Summary
Fine-tuning of transcriptional responses can be critical for long-term outcomes in response to an environmental challenge. The circadian protein Nocturnin belongs to a family of proteins that include exonucleases, endonucleases, and phosphatases and is most closely related to the CCR4 family of deadenylases that regulate the cellular transcriptome via control of poly(A) tail length of RNA transcripts. In this study, we investigate the role of Nocturnin in regulating the transcriptional response and downstream metabolic adaptations during cold exposure in brown adipose tissue. We find that Nocturnin exhibits dual localization within the cytosol and mitochondria, and loss of Nocturnin causes changes in expression of networks of mRNAs involved in mitochondrial function. Furthermore, Nocturnin−/− animals display significantly elevated levels of tricarboxylic acid cycle intermediates, indicating that they have distinct metabolic adaptations during a prolonged cold exposure. We conclude that cold-induced stimulation of Nocturnin levels can regulate long-term metabolic adaptations to environmental challenges.
Subject Areas: Biological Sciences, Cell Biology, Metabolomics, Transcriptomics
Graphical Abstract
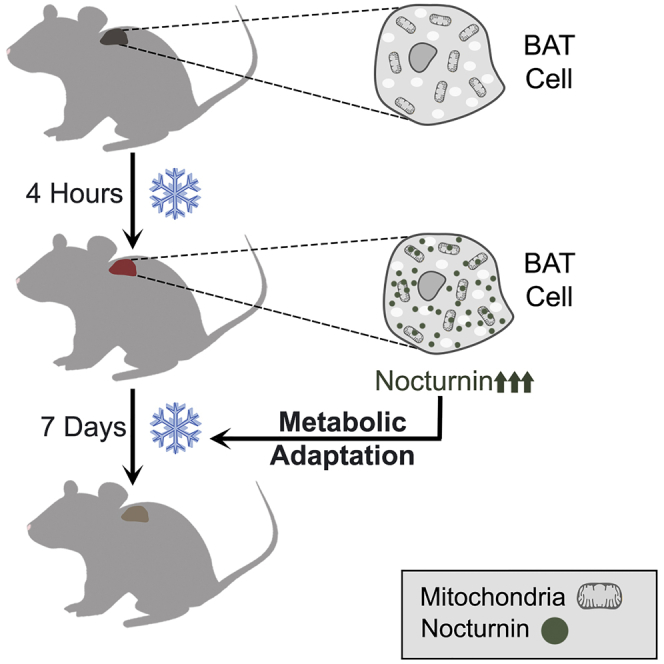
Highlights
-
•
Nocturnin localizes to both the cytosol and the mitochondria
-
•
Nocturnin is robustly induced in response to cold exposure in brown fat
-
•
Regulation of mitochondrial metabolic genes is altered in Nocturnin−/− brown fat
-
•
Nocturnin regulates long-term metabolic adaptation to cold exposure in brown fat
Biological Sciences; Cell Biology; Metabolomics; Transcriptomics.
Introduction
Circadian rhythms allow organisms to adapt to daily alterations in the environment, including changes in energy demand, and are critical for metabolic homeostasis. Indeed, disruptions in circadian rhythms are often correlated with metabolic disorders (Brum et al., 2015, Karlsson et al., 2001, Lamia et al., 2008, Marcheva et al., 2010, Mukherji et al., 2015, Oishi et al., 2006, Rorbach and Minczuk, 2012, Rudic et al., 2004, Turek et al., 2005). Nocturnin (Noct, Ccrn4l) is widely conserved among eukaryotes and is expressed ubiquitously in many mammalian tissues (Baggs and Green, 2003, Wang et al., 2001). As a highly rhythmic output gene of the core circadian clock machinery (Oishi et al., 2003), Nocturnin mRNA levels peak at early night in mice. However, it has also been identified as an immediate-early gene, and it is acutely induced in response to numerous stimuli, including serum deprivation, lipopolysaccharide, and peroxisome proliferator-activated receptor γ (PPARγ) agonist rosiglitazone (Garbarino-Pico et al., 2007, Kawai et al., 2010b, Niu et al., 2011). Nocturnin can also be induced by nutrient and metabolic cues, such as high-fat diet and olive oil gavage (Douris et al., 2011, Green et al., 2007, Stubblefield et al., 2018). Analysis of Nocturnin−/− animals has provided evidence for a role in numerous physiologic processes, including lipid metabolism (Green et al., 2007, Stubblefield et al., 2018), adipogenesis (Kawai et al., 2010b), osteogenesis (Kawai et al., 2010a), and immune function (Niu et al., 2011).
Nocturnin belongs to a family of proteins that include exonucleases, endonucleases, and phosphatases and is most closely related to the CCR4 family of deadenylases, which remove poly(A) tails from mRNAs (Baggs and Green, 2003, Godwin et al., 2013). This potential function and its capacity to respond to environmental cues make Nocturnin a candidate for fine-tuning transcriptional responses to metabolic challenges. By shortening the length of mRNA poly(A) tails, Nocturnin is predicted to destabilize its target mRNAs. RNA sequencing (RNA-seq) analysis of Nocturnin−/− mouse liver revealed increased amplitudes in the daily rhythms of many enzymes regulating triglyceride and cholesterol synthesis, as well as an increased amplitude in response to a fasting-refeeding challenge (Stubblefield et al., 2018). This has led to the model that modulation of Nocturnin levels allows for post-transcriptional regulation of metabolic flux. In this study, we investigate the role of Nocturnin in mediating metabolic adaptation in brown adipose tissue (BAT). We find that Nocturnin exhibits dual localization within cells, with a portion of the protein targeted to the mitochondrion. Nocturnin is acutely induced in BAT in response to cold exposure, and a combination of transcriptomic and metabolic analyses indicate that it regulates long-term metabolic adaptation to cold exposure.
