 6). (E) The content of MDA, NADPH oxidase activity and total SOD activity in the myocardium (n = 6). (F) Relative mRNA levels of p67phox, Gp91phox and Sod2 (n = 6). Values represent the mean±SEM. *P< 0.05 versus NS+Vehicle; #P< 0.05 versus DOX+Vehicle.
3.3. Matrine attenuated DOX-induced cardiomyocyte apoptosis
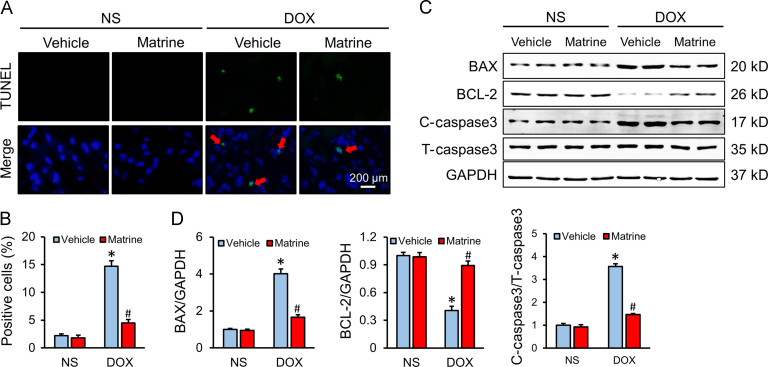
Cardiomyocyte apoptosis is an important event in the process of DOX-induced cardiotoxicity and contributes to the progression of cardiac dysfunction. Consistently, we found that DOX injection resulted in distinct cardiomyocyte apoptosis, which was significantly alleviated in mice with matrine protection (Fig. 3A and B). The protective effect on apoptosis was further confirmed by Western blot data showing that matrine decreased the expression of BAX, C-caspase3 and increased the BCL-2 level after DOX treatment (Fig. 3C and D).
Figure 3.
Matrine attenuated DOX-induced cardiomyocyte apoptosis. (A) and (B) Representative TUNEL staining images and the quantitative results for apoptosis in heart tissues (n = 6). (C) and (D) Western blot and statistical results in the indicated groups (n = 6). Red arrows indicate TUNEL positive staining. Values represent the mean±SEM. *P< 0.05 versus NS+Vehicle; #P< 0.05 versus DOX+Vehicle.
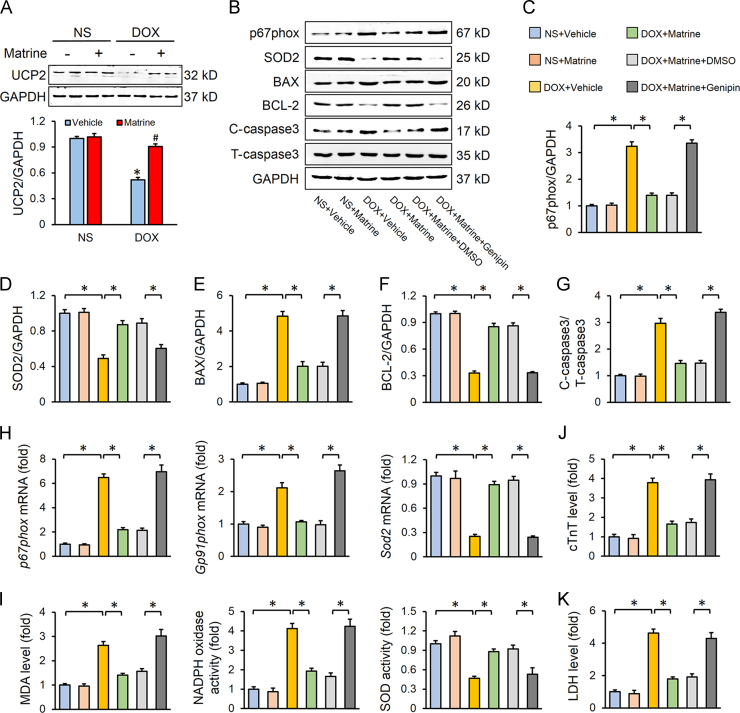
3.4. UCP2 was responsible for matrine-mediated beneficial effect on DOX-induced cardiotoxicity
UCP2 is known to restrain ROS generation, and UCP2 knockdown exacerbated DOX-induced oxidative stress and cell death40. Consistent with previous studies, we observed that myocardial expression of UCP2 was downregulated by DOX, which was alleviated in mice with matrine protection (Fig. 4A). To further elucidate the role of UCP2 in matrine-mediated protective effects in vivo, we treated mice with genipin, an inhibitor of UCP2. As shown in Fig. 4B–G, UCP2 inhibition almost completely abolished matrine-induced downregulation of p67phox, BAX, C-caspase3 and upregulation of SOD2, BCL-2, which was further verified by alterations of p67phox, Gp91phox and Sod2 in mRNA level (Fig. 4H). In addition, we found that the inhibitory effect of matrine on the abnormal MDA level, NADPH oxidase activity and total SOD activity was also abrogated in the presence of genipin (Fig. 4I). DOX-triggered myocardial injury, as assessed by serum levels of cTnT and LDH, was prevented in mice with matrine treatment, but not in mice treated with matrine together with genipin (Fig. 4J–K).
