Abstract
Purpose
This study was conducted to evaluate the validity of drug promotion materials (DPMs) in Ethiopia.
Methods
A cross sectional document review was done. DPMs were evaluated for fulfilment of the World Health Organization’s (WHO) criteria for ethical promotion of drugs. They were also evaluated for font size, type of formulation, claims made, pictures depicted, retrievability and source of references used.
Results
A total of 235 DPMs were collected from the community and hospital pharmacies. Documents promoting devices and equipment, orthopedic appliances, reminder cards and drug lists were excluded, leaving 173 promotional materials. Antimicrobials were the most promoted drugs (27.2%) followed by respiratory drugs (11.0%) and gastrointestinal drugs (9.8%). Brand name was written in all of the DPMs while approved generic names, indication and active ingredient per dosage form were written in 94.8%, 92.5% and 62.4% respectively. Side effects and contraindications were written in 27.2% and 18.5% of the DPMs. A total of 223 claims were made. Efficacy was the dominant claim (62.3%) followed by safety (8.5%). Pictorial demonstrations were used in 84.4% of the DPMs. Almost half of the pictures depicted, 47.3%, were the cover of the drug products. Only 48.6% of the DPMs has supported their claims with references. Review articles account for 23.3% of the references. Only 5.8% of the journal articles were published after the year 2013.
Conclusion
We conclude that the design and content of studied drug promotional materials are most effective as sales materials rather than thorough informational vehicles. The WHO and Food, Medicine and Health Care Administration and Control Authority of Ethiopia recommendations are rarely met.
Keywords: WHO, drug promotion, ethical drug promotion, promotional information
Introduction
When medicines are used rationally, patients receive appropriate treatment, in doses that meet their individual requirements, for an adequate period of time, with the justifiable lowest possible price.1 Half of the medications used globally are prescribed irrationally and bought inappropriately by patients which can lead to poor treatment outcomes, adverse drug reactions and wastage of resources.2 The huge number of products on the market and easy accessibility of commercial drug information through a network of medical representatives make the selection of the right drug and its proper use an increasingly difficult task.3,4 Drug promotion should lay the foundation for proper behavior concerning the promotion of medicinal drugs, consistent with the search for truthfulness and righteousness.5
The World Health Organizations’ (WHO) general assembly held a meeting of experts of the pharmaceutical sector, representatives of governments and consumers’ organizations in 1985. The assembly discussed the approaches which help to ensure the rational use of drugs especially by increasing medication knowledge and flow of information. During that meeting, the WHO requested member countries to develop national drug policy which includes strategies to improve the objectivity and completeness of drug product information and making it accessible to those who need it.1 Three years later, in 1988, the WHO developed the “Ethical criteria for medicinal drug promotion” with the purpose of assisting and encouraging the improvement of health care via the rational use of drug products.5
According to WHO, medicinal drug promotion refers to “all informational and persuasive activities by manufacturers and distributors, the effect of which is to induce the prescription, supply, purchase, and/or use of medicinal drugs”.5
Materials promoting drugs which are distributed by the company’s medical representatives are important source of information to physicians.6,7 Even though most of drug promotional materials (DPMs) aim is to raise awareness, because of the incompleteness of the information they provide to draw conclusions, wrong and misleading information is not uncommon in materials used for drug promotion.8 Erroneous and deficiency of medication information might cause serious damages including disability and death.9 Studies revealed most promotional materials do not fulfill WHO criteria for promotion of pharmaceutical products.10–12 In developing countries like Ethiopia, there is insufficient drug information service that goes in line with the flourishing pharmaceutical market.13 When pharmaceutical companies compete to have a better market share and aggressively promote their drug products there is every possibility of the promotion being unethical.14
In Ethiopia, there is a pharmaceutical and medical device promotion guideline that regulates the contents of the promotional materials provided by the pharmaceutical companies. Pharmaceutical companies use medical representatives who are pharmacists by profession to distribute DPMs to physicians and other health professionals. The criteria’s set by Food, Medicine and Health Care Administration and Control Authority of Ethiopia (FMHACA) for DPMs are adopted from WHO.15 However, as of now, there is no study from Ethiopia which has evaluated the validity of the promotional materials provided by the pharmaceutical companies. Hence, the present study was conducted with the purpose of evaluating the validity of drug promotional materials in Ethiopia.
