Abstract
Preferential treatment of kin is widespread across social species and is considered a central prerequisite to the evolution of cooperation through kin selection. Though it is well known that, among most social mammals, females will remain within their natal group and often bias social behaviour towards female maternal kin, less is known about the fitness consequences of these relationships. We test the fitness benefits of living with maternal sisters, measured by age-specific female reproduction, using an unusually large database of a semi-captive Asian elephant (Elephas maximus) population. This study system is particularly valuable to an exploration of reproductive trends in a long-lived mammal, because it includes life-history data that span multiple generations, enabling a study of the effects of kinship across a female's lifespan. We find that living near a sister significantly increased the likelihood of annual reproduction among young female elephants, and this effect was strongest when living near a sister 0–5 years younger. Our results show that fitness benefits gained from relationships with kin are age-specific, establish the basis necessary for the formation and maintenance of close social relationships with female kin, and highlight the adaptive importance of matriliny in a long-lived mammal.
This article is part of the theme issue ‘The evolution of female-biased kinship in humans and other mammals'.
Keywords: kinship, Asian elephant, fertility, matriliny
1. Introduction
Among social mammals, there is widespread evidence that individuals prefer kin over non-kin as social partners [1–3]. Generally, these preferences are explained by kin selection theory [4], whereby kin-biased cooperation and affiliation are predicted when the inclusive fitness benefits (direct fitness via an individual's own reproduction and indirect fitness via the reproduction of relatives) outweigh the costs of these behaviours. In particular, within matrilocal societies, where females remain in their natal group and males disperse [5,6], females may live in the same social group throughout their lives and are therefore expected to bias altruistic behaviours towards close maternal relatives [1,7]. Empirical observations support these predictions and indicate that most female mammals maintain closer proximity and spatial associations with female maternal kin, suggesting female-biased kinship serves an important adaptive function (see reviews [1,2]).
The most complete information on the measurable fitness consequences of female kinship comes from studies on small, short-lived mammals, which show that the presence of maternal kin enhances female reproduction (see review [2]). For example, in house mice (Mus domesticus), females housed with sisters experienced shorter inter-birth intervals and produced more offspring per litter with greater overall weight, than those housed with non-kin [8]. Among Kalahari meerkats (Suricata suricatta), offspring weight and survival were directly related to the number of maternal kin present [9]. Such studies confirm the reproductive benefits that may be gained through associating with female relatives among short-lived mammals.
When examining the evolutionary consequences of female-biased kinship, however, it is crucial to distinguish between the selection pressures experienced by short- and long-lived mammals. Life-history differences may have significant implications for the adaptive function of kin-biased behaviours. Compared with short-lived species, long-lived mammals experience longer generation times and slower rates of reproduction [10], and suffer from senescence in survival at a relatively younger age [11]. These different life-histories may be linked to different reproduction and survival strategies that must be flexibly used across a long lifespan. For example, because long-lived mammals are characterized by prolonged periods of immaturity [10], mothers are particularly dependent upon the help of relatives. As such, additional investment in youngsters from non-mothers may play a particularly important role in long-lived species, where extra help may enhance the development of immatures [12,13] and lead to higher reproductive success of mothers [12]. It is therefore important to examine the evolutionary consequences of kinship in long-lived mammals to broaden our understanding of the adaptive function of female-biased kinship.
Little research has addressed the fitness benefits associated with maternal kinship in long-lived mammals, however. This basic gap in our knowledge is primarily due to the difficulty of recording the breadth of information, across multiple generations, that is needed to comprehensively analyse the proximate and ultimate effects of kinship. From the few studies that have been able to access longitudinal records of wild or free-ranging long-lived mammals, powerful empirical evidence highlights the need to further explore the evolutionary significance of maternal relatives, particularly that associating with maternal relatives increases care for dependent young [14,15] while also decreasing infant mortality [16,17]. More generally, the presence of close female maternal kin (mothers and sisters) has been found to significantly improve female reproductive success (non-human primates [18–20], cetaceans [21,22] and elephants [12,23]). These results parallel those from human studies, which show that female reproductive success improves with help from mothers and pre-reproductive daughters [24–26].
There are several possible mechanisms driving such improved reproduction when living near maternal relatives. In long-lived mammals, consistent social interactions have been shown to have significant effects (positive or negative) across a range of traits, from physiological (e.g. body condition, stress) to social (e.g. social status) [2,3,27,28]. It is therefore possible the presence (or the absence) of kin, as social partners [1,2], may have direct effects on a female's health and, consequently, her lifetime reproduction success. For example, a study of wild African elephant (Loxodonta africana) herds disturbed by poaching found that females had higher faecal glucocorticoid concentrations (indicative of stress) and lower reproductive output when living in herds with unrelated conspecifics, as compared with females living with relatives [29]. In addition, positive associations with relatives may shorten a female's inter-birth interval by decreasing her required investment in offspring [30], resulting in earlier weaning and earlier re-start of reproductive cycling [31].
While these studies have made important contributions to our understanding of the adaptive value of female-biased kinship, three notable shortcomings constrain interpretations about the evolutionary trends of matriliny. First, studies on wild populations are unable to tease apart the cause and effect of the presence of kin. Large families will be able to maintain access to large areas of resources and have access to a large number of potential helpers, both of which may contribute to a female's reproductive success. Our understanding of the adaptive consequences of kinship would therefore benefit from the ability to control for confounding factors, such as family size and resource availability across different locations, in a species' native environment.
Second, previous work has done little to address the effects of maternal relatives on reproductive success across different stages of life in long-lived mammals, despite the changing needs of individuals as they age. For example, because primiparous females experience a greater risk of pregnancy loss/stillbirth [32] and offspring mortality [32–34], young, inexperienced females may benefit more from supporting kin networks than their older, experienced conspecifics. It is therefore possible that the fitness benefits accrued by living near kin are age-specific, changing over an individual's lifespan, but this has yet to be thoroughly studied (but see [12,35,36]).
Finally, the age of the maternal relative should be considered, yet few studies have considered how optimal strategies for an individual may change over time (but see [35,37,38]). A careful analysis of these strategies requires an examination of siblings over different life stages, which will enable an examination of how an individual may alter its behaviour across its lifespan towards the same relative. Explanations of the evolution of non-parental investment in social mammals include two, non-mutually exclusive hypotheses for how kin may maximize their fitness. One explanation posits that assisting in the care of young may yield direct benefits by providing experience that enables females to become more successful parents [39–42]. In this interpretation, younger, inexperienced females gain future reproductive benefits through helping older sisters. A second hypothesis focuses on indirect fitness benefits, theorizing that females may assist the reproductive efforts of sisters if it significantly improves sister reproductive success [4,30]. It is possible that older females may gain indirect fitness benefits through helping a younger, more fertile sister when they are not investing in their own reproductive efforts. Alternatively, if resources are limited, an older sibling with more experience might benefit more from investing in her own reproductive efforts than those of a younger sister [30]. Indeed, though females may experience differing selective pressures to invest in a sibling's reproductive efforts across different life stages, the consequences of these strategies remain largely unknown.