Results
Nocturnin Localizes to Both the Mitochondria and Cytosol
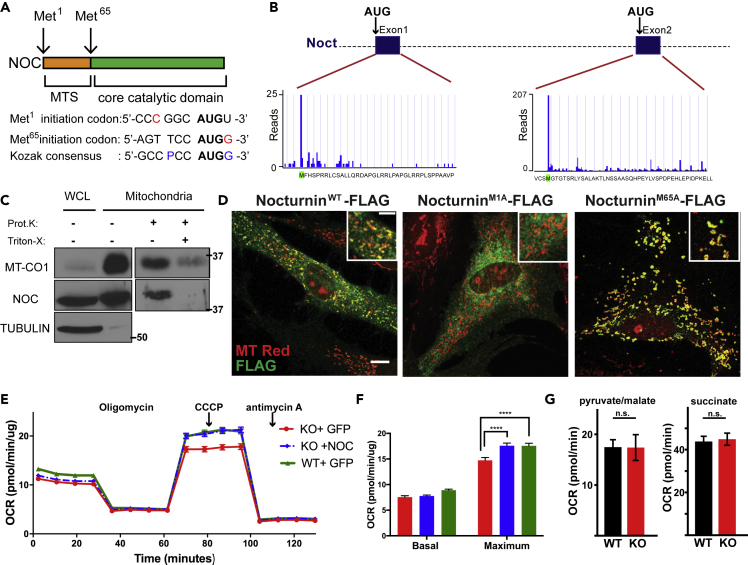
Analysis of Nocturnin's mRNA sequence reveals two potential in-frame translation initiation codons near the 5′ end in exons 1 and 2, separated by 64 amino acids (Figure 1A). Both start codons are surrounded by sequences that diverge from the canonical Kozak sequence. From publicly available quantitative translation initiating ribosome profiling data (Crappe et al., 2015, Fijalkowska et al., 2017, Gao et al., 2015, Gawron et al., 2016, Ji et al., 2015, Raj et al., 2016, Zhang et al., 2017), we found that global aggregate from human cell lines indicated peaks of ribosome occupancy at both methionines, referred to as Met1 and Met65 (numbering based on Mus musculus sequence) (Figure 1B). In addition, elongating ribosomes are readily observed in exon 1, before Met65 (Figure S1A). Analysis of Nocturnin's amino acid sequence (starting at Met1) predicts a putative mitochondrial targeting sequence within the first 65 amino acids, as determined by multiple prediction programs (Figures 1A and S1B). Thus, we hypothesized that the choice of start codon determines Nocturnin's subcellular localization. We carried out biochemical fractionation of HEK293 cells to separate the mitochondrial and cytosolic fractions, followed by a Proteinase K protection assay on the mitochondrial fraction (Figure 1C). A portion of Nocturnin was significantly enriched in the mitochondrial fraction and protected from degradation upon Proteinase K treatment, confirming that Nocturnin localizes within the mitochondrial membrane.
Figure 1.
Nocturnin Localizes to Both the Mitochondria and Cytosol via the Use of Alternative Translation Initiation Sites
(A) Nocturnin contains two potential initiation codons (Met1 and Met65), separated by a mitochondrial targeting sequence. Nucleotides in red show the critical components of the Kozak sequence: a purine (A or G) at position −3 and a G following the AUG start codon.
(B) Quantitative translation initiation sequencing (QTI-seq) analysis of human Nocturnin; graphs and data reproduced from GWIPS-viz database (Michel et al., 2014).
(C) Proteinase K (Prot.K) protection assay of the mitochondrial fraction from HEK293 cells. Mitochondrial fractions were treated with Proteinase K (100 μg/mL) or Triton X-100 (1%), and samples were blotted for MT-CO1 (mitochondrial) and TUBULIN (cytoplasmic) as controls. WCL, whole-cell lysate. Molecular weight markers are indicated in kilodaltons.
(D) Nocturnin−/− MEFs expressing exogenous versions of Nocturnin were stained with MitoTracker (red; mitochondria), FLAG antibody (green; NOCTURNIN). Scale bar, 21μM.
(E) Oxygen consumption rates (normalized to protein concentration) in cells of the indicated genotype. Oligomycin, CCCP (carbonyl cyanide m-chlorophenylhydrazone), and antimycin A were injected at the indicated times.
(F) Bar graph showing averages for basal and maximum respiration. A representative experiment from three independent experiments is shown.
(G) State 4 respiration from permeabilized cells of the indicated genotype in response to complex I (pyruvate/malate) or complex II (succinate) substrates.
N = 16–23 per group, ****p < 0.0001, p values are calculated by two-way ANOVA followed by Tukey's post-hoc test. Data are represented as mean ± SEM; n.s., not significant. See also Figure S1.
We transduced Nocturnin−/− mouse embryonic fibroblasts (MEFs) with lentivirus expressing FLAG-tagged Nocturninwt, as well as FLAG-tagged NocturninM1A or NocturninM65A, to force translation initiation at the second or first start codon, respectively (Figure 1D). NocturninM1A localized to the cytoplasm exclusively, whereas NocturninM65A exhibited predominant mitochondrial localization (as detected by overlap with MitoTracker Red). Cells expressing wild-type Nocturnin exhibited both cytoplasmic and mitochondrial localization. Thus the choice of initiation codon is sufficient to determine Nocturnin's subcellular localization.
