Summary
Conventional printing is worth revisiting because of its established procedures in meeting the surging demand of manufacturing printed electronics, 3D products, etc. However, one goal in penetrating printing into these is to control pattern transfer with no limitation of wettability. Here we introduce a miscible liquid-liquid transfer printing mechanism that can synchronize material preparation and material patterning with desirable properties including limitless selection of raw materials, corrosion resistance, no wetting constraint, and ability to prepare large-area defect-free materials for multi-function applications. Theoretical modeling and experiments demonstrate that donor liquid could be used to make patterns within the bulk of a receiver material, allowing the obtained intrinsically patterned functional materials to be resistant to harsh conditions. Different from current liquid printing technologies, this printing approach enables stable and defect-free material preparation and is expected to prove useful in flexible display, soft electronics, 4D printing, and beyond.
Subject Areas: Interface Science, Surface Property, Materials Property, Materials Design
Graphical Abstract
Highlights
-
•
A transfer printing mechanism based on miscible liquid-liquid interfaces
-
•
The material preparation and defect-free patterning can be synchronized
-
•
This printing mechanism is free of wetting constraint
Interface Science; Surface Property; Materials Property; Materials Design
Introduction
Printing has played a crucial role in promoting the development of human civilization (Fukuda and Someya, 2017, Kumar, 2015, Tian et al., 2013). Also, beyond publishing and transacting, nowadays “printing” is endowed with more roles in fulfilling myriad demands such as fabricating delicate structures and patterned functional materials (Feng et al., 2019, Fukuda and Someya, 2017, Kim et al., 2017, Parra-Cabrera et al., 2018, Raut and Al-Shamery, 2018, Schwartz and Boydston, 2019, Su et al., 2018). However, although printing holds great promises for fabricating and manufacturing patterned functional materials (Sun et al., 2015, Xia and Whitesides, 1998, Zhou and Song, 2011), the realization of functional properties of patterned materials such as tunability and mechanical and electrical stabilities heavily relies on not only the pattern delivering technologies but also the compatibility of the “donor patterns” and receiver materials (Carlson et al., 2012, Derby, 2010, Hoon et al., 2018, Hwang et al., 2010, Kumar, 2015, Lee et al., 2016, Liu et al., 2014, Meitl et al., 2006, Sun et al., 2015, Tian et al., 2013).
Driven by the demand of speed and precision in processes, and inspired from interface manipulation (Sun et al., 2015, Tumbleston et al., 2015, Villar et al., 2013), rapid progress has been made in liquid printing technologies based on immiscible interfaces, such as green plate-making technology (Bao et al., 2014, Zhou and Song, 2011), inkjet printing of concave microstructures (Bao et al., 2015), inkjet printing of embedded circuits (Jiang et al., 2016) and microchannels (Guo et al., 2015), all-liquid printing of microchannels (Feng et al., 2019), 3D microstructure fabrication via dynamic dewetting surfaces (Wang et al., 2015), and 3D printing of droplet networks or threads (Forth et al., 2018, Villar et al., 2013). However, even that the immiscible interfaces (Zeng et al., 2009) provide convenience in shaping delicate structures, it is limited in uniformity and integrity in fabricating intrinsically patterned materials, leading to compromises of functional properties. Moreover, another difficulty in optimizing the properties of patterned functional materials is that, an independent patterning process usually follows the material preparation (Lee et al., 2010). Therefore the integration of these two will benefit by saving time, ensuring structural integrity, and potentially enabling more application for intelligent manufacturing.
It is most commonly thought that liquid-liquid interfaces refer to the interfaces in immiscible systems (Hou et al., 2015, Zeng et al., 2009), due to their clear contact line and sustained phase boundaries. However, for printing applications based on immiscible systems, their material selections could be restricted, and therefore most of them are derived from oil-aqueous solution systems (Forth et al., 2018, Villar et al., 2013). The miscible liquid-liquid interfaces have proved their usefulness in numerous industrial applications such as oil recovery and oil extraction (Vorobev, 2014). Distinct from those in immiscible systems, the dynamics involved with miscible liquid-liquid interfaces, even after agitating, are time variant and the interfaces in between would eventually disappear, leading to the whole homogeneity of mixture (Bai et al., 2018). This whole homogeneity is highly desirable in many manufacturing processes and can hardly be achieved in the immiscible systems. Also, the time-variant interfaces provide us with more possibilities in synchronizing multifunction. Here we show a liquid transfer printing approach, which employs the miscible liquid-liquid interfacial contact, through interfacial diffusion, and solidification, taking advantage of time variability of miscible interface, to achieve synchronization of designated material preparation with stable transfer printing without interfacial wettability restriction.