Here, we investigate the reproductive effects of living near maternal sisters across a female's lifetime in semi-captive Asian elephants (Elephas maximus). The majority of work, to date, on the effects of relatives on female fitness has focused on short-term measures, such as individual offspring growth, condition or survival to breeding age [2]. Consequently, potential effects of relatives on other fitness outcomes, such as annual reproductive output across an individual's lifespan, are not well studied, despite their key importance to a female's lifetime reproductive success. This is particularly true for long-lived mammals which reproduce at a comparatively slower rate [10]. We focus our analysis on maternal sisters for two reasons. First, building on previous work demonstrating the importance of mothers on female reproduction in Asian elephants [12], we seek to expand our understanding of the impact of maternal relatives on sisters. Second, a focus on siblings enables a comparative exploration of the potential benefits or costs of living near maternal relatives across different ages (i.e. older and younger than the focal individual), while holding the type of relatedness constant.
Our study population of elephants offers a particularly good opportunity to effectively address such questions about the adaptive effects of female-biased kinship on reproduction. Asian elephant females live within multi-generational, matrilocal herds [14], which may facilitate the evolution of nepotistic behaviours among female kin. In addition, their long lifespans enable the development of complex and enduring social relationships among female relatives and provide an opportunity to explore the age-specific effects of living near kin. Because elephants, like other long-lived mammals, are characterized by extended periods of immaturity [10], additional investment from female kin may play a particularly important role in these social systems. As such, elephants may provide a useful comparison with other long-lived, social mammalian species, to illuminate the evolutionary mechanisms of female-biased kinship.
We use one of the world's largest, most comprehensive datasets on semi-captive Asian elephants, employed in the timber logging industry, to examine the relationship between maternal kinship and reproductive success in a long-lived mammal. This longitudinal dataset, generated by the Myanma Timber Enterprise (MTE), includes comprehensive demographic information tracked across several generations, enabling a study of fitness benefits over a female's lifetime. We are therefore able to conduct a time–event analysis to investigate the association between a female's annual reproductive output and (1) the presence of a maternal sister and (2) sister age difference. For both analyses, we also consider age-specific effects to explore the importance of maternal relatives across a female's lifetime. A particularly valuable feature of this population is that we may test questions related to kinship while teasing apart critical environmental influences due the unique conditions of the population. While these semi-captive elephants live within their natural habitat and experience natural birth and death rates, unlike their captive counterparts [43], the elephants are employed for sustainable forestry work. Depending on MTE's timber harvesting needs, family members are either kept in their original natal group or relocated. These conditions present a ‘natural experiment’ where some individuals continue to live near relatives while others live without kin. In this way, we may avoid the confounding influence on female reproductive success of factors such as group size, location and inherent differences in mortality and genetic quality. We aim to gain insight into the possible selective pressures driving female-biased kinship in a long-lived, social mammal.
2. Methods
(a). Study population
The timber camps of Myanmar contain the world's largest (N ≈ 5000) remaining semi-captive population of elephants [44]. For over a century, the Extraction Department, Myanma Timber Enterprise, has kept records of each animal's permanently marked identification (ID) number and name, origin (wild-caught or captive-born), date and place of birth, mother's ID number and name, age or year of taming, birth dates and ID numbers of all offspring, date of death or last known date alive and cause of death.
The elephants live within their native forest habitat, distributed across the country, and are used during the day as riding, transport and draft animals, following strict set working hours, working days per year and tonnage per individual. During the night, however, the elephants forage in the forest, unsupervised, and may interact and mate with both wild and tame conspecifics. Breeding rates are natural (without human intervention), and calves are cared for and nursed by the biological mother until lactation no longer supports their demands (approx. age 4). Calves are then separated from the mother and tamed, after which they may return to their natal group or may be relocated, depending upon timber harvesting needs.
(b). The sample
This study included 475 captive-born females, born since 1959 and surviving to at least age 12 (marking the beginning of a female's true reproductive career). No twins were included in this study. Maternal siblings were determined by shared mother ID and only sisters over the age of 5 were considered in the analysis: before this age, individuals are still being nursed and are dependent on their mothers.
The timber landscape is composed of many townships where different logging camps and working groups reside. Based on the ability of the elephants to roam and interact with conspecifics during their free time, relatives living in the same township were considered to be ‘near’ one another whereas those living in disparate townships were considered ‘far’; those living within the same township are more likely to have the opportunity to engage in affiliative behaviours outside working hours compared with those living in different townships. Ultimately, the analysis included individuals from 30 townships across the entire country.
(c). Statistical analysis
All analyses were conducted with R (version 3.4.4). Codes for data analysis can be found in the electronic supplementary material.
(i). Does the presence of a maternal sister have an age-specific effect on female reproduction?
We studied age-specific effects of sister presence on female age-specific reproductive rate by focusing our analysis on three separate life stages. Age-specific fertility in female elephants generally shows a reversed U-shape curve, as seen in humans [45]. However, Hayward et al. [46] found that this curve of age-specific reproductive probability follows a ‘life stages’ pattern in the population: low annual breeding success at ages 5–11; a rapid increase between ages 12 and 21; little change from age 22 to age 43; and a steep decline from 44 onwards. Because the distribution of breeding success follows different, and distinct, distributions between life stages, an analysis that cuts these different life stages into three separate models, treating age as a linear term, is more effective and is a better fit to the data than one global model that includes all ages and treats age as a linear, quadratic or higher-order polynomial term [46]. Therefore, our analysis focused on the latter three stages owing to our interest in breeding success rates.
