Abstract
Hyperactivation, which is defined as a higher level of activation in patients compared to cognitively unimpaired older adults (controls; CTL), might represent an early signature of Alzheimer's Disease (AD). The goal of this study was to assess the presence and location of hyperactivation in individuals with mild cognitive impairment (MCI) who were later diagnosed with dementia, examine how hyperactivation changes longitudinally, and whether it is related to time before dementia. Forty participants, 26 with MCI and 14 CTL were enrolled in the study. Magnetic resonance imaging was used to measure functional activation while participants encoded word-pairs as well as cortical thickness and regional brain volume at study entry (Y0) and two years later (Y2). Clinical follow-up was completed every two years following study entry to identify progressors (pMCI), that is, individuals who later received a diagnosis of dementia. Task-related activation was assessed in pMCI in both hippocampi and in regions showing greater cortical thinning from Y0 to Y2 compared to CTLs. Hyperactivation was found in pMCI individuals in the right supramarginal gyrus. Persons with pMCI also showed hypoactivation in the left hippocampus and left pars opercularis. Both hyper- and hypoactivation were present at Y0 and Y2 and did not change longitudinally. Activation was not associated with time before dementia diagnosis. Smaller volume and thinner cortical thickness were associated with shorter time to diagnosis in the left hippocampus and left pars opercularis. In conclusion, hyperactivation was found in individuals who later progressed to dementia, confirming that it might represent an early biomarker to identify individuals in the prodromal phase of AD and that its understanding could contribute to elucidate the key brain mechanisms that precede dementia.
Keywords: Mild cognitive impairment, Alzheimer's disease, Task-related hyperactivation, Longitudinal fMRI, Episodic memory
Highlights
-
•
Hyperactivation of the right parietal region was found in MCI progressors.
-
•
Hypoactivation was found in the hippocampal and frontal regions.
-
•
This overall pattern was specific to MCI who progressed to dementia.
1. Introduction
Alzheimer's Disease (AD) is progressive and its onset probably occurs 20 to 30 years prior to clinical diagnosis (Jansen et al., 2015; Villemagne et al., 2013). Thus, studying the prodromal phase of AD is of a tremendous importance to contribute to its early diagnosis and better understand its early effects on the brain. Persons meeting criteria for Mild cognitive impairment (MCI) have a high likelihood of progressing to dementia (Gauthier et al., 2006; Petersen et al., 1999, Petersen et al., 2001; Winblad et al., 2004) thus, making it a suitable target population to study the early phase of the disease.
A number of studies have observed hyperactivation in MCI individuals, that is, higher level of brain activation than what is found in cognitively unimpaired older adults (controls; CTL) (Celone et al., 2006; Clément and Belleville, 2010, Clément and Belleville, 2012; Clément et al., 2010, Clément et al., 2013; Putcha et al., 2011). This is in contrast with studies of persons with dementia who most often reported hypoactivation i.e., lower levels of activation in patients than in CTLs (Golby et al., 2005; Hämäläinen et al., 2007; Machulda et al., 2003; Mandzia et al., 2002; Rombouts et al., 2000; Small et al., 1999). Thus, the presence of hyperactivation might represent an early signature of the disease. It might also reflect key mechanisms regarding how the early neuropathology of AD leads to clinical symptoms of dementia (Clément and Belleville, 2010, Clément and Belleville, 2012; Clément et al., 2010; Leal et al., 2017; Mutlu et al., 2017; Sperling et al., 2010). However, a few studies have also observed hypoactivation in MCI and therefore, it is critical to better understand the conditions that lead to hyperactivation and the reasons for such discrepancy (Johnson et al., 2006; Hampstead et al., 2011; Hanseeuw et al., 2011; Machulda et al., 2003, Machulda et al., 2009).
The finding of both hypoactivation and hyperactivation in MCI might have a number of possible explanations. First, not all MCI progress to dementia and very few studies about hyperactivation have followed this group over time to separate progressors from stable MCI (sMCI). If hyperactivation is specific to progressors, including non-progressors might contribute to reduce or hinder the effect.
It has also been proposed that task-related activation follows a non-linear inverse U-shape trajectory with disease progression (Clément and Belleville, 2010, Clément and Belleville, 2012; Gregory et al., 2017; Prvulovic et al., 2005). One account is that increased compensatory activation would occur when neural loss is mild but would no longer be possible when the neuronal insult becomes more important, producing hypoactivation and cognitive breakdown (Prvulovic et al., 2005). Another account proposes that early amyloid accumulation would increase the production and inhibit recapture of glutamate which would result in hyperactivity (Bero et al., 2011; Busche et al., 2012; Busche and Konnerth, 2015; Jagust, 2009). Aberrant synaptic activity would contribute to an increase in amyloid and tau production, which would lead to increased neuronal death (Esposito et al., 2013; Lee et al., 2005; Wu et al., 2016), and this whole pattern would account for the inverse U-shape activation. The hypothesis of an inverse U-shape pattern was partly supported by transversal studies from Clément and Belleville, 2010, Clément and Belleville, 2012, and Clément et al. (2013). They observed hyperactivation in early MCI and hypoactivation in late MCI when participants completed tasks known to be impaired in MCI (associative memory: Clément and Belleville, 2010; recollection: Clément and Belleville, 2012; working memory and divided attention: Clément et al., 2013). Therefore, prior findings suggest that task-related hyperactivation characterizes the earliest phase of MCI and that it is followed by hypoactivation as patients progress to dementia. However, these studies relied on a transversal design where they compared groups of “early” vs. “late” MCI persons based on their scores on a clinical scale. This has limitations because combining patients at different disease stages might reflect interindividual differences in activation and conceal genuine changes caused by the progression of the disease. Therefore, the effect of hyperactivation can be best assessed with longitudinal studies where intraindividual change is privileged over interindividual differences. Furthermore, only a longitudinal follow-up can exclude MCI persons who will not progress to dementia.
Very few studies used a longitudinal design to measure brain activation changes in MCI persons. Two studies reported that higher hippocampal task-related activation at baseline preceded decrease of activation and cognitive decline in MCI individuals (Huijbers et al., 2015; O'brien et al., 2010). This supports the descending phase of the inverse U-shape of activation co-occurring with cognitive breakdown. However, these studies only assessed activation in the hippocampus. To determine whether this longitudinal pattern of hyperactivation is specific to the hippocampus or whether it is also observed in cortical regions might help contribute to understanding the source of hyperactivation and its relation to cognition. Moreover, although these previous longitudinal studies involved a follow-up, they did not separate their group to examine if hyperactivation was only found in MCI individuals who later developed dementia.
In summary, hyperactivation has great potential as an early signature of AD and in accounting for the dynamic of brain changes with the disease. However, it is critical to confirm its presence in MCI later progressing to dementia and to determine its localization and temporal pattern. Thus, a first objective was to assess whether hyperactivation is present in MCI individuals who later progressed to dementia (pMCI). MCI participants received a clinical assessment over many years following recruitment which allowed to identify pMCI and examine hyperactivation in that group. A second objective was to assess whether hyperactivation is found only in the hippocampus or if it is also observed in cortical regions. We used a region of interest (ROI) approach and assessed task-related activation only in regions showing cortical thinning over a two-year period. This approach was selected for several reasons. First, our study is based on the model that increased activation occurs in regions that suffer mild neural loss and that as the damage becomes more important, recruitment is no longer possible and hypoactivation occurs. Hence, regions with structural impairment are those that should preferentially show altered fMRI activity i.e., hyperactivation in the early disease phase followed by hypoactivation (Clément and Belleville, 2010, Clément and Belleville, 2012; Gregory et al., 2017; Prvulovic et al., 2005). Based on this model, one should select brain regions according to the likelihood that they will have suffered structural impairment. This has the additional pragmatic advantage that it reduces the number of regions examined and the likelihood of type I error which might occur due to multiple comparisons. The latter is a well-recognized risk in fMRI studies inherent to voxel-wise whole-brain between-group comparisons. Additionally, the approach is consistent with influential and seminal studies which have focused on brain regions known to be structurally impaired in early AD and have found increased activation in individuals in the prodromal phase of AD (Dickerson et al., 2004; Huijbers et al., 2015; O'Brien et al., 2010; Putcha et al., 2011). Task-related activation was also assessed in the hippocampus where AD-related structural changes are known to occur very early in the disease process. A third objective was to study how hyperactivation changes over time by measuring activation twice over a two-year period. pMCI are expected to show hyperactivation, that is, larger task-related activation than CTLs in both hippocampi and in structurally-impaired cortical regions. Hyperactivation is expected to decrease with time.
A secondary objective was to assess whether task-related activation relates with time before the clinical diagnosis of dementia. This was done because even though we used a longitudinal design, different entry points might prevent us from observing activation changes, as some individuals may be in the ascending portion of the inverse U-shape function, and others in the descending one. Examining activation as a function of time to dementia diagnosis might provide more precise information regarding the position of the participants on the MCI-to-dementia continuum. We also assessed the relationship between hippocampal volume/cortical thickness and time to diagnosis to support the validity of the measure.