Nocturnin's localization to the mitochondrion suggests a potential role in mitochondrial function, and mitochondrial mRNAs are exclusively utilized for the production of components of the electron transport chain. Oxygen consumption measurements in Nocturnin−/− MEFs revealed no significant differences in basal respiration, but a mild reduction in uncoupled respiration (induced by CCCP [carbonyl cyanide m-chlorophenylhydrazone]) (Figures 1E and 1F). Stable re-expression of Nocturnin (Figure S1C) (in Nocturnin−/− MEFs) was sufficient to rescue the defect in uncoupled respiration (Figures 1E and 1F). We therefore used permeabilized cells to assess organellar function in wild-type and Nocturnin−/− MEFs. Analysis of state 4 (uncoupled) mitochondrial respiration (Figure 1G) revealed no defects in complex I (pyruvate/malate) or complex II (succinate) stimulated respiration, indicating that the mild respiratory defect observed in intact cells is due to metabolic alterations, as opposed to inherent reductions in mitochondrial function.
Nocturnin mRNA Is Acutely Induced in Brown Adipose Tissue Following Cold Exposure
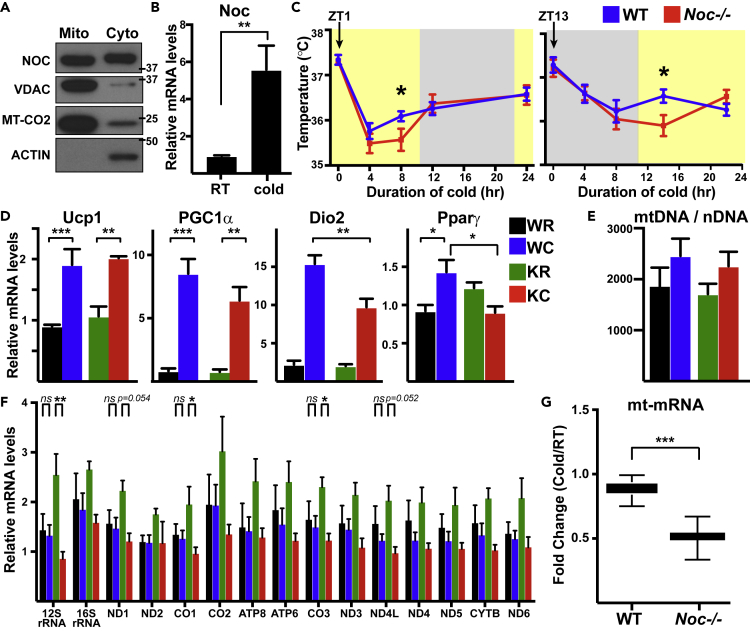
The above results suggest that Nocturnin does not play a major role in regulating mitochondrial function in cultured MEFs but may be specifically important to metabolic control of the uncoupled state. In vivo, BAT is well known to use uncoupled mitochondrial respiration to generate heat in response to cold exposure via the rapid induction of mitochondrial uncoupling proteins (e.g., UCP-1). In this situation, nutrients (e.g., glucose, fatty acid) are rapidly oxidized within the tricarboxylic acid (TCA) cycle, providing substrates for the electron transport chain. Like HEK293 cells, biochemical fractionation of BAT indicated that Nocturnin is present in both cytoplasmic and mitochondrial compartments (Figure 2A). Furthermore, an acute cold-exposure protocol in mice (6°C for 4 h) revealed that Nocturnin transcripts are strongly induced in BAT (Figure 2B). We therefore examined the role of Nocturnin during cold exposure.
Figure 2.
Nocturnin Is Induced in BAT in Response to Acute Cold Exposure
(A) Biochemical fractionation of mitochondrial and cytoplasmic compartments in wild-type BAT, blotted for NOC, VDAC (mitochondrial outer membrane), MT-CO2 (mitochondrial matrix), and ACTIN (cytoplasm) proteins. Molecular weight markers are indicated in kilodaltons.
(B) qRT-PCR analysis of BAT samples from wild-type and Nocturnin−/− littermates kept at room temperature (RT) or exposed to cold (6°C) for 4 h.
(C) Core body temperature of mice during prolonged cold exposure at 6°C; animals placed at cold at ZT1 (left) or ZT13 (right) (N = 8–10/genotype, yellow and gray shading indicating day and night, respectively).
(D) Transcript levels of Ucp1, Pparγ, PGC1α, and Dio2 genes normalized to B2M in BAT tissue of the indicated genotype and condition (N = 3–6). WR, wild-type room temperature; WC, wild-type cold; KR, Nocturnin−/− room temperature; KC, Nocturnin−/− cold.
(E) mtDNA copy numbers in BAT of mice kept at RT or cold (6°C, 4h) (N = 4–5).
(F) qRT-PCR analysis of mitochondrial-encoded genes in BAT. Transcript levels were normalized to mtDNA copy number. (N = 3–5).
(G) Average fold changes for mtDNA genes in response to a 4-h cold exposure in the indicated genotypes.
*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 as analyzed by two-way analysis of variance (ANOVA) with a Tukey's post-hoc test (A, D, and F) or two-tailed Student's t test (C and G). Data are represented as mean ± SEM; ns, not significant.