Because sibling presence is not constant throughout a female's lifetime, we used discrete time–event analysis, with constant and time-varying variables for each year of a females' life. As such, annual breeding success was the dependent variable (binary: 0 = did not produce a calf in the given year; 1 = produced at least one calf), and was analysed using binomial generalized linear mixed-effect models (GLMMs) with a logit-link function through the ‘glmer’ function in the ‘lme4’ package [47]. The main term of interest was sibling presence (time-varying: 0 = no female sibling living within the same township that year, 1 = at least one female sibling present in the same township that year). We chose a binomial approach over continuous number of sisters because few females had more than one sibling present at a given time. We controlled for the following variables, known to potentially influence female reproductive rates, as fixed effects: mother living status [12] (time-varying, categorical: 0 = mother alive, 1 = mother dead, 2 = mother's status unknown); mother origin [45] (0 = wild-born, 1 = captive-born); female birth order [45] (0 = not first-born, 1 = first-born or only-born). In addition, we included a linear term for female age (time-varying, continuous) based on previous work on age-specific reproduction in female elephants in this population [46]. To improve model convergence and interpretation, age was re-scaled to 0–40 (i.e. where the youngest age included in the data (here, age 12) was labelled ‘0’ and the oldest age (here, age 50) was labelled ‘40’. An interaction term, sister nearby × female age, was also included as a fixed effect term to explore whether the effect of a sister's presence changes with female age. Including this interaction term allowed us to not only evaluate the effects of sister presence, but also reveal possible disproportionate effects of such a presence on female reproduction across a life stage.
To adjust for any temporal or spatial variation in birth and death rates across Myanmar, we included ‘year’ (N = 60) and ‘ecological division’ (N = 4) as random terms. The logging townships were divided into four larger areas representing different ecological landscapes, based on metres elevation from sea level: coastal, central, mountainous and northern, using topological data provided by Myanmar Information Management Unit (map ID: MIMU001, 2007). Finally, we included mother ID as a random term to adjust for genetic and maternal effects (e.g. inherited fitness; N = 336) between maternal sisters.
For each life stage, we followed the same statistical methods, using the same fixed and random effects. However, for the oldest life stage, because the variance and standard deviation for all random effects (year, mother ID and ecological division) were null (likely owing to the small sample size of this age group), we used binomial generalized linear models (GLMs) with a logit-link function without random terms, as opposed to GLMMs. Also, owing to the extreme discrepancy between the number of females living with and without a sister nearby in the oldest female age group, we were unable to include squared age and the interaction term of female sibling nearby × female linear age. Instead, both terms were included as separate main effects only.
Female age and sister nearby variables were kept in the final models as they were the variables of main interest in the models. All other fixed terms were retained in models only if they improved explanatory power, determined using Akaike information criteria (AIC) [48]. To do this, we used the drop1() function in R to examine each individual fixed effect, where a likelihood ratio test was conducted between the full model and a model without a particular variable (i.e. ‘single term deletions'). To obtain odds ratios (ORs) instead of coefficients on the logit scale, the regression coefficients and the 95% confidence intervals (CIs) were exponentiated for each final model of interest.
We first tested whether young, inexperienced females (ages 12–21, N = 475) are more likely to reproduce when living near a maternal sister. Within this subset, there were 4389 observation years (not near a sister, N = 3233; near a sister, N = 1156), with 247 births, where each female's reproductive output during the observation period ranged from 0 to 4 calves (mean 0.41 ± 0.69). For each year during this life stage, 0–4 maternal sisters lived nearby (mean 0.26 ± 0.59).
We then investigated the reproductive effects of living near a sister in middle aged females (N = 391, ages 22–43). This subset contained 4878 observation years (not near a sister, N = 3806; near a sister, N = 1072), with 404 births, where each female's reproductive output ranged from 0 to 7 calves (mean 1.04 ± 1.3). For each year during this life stage, 0–4 maternal sisters lived nearby (mean 0.21 ± 0.55).
Finally, we examined the reproductive effects of living near a sister in the oldest age group of females (N = 89, ages 44–50). This subset contained 407 observation years (not near a sister, N = 353; near a sister, N = 54), with 22 births, where each female's reproductive output ranged from 0 to 2 calves (mean 0.26 ± 0.55). For this stage of life, 0–2 maternal sisters lived nearby, annually (mean 0.08 ± 0.31).
(ii). Does the age difference between sisters have an age-specific effect on female reproduction?
We next tested for the effect of sibling age difference on female reproduction. This was carried out in a separate analysis to above, because we here consider only females who lived near at least one sister at some point in their lifetime (N = 151, ages 12–50). We again examine age-specific effects on female reproduction by focusing our analyses on separate life stages (young females, aged 12–21; middle-aged females, aged 22–43). It should be noted we were unable to examine the older female group (ages 44–50) owing to the previously noted small sample size. Like the previous models, we included mother ID as a random effect, necessitating the use of a GLMM. However, we were unable to include the random effects of year and division owing to smaller sample size. We considered the same fixed effects as in the previous models but instead of the interaction term sister nearby × female age, we included interactions between female age and each sibling age difference variable to test the reproductive effects of particular sibling changes with focal female age. Again, we used this interaction to explore the age-specific reproductive effects of sibling age difference. Female age was kept in the final models but other terms were retained in models only if they improved explanatory power, determined using AIC [48].
We first tested if young females (N = 151, ages 12–21) were more likely to reproduce when living near a sister with a particular age difference. Within this subset, there were 1334 observation years, with 93 births, where each female's reproductive output ranged from 0 to 3 calves (mean 0.37 ± 0.68). For each year during this life stage, 0–4 maternal sisters lived nearby (mean 1.04 ± 0.7). Sibling ages were categorized: older sisters (by observation years: 0–5 years (N = 258), 6–10 years (N = 251), 11–15 years (N = 125)) and younger sisters (by observation years: 0–5 years (N = 288), 6–10 years (N = 301), 11–15 years (N = 111)).
We then tested the effect of sibling age difference on reproduction in middle-aged females (N = 118, ages 22–43). Within this subset, there were a total of 1316 observation years, with 119 births, where each female's reproductive output ranged from 0 to 5 calves (mean 0.65 ± 1.1). For each year during this life stage, 0–4 maternal sisters lived nearby (mean 1.02 ± 0.74). Sibling ages were again categorized: older sisters (by observation years: 0–5 years (N = 173), 6–10 years (N = 154), 11–15 years (N = 46)) and younger sisters (by observation years: 0–5 years (N = 246), 6–10 years (N = 335), 11–15 years (N = 218)).
3. Results
(a). Does the presence of a maternal sister have an age-specific effect on female reproduction?
We found that a sister's presence significantly increased a young female's (age 12–21) annual chances of reproduction, but, notably, the interaction between sibling presence and age had a significant negative effect (table 1). For example, for a female at age 13, the chances of reproducing increased from 1 to 3% when a sister was nearby. By contrast, a 19 year old female only increased her annual chances of reproduction in a given year by 0.06% when living near a sister (figure 1). Having a living mother also increased the chances of reproduction (table 1). These results were not confounded by year (S2 = 0.07 ± 0.27), location (S2 = 0.053 ± 0.23), mother identity (S2 = 0.71 ± 0.84) or other non-significant terms that were controlled for.
Table 1.
