Abstract
Bariatric surgery is the most effective and durable treatment for morbid obesity, with an unexplained yet beneficial side effect of restoring insulin sensitivity and improving glycemia, often before weight loss is observed. Among the many contributing mechanisms often cited, the altered handling of intestinal bile acids is of considerable therapeutic interest. Here, we review a growing body of literature examining the metabolic effects of bile acids ranging from their physical roles in dietary fat handling within the intestine to their functions as endocrine and paracrine hormones in potentiating responses to bariatric surgery. The roles of 2 important bile acid receptors, Takeda G-protein coupled receptor (also known as G-protein coupled bile acid receptor) and farnesoid X receptor, are highlighted as is downstream signaling through glucagon-like polypeptide 1 and its cognate receptor. Additional improvements in other phenotypes and potential contributions of commensal gut bacteria, such as Akkermansia muciniphila, which are manifest after Roux-en-Y gastric bypass and other emulations, such as gallbladder bile diversion to the ileum, are also discussed.
Keywords: Bariatric Surgery, Bile Acids, Type 2 Diabetes, Roux-en Y Gastric Bypass, Glucagon-Like Polypeptide 1 (GLP-1)
Abbreviations used in this paper: BA, bile acids; CA, cholic acid; CDCA, chenodeoxycholic acid; FGF, fibroblast growth factor; FXR, farnesoid X receptor; GB, gallbladder; GB-IL, bile diversion from the gallbladder to the terminal ileum; GLP, glucagon-like polypeptide; GPBAR, G-protein coupled BA receptor; MCA, muricholic acid; RYGB, Roux-en-Y gastric bypass; T2D, type 2 diabetes; TAG, triacylglycerol; UDCA, ursodeoxycholate acid
Summary.
Although surgical treatment of obesity is becoming widely accepted, the mechanisms of how these operations mediate their beneficial effects remain elusive. Changes in bile acid handling after bariatric surgery hallmark these procedures and likely contribute to the efficacy of these metabolic operations. Establishing the crosstalk and intracellular signaling influenced by bile acids may lead to new insights into the pathogenesis and treatment for numerous diseases.
Over the past 2 decades, bile acids (BAs) have gained a greater visibility and notoriety for their roles in regulating metabolism. Once only appreciated for their role in facilitating the availability of dietary fat,1 BAs are now known to exert hormonal functions throughout the body via nuclear and membrane receptors. Here, we review the expansive list of BA-sensitive signaling pathways, with a focus on intestinal and liver metabolism as regulated by BA availability. We aim to highlight the mechanisms by which BA signaling networks modulate complex physiologic events and explore potential opportunities for BA manipulating interventions that might improve obesity or related disease, such as type 2 diabetes (T2D), hyperlipidemia, and nonalcoholic fatty liver disease.
Bile Acid Synthesis and Enterohepatic Circulation
BAs are physiologic surfactants and cell signaling molecules that are synthesized from a cholesterol precursor in the liver. BA synthesis is facilitated by 2 distinct enzymatic pathways. The classical pathway contributes approximately 75% of the total BA pool and is regulated by cholesterol 7α-hydroxylase.2 The alternate (acidic pathway) is responsible for the remaining, approximately 25% of BA synthesis and is regulated by sterol-27 hydroxylase.3 Chenodeoxycholic acid (CDCA) and cholic acid (CA) in humans, and α-muricholic acid (MCA), β-MCA in mice, are the 2 major primary BAs; they are conjugated to either taurine (in mice) or glycine (in humans) in the liver, before they are actively secreted into the canalicular space of the liver, where they are concentrated over 100-fold in the fasting state before being stored in the gallbladder (GB) and secreted in the duodenum after stimulation by food.4 Secondary BAs, such as lithocholic acid and deoxycholic acid, are formed through additional reactions including bile salt hydrolase and 7α-dehydroxylase present in commensal gut bacteria.5, 6 These gut bacteria additionally oxidize, sulfonate, and dehydroxylate BAs to form a diverse array of other BA species that vary in structure, function, and hydrophobicity. Most of the BAs remain in the gut lumen until they reach the terminal ileum4 where uptake into the enterocyte occurs via the apical sodium-dependent BA transporter and basolateral transporters OSTα/β.7 BAs are then transported back to the liver via portal circulation where they are reabsorbed and then enter hepatic portal circulation in a process repeated 8–10 times per day. In a 70-kg human the sum of dietary cholesterol intake (5 mg/day/kg) and cholesterol synthesis (10 mg/day/kg) is nearly equal to fecal neutral sterol (8 mg/day/kg) and fecal acidic sterol (7 mg/day/kg) secretion. In mice, cholesterol intake (30 mg/day/kg), cholesterol synthesis (160 mg/day/kg), and sterol excretion (60 mg/day/kg for neutral sterols and 50 mg/day/kg for acidic sterols) are considerably higher.8 BA malabsorption can cause congenital diarrhea, steatorrhea, and reduced plasma cholesterol levels. The eventual loss of BAs in feces serves as the primary mechanism for cholesterol excretion from the body.
Bile Acid Regulation of Dietary Fat Availability
Dietary fat in the Western diet accounts for nearly 40% of calories ingested per day, and most (∼95%) of the dietary lipids is derived from triacylglycerol (TAG), approximately 5% from phospholipids and less than 0.5% from cholesteryl esters.9 BA concentrations in the intestine range from 10 mM in the duodenum to 2 mM in the ileum,10 and these salts (predominantly sodium and potassium in most of the body) play a vital role in intestinal fat absorption. It is noteworthy that conjugated BAs have lower pKa values than the unconjugated acids and are therefore more ionized and exhibit greater water solubility at alkaline pH of intestinal chyme. In response to a meal, cholecystokinin stimulates GB contraction releasing bile into the duodenum. Dietary fats (mainly TAG and phospholipids) in the intestinal lumen are solubilized into micelles through the coordinated actions of BAs and various lipases (lingual, gastric, and most importantly pancreatic lipases)11 at the TAG droplet-water interface.12 Dietary TAGs are hydrolyzed by intestinal lipases generating monoacylglycerols13 sensed by GPR119. Fatty acids are sensed by free fatty acid receptors 1–4, which are absorbed by passive diffusion and specific transporters, such as CD36, across the brush border of enterocytes.14 These products are re-esterified to diacylglycerols and TAGs before being assembled into apolipoprotein B-48-containing chylomicron particles used for export to peripheral tissues.14
In the intestine, TAG synthesis is thought to occur mainly through the monoacylglycerols pathway, where monoacylglycerol acyltransferase joins monoacylglycerols and fatty acid–coenzyme A to form diacylglycerols.15 Diacylglycerols and fatty acid–coenzyme A are covalently joined to form TAG through the actions of DGAT1 and DGAT2. Dgat1 and Dgat2 are highly expressed in mouse intestinal tissue,16 with DGAT2 predominating lipid processing.17 In humans, DGAT1 is the only highly expressed enzyme in the intestine, with DGAT2 being expressed mainly in the liver. The coordinated roles of Dgat1 and Dgat2 in intestinal TAG synthesis are not completely understood, but recent studies in mice with intestine-specific deletion of individual isoforms, Dgat1 (Dgat1Int) or Dgat2 (Dgat2Int), suggest different and nonredundant roles in regulating chylomicrons and cytoplasmic lipid droplets.18 The influence of BAs on the activity and/or localization of these enzymes is unknown; however, humans with DGAT1 deficiency exhibit BA diarrhea and may exhibit altered BA metabolism/composition,19 although fecal BA measurements have not yet been reported in these patients.