Figure 4.
UCP2 was responsible for matrine-mediated beneficial effect on DOX-induced cardiotoxicity. (A) UCP2 expression level in murine hearts with or without DOX or matrine (n = 6; *P< 0.05 versus NS+Vehicle, #P< 0.05 versus DOX+Vehicle). (B)–(G) Western blot and statistical results in the indicated groups (n = 6). (H) Relative mRNA levels of p67phox, Gp91phox and Sod2 (n = 6). (I) The content of MDA, NADPH oxidase activity and total SOD activity in the myocardium (n = 6). (J)–(K) Plasma levels of cTnT and LDH (n = 6). Values represent the mean±SEM. *P< 0.05 versus the matched groups.
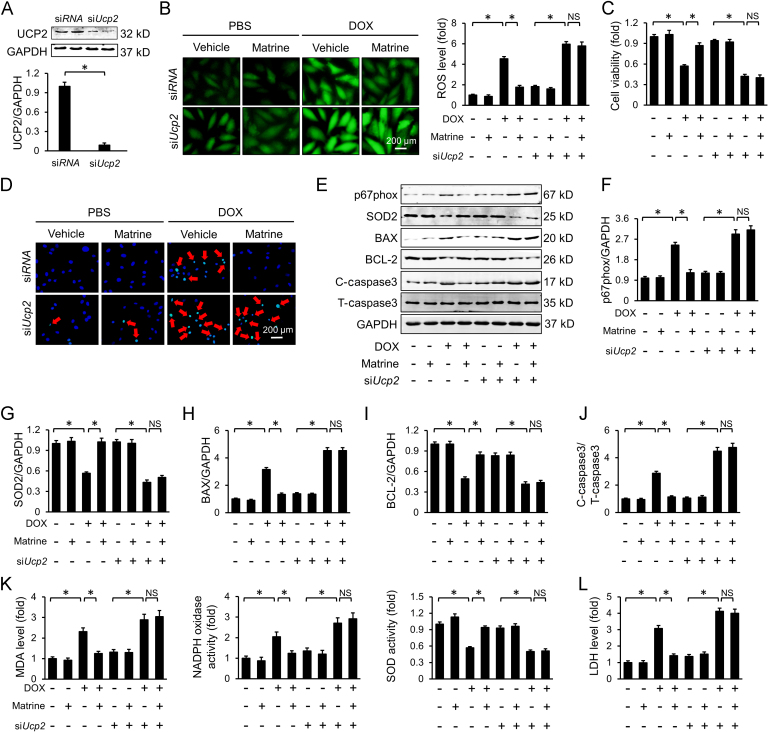
3.5. Knockdown of UCP2 blunted the protective effects of matrine in vitro
To investigate whether Ucp2 deficiency could blunt the protective effects of matrine on oxidative stress and cardiomyocyte apoptosis in vitro, we knocked down the expression of UCP2 in H9C2 cells via siUcp2 and the efficiency was confirmed by Western blot (Fig. 5A). Consistent with the data in vivo, we found that the inhibitory effect of matrine on ROS generation was blunted after the knockdown of UCP2 (Fig. 5B). Besides, matrine lost its effect on DOX-induced cardiomyocyte apoptosis in Ucp2-knockdown cells, as confirmed by the cell viability assay and TUNEL staining (Fig. 5C–D). Western blot results further confirmed that matrine-induced downregulation of p67phox, BAX, and C-caspase3, as well as upregulation of SOD2 and BCL-2, was counteracted in the absence of UCP2 (Fig. 5E–J). In line with the molecular alterations, we found that UCP2 knockdown negated matrine-mediated beneficial effects on lipid peroxidation and oxidative damage in DOX-treated H9C2 cells, as evidenced by the unaltered MDA, NADPH oxidase, SOD activity and LDH level (Fig. 5K–L). These results suggested that matrine alleviated DOX-induced oxidative stress and cardiomyocyte apoptosis via preserving the expression of UCP2.
Figure 5.
Knockdown of UCP2 blunted the protective effects of matrine in vitro. (A) Western blot for UCP2 and the quantitative data for H9C2 cells (n = 6). (B) Representative DCFH-DA images and the statistical results. (C) CCK-8 assay for cell viability (n = 5). (D) Representative images of TUNEL in H9C2 cells (n = 5). (E)–(J) Western blot and statistical results (n = 6). (K) The content of MDA, NADPH oxidase activity and total SOD activity in H9C2 cells (n = 5). (L) LDH levels in H9C2 cells (n = 5). Red arrows indicate TUNEL positive staining. Values represent the mean±SEM. *P< 0.05 versus the matched groups. NS indicates no statistical difference.