Time–event model of maternal sister presence on young female reproduction (ages 12–21) in Asian elephants. Terms retained and rejected in the final model (determined using AIC) are shown above and below the intercept, respectively. Mother's identity, year and location were fitted as random terms.
| term | estimate | s.e. | Z-value | p-value |
|---|---|---|---|---|
| sister nearby (0 = far) | 0.8 | 0.33 | 2.35 | <0.05 |
| female age | 0.23 | 0.03 | 7.6 | <0.001 |
| mother's living status (0 = alive) | −0.39 | 0.19 | −2.02 | <0.05 |
| sister nearby × Female age | −0.1 | 0.05 | −1.93 | <0.05 |
| intercept of Full Model | −4.29 | 0.25 | −17.22 | <0.001 |
| birth order (0 = not first-born) | 0.2 | 0.18 | 1.03 | 0.3 |
| mother's origin (0 = wild-born) | −0.2 | 0.19 | −1.06 | 0.29 |
Figure 1.
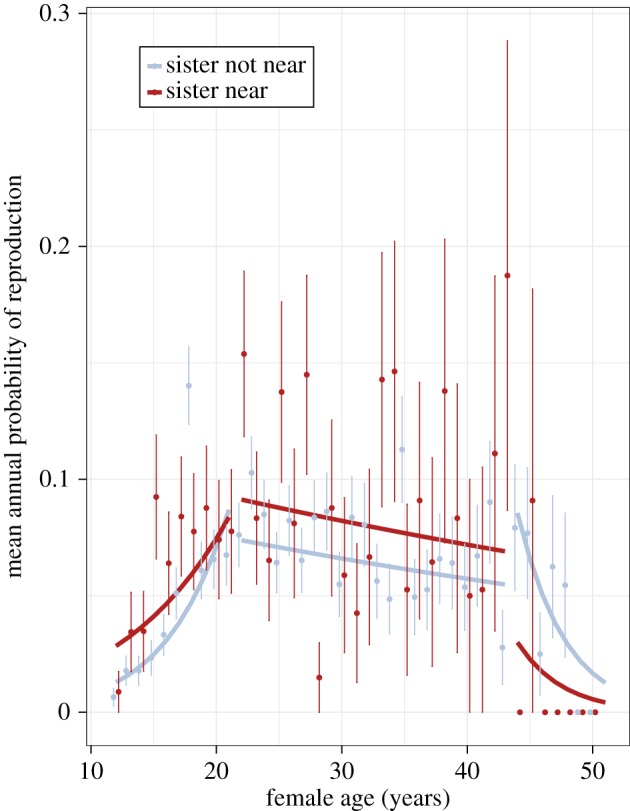
Presence of maternal sister and mean annual probability of female reproduction in Asian elephants. Sister presence improves female reproduction with an age-specific effect whereby younger females benefit from the presence of such relatives. Each line grouping represents predictions made by the three final models (tables 1–3), broken down by agegroup (ages 12–21: N = 475; ages 22–43: N = 391; ages 44–50: N = 89). Each point represents the mean of the annual probability of reproduction from the raw data. Error bars represent 95% standard error. (Online version in colour.)
We found that only maternal living status was able to significantly predict annual female reproduction among middle-aged females (ages 22–43), with those females with living mothers having increased annual chances of reproduction compared with those whose mothers were already deceased (table 2). Middle-aged females were characterized by a constant probability of reproduction, without age-dependent effects, and we did not find that a sister's presence had a significant effect on the probability of annual female reproduction although the probability of reproducing was higher when the sister was nearby (OR: 1.29, CI: 0.86, 1.93; figure 1). These results were not confounded by year (S2 = 0.09 ± 0.31), location (S2 = 0.05 ± 0.22), mother identity (S2 = 0.22 ± 0.47) or other non-significant terms that were controlled for.
Table 2.
Time–event model of maternal sister presence on middle-aged female reproduction (ages 22–43) in Asian elephants. Terms retained and rejected in the final model (determined using AIC) are shown above and below the intercept, respectively.
| term | estimate | s.e. | Z-value | p-value |
|---|---|---|---|---|
| sister nearby (0 = far) | 0.21 | 0.15 | 1.42 | 0.15 |
| mother's living status (0 = alive) | −0.25 | 0.12 | −1.98 | <0.05 |
| female age | −0.01 | 0.01 | −1.56 | 0.12 |
| intercept of full model | −2.5 | 0.2 | −12.29 | <0.001 |
| sister nearby × female age | 0.004 | 0.02 | 0.21 | 0.83 |
| birth order (0 = not first-born) | −0.14 | 0.13 | −1.02 | 0.31 |
| mother's origin (0 = wild-born) | 0.11 | 0.13 | 0.87 | 0.39 |
In older females (ages 44–50), the probability of reproduction decreased clearly with age, but sister presence, mother's living status, birth order and mother origin terms were non-significant (table 3 and figure 1).
Table 3.
Time–event model of maternal sister presence on older female reproduction (ages 44–50) in Asian elephants. Terms retained and rejected in the final model (determined using AIC) are shown above and below the intercept, respectively.
| term | estimate | s.e. | Z-value | p-value |
|---|---|---|---|---|
| female age | −0.3 | 0.14 | −2.14 | <0.05 |
| sister nearby (0 = far) | −1.3 | 1.04 | −1.21 | 0.22 |
| intercept of Full Model | −3.36 | 1.1 | −3.04 | <0.01 |
| mother's living status (0 = dead) | 1.14 | 1.08 | 1.06 | 0.3 |
| birth order (0 = not first-born) | −0.02 | 0.52 | −0.05 | 0.96 |
| mother's origin (0 = wild-born) | 0.79 | 0.53 | 1.5 | 0.14 |
(b). Does the age difference between sisters have an age-specific effect on female reproduction?
We found that having a sister 0–5 years younger living nearby significantly improved the likelihood of annual reproduction of young, inexperienced females (table 4 and figure 2) and that the effect on younger females diminished with age. For example, the chances of reproduction for a female at age 14 increased from 2 to 8% when a sister 0–5 years younger was nearby. On the other hand, a female at age 17 only increased her chances of reproduction by 2% when living near a sister 0–5 years younger. These results were not confounded by mother identity (S2 = 0.44 ± 0.67) or other non-significant terms that were controlled for.
Table 4.