Methods
A cross sectional document review was done. The primary goal of this study was to compare the contents of DPMs against the WHO criteria for ethical drug promotion. Additionally, DPMs were analyzed for parameters which are not included in the WHO’s list as explained below. All promotional materials of the companies which promote their product in Dessie at the time of the data collection period were considered for this study. Dessie is a city located 401 kilometers north of the capital, Addis Ababa. The city is home to one governmental teaching referral hospital, four general private hospitals, eight health centers and twenty-seven private clinics. The teaching referral hospital is where Wollo University teaches health sciences including medicine, pharmacy and nursing. Four health science colleges, one governmental and three private, are also found in the city. Only drugs which are on the list of the FMHACA are sold in the Ethiopian market. Nationwide treatment guidelines are available although regulations are not stringent. The Ethiopian formulary and treatment guidelines are available at the official website of FMHACA ( http://www.fmhaca.gov.et/ ).
DPMs were collected from the community and hospital pharmacies of Dessie town from May to July of 2018. Duplicates were checked and discarded. Each DPL was evaluated according to WHO criteria for fulfilment of each of the following parameters.5
The name(s) of the active ingredient(s) using either international nonproprietary names (INN) or the approved generic name of the drug
The brand name
Content of active ingredient(s) per dosage form
Name of inactive ingredients known to cause problems (for example, inactive ingredients like Lactose, peanut oil, gluten and chemical dyes)
Indication(s) for use
Dosage form or regimen
Side‑effects and major adverse drug reactions
Precautions, contra‑indications, and warnings
Major interactions
Name and address of manufacturer or distributor
Reference to scientific literature as appropriate.
In addition to the above WHO criteria, promotional materials were evaluated for font size, type of formulation, type and frequency of claims made, pictures depicted and cost. The claims made in the promotional materials were categorized into seven groups according to Mali et al.,10 efficacy, safety, cost, convenience, pharmacokinetic property, pharmaceutical property and extravagant emotional claims. The references used by the DPMs were evaluated for retrievability, type of source and year of publication. The data were feed to SPSS version 20.0, descriptive statistics produced and expressed as a percentage.
Results
A total of 235 drug promotional materials were collected from the community and hospital pharmacies. Documents promoting devices and equipment, orthopedic appliances, reminder cards and drug lists were excluded, leaving 173 promotional materials. Majority of the materials (61.8%) promote a single drug formulation whereas 38.2% of them contain fixed mixed dose formulations.
Class of drugs
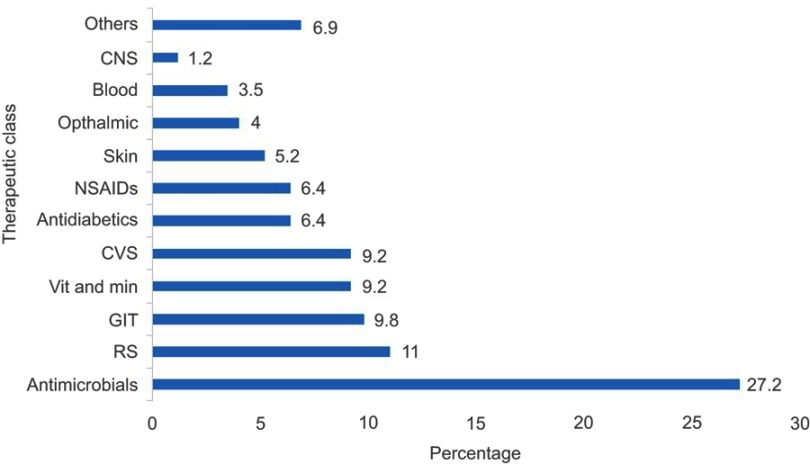
Category of drugs presented in the promotional literatures were (Figure 1): Antimicrobials (27.2%), respiratory drugs (11.0%), gastrointestinal drugs (9.8%), cardiovascular drugs (9.2%), vitamins and minerals (9.2%), non-steroidal ant-inflammatory drugs (6.4%) and antidiabetics (6.4%), skin (5.2%), ophthalmic (4.0%), blood (3.5%), central nervous system (1.2%) and others (6.9%).