2. Methods
2.1. Participants
Forty participants, 26 persons with MCI and 14 CTLs, were recruited for this study.1 All participants were native French speakers and right-handed. Participants with MCI were recruited from memory clinics and met the criteria for single or multiple domains amnestic MCI (Petersen et al., 2001; Petersen et al., 1999; Winblad et al., 2004), in that 1) they worried about their memory, 2) they performed at least 1.5 standard deviation below age- and education-adjusted norms on neuropsychological memory tests, 3) they did not show global cognitive impairment on the basis of the Mini-Mental State Evaluation (MMSE, adjusted for age and education; Folstein et al., 1975), and 4) they were not impaired in their activities of daily living on the basis of the Functional Autonomy Measurement System (Hébert et al., 1988) and clinical interview. At baseline and follow-up, individuals with MCI underwent a neuropsychological assessment to measure their episodic memory (RL/RI-16, free and cued word recall task (Buschke, 1984; Van der Linden et al., 2004), 20-min delayed recall of the Rey Complex Figure (Rey, 1959), executive functions (third plate of the Stroop-Victoria (Regard, 1981) and copy of the Rey Complex Figure), visuospatial processing (Benton Judgment of line orientation; Benton et al., 1994), speed of information processing (Coding of the WAIS-III; Wechsler, 1997), language (Boston Naming Test; Kaplan et al., 2001), and global cognitive functions (Mattis Dementia Rating Scale; Mattis, 1976). They also underwent an extensive medical, neurological and neuroradiological examination to exclude the existence of any systemic, neurological, or psychiatric condition that could account for the cognitive impairments. MCI individuals received the same clinical assessment every two years following recruitment to identify whether they progressed to dementia according to the NINCDS-ADRDA (McKhann et al., 1984) and DSM-IV criteria (American Psychiatric Association, 2004). The two-year follow-up was continued for up to 6 years.
CTL older adults received an abbreviated neuropsychological assessment covering episodic memory (RL/RL-16, free and cued word recall task), speed of information processing (Coding sub-test of the WAIS-III), and global cognitive functions (MDRS, MMSE) at entry of the study to characterize their cognition. CTLs were followed over the two-year period of the study.
2.2. General procedure
At baseline (Y0), a first session was used to provide informed consent and to complete the clinical and neuropsychological assessment. One week later, participants were familiarized with the MRI procedure and task, with a simulator that imitates the MRI environment. This ensured that participants understood the task and were comfortable with the scanning procedure and environment. The MRI examination was done in a separate session which took place one week following simulation. Longitudinal follow-up (Y2) was done approximatively two years following the first MRI session (18 to 30 months later) with the same MRI and clinical procedure as for Y0. Follow-up assessments were repeated on Y4 and Y6 following initial recruitment using the clinical and neuropsychological assessment only. The study was approved by the Comité mixte d'éthique de la recherche du Regroupement Neuroimagerie/Québec (CMER-RNQ) ethic committee.
2.3. fMRI memory task
Participants were asked to memorize 16 lists of nine concrete word pairs. Following the encoding of one list, participants were shown eight word-pairs and were asked to indicate whether the pair was part of the learning list or not. Retrieval lists included four pairs that were part of the learning list and four new pairs. Half of the new pairs were made up of an old and a new word and half were made up of old words that were rearranged to make new pairs. All words were one- or two-syllables long and the different lists were matched as much as possible for mean frequency, average word length and semantic relatedness.
The task was programmed on E-prime (Psychology Software Tool, Pittsburgh, Pennsylvania, 2016) and stimuli were projected onto a mirror. Pairs were presented sequentially at a rate of 4 s (s) per pair. Before each block of encoding, a brief instruction to memorize the word pairs was presented. Scanning was done in two separate runs. Each run was composed of four alternating series of cross fixation (20s), encoding instructions (4 s), encoding (36 s), retrieval instructions (4 s), and retrieval phase (40s). Only the encoding data is presented here.
2.4. Data acquisition
MRI sessions were performed using a SIEMENS 3 T Magnetom TRIO System (Erlangen, Germany) at the Unité de Neuroimagerie Fonctionnelle (UNF) of the Institut universitaire de gériatrie de Montréal. The structural images were obtained with a sagittal T1-weighted three-dimensional MPRAGE sequence at the end of the scan session (Time of repetition (TR)/Time of echo (TE) = 1950/3.93 milliseconds (ms), flip angle = 15o; 176 slices, voxel size = 1 × 1 × 1 mm (mm), field of view (FOV) = 256 mm, matrix = 256 × 256). Functional MR images were acquired using gradient-echo echo-planar imaging sequences (GE-EPI) sensitive to blood oxygen level-dependent (BOLD) contrast (TR/TE = 2000/30 ms, flip angle = 90o, 31 interleaved slices, voxel size = 3.75 × 3.75 × 5 mm with a gap of 1 mm, FOV = 240 mm, matrix = 64 × 64).
2.5. MRI image processing
Longitudinal data were analyzed using the FreeSurfer 5.3 longitudinal pipeline (Reuter et al., 2012), which consists in the normalization of all scans belonging to a subject into an individual template instead of individual sessions. Cortical reconstruction and volumetric segmentation (Dale et al., 1999) included motion correction of individual T1-weighted images, removal of non-brain tissue using a hybrid watershed/surface deformation procedure, automated transformation into the Talairach stereotaxic space, segmentation of the cortical and subcortical gray and white matter volumetric structures (Desikan et al., 2006; Fischl et al., 2004), intensity normalization (Sled et al., 1998), tessellation of the boundary between gray and white matter, and an automated topology correction (Ségonne et al., 2004). Individual data were inspected at each step and manual corrections were applied when necessary. The preprocessing stream was re-run for each edited step and re-examined to ensure that image quality was optimal. Hippocampal volumetric data were derived according to the Desikan-Killany atlas (Desikan et al., 2006) and were corrected as a function of the total intracranial volume (ICV; Raw hippocampal volume/Intracranial volume X 100).
2.6. fMRI image processing
Prior to preprocessing, fMRI images for each subject were first corrected for movements using “BadSlice correction” included in the “Artrepair” software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html). Images were then preprocessed and analyzed using Statistical Parametric Mapping (SPM12; http://www.fil.ion.ucl.ac.uk/spm). Functional data were realigned to the median image acquired in the session, and a mean image was created for each subject. Realigned volumes were then normalized into Montreal Neurological Institut (MNI) stereotaxic space and spatially smoothed with an 8 mm Full width at half maximum (FWHM) Gaussian kernel. Data were modelled with the canonical dynamic response function, and a high pass filter of 208 s was used in order to exclude low-frequency variations.
2.7. Statistical analysis
Since the main focus of the paper was to assess task-related activation in pMCI, analyses were first performed on this subgroup. They were then repeated on all MCI to facilitate comparison with published data that do not separate pMCI and sMCI though the entire group is not a focus of our paper.
Behavioral performance was measured with a memory score which takes into consideration both hits and false alarms: ((hit rates/total stimuli) – (false alarm/total stimuli)). Performance was analyzed with a mixed analysis of variance (ANOVA) using Group (pMCI/all MCI, CTL) as a between-subject factor, and Time (Y0, Y2) as a within-subject factor. All behavioral analyses were done using the Statistical Package for Social Sciences (SPSS) v.25.0.
Structural brain analyses were conducted in the QDEC interface of FreeSurfer 5.3 to identify cortical regions with cortical thinning. This method was used since it is well suited to assess longitudinal cortical thickness changes between two groups and because the analysis can simplify the models to a paired analysis when there are only two time points (Reuter et al., 2012). A General Linear Model (GLM) with a Monte Carlo simulation correction with a threshold set at p < .005 with a smoothing of 10 mm FWHM was used to test slope differences in thickness from Y0 to Y2 between CTL and MCI (pMCI/all MCI) individuals. Hippocampal volumes were extracted from FreeSurfer and exported in SPSS. Hippocampal volume was analyzed using Group (pMCI/all MCI, CTL) as a between-subject factor, and Hemisphere (left, right) and Time (Y0, Y2) as within-subject factors.
The fMRI design was a block design in order to maximize statistical power (Liu and Frank, 2004). The instruction blocks were modelled as a condition of no interest. Within-group voxel-wise comparisons were first performed for the “encoding” vs. “cross fixation” contrast using random effect models at both times of measure in order to assess regions activated by the task. This was done with a threshold of p < .05 and family-wise correction (FWE). Functional ROI spheres were then created using the toolbox MARSeille Boîte À Région d'intérêt (Marsbar) (http://marsbar.sourceforge.net) on regions showing steeper slope in cortical thickness in pMCI compared to CTL from Y0 to Y2 on the basis of the QDEC analysis using their peak coordinates. Since MRI and fMRI analyses were done using the Talairach and MNI templates respectively, we assessed the correspondence between coordinates using the Yale BioImage Suite Package application (http://sprout022.sprout.yale.edu/mni2tal/mni2tal.html) to build ROIs. Hippocampi ROIs were built using the PickAtlas toolbox (Maldjian et al., 2003). Functional betas values obtained via ROI analyses were then extracted from MarsBar and exported in SPSS. Between-group differences in brain activation values derived from the ROIs were directly assessed with mixed ANOVAs using Group (pMCI/all MCI, CTL) as a between-subject factor, and Time (Y0, Y2) as a within-subject factor and followed by simple effects in the case of significant interactions.