Nocturnin expression is robustly circadian with peak mRNA and protein levels at the onset of night (ZT12) in murine liver (Garbarino-Pico et al., 2007, Sinturel et al., 2017, Stubblefield et al., 2018, Wang et al., 2001, Zhang et al., 2014). In brown fat, Nocturnin mRNA is also circadian, with peak levels at ZT12 (Zhang et al., 2014). We therefore examined thermogenic responses in mice following cold exposure at two opposite phases of the circadian cycle. WT and Nocturnin−/− mice were put in cold chambers at 1 h after light onset (ZT1) or 1 h after dark onset (ZT13), and temperatures were measured every 4 h for the following 12 h. We observed a mild deficit in thermogenic recovery in the Nocturnin−/− mice in both conditions (Figure 2C). Thus, Nocturnin is not strictly required for cold-temperature adaptation, although adaptation mechanisms appear less robust in Nocturnin−/− animals. Consistent with this, induction of known cold-responsive genes was largely intact in Nocturnin−/− mice, although some genes (e.g., Pparγ) exhibited reduced responsiveness (Figure 2D).
Nocturnin's putative function as a deadenylase suggests a role in post-transcriptional regulation of mRNA stability via control of poly(A) tail length (Baggs and Green, 2003). Twelve of the 13 mRNAs (ND6 is the exception) encoded by the mitochondrial genome are polyadenylated with poly(A) tails shorter than those found on nuclear-encoded mRNAs in the cytoplasm, although the exact role of these tails is still being debated (Rorbach and Minczuk, 2012). We therefore measured levels of mitochondrial RNA (mtRNA) species in the two genotypes at room temperature and following cold exposure. There were no apparent defects in mitochondrial genome content in Nocturnin−/− BAT (Figure 2E), suggesting that mitochondrial biogenesis is unchanged. However, whereas levels of mtRNA species did not change significantly in response to cold exposure in the WT mice, the mtRNAs in the Nocturnin−/− BAT were modestly affected by temperature, with globally elevated levels in room temperature-housed mice and global decreases following cold exposure (Figures 2F and 2G).
Regulation of Mitochondrial Metabolic Genes Is Altered in Nocturnin−/− BAT
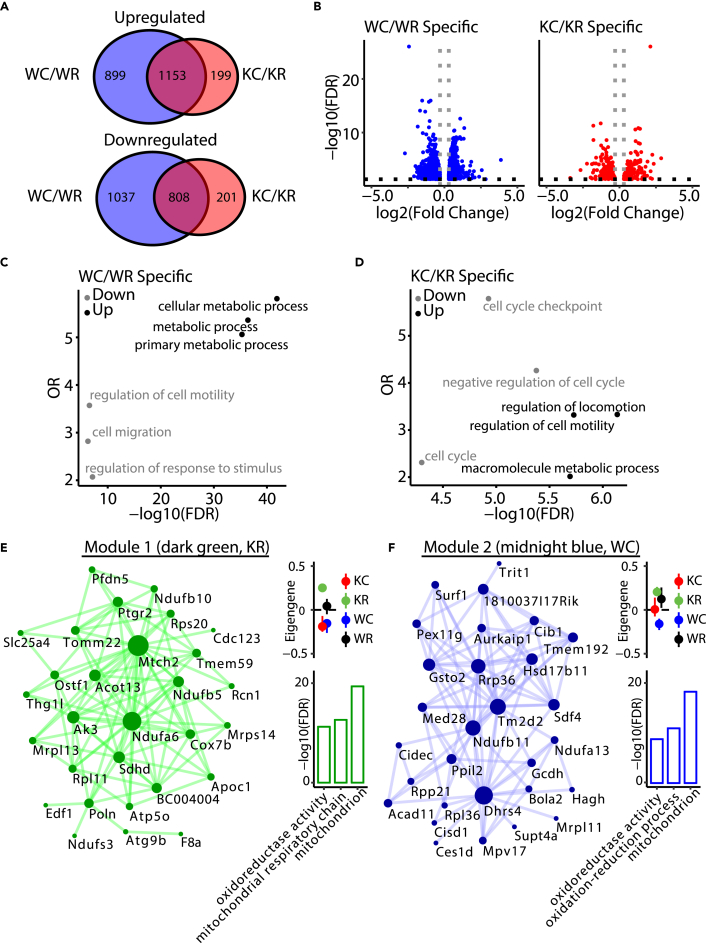
The acute induction of Nocturnin in response to cold (Figure 2B) suggests that it may regulate the overall transcriptional response to cold exposure. We performed mRNA-seq analysis on BAT isolated from wild-type and Nocturnin−/− littermates at room temperature or after a 4-h cold challenge. Differential expression analysis between the two temperatures revealed some changes in the transcriptional response to cold between wild-type and Nocturnin−/− animals (Figures 3A and 3B and Table S1). Many of the mRNAs that showed altered levels in response to temperature were not different between genotypes, and among those genes that were uniquely regulated in either wild-type or Nocturnin−/− animals, similar gene ontology pathways were represented (Figures 3C and 3D, Table S1). Thus the overall transcriptional response to acute cold exposure is largely conserved in Nocturnin−/− animals, with only subtle changes.
Figure 3.
Altered Expression of Mitochondrial Metabolic Genes in Nocturnin−/− BAT Following Cold Exposure
(A) Venn diagrams of common and unique genes that are differentially expressed following 4-h cold exposure in wild-type (WC/WR) and Nocturnin−/− (KC/KR) BAT. Top: Venn diagram of upregulated genes. Bottom: Venn diagram, downregulated genes. Blue, WT; red, Nocturnin−/−. WR, wild-type room temperature; WC, wild-type cold; KR, Nocturnin−/− room temperature; KC, Nocturnin−/− cold.
(B) Volcano plot for uniquely changing genes in WT and Nocturnin−/− BAT following 4-h cold exposure. y axis represents –log10(FDR). x axis represent log2(fold change) from differential expression analysis statistics.