Results and Discussion
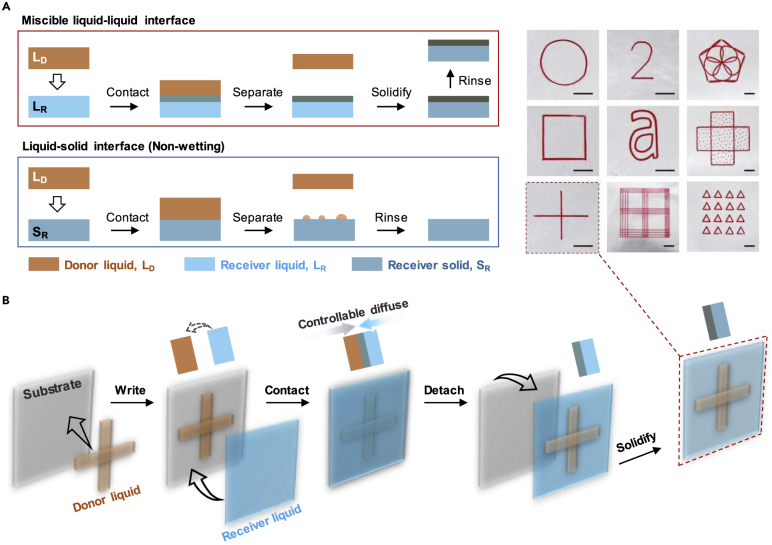
Figure 1A contrasts the printing concept at different interfaces. For the liquid-liquid interface, the donor liquid starts to migrate into the receiver liquid after the contact between them, along with the solidification of the receiver, whereas for the liquid-solid interface (non-wetting system), the donor stays on the surface of the receiver. Owing to its high tunability in preparing dynamic liquid-liquid interface (Bai et al., 2018, Goncalves et al., 2018, Jaworek, 2007, Rietveld et al., 2006), electrospray was selected to demonstrate our liquid-liquid printing concept. Figure 1B shows a typical process of our liquid-liquid printing for synchronization of material preparation. Compared with the conventional printing, our liquid-liquid printing excels in non-wetting systems (Video S1). When we chose a red dye aqueous rhodamine B solution (RB) as donor, and PVDF (acetone-DMAC) (polyvinylidene fluoride with acetone-dimethylacetamide) as receiver, RB could not sustain on the surface of solid PVDF, due to the hydrophobic surface of the solid PVDF. However, liquid-liquid printing has no such limitation, because the liquid interface could let the aqueous dye into the liquid PVDF (acetone-DMAC) before its solidification. Thus our liquid-liquid printing approach with the unrestricted wetting condition would expand the scope of printing materials.
Figure 1.
Liquid-Liquid Printing Mechanism for Synchronization of Material Preparation and Material Patterning
(A) Different behaviors of mass transfer at liquid-liquid and liquid-solid interfaces. SR is the solid state of the receiver liquid (LR), whereas SD is the solid state of the donor liquid (LD). For liquid-liquid interface, the donor liquid could spontaneously diffuse into the bulk of the receiver liquid. For liquid-solid interface, the donor could only stay on the surface of the receiver. See more details in Figure S1 and Table S1.
(B) Schematic illustration of a liquid-liquid printing process. See more details in Figure S2. Top right inset, intricately designed patterns with high resolution. The donor liquid is neutral red ink, and the receiver liquid is PVDF (acetone-DMAC) (Figure S3). Scale bars, 5 mm.
The liquid-liquid printing process was performed with an electrospray machine. For liquid-liquid printing, the donor is aqueous ink and the receiver is PVDF (acetone-DMAC). For liquid-solid printing, the donor is aqueous ink and the receiver is hydrophobic PVDF.