Bile Acid Receptors
In lean and fasted humans, plasma BA concentrations are very low and hence most receptors are not activated. However, in metabolic stress or in the postprandial period, BA levels increase and composition changes resulting in the activation of various membrane bound and nuclear receptors. The quintessential membrane-bound receptor is a G-protein coupled BA receptor 1 (GPBAR1; TGR5),20, 21 which is involved with rapid and dose-dependent elevation of intracellular cAMP levels.20 The most prototypical nuclear receptor is farnesoid X receptor (FXR; also known as NR1H4)22; other nuclear receptors include vitamin D receptor (NR1H1),23 pregnane X receptor (NR1H2),24, 25 and constitutive androstane receptor (HR1H3). Other receptors include muscarinic receptors,26 active voltage-receptors (BKCA), calcium and chlorine channels,27 tyrosine kinase coupled receptors, and phospholipases (NAPE-PLD).28 BAs species bind these receptors with varying affinities and with a multitude of pathophysiological and pharmacologic effects. It has also been shown that conjugated BAs also activate sphingosine-1-phosphate receptor 2 leading to activation of the ERK1/2 and AKT signaling pathways.29
TGR5
TGR5 (encoded by GPBAR1) is a BA receptor that is a key mediator of the nongenomic actions of BAs. TGR5 is not expressed in hepatocytes, but is localized to sinusoidal endothelial cells,30 monocytes,20 enteroendocrine cells,31, 32 adipose tissue,21, 33 smooth muscle,34 skeletal muscle,21 pancreas,35 and the central nervous system.36 BAs activate TGR5 with a potency order of lithocholic acid>deoxycholic acid>CDCA>CA. TGR5-/- mice have mildly reduced BA pools,37, 38 impaired glucose tolerance,31 and amplified inflammatory responses to partial hepatectomy, CA-enriched feeding, or bile duct ligation injury.39 Through kinase signaling pathways, TGR5 activation stimulates GB filling,40 modulates energy expenditure,21 suppresses hepatic glycogenolysis, and reduces inflammation and inflammatory macrophage activation.20, 41, 42, 43, 44, 45 TGR5 also maintains intestinal epithelial barrier integrity and maintains intestinal homeostasis.46
Farnesoid X receptor
FXR is a nuclear BA sensor critical in regulating BA synthesis and transport. The receptor also serves as a critical regulator of glucose, lipid, and amino acid metabolism.47, 48 Such features make it an attractive therapeutic target for T2D, dyslipidemia, BA disorders (inflammatory bowel disease, cholangitis), and nonalcoholic fatty liver disease.49, 50, 51 FXR is expressed as 4 different isoforms in humans52 and mice.53 FXR isoforms α1-α2 differ in the function of the N-terminal activation function domain and in an alternative splicing event giving rise to a 4 amino acid insertion (methionine-tyrosine-threonine-glycine) between the DNA binding domain and the hinge domain that connects the DNA binding domain with the ligand binding domain. In humans, FXRβ is highly expressed in small and large intestine and kidney, whereas FXRα is highly expressed in adrenal and liver.52 FXR subsequently heterodimerizes with RXR and binds to FXR responsive element motifs, namely IR-1, depending on pathophysiologic or metabolic state.
Access to the FXR nuclear receptor is facilitated by many BA transporters and by passive diffusion. Reciprocally, FXR controls absorption of BAs via apical and basolateral transporters in both the liver and the intestine54 and these are essential for the function of the enterohepatic circulation. When bound by BAs (6α-ECDCA>CDCA>deoxycholic acid>CA>lithocholic acid relative potency),55, 56 nuclear FXR changes conformation to release corepressors, recruit coactivators, and drive target gene transcription programs. Other bile secondary acids, such as ursodeoxycholate acid (UDCA), are antagonistic.57 In the liver induction of small heterodimer partner inactivates liver receptor homolog 1 and liver x receptor alpha, leading to inhibition of CYP7A1 expression and suppression of BA synthesis.58, 59, 60 Fibroblast growth factor 15/19 (FGF15 in mouse; FGF19 in humans) is an atypical FGF produced by the intestines and released into circulation that acts on FGFR4 and Shp2 in the liver to downregulate cholesterol 7α-hydroxylase expression.58 These negative feedback mechanisms are the primary means of regulating hepatic BA synthesis.58, 61, 62 FGF15/19 also activates hepatic FGFR4/βKlotho decreasing hepatic lipogenesis,63 increasing glycogenesis,64 and promoting gluconeogenesis. Hepatic FXR activity can additionally be modulated by post-translational modifications including O-GlcNacylation,65 methylation,66 acetylation,67 phosphorylation,68, 69 and SUMOylation.69, 70 The presence and impact of these modifications on intestinal FXR function is unknown.
Bile Acid Regulation of Metabolism
By examining loss-of-function and gain-of-function of TGR5, it was discovered that the TGR5 pathway is essential in glucose homeostasis.31 TGR5 stimulates cAMP synthesis and activation of the MAPK pathway induces secretion of glucagon-like polypeptide 1 (GLP-1).20, 31, 71 GLP-1 is a hormone that has been shown to promote satiety, optimize nutrient absorption, stimulate the secretion of insulin, and impede gastric emptying.72, 73 Katsuma et al74 showed that BAs interact with TGR5 to stimulate the secretion of GLP-1 in a murine enteroendocrine cell line STC-1. The promotion of GLP-1 secretion caused by BAs via TGR5 is caused by accumulation of intracellular cAMP within the STC-1 cells. BAs stimulate the release of GLP-1 in a dose-dependent manner.74, 75 In TGR5 knockout mice, there is no significant increase in secretion of GLP-1 when BAs are introduced suggesting that TGR5 is necessary for BAs to stimulate the release of GLP-1 from intestinal L-cells.75 TGR5 mediates the release of GLP-1 in L-cells through modulating mitochondrial oxidative phosphorylation, which causes the closing and opening of KATP/Cav channels and changes in the ATP/ADP ratio.31
Using ileal organoids, Goldspink et al76 discovered that there is an elevation of L-cell cAMP concentrations and increase in L-type Ca2+ currents when administering the BA taurodeoxycholic acid and TGR5 agonist GPBAR-A individually leading to increased secretion of GLP-1. Similar results were achieved with administration of large amounts TAK-875, a free fatty acid receptor 1 agonist. Administration of a combination of TAK-875 and GPBAR-A causes a synergistic increase in Ca2+ response along with a synergistic stimulation of GLP-1 secretion from L-cells. In human studies, cholecystokinin-induced GB emptying results in significant GLP-1 secretion, which is abrogated with the use colesevelam, a BA sequestrant.77 Conjugated BAs released in the ileocolonic region in obese patients causes a statistically significant increase in postprandial GLP-1.78 GLP-2 is another proglucagon polypeptide secreted by L-cells, which helps in intestinal growth76 and nutrient absorption.73 Patel et al73 showed that GLP-2R plays a role in increasing circulating GLP-1 and BA levels, but despite markedly elevated levels of GLP-2 after vertical sleeve gastrectomy in mice, GLP-2R does not seem to play a vital role in reducing weight loss and glycemia postoperatively.
In the liver, FXR activation not only reduces BA synthesis but also reduces the expression of several genes mediating free fatty acid synthesis, including sterol responsive element binding protein 1 c, thereby attenuating de novo lipogenesis.63, 79, 80 FXR also represses the expression of microsomal triglyceride transfer protein79 and apolipoprotein B,81 thereby blunting very-low-density lipoprotein secretion. Hypercholesterolemia is promoted through FXR-mediated inhibition of BA synthesis and the resulting accumulation of the cholesterol precursor.82 Furthermore, FXR increases the expression of apolipoprotein C-II and decreases the expression of apo C-III, increasing the activity of lipoprotein lipase and consequently reducing triglyceride uptake by peripheral tissues. Consistent with these observations, mice deficient in FXR exhibit increased plasma lipids and cholesterol and increased hepatic steatosis.48, 83, 84, 85, 86 Recent studies also demonstrate a central role for BA stimulation of FXR and the release of FGF15/19 in transintestinal cholesterol excretion by increasing the hydropholicity of the BA pool stimulating cholesterol efflux through the sterol-exporting heterodimer adenosine triphosphate binding cassette subfamily G member 5/8.87
With respect to hepatic carbohydrate metabolism, responses to activated FXR seem to depend on the prevailing metabolic state. During fasting FXR activation enhances hepatic glucose production by promoting the PKA-mediated phosphorylation of cAMP regulatory element binding protein and blunting the FOXA2 stimulation of small heterodimer partner.88 Not surprisingly, FXR-/- mice develop transient impairments in adaptive responses to fasting that include reduced hepatic gluconeogenesis and impaired glycogenolysis resulting in transient, fasting-induced hypoglycemia.83, 89 In the postprandial state FXR agonism reduces hepatic glucose production by repressing the expression of Pck1 and G6pc that are elevated in obesity and T2D models.82, 83, 90, 91 Such differences may be attributable to the concomitant actions of intestinal FGF-15/19, released in the fed state.92 FGF15/19 acts on the liver to decrease glycemia and increase glycogenesis through a mechanism involving inactivation of the transcription factor cAMP regulatory element binding protein and the blunted expression of peroxisome proliferator-activated receptor γ coactivator-1α.64 Studies in FXR-/- mice further suggest these actions may additionally be mediated by small heterodimer partner, a direct FXR target and gluconeogenic driver.21, 93
Metabolic Effects of Manipulating Intestinal Bile Acid Availability
Dyslipidemia is more than 2 times more prevalent with T2D than in people without.94, 95 Although statins are among mainstay therapies in treating dyslipidemia, BA sequestrant therapy has long proven effective in reducing low-density lipoprotein levels and improving glycemic control.96, 97, 98 The sequestrant works by mechanisms that are additive to the actions of other glucose-lowering drugs, such as metformin.99, 100 Inhibition of ileal BA uptake by resins and luminal exposure to perfused BAs96, 101, 102, 103, 104, 105 increases L-cell secretion and improves glycemic control through TGR5-FGF15/19106 and FXR-LXRα107 axes. To more selectively modulate FXR and minimize undesirable side effects, novel strategies have been taken to develop tissue-specific FXR agonists. The gut-restricted FXR agonist fexaramine increased thermogenesis, adipose tissue browning, and insulin sensitivity, and reduced weight gain.108 These beneficial effects were mediated by increased FGF15 production leading to alterations in BA composition.