(C and D) Functional enrichment analyses of uniquely differentially expressed genes in (C) WT (# of genes = 1,935) and (D) Nocturnin−/− (# of genes = 399). Downregulated pathways are shown in gray; upregulated pathways are shown in black. y axis represents odds ratios (OR). x axis represents –log10(FDR). The top three most significant categories are shown.
(E and F) Visualization of the top 100 connections ranked by weighted topological overlap values for module 1 (dark green, KR correlated) and module 2 (midnight blue, WC correlated). Node size corresponds to the number of edges (degree). Side dot plots with standard errors demonstrate the association of the modules detected with genotype and temperature. Standard errors are calculated based on the eigengene across samples. Dots represent the mean eigengene for that module. Side bar plots show the top three gene ontology groups of the module based on –log10(FDR). FDR, false discovery rate.
To determine whether there might be gene expression changes driven by the intersection of genotype and temperature at the network level, we performed weighted gene co-expression network analysis (Fontenot et al., 2017, Langfelder and Horvath, 2008) (Table S2). We identified two modules with gene expression patterns driven by samples with a specific combination of genotype and temperature. Module 1 (dark green, Figure 3E) represents genes most highly correlated in the Nocturnin−/− BAT room-temperature samples (see eigengene inset). Module 2 (midnight blue, Figure 3F) represented genes most highly correlated in wild-type BAT cold samples. Interestingly, whereas the dark green and midnight blue modules contain completely independent genes, ontology analysis indicates that both modules are highly enriched in genes linked to mitochondrial function (Figures 3E and 3F, Table S2).
Metabolomics Analysis Reveals Altered Long-Term Metabolic Adaptation in Nocturnin−/− BAT
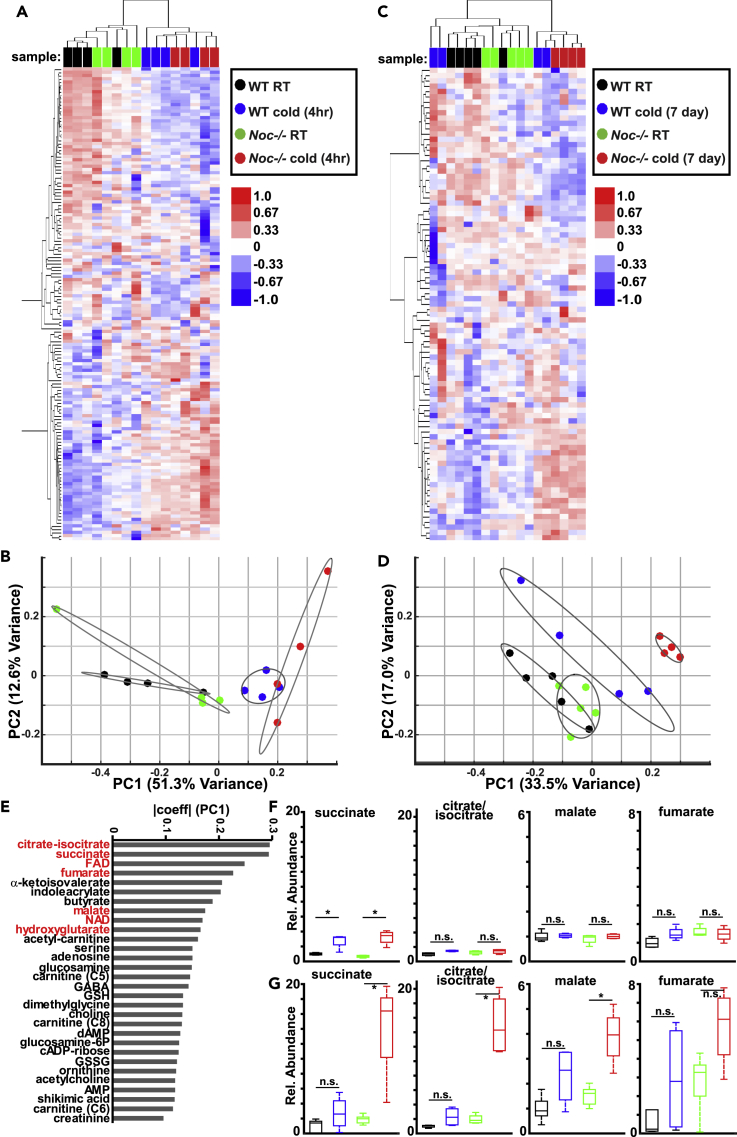
The network analysis above suggests a metabolic role for Nocturnin induction during cold exposure. We therefore performed targeted metabolomics measurements in BAT from wild-type and Nocturnin−/− mice in response to a 4-h cold exposure (Figure 4A, Table S3). Unsupervised hierarchical clustering and principal-component analysis (PCA) reliably separated room-temperature and cold-exposure samples but could not distinguish wild-type and Nocturnin−/− genotypes (Figures 4A and 4B). These results indicate that the overall metabolic response to acute cold exposure is largely maintained in Nocturnin−/− BAT.
Figure 4.
Tricarboxylic Acid Cycle Intermediates Are Altered in Nocturnin−/− BAT in Response to Prolonged Cold Exposure
(A) Heatmap representing hierarchical clustering of normalized metabolite abundances from the indicated genotypes, subject to room temperature (RT) or a 4-h cold exposure. Color bar reflects log-transformed abundance levels. Sample identity is indicated at the top of each column.
(B) Principal-component analysis of wild-type and Nocturnin−/− BAT subject to RT or a 4-h cold exposure. Ovals represent 95% confidence intervals. Contribution of each principal component to total variance is indicated. Same color scheme as (A).