Figure 1.
Category of drugs presented in the promotional material (n=173).
Abbreviations: CNS, central nervous system; CVS, cardiovascular; RS, respiratory system; GIT, gastrointestinal; Vit and Min, vitamins and minerals; NSAIDs, non-steroidal anti-inflammatory drugs.
Fulfillment of WHO criteria
None of the studied DPMs fulfilled the WHO’s criteria for the ethical promotion of drugs as presented in Table 1. All of the DPMs have had the brand name written while approved generic names were written in 94.8% of the DPMs. However, only 3.5% of the DPMs had presented the generic name and brand name in similar fonts. In the rest of them, the generic names were written in small fonts while they use large fonts for brand names. Majority of the DPMs also displayed information for indication (92.5%) and active ingredient per dosage form (62.4%). Mechanism of action of the drug and inactive ingredients known to cause problems were written only in 16.2% and 4.6% of the DPMs respectively.
Table 1.
Evaluation of promotional material as per World Health Organization criteria (n=173)
| Criteria | Percentage of promotional material that contains the information |
|---|---|
| Generic name | 94.8 |
| Brand name | 100 |
| Active ingredient per dosage form | 62.4 |
| Inactive ingredients known to cause problems | 4.6 |
| Mechanism of action | 16.2 |
| Indications | 92.5 |
| Regimen | 49.7 |
| Route of administration | 52.6 |
| References | 48.6 |
| Dosage form | 83.8 |
| Storage conditions | 13.3 |
| Pack sizes | 20.8 |
| Description of the product and package | 5.2 |
| Legal categorya | 0 |
| Manufacturer/distributor’s name | 93.1 |
| Manufacturer/distributor’s address | 47.9 |
Note: aNarcotic or other controlled drug, prescription or non-prescription.
Many of the DPMs included information regarding the type of dosage form (83.3%) and manufacturer/Distributor’s name (93.1%). However, the manufacturer/Distributor’s address was written only in 47.9% of the DPMs. None of the DPMs provided the information for the legal category of the promoted drugs (Table 1).
Safety information
Based on the findings of this study, inclusion of information about drug safety was inadequate (Table 2). Information regarding side effects, precautions, contraindications were written only in 27.2%, 18.5% and 18.5% of the DPMs respectively. Potential drug interaction and overdose were mentioned occasionally, 16.2% and 10.1% of the DPMs respectively. Similarly, special situations like hepatic and renal failure which require dose adjustments were written only in 13.9% of the DPMs. Only 6.4% (n=47) of the DPMs displayed the side effects of the drugs in similar font with their indication. The rest of the DPMs used small fonts, commonly at the bottom of the pages. All of the DPMs used small fonts to write other safety information.
Table 2.
Evaluation of promotional material as per World Health Organization criteria, safety information (n=173)
| Type of safety information | Percentage of promotional material that contains the information |
|---|---|
| Side effects | 27.2 |
| Precautions | 18.5 |
| Contraindications | 18.5 |
| Pregnancy category | 15.6 |
| Drug interaction | 16.2 |
| Overdose | 10.1 |
| Special situationsa | 13.9 |
Note: aEg, renal, hepatic, cardiac, or nutritional insufficiencies that require either increased or reduced dosage.