To assess the relationship between task-related activation and hippocampal volume/cortical thickness, bivariate Pearson correlation were computed between ROI activation betas values, hippocampal volume, cortical thickness derived from ROIs (at Y0 and Y2) and time to diagnosis (in months, at Y0 and Y2).
3. Results
3.1. Clinical follow-up
Mean follow-up length in MCI individuals was 44.31 months (minimum of 24.67 months and maximum of 74.66 months). Thirteen MCI progressed to dementia. The mean time between the first scan and diagnosis was 33.64 months (SD = 22.03 months; range: 5–72 months). None of the CTLs met criteria for MCI or AD at Y2. Seven MCI persons and 4 CTLs dropped out of the study between Y0 and Y2 and were not included in the analyses.
3.2. Sociodemographic and neuropsychological data
Participants' demographic and clinical data at Y0 are presented in Table 1 and are shown for the initial sample (n = 40) and for participants who remained in the study over the two-year follow-up (final sample; n = 29; 10 CTLs, 13 pMCI and 6 sMCI). Only the final sample was used for analyses. Independent-sample t-tests and chi-square analyses indicated that the final groups were comparable (pMCI/all MCI vs. CTL) for age, education, and gender distribution. Persons with pMCI performed significantly lower than CTLs on global clinical scales (MDRS, MMSE), as well as on measures of episodic memory (RL-RI 16 3rd free recall and delayed recall), and executive functions (coding WAIS-III. Of note, the initial versus final groups were comparable on these aforementioned measures, suggesting that the survival bias was unlikely to have impacted our findings.
Table 1.
Demographic and clinical characteristics of participants (mean, with standard deviations in parentheses) at Y0.
| Initial sample |
Final sample |
|||||||
|---|---|---|---|---|---|---|---|---|
| CTL | MCI | pMCI | sMCI | CTL | MCI | pMCI | sMCI | |
| Sex (f, m) | 8, 6 | 15, 11 | 7, 6 | 3, 3 | 6, 4 | 10, 9 | 7, 5 | 3, 3 |
| Age | 67.21 (6.80) | 68.32 (8.61) | 69.42 (7.25) | 67.00 (12.30) | 65.70 (6.96) | 68.61 (8.94) | 69.42 (7.25) | 67.00 (12.30) |
| Education | 14.57 (3.76) | 14.56 (3.92) | 15.17 (4.55) | 14.67 (3.88) | 13.80 (3.39) | 15.00 (4.23) | 15.17 (4.55) | 14.67 (3.88) |
| MDRS | 140.33 (2.65) | 134.96 (4.99)b | 134.00 (4.55)c | 140.33 (2.75) | 140.50 (2.88) | 136.11 (5.03)a | 134.00 (4.55)b | 140.33 (2.75) |
| MMSE | 29.29 (1.14) | 27.57 (1.97)b | 27.17 (2.08)b | 29.17 (1.17) | 29.40 (0.70) | 27.83 (2.04)b | 27.17 (2.08)b | 29.17 (1.17) |
| SMAF | – | −1.05 (1.05) | −0.92 (1.02) | −1.00 (1.00) | – | −0.94 (0.98) | −0.92 (1.02) | −1.00 (1.00) |
| Boston Naming Test | – | 13.22 (1.62) | 12.75 (1.71) | 14.33 (1.21) | – | 13.28 (1.71) | 12.75 (1.71) | 14.33 (1.21) |
| Coding (WAIS-III) | 11.29 (2.30) | 9.57 (2.61) | 9.75 (2.60)a | 10.67 (2.25) | 11.00 (2.58) | 10.06 (2.46) | 9.75 (2.60) | 10.67 (2.25) |
| Benton Judgment of line orientation | – | 23.78 (3.86) | 23.83 (2.69) | 25.67 (3.88) | – | 24.44 (3.15) | 23.83 (2.69) | 25.67 (3.88) |
| Rey Complex Figure | ||||||||
| Copy (score) | – | 30.59 (3.52) | 31.00 (3.23) | 30.92 (2.84) | – | 30.97 (3.02) | 31.00 (3.23) | 30.92 (2.84) |
| Immediate recall (score) | – | 10.23 (6.16) | 10.58 (6.24) | 13.90 (5.46) | – | 11.56 (6.05) | 10.58 (6.24) | 13.90 (5.46) |
| Delayed recall (score) | – | 10.57 (6.26) | 10.92 (5.49) | 15.08 (5.55) | – | 12.31 (5.72) | 10.92 (5.49) | 15.08 (5.55) |
| Stroop (3rd plate) | ||||||||
| Time | – | 31.40 (8.06) | 31.78 (8.54) | 29.13 (7.53) | – | 30.90 (8.09) | 31.78 (8.54) | 29.13 (7.53) |
| Errors | – | 1.22 (2.04) | 1.50 (2.02) | 0.50 (0.84) | – | 1.17 (1.76) | 1.50 (2.02) | 0.50 (0.84) |
| RL/RI 16 | ||||||||
| 3rd immediate free recall | 12.21 (2.33) | 7.43 (3.40)c | 6.92 (2.31)c | 11.33 (2.07) | 12.40 (2.67) | 8.39 (3.05)b | 6.92 (2.31)c | 11.33 (2.07) |
| Delayed free recall | 12.71 (2.40) | 7.09 (4.01)c | 6.33 (3.63)c | 11.50 (1.87) | 12.30 (1.00) | 8.06 (3.98)b | 6.33 (3.63)c | 11.50 (1.87) |
impairment relative to CTLs at p < .05.
impairment relative to CTLs at p < .01.
impairment relative to CTLs at p < .001.
3.3. Behavioral performance during fMRI
Performances on the memory task used during the fMRI scan are shown in Table 2. The analysis of the memory score in pMCI versus CTLs indicated a significant Group effect, F(1, 19) = 34.043, p < .001, η2 = 0.642, with no Time, or Group x Time interaction, both F < 1. Overall, CTLs showed better memory performance than pMCI persons. The same analysis with all MCI (sMCI + pMCI) also indicated a Group effect, F(1, 25) = 15.398, p < .01, η2 = 0.381, CTLs showing better performance than all MCI, but no Time effect, F(1, 25) = 1.494, p = .233, or Group x Time interaction, F(1, 25) = 1.042, p = .317 (Fig. 1).
Table 2.
Scores on the memory task (mean, with standard deviations in parentheses).
| CTL | MCI | pMCI | sMCI | |
|---|---|---|---|---|
| T1 | ||||
| Memory score | 0.70 (0.22) | 0.34 (0.28)a | 0.25 (0.23)a | 0.54 (0.29) |
| T2 | ||||
| Memory score | 0.68 (0.22) | 0.26 (0.27)a | 0.18 (0.17)a | 0.43 (0.36) |
Group effect, with impairment relative to CTLs at p < .05.
Fig. 1.
Time to dementia for the 13 pMCI participants included in the study. Time 0 represents the year at which diagnosis was received for each participant. Dots indicates the Y0 and Y2 scans.
3.4. Structural MRI analyses
3.4.1. Analysis of cortical thinning for ROI selection
Comparison of pMCI and CTLs. One pMCI subject had to be discarded from neuroimaging analyses due to poor image quality. The QDEC analysis comparing CTLs to the pMCI group between Y0 and Y2 revealed five regions that showed more cortical thinning in pMCI than in CTL individuals (see Fig. 2 and Table 3): the right supramarginal (BA40), right pars orbitalis (BA47), left pars opercularis (BA45), the left superior frontal gyrus (BA10) and the left lateral occipital gyrus (BA18). Thus, those regions were used as ROIs for functional analyses in addition to the hippocampi.
Fig. 2.
Maps showing regions with significantly different thickness slopes from Y0 to Y2 between the pMCI and CTL using the general linear model at each vertex across the entire cortical mantle. Differences are expressed in Z scores, with the blue indicating a significantly steeper slope difference in the pMCI group than in the CTL group. Maps are presented on the pial cortical surface of an average brain with sulci in dark gray color and gyris in light gray color. Non-cortical regions (i.e. thalamus, basal ganglia) were not included in the analysis. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Cluster sizes, peak Talairach coordinates, and corresponding Z-scores for clusters showing a steeper cortical thickness slope from Y0 to Y2 in the pMCI group compared to CTLs with Monte Carlo simulation correction set at 0.005.
| Cluster size (mm2) | x | y | z | Z score | |
|---|---|---|---|---|---|
| Right supramarginal (BA40) | 326.02 | 52.6 | −36.5 | 42.9 | −4.162 |
| Right pars orbitalis (BA47) | 9009.80 | 44.3 | 39.2 | −13.0 | −8.809 |
| Left pars opercularis (BA45) | 1095.03 | −53.2 | 21.8 | 9.3 | −5.757 |
| Left superior frontal gyrus (BA10) | 4499.26 | −7.9 | 58.8 | −1.7 | −8.438 |
| Left lateral occipital gyrus (BA18) | 284.21 | −15.3 | −98.7 | 4.0 | −3.181 |
Comparison of all MCI and CTLs. There was no region showing cortical thinning between Y0 and Y2 when comparing the whole MCI group to CTLs.