Obeticholic acid is a semisynthetic FXR-agonist that in the liver inhibits BA synthesis and promotes BA efflux, inhibits inflammation, and reduces fibrosis.109 In enterocytes, obeticholic acid stimulates FGF-15/19 release and inhibits intestinal inflammation.110 Interestingly, antagonism of FXR also has metabolic benefits. Oral administration of the antioxidant tempol reduced Lactobacillus bile salt hydrolase activity leading to accumulation of T-β-MCA, an FXR antagonist.111 Obese, high-fat-diet fed mice treated with tempol exhibited reduced obesity and improved insulin resistance. Because T-β-MCA is rapidly metabolized by bacteria through the actions of bile salt hydrolase, a variant of this BA, glycine-β-MCA, was developed and tested. G-β-MCA was tested in high-fat-fed mice and revealed to be a potent intestinal FXR antagonist resulting in decreased serum ceramide levels blunting obesity, insulin resistance, and development of fatty liver.112 These observed metabolic improvements were associated with white adipose tissue beiging and increased energy expenditure and were solely caused by inhibition of FXR signaling in the intestine. Interestingly, intestine-specific Fxr-null mice were unresponsive to the beneficial effects of Gly-β-MCA. Collectively, these data suggest a complex interplay between BAs, gut bacteria, and intestinal BA receptor signaling. Further studies are needed to clarify tissue-specific BA signaling pathways and how such pathways can be modulated to achieve therapeutic effect.
Bariatric Surgery
Bariatric surgery is the most effective and durable treatment for class III or higher obesity (body mass index ≥35 kg/m2) with and without diabetes.113 Currently, the 2 most popular bariatric procedures are Roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy, both effectively promote weight loss. These bariatric operations reduce satiety, alter food preference, and improve nutrient handing with the beneficial side effect of improving insulin sensitivity before significant weight loss.114, 115 We and others have shown that these metabolic improvements occur as early as 1 week post-surgery before significant weight loss; we attributed these improvements to caloric restriction.116, 117 Data from our longitudinal study showed that the average weight loss in bariatric subjects undergoing RYGB was 10% at 1 month,118 27% at 6 months, 34% at 1 year,119 33% at 2 years,120 and 27% at 5 years.
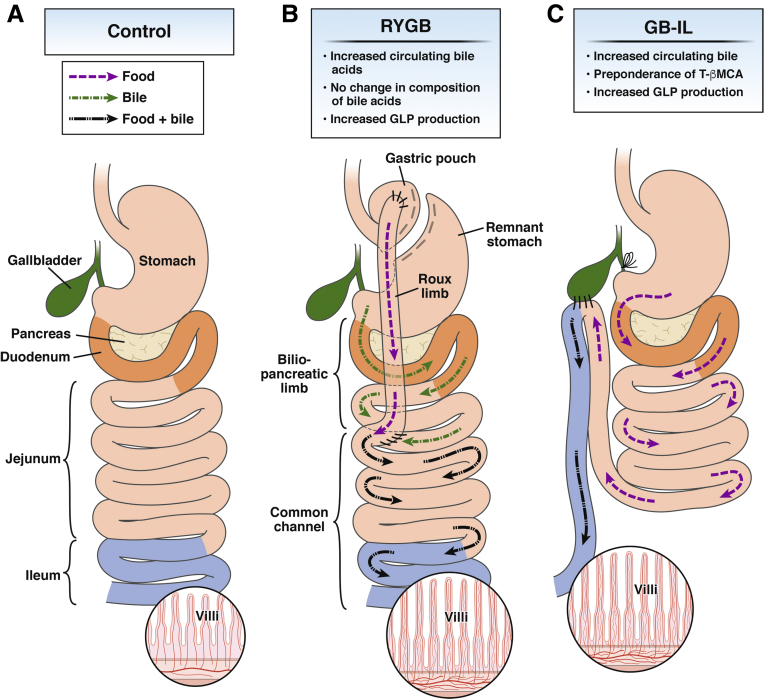
RYGB remodels the digestive tract by interrupting the stomach forming a small and vertical-oriented gastric pouch (≤30 mL), with the upper pouch reanastomosed to jejunum; bowel continuity is restored by jejunojejunostomy (Figure 1). The newly created digestive tract bypasses a major portion of the stomach, the duodenum, and the proximal jejunum, leading to decreased food intake and nutrient absorption. Following RYGB bile and pancreatic secretions drain through the foregut and meet with chime in the mid to distal jejunum at the site of the newly created jejunojejunostomy, where bowel continuity is restored. Hallmark of bariatric procedures is a chronic elevation in systemic BAs. Although increases in serum BAs are evident in the fasted state, increases are most predominant in the early postprandial period, particularly after RYGB. We measured by liquid chromatography–mass spectrometry the plasma BAs in class III obese (body mass index ≥40 kg/m2), preoperatively and longitudinally up to 2 years after RYGB.121 We observed bimodal significant increases in total BAs 1 month and 2 years after surgery. These increases were consistent with improvements in glucose tolerance and insulin sensitivity. The early changes (at 1 month) were characterized by significant increases in the secondary BA, UCDA, and conjugates GUDCA and TUDCA, whereas the increases at 2 years were caused by significant increases in CA and CDCA. Several hypotheses have been put forth to explain these improvements. The foregut hypothesis suggests that exclusion of the upper small intestine prevents secretion of “inhibitory” signals that promote insulin resistance and formation of T2D.117 A second hypothesis proposes that enhanced glucose use in the Roux (alimentary) limb favorably alters whole-body glucose disposal.122 A third hypothesis implicates the hindgut in modulating intestinal sodium-glucose cotransport in mediating the improvement in glucose tolerance and insulin sensitivity.123 A fourth hypothesis implicates intestinal satiety factors, such as oleoylethanolaminde and BAs acting on brain dopaminergic circuits to impart satiety.124 Recent evidence obtained by our group125, 126, 127 and others128, 129 supports a central role for BAs in each of these hypotheses.
Figure 1.
Comparison of anatomic features and the flow of food and bile before (Control) and after RYGB and GB-IL. (A) In response to a meal, gallbladder bile and pancreatic juices are released into the duodenum (orange) where they aide the breakdown and absorption of dietary fat as it traverses the small intestine (jejunum and ileum). Bile acids are reabsorbed in the terminal ileum (blue) in a processed termed enterohepatic circulation. (B) After RYGB, ingested food (purple dotted) and bile (green interrupted arrows) form mix (black broken arrows) delaying lipid absorption to the proximal/mid jejunum. (C) In bile diversion to the ileum the mixing of nutrients and bile is delayed until the terminal ileum.
Patients who have undergone either laparoscopic RYGB or laparoscopic sleeve gastrectomy have a significant increase in the secretion of GLP-1 and PYY by 1 week postoperatively.130 Kohli et al131 discovered that patients after RYGB have a positive correlation between the postprandial levels of BAs and GLP-1. The data generated by us121, 125, 126 and others128, 129, 131, 132 show that both bariatric procedures, RYGB and vertical sleeve gastrectomy, are associated with enhanced delivery of BAs to distal segments of the small and large intestine, to the sites where BA-responsive enteroendocrine cells are enriched, thus eliciting amplified hormonal secretory responses. These include increased GLP-1, PYY, and FGF15/19 release, all of which have insulin-sensitizing effects in the liver and peripheral tissue (eg, skeletal muscle and adipose).
Bile Diversion
To understand the role of BAs, we developed a murine mouse model connecting the GB to specific segments of the small intestine (eg, duodenum vs mid- or distal-jejunum vs terminal ileum), without stomach or intestinal remodeling (Figure 1). We recently showed that specific intestinal segment exposure to BAs leads to distinct site-specific metabolic changes collectively recapitulating all of the metabolic and physiologic improvements observed with RYGB.126 Bile diversion from the GB to the terminal ileum (GB-IL) in obese, high-fat-fed mice resulted in weight loss, fat malabsorption, and improved glucose tolerance identical to those observed with RYGB.126 Mice also exhibited marked adaptations in their gut microbiomes with blooming of mucin-degrading bacterial species, such as Akkermansia muciniphila. Although there were clear metabolic effects after GB-IL in obese mice, the confounding effects of weight loss, reduced adiposity, and fat malabsorption in this animal model limited a direct understanding of the effects of BAs on improvements in insulin sensitivity and glucose handling.