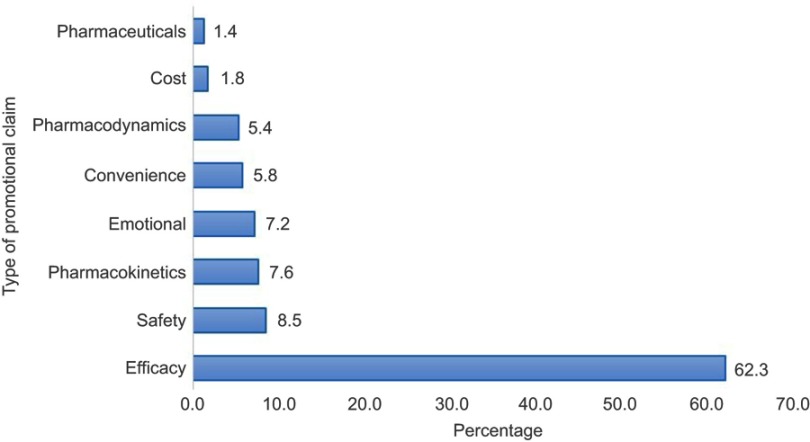
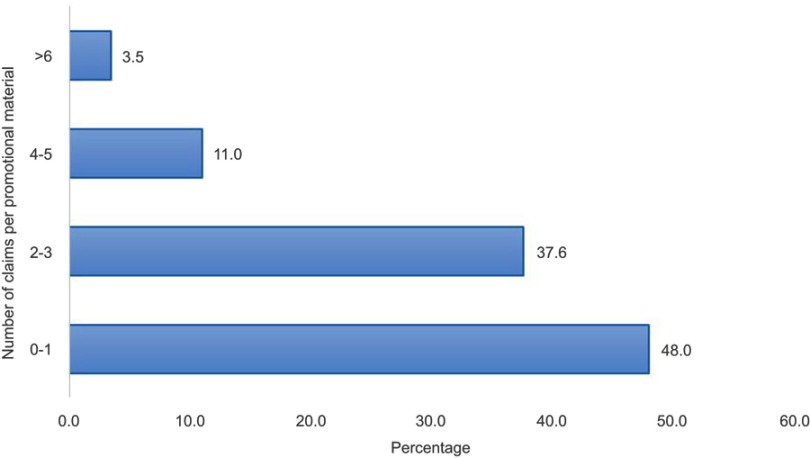
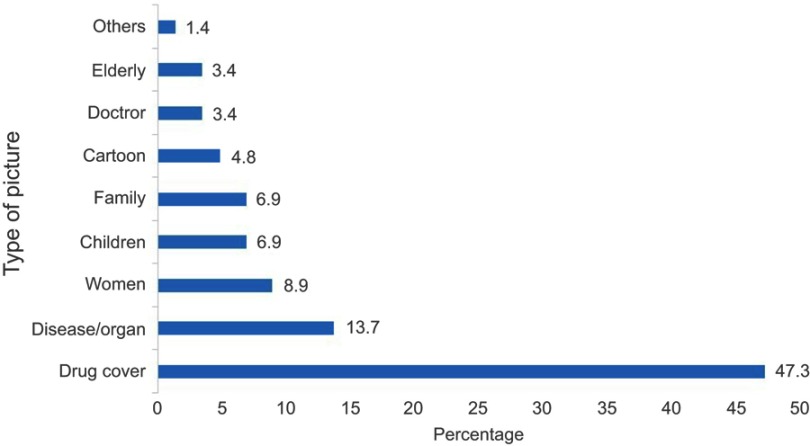
The type and frequency of claims made are represented in Figure 2. Of the 173 promotional materials, 223 claims were made. Better efficacy of the promoted product than competitors was the dominant claim, 62.3%. Safety, pharmacokinetics and emotional exaggerated claims were made in 8.5%, 7.6% and 7.2% of the DPL respectively. A lower price of the specific products was also claimed in 1.8% of the DPMs though none of these did present their current cost. The number of claims per advertisement are presented in Figure 3. Forty-eight percent of the DPMs did have a single claim whereas 37.6% of them made 2–3 claims per advertisement. This was followed by 11.0% of the DPMs which made 4–5 claims. Only 3.5% of them made six and more than six claims per promotional material. Pictorial demonstrations were used in 84.4% of the DPMs (Figure 4). Majority of the DPMs, 47.3%, displayed the cover of their products followed by pictures of the disease/organ (13.7%) and women (8.9%).
Figure 2.
Categorization of promotional claims (n=223).
Figure 3.
Graph depicting variation in number of claims per promotional material (n=173).
Figure 4.
Categorization of pictorial contents of the drug promotional materials (n=146).
References used by the DPMs
DPMs were analyzed for type and retrievability of the references they used (Table 3). Only 48.6% of the DPMs supported their claims with references. A total of 249 references were used. Two hundred and three of the references were journal articles. Of all the journal articles, 93.6% were retrieved. Those articles were evaluated and grouped based on their study design to indicate the type of evidence being used by the DPMs. Articles were also evaluated for the year of publication. Review articles account for 23.3% of the references. RCT, RPCT, NRCT and meta-analysis constitute 17.3%, 8.8%, 6.4% and 4.4% of the journal articles, respectively. Treatment guidelines (5.6%) and package inserts (4.8%) were also mentioned as sources of information. Of the total articles, 30.0% were published before the year 1999 (Table 4). Twenty-five percent of the articles were from the years between 2004–2008 followed by 21.6% of the articles from the years between 1999–2003. Only 5.8% of the articles were from journals issued after the year 2013.