(C) Heatmap representing hierarchical clustering of normalized metabolite abundances from the indicated genotypes, subject to RT or a 7-day cold exposure. Color bar reflects log-transformed abundances. Sample identity is indicated at the top of each column.
(D) Principal-component analysis of wild-type and Nocturnin−/− BAT subject to RT or a 7-day cold exposure. Same color scheme as (C). Ovals represent 95% confidence intervals. Contribution of each principal component to total variance is indicated.
(E) Contribution of the top 30 metabolites contributing to principal component 1 (PC1) for the principal component analysis of 7-day metabolomics shown in (D). Mitochondrial TCA-related metabolites are shown in red. Absolute magnitude of coefficients for each metabolite for PC1 (7-day) are plotted.
(F) Normalized abundances for select TCA metabolites in wild-type and Nocturnin−/− BAT subject to RT or a 4-h cold exposure. Boxplots display the median, 25th and 75th percentiles, and the range for metabolite levels. Same color scheme as (A). N = 4/genotype. *p < 0.05, as analyzed by analysis of variance (ANOVA) with a Tukey's post-hoc test; n.s., not significant.
(G) Same as (F), but for BAT subject to RT or a 7-day cold exposure. Same color scheme as (B). N = 4–5/genotype.
*p < 0.05, as analyzed by analysis of variance (ANOVA) with a Tukey's post-hoc test.
See also Figures S2 and S3 and Table S3.
As Nocturnin's putative function invokes regulation of mRNA stability, we hypothesized that its induction during cold exposure may play a role during longer-term adaptations. To examine this possibility, we assessed animals subject to a prolonged cold exposure (7 days). Nocturnin−/− animals did not display any defects in core body temperature or body weight during this challenge (Figures S2A and S2B). Despite this, steady-state metabolomics revealed striking differences between wild-type and Nocturnin−/− BAT (Figures 4C and 4D, Table S3): Unsupervised hierarchical clustering and PCA revealed that Nocturnin−/− BAT clusters distinctly from other conditions after a 7-day cold exposure. Interestingly, 7-day cold wild-type BAT appears to occupy an intermediate position between room-temperature groups and 7-day cold Nocturnin−/− BAT (Figures 4C and 4D), indicating that long-term metabolic adaptation in Nocturnin−/− BAT is distinct and more profound than in wild-type BAT. To assess the relevant metabolic pathways mediating separation of 7-day cold Nocturnin−/− BAT, we focused on principal component 1 (PC1) in the PCA, which reliably separates these groups. Of the top 10 metabolites contributing to PC1, seven reflect intermediates in the mitochondrial TCA cycle (Figure 4E). This pattern was specific to the 7-day analysis (Figures S3A and S3B). We therefore compared patterns of TCA metabolite abundances in 4-h and 7-day cold-exposure cohorts. BAT is known to readily use succinate as a key mitochondrial fuel source during acute cold exposure (Mills et al., 2018), and Nocturnin−/− animals maintained this metabolic signature (Figure 4F), consistent with their overall ability to maintain thermogenesis. However, succinate levels in wild-type BAT were relatively blunted after 7 days, whereas Nocturnin−/− BAT displayed continued striking increases in succinate levels, accompanied by similar increases in other TCA cycle metabolites (Figure 4G). Consistent with this model, Slc25a10, the mitochondrial dicarboxylate carrier responsible for succinate entry into the mitochondria, is specifically downregulated in wild-type, but not Nocturnin−/−, animals after 4-h cold exposure (Figure S2C). Thus, we propose that whereas BAT metabolism is profoundly altered in response to an acute cold exposure, the induction of Nocturnin serves to suppress the acute metabolic response in the case of long-term cold exposure, thereby mediating long-term adaptation.
Discussion
Our results indicate that the induction of Nocturnin is non-essential during cold exposure in mice, but plays a role in regulating long-term metabolic adaptation of BAT. Mechanistically, the precise function and targets of Nocturnin are unknown, although it has been implicated in de-stabilizing mRNAs (via control of poly(A) tail lengths) as well as circadian control of metabolic enzymes. Tuning of transcriptional responses via regulation of mRNA stability may be important for the long-term outcome to an environmental challenge, and this role for Nocturnin may underlie its role in long-term metabolic adaptation. Alternatively, Nocturnin may have a more direct function in controlling metabolic activity. In addition, dual localization within the cytosol and mitochondria allows Nocturnin to potentially coordinate metabolism in different compartments. Studies examining BAT with exclusively localized mitochondrial versus cytosolic Nocturnin will be need to validate this model. However, the relatively subtle changes in gene expression observed in Nocturnin−/− animals suggest that loss of this gene is having an indirect effect on mitochondrial metabolism rather than targeting a few specific transcripts.
In the setting of cold exposure, BAT must rapidly oxidize nutrients within the mitochondrion to generate heat and maintain body temperature. Recent reports indicate that muscle-derived succinate serves as an important substrate during the acute cold response and that exogenous administration of succinate is sufficient to promote thermogenesis and weight loss (Mills et al., 2018). Acute utilization of succinate appears intact in Nocturnin−/− animals, whereas wild-type animals appear to downregulate succinate oxidation in the long term, perhaps shifting to alternative carbon sources. We hypothesize then that Nocturnin induction serves to limit the amplitude of the initial cold response. In this model, Nocturnin's role in multiple compartments allows for fine-tuning of metabolic adaptations during a prolonged environmental challenge. Despite the striking differences observed in metabolite levels, we observed no overt phenotypes related to body weight or core body temperature in Nocturnin−/− animals subject to prolonged cold. Importantly, steady-state metabolite levels are not directly indicative of pathway flux, and future experiments using in vivo isotope tracing techniques will be required to validate specific mechanisms of metabolic adaptation.