Time–event model of maternal sister age difference on young female reproduction (ages 12–21) in Asian elephants. Terms retained and rejected in the final model (determined using AIC) are shown above and below the intercept, respectively. Mother's identity was fitted as a random term.
| term | estimate | s.e. | Z-value | p-value |
|---|---|---|---|---|
| female age | 0.17 | 0.04 | 3.65 | <0.001 |
| female age × age difference: 0–5 years younger | −0.16 | 0.08 | −1.92 | 0.05 |
| age difference: 0–5 years younger | 1.4 | 0.47 | 3.03 | <0.001 |
| intercept of full model | −3.84 | 0.5 | −8.3 | <0.001 |
| mother's origin (0 = wild-born) | 0.26 | 0.24 | 1.09 | 0.27 |
| mother's living status (0 = dead) | −0.2 | 0.33 | −0.62 | 0.54 |
| birth order (0 = not first-born) | 0.21 | 0.28 | 0.76 | 0.45 |
| age difference: 6–10 years younger | 0.15 | 0.64 | 0.25 | 0.8 |
| age difference: 11–15 years younger | 2.4 | 1.53 | 1.58 | 0.11 |
| age difference: 0–5 years older | −0.27 | 0.71 | −0.38 | 0.7 |
| age difference: 6–10 years older | 0.03 | 0.64 | 0.05 | 0.96 |
| age difference: 11–15 years older | −0.29 | 1.03 | −0.29 | 0.77 |
| female age × age difference: 6–10 years younger | 0.004 | 0.1 | 0.04 | 0.96 |
| female age × age difference: 11–15 years younger | −0.4 | 0.23 | −1.57 | 0.12 |
| female age × age difference: 0–5 years older | 0.05 | 0.12 | 0.45 | 0.65 |
| female age × age difference: 6–10 years older | −0.08 | 0.12 | −0.65 | 0.51 |
| female age × age difference: 11–15 years older | −0.12 | 0.22 | −0.55 | 0.58 |
Figure 2.
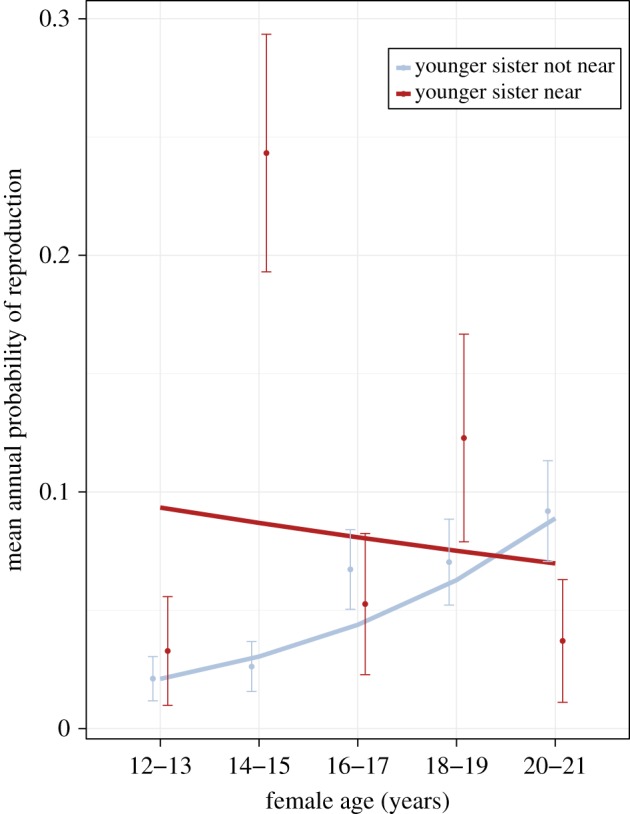
Presence of maternal sister (0–5 years younger) and mean annual probability of young female reproduction in Asian elephants. Young females (N = 151) living near a sister 0–5 years their junior are more likely to reproduce, compared with young females without a sibling in this age range present. This effect is age-specific and diminishes over time as females age. Lines represent final model predictions (table 4) and points represent the mean of the annual probability of reproduction from the raw data. Error bars represent 95% standard error. (Online version in colour.)
We did not find any of our predictor terms to have a significant effect on middle-aged female reproduction (table 5).
Table 5.
Time–event model of maternal sister age difference on middle-aged female reproduction (ages 22–43) in Asian elephants. Terms retained and rejected in the final model (determined using AIC) are shown above and below the intercept, respectively. Mother's identity was fitted as a random term.
| term | estimate | s.e. | Z-value | p-value |
|---|---|---|---|---|
| female age | −0.2 | 0.02 | −1.4 | 0.16 |
| age difference: 11–15 years older | −6.19 | 3.5 | −1.7 | 0.07 |
| female age × age difference: 11–15 years older | 0.3 | 0.18 | 1.9 | 0.06 |
| intercept of full model | −2.04 | 0.54 | −3.8 | <0.01 |
| mother's origin (0 = wild-born) | 0.28 | 0.21 | 1.32 | 0.18 |
| mother's living status (0 = dead) | −0.19 | 0.23 | −0.8 | 0.4 |
| birth order (0 = not first-born) | −0.01 | 0.25 | −0.05 | 0.96 |
| age difference: 0–5 years younger | −0.63 | 1.27 | −0.49 | 0.62 |
| age difference: 6–10 years younger | −1.5 | 1.17 | −1.3 | 0.18 |
| age difference: 11–15 years younger | −0.8 | 1.4 | −0.5 | 0.56 |
| age difference: 0–5 years older | 1.79 | 1.3 | 1.3 | 0.18 |
| age difference: 6–10 years older | 1.3 | 1.9 | 0.67 | 0.5 |
| female age × age difference: 0–5 years younger | 0.02 | 0.04 | 0.46 | 0.64 |
| female age × age difference: 6–10 years younger | 0.05 | 0.04 | 1.29 | 0.19 |
| female age × age difference: 11–15 years younger | 0.02 | 0.05 | 0.5 | 0.62 |
| female age × age difference: 0–5 years older | −0.05 | 0.05 | −1.03 | 0.3 |
| female age × age difference: 6–10 years older | −0.04 | 0.07 | −0.6 | 0.55 |
4. Discussion
Though it is well established that relationships with relatives are a fundamental and universal aspect of mammalian sociality [1], less is known about the long-term fitness consequences of female-biased kinship, particularly among long-lived mammals [2]. Here, we measured the effects of maternal sister presence on female reproduction across a lifespan in a semi-captive population of Asian elephants. We report that living near a sister significantly increased the likelihood of annual female elephant reproduction among young individuals (ages 12–21). Upon further exploration of this effect, we found that living near a sister 0–5 years younger is associated with a higher likelihood of young female reproduction. These findings have implications for our understanding of the fitness consequences of relationships with female relatives, as well as the selective pressures driving these social bonds.