3.4.2. Hippocampal volume analysis
Comparison of pMCI and CTLs. The analysis of hippocampal volume (see Table 4) indicated a significant Group effect when comparing pMCI to CTLs, F(1, 20) = 7.617, p < .05, η2 = 0.276, due to smaller hippocampal volumes in pMCI than CTLs. The Hemisphere effect was also significant, F(1, 20) = 119.073, p < .001, η2 = 0.856, and this was qualified by a Group x Hemisphere interaction, F(1, 20) = 4.796, p < .05, η2 = 0.193. The interaction was due to the fact that pMCI have larger left than right hippocampus volume, while this was not found in CTLs. None of the other effects were significant: Time effect, F(1, 20) = 1.643, p = .215, Group X Time, F < 1, Group x Hemisphere x Time interactions, F(1, 20) = 1.939, p = .179.
Table 4.
Hippocampal volumes (corrected for intracranial volume) for the CTLs, all MCI, pMCI, and sMCI groups (mean, with standard deviations in parentheses).
| CTL | MCI | pMCI | sMCI | |
|---|---|---|---|---|
| T1 | ||||
| Left hippocampus | 0.27 (0.03) | 0.24 (0.06) | 0.23 (0.06) | 0.28 (0.04) |
| Right hippocampus | 0.28 (0.03) | 0.26 (0.05) | 0.24 (0.06) | 0.28 (0.04) |
| T2 | ||||
| Left hippocampus | 0.23 (0.03) | 0.20 (0.06) | 0.18 (0.05) | 0.24 (0.04) |
| Right hippocampus | 0.24 (0.03) | 0.20 (0.06) | 0.18 (0.05) | 0.25 (0.04) |
Comparison of all MCI and CTLs. When comparing all MCI to CTLs, there was no Group or Time effect, nor Group x Time interaction.
4. fMRI analyses
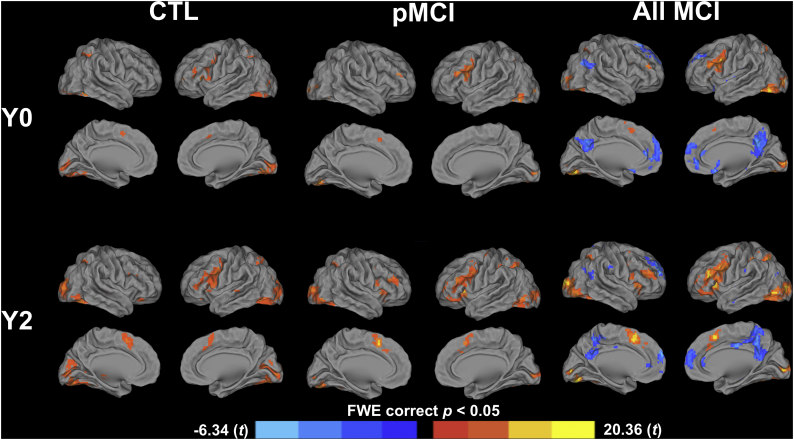
4.1. Within-group whole-brain activation
Activation at Y0. The areas of activation during the memory task are presented in Table 5 and activation maps are shown in Fig. 3. At Y0, all groups (CTL, pMCI, all MCI) activated the occipital lobe bilaterally, the left inferior (pars opercularis and pars triangularis) and middle gyri, the left precuneus, and the left inferior parietal lobe. In addition to common areas of activation, CTLs activated the right inferior and superior parietal lobes and the left cerebellum. The group of pMCI additionally activated the left superior parietal lobe and the right cerebellum in addition to common areas of activation. When combined, all MCI individuals also activated the right angular gyrus, and the right inferior and superior parietal lobes, and deactivated the right superior and middle temporal lobes, the posterior cingulate and the precuneus bilaterally, in the anterior cingulate, and in the left superior and medial frontal gyri.
Table 5.
Cluster size, peak voxel MNI coordinates, and corresponding t-values for clusers associated with encoding at Y0 and Y2 for the CTL, pMCI, and all MCI (p < .05, FWE corrected).
| Y0 | Cluster size | x | y | z | t-value |
|---|---|---|---|---|---|
| CTL group: Encoding > Visual fixation | |||||
| Left cerebellum anterior lobe | 25 | 0 | −58 | −34 | 8.59 |
| Right occipital lobe (18) | 721 | 45 | −55 | −13 | 12.41 |
| Left occipital lobe (18) | 505 | −15 | −85 | 19 | 9.06 |
| Left inferior and middle frontal gyri (10, 46) | 63 | −39 | 50 | 8 | 13.06 |
| Left inferior frontal gyrus (pars opercularis and triangularis; 6, 9, 44, 45) | 122 | −51 | 20 | 8 | 8.92 |
| Left precuneus and inferior parietal lobes (7, 19) | 73 | −27 | −64 | 38 | 5.93 |
| Right inferior and superior parietal lobes (7, 19, 40) | 86 | 24 | −61 | 32 | 10.20 |
| Left supplementary motor area (6, 8, 32) | 19 | 9 | 14 | 47 | 6.60 |
| pMCI group: Encoding > Visual fixation | |||||
| Right cerebellum posterior lobe | 21 | 33 | −64 | −31 | 5.18 |
| Left occipital lobe (18) | 298 | −24 | −76 | −13 | 9.89 |
| Right occipital lobe (18) | 197 | 18 | −94 | −1 | 10.67 |
| Left inferior (pars opercularis and triangularis) and middle gyri (6, 9, 44, 45, 46) | 181 | −48 | 11 | 20 | 7.73 |
| Left precuneus and inferior and superior parietal lobes (7, 19) | 78 | −27 | −76 | 41 | 7.97 |
| Left supplementary motor area (6. 8, 32) | 16 | −3 | 11 | 50 | 5.67 |
| Whole MCI group: Encoding > Visual fixation | |||||
| Right occipital lobe (18) | 557 | 18 | −94 | 2 | 6.47 |
| Left occipital lobe (18) | 642 | −24 | −76 | −13 | 6.62 |
| Left inferior (pars opercularis and triangularis) and middle gyri (6, 9, 44, 45, 46) | 381 | −39 | 5 | 32 | 5.26 |
| Right angular gyrus and inferior and superior parietal lobes (7, 40, 19) | 102 | 24 | −61 | 50 | 5.00 |
| Left precuneus and inferior and superior parietal lobes (7, 19) | 179 | −27 | −76 | 41 | 5.37 |
| Whole MCI group: Encoding < Visual fixation | |||||
| Right superior and middle temporal lobes (39, 40) | 166 | 51 | −61 | 23 | −4.98 |
| Posterior cingulate cortex and precuneus bilaterally (7, 31) | 658 | 0 | −61 | 47 | −5.19 |
| Anterior cingulate cortex and left superior and medial frontal gyri (9, 10) | 753 | −15 | 56 | 23 | −5.79 |
| Y2 | Cluster size | x | y | z | t-value |
| CTL group: Encoding > Visual fixation | |||||
| Right cerebellum and occipital lobes bilaterally (18) | 2702 | 30 | −85 | −4 | 19.81 |
| Right inferior frontal gyrus (47) | 36 | 24 | 29 | −10 | 7.16 |
| Left inferior (pars opercularis and triangularis) and middle gyri (6, 9, 44, 45, 46) | 743 | −42 | −1 | 26 | 20.36 |
| Left precuneus and superior parietal lobe (7, 19) | 169 | −36 | −43 | 32 | 11.92 |
| Left supplementary motor area (6, 8, 32) | 218 | −9 | 11 | 50 | 8.81 |
| pMCI group: Encoding > Visual fixation | |||||
| Right cerebellum and occipital lobe (18, 19) | 670 | 18 | −88 | 5 | 10.28 |
| Left occipital lobe (18, 19) | 704 | −27 | −82 | −19 | 15.35 |
| Left inferior gyrus (pars opercularis, triangularis, and orbitalis; 6, 9, 13, 45, 46, 47) | 757 | −39 | 26 | 17 | 11.50 |
| Right inferior frontal gyrus (pars opercularis; 13, 45, 47) | 131 | 42 | 17 | 5 | 8.92 |
| Left frontal middle gyrus (10, 46) | 53 | −39 | 50 | 11 | 8.24 |
| Right inferior (pars triangularis) and middle gyri (10, 46) | 135 | 33 | 32 | 17 | 10.36 |
| Right supramarginal gyrus and inferior parietal lobe (7, 40) | 166 | 36 | −49 | 38 | 8.85 |
| Left precuneus and inferior and superior parietal lobes (7, 40) | 265 | −27 | −61 | 38 | 8.94 |
| Whole MCI group: Encoding > Visual fixation | |||||
| Right cerebellum and occipital lobe (18, 19) | 1015 | 18 | −88 | 5 | 5.76 |
| Left occipital lobe (18, 19) | 953 | −27 | −82 | −19 | 6.42 |
| Left inferior (pars opercularis and triangularis) and middle gyri (6, 13, 45, 46, 47) | 1071 | −39 | 26 | 17 | 6.05 |
| Right inferior (pars opercularis and triangularis) and middle gyri (46, 47) | 468 | 33 | 32 | 17 | 5.35 |
| Left putamen | 109 | −21 | −1 | 5 | 4.88 |
| Right angular gyrus and inferior and superior parietal lobes (7, 40) | 322 | 36 | −49 | 38 | 5.28 |
| Left supplementary motor area (6, 8, 32) | 313 | −6 | 11 | 53 | 5.62 |
| Left precuneus and inferior and superior parietal lobes (7, 40) | 429 | −27 | −61 | 38 | 5.72 |
| Whole MCI group: Encoding < Visual fixation | |||||
| Right superior and middle temporal lobes (22, 39, 40) | 145 | 57 | −52 | 17 | −5.28 |
| Posterior and middle cingulate cortices and precuneus bilaterally (5, 7, 24, 31, 35) | 915 | −6 | −40 | 47 | −6.13 |
| Anterior cingulate cortex and superior frontal gyrus bilaterally (9, 10) | 613 | −6 | 56 | 26 | −6.34 |
Fig. 3.