Table 3.
Classification of references as per its source (n=249)
| Serial Number | Type of references | Percentage of references (n=249) | ||
|---|---|---|---|---|
| 1 | Journal article | Research article | RCT | 17.3 |
| RPCT | 8.8 | |||
| NRCT | 6.4 | |||
| Survey | 2.4 | |||
| CCT | 2.8 | |||
| Preclinical studies | In-vitro study | 4.4 | ||
| Animal studies | 1.2 | |||
| Review article | Review | 23.3 | ||
| Meta-analysis | 4.4 | |||
| Journal article not retrievable | 5.2 | |||
| 2 | Treatment guidelines | 5.6 | ||
| 3 | Leaflets (package inserts) | 4.8 | ||
| 4 | Data on file | 3.6 | ||
| 5 | Websites | 3.2 | ||
| 6 | Books | 2.8 | ||
| 7 | Regulatory body approval data | 2.8 | ||
| 8 | Others | 2.0 | ||
Note: The category ‘others’ include expert opinions and letter to editors.
Abbreviations: RCT, Randomized control trial; RPCT, Randomized placebo control trial; NRCT, Non Randomized control trial; CCT, Case-control study.
Table 4.
Classification of journal articles as per their publication year (n=190)
| Year of publication | Percentage of journal articles (n=190) |
|---|---|
| After 2013 | 5.8 |
| 2009–2013 | 17.9 |
| 2004–2008 | 24.7 |
| 1999–2003 | 21.6 |
| Before 1999 | 30.0 |
Discussion
This study was done to evaluate the validity of the DPMs used at Dessie as to WHO criteria for ethical drug promotion.5 A study from northern Ethiopia has indicated that prescribing decisions of physicians are influenced by medical representatives. This same study has also pointed out that DPMs were the most widely used mode of promotion next to face to face talking.16 Thus, DPMs must present the most accurate possible data and needs to be regulated. According to this study, none of the DPMs analyzed has fulfilled the WHO criteria. This was similar to studies from other parts of the world.10,17
The result of this study shows that antibiotics are the most commonly promoted drugs (27.2%). This might be due to three reasons. The first one is infectious diseases are the primary cause of death in Ethiopia according to WHO’s report from 2012; lower respiratory infections were reported as the leading cause of death.18 The second reason is that antibiotics are prescription only drugs. The third justification goes to the culture of inappropriate use of antibiotics which has been reported in different parts of the country including the largest tertiary teaching hospital of the country.19–22 Inappropriate use of drugs could prevent or delay patients from getting desired therapeutic outcomes.23 In particular with antibiotics, their misuse and overuse are associated with the emergence of resistance and increased health costs.24
In this study, the brand name was written in all of the DPMs. Majority of them also displayed information about the generic name (94.8%) and indications (92.5%) of the promoted drugs which are similar to a study from India.11 However, the safety information like side effects, precautions, and drug interactions were found to be overlooked despite the fact that side effects and drug interactions have been described as drug-related problems in different parts of the country.25–27 Such neglects have also been reported in other studies.28,29 Fonts used to write the safety information were small in size and usually at the bottom of the page. Only 6.4% (n=47) of the side effects and none of the other safety information were written in similar fonts with the indication of the drugs. This makes the information to be unnoticed and at worst cases, it might create the perception that those information are not crucial. This combined with the type of claims made by the pharmaceutical companies might say a lot about their motives. Of all the claims made, efficacy was the dominant one, 62.3%. Only 8.5% of the claims were about the better safety profile of the products. This shows the primary purpose of the manufacturers and/or distributors is to sell their products not to convey information. The DPMs has displayed a total of 146 pictures. Almost half of the pictures, 47.3%, were the cover of the product being promoted. However, this space could have been used to inscribe the much-needed safety information of the drugs.