In more recent studies, we performed a series of bile diversion studies in normal-weight, chow-fed mice. Lean GB-IL mice maintained on low-fat diet exhibited no weight loss, reductions in food intake, or fecal fat loss but showed significant improvements in glucose tolerance associated with marked increases in circulating BAs. These improvements were associated with significantly increased lymphatic GLP-1 levels in the fasting period suggesting a direct role for BAs in augmenting fasting intestinal incretin tone. The improvements in oral glucose tolerance were precluded by exendin-9, an antagonist of the GLP-1 receptor. They were also abrogated in GLP-1 receptor knockout mice, thus providing direct evidence linking GLP-1 and its receptor to these metabolic improvements. Intestinal-specific Fxr null (FxrΔ/E) mice on high-fat diet but not Tgr5-/- mice after GB-IL were resistant to the observed metabolic improvements following this procedure. These observations demonstrate that FXR signaling in the intestine has a dominant downstream effect on the clinical and metabolic improvements observed after bariatric surgery.125 Collectively, these studies, highlight the metabolic benefits of FXR agonism and antagonism in different disease models and suggests that differential targeting of FXR signaling in the intestine could be a novel approach for development of antiobesity drugs and needs to be further examined. These data also suggest that intraluminal nascent BAs play an important role in the metabolic improvements observed with RYGB, and that these improvements seem to be site specific in nature.
Although the role of bile and BAs on enterocyte TAG synthesis is relatively unknown, our recent studies in mice with GB-IL suggests that bile may interfere with fatty acid absorption in the terminal ileum. GB bile is rich in phospholipids and provides the main source of lipid for intestinal chylomicron assembly and secretion into lymph.133 Because phospholipid biosynthesis is tightly coupled to production of cellular membranes and intestinal phospholipid synthesis is required for phospholipid monolayers in endoplasmic reticulum, Golgi and lipid droplet membranes, the lipid inclusions we observed in GB-IL ileocytes could have resulted from impaired phospholipid handling as well.
Summary
Overall, metabolic benefits of altering intestinal BA availability include weight-dependent and weight-independent effects (Figure 2). Bile diversion increases circulating BAs and improves glucose tolerance without altering body weight. This improved glucose homeostasis is typically attributed to effects of weight loss when observed clinically, but our findings suggest the weight-independent effects of bariatric surgery on glucose metabolism are driven by BAs. These findings implicate BAs as novel therapeutics for obesity and T2D, and adjuvant therapies in poor responders to bariatric surgery. With the continued development and greater availability of low-cost, high-throughput screening technologies for identifying risk and predicting response to therapy it may one day be routine to tailor bariatric procedures or suggest alternative, more effective procedures to those for whom it is warranted.
Figure 2.
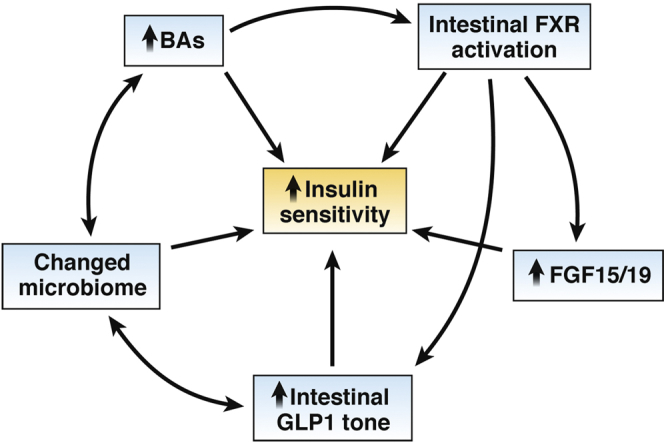
Relationships between observations after bile diversion to the ileum and enhanced insulin sensitivity.
Acknowledgments
All authors contributed equally to the drafting and critical revisions of the manuscript.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health, specifically grants DK059637 (Vanderbilt Mouse Metabolic Phenotyping Center), DK020593 (Vanderbilt Diabetes Research and Training Center), DK058404 (Vanderbilt Digestive Disease Research Center), F32 DK103474 (V.L.A.), and R01 DK105847 (N.N.A. and C.R.F.). This work was also supported by a Research Grant from the Society of American Gastrointestinal and Endoscopic Surgeons (V.L.A.).
References
- 1.Borgstrom B., Dahlqvist A., Lundh G., Sjovall J. Studies of intestinal digestion and absorption in the human. J Clin Invest. 1957;36:1521–1536. doi: 10.1172/JCI103549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li-Hawkins J., Gafvels M., Olin M., Lund E.G., Andersson U., Schuster G., Bjorkhem I., Russell D.W., Eggertsen G. Cholic acid mediates negative feedback regulation of bile acid synthesis in mice. J Clin Invest. 2002;110:1191–1200. doi: 10.1172/JCI16309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pandak W.M., Bohdan P., Franklund C., Mallonee D.H., Eggertsen G., Bjorkhem I., Gil G., Vlahcevic Z.R., Hylemon P.B. Expression of sterol 12alpha-hydroxylase alters bile acid pool composition in primary rat hepatocytes and in vivo. Gastroenterology. 2001;120:1801–1809. doi: 10.1053/gast.2001.24833. [DOI] [PubMed] [Google Scholar]
- 4.Farkkila M., Miettinen T.A. Lipid metabolism in bile acid malabsorption. Ann Med. 1990;22:5–13. doi: 10.3109/07853899009147233. [DOI] [PubMed] [Google Scholar]
- 5.Duboc H., Rajca S., Rainteau D., Benarous D., Maubert M.A., Quervain E., Thomas G., Barbu V., Humbert L., Despras G., Bridonneau C., Dumetz F., Grill J.P., Masliah J., Beaugerie L., Cosnes J., Chazouilleres O., Poupon R., Wolf C., Mallet J.M., Langella P., Trugnan G., Sokol H., Seksik P. Connecting dysbiosis, bile-acid dysmetabolism and gut inflammation in inflammatory bowel diseases. Gut. 2013;62:531–539. doi: 10.1136/gutjnl-2012-302578. [DOI] [PubMed] [Google Scholar]
- 6.Ridlon J.M., Harris S.C., Bhowmik S., Kang D.J., Hylemon P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes. 2016;7:22–39. doi: 10.1080/19490976.2015.1127483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jahnel J., Fickert P., Hauer A.C., Hogenauer C., Avian A., Trauner M. Inflammatory bowel disease alters intestinal bile acid transporter expression. Drug Metab Dispos. 2014;42:1423–1431. doi: 10.1124/dmd.114.058065. [DOI] [PubMed] [Google Scholar]
- 8.Dietschy J.M., Turley S.D. Control of cholesterol turnover in the mouse. J Biol Chem. 2002;277:3801–3804. doi: 10.1074/jbc.R100057200. [DOI] [PubMed] [Google Scholar]
- 9.US Department of Healrth and Human Services; US Department of Agriculture . 8th ed. US Dept of Health and Human Services; Washington, DC: December 2015. 2015-2020 Dietary Guidelines for Americans. Available at: http://www.health.gov/DietartyGuidelines. Accessed December 16, 2018. [Google Scholar]
- 10.Northfield T.C., McColl I. Postprandial concentrations of free and conjugated bile acids down the length of the normal human small intestine. Gut. 1973;14:513–518. doi: 10.1136/gut.14.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeNigris S.J., Hamosh M., Kasbekar D.K., Lee T.C., Hamosh P. Lingual and gastric lipases: species differences in the origin of prepancreatic digestive lipases and in the localization of gastric lipase. Biochim Biophys Acta. 1988;959:38–45. doi: 10.1016/0005-2760(88)90147-6. [DOI] [PubMed] [Google Scholar]
- 12.Wang T.Y., Liu M., Portincasa P., Wang D.Q. New insights into the molecular mechanism of intestinal fatty acid absorption. Eur J Clin Invest. 2013;43:1203–1223. doi: 10.1111/eci.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Storch J., Zhou Y.X., Lagakos W.S. Metabolism of apical versus basolateral sn-2-monoacylglycerol and fatty acids in rodent small intestine. J Lipid Res. 2008;49:1762–1769. doi: 10.1194/jlr.M800116-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abumrad N.A., Davidson N.O. Role of the gut in lipid homeostasis. Physiol Rev. 2012;92:1061–1085. doi: 10.1152/physrev.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coleman R.A., Haynes E.B. Monoacylglycerol acyltransferase. Evidence that the activities from rat intestine and suckling liver are tissue-specific isoenzymes. J Biol Chem. 1986;261:224–228. [PubMed] [Google Scholar]
- 16.Grigor M.R., Bell R.M. Separate monoacylglycerol and diacylglycerol acyltransferases function in intestinal triacylglycerol synthesis. Biochim Biophys Acta. 1982;712:464–472. doi: 10.1016/0005-2760(82)90273-9. [DOI] [PubMed] [Google Scholar]