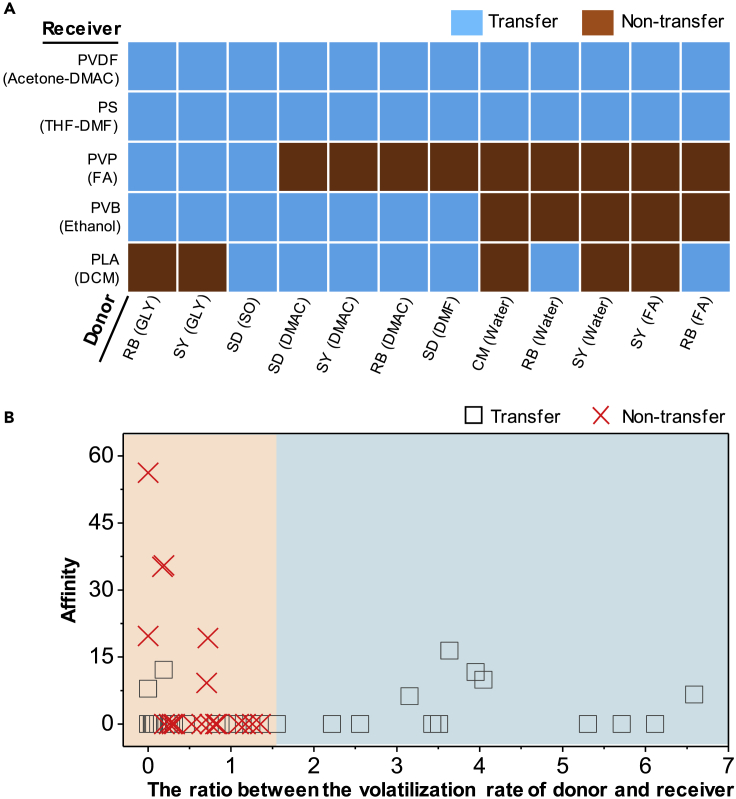
Figure 2A shows our data matrix from printing experiments of 60 pairs of different donor and receiver liquids, among which 41 work well. We found that when the receiver liquids are highly volatile, it is difficult for pattern transfer between the interfaces because of the quicker evaporation of the solvents, which leaves behind the solid-solid or the solid-liquid contact rather than the liquid-liquid contact. The affinity between the two liquids depends on their mutual solubility. For example, carmine with deionized (DI) water could not be used as the donor material to make patterns inside the receiver liquid of polylactic acid (PLA) with dichloromethane (DCM), as they are immiscible liquids. However, when the soluble receiver liquid, such as polystyrene with tetrahydrofuran-dimethylformamide was selected, it worked. Deriving from the above, it is thought that the two factors accountable to realize the synchronization of material preparation and material patterning by liquid-liquid printing are volatility and affinity.
Figure 2.
Data Matrix of 60 Pairs of Different Donor and Receiver Liquids
(A) The printing experimental results on 60 pairs of different donor and receiver liquids. The solvents are in the brackets following by the solutes. For example, donor RB (GLY) means GLY (GLYglycerin) is the donor solvent and RB is the donor solute. Other solvents include shell oil (SO), dimethylacetamide (DMAC), dimethylformamide (DMF), formic acid (FA), dichloromethane (DCM), and tetrahydrofuran (THF). The corresponding solutes include polystyrene (PS), polylactic acid (PLA), polyvinyl butyral (PVB), polyvinylpyrrolidone (PVP), sunset yellow (SY), carmine (CM) and Sudan red (SD).
(B) The ratio between the volatilization rate of the donor and receiver with regard to the affinity between the donor and receiver. Volatilization rate is the mass reduction of the liquids divided by the elapsed time whereby the liquid is vaporized (Table S2). The affinity is obtained by dividing the value of receding angle by the solubility level of the donor solute in the receiver solvent (Tables S3 and S4). The experiments show that in the cyan shading area, the pattern transfers at the liquid-liquid interface have higher possibilities to be achieved, whereas in the pink shading area some of them may not be achieved.
The ratio between the volatilization rates of the donor and receiver liquids was further investigated with regard to a certain affinity between the two liquids (Figure 2B). It is noted that the slower the receiver liquid volatilizes, the easier the donor can deliver into it. When the ratio is above 1.55, meaning the volatilization rate of the donor liquid is at least 1.55 times the volatilization rate of receiver, pattern transfer at the liquid-liquid interface could be well achieved. For instance, when RB is used as a donor liquid, if we choose polyvinylpyrrolidone with formic acid as the receiver liquid, the ratio of volatilization of which is 0.72, the donor liquid material cannot deliver into the receiver; if we choose the liquid PVDF (acetone-DMAC) as the receiver liquid, the ratio of which is 4.00, the pattern of donor liquid can be printed into the receiver. Incomplete transfer can occur in the immiscible systems due to the solvent extraction effect. For example, the donor liquid RB can also be partly transferred into the receiver liquid PLA with DCM, because of the higher solubility of RB in dichloromethane than DI water.