The presence of a maternal sister was positively and significantly associated with annual female reproduction. Several mechanisms may be driving this difference in birth rates across individuals. For instance, because pregnancy failures are common among Asian elephants [32], it is possible that a sister's presence may protect a female from potential stressors that trigger such losses. Indeed, among long-lived mammals, consistent social interactions are associated with numerous health benefits (see review by Silk [3] and Kikusui et al. [27]) and close kin are often preferred social partners [1,2]. The loss of such relatives is associated with stress and negative health effects [49]. Among wild African elephants, for example, sociality is associated with improved body condition [28,50] and kinship is a strong predictor of female social relationships [51]. Similar findings have been reported in humans: greater social integration is associated with reduced mortality and better physical and mental health [27], particularly for women [52]. In their study on the effects of mother's presence on human female reproduction, Lahdenperä et al. [24] found that females not only experienced higher reproductive output when living near their mothers, but also produced more offspring throughout their lifetimes, and with higher survival rates. Considering the numerous benefits that may be gained through social ties with female kin across mammalian species, the ability to associate with a maternal sister may therefore have positive effects on female fertility and reproduction in Asian elephants.
It should be noted, however, that we found an age-specific effect throughout our analyses where young females were more likely than their older conspecifics to reproduce within a given year when living near a sister. While the proximate mechanism driving enhanced reproduction may be related to improved health generated by sociality [3,27], such disproportional benefits accrued across a lifespan may also be the result of two age-specific biological and behavioural phenomena. First, young females often have lower bodily resources available for mobilization during pregnancy and lactation [53], perhaps owing to the trade-offs faced by such youngsters between allocating energy to reproduction and to their own continued development [54]. This paradox is exacerbated by an extended period of immature development, which significantly increases the energetic burden placed on a mother [10]; females in our population gained height on average until age 15 and weight until age 35 [55]. Second, parenting experience may be critical to successfully rear offspring [39–42], positioning young, inexperienced females at a disadvantage. Indeed, offspring of young, inexperienced females often suffer greater mortality [32–34]; assistance from relatives may therefore be particularly valuable to young females. Similar results to the ones reported here have been found among Asian elephants, where a maternal grandmothers’ presence is associated with improved grand-calf survival and increased reproductive output of the daughter, and this effect was particularly strong among young females [12]. The combined theoretical and empirical support presented here suggests both experience and energetic demands may play an important role in mediating relationships with female maternal relatives, particularly early in the female reproductive career.
We also found that the presence of a sibling 0–5 years younger is associated with a higher likelihood of female reproduction. This result may be interpreted through an understanding of common explanations of alloparental care, often where behavioural decisions may be simplified to assisting the reproductive efforts of relatives versus seeking direct fitness benefits via reproductive opportunities [30]. In our analysis, the grouping of sisters 0–5 years younger than the ‘young female group’ ranged in age from 7 to 16, whereas peak probability of reproduction is seen among females aged 18–22. Because these younger sisters are not within the age range of peak reproduction, rather than risk seeking out breeding opportunities themselves, they may benefit more through assisting the reproductive efforts of their older sisters by promoting their annual reproductive output, and perhaps ultimately gaining both parenting experience and indirect fitness benefits through helping related infants [39–42]. Alternatively, a maternal effect may generate these results, whereby females with shorter inter-birth intervals are in better condition and have daughters with relatively higher reproductive output. This is unlikely, though, because we only see an effect of younger siblings on young female annual reproductive probability, whereas a maternal effect would predict a high reproductive output across a female's reproductive career. We would expect a similar effect in the presence of 0–5 years older siblings, but this was not found. Furthermore, we include mother ID as a random effect to help control for such heritable differences.
While it is also possible that older sisters may gain indirect fitness benefits from promoting their younger sisters' reproduction, we did not find any evidence of this effect in our study. These results may be explained by competition between two potentially reproductive females. If resources are limited, for example, an older sibling with more experience might invest more in her own reproductive efforts than those of a younger sister [30]. Alternatively, though older sisters may not promote annual female reproduction, they may provide investment in other ways not measured here, such as direct care for the offspring. Nonetheless, our findings establish that sibling effects on female fertility are not uniform over time, but, rather, differ across an individual's lifespan.
Some caveats must be noted when interpreting these results. First, females in these groups live near other types of kin and non-kin, which may also contribute to their reproduction. In social mammals, while mothers and sisters are generally considered preferred social partners [1,2], other maternal relatives may also serve as allies in these groups, such as aunts and cousins. Generally, however, such categories of kin are considered ‘distant relatives’ and are not typically associated with the fitness benefits gained through social integration [2,7]. Second, our location data do not consider the potential for multiple transfers of the focal female or her sister, and mainly list the first allocated working location of a given individual. However, this would most likely underestimate (and not over-estimate) the effects of sisters on female reproductive rate by causing greater variation in the dataset and weaker statistical significance (e.g. among middle-aged females where clear positive effects of sisters were visible). Despite not knowing the specific transfer patterns, we are nonetheless still able to conclude that females living with and without sisters experience different annual rates of reproduction.
Our study demonstrates the adaptive value of female-biased kinship in a long-lived species and provides support to a greater body of knowledge, suggesting that social bonds with female maternal relatives improve female fertility and fitness. In humans, general sociality is tied to enhanced physical and mental health [45], and this effect is particularly strong among females [52]. Furthermore, human female reproductive success significantly improves with help from close female maternal kin, such as mothers and pre-reproductive daughters [24–26]. Our results build on these findings, showing that fitness benefits gained from relationships with kin are age-specific for both the female and her relatives in Asian elephants. It is therefore possible that, among long-lived mammals, the selective pressures for female-biased kinship change across an individual's lifespan, where the motivation to nurture social bonds with female relatives is age-dependent, resulting in different fitness benefits. Overall, this study establishes a basis necessary for the formation and maintenance of close social relationships with female kin among social, long-lived mammals.
Supplementary Material
Acknowledgements
We are grateful to the Ministry of Natural Resources and Environment Conservation, the Government of the Union of Myanmar for their permission to work with the Myanma Timber Enterprise, and all the vets and officers involved in the data collection. Specifically, we thank Thuzar Thwin for collecting the location data. We thank all members of the Myanmar Timber Elephant Project for their help and support, as well as Simon N. Chapman, John Jackson and Robert Lynch for their assistance with data formatting, analysis and graphic composition.
Data accessibility
The data used for this analysis were generated by the Myanma Timber Enterprise and are the property of the Myanmar Government. While we are unable to authorize public use of the data, specific requests for re-analysis purposes may be directed to V.L. The materials supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
E.C.L., V.L. and M.L. conceived and designed the paper. E.C.L. performed all analyses and wrote the paper with contributions from all authors. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing financial interests.
Funding
This study was funded by the European Research Council (V.L. and E.C.L.), the Kone Foundation (M.L.) and the Academy of Finland (V.L.).