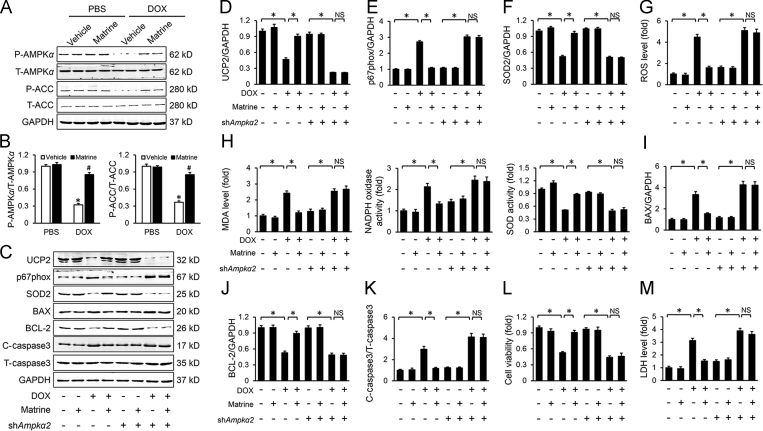
3.6. Matrine prevented DOX-induced downregulation of UCP2 via activating AMPKα
We next explored the possible mechanism by which matrine prevented DOX-induced downregulation of UCP2. Previous studies implied that AMPKα was inactivated in response to DOX treatment41. More importantly, Ampkα silence reduced UCP2 level, whereas Ampkα activation increased the expression of UCP242. Therefore, we speculated that matrine might prevent DOX-induced downregulation of UCP2 via activating AMPKα. As expected, we found that matrine treatment restored the decreased AMPKα activity induced by DOX incubation in H9C2 cells, which was further assessed by the increased phosphorylation of ACC (Fig. 6A and B). To gain evidence that AMPKα was responsible for the upregulation of UCP2, we knocked down Ampkα with adenoviruses in H9C2 cells, and the efficiency was evidenced by Western blot (Supporting Information Fig. S1). As shown in Fig. 6C and D, Ampkα knockdown abolished the restoration of UCP2 level by matrine in vitro. In line with the downregulation of UCP2, we found that Ampkα knockdown significantly abrogated matrine-mediated inhibitory effect on ROS generation and oxidative stress level (Fig. 6E–G), as well as the content of MDA, NADPH oxidase activity and total SOD activity (Fig. 6H). Besides, the protective effect of matrine on cardiomyocyte injury was also blunted, as evaluated by the cell viability, apoptosis-related proteins and serum LDH level (Fig. 6I–M). Therefore, we concluded that matrine prevented DOX-induced downregulation of UCP2 via activating AMPKα.
Figure 6.
Matrine prevented DOX-induced downregulation of UCP2 via activating AMPKα. (A) and (B) Western blot and statistical results (n = 6). (C)–(F) Western blot and statistical results (n = 6). (G) Statistical results of intracellular ROS production detected by DCFH-DA in H9C2 cells (n = 5). (H) The content of MDA, NADPH oxidase activity and total SOD activity in H9C2 cells (n = 5). (I)–(K) Western blot and statistical results (n = 6). (L) Cell viability by CCK-8 (n = 5). (M) Relative LDH levels (n = 5). Values represent the mean±SEM. *P< 0.05 versus the matched groups. NS indicates no statistical difference.
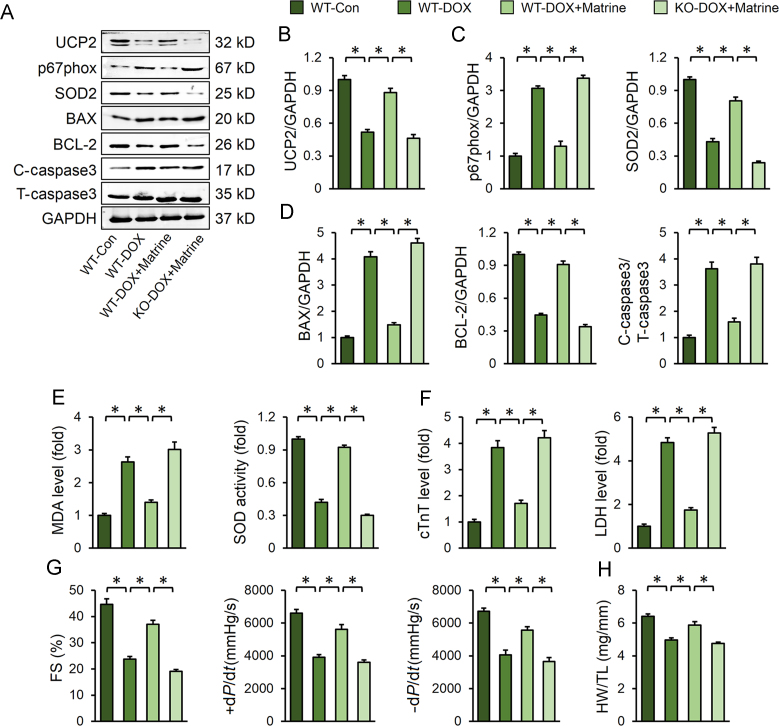
3.7. Ampkα deficiency abolished the protective effect of matrine in vivo
To further investigate whether matrine exerted its protective effects through AMPKα/UCP2 pathway axis in vivo, Ampkα2 KO mice were used. Consistent with the data in vitro, we observed that Ampkα deletion suppressed matrine-induced upregulation of UCP2 in murine hearts (Fig. 7A). Besides, matrine-mediated downregulation of p67phox, BAX, and C-caspase3, as well as upregulation of SOD2 and BCL-2, was all reversed by Ampkα deletion (Fig. 7C and D). In line with the molecular alterations, matrine lost its protective effects on oxidative stress and cardiomyocyte apoptosis in Ampkα-deficient mice, as evidenced by the MDA level, SOD activity and serum myocardial injury markers (Fig. 7E–F). More importantly, we found that matrine could attenuate DOX-induced cardiac dysfunction in wild type mice, but not in Ampkα-deficient mice (Fig. 7G). The protective effect of matrine on HW/TL was also abolished in Ampkα-deficient mice (Fig. 7H). Collectively, these results suggested that the protective effects of matrine on oxidative stress and cardiomyocyte apoptosis were dependent on the activation of AMPKα/UCP2 axis.