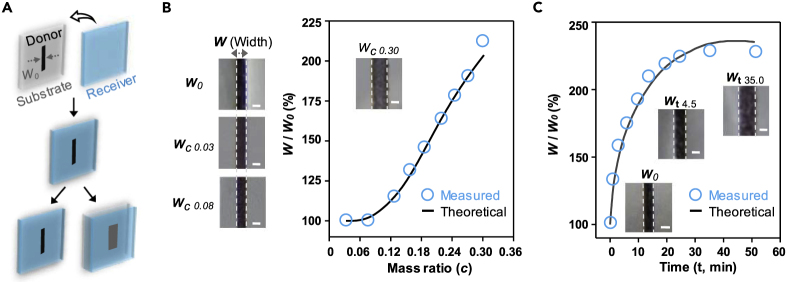
The diffusivity property on the miscible liquid-liquid interfaces between two materials plays a big part in controlling the size of patterns obtained through our liquid-liquid printing mechanism, as after the contact, the donor liquid patterns tend to expand. Two parameters are employed to realize accurate sizes of the patterns. One is the mass ratio of the receiver solvent to the overall receiver (c) and the other is the contact time between the donor and the receiver. Here we selected a line pattern to demonstrate how to obtain the original or the extended width (Figure 3A). Figure 3B illustrates the ratio of the width change before and after printing by increasing c. When c is below 0.08, the ratio is at 1 to achieve the original pattern size printing, whereas when c is above 0.08, the ratio increases rapidly with the increase of c.
Figure 3.
Pattern Size Tuning of Liquid-Liquid Printing
(A) Schematic diagram of the diffusion model. W0 is the original width of the pattern line.
(B) Mass ratio of the receiver solvent to the overall receiver, i.e., c, regulates the printing line width. It is determined by mass flow rate (Table S6). The liquid receiver is PVDF (acetone-DMAC) (Figure S4A), whereas the liquid donor is black neutral pen ink (Figure S4B). Wc is the width of the line for a special c. The blue circles are experimental data for the ratio between Wc and W0, whereas the black curve represents the model results. Scale bars, 0.5 mm. The experiments are conducted at 25°C and 35% ± 3% relative humidity.
(C) The diffusion time regulates the printing line width. Wt is the width of the line at a certain time (t). The blue circles are experimental data for the ratio between Wt and W0, whereas the black curve represents the theoretical simulation results. Scale bars, 0.5 mm.
A theoretical model is established on the underlying mechanism of quality and controllable mass transfer under our liquid-liquid printing system. The donor liquid (LD) that is composed of solvent (LDS) and solute (ink pigment) is mixed with the receiver liquid (LR) that is composed of solvent (LRS) and solute (polymers, LRP) in the vicinity of contact. First, we studied the diffusion of the donor solvent in the receiver. When the mass ratio of the receiver solvent to the overall receiver (c) is low, the diffusion of the donor solvent is primarily dominated by diffusing through the polymers in the receiver, whereas when c is high, the donor solvent can diffuse into the receiver easier with the facility of the solvent in the receiver. Therefore, the diffusivity of the donor solvent in the receiver is defined as follows:
| (Equation 1) |
where D1solv is the diffusion coefficient of the pure donor solvent in the receiver solvent LRS, D1poly is the diffusion coefficient of the pure donor solvent in the receiver polymer LRP, and ccri is the critical mass ratio of the receiver solvent to the overall receiver for the receiver solvent to start promoting the diffusion of the donor solvent (Table S5). Next, we studied the diffusion of the donor ink solute in the receiver, for which we referred to a theoretical model proposed by Kunii (Kunii et al., 1995), and define the diffusivity of the ink solute as
| (Equation 2) |
where g is the density of the donor solvent and f is the density of the donor ink solute and Z0 and Z1 are two constants determining the drag of solvent on solute. When g/f is smaller than Z0, the flow of the donor liquid is not sufficient enough to accelerate the flow of the ink solute in the donor into the receiver. In this case, the diffusion coefficient of the ink solute is D0 (c), which has a similar form as Equation 1. If g/f is bigger than Z1, the donor ink solute will move with the donor liquid. However, due to the resistance of the polymers, the diffusion coefficient of the ink solute in the donor is smaller than that of the donor liquid by a factor r that is smaller than 1. If g/f is in between, we use a cosine function as the transition curve for the diffusion coefficient of the ink solute. As shown in Figure 3B, as the solvent content in the receiver c increases, it promotes more ink solute to diffuse into the donor, and thus the width of the print is wider. This model also captures the kinetic process. As shown in Figure 3C, the width of the print is plotted as a function of time. As time increases, the width of the print increases and eventually reaches a plateau value. The experimental results and theoretical predictions agree well, and this model can be used for the quantitative system design.