- 17.Schlegel C., Lapierre L.A., Weis V.G., Williams J.A., Kaji I., Pinzon-Guzman C., Prasad N., Boone B., Jones A., Correa H., Levy S.E., Han X., Wang M., Thomsen K., Acra S., Goldenring J.R. Reversible deficits in apical transporter trafficking associated with deficiency in diacylglycerol acyltransferase. Traffic. 2018;19:879–892. doi: 10.1111/tra.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hung Y.H., Carreiro A.L., Buhman K.K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim Biophys Acta Mol Cell Biol Lipids. 2017;1862:600–614. doi: 10.1016/j.bbalip.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haas J.T., Winter H.S., Lim E., Kirby A., Blumenstiel B., DeFelice M., Gabriel S., Jalas C., Branski D., Grueter C.A., Toporovski M.S., Walther T.C., Daly M.J., Farese R.V., Jr. DGAT1 mutation is linked to a congenital diarrheal disorder. J Clin Invest. 2012;122:4680–4684. doi: 10.1172/JCI64873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamata Y., Fujii R., Hosoya M., Harada M., Yoshida H., Miwa M., Fukusumi S., Habata Y., Itoh T., Shintani Y., Hinuma S., Fujisawa Y., Fujino M. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe M., Houten S.M., Mataki C., Christoffolete M.A., Kim B.W., Sato H., Messaddeq N., Harney J.W., Ezaki O., Kodama T., Schoonjans K., Bianco A.C., Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- 22.Makishima M., Okamoto A.Y., Repa J.J., Tu H., Learned R.M., Luk A., Hull M.V., Lustig K.D., Mangelsdorf D.J., Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362–1365. doi: 10.1126/science.284.5418.1362. [DOI] [PubMed] [Google Scholar]
- 23.Makishima M., Lu T.T., Xie W., Whitfield G.K., Domoto H., Evans R.M., Haussler M.R., Mangelsdorf D.J. Vitamin D receptor as an intestinal bile acid sensor. Science. 2002;296:1313–1316. doi: 10.1126/science.1070477. [DOI] [PubMed] [Google Scholar]
- 24.Staudinger J.L., Goodwin B., Jones S.A., Hawkins-Brown D., MacKenzie K.I., LaTour A., Liu Y., Klaassen C.D., Brown K.K., Reinhard J., Willson T.M., Koller B.H., Kliewer S.A. The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A. 2001;98:3369–3374. doi: 10.1073/pnas.051551698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie W., Radominska-Pandya A., Shi Y., Simon C.M., Nelson M.C., Ong E.S., Waxman D.J., Evans R.M. An essential role for nuclear receptors SXR/PXR in detoxification of cholestatic bile acids. Proc Natl Acad Sci U S A. 2001;98:3375–3380. doi: 10.1073/pnas.051014398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raufman J.P., Chen Y., Cheng K., Compadre C., Compadre L., Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol. 2002;457:77–84. doi: 10.1016/s0014-2999(02)02690-0. [DOI] [PubMed] [Google Scholar]
- 27.Li Q., Dutta A., Kresge C., Bugde A., Feranchak A.P. Bile acids stimulate cholangiocyte fluid secretion by activation of transmembrane member 16A Cl(-) channels. Hepatology. 2018;68:187–199. doi: 10.1002/hep.29804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Margheritis E., Castellani B., Magotti P., Peruzzi S., Romeo E., Natali F., Mostarda S., Gioiello A., Piomelli D., Garau G. Bile acid recognition by NAPE-PLD. ACS Chem Biol. 2016;11:2908–2914. doi: 10.1021/acschembio.6b00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studer E., Zhou X., Zhao R., Wang Y., Takabe K., Nagahashi M., Pandak W.M., Dent P., Spiegel S., Shi R., Xu W., Liu X., Bohdan P., Zhang L., Zhou H., Hylemon P.B. Conjugated bile acids activate the sphingosine-1-phosphate receptor 2 in primary rodent hepatocytes. Hepatology. 2012;55:267–276. doi: 10.1002/hep.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keitel V., Reinehr R., Gatsios P., Rupprecht C., Gorg B., Selbach O., Haussinger D., Kubitz R. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45:695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 31.Thomas C., Gioiello A., Noriega L., Strehle A., Oury J., Rizzo G., Macchiarulo A., Yamamoto H., Mataki C., Pruzanski M., Pellicciari R., Auwerx J., Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Habib A.M., Richards P., Rogers G.J., Reimann F., Gribble F.M. Co-localisation and secretion of glucagon-like peptide 1 and peptide YY from primary cultured human L cells. Diabetologia. 2013;56:1413–1416. doi: 10.1007/s00125-013-2887-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson P.A., Olsson M., Andersson-Assarsson J.C., Taube M., Pereira M.J., Froguel P., Jacobson P. The TGR5 gene is expressed in human subcutaneous adipose tissue and is associated with obesity, weight loss and resting metabolic rate. Biochem Biophys Res Comm. 2013;433:563–566. doi: 10.1016/j.bbrc.2013.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rajagopal S., Kumar D.P., Mahavadi S., Bhattacharya S., Zhou R., Corvera C.U., Bunnett N.W., Grider J.R., Murthy K.S. Activation of G protein-coupled bile acid receptor, TGR5, induces smooth muscle relaxation via both Epac- and PKA-mediated inhibition of RhoA/Rho kinase pathway. Am J Physiol Gastrointest Liver Physiol. 2013;304:G527–G535. doi: 10.1152/ajpgi.00388.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar D.P., Rajagopal S., Mahavadi S., Mirshahi F., Grider J.R., Murthy K.S., Sanyal A.J. Activation of transmembrane bile acid receptor TGR5 stimulates insulin secretion in pancreatic beta cells. Biochem Biophys Res Commun. 2012;427:600–605. doi: 10.1016/j.bbrc.2012.09.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poole D.P., Godfrey C., Cattaruzza F., Cottrell G.S., Kirkland J.G., Pelayo J.C., Bunnett N.W., Corvera C.U. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maruyama T., Tanaka K., Suzuki J., Miyoshi H., Harada N., Nakamura T., Miyamoto Y., Kanatani A., Tamai Y. Targeted disruption of G protein-coupled bile acid receptor 1 (Gpbar1/M-Bar) in mice. J Endocrinol. 2006;191:197–205. doi: 10.1677/joe.1.06546. [DOI] [PubMed] [Google Scholar]
- 38.Vassileva G., Golovko A., Markowitz L., Abbondanzo S.J., Zeng M., Yang S., Hoos L., Tetzloff G., Levitan D., Murgolo N.J., Keane K., Davis H.R., Jr., Hedrick J., Gustafson E.L. Targeted deletion of Gpbar1 protects mice from cholesterol gallstone formation. Biochem J. 2006;398:423–430. doi: 10.1042/BJ20060537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pean N., Doignon I., Garcin I., Besnard A., Julien B., Liu B., Branchereau S., Spraul A., Guettier C., Humbert L., Schoonjans K., Rainteau D., Tordjmann T. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58:1451–1460. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- 40.Li T., Holmstrom S.R., Kir S., Umetani M., Schmidt D.R., Kliewer S.A., Mangelsdorf D.J. The G protein-coupled bile acid receptor, TGR5, stimulates gallbladder filling. Mol Endocrinol. 2011;25:1066–1071. doi: 10.1210/me.2010-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keitel V., Donner M., Winandy S., Kubitz R., Haussinger D. Expression and function of the bile acid receptor TGR5 in Kupffer cells. Biochem Biophys Res Commun. 2008;372:78–84. doi: 10.1016/j.bbrc.2008.04.171. [DOI] [PubMed] [Google Scholar]
- 42.Maruyama T., Miyamoto Y., Nakamura T., Tamai Y., Okada H., Sugiyama E., Itadani H., Tanaka K. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y.D., Chen W.D., Wang M., Yu D., Forman B.M., Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632–1643. doi: 10.1002/hep.22519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lou G., Ma X., Fu X., Meng Z., Zhang W., Wang Y.D., Van Ness C., Yu D., Xu R., Huang W. GPBAR1/TGR5 mediates bile acid-induced cytokine expression in murine Kupffer cells. PLoS One. 2014;9:e93567. doi: 10.1371/journal.pone.0093567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perino A., Pols T.W., Nomura M., Stein S., Pellicciari R., Schoonjans K. TGR5 reduces macrophage migration through mTOR-induced C/EBPbeta differential translation. J Clin Invest. 2014;124:5424–5436. doi: 10.1172/JCI76289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cipriani S., Mencarelli A., Chini M.G., Distrutti E., Renga B., Bifulco G., Baldelli F., Donini A., Fiorucci S. The bile acid receptor GPBAR-1 (TGR5) modulates integrity of intestinal barrier and immune response to experimental colitis. PLoS One. 2011;6:e25637. doi: 10.1371/journal.pone.0025637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuipers F., Bloks V.W., Groen A.K. Beyond intestinal soap: bile acids in metabolic control. Nat Rev Endocrinol. 2014;10:488–498. doi: 10.1038/nrendo.2014.60. [DOI] [PubMed] [Google Scholar]