One t-test maps of activation during the encoding of word-pairs by the CTL, pMCI, and all MCI groups at Y0 and Y2. Contrasts are expressed in t scores with the orange and yellow indicating significantly higher activation than baseline and the blue indicating significantly lower activation than baseline (deactivation). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Activation at Y2. At Y2, all groups activated the occipital lobes bilaterally, the right cerebellum, the left inferior frontal gyrus bilaterally, the left middle frontal gyrus, the left precuneus, the left superior parietal lobe, and the supplementary motor area. In addition to common areas of activation, the pMCI group activated the right middle frontal gyrus, the inferior parietal lobe bilaterally, and the right supramarginal gyrus. When combined, MCI individuals additionally activated the left putamen, the right angular gyrus, the right inferior and superior parietal lobes, and the left inferior parietal lobe and deactivated the right superior and middle temporal lobes, the anterior, posterior and middle cingulate cortices, the precuneus bilaterally, and the superior frontal gyrus bilaterally.
4.2. Between group ROI-based activations
Comparison of pMCI and CTLs. Groups were directly compared on brain activation derived from the hippocampi and five cortical regions showing cortical thinning: the right supramarginal (BA40), right pars orbitalis (BA47), left pars opercularis (BA45), left superior frontal gyrus (BA10) and the left lateral occipital gyrus (BA18). Fig. 4 shows activations in all ROIs for the pMCI and CTL groups. The analyses that assessed activation of the right supramarginal gyrus in pMCI and CTLs indicated a main Group effect, F(1, 20) = 6.495, p < .05, η2 = 0.245, due to larger activation in pMCI than in CTLs but no Time effect, or Group X Time interaction, F < 1 in both cases. There was also a significant Group, F(1, 20) = 5.508, p < .05, η2 = 0.216, and Time effect, F(1, 20) = 7.786, p < .05, η2 = 0.280, in the left opercularis, but no interaction, F < 1. pMCI showed a lower level of activation than CTLs and activation increased from Y0 to Y2 for both groups. Analysis of the left lateral occipital gyrus revealed a Time effect, F(1, 20) = 12.019, p < 0.01, η2 = 0.375, as activation increased from Y0 to Y2. There was no Group effect, F(1, 20) = 1.181, p = .290, nor Group X Time interaction, F(1, 20) = 2.523, p = .128. There were no effects or interactions in the right pars orbitalis (Group and Time, F < 1; Group x Time interaction, F(1, 20) = 3.149, p = .093), or in the left superior frontal gyrus (Group, Time, Group x Time interaction, all F < 1).
Fig. 4.
Task-related activation comparisons between CTLs and pMCI from Y0 to Y2 in the four ROIs derived from the QDEC analysis and in both hippocampi. Significant group differences were only found in the right supramarginal gyrus, left pars opercularis, and the left hippocampus, with no Time effect nor Group X Time interaction. None of these effects were significant in the right hippocampus, left superior frontal gyrus, and left pars orbitalis.
A significant Group effect was found in the left hippocampus, F(1, 20) = 6.834, p < .05, η2 = 0.255, with lower levels of activation in pMCI persons than in CTLs. There was also a Time effect, F(1, 20) = 4.934, p < 0.05, η2 = 0.198, as the level of activation increased from Y0 to Y2 in both groups. There was no Group x Time interaction, F < 1. The Group effect in the right hippocampus just missed significance, F(1, 20) = 3.601, p = .07, and the Time and Group x Time interaction was not significant, both F < 1.
Comparison of all MCI and CTLs. When comparing activation in the whole MCI group and CTLs, the analysis indicated a significant Group effect in the left pars opercularis, F(1, 25) = 4.952, p < .05, η2 = 0.160, as MCI showed less activation than CTLs, and a Time effect, F(1, 25) = 7.558, p < .05, η2 = 0.225, as activation increased from Y0 to Y2, but no Group x Time interaction, F < 1. A Time effect was found significant for the left lateral occipital gyrus, F(1, 25) = 7.974, p < .01, η2 = 0.235, as activation increased from Y0 to Y2. There was no Group effect, F(1, 25) = 3.856, p = 0.06, nor Group X Time interaction, F < 1. None of the other cortical regions showed a significant effect: the superior frontal area (Group, F(1, 25) = 1.054, p = .396, Time and Group x Time interaction, both F < 1), right pars orbitalis (Group, Time, both F < 1, Group x Time interaction, F(1, 25) = 2.571, p = .124), right supramarginal gyrus (Group, F(1, 25) = 3.241, p = .08, Time and Group x Time interaction, both F < 1).
The analysis of activation in the left hippocampus, indicated a significant Group, F(1, 25) = 5.285, p < .05, η2 = 0.169, and Time effects, F(1, 25) = 4.934, p < .05, η2 = 0.198, but no Group x Time interaction, F < 1. MCI showed less activation than CTLs, and activation increased from Y0 to Y2. None of the effects were significant for activation in the right hippocampus (Group, F(1, 25) = 2.386, p = .135, Time and Group x Time interaction, both F < 1).
4.3. Correlational analyses
No correlation was found significant between activation (betas values) and time to dementia (months) in any of the cortical ROIs or hippocampi (p ranging from 0.174 to 0.874; see Fig. 5). However, we found negative correlations between the volume of the left hippocampus and time to dementia, r = −0.547, p < .01, r2 = 0.30, and between thickness of the left pars opercularis and time to dementia, r = −0.534, p < .01, r2 = 0.29. In both cases, smaller volume/thickness are associated with closer time to dementia.
Fig. 5.
Relation between morphological measures (hippocampal volume, cortical thickness; upper row), task-related activation betas values (lower row) and time to diagnosis in regions showing group differences in task-related activation in the pMCI group. Each pMCI subject is depicted in relation to its individual time to diagnosis with its Y0 and Y2 connected by a line.
5. Discussion
The innovative aspect of this study is that we relied on a longitudinal design to assess task-related brain activation in persons with MCI. This allows for the identification of individuals with MCI who later progressed to dementia and to assess whether activation changes over a two-year period. We also examined task-related activation beyond the hippocampus to include structurally-damaged cortical regions. Our study confirms that hyperactivation is an early hallmark of AD, as we observed larger activation than CTLs in the right supramarginal gyrus of MCI who were confirmed to later progress to dementia. Interestingly, we also found hypoactivation in the left hippocampus and pars opercularis, indicating that hyper- and hypoactivation can co-exist during the disease progression. There were no activation changes after two years, and task-related activation did not relate to time before the clinical diagnosis of dementia. This suggests that hyperactivation is relatively stable when examined over a relatively short period. In contrast, hippocampal volume and cortical thickness showed change over time, and these changes were associated with shorter time to diagnosis. Each of these main findings will be discussed in the following section in relation to our research objectives.
Our first objective was to assess whether task-related hyperactivation was found when examined in a group of pMCI individuals, that is, in individuals who were confirmed to later progress to dementia. Examining hyperactivation only in pMCI is of a great importance to understand the early mechanisms that are truly associated with neurodegenerative processes and to identify individuals that are more likely to develop dementia. Our results indicated that this was indeed the case, as hyperactivation was found to be present in pMCI. We also assessed activation using the whole MCI sample that is, including both stable and pMCI. This was done to compare our results with the literature, where most studies included MCI individuals irrespective of whether they will later progress to dementia or not. Interestingly, the right parietal hyperactivation was no longer significant when using the larger group. It is likely that including stable MCI contributes to reducing the effect, which might partly explain the discrepancies observed in the literature, where some studies failed to observe hyperactivation in MCI. Including stable MCI might indeed impede the possibility to examine task-related hyperactivation. Of note, there was a conspicuous absence of task-induced deactivation in the CTL group, a result similar to a large number of prior studies in older adults (Lustig et al., 2003; Hansen et al., 2014; Li et al., 2015; Miller et al., 2008b; Persson et al., 2007). Absence of task-induced deactivation was also present in pMCI, consistent with other prior studies (Balardin et al., 2015; Petrella et al., 2007; Pihlajamäki and Sperling, 2009). Note that a few studies did not find failure to deactivate in MCI (see Gould et al., 2006; Kochan et al., 2011). This discrepancy may be due to an effect of disease severity (Celone et al., 2006; Pihlajamäki and Sperling, 2009; Sperling et al., 2010) or to the fact that few prior studies have examined whether their at-risk individuals actually progressed to dementia. They might thus have included a heterogeneous group of individuals. Nonetheless, it is reassuring that we did not find deactivation, as it indicates that hyperactivation cannot be merely explained by reduced deactivation in pMCI.