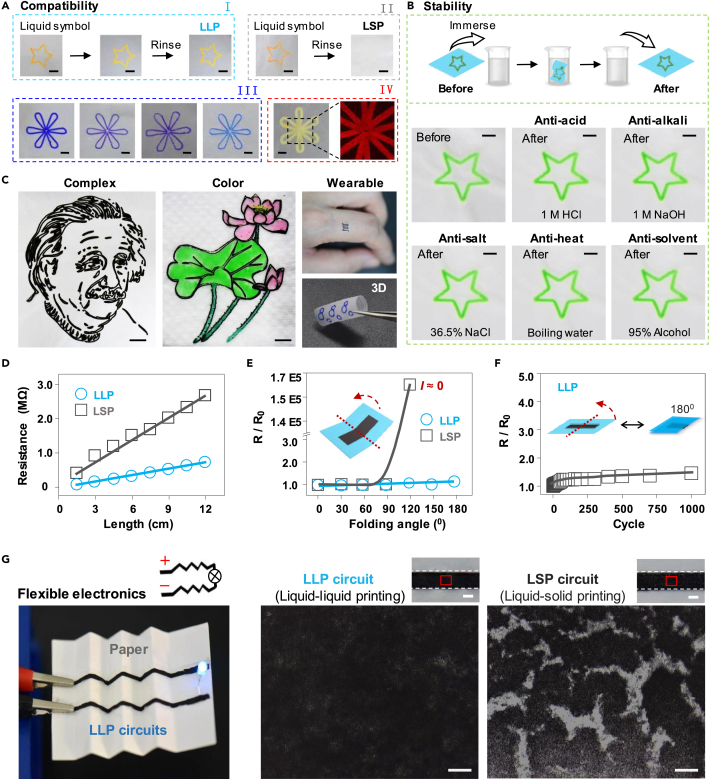
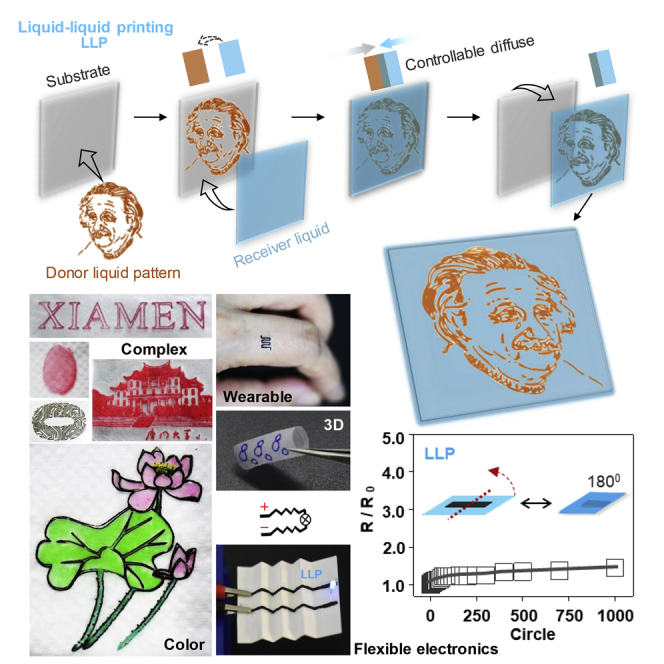
Our liquid-liquid printing can be used to prepare universally durable patterned materials (Figure 4A and S5, and Video S1), and these prepared materials are very stable, even under harsh conditions (Figure 4B). We further demonstrate its application scopes by realizing complex printing (Figures 4C, S6, and S7A), colorful printing (Figures 4C and S7B), multifunctional printing (Figures S7C–S7E), and 3D printing (Figures 4C and S8). Our approach also shows promise in making flexible circuits (Video S2). Compared with the conventional circuits' liquid-solid printing (LSP), the circuits' liquid-liquid printing (LLP) shows lower resistivity (Figures 4D and S9), better mechanical flexibility (Figure 4E), and durability (Figure 4F) during the folding process. Because of the dewetting property of the aqueous ink donor on the hydrophobic receiver solid, LSP left behind many cracks on the surface of the final products, whereas LLP left behind a smooth surface without cracks (see Figures 4G, S10, and S11). Moreover, our approach can be used to prepare waterproof circuits (Figure S12, Video S2). Therefore, this printing approach opens possibilities in building stable, defect-free, non-wetting, waterproof printing technology for huge demand in deformable circuits.
Figure 4.