References
- 1.Smith JE. 2014. Hamilton's legacy: kinship, cooperation and social tolerance in mammalian groups. Anim. Behav. 92, 291–304. ( 10.1016/j.anbehav.2014.02.029) [DOI] [Google Scholar]
- 2.Silk JB. 2007. The adaptive value of sociality in mammalian groups. Phil. Trans. R. Soc. B 362, 539–559. ( 10.1098/rstb.2006.1994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silk JB. 2014. Evolutionary perspectives on the links between close social bonds, health, and fitness. In Sociality, hierarchy, health: comparative biodemography: a collection of papers (ed. M Weinstein, MA Lane), §6. Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- 4.Hamilton WD. 1964. The genetical evolution of social behavior, I and II. J. Theor. Biol. 7, 1–52. ( 10.1016/0022-5193(64)90038-4) [DOI] [PubMed] [Google Scholar]
- 5.Clutton-Brock TH, Lukas D. 2012. The evolution of social philopatry and dispersal in female mammals. Mol. Ecol. 21, 472–492. ( 10.1111/j.1365-294X.2011.05232.x) [DOI] [PubMed] [Google Scholar]
- 6.Greenwood PJ. 1980. Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162. ( 10.1016/S0003-3472(80)80103-5) [DOI] [Google Scholar]
- 7.Silk JB. 2002. Kin selection in primate groups. Int. J. Primatol. 23, 849–875. ( 10.1023/A:1015581016205) [DOI] [Google Scholar]
- 8.König B. 1994. Fitness effects of communal rearing in house mice: the role of relatedness versus familiarity. Anim. Behav. 48, 1449–1457. ( 10.1006/anbe.1994.1381) [DOI] [Google Scholar]
- 9.Russell AF, Clutton-Brock TH, Brotherton PN, Sharpe LL, Mcilrath G, Dalerum FD, Cameron EZ, Barnard JA. 2002. Factors affecting pup growth and survival in co-operatively breeding meerkats Suricata suricatta. J. Anim. Ecol. 71, 700–709. ( 10.1046/j.1365-2656.2002.00636.x) [DOI] [Google Scholar]
- 10.Carey JR. 2003. Life span: a conceptual overview. New York, NY: Population Council. [Google Scholar]
- 11.Turbill C, Ruf T. 2010. Senescence is more important in the natural lives of long- than short-lived mammals. PLoS ONE 5, e12019 ( 10.1371/journal.pone.0012019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lahdenperä M, Mar KU, Lummaa V. 2016. Nearby grandmother enhances calf survival and reproduction in Asian elephants. Sci. Rep. 6, 27213 ( 10.1038/srep27213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee PC. 1987. Allomothering among African elephants. Anim. Behav. 35, 278–291. ( 10.1016/S0003-3472(87)80234-8) [DOI] [Google Scholar]
- 14.McComb K, Moss C, Durant SM, Baker L, Sayialel S. 2001. Matriarchs as repositories of social knowledge in African elephants. Science 292, 491–494. ( 10.1126/science.1057895) [DOI] [PubMed] [Google Scholar]
- 15.Christal J, Whitehead H. 2001. Social affiliations within sperm whale (Physeter macrocephalus) groups. Ethology 107, 323–340. ( 10.1046/j.1439-0310.2001.00666.x) [DOI] [Google Scholar]
- 16.Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. 2009. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc. R. Soc. B 276, 3099–3104. ( 10.1098/rspb.2009.0681) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silk JB, Alberts SC, Altmann J. 2003. Social bonds of female baboons enhance infant survival. Science 302, 1231–1234. ( 10.1126/science.1088580) [DOI] [PubMed] [Google Scholar]
- 18.Fairbanks LA, McGuire MT. 1986. Age, reproductive value, and dominance-related behaviour in vervet monkey females: cross-generational influences on social relationships and reproduction. Anim. Behav. 34, 1710–1721. ( 10.1016/S0003-3472(86)80258-5) [DOI] [Google Scholar]
- 19.Borries C. 1988. Patterns of grandmaternal behaviour in free-ranging Hanuman langurs (Presbytis entellus). Hum. Evol. 3, 239–259. ( 10.1007/BF02435856) [DOI] [Google Scholar]
- 20.Pavelka MSM, Fedigan LM, Zohar S. 2002. Availability and adaptive value of reproductive and postreproductive Japanese macaque mothers and grandmothers. Anim. Behav. 64, 407–414. ( 10.1006/anbe.2002.3085) [DOI] [Google Scholar]
- 21.Foster EA, Franks DW, Mazzi S, Darden SK, Balcomb KC, Ford JKB, Croft DP. 2012. Adaptive prolonged postreproductive life span in killer whales. Science 337, 1313 ( 10.1126/science.1224198) [DOI] [PubMed] [Google Scholar]
- 22.Frère CH, Krützen M, Mann J, Connor RC, Bejder L, Sherwin WB. 2010. Social and genetic interactions drive fitness variation in a free-living dolphin population. Proc. Natl Acad. Sci. USA 107, 19 949–19 954. ( 10.1073/pnas.1007997107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss CJ, Croze H, Lee PC. 2011. The Amboseli elephants: a long-term perspective on a long-lived mammal. Chicago, IL: University of Chicago Press. [Google Scholar]
- 24.Lahdenperä M, Lummaa V, Helle S, Tremblay M, Russell AF. 2004. Fitness benefits of prolonged post-reproductive lifespan in women. Nature 428, 178–181. ( 10.1038/nature02367) [DOI] [PubMed] [Google Scholar]
- 25.Hawkes K. 2004. Human longevity: the grandmother effect. Nature 428, 128–129. ( 10.1038/428128a) [DOI] [PubMed] [Google Scholar]
- 26.Sear R, Mace R. 2008. Who keeps children alive? A review of the effects of kin on child survival. Evol. Hum. Behav. 29, 1–18. ( 10.1016/j.evolhumbehav.2007.10.001) [DOI] [Google Scholar]
- 27.Kikusui T, Winslow JT, Mori Y. 2006. Social buffering: relief from stress and anxiety. Phil. Trans. R. Soc. B 361, 2215–2228. ( 10.1098/rstb.2006.1941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meehan CL, Mench JA, Carlstead K, Hogan JN. 2016. Determining connections between the daily lives of zoo elephants and their welfare: an epidemiological approach. PLoS ONE 11, e0158124 ( 10.1371/journal.pone.0158124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gobush KS, Mutayoba BM, Wasser SK. 2008. Long-term impacts of poaching on relatedness, stress physiology, and reproductive output of adult female African elephants. Conserv. Biol. 22, 1590–1599. ( 10.1111/j.1523-1739.2008.01035.x) [DOI] [PubMed] [Google Scholar]