A second objective was to assess the location of these hyperactivations and more precisely, whether hyperactivation is present in cortical regions. Most fMRI studies reporting hyperactivation have focused on the hippocampus, so it is important to investigate whether the hyperactivation phenomenon also occurs in cortical regions. Our finding of hyperactivation in the right supramarginal gyrus indicates that hyperactivation can be found in other regions that are vulnerable to AD. Contrary to prior studies, we did not observe hippocampal hyperactivation. We rather observed hypoactivation in the left hippocampus in the pMCI group and neither hyper- nor hypoactivation difference in the right hippocampus. This is in opposition to the data reported by a number of previous studies (Dickerson et al., 2004; Huijbers et al., 2015; Miller et al., 2008a; Putcha et al., 2011; O'Brien et al., 2010) where hyperactivation was reported in the hippocampus. This is not entirely incompatible with the model, however. One interpretation for the lack of hyperactivation in the hippocampus is that MCI individuals in our sample are more severely impaired than those included in previous studies. It is interesting to highlight that our pMCI were first scanned on average 33 months prior to diagnosis. This is quite close to diagnosis considering that the disease progresses over about 20 years, and it is possible that hyperactivation occurs at different times for different brain regions. Importantly, few prior studies have examined pMCI separated from stable MCI and these have not reported time to diagnosis. It is therefore not possible to determine at which stage participants were in those earlier studies. Hyperactivation might have been present in the hippocampus of our participants at some point prior to study entry. It is also possible that previous studies included a mixture or progressors and stable MCI and that stable MCI may have contributed to increase the group level of hippocampal activation given that they might not be affected by AD.
The fact that we found hyperactivation in the right parietal area is not trivial. Indeed, it is in line with a study from our team that reported that increased parietal activation was positively correlated with memory improvement following cognitive training in persons with MCI (Belleville et al., 2011). In the same vein, Elman et al. (2014) reported that larger parietal activation was associated with better cognition in older adults with high amyloid deposition. These authors have proposed that activation in this region can support compensatory mechanisms in older adults suffering from early AD. It is interesting to note that CTLs recruited the left parietal area homologous to the right parietal region recruited by MCI. Thus, it appears that pMCI recruited an alternative region that is not typically involved in the task. Interestingly, this new recruitment is controlateral to the same region recruited in the left hemisphere by CTLs. This result is consistent with studies indicating that older adults recruit regions that are controlateral to the ones recruited by younger adults (Logan and Buckner, 2001; Reuter-Lorenz et al., 2000; Stebbins et al., 2002). It is also consistent with some prior studies from our lab that found similar controlateral recruitments in MCI when compared to older CTLs (Clément and Belleville, 2010, Clément and Belleville, 2012). This pattern is consistent with the hemispheric asymmetry reduction in older adults (HAROLD; Cabeza, 2002), which suggests that neural compensation occurs by recruiting brain areas that are controlateral to those normally recruited by a task. Interestingly, parietal hyperactivation in pMCI co-occurred with hypoactivation of the left hippocampus and inferior frontal gyrus, two regions activated by healthy controls and typically involved in verbal memory (Daselaar et al., 2003; Duverne et al., 2008; Miller et al., 2008a). This suggests that parietal hyperactivation may result from a shift of activation from impaired, underrecruited prefrontal areas within the memory network to more posterior regions. Hence the recruitment of alternative regions such as the right parietal area might reflect compensatory mechanisms in response to the effects of neuropathology on the function of specialized regions. We must acknowledge that performance is quite low in pMCI in spite of putative compensation processes. The presence of compensation mechanisms does not guarantee that the newly deployed or increased neural resources will totally eliminate the gap between task demands and available resources, and in fact, it is unlikely to be the case in most circumstances, especially in individuals with severe clinical impairments (Cabeza et al., 2018). Thus, there are occurrences where compensation occurs but is only partially successful and insufficient to normalize performance. It is possible that the pMCI individuals in our study recruited the right supramarginal gyrus in an “attempted/incomplete compensation”, but were unable to equal their healthy counterparts' performance. There is presently no gold standard that would allow us to determine the amount of impairment expected in the presence of a given brain atrophy and hence to quantify the extent of successful compensation if any.
Our third and last objective was to assess the hyperactivation trajectory. This was done by looking at how hyperactivation changes over a two-year period and whether it interacts with group membership and by examining its relationship with clinical symptoms and time before dementia. Surprisingly, we found that both hyperactivation and hypoactivation were stable over the two-year follow-up that was used here. We have to remain prudent in interpreting this lack of longitudinal change, as it might be explained by our relatively small sample size. It might also be explained by interindividual variability in the temporality of the inverse U-shape. As patients are at different stages of the continuum, some might show increased activation whereas others might show decreased activation. However, if that was the case, one would expect a correlation between activation and time to dementia, which was not found here. Another hypothesis is that change in task-related activation might take place on a longer timeframe than a two-year period and our test-retest length might not have been sufficient to capture it. This stresses the importance to study hyperactivation on a longer period of time to better determine its trajectory and its effect on the brain and cognition. Activation in the left opercularis increased over time, but the Group effect remained significant in the absence of Group X Time interaction. This means that the increased activation is present to a similar degree in pMCI and CTLs with the result that activation in pMCI remains hypoactive when comparing their activation to that of CTLs. Importantly, hippocampal volume and volume of the left pars opercularis regions were found to be negatively correlated with time to dementia, confirming that time to dementia was a sound measure of disease severity.
Overall our results are partly consistent with the cascading network model (Jones et al., 2015, Jones et al., 2017). This model proposes that early disruption of tau-related networks would lead to a compensatory load shift to posterior areas that are more prone to amyloid accumulation, until these latter regions would meet amyloid saturation. Since the hippocampus is an early site of tau accumulation (Braak and Braak, 1991; Schwarz et al., 2018; Villemagne et al., 2013), hypoactivation found in this region might result from excessive tau pathology while right parietal hyperactivation might be indicative of a compensatory load shift toward more posterior regions. However, it should be acknowledged that this interpretation remains speculative since we did not measure amyloid level or tau in our study. It is also important to keep in mind that the compensatory and excitotoxic accounts might not be mutually exclusive as early compensatory increased neuronal activity might contribute to neuropathology propagation (Huijbers et al., 2015, Huijbers et al., 2018; Schultz et al., 2017).
Our study has limitations which must be recognized and addressed. Although focusing on MCI persons who progressed to dementia is a strength and reduced within-group heterogeneity, it also negatively impacted our sample size since we only examined those who were retained at follow-up and who progressed to dementia. Also, we did not include markers of amyloid and/or tau pathology in our participants and hence cannot conclude with certainty about the etiology of their cognitive symptomatology. We only used two longitudinal points which does not allow a measure of non-linear pattern of changes. Using non-linear models would have more directly tested the postulated inverse U-shape trajectory of task-related activation. We used a block design and therefore, we did not assess activation for correct vs. incorrect responses. Of note is the fact that several studies have found hyperactivation using a block design (Clément et al., 2010; Clément and Belleville, 2010; Clément et al., 2013; Erk et al., 2011; Gordon et al., 2015; Rodda et al., 2009, Rodda et al., 2011; Yetkin et al., 2006) and therefore, we believe that such a design is appropriate to detect the presence of hyperactivation in MCI. We did not control for the potential effect of reduced behavioral performance on patterns of brain activation, as statistically controlling for group differences in performance controls for the clinical effect and therefore would result in potentially removing group effect in activation. Finally, partial volume effect could have introduced potential noise in fMRI signal.
6. Conclusion
To conclude, our findings show that task-related hyperactivation is present in structurally impaired regions when examining MCI individuals with a confirmed progression to dementia and that hyperactivation can co-occur with hypoactivation. Hypoactivation is deemed to reflect a failure to activate regions typically implicated in episodic memory. In turn, the hyperactivation of the right supramarginal gyrus which was found here might represent a shift in activation to compensate for the harmful consequences of neuropathology. Larger longitudinal studies with longer follow-up and additional time points will be required to underpin the complex relation between activation and cognition. Further studies will also be needed to determine how hyperactivation can contribute to optimize prediction of future dementia in combination with other neuroimaging markers, biomarkers and/or cognition. It will also be important to prove its value as a “pre-clinical” marker when cognitive symptoms are absent of very subtle in order to identify preclinical AD.