Advantages and Applications of Liquid-Liquid Printing
(A) Compatibility. The liquid-liquid printing (LLP) symbol could withstand surface rinsing (I), whereas the same liquid-solid printing (LSP) symbol disappeared after surface rinsing (II). LLP is compatible with various donor liquids (III). From left, oily gel donor, ballpoint pen ink donor, and two aqueous gel donors. A fluorescent donor (IV). The right inset is a magnified fluorescence photograph. Scale bars, 5 mm.
(B) Stability. Schematic illustration (top) of the treatment process and optical images (middle and bottom) of the LLP stars before and after immersing into the strong acid, strong alkali, salt brine, boiling water, and the organic solvent. Scale bars, 3 mm.
(C) Examples of intricately designed patterns by liquid-liquid printing: complex, colorful, wearable, and three-dimensional (3D) products. Scale bars, 5 mm.
(D) The length-resistance tests of the conductive circuits prepared by LLP and LSP.
(E) Relative resistance of printing circuits folded under various folding angles. The inset is the schematic illustration of the bending tests. The LLP circuits (thickness 54 μm) achieve better mechanical flexibility, whereas the LSP circuits (thickness 64 μm) broke when bending to 120°.
(F) The relative resistance change of the LLP flexible circuits as a function of the folding times under 180° folding angle.
(G) A light-emitting diode device connected by LLP flexible and transparent circuits, which were supported on a paper (left). Zoomed-in view of the surface morphology of the LLP and LSP circuits (right). LLP circuits show a smooth surface after drying, whereas LSP circuits have many cracks on the surface. Scale bars, 1 mm (top) and 100 μm (bottom).
To prepare waterproof circuit, the resulting LLP circuit was peeled off from the substrate and turned over for another electrospray process. Therefore the resulting waterproof circuit was sandwiched between two layers of PVDF.
Conclusion
In summary, we show a new liquid-liquid printing capable of achieving the synchronization of material preparation and durable material pattern without wetting constraint. This mechanism realizes a controllable pattern transfer by miscible liquid-liquid interfacial contact, diffusion, and solidification. By our experimental results and theoretical modeling, there are 60 combination experiments with 41 combinations of liquid-liquid printing that work well. The main reasons for the remaining unworkable combinations are two key factors: volatility and affinity. We found the critical value of liquid-liquid printing as the ratio of liquid volatilization rate is 1.55. Controllable printing is expected to be achieved by liquid-liquid interface behavior. It was assumed that two effective ways in our system to get controllable sizes of patterns are by utilizing the mass ratio of the receiver solvent and the diffusion time. Our approach is applicable to miscible liquid-liquid system and breaks the limitation of printing materials in the non-wetting system. Moreover, it has great potentials in defect-free material preparations for many applications such as durable and deformable electrical circuits, flexible and wearable devices, electronic displays, and many other applications beyond publishing, packaging, and manufacturing.
Limitations of Study
When the volatilization rate of the donor liquid is at least 1.55 times that of the receiver, pattern transfer at the liquid-liquid interface can be ensured. This is obtained through 60 pairs of donor and receiver liquids in our study, so we do not know if the ratio can vary with more different samples.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Key R&D Program of China (2018YFA0209500), the National Natural Science Foundation of China (21673197, 21621091, 51706191, 21808191), Young Overseas High-level Talents Introduction Plan, the 111 Project (B16029), the Fundamental Research Funds for the Central Universities in China (20720190037, 20720170050), the Natural Science Foundation of Fujian Province of China (No. 2018J06003), and Special Project of Strategic Emerging Industries from Fujian Development and Reform Commission. The authors thank W. Chen, Y. Chi, Y. Fan, and S. Chen for discussion and help.
Author Contributions
X.H. conceived the idea. X.H. and L.M. designed the research. L.M., H.P., and F.W. performed the experiments. Y.H. and H.Z. built the mathematical model. X.H., L.M., H.P., Y.H., H.Z., F.W., Z.S., M.W., M.Z., S.W., and X.C. analyzed and interpreted the results. X.H., L.M., H.Z., and X.C. drafted the manuscript, and all authors contributed to the writing of the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: September 27, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.07.017.