Limitations of the Study
It is important to mention that energy balance and temperature are tightly regulated via hypothalamus and systemic cues, and the lack of obvious phenotypes in Nocturnin−/− animals could be due to compensatory mechanisms such as browning of white adipose tissue or muscle-based thermogenesis. Whether the phenotypes we observe are due to Nocturnin function in the mitochondria, the cytoplasm, or both cannot be determined by the experiments proposed here and will require the generation of mice expressing specific isoforms. In addition, a significant limitation of our study is that our comparisons were made between subjects at 6°C and room temperature, as opposed to thermoneutral animals (∼30°C). In addition, rectal temperature measurements predominantly reflect whole-body temperature and are subject to handling-induced stress, whereas telemetry measurements of BAT temperature will provide a more robust and direct measurement of BAT function. In the future, a detailed phenotypic analysis of BAT in Nocturnin−/− mice will be important to evaluate the functional importance of Nocturnin during long-term stressors.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We would like to acknowledge all Green lab and Mishra lab members for helpful discussions. We thank Peng Gao for sharing the pLJM1-mNOC plasmid. We acknowledge Kfir Lapid for sharing resources and helpful tips on temperature measurements, Shin Yamazaki for helpful suggestions for imaging, Katie Regan for creating the graphical abstract, and Lauren Zacharias and the UT Southwestern CRI Metabolomics Facility. This work was supported by the National Institutes of Health grants R01 GM112991 and R35 GM127122 (C.B.G.).
Author Contributions
Y.O. performed the experimental design, data collection and analysis, as well as manuscript preparation. I.L. performed the Proteinase K treatment experiment. K.S. prepared the mRNA-seq libraries and helped with mouse husbandry and genotyping. G.K. performed the mapping analysis of the mRNA-seq data. S.B. and G.K. performed bioinformatics analysis of the mRNA-seq data including the WGCNA analysis. B.B. performed the metabolomics measurements and analysis. J.J.S. and I.L. helped with tissue collections. P.M. and C.B.G. provided supervision on experimental design, data interpretation, and manuscript preparation.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.016.
Contributor Information
Prashant Mishra, Email: prashant.mishra@utsouthwestern.edu.
Carla B. Green, Email: carla.green@utsouthwestern.edu.
Data and Code Availability
All data supporting the findings of this study are available within the article, extended data files, or available from the corresponding author upon reasonable request. The NCBI Gene Expression Omnibus (GEO) accession number for the RNA-seq data reported in this study is GSE133050 (token: ymregeultsvtih).
Supplemental Information
References
- Baggs J.E., Green C.B. Nocturnin, a deadenylase in Xenopus laevis retina: a mechanism for posttranscriptional control of circadian-related mRNA. Curr. Biol. 2003;13:189–198. doi: 10.1016/s0960-9822(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Brum M.C., Filho F.F., Schnorr C.C., Bottega G.B., Rodrigues T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015;7:45. doi: 10.1186/s13098-015-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crappe J., Ndah E., Koch A., Steyaert S., Gawron D., De Keulenaer S., De Meester E., De Meyer T., Van Criekinge W., Van Damme P. PROTEOFORMER: deep proteome coverage through ribosome profiling and MS integration. Nucleic Acids Res. 2015;43:e29. doi: 10.1093/nar/gku1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N., Kojima S., Pan X., Lerch-Gaggl A.F., Duong S.Q., Hussain M.M., Green C.B. Nocturnin regulates circadian trafficking of dietary lipid in intestinal enterocytes. Curr. Biol. 2011;21:1347–1355. doi: 10.1016/j.cub.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijalkowska D., Verbruggen S., Ndah E., Jonckheere V., Menschaert G., Van Damme P. eIF1 modulates the recognition of suboptimal translation initiation sites and steers gene expression via uORFs. Nucleic Acids Res. 2017;45:7997–8013. doi: 10.1093/nar/gkx469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot M.R., Berto S., Liu Y., Werthmann G., Douglas C., Usui N., Gleason K., Tamminga C.A., Takahashi J.S., Konopka G. Novel transcriptional networks regulated by CLOCK in human neurons. Genes Dev. 2017;31:2121–2135. doi: 10.1101/gad.305813.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X., Wan J., Liu B., Ma M., Shen B., Qian S.B. Quantitative profiling of initiating ribosomes in vivo. Nat. Methods. 2015;12:147–153. doi: 10.1038/nmeth.3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbarino-Pico E., Niu S., Rollag M.D., Strayer C.A., Besharse J.C., Green C.B. Immediate early response of the circadian polyA ribonuclease nocturnin to two extracellular stimuli. RNA. 2007;13:745–755. doi: 10.1261/rna.286507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gawron D., Ndah E., Gevaert K., Van Damme P. Positional proteomics reveals differences in N-terminal proteoform stability. Mol. Syst. Biol. 2016;12:858. doi: 10.15252/msb.20156662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin A.R., Kojima S., Green C.B., Wilusz J. Kiss your tail goodbye: the role of PARN, Nocturnin, and Angel deadenylases in mRNA biology. Biochim. Biophys. Acta. 2013;1829:571–579. doi: 10.1016/j.bbagrm.