- 30.Emlen ST. 1995. An evolutionary theory of the family. Proc. Natl Acad. Sci. USA 92, 8092–8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kachel AF, Premo LS, Hublin J-J. 2011. Modeling the effects of weaning age on length of female reproductive period: implications for the evolution of human life history. Am. J. Hum. Biol. 23, 479–487. ( 10.1002/ajhb.21157) [DOI] [PubMed] [Google Scholar]
- 32.Mar KU, Lahdenperä M, Lummaa V. 2012. Causes and correlates of calf mortality in captive Asian elephants (Elephas maximus). PLoS ONE 7, e32335 ( 10.1371/journal.pone.0032335) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silk JB. 1988. Maternal investment in captive bonnet macaques (Macaca radiata). Am. Nat. 132, 1–19. ( 10.1086/284834) [DOI] [Google Scholar]
- 34.Smuts B, Nicolson N. 1989. Reproduction in wild female olive baboons. Am. J. Primatol. 19, 229–246. ( 10.1002/ajp.1350190405) [DOI] [PubMed] [Google Scholar]
- 35.Nitsch A, Faurie C, Lummaa V. 2013. Are elder siblings helpers or competitors? Antagonistic fitness effects of sibling interactions in humans. Proc. R. Soc. B 280, 20122313 ( 10.1098/rspb.2012.2313) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brent LJN, Ruiz-Lambides A, Platt ML. 2017. Family network size and survival across the lifespan of female macaques. Proc. R. Soc. B 284, 20170515 ( 10.1098/rspb.2017.0515) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparkman AM, Adams J, Beyer A, Steury TD, Waits L, Murray DL. 2010. Helper effects on pup lifetime fitness in the cooperatively breeding red wolf (Canis rufus). Proc. R. Soc. B 278, 1381–1389. ( 10.1098/rspb.2010.1921) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nitsch A, Faurie C, Lummaa V. 2014. Alloparenting in humans: fitness consequences of aunts and uncles on survival in historical Finland. Behav. Ecol. 25, 424–433. ( 10.1093/beheco/art126) [DOI] [Google Scholar]
- 39.Margulis SW, Nabong M, Alaks G, Walsh A, Lacy RC. 2005. Effects of early experience on subsequent parental behaviour and reproductive success in oldfield mice, Peromyscus polionotus. Anim. Behav. 69, 627–634. ( 10.1016/j.anbehav.2004.04.021) [DOI] [Google Scholar]
- 40.Stone AI, Mathieu D, Griffin L, Bales KL. 2010. Alloparenting experience affects future parental behavior and reproductive success in prairie voles (Microtus ochrogaster). Behav. Processes 83, 8–15. ( 10.1016/j.beproc.2009.08.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salo AL, French JA. 1989. Early experience, reproductive success, and development of parental behaviour in Mongolian gerbils. Anim. Behav. 38, 693–702. ( 10.1016/S0003-3472(89)80015-6) [DOI] [Google Scholar]
- 42.Fairbanks LA. 1990. Reciprocal benefits of allomothering for female vervet monkeys. Anim. Behav. 40, 553–562. ( 10.1016/S0003-3472(05)80536-6) [DOI] [Google Scholar]
- 43.Clubb R, Rowcliffe M, Lee P, Mar KU, Moss C, Mason GJ. 2008. Compromised survivorship in zoo elephants. Science 322, 1649 ( 10.1126/science.1164298) [DOI] [PubMed] [Google Scholar]
- 44.Leimgruber P, Senior B, Aung M, Songer MA, Mueller T, Wemmer C, Ballou JD. 2008. Modeling population viability of captive elephants in Myanmar (Burma): implications for wild populations. Anim. Conserv. 11, 198–205. ( 10.1111/j.1469-1795.2008.00172.x) [DOI] [Google Scholar]
- 45.Lahdenperä M, Mar KU, Lummaa V. 2014. Reproductive cessation and post-reproductive lifespan in Asian elephants and pre-industrial humans. Front. Zool. 11, 54 ( 10.1186/s12983-014-0054-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hayward AD, Mar KU, Lahdenperä M, Lummaa V. 2014. Early reproductive investment, senescence and lifetime reproductive success in female Asian elephants. J. Evol. Biol. 27, 772–783. ( 10.1111/jeb.12350) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bates D, Maechler M, Bolker B, Walker S. 2014. lme4: Linear mixed-effects models using Eigen and S4. R package version 1.1-7. See https://CRAN.R-project.org/package=lme4.
- 48.Burnham KP, Anderson DR. 2004. Multimodel inference: understanding AIC and BIC in model selection. Sociol. Methods Res. 33, 261–304. ( 10.1177/0049124104268644) [DOI] [Google Scholar]
- 49.Brown JL, Paris S, Prado-Oviedo NA, Meehan CL, Hogan JN, Morfeld KA, Carlstead K. 2016. Reproductive health assessment of female elephants in North American zoos and association of husbandry practices with reproductive dysfunction in African elephants (Loxodonta africana). PLoS ONE 11, e0145673 ( 10.1371/journal.pone.0145673) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pinter-Wollman N, Isbell LA, Hart LA. 2009. The relationship between social behaviour and habitat familiarity in African elephants (Loxodonta africana). Proc. R. Soc. B 276, 1009–1014. ( 10.1098/rspb.2008.1538) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Archie EA, Moss CJ, Alberts SC. 2006. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc. R. Soc. B 273, 513–522. ( 10.1098/rspb.2005.3361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. 2000. Biobehavioral responses to stress in females: tend-and-befriend, not fight-or-flight. Psychol. Rev. 107, 411 ( 10.1037/0033-295X.107.3.411) [DOI] [PubMed] [Google Scholar]
- 53.Dufour DL, Sauther ML. 2002. Comparative and evolutionary dimensions of the energetics of human pregnancy and lactation. Am. J. Hum. Biol. 14, 584–602. ( 10.1002/ajhb.10071) [DOI] [PubMed] [Google Scholar]
- 54.Stearns SC. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268. ( 10.2307/2389364) [DOI] [Google Scholar]
- 55.Mumby HS, Chapman SN, Crawley JA, Mar KU, Htut W, Soe AT, Aung HH, Lummaa V. 2015. Distinguishing between determinate and indeterminate growth in a long-lived mammal. BMC Evol. Biol. 15, 214 ( 10.1186/s12862-015-0487-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this analysis were generated by the Myanma Timber Enterprise and are the property of the Myanmar Government. While we are unable to authorize public use of the data, specific requests for re-analysis purposes may be directed to V.L. The materials supporting this article have been uploaded as part of the electronic supplementary material.