Figure 7.
Ampkα deficiency abolished the protective effect of matrine in vivo. (A)–(D) Representative Western blot images and statistical results (n = 6). (E) The content of MDA and total SOD activity (n = 6). (F) Plasma levels cTnT and LDH (n = 6). (G) Heart function evaluated by echocardiography and hemodynamic analysis of mice (n = 8). (H) Statistical results of the HW/TL (n = 12). Values represent the mean±SEM. *P< 0.05 versus the matched groups.
4. Discussion
DOX significantly increased the survival rate of cancer patient; nevertheless, many survivors suffer from DOX-induced cardiotoxicity with left ventricular dysfunction and heart failure, which markedly reduced the quality of life for survivors1. Thus, a promising pharmacological therapy for DOX-induced cardiotoxicity is urgently need to be addressed. In this study, we determined that matrine attenuated DOX-induced cardiotoxicity via inhibiting oxidative stress and cardiomyocyte apoptosis. Mechanistically, we found that matrine suppressed UCP2 downregulation in DOX-treated murine hearts, thus exerted beneficial effects on oxidative stress and cardiomyocyte apoptosis. And we also verified that AMPKα activation was responsible for restoring the expression of UCP2. Either AMPKα deletion or UCP2 inhibition with genetic or pharmacological methods abrogated matrine-mediated beneficial effects on oxidative stress and cardiomyocyte apoptosis both in vivo and in vitro. Therefore, our present study identified matrine as a promising therapeutic agent against DOX-induced cardiotoxicity.
Increased oxidative damage due to ROS overproduction has been actually implicated in the progression of DOX-induced cardiotoxicity43. Excessive ROS generation may cause DNA damage, mitochondrial dysfunction and protein synthesis inhibition, which were linked to the activation of apoptotic cascades and ultimately contribute to heart failure44. Zhu et al.45 found that dietary nitrate supplementation decreased oxidative damage and protected against DOX-induced cardiomyopathy in mice. Moreover, Zhang et al.43 previously defined topoisomerase-II β (Top2β) as an essential driver of DOX-induced oxidative stress and cardiomyocyte apoptosis. DOX application suppressed DNA replication and resulted in cell cycle arrest and apoptosis of cancer cells when binding to Top2α. However, when DOX bound to Top2β, it increased ROS generation, impaired mitochondrial function and triggered cardiomyocyte apoptosis for the absence of Top2α and the abundance of Top2β within the heart. These findings collectively suggested that oxidative damage was responsible for the occurrence of DOX-induced cardiac dysfunction. Accordingly, targeting oxidative stress, many cardioprotective agents including N-acetylcystiene, angiotensin converting enzyme inhibitors and statins have been tested in clinical trials with some efficacy for recovery of cardiac function but are not used prophylactically. Dexrazoxane is the only cardioprotective agent proven to be effective in the prevention and treatment of DOX-induced cardiotoxicity46., 47.. It is regarded as a derivative of EDTA and can decrease ROS generation through its iron chelating activity. However, long-term use of high-doses of dexrazoxane resulted in severe side effects, such as hepatic toxicity, early death, etc. Furthermore, dexrazoxane also depletes Top2α and may reduce its chemotherapeutic capacities48. In the present study, we found that matrine prevented DOX-induced oxidative stress and cardiomyocyte apoptosis both in vivo and in vitro, and matrine administration attenuated cardiac dysfunction in response to DOX insult. More importantly, matrine has been clinically used for the treatment of hepatic tumors in the form of capsule or injection solution with little adverse effects (China Food and Drug Administration approvals H20010242 or H20044669; China Food and Drug Administration, 2014)29., 49.. In addition, numerous studies implied that matrine or its derivative WM130 showed beneficial effects on other cancer cells in basic experiment50., 51., 52.. All these data proved that matrine could be an effective and safe therapeutic agent against DOX-induced cardiotoxicity.