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green C.B., Douris N., Kojima S., Strayer C.A., Fogerty J., Lourim D., Keller S.R., Besharse J.C. Loss of Nocturnin, a circadian deadenylase, confers resistance to hepatic steatosis and diet-induced obesity. Proc. Natl. Acad. Sci. U S A. 2007;104:9888–9893. doi: 10.1073/pnas.0702448104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Z., Song R., Regev A., Struhl K. Many lncRNAs, 5'UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife. 2015;4:e08890. doi: 10.7554/eLife.08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson B., Knutsson A., Lindahl B. Is there an association between shift work and having a metabolic syndrome? Results from a population based study of 27 485 people. Occup. Environ. Med. 2001;58:747–752. doi: 10.1136/oem.58.11.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Delany A.M., Green C.B., Adamo M.L., Rosen C.J. Nocturnin suppresses igf1 expression in bone by targeting the 3' untranslated region of igf1 mRNA. Endocrinology. 2010;151:4861–4870. doi: 10.1210/en.2010-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M., Green C.B., Lecka-Czernik B., Douris N., Gilbert M.R., Kojima S., Ackert-Bicknell C., Garg N., Horowitz M.C., Adamo M.L. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc. Natl. Acad. Sci. U S A. 2010;107:10508–10513. doi: 10.1073/pnas.1000788107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia K.A., Storch K.F., Weitz C.J. Physiological significance of a peripheral tissue circadian clock. Proc. Natl. Acad. Sci. U S A. 2008;105:15172–15177. doi: 10.1073/pnas.0806717105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheva B., Ramsey K.M., Buhr E.D., Kobayashi Y., Su H., Ko C.H., Ivanova G., Omura C., Mo S., Vitaterna M.H. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A.M., Fox G., M Kiran A., De Bo C., O'Connor P.B., Heaphy S.M., Mullan J.P., Donohue C.A., Higgins D.G., Baranov P.V. GWIPS-viz: development of a ribo-seq genome browser. Nucleic Acids Res. 2014;42(Database issue):D859–D864. doi: 10.1093/nar/gkt1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.L., Pierce K.A., Jedrychowski M.P., Garrity R., Winther S., Vidoni S., Yoneshiro T., Spinelli J.B., Lu G.Z., Kazak L. Accumulation of succinate controls activation of adipose tissue thermogenesis. Nature. 2018;560:102–106. doi: 10.1038/s41586-018-0353-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherji A., Kobiita A., Damara M., Misra N., Meziane H., Champy M.F., Chambon P. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc. Natl. Acad. Sci. U S A. 2015;112:E6691–E6698. doi: 10.1073/pnas.1519807112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu S., Shingle D.L., Garbarino-Pico E., Kojima S., Gilbert M., Green C.B. The circadian deadenylase Nocturnin is necessary for stabilization of the iNOS mRNA in mice. PLoS One. 2011;6:e26954. doi: 10.1371/journal.pone.0026954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K., Atsumi G., Sugiyama S., Kodomari I., Kasamatsu M., Machida K., Ishida N. Disrupted fat absorption attenuates obesity induced by a high-fat diet in Clock mutant mice. FEBS Lett. 2006;580:127–130. doi: 10.1016/j.febslet.2005.11.063. [DOI] [PubMed] [Google Scholar]
- Oishi K., Miyazaki K., Kadota K., Kikuno R., Nagase T., Atsumi G., Ohkura N., Azama T., Mesaki M., Yukimasa S. Genome-wide expression analysis of mouse liver reveals CLOCK-regulated circadian output genes. J. Biol. Chem. 2003;278:41519–41527. doi: 10.1074/jbc.M304564200. [DOI] [PubMed] [Google Scholar]
- Raj A., Wang S.H., Shim H., Harpak A., Li Y.I., Engelmann B., Stephens M., Gilad Y., Pritchard J.K. Thousands of novel translated open reading frames in humans inferred by ribosome footprint profiling. Elife. 2016;5:e13328. doi: 10.7554/eLife.13328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rorbach J., Minczuk M. The post-transcriptional life of mammalian mitochondrial RNA. Biochem. J. 2012;444:357–373. doi: 10.1042/BJ20112208. [DOI] [PubMed] [Google Scholar]
- Rudic R.D., McNamara P., Curtis A.M., Boston R.C., Panda S., Hogenesch J.B., Fitzgerald G.A. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2004;2:e377. doi: 10.1371/journal.pbio.0020377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinturel F., Gerber A., Mauvoisin D., Wang J., Gatfield D., Stubblefield J.J., Green C.B., Gachon F., Schibler U. Diurnal oscillations in liver mass and cell size accompany ribosome assembly cycles. Cell. 2017;169:651–663.e14. doi: 10.1016/j.cell.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubblefield J.J., Gao P., Kilaru G., Mukadam B., Terrien J., Green C.B. Temporal control of metabolic amplitude by nocturnin. Cell Rep. 2018;22:1225–1235. doi: 10.1016/j.celrep.2018.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turek F.W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D.R. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Osterbur D.L., Megaw P.L., Tosini G., Fukuhara C., Green C.B., Besharse J.C. Rhythmic expression of Nocturnin mRNA in multiple tissues of the mouse. BMC Dev. Biol. 2001;1:9. doi: 10.1186/1471-213X-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., He D., Xu Y., Hou J., Pan B.F., Wang Y., Liu T., Davis C.M., Ehli E.A., Tan L. Genome-wide identification and differential analysis of translational initiation. Nat. Commun. 2017;8:1749. doi: 10.1038/s41467-017-01981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Lahens N.F., Ballance H.I., Hughes M.E., Hogenesch J.B. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc. Natl. Acad. Sci. U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data supporting the findings of this study are available within the article, extended data files, or available from the corresponding author upon reasonable request. The NCBI Gene Expression Omnibus (GEO) accession number for the RNA-seq data reported in this study is GSE133050 (token: ymregeultsvtih).