Disclosure statement
All authors disclose no actual or potential conflict of interests in relation to this research.
Acknowledgments
This work was supported by grants from the Canadian Institutes for Health Research (CIHR, project and Foundation grants) and the Canada Research Chair on Cognitive neuroscience of aging and brain plasticity (#950-232074) to SB. NCL was supported by a CIHR doctoral scholarship (#395361). We would like to thank Nadia Jaffer and the CRIUGM banque de Participants for their help with recruitment, Émilie Lepage for her help in testing the participants and Gabrielle Ciquier for English editing.
Footnotes
The participants were part of a larger group initially described in Clément and Belleville, 2010, Clément and Belleville, 2012.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders; 2004. Diagnostic Statistical Manual IV-TR (2000) [Google Scholar]
- Balardin J.B., Batistuzzo M., Moraes Martin M.D.G., Sato J., Smid J., Porto C.…Miotto E.C. Differences in prefrontal cortex activation and deactivation during strategic episodic verbal memory encoding in mild cognitive impairment. Front. Aging Neurosci. 2015;7:147. doi: 10.3389/fnagi.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S., Clement F., Mellah S., Gilbert B., Fontaine F., Gauthier S. Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain. 2011;134(6):1623–1634. doi: 10.1093/brain/awr037. [DOI] [PubMed] [Google Scholar]
- Benton A.L., Sivan A.B., de Hamsher K., Varney N.R. Oxford University Press; USA: 1994. Contributions to neuropsychological assessment: A clinical manual. [Google Scholar]
- Bero A.W., Yan P., Roh J.H., Cirrito J.R., Stewart F.R., Raichle M.E.…Holtzman D.M. Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 2011;14(6):750. doi: 10.1038/nn.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Busche M.A., Konnerth A. Neuronal hyperactivity–A key defect in Alzheimer's disease? Bioessays. 2015;37(6):624–632. doi: 10.1002/bies.201500004. [DOI] [PubMed] [Google Scholar]
- Busche M.A., Chen X., Henning H.A., Reichwald J., Staufenbiel M., Sakmann B., Konnerth A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. 2012;109(22):8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschke H. Cued recall in amnesia. J. Clin. Exp. Neuropsychol. 1984;6(4):433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging. 2002;17(1):85. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Albert M., Belleville S., Craik F.I., Duarte A., Grady C.L.…Rugg M.D. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018;1 doi: 10.1038/s41583-018-0068-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone K.A., Calhoun V.D., Dickerson B.C., Atri A., Chua E.F., Miller S.L.…Albert M.S. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J. Neurosci. 2006;26(40):10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément F., Belleville S. Compensation and disease severity on the memory-related activations in mild cognitive impairment. Biol. Psychiatry. 2010;68(10):894–902. doi: 10.1016/j.biopsych.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Clément F., Belleville S. Effect of disease severity on neural compensation of item and associative recognition in mild cognitive impairment. J. Alzheimers Dis. 2012;29(1):109–123. doi: 10.3233/JAD-2012-110426. [DOI] [PubMed] [Google Scholar]
- Clément F., Belleville S., Mellah S. Functional neuroanatomy of the encoding and retrieval processes of verbal episodic memory in MCI. Cortex. 2010;46(8):1005–1015. doi: 10.1016/j.cortex.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Clément F., Gauthier S., Belleville S. Executive functions in mild cognitive impairment: emergence and breakdown of neural plasticity. Cortex. 2013;49(5):1268–1279. doi: 10.1016/j.cortex.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Daselaar S., Veltman D.J., Rombouts S.A.R.B., Raaijmakers J.G.W., Jonker C. Neuroanatomical correlates of episodic encoding and retrieval in young and elderly subjects. Brain. 2003;126(1):43–56. doi: 10.1093/brain/awg005. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D.…Albert M.S. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Dickerson B.C., Salat D.H., Bates J.F., Atiya M., Killiany R.J., Greve D.N.…Sperling R.A. Medial temporal lobe function and structure in mild cognitive impairment. Ann. Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S., Motamedinia S., Rugg M.D. The relationship between aging, performance, and the neural correlates of successful memory encoding. Cereb. Cortex. 2008;19(3):733–744. doi: 10.1093/cercor/bhn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman J.A., Oh H., Madison C.M., Baker S.L., Vogel J.W., Marks S.M.…Jagust W.J. Neural compensation in older people with brain amyloid-β deposition. Nat. Neurosci. 2014;17(10):1316. doi: 10.1038/nn.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S., Spottke A., Meisen A., Wagner M., Walter H., Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch. Gen. Psychiatry. 2011;68(8):845–852. doi: 10.1001/archgenpsychiatry.2011.80. [DOI] [PubMed] [Google Scholar]
- Esposito Z., Belli L., Toniolo S., Sancesario G., Bianconi C., Martorana A. Amyloid β, glutamate, excitotoxicity in Alzheimer's disease: are we on the right track? CNS Neurosci. Ther. 2013;19(8):549–555. doi: 10.1111/cns.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Van Der Kouwe A., Destrieux C., Halgren E., Ségonne F., Salat D.H.…Caviness V. Automatically parcellating the human cerebral cortex. Cereb. Cortex. 2004;14(1):11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gauthier S., Reisberg B., Zaudig M., Petersen R.C., Ritchie K., Broich K.…Cummings J.L. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- Golby A., Silverberg G., Race E., Gabrieli S., O'shea J., Knierim K.…Gabrieli J. Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory. Brain. 2005;128(4):773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- Gordon B.A., Zacks J.M., Blazey T., Benzinger T.L., Morris J.C., Fagan A.M.…Balota D.A. Task-evoked fMRI changes in attention networks are associated with preclinical Alzheimer’s disease biomarkers. Neurobiol. Aging. 2015;36(5):1771–1779. doi: 10.1016/j.neurobiolaging.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R.L., Brown R.G., Owen A.M., Bullmore E.T., Howard R.J. Task-induced deactivations during successful paired associates learning: an effect of age but not Alzheimer's disease. Neuroimage. 2006;31(2):818–831. doi: 10.1016/j.neuroimage.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Gregory S., Long J.D., Klöppel S., Razi A., Scheller E., Minkova L.…Roos R.A. Operationalizing compensation over time in neurodegenerative disease. Brain. 2017;140(4):1158–1165. doi: 10.1093/brain/awx022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämäläinen A., Pihlajamäki M., Tanila H., Hänninen T., Niskanen E., Tervo S.…Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol. Aging. 2007;28(12):1889–1903. doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hampstead B.M., Stringer A.Y., Stilla R.F., Amaraneni A., Sathian K. Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia. 2011;49(9):2349–2361. doi: 10.1016/j.neuropsychologia.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw B., Dricot L., Kavec M., Grandin C., Seron X., Ivanoiu A. Associative encoding deficits in amnestic mild cognitive impairment: a volumetric and functional MRI study. Neuroimage. 2011;56(3):1743–1748. doi: 10.1016/j.neuroimage.2011.03.034. [DOI] [PubMed] [Google Scholar]
- Hansen N.L., Lauritzen M., Mortensen E.L., Osler M., Avlund K., Fagerlund B., Rostrup E. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain's default mode network. Hum. Brain Mapp. 2014;35(9):4488–4498. doi: 10.1002/hbm.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert R., Carrier R., Bilodeau A. The Functional Autonomy Measurement System (SMAF): description and validation of an instrument for the measurement of handicaps. Age Ageing. 1988;17(5):293–302. doi: 10.1093/ageing/17.5.293. [DOI] [PubMed] [Google Scholar]
- Huijbers W., Mormino E.C., Schultz A.P., Wigman S., Ward A.M., Larvie M.…Sperling R.A. Amyloid-β deposition in mild cognitive impairment is associated with increased hippocampal activity, atrophy and clinical progression. Brain. 2015;138(4):1023–1035. doi: 10.1093/brain/awv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W., Schultz A.P., Papp K.V., LaPoint M.R., Hanseeuw B., Chhatwal J.P.…Sperling R.A. Tau accumulation in clinically normal older adults is associated with hippocampal hyperactivity. J. Neurosci. 2018:1397–1398. doi: 10.1523/JNEUROSCI.1397-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagust W. Amyloid+ activation= Alzheimer's? Neuron. 2009;63(2):141–143. doi: 10.1016/j.neuron.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R.…Alexander M. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. Jama. 2015;313(19):1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.C., Schmitz T.W., Moritz C.H., Meyerand M.E., Rowley H.A., Alexander A.L.…Asthana S. Activation of brain regions vulnerable to Alzheimer's disease: the effect of mild cognitive impairment. Neurobiol. Aging. 2006;27(11):1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Knopman D.S., Gunter J.L., Graff-Radford J., Vemuri P., Boeve B.F.…Jack C.R., Jr. Cascading network failure across the Alzheimer’s disease spectrum. Brain. 2015;139(2):547–562. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.T., Graff-Radford J., Lowe V.J., Wiste H.J., Gunter J.L., Senjem M.L.…Petersen R.C. Tau, amyloid, and cascading network failure across the Alzheimer's disease spectrum. Cortex. 2017;97:143–159. doi: 10.1016/j.cortex.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E., Goodglass H., Weintraub S. 2001. Boston Naming Test. Pro-ed. [Google Scholar]