UCP2 is increasingly recognized as an important target to restrain oxidative damage in the pathologic processes of various cardiovascular diseases, including atherosclerosis, hypertension and cardiac injury53. UCP2 exerted its protective effects on oxidative stress via uncoupling of respiration, that is, uncoupling oxygen consumption from ATP synthesis54. Previously, Teshima et al.55 found that UCP2 overexpression reduced ROS production in mitochondria and protected cardiomyocytes from oxidative stress-induced cell death. In addition to its antioxidant effect, Turner et al.10 also demonstrated that UCP2 overexpression markedly inhibited mitochondrial Ca2+ uptake and significantly prolonged the contractile effect. Recent studies indicated that myocardial UCP2 expression was downregulated in response to DOX and Ucp2 deficiency further exacerbated DOX-induced oxidative stress and cardiomyocyte apoptosis40. In this study, we observed that matrine alleviated oxidative damage and cardiomyocyte apoptosis via preventing DOX-induced downregulation of UCP2, whereas pharmacological inhibition or genetic deletion of UCP2 abolished the beneficial effects both in vivo and in vitro. UCP2 protein expression has been proved to be regulated at multiple levels, and we found that AMPKα activation was responsible for matrine-mediated upregulation of UCP2, which is consistent with previous studies56., 57.. Long-chain fatty acid was the first described transcriptional activators of Ucp2. Reilly et al.58 and Samec et al.59 found that polyunsaturated fatty acids triggered UCP2 upregulation in 3T3-L1 preadipocytes. One possible candidate for transcriptional activation of Ucp2 by long-chain fatty acid is peroxisomal proliferators-activated receptors (PPARs); however, no such PPAR response elements have been annotated within or in vicinity of the Ucp2 gene. All this suggested that PPAR-mediated regulation of UCP2 appeared to be indirect60. AMPKα is a key controller of energy metabolism and survival during cellular stress, and plays a critical role in regulating lipid metabolism via PPAR-dependent or PPAR-independent manners61. Despite the exact mechanism through which AMPKα activation increases UCP2 expression remains unclear, previous studies also indicated that AMPKα activation increased the expression of UCP2, which is in line with our present study.
5. Conclusions
The present study found that matrine administration attenuated DOX-induced cardiotoxicity via suppressing oxidative stress and cardiomyocyte apoptosis. Matrine treatment resulted in AMPKα activation and subsequently upregulated UCP2 expression to restrain ROS generation and oxidative damage. Our data identified matrine as a promising therapeutic agent for the treatment of DOX-induced cardiotoxicity.
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (Nos. 81470516 and 81500184), the Key Project of the National Natural Science Foundation (No. 81530012, China), the Fundamental Research Funds for the Central Universities (Nos. 2042017kf0085 and 2042015kf0073, China) and Scientific Action Plans for the Prevention and Treatment of Major Diseases-Cardiovascular Diseases (2016ZX-008-01, China).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supporting data associated with this article can be found in the online version at doi:10.1016/j.apsb.2019.03.003.
Contributor Information
Wei Deng, Email: vivideng1982@whu.edu.cn.
Qizhu Tang, Email: qztang@whu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Carvalho C., Santos R.X., Cardoso S., Correia S., Oliveira P.J., Santos M.S. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16:3267–3285. doi: 10.2174/092986709788803312. [DOI] [PubMed] [Google Scholar]
- 2.Li M., Sala V., De Santis M.C., Cimino J., Cappello P., Pianca N. Phosphoinositide 3-Kinase γ inhibition protects from anthracycline cardiotoxicity and reduces tumor growth. Circulation. 2018;138:696–711. doi: 10.1161/CIRCULATIONAHA.117.030352. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka S., Tatsumi T., Shiraishi J., Mano A., Keira N., Matoba S. Amlodipine inhibits doxorubicin-induced apoptosis in neonatal rat cardiac myocytes. J Am Coll Cardiol. 2003;41:870–878. doi: 10.1016/s0735-1097(02)02935-2. [DOI] [PubMed] [Google Scholar]
- 4.Pacher P., Liaudet L., Bai P., Mabley J.G., Kaminski P.M., Virag L. Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation. 2003;107:896–904. doi: 10.1161/01.cir.0000048192.52098.dd. [DOI] [PubMed] [Google Scholar]