Pharmaceutical companies are expected to provide references as an evidence to their claims. Hence, health care providers could cross check that. However, only 48.6% of the DPMs has done that. This is similar to studies from other parts of the world.17,30 Majority of the references used were review articles, 27.7%. Of these review articles, only 15.9% were a meta-analysis. The rest (84.1%) were narrative reviews which describe the science of a given drug or condition rather than delivering a conclusive answer about a specific medical question.
We conclude that the design and content of studied DPMs are most effective as sales materials rather than thorough informational vehicles. By limiting or de-emphasizing content related to safety, adverse drug effects and other concerns, these materials seem to be primarily advertisements. The WHO and FMHACA recommendations are rarely met. We believe the potential risk to patient health by reliance on these incomplete materials is a significant public health concern. Physicians and pharmacists should be provided with additional training and information about drug selection and use. Audit of promotional materials for both essential and non-essential drugs should be considered.
Limitation of the study
All the groupings shown in the results of this study were done by the first author which might cause bias. DPMs were collected irrespective of their publication year.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.World Health Organization. The Rational Use of Drugs: Report of the Conference of Experts. Nairobi, Kenya: World Health Organization; 1985. November 25-29. [Google Scholar]
- 2.World Health Organization. Promoting rational use of medicines: core components. No. WHO/EDM/2002.3; 2002. September Geneva: World Health Organization. [Google Scholar]
- 3.Gopalakrishnan S, Murali R. India: campaign to tackle unethical promotion. Essent Drugs Monit. 2002;31:22. [Google Scholar]
- 4.Marco CA, Moskop JC, Solomon RC, Geiderman JM, Larkin GL. Gifts to physicians from the pharmaceutical industry : an ethical analysis. Ann Emerg Med. 2002;48(5):513–521. doi: 10.1016/j.annemergmed.2005.12.013 [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Ethical Criteria for Medicinal Drug Promotion. WHA41.17. Geneva: World Health Organization; 1988. Available from: http://apps.who.int/iris/handle/10665/38125. Accessed July01, 2019 [DOI] [PubMed] [Google Scholar]
- 6.Stryer D, Bero LA. Characteristics of materials distributed by drug companies. J Gen Intern Med. 1995;11:575–583. doi: 10.1007/BF02599024 [DOI] [PubMed] [Google Scholar]
- 7.Saxena D, Yadav P, Kantharia ND. Drug promotional literature distributed by pharmaceutical companies : do they provide enough information to ascertain their validity? J Pharmcol Pharmacother. 2011;2(3):192–194. doi: 10.4103/0976-500X.83288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler MG, Lew P, Brian CS. The accuracy of drug information from pharmaceutical sales representatives. JAMA. 1995;273(16):1296–1298. [PubMed] [Google Scholar]
- 9.Shikvar YM. Clinical information in drug package inserts in India. J Postgr Med. 2009;55(2):104–107. doi: 10.4103/0022-3859.52840 [DOI] [PubMed] [Google Scholar]
- 10.Mali SN, Dudhgaonkar S, Bachewar NP. Evaluation of rationality of promotional drug literature using World Health Organization guidelines. Indian J Pharmacol. 2010;42(5):267–272. doi: 10.4103/0253-7613.70020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganashree P, Bhuvana K, Sarala N. Critical review of drug promotional literature using the World Health Organization guidelines. J Res Pharm Pract. 2016;5(3):162–165. doi: 10.4103/2279-042X.185711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mikhael EM. Evaluating the reliability and accuracy of the promotional brochures for the generic pharmaceutical companies in Iraq using World Health Organization guidelines. J Pharm Bioallied Sci. 2015;7(1):65–68. doi: 10.4103/0975-7406.148781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashenef A, Reshid E, Yilma Z, Melaku T, Chane T. Assessment of the use and status of new drug information centers in a developing Country, Ethiopia: the case of public university hospital drug information centers. Biomed Res Int. 2018. doi: 10.1155/2018/3840976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lexchin J. Deception by design: pharmaceutical promotion in the third world. Can Med Assoc J. 1995;153(12):1753. [Google Scholar]