- 48.Sinal C.J., Tohkin M., Miyata M., Ward J.M., Lambert G., Gonzalez F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 49.Kowdley K.V., Luketic V. An international study evaluating the farnesoid X receptor agonist obeticholic acid as monotherapy in PBC. J Hepatol. 2011;54:S13. [Google Scholar]
- 50.Mudaliar S., Henry R.R., Sanyal A.J., Morrow L., Marschall H.U., Kipnes M., Adorini L., Sciacca C.I., Clopton P., Castelloe E., Dillon P., Pruzanski M., Shapiro D. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574–582. doi: 10.1053/j.gastro.2013.05.042. [DOI] [PubMed] [Google Scholar]
- 51.Neuschwander-Tetri B.A., Loomba R., Sanyal A.J., Lavine J.E., Van Natta M.L., Abdelmalek M.F., Chalasani N., Dasarathy S., Diehl A.M., Hameed B., Kowdley K.V., McCullough A., Terrault N., Clark J.M., Tonascia J., Brunt E.M., Kleiner D.E., Doo E., Network N.C.R. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956–965. doi: 10.1016/S0140-6736(14)61933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huber R.M., Murphy K., Miao B., Link J.R., Cunningham M.R., Rupar M.J., Gunyuzlu P.L., Haws T.F., Kassam A., Powell F., Hollis G.F., Young P.R., Mukherjee R., Burn T.C. Generation of multiple farnesoid-X-receptor isoforms through the use of alternative promoters. Gene. 2002;290:35–43. doi: 10.1016/s0378-1119(02)00557-7. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y., Kast-Woelbern H.R., Edwards P.A. Natural structural variants of the nuclear receptor farnesoid X receptor affect transcriptional activation. J Biol Chem. 2003;278:104–110. doi: 10.1074/jbc.M209505200. [DOI] [PubMed] [Google Scholar]
- 54.Dawson P.A., Karpen S.J. Intestinal transport and metabolism of bile acids. J Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Claudel T., Staels B., Kuipers F. The Farnesoid X receptor: a molecular link between bile acid and lipid and glucose metabolism. Arterioscler Thromb Vasc Biol. 2005;25:2020–2030. doi: 10.1161/01.ATV.0000178994.21828.a7. [DOI] [PubMed] [Google Scholar]
- 56.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 57.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K., Angelin B., Hyotylainen T., Oresic M., Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Li S., Hsu D.D., Li B., Luo X., Alderson N., Qiao L., Ma L., Zhu H.H., He Z., Suino-Powell K., Ji K., Li J., Shao J., Xu H.E., Li T., Feng G.S. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab. 2014;20:320–332. doi: 10.1016/j.cmet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kong B., Wang L., Chiang J.Y., Zhang Y., Klaassen C.D., Guo G.L. Mechanism of tissue-specific farnesoid X receptor in suppressing the expression of genes in bile-acid synthesis in mice. Hepatology. 2012;56:1034–1043. doi: 10.1002/hep.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L., Lee Y.K., Bundman D., Han Y., Thevananther S., Kim C.S., Chua S.S., Wei P., Heyman R.A., Karin M., Moore D.D. Redundant pathways for negative feedback regulation of bile acid production. Dev Cell. 2002;2:721–731. doi: 10.1016/s1534-5807(02)00187-9. [DOI] [PubMed] [Google Scholar]
- 61.Xie M.H., Holcomb I., Deuel B., Dowd P., Huang A., Vagts A., Foster J., Liang J., Brush J., Gu Q., Hillan K., Goddard A., Gurney A.L. FGF-19, a novel fibroblast growth factor with unique specificity for FGFR4. Cytokine. 1999;11:729–735. doi: 10.1006/cyto.1999.0485. [DOI] [PubMed] [Google Scholar]
- 62.Inagaki T., Choi M., Moschetta A., Peng L., Cummins C.L., McDonald J.G., Luo G., Jones S.A., Goodwin B., Richardson J.A., Gerard R.D., Repa J.J., Mangelsdorf D.J., Kliewer S.A. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 63.Bhatnagar S., Damron H.A., Hillgartner F.B. Fibroblast growth factor-19, a novel factor that inhibits hepatic fatty acid synthesis. J Biol Chem. 2009;284:10023–10033. doi: 10.1074/jbc.M808818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kir S., Beddow S.A., Samuel V.T., Miller P., Previs S.F., Suino-Powell K., Xu H.E., Shulman G.I., Kliewer S.A., Mangelsdorf D.J. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;331:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berrabah W., Aumercier P., Gheeraert C., Dehondt H., Bouchaert E., Alexandre J., Ploton M., Mazuy C., Caron S., Tailleux A., Eeckhoute J., Lefebvre T., Staels B., Lefebvre P. Glucose sensing O-GlcNAcylation pathway regulates the nuclear bile acid receptor farnesoid X receptor (FXR) Hepatology. 2014;59:2022–2033. doi: 10.1002/hep.26710. [DOI] [PubMed] [Google Scholar]
- 66.Balasubramaniyan N., Ananthanarayanan M., Suchy F.J. Direct methylation of FXR by Set7/9, a lysine methyltransferase, regulates the expression of FXR target genes. Am J Physiol Gastrointest Liver Physiol. 2012;302:G937–G947. doi: 10.1152/ajpgi.00441.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kemper J.K., Xiao Z., Ponugoti B., Miao J., Fang S., Kanamaluru D., Tsang S., Wu S.Y., Chiang C.M., Veenstra T.D. FXR acetylation is normally dynamically regulated by p300 and SIRT1 but constitutively elevated in metabolic disease states. Cell Metab. 2009;10:392–404. doi: 10.1016/j.cmet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gineste R., Sirvent A., Paumelle R., Helleboid S., Aquilina A., Darteil R., Hum D.W., Fruchart J.C., Staels B. Phosphorylation of farnesoid X receptor by protein kinase C promotes its transcriptional activity. Mol Endocrinol. 2008;22:2433–2447. doi: 10.1210/me.2008-0092. [DOI] [PubMed] [Google Scholar]
- 69.Hu X., Zhang Q., Zheng J., Kong W., Zhang H.H., Zeng T.S., Zhang J.Y., Min J., Wu C., Chen L.L. Alteration of FXR phosphorylation and sumoylation in liver in the development of adult catch-up growth. Exp Biol Med (Maywood) 2017;242:297–304. doi: 10.1177/1535370216641788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Balasubramaniyan N., Luo Y., Sun A.Q., Suchy F.J. SUMOylation of the farnesoid X receptor (FXR) regulates the expression of FXR target genes. J Biol Chem. 2013;288:13850–13862. doi: 10.1074/jbc.M112.443937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duboc H., Tache Y., Hofmann A.F. The bile acid TGR5 membrane receptor: from basic research to clinical application. Dig Liver Dis. 2014;46:302–312. doi: 10.1016/j.dld.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye J., Hao Z., Mumphrey M.B., Townsend R.L., Patterson L.M., Stylopoulos N., Munzberg H., Morrison C.D., Drucker D.J., Berthoud H.R. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol. 2014;306:R352–R362. doi: 10.1152/ajpregu.00491.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Patel A., Yusta B., Matthews D., Charron M.J., Seeley R.J., Drucker D.J. GLP-2 receptor signaling controls circulating bile acid levels but not glucose homeostasis in Gcgr(-/-) mice and is dispensable for the metabolic benefits ensuing after vertical sleeve gastrectomy. Mol Metab. 2018 doi: 10.1016/j.molmet.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katsuma S., Hirasawa A., Tsujimoto G. Bile acids promote glucagon-like peptide-1 secretion through TGR5 in a murine enteroendocrine cell line STC-1. Biochem Biophys Res Commun. 2005;329:386–390. doi: 10.1016/j.bbrc.2005.01.139. [DOI] [PubMed] [Google Scholar]
- 75.Brighton C.A., Rievaj J., Kuhre R.E., Glass L.L., Schoonjans K., Holst J.J., Gribble F.M., Reimann F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein–coupled bile acid receptors. Endocrinology. 2015;156:3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Goldspink D.A., Lu V.B., Billing L.J., Larraufie P., Tolhurst G., Gribble F.M., Reimann F. Mechanistic insights into the detection of free fatty and bile acids by ileal glucagon-like peptide-1 secreting cells. Mol Metab. 2018;7:90–101. doi: 10.1016/j.molmet.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bronden A., Alber A., Rohde U., Gasbjerg L.S., Rehfeld J.F., Holst J.J., Vilsboll T., Knop F.K. The bile acid-sequestering resin sevelamer eliminates the acute GLP-1 stimulatory effect of endogenously released bile acids in patients with type 2 diabetes. Diabetes Obes Metab. 2018;20:362–369. doi: 10.1111/dom.13080. [DOI] [PubMed] [Google Scholar]