- 5.Wang W., Fang H., Groom L., Cheng A., Zhang W., Liu J. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Battogtokh G., Choi Y.S., Kang D.S., Park S.J., Shim M.S., Huh K.M. Mitochondria-targeting drug conjugates for cytotoxic, anti-oxidizing and sensing purposes: current strategies and future perspectives. Acta Pharm Sin B. 2018;8:862–880. doi: 10.1016/j.apsb.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krauss S., Zhang C.Y., Lowell B.B. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X., Zhang X., Zhang Y., Liu M., Jin J., Yan J. Mitochondrial uncoupler triclosan induces vasorelaxation of rat arteries. Acta Pharm Sin B. 2017;7:623–629. doi: 10.1016/j.apsb.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brand M.D., Esteves T.C. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Turner J.D., Gaspers L.D., Wang G., Thomas A.P. Uncoupling protein-2 modulates myocardial excitation-contraction coupling. Circ Res. 2010;106:730–738. doi: 10.1161/CIRCRESAHA.109.206631. [DOI] [PubMed] [Google Scholar]
- 11.Horimoto M., Fulop P., Derdak Z., Wands J.R., Baffy G. Uncoupling protein-2 deficiency promotes oxidant stress and delays liver regeneration in mice. Hepatology. 2004;39:386–392. doi: 10.1002/hep.20047. [DOI] [PubMed] [Google Scholar]
- 12.Lee K.U., Lee I.K., Han J., Song D.K., Kim Y.M., Song H.S. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005;96:1200–1207. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- 13.Chen K., Xu Z., Liu Y., Wang Z., Li Y., Xu X. Irisin protects mitochondria function during pulmonary ischemia/reperfusion injury. Sci Transl Med. 2017;9:1–32. doi: 10.1126/scitranslmed.aao6298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhamrait S.S., Stephens J.W., Cooper J.A., Acharya J., Mani A.R., Moore K. Cardiovascular risk in healthy men and markers of oxidative stress in diabetic men are associated with common variation in the gene for uncoupling protein 2. Eur Heart J. 2004;25:468–475. doi: 10.1016/j.ehj.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Yao F., Zhang M., Chen L. 5′-Monophosphate-activated protein kinase (AMPK) improves autophagic activity in diabetes and diabetic complications. Acta Pharm Sin B. 2016;6:20–25. doi: 10.1016/j.apsb.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinberg G.R., Kemp B.E. AMPK in health and disease. Physiol Rev. 2009;89:1025–1078. doi: 10.1152/physrev.00011.2008. [DOI] [PubMed] [Google Scholar]
- 17.Li J., Zhong L., Wang F., Zhu H. Dissecting the role of AMP-activated protein kinase in human diseases. Acta Pharm Sin B. 2017;7:249–259. doi: 10.1016/j.apsb.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon S.M., Chandel N.S., Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morell M., Burgos J.I., Gonano L.A., Vila P.M. AMPK-dependent nitric oxide release provides contractile support during hyperosmotic stress. Basic Res Cardiol. 2017;113:7–18. doi: 10.1007/s00395-017-0665-7. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.W., Lee S.M., Shin S.M., Hwang S.J., Brooks J.S., Kang H.E. Efficacy of sauchinone as a novel AMPK-activating lignan for preventing iron-induced oxidative stress and liver injury. Free Radic Biol Med. 2009;47:1082–1092. doi: 10.1016/j.freeradbiomed.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Ceolotto G., Gallo A., Papparella I., Franco L., Murphy E., Iori E. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 22.Xu W.H., Hu H.G., Tian Y., Wang S.Z., Li J., Li J.Z. Bioactive compound reveals a novel function for ribosomal protein S5 in hepatic stellate cell activation and hepatic fibrosis. Hepatology. 2014;60:648–660. doi: 10.1002/hep.27138. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., Xu M., Gao Y., Deng Z., Cao H., Zhang W. Matrine induces caspase-independent program cell death in hepatocellular carcinoma through bid-mediated nuclear translocation of apoptosis inducing factor. Mol Cancer. 2014;13:59–70. doi: 10.1186/1476-4598-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu J., Wang K.Q., Xu W.H., Li Y.H., Qi Y., Wu H.Y. The matrine derivate MASM prolongs survival, attenuates inflammation, and reduces organ injury in murine established lethal sepsis. J Infect Dis. 2016;214:1762–1772. doi: 10.1093/infdis/jiw445. [DOI] [PubMed] [Google Scholar]