- Kochan N.A., Breakspear M., Valenzuela M., Slavin M.J., Brodaty H., Wen W.…Sachdev P.S. Cortical responses to a graded working memory challenge predict functional decline in mild cognitive impairment. Biol. Psychiatry. 2011;70(2):123–130. doi: 10.1016/j.biopsych.2011.03.006. [DOI] [PubMed] [Google Scholar]
- Leal S.L., Landau S.M., Bell R.K., Jagust W.J. Hippocampal activation is associated with longitudinal amyloid accumulation and cognitive decline. Elife. 2017;6 doi: 10.7554/eLife.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.G., Perry G., Moreira P.I., Garrett M.R., Liu Q., Zhu X.…Smith M.A. Tau phosphorylation in Alzheimer's disease: pathogen or protector? Trends Mol. Med. 2005;11(4):164–169. doi: 10.1016/j.molmed.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Li H.J., Hou X.H., Liu H.H., Yue C.L., Lu G.M., Zuo X.N. Putting age-related task activation into large-scale brain networks: a meta-analysis of 114 fMRI studies on healthy aging. Neurosci. Biobehav. Rev. 2015;57:156–174. doi: 10.1016/j.neubiorev.2015.08.013. [DOI] [PubMed] [Google Scholar]
- Liu T.T., Frank L.R. Efficiency, power, and entropy in event-related fmri with multiple trial types: Part i: Theory. NeuroImage. 2004;21(1):387–400. doi: 10.1016/j.neuroimage.2003.09.030. [DOI] [PubMed] [Google Scholar]
- Logan J.M., Buckner R.L. Paper presented at the Eighth Annual Meeting of the Cognitive Neuroscience Society, New York, NY. 2001, April. Age-related changes in neural correlates of encoding. [Google Scholar]
- Lustig C., Snyder A.Z., Bhakta M., O'Brien K.C., McAvoy M., Raichle M.E.…Buckner R.L. Functional deactivations: change with age and dementia of the Alzheimer type. Proc. Natl. Acad. Sci. 2003;100(24):14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda M.M., Ward H.A., Borowski B., Gunter J.L., Cha R.H., O’brien P.C.…Ivnik R.J. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda M.M., Senjem M.L., Weigand S.D., Smith G.E., Ivnik R.J., Boeve B.F.…Jack C.R. Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. J. Int. Neuropsychol. Soc. 2009;15(3):372–382. doi: 10.1017/S1355617709090523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mandzia J., Black S., Grady C., McAndrews M.P., Graham S. Encoding and retrieval in aging and memory loss, a fMRI study. Brain Cogn. 2002;49(2):225–228. [PubMed] [Google Scholar]
- Mattis S. 1976. Dementia Rating Scale H Geriatric psychiatry: A Handbook for Psychiatrist and Primary Care Physicians. [Google Scholar]
- McKhann G., Drachman D., Folstein M., Katzman R., Price D., Stadlan E.M. Clinical diagnosis of Alzheimer's disease Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller S.L., Fenstermacher E., Bates J., Blacker D., Sperling R.A., Dickerson B.C. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J. Neurol. Neurosurg. Psychiatry. 2008;79(6):630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.L., Celone K., DePeau K., Diamond E., Dickerson B.C., Rentz D.…Sperling R.A. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc. Natl. Acad. Sci. 2008;105(6):2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutlu J., Landeau B., Gaubert M., de La Sayette V., Desgranges B., Chételat G. Distinct influence of specific versus global connectivity on the different Alzheimer’s disease biomarkers. Brain. 2017;140(12):3317–3328. doi: 10.1093/brain/awx279. [DOI] [PubMed] [Google Scholar]
- O'brien J.L., O'keefe K.M., LaViolette P.S., DeLuca A.N., Blacker D., Dickerson B.C., Sperling R.A. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology. 2010;74(24):1969–1976. doi: 10.1212/WNL.0b013e3181e3966e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson J., Lustig C., Nelson J.K., Reuter-Lorenz P.A. Age differences in deactivation: a link to cognitive control? J. Cogn. Neurosci. 2007;19(6):1021–1032. doi: 10.1162/jocn.2007.19.6.1021. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch. Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen R.C., Doody R., Kurz A., Mohs R.C., Morris J.C., Rabins P.V.…Winblad B. Current concepts in mild cognitive impairment. Arch. Neurol. 2001;58(12):1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Petrella J.R., Wang L., Krishnan S., Slavin M.J., Prince S.E., Tran T.T.T., Doraiswamy P.M. Cortical deactivation in mild cognitive impairment: high-field-strength functional MR imaging. Radiology. 2007;245(1):224–235. doi: 10.1148/radiol.2451061847. [DOI] [PubMed] [Google Scholar]
- Pihlajamäki M., Sperling R.A. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals. Behav. Neurol. 2009;21(1-2):77–91. doi: 10.3233/BEN-2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prvulovic D., Van de Ven V., Sack A.T., Maurer K., Linden D.E. Functional activation imaging in aging and dementia. Psychiatry Res. Neuroimaging. 2005;140(2):97–113. doi: 10.1016/j.pscychresns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Putcha D., Brickhouse M., O'Keefe K., Sullivan C., Rentz D., Marshall G.…Sperling R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer's disease signature regions in non-demented elderly adults. J. Neurosci. 2011;31(48):17680–17688. doi: 10.1523/JNEUROSCI.4740-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard M. University of Victoria; Canada: 1981. Cognitive Rigidity and Flexibility: A Neuropsychological Study. Unpublished Ph.D. dissertation. [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Jonides J., Smith E.S., Hartley A., Miller A., Marshuetz C. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J. Cogn. Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Rey A. Éditions Centre de psychologie appliquée; Paris: 1959. Manual of Copy and Memory Reproduction Test of Complex Geometric Figures. [Google Scholar]
- Rodda J.E., Dannhauser T.M., Cutinha D.J., Shergill S.S., Walker Z. Subjective cognitive impairment: increased prefrontal cortex activation compared to controls during an encoding task. Int. J. Geriatr. Psychiatr. 2009;24(8):865–874. doi: 10.1002/gps.2207. [DOI] [PubMed] [Google Scholar]
- Rodda J., Dannhauser T., Cutinha D.J., Shergill S.S., Walker Z. Subjective cognitive impairment: functional MRI during a divided attention task. Eur. Psychiatr. 2011;26(7):457–462. doi: 10.1016/j.eurpsy.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Rombouts S.A., Barkhof F., Veltman D.J., Machielsen W.C., Witter M.P., Bierlaagh M.A.…Scheltens P. Functional MR imaging in Alzheimer's disease during memory encoding. Am. J. Neuroradiol. 2000;21(10):1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Schultz A.P., Chhatwal J.P., Hedden T., Mormino E.C., Hanseeuw B.J., Sepulcre J.…Sperling R.A. Phases of hyper and hypo connectivity in the Default Mode and Salience networks track with amyloid and Tau in clinically normal individuals. J. Neurosci. 2017:3263–3626. doi: 10.1523/JNEUROSCI.3263-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz A.J., Shcherbinin S., Slieker L.J., Risacher S.L., Charil A., Irizarry M.C.…Miller B.B. Topographic staging of tau positron emission tomography images. Alzheimer's Dement. 2018;10:221–231. doi: 10.1016/j.dadm.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17(1):87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Small S.A., Perera G.M., DeLaPaz R., Mayeux R., Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann. Neurol. 1999;45(4):466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sperling R.A., Dickerson B.C., Pihlajamaki M., Vannini P., LaViolette P.S., Vitolo O.V.…Johnson K.A. Functional alterations in memory networks in early Alzheimer’s disease. NeuroMolecular Med. 2010;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stebbins G.T., Carrillo M.C., Dorman J., Dirksen C., Desmond J., Turner D.A. Aging effects on memory encoding in the frontal lobes. Psychol. Aging. 2002;17:44–55. doi: 10.1037//0882-7974.17.1.44. [DOI] [PubMed] [Google Scholar]
- Van der Linden M., Coyette F., Poitrenaud J., Kalafat M., Calicis F., Wyns C., Adam S. 2004. II. L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI-16) [Google Scholar]
- Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O.…Ames D. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1997. Wechsler Adult Intelligence S-III. [Google Scholar]
- Winblad B., Palmer K., Kivipelto M., Jelic V., Fratiglioni L., Wahlund L.O.…Arai H. Mild cognitive impairment–beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wu J.W., Hussaini S.A., Bastille I.M., Rodriguez G.A., Mrejeru A., Rilett K.…Herman M. Neuronal activity enhances tau propagation and tau pathology in vivo. Nat. Neurosci. 2016;19(8):1085. doi: 10.1038/nn.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yetkin F.Z., Rosenberg R.N., Weiner M.F., Purdy P.D., Cullum C.M. FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur. Radiol. 2006;16(1):193–206. doi: 10.1007/s00330-005-2794-x. [DOI] [PubMed] [Google Scholar]