- 15.Food, Medicine & Health Care Administration & Control Authority of Ethiopia. Over the counter medicines list for ethiopia; 2012. June Available from: http://www.fmhaca.gov.et/publication/over-the-counter-medicines-list-for-ethiopia-2nd-edition/. Accessed December 10, 2018. doi: 10.1094/PDIS-11-11-0999-PDN [DOI]
- 16.Workneh BD, Gebrehiwot MG, Bayo TA, et al. Influence of medical representatives on prescribing practices in Mekelle, Northern Ethiopia. PLoS One. 2016;11(6):e0156795. doi: 10.1371/journal.pone.0156795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas PP, Bhave AL. Critical appraisal of Drug Promotional Literatures (DPLs) as per World Health Organization (WHO) guidelines. Int J Basic Clin Pharmacol. 2018;7(2):238–243. doi: 10.18203/2319-2003.ijbcp20180092 [DOI] [Google Scholar]
- 18.World Health Organization.Ethiopia: WHO statistical profile; 2012. Available from: https://www.who.int/gho/mortality_burden_disease/en/.doi: 10.1094/PDIS-11-11-0999-PDN [DOI] [Google Scholar]
- 19.Erku DA, Mekuria AB, Belachew SA. Inappropriate use of antibiotics among communities of Gondar town, Ethiopia: a threat to the development of antimicrobial resistance. Antimicrob Resist Infect Control. 2017;6(112):1–7. doi: 10.1186/s13756-017-0272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebeyehu E, Bantie L, Azage M. Inappropriate use of antibiotics and its associated factors among urban and rural communities of Bahir Dar City administration, Northwest Ethiopia. PLoS One. 2015;10(9):e0138179. doi: 10.1371/journal.pone.0138179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutema G, Håkonsen H, Engidawork E, Toverud E. Multiple challenges of antibiotic use in a large hospital in Ethiopia – a ward-specific study showing high rates of hospital- acquired infections and ineffective prophylaxis. BMC Health Serv Res. 2018;18:326. doi: 10.1186/s12913-018-3107-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tulu S, Tadesse T, Gube AA. Assessment of antibiotic utilization pattern in treatment of acute diarrhoea diseases in bishoftu general hospital, Oromia Ethiopia. Adv Med. 2018. doi: 10.1155/2018/2376825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayele Y, Melaku K, Dechasa M, Ayalew MB, Horsa BA. Assessment of drug related problems among type 2 diabetes mellitus patients with hypertension in Hiwot fana specialized university hospital, Harar, Eastern Ethiopia. BMC Res Notes. 2018;11(1):728. doi: 10.1186/s13104-018-3838-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yadesa TM, Gudina EK, Angamo MT. Antimicrobial use-related problems and predictors among hospitalized medical in- patients in Southwest Ethiopia: prospective observational study. PLoS One. 2015;9(10):12 e0138385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Birarra MK, Heye TM, Shibeshi W. Assessment of drug-related problems in pediatric ward of zewditu memorial referral hospital, addis ababa, Ethiopia. Int J Clin Pharm. 2018;39(5):1039–1046. doi: 10.1007/s11096-017-0504-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dedefo MG, Mitike AH, Angamo MT. Incidence and determinants of medication errors and adverse drug events among hospitalized children in West Ethiopia. BMC Pediatr. 2016;16(1):81. doi: 10.1186/s12887-016-0619-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ayalew MB, Megersa TN, Mengistu YT. Drug-related problems in medical wards of tikur anbessa specialized hospital, Ethiopia. J Res Pharm Pract. 2015;4(4):216–221. doi: 10.4103/2279-042X.167048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parli K, Reema R, Devang R, Supriya M. Evaluation of promotional drug literature provided by medical representative at a tertiary care hospital. Int J Pharm Sci Res. 2017;8(4):1744–1750. [Google Scholar]
- 29.Hoovinahole S, Kamath A. A study of adherence of drug promotional literatures from various clinical specialties to the World Health Organization ethical criteria for drug promotion. J Pharm Negat Results. 2016;7(1):37–41. doi: 10.4103/0976-9234.177063 [DOI] [Google Scholar]
- 30.Sekar P, Punnagai K, David DC. Evaluation of the rationality of claims made in drug promotional literature in west chennai. Asian J Pharm Clin Res. 2015;8(5):107–109. [Google Scholar]