- 25.Liu N., Kan Q.C., Zhang X.J., Xv Y.M., Zhang S., Zhang G.X. Upregulation of immunomodulatory molecules by matrine treatment in experimental autoimmune encephalomyelitis. Exp Mol Pathol. 2014;97:470–476. doi: 10.1016/j.yexmp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z.W., Wang J.K., Qiu C., Guan G.C., Liu X.H., Li S.J. Matrine pretreatment improves cardiac function in rats with diabetic cardiomyopathy via suppressing ROS/TLR-4 signaling pathway. Acta Pharmacol Sin. 2015;36:323–333. doi: 10.1038/aps.2014.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X., Ma Z.G., Yuan Y.P., Xu S.C., Wei W.Y., Song P. Rosmarinic acid attenuates cardiac fibrosis following long-term pressure overload via AMPKα/Smad3 signaling. Cell Death Dis. 2018;9:102–116. doi: 10.1038/s41419-017-0123-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma Z.G., Yuan Y.P., Xu S.C., Wei W.Y., Xu C.R., Zhang X. CTRP3 attenuates cardiac dysfunction, inflammation, oxidative stress and cell death in diabetic cardiomyopathy in rats. Diabetologia. 2017;60:1126–1137. doi: 10.1007/s00125-017-4232-4. [DOI] [PubMed] [Google Scholar]
- 29.Zeng X.Y., Wang H., Bai F., Zhou X., Li S.P., Ren L.P. Identification of matrine as a promising novel drug for hepatic steatosis and glucose intolerance with HSP72 as an upstream target. Br J Pharmacol. 2015;172:4303–4318. doi: 10.1111/bph.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dando I., Pacchiana R., Pozza E.D., Cataldo I., Bruno S., Conti P. UCP2 inhibition induces ROS/Akt/mTOR axis: role of GAPDH nuclear translocation in genipin/everolimus anticancer synergism. Free Radic Biol Med. 2017;113:176–189. doi: 10.1016/j.freeradbiomed.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N., Yang Z., Xiang S.Z., Jin Y.G., Wei W.Y., Bian Z.Y. Nobiletin attenuates cardiac dysfunction, oxidative stress, and inflammatory in streptozotocin: induced diabetic cardiomyopathy. Mol Cell Biochem. 2016;417:87–96. doi: 10.1007/s11010-016-2716-z. [DOI] [PubMed] [Google Scholar]
- 32.Guo S., Gao C., Xiao W., Zhang J., Qu Y., Li J. Matrine protects cardiomyocytes from ischemia/reperfusion injury by regulating HSP70 expression via activation of the JAK2/STAT3 pathway. Shock. 2018;50:664–670. doi: 10.1097/SHK.0000000000001108. [DOI] [PubMed] [Google Scholar]
- 33.Yuan Y.P., Ma Z.G., Zhang X., Xu S.C., Zeng X.F., Yang Z. CTRP3 protected against doxorubicin-induced cardiac dysfunction, inflammation and cell death via activation of Sirt1. J Mol Cell Cardiol. 2018;114:38–47. doi: 10.1016/j.yjmcc.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Ma Z.G., Dai J., Wei W.Y., Zhang W.B., Xu S.C., Liao H.H. Asiatic acid protects against cardiac hypertrophy through activating AMPKα signalling pathway. Int J Biol Sci. 2016;12:861–871. doi: 10.7150/ijbs.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao H.H., Zhu J.X., Feng H., Ni J., Zhang N., Chen S. Myricetin possesses potential protective effects on diabetic cardiomyopathy through inhibiting IκBα/NF-κB and Enhancing Nrf2/HO-1. Oxid Med Cell Longev. 2017;2017:1–14. doi: 10.1155/2017/8370593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei W.Y., Ma Z.G., Zhang N., Xu S.C., Yuan Y.P., Zeng X.F. Overexpression of CTRP3 protects against sepsis-induced myocardial dysfunction in mice. Mol Cell Endocrinol. 2018;476:27–36. doi: 10.1016/j.mce.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Wang Z., Wang M., Liu J., Ye J., Jiang H., Xu Y. Inhibition of TRPA1 attenuates doxorubicin-induced acute cardiotoxicity by suppressing oxidative stress, the inflammatory response, and endoplasmic reticulum stress. Oxid Med Cell Longev. 2018;2018:1–9. doi: 10.1155/2018/5179468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons J.P., Schols A.M., Buurman W.A., Wouters E.F. Weight loss and low body cell mass in males with lung cancer: relationship with systemic inflammation, acute-phase response, resting energy expenditure, and catabolic and anabolic hormones. Clin Sci. 1999;97:215–223. [PubMed] [Google Scholar]
- 39.Kratz F., Roth T., Fichiner I., Schumacher P., Fiebig H.H., Unger C. In vitro and in vivo efficacy of acid-sensitive transferrin and albumin doxorubicin conjugates in a human xenograft panel and in the MDA-MB-435 mamma carcinoma model. J Drug Target. 2000;8:305–318. doi: 10.3109/10611860008997908. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y., Lei T., Yuan J., Wu Y., Shen X., Gao J. GCN2 deficiency ameliorates doxorubicin-induced cardiotoxicity by decreasing cardiomyocyte apoptosis and myocardial oxidative stress. Redox Biol. 2018;17:25–34. doi: 10.1016/j.redox.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang S., Song P., Zou M.H. Inhibition of AMP-activated protein kinase alpha (AMPKα) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: role of p53 and SIRT1. J Biol Chem. 2012;287:8001–8012. doi: 10.1074/jbc.M111.315812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu L., Liu J., Gao Y., Yu X., Xu G., Huang Y. Uncoupling protein-2 mediates the protective action of berberine against oxidative stress in rat insulinoma INS-1E cells and in diabetic mouse islets. Br J Pharmacol. 2014;171:3246–3254. doi: 10.1111/bph.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang S., Liu X., Bawa-Khalfe T., Lu L.S., Lyu Y.L., Liu L.F. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 44.Tsutsui H., Ide T., Shiomi T., Kang D., Hayashidani S., Suematsu N. 8-oxo-dGTPase, which prevents oxidative stress-induced DNA damage, increases in the mitochondria from failing hearts. Circulation. 2001;104:2883–2885. doi: 10.1161/hc4901.101347. [DOI] [PubMed] [Google Scholar]