Supplemental Information
References
- Bai X.-J., Chen D., Li L.-L., Shao L., He W.-X., Chen H., Li Y.-N., Zhang X.-M., Zhang L.-Y., Wang T.-Q. Fabrication of MOF thin films at miscible liquid–liquid interface by spray method. ACS Appl. Mater. Interfaces. 2018;10:25960–25966. doi: 10.1021/acsami.8b09812. [DOI] [PubMed] [Google Scholar]
- Bao B., Jiang J., Li F., Zhang P., Chen S., Yang Q., Wang S., Su B., Jiang L., Song Y. Fabrication of patterned concave microstructures by inkjet imprinting. Adv. Funct. Mater. 2015;25:3286–3294. [Google Scholar]
- Bao B., Su B., Wang S., Chen S., Wu L., Jia Z., Song Y., Jiang L. Stretching velocity-dependent dynamic adhesion of the water/oil interfaces for high quality lithographic printing. Adv. Mater. Interfaces. 2014;1:1400080. [Google Scholar]
- Carlson A., Bowen A.M., Huang Y., Nuzzo R.G., Rogers J.A. Transfer printing techniques for materials assembly and micro/nanodevice fabrication. Adv. Mater. 2012;24:5284–5318. doi: 10.1002/adma.201201386. [DOI] [PubMed] [Google Scholar]
- Derby B. Inkjet printing of functional and structural materials: fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010;40:395–414. [Google Scholar]
- Feng W., Chai Y., Forth J., Ashby P.D., Russell T.P., Helms B.A. Harnessing liquid-in-liquid printing and micropatterned substrates to fabricate 3-dimensional all-liquid fluidic devices. Nat. Commun. 2019;10:1095. doi: 10.1038/s41467-019-09042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forth J., Liu X., Hasnain J., Toor A., Miszta K., Shi S., Geissler P.L., Emrick T., Helms B.A., Russell T.P. Reconfigurable printed liquids. Adv. Mater. 2018;30:1707603. doi: 10.1002/adma.201707603. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Someya T. Recent progress in the development of printed thin-film transistors and circuits with high-resolution printing technology. Adv. Mater. 2017;29:1602736. doi: 10.1002/adma.201602736. [DOI] [PubMed] [Google Scholar]
- Goncalves R., Cardoso V.F., Pereira N., Oliveira J., Nunes-Pereira J., Costa C.M., Lanceros-Mendez S. Evaluation of the physicochemical properties and active response of piezoelectric poly(vinylidene fluoride-co-trifluoroethylene) as a function of its microstructure. J. Phys. Chem. C. 2018;122:11433–11441. [Google Scholar]
- Guo Y., Li L., Li F., Zhou H., Song Y. Inkjet print microchannels based on a liquid template. Lab Chip. 2015;15:1759–1764. doi: 10.1039/c4lc01486c. [DOI] [PubMed] [Google Scholar]
- Hoon Y., Minho S., Kahyun S., Insol H., Kangseok L., Chaenyung C., Tae-il K., Eui J.H. Wet-responsive, reconfigurable, and biocompatible hydrogel adhesive films for transfer printing of nanomembranes. Adv. Funct. Mater. 2018;28 [Google Scholar]
- Hou X., Hu Y., Grinthal A., Khan M., Aizenberg J. Liquid-based gating mechanism with tunable multiphase selectivity and antifouling behaviour. Nature. 2015;519:70–73. doi: 10.1038/nature14253. [DOI] [PubMed] [Google Scholar]
- Hwang J.K., Cho S., Dang J.M., Kwak E.B., Song K., Moon J., Sung M.M. Direct nanoprinting by liquid-bridge-mediated nanotransfer moulding. Nat. Nanotechnol. 2010;5:742–748. doi: 10.1038/nnano.2010.175. [DOI] [PubMed] [Google Scholar]
- Jaworek A. Electrospray droplet sources for thin film deposition. J. Mater. Sci. 2007;42:266–297. [Google Scholar]
- Jiang J., Bao B., Li M., Sun J., Zhang C., Li Y., Li F., Yao X., Song Y. Fabrication of transparent multilayer circuits by inkjet printing. Adv. Mater. 2016;28:1420–1426. doi: 10.1002/adma.201503682. [DOI] [PubMed] [Google Scholar]
- Kim J., Kumar R., Bandodkar A.J., Wang J. Advanced materials for printed wearable electrochemical devices: a review. Adv. Electron. Mater. 2017;3:1600260. [Google Scholar]
- Kumar S. Liquid transfer in printing processes: liquid bridges with moving contact lines. Annu. Rev. Fluid Mech. 2015;47:67–94. [Google Scholar]
- Kunii, T.L., Nosovskij, G.V., and Hayashi, T.. (1995). A diffusion model for computer animation of diffuse ink painting. In Proceedings of the Computer Animation (IEEE Computer Society), pp. 98.