- 78.G.C. M, Davis J., Bonis A.N., Khemani D., Gedulin B., Vella A., Camilleri M., Acosta A. Effects of ileo-colonic delivery of conjugated bile acids on glucose metabolism, GLP-1, and body weight in patients with obesity and type 2 diabetes mellitus: a randomized controlled trial. Diabetes. 2018;67(Suppl 1) [Google Scholar]
- 79.Hirokane H., Nakahara M., Tachibana S., Shimizu M., Sato R. Bile acid reduces the secretion of very low density lipoprotein by repressing microsomal triglyceride transfer protein gene expression mediated by hepatocyte nuclear factor-4. J Biol Chem. 2004;279:45685–45692. doi: 10.1074/jbc.M404255200. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y., Lee F.Y., Barrera G., Lee H., Vales C., Gonzalez F.J., Willson T.M., Edwards P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Watanabe M., Houten S.M., Wang L., Moschetta A., Mangelsdorf D.J., Heyman R.A., Moore D.D., Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y., Lee F.Y., Barrera G., Lee H., Vales C., Gonzalez F.J., Willson T.M., Edwards P.A. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. PNAS. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T.H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J.C., Gonzalez F.J., Kuipers F., Staels B. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem. 2006;281:11039–11049. doi: 10.1074/jbc.M510258200. [DOI] [PubMed] [Google Scholar]
- 84.Bjursell M., Wedin M., Admyre T., Hermansson M., Bottcher G., Goransson M., Linden D., Bamberg K., Oscarsson J., Bohlooly Y.M. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One. 2013;8:e64721. doi: 10.1371/journal.pone.0064721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lambert G., Amar M.J., Guo G., Brewer H.B., Jr., Gonzalez F.J., Sinal C.J. The farnesoid X-receptor is an essential regulator of cholesterol homeostasis. J Biol Chem. 2003;278:2563–2570. doi: 10.1074/jbc.M209525200. [DOI] [PubMed] [Google Scholar]
- 86.Prawitt J., Abdelkarim M., Stroeve J.H., Popescu I., Duez H., Velagapudi V.R., Dumont J., Bouchaert E., van Dijk T.H., Lucas A., Dorchies E., Daoudi M., Lestavel S., Gonzalez F.J., Oresic M., Cariou B., Kuipers F., Caron S., Staels B. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–1871. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.de Boer J.F., Schonewille M., Boesjes M., Wolters H., Bloks V.W., Bos T., van Dijk T.H., Jurdzinski A., Boverhof R., Wolters J.C., Kuivenhoven J.A., van Deursen J.M., Oude Elferink RPJ. Moschetta A., Kremoser C., Verkade H.J., Kuipers F., Groen A.K. Intestinal farnesoid X receptor controls transintestinal cholesterol excretion in mice. Gastroenterology. 2017;152:1126–1138. doi: 10.1053/j.gastro.2016.12.037. [DOI] [PubMed] [Google Scholar]
- 88.Ploton M., Mazuy C., Gheeraert C., Dubois V., Berthier A., Dubois-Chevalier J., Marechal X., Bantubungi K., Diemer H., Cianferani S., Strub J.M., Helleboid-Chapman A., Eeckhoute J., Staels B., Lefebvre P. The nuclear bile acid receptor FXR is a PKA- and FOXA2-sensitive activator of fasting hepatic gluconeogenesis. J Hepatol. 2018 doi: 10.1016/j.jhep.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 89.van Dijk T.H., Grefhorst A., Oosterveer M.H., Bloks V.W., Staels B., Reijngoud D.J., Kuipers F. An increased flux through the glucose 6-phosphate pool in enterocytes delays glucose absorption in Fxr-/- mice. J Biol Chem. 2009;284:10315–10323. doi: 10.1074/jbc.M807317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mencarelli A., Renga B., D'Amore C., Santorelli C., Graziosi L., Bruno A., Monti M.C., Distrutti E., Cipriani S., Donini A., Fiorucci S. Dissociation of intestinal and hepatic activities of FXR and LXRalpha supports metabolic effects of terminal ileum interposition in rodents. Diabetes. 2013;62:3384–3393. doi: 10.2337/db13-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cipriani S., Mencarelli A., Palladino G., Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Potthoff M.J., Boney-Montoya J., Choi M., He T., Sunny N.E., Satapati S., Suino-Powell K., Xu H.E., Gerard R.D., Finck B.N., Burgess S.C., Mangelsdorf D.J., Kliewer S.A. FGF15/19 regulates hepatic glucose metabolism by inhibiting the CREB-PGC-1alpha pathway. Cell Metab. 2011;13:729–738. doi: 10.1016/j.cmet.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park M.J., Kong H.J., Kim H.Y., Kim H.H., Kim J.H., Cheong J.H. Transcriptional repression of the gluconeogenic gene PEPCK by the orphan nuclear receptor SHP through inhibitory interaction with C/EBPalpha. Biochem J. 2007;402:567–574. doi: 10.1042/BJ20061549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Emerging Risk Factors C., Sarwar N., Gao P., Seshasai S.R., Gobin R., Kaptoge S., Di Angelantonio E., Ingelsson E., Lawlor D.A., Selvin E., Stampfer M., Stehouwer C.D., Lewington S., Pennells L., Thompson A., Sattar N., White I.R., Ray K.K., Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rao Kondapally Seshasai S., Kaptoge S., Thompson A., Di Angelantonio E., Gao P., Sarwar N., Whincup P.H., Mukamal K.J., Gillum R.F., Holme I., Njolstad I., Fletcher A., Nilsson P., Lewington S., Collins R., Gudnason V., Thompson S.G., Sattar N., Selvin E., Hu F.B., Danesh J. Emerging Risk Factors C. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garg S.K., Ritchie P.J., Moser E.G., Snell-Bergeon J.K., Freson B.J., Hazenfield R.M. Effects of colesevelam on LDL-C, A1c and GLP-1 levels in patients with type 1 diabetes: a pilot randomized double-blind trial. Diabetes Obes Metab. 2011;13:137–143. doi: 10.1111/j.1463-1326.2010.01320.x. [DOI] [PubMed] [Google Scholar]
- 97.Yamakawa T., Takano T., Utsunomiya H., Kadonosono K., Okamura A. Effect of colestimide therapy for glycemic control in type 2 diabetes mellitus with hypercholesterolemia. Endocr J. 2007;54:53–58. doi: 10.1507/endocrj.k05-098. [DOI] [PubMed] [Google Scholar]
- 98.Zieve F.J., Kalin M.F., Schwartz S.L., Jones M.R., Bailey W.L. Results of the glucose-lowering effect of WelChol study (GLOWS): a randomized, double-blind, placebo-controlled pilot study evaluating the effect of colesevelam hydrochloride on glycemic control in subjects with type 2 diabetes. Clin Ther. 2007;29:74–83. doi: 10.1016/j.clinthera.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 99.Bays H.E., Goldberg R.B., Truitt K.E., Jones M.R. Colesevelam hydrochloride therapy in patients with type 2 diabetes mellitus treated with metformin: glucose and lipid effects. Arch Intern Med. 2008;168:1975–1983. doi: 10.1001/archinte.168.18.1975. [DOI] [PubMed] [Google Scholar]
- 100.Fonseca V.A., Rosenstock J., Wang A.C., Truitt K.E., Jones M.R. Colesevelam HCl improves glycemic control and reduces LDL cholesterol in patients with inadequately controlled type 2 diabetes on sulfonylurea-based therapy. Diabetes Care. 2008;31:1479–1484. doi: 10.2337/dc08-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen L., McNulty J., Anderson D., Liu Y., Nystrom C., Bullard S., Collins J., Handlon A.L., Klein R., Grimes A., Murray D., Brown R., Krull D., Benson B., Kleymenova E., Remlinger K., Young A., Yao X. Cholestyramine reverses hyperglycemia and enhances glucose-stimulated glucagon-like peptide 1 release in Zucker diabetic fatty rats. J Pharmacol Exp Ther. 2010;334:164–170. doi: 10.1124/jpet.110.166892. [DOI] [PubMed] [Google Scholar]
- 102.Shang Q., Saumoy M., Holst J.J., Salen G., Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol. 2010;298:G419–G424. doi: 10.1152/ajpgi.00362.2009. [DOI] [PubMed] [Google Scholar]
- 103.Kobayashi M., Ikegami H., Fujisawa T., Nojima K., Kawabata Y., Noso S., Babaya N., Itoi-Babaya M., Yamaji K., Hiromine Y., Shibata M., Ogihara T. Prevention and treatment of obesity, insulin resistance, and diabetes by bile acid-binding resin. Diabetes. 2007;56:239–247. doi: 10.2337/db06-0353. [DOI] [PubMed] [Google Scholar]
- 104.Adrian T.E., Ballantyne G.H., Longo W.E., Bilchik A.J., Graham S., Basson M.D., Tierney R.P., Modlin I.M. Deoxycholate is an important releaser of peptide YY and enteroglucagon from the human colon. Gut. 1993;34:1219–1224. doi: 10.1136/gut.34.9.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adrian T.E., Gariballa S., Parekh K.A., Thomas S.A., Saadi H., Al Kaabi J., Nagelkerke N., Gedulin B., Young A.A. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia. 2012;55:2343–2347. doi: 10.1007/s00125-012-2593-2. [DOI] [PubMed] [Google Scholar]