- 45.Zhu S.G., Kukreja R.C., Das A., Chen Q., Lesnefsky E.J., Xi L. Dietary nitrate supplementation protects against doxorubicin-induced cardiomyopathy by improving mitochondrial function. J Am Coll Cardiol. 2011;57:2181–2189. doi: 10.1016/j.jacc.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chakrabarti K.B., Hopewell J.W., Wilding D., Plowman P.N. Modification of doxorubicin-induced cardiotoxicity: effect of essential fatty acids and ICRF-187 (dexrazoxane) Eur J Cancer. 2001;37:1435–1442. doi: 10.1016/s0959-8049(01)00145-9. [DOI] [PubMed] [Google Scholar]
- 47.Ichikawa Y., Ghanefar M., Bayeva M., Wu R., Khechaduri A., Naga P.S. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Investig. 2014;124:617–630. doi: 10.1172/JCI72931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao V.A. Iron chelators with topoisomerase-inhibitory activity and their anticancer applications. Antioxid Redox Signal. 2013;18:930–955. doi: 10.1089/ars.2012.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J., Zhu M., Shi R., Yang M. Radix Sophorae flavescentis for chronic hepatitis B: a systematic review of randomized trials. Am J Chin Med. 2003;31:337–354. doi: 10.1142/S0192415X03001107. [DOI] [PubMed] [Google Scholar]
- 50.Cho Y.R., Lee J.H., Kim J.H., Lee S.Y., Yoo S., Jung M.K. Matrine suppresses KRAS-driven pancreatic cancer growth by inhibiting autophagy-mediated energy metabolism. Mol Oncol. 2018;12:1203–1215. doi: 10.1002/1878-0261.12324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou Y.J., Guo Y.J., Yang X.L., Ou Z.L. Anti-cervical cancer role of matrine, oxymatrine and sophora flavescens alkaloid gels and its mechanism. J Cancer. 2018;9:1357–1364. doi: 10.7150/jca.22427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D., Cao Y., Zheng L., Lv D., Chen L., Xing X. Identification of annexin A2 as a target protein for plant alkaloid matrine. Chem Commun. 2017;53:5020–5023. doi: 10.1039/c7cc02227a. [DOI] [PubMed] [Google Scholar]
- 53.Tian X.Y., Ma S., Tse G., Wong W.T., Huang Y. Uncoupling protein 2 in cardiovascular health and disease. Front Physiol. 2018;9:1060–1073. doi: 10.3389/fphys.2018.01060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blanc J., Alves-Guerra M.C., Esposito B., Rousset S., Gourdy P., Ricquier D. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 55.Teshima Y., Akao M., Jones S.P., Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Nishi M., Doi A., Shono T., Furukawa Y., Shimada T. Ghrelin inhibits insulin secretion through the AMPK-UCP2 pathway in beta cells. FEBS Lett. 2010;584:1503–1508. doi: 10.1016/j.febslet.2010.02.069. [DOI] [PubMed] [Google Scholar]
- 57.Liu L., Liu J., Tian X.Y., Wong W.T., Lau C.W., Xu A. Uncoupling protein-2 mediates DPP-4 inhibitor-induced restoration of endothelial function in hypertension through reducing oxidative stress. Antioxid Redox Signal. 2014;21:1571–1581. doi: 10.1089/ars.2013.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Reilly J.M., Thompson M.P. Dietary fatty acids up-regulate the expression of UCP2 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 2000;277:541–545. doi: 10.1006/bbrc.2000.3705. [DOI] [PubMed] [Google Scholar]
- 59.Samec S., Seydoux J., Dulloo A.G. Interorgan signaling between adipose tissue metabolism and skeletal muscle uncoupling protein homologs: is there a role for circulating free fatty acids? Diabetes. 1998;47:1693–1698. doi: 10.2337/diabetes.47.11.1693. [DOI] [PubMed] [Google Scholar]
- 60.Donadelli M., Dando I., Fiorini C., Palmieri M. UCP2, a mitochondrial protein regulated at multiple levels. Cell Mol Life Sci. 2014;71:1171–1190. doi: 10.1007/s00018-013-1407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kewalramani G., An D., Kim M.S., Ghosh S., Qi D., Abrahani A. AMPK control of myocardial fatty acid metabolism fluctuates with the intensity of insulin-deficient diabetes. J Mol Cell Cardiol. 2007;42:333–342. doi: 10.1016/j.yjmcc.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material