- Lee C.H., Kim D.R., Zheng X.L. Fabricating nanowire devices on diverse substrates by simple transfer-printing methods. Proc. Natl. Acad. Sci. U S A. 2010;107:9950–9955. doi: 10.1073/pnas.0914031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Um D.-S., Lee Y., Lim S., Kim H.-j., Ko H. Octopus-inspired smart adhesive pads for transfer printing of semiconducting nanomembranes. Adv. Mater. 2016;28:7457–7465. doi: 10.1002/adma.201601407. [DOI] [PubMed] [Google Scholar]
- Liu M., Wang J., He M., Wang L., Li F., Jiang L., Song Y. Inkjet printing controllable footprint lines by regulating the dynamic wettability of coalescing ink droplets. ACS Appl. Mater. Interfaces. 2014;6:13344–13348. doi: 10.1021/am5042548. [DOI] [PubMed] [Google Scholar]
- Meitl M.A., Zhu Z.T., Kumar V., Lee K.J., Feng X., Huang Y.Y., Adesida I., Nuzzo R.G., Rogers J.A. Transfer printing by kinetic control of adhesion to an elastomeric stamp. Nat. Mater. 2006;5:33–38. [Google Scholar]
- Parra-Cabrera C., Achille C., Kuhn S., Ameloot R. 3D printing in chemical engineering and catalytic technology: structured catalysts, mixers and reactors. Chem. Soc. Rev. 2018;47:209–230. doi: 10.1039/c7cs00631d. [DOI] [PubMed] [Google Scholar]
- Raut N.C., Al-Shamery K. Inkjet printing metals on flexible materials for plastic and paper electronics. J. Mater. Chem. C. 2018;6:1618–1641. [Google Scholar]
- Rietveld I.B., Kobayashi K., Yamada H., Matsushige K. Electrospray deposition, model, and experiment: toward general control of film morphology. J. Phys. Chem. B. 2006;110:23351–23364. doi: 10.1021/jp064147+. [DOI] [PubMed] [Google Scholar]
- Schwartz J.J., Boydston A.J. Multimaterial actinic spatial control 3D and 4D printing. Nat. Commun. 2019;10:791. doi: 10.1038/s41467-019-08639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su M., Huang Z.D., Li Y.F., Qian X., Li Z., Hu X.T., Pan Q., Li F.Y., Li L.H., Song Y.L. A 3D self-shaping strategy for nanoresolution multicomponent architectures. Adv. Mater. 2018;30:8. doi: 10.1002/adma.201703963. [DOI] [PubMed] [Google Scholar]
- Sun J., Bao B., He M., Zhou H., Song Y. Recent advances in controlling the depositing morphologies of inkjet droplets. ACS Appl. Mater. Interfaces. 2015;7:28086–28099. doi: 10.1021/acsami.5b07006. [DOI] [PubMed] [Google Scholar]
- Tian D.L., Song Y.L., Jiang L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013;42:5184–5209. doi: 10.1039/c3cs35501b. [DOI] [PubMed] [Google Scholar]
- Tumbleston J.R., Shirvanyants D., Ermoshkin N., Janusziewicz R., Johnson A.R., Kelly D., Chen K., Pinschmidt R., Rolland J.P., Ermoshkin A. Continuous liquid interface production of 3D objects. Science. 2015;347:1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- Villar G., Graham A.D., Bayley H. A tissue-like printed material. Science. 2013;340:48–52. doi: 10.1126/science.1229495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobev A. Dissolution dynamics of miscible liquid/liquid interfaces. Curr. Opin. Colloid Interface Sci. 2014;19:300–308. [Google Scholar]
- Wang L., Li F., Kuang M., Gao M., Wang J., Huang Y., Jiang L., Song Y. Interface manipulation for printing three-dimensional microstructures under magnetic guiding. Small. 2015;11:1900–1904. doi: 10.1002/smll.201403355. [DOI] [PubMed] [Google Scholar]
- Xia Y.N., Whitesides G.M. Soft lithography. Annu. Rev. Mater. Sci. 1998;28:153–184. [Google Scholar]
- Zeng H., Tian Y., Zhao B., Tirrell M., Israelachvili J. Friction at the liquid/liquid interface of two immiscible polymer films. Langmuir. 2009;25:4954–4964. doi: 10.1021/la804020k. [DOI] [PubMed] [Google Scholar]
- Zhou H.H., Song Y.L. Green plate making technology based on nano-materials. Adv. Mater. Res. 2011;174:447–449. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The liquid-liquid printing process was performed with an electrospray machine. For liquid-liquid printing, the donor is aqueous ink and the receiver is PVDF (acetone-DMAC). For liquid-solid printing, the donor is aqueous ink and the receiver is hydrophobic PVDF.
To prepare waterproof circuit, the resulting LLP circuit was peeled off from the substrate and turned over for another electrospray process. Therefore the resulting waterproof circuit was sandwiched between two layers of PVDF.