- 106.Beysen C., Murphy E.J., Deines K., Chan M., Tsang E., Glass A., Turner S.M., Protasio J., Riiff T., Hellerstein M.K. Effect of bile acid sequestrants on glucose metabolism, hepatic de novo lipogenesis, and cholesterol and bile acid kinetics in type 2 diabetes: a randomised controlled study. Diabetologia. 2012;55:432–442. doi: 10.1007/s00125-011-2382-3. [DOI] [PubMed] [Google Scholar]
- 107.Herrema H., Meissner M., van Dijk T.H., Brufau G., Boverhof R., Oosterveer M.H., Reijngoud D.J., Muller M., Stellaard F., Groen A.K., Kuipers F. Bile salt sequestration induces hepatic de novo lipogenesis through farnesoid X receptor- and liver X receptor alpha-controlled metabolic pathways in mice. Hepatology. 2010;51:806–816. doi: 10.1002/hep.23408. [DOI] [PubMed] [Google Scholar]
- 108.Fang S., Suh J.M., Reilly S.M., Yu E., Osborn O., Lackey D., Yoshihara E., Perino A., Jacinto S., Lukasheva Y., Atkins A.R., Khvat A., Schnabl B., Yu R.T., Brenner D.A., Coulter S., Liddle C., Schoonjans K., Olefsky J.M., Saltiel A.R., Downes M., Evans R.M. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Verbeke L., Farre R., Trebicka J., Komuta M., Roskams T., Klein S., Elst I.V., Windmolders P., Vanuytsel T., Nevens F., Laleman W. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286–2298. doi: 10.1002/hep.26939. [DOI] [PubMed] [Google Scholar]
- 110.Ubeda M., Lario M., Munoz L., Borrero M.J., Rodriguez-Serrano M., Sanchez-Diaz A.M., Del Campo R., Lledo L., Pastor O., Garcia-Bermejo L., Diaz D., Alvarez-Mon M., Albillos A. Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J Hepatol. 2016;64:1049–1057. doi: 10.1016/j.jhep.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 111.Li F., Jiang C., Krausz K.W., Li Y., Albert I., Hao H., Fabre K.M., Mitchell J.B., Patterson A.D., Gonzalez F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang C., Xie C., Lv Y., Li J., Krausz K.W., Shi J., Brocker C.N., Desai D., Amin S.G., Bisson W.H., Liu Y., Gavrilova O., Patterson A.D., Gonzalez F.J. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat Commun. 2015;6:10166. doi: 10.1038/ncomms10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pories W.J., Swanson M.S., MacDonald K.G., Long S.B., Morris P.G., Brown B.M., Barakat H.A., deRamon R.A., Israel G., Dolezal J.M., Dohm L. Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus. Ann Surg. 1995;222:339–350. doi: 10.1097/00000658-199509000-00011. discussion 50–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Buchwald H., Avidor Y., Braunwald E., Jensen M.D., Pories W., Fahrbach K., Schoelles K. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 115.Buchwald H., Estok R., Fahrbach K., Banel D., Jensen M.D., Pories W.J., Bantle J.P., Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 116.Isbell J.M., Tamboli R.A., Hansen E.N., Saliba J., Dunn J.P., Phillips S.E., Marks-Shulman P.A., Abumrad N.N. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care. 2010;33:1438–1442. doi: 10.2337/dc09-2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hansen E.N., Tamboli R.A., Isbell J.M., Saliba J., Dunn J.P., Marks-Shulman P.A., Abumrad N.N. Role of the foregut in the early improvement in glucose tolerance and insulin sensitivity following Roux-en-Y gastric bypass surgery. Am J Physiol Gastrointest Liver Physiol. 2011;300:G795–G802. doi: 10.1152/ajpgi.00019.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dunn J.P., Abumrad N.N., Breitman I., Marks-Shulman P.A., Flynn C.R., Jabbour K., Feurer I.D., Tamboli R.A. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012;35:137–142. doi: 10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fabbrini E., Tamboli R.A., Magkos F., Marks-Shulman P.A., Eckhauser A.W., Richards W.O., Klein S., Abumrad N.N. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tamboli R.A., Breitman I., Marks-Shulman P.A., Jabbour K., Melvin W., Williams B., Clements R.H., Feurer I.D., Abumrad N.N. Early weight regain after gastric bypass does not affect insulin sensitivity but is associated with elevated ghrelin. Obesity (Silver Spring) 2014 doi: 10.1002/oby.20776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Albaugh V.L., Flynn C.R., Cai S., Xiao Y., Tamboli R.A., Abumrad N.N. Early increases in bile acids post Roux-en-Y gastric bypass are driven by insulin-sensitizing, secondary bile acids. J Clin Endocrinol Metab. 2015;100:E1225–E1233. doi: 10.1210/jc.2015-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Saeidi N., Meoli L., Nestoridi E., Gupta N.K., Kvas S., Kucharczyk J., Bonab A.A., Fischman A.J., Yarmush M.L., Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–410. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Baud G., Daoudi M., Hubert T., Raverdy V., Pigeyre M., Hervieux E., Devienne M., Ghunaim M., Bonner C., Quenon A., Pigny P., Klein A., Kerr-Conte J., Gmyr V., Caiazzo R., Pattou F. Bile diversion in Roux-en-Y gastric bypass modulates sodium-dependent glucose intestinal uptake. Cell Metab. 2016;23:547–553. doi: 10.1016/j.cmet.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 124.Hankir M.K., Seyfried F., Hintschich C.A., Diep T.A., Kleberg K., Kranz M., Deuther-Conrad W., Tellez L.A., Rullmann M., Patt M., Teichert J., Hesse S., Sabri O., Brust P., Hansen H.S., de Araujo I.E., Krugel U., Fenske W.K. Gastric bypass surgery recruits a gut PPAR-alpha-striatal D1R pathway to reduce fat appetite in obese rats. Cell Metab. 2017;25:335–344. doi: 10.1016/j.cmet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 125.Albaugh V.L., Banan B., Antoun J., Xiong Y., Guo Y., Ping J., Alikhan M., Clements B.A., Abumrad N.N., Flynn C.R. Role of bile acids and GLP-1 in mediating the metabolic improvements of bariatric surgery. Gastroenterology. 2018 doi: 10.1053/j.gastro.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Flynn C.R., Albaugh V.L., Cai S., Cheung-Flynn J., Williams P.E., Brucker R.M., Bordenstein S.R., Guo Y., Wasserman D.H., Abumrad N.N. Bile diversion to the distal small intestine has comparable metabolic benefits to bariatric surgery. Nat Commun. 2015;6:7715. doi: 10.1038/ncomms8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reddy I.A., Smith N.K., Erreger K., Ghose D., Saunders C., Foster D.J., Turner B., Poe A., Albaugh V.L., McGuinness O., Hackett T.A., Grueter B.A., Abumrad N.N., Flynn C.R., Galli A. Bile diversion, a bariatric surgery, and bile acid signaling reduce central cocaine reward. PLoS Biol. 2018;16:e2006682. doi: 10.1371/journal.pbio.2006682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kohli R., Setchell K.D., Kirby M., Myronovych A., Ryan K.K., Ibrahim S.H., Berger J., Smith K., Toure M., Woods S.C., Seeley R.J. A surgical model in male obese rats uncovers protective effects of bile acids post-bariatric surgery. Endocrinology. 2013;154:2341–2351. doi: 10.1210/en.2012-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R., Wilson-Perez H.E., Sandoval D.A., Kohli R., Backhed F., Seeley R.J. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Steinert R.E., Peterli R., Keller S., Meyer-Gerspach A.C., Drewe J., Peters T., Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 2013;21:E660–E668. doi: 10.1002/oby.20522. [DOI] [PubMed] [Google Scholar]
- 131.Kohli R., Bradley D., Setchell K.D., Eagon J.C., Abumrad N., Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab. 2013;98:E708–E712. doi: 10.1210/jc.2012-3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pournaras D.J., Glicksman C., Vincent R.P., Kuganolipava S., Alaghband-Zadeh J., Mahon D., Bekker J.H., Ghatei M.A., Bloom S.R., Walters J.R., Welbourn R., le Roux C.W. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153:3613–3619. doi: 10.1210/en.2011-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tso P., Kendrick H., Balint J.A., Simmonds W.J. Role of biliary phosphatidylcholine in the absorption and transport of dietary triolein in the rat. Gastroenterology. 1981;80:60–65. [PubMed] [Google Scholar]