Abstract
Background
Osteoarthritis (OA) is a common joint disease, which is characterized by degradation of articular cartilage. Evidence indicated that miR-23b-3p was upregulated in cartilage tissues of a patient with OA. However, the mechanism by which miR-23b-3p regulates the occurrence and development of OA remains unclear. Thus, this study aimed to investigate the role of miR-23b-3p in the progression of OA.
Methods
In this study, qRT-PCR was used to measure the expression of miR-23b-3p in OA tissue samples and normal controls, respectively. Western blotting assay was performed to detect the levels of collagen II, aggrecan, Bax and active caspase 3 in CHON-001 cells. In addition, the dual-luciferase reporter system assay was used to detect the interaction between miR-23b-3p and COL11A2 in OA.
Results
The levels of miR-23b-3p were upregulated, while the expressions of collagen II and aggrecan were decreased in OA tissues and in IL-1β-treated CHON-001 cells. In addition, IL-1β significantly induced apoptosis of CHON-001 cells via increasing the levels of Bax and active caspase 3. However, downregulation of miR-23b-3p markedly inhibited IL-1β-induced apoptosis in CHON-001 cells via increasing the collagen II and aggrecan levels and decreasing Bax and active caspase 3 expressions. Meanwhile, dual-luciferase assay showed that COL11A2 was the direct target of miR-23b-3p in CHON-001 cells. Overexpression of miR-23b-3p markedly decreased the level of COL11A2 in cells. Moreover, downregulation of miR-23b-3p alleviated synovitis/cartilage destruction and reduced Osteoarthritis Research Society International scores and subchondral bone thickness in vivo.
Conclusion
Downregulation of miR-23b-3p could alleviate the progression of OA through upregulating COL11A2 in vivo and in vitro. Therefore, downregulation of miR-23b-3p might be a potential therapeutic strategy for the treatment of OA.
Keywords: microRNA-23b-3p, osteoarthritis, IL-1β, COL11A2
Introduction
Osteoarthritis (OA) is the most prevalent form of arthropathy in older people worldwide, which is characterized by articular cartilage damage and degradation.1 In addition, OA is a chronic disease of joint accompanied by arthrocele, pain, and loss of function.2 Among all OA cases, knee, hip, and hand joints are the most common locations in patients with OA.3 It has been reported that the ratio of population aged ≥45 with OA might rise to 30% by 2032.4 The number of patients with OA will increase as the population ages, which bring the enormously social and economic burden for patients and society.5 Articular cartilage is composed of chondrocytes and the extracellular matrix (ECM).6 However, the catabolic abilities of chondrocytes were abnormal in patients with OA, which led to the degradation of ECM eventually.7 At present, autologous chondrocyte implantation (ACI) is the most effective technique to repair cartilage lesions clinically.8 However, there are some limitations of ACI including high donor-to-donor variability and lack of integration of the grafts.9 Therefore, it is very important to explore more effective therapeutic strategies for the treatment of OA.
Collagen is the primary structural composition of cartilage, which is associated with severe skeletal dysplasias and OA.10 COL11A2 is a member of the fibrillar collagen subgroup, which encodes α chains of type XI collagen.11 It has been reported that COL11A2 may associate with pain sensitivity in OA.12
miRNAs are small non-coding RNAs which participate in multiple biological processes.13 Recently, several studies reported that miRNAs are involved in chondrogenesis and cartilage degradation.14,15 It has been reported that miR-23b-3p was upregulated in cartilage tissues of a patient with OA.16 Recently, a study indicated that silencing of miR-23b could aggravate LPS-induced inflammatory injury in OA cells by targeting PDCD4.13 In addition, miR-23b enhanced the autologous restorative possibility for degenerative arthritis by targeting PRKACB.17 However, the mechanism by which miR-23b-3p regulates the occurrence and development of OA remains unclear. Therefore, this study aimed to investigate the role of miR-23b-3p in the progression of OA in vitro and in vivo.
Materials and methods
Cell culture
Human chondrocyte cell line CHON-001 (American Type Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640 medium, supplemented with 10% FBS and 2 mM glutamine (Sigma-Aldrich, St. Louis, MO, USA) at 37°C. CHON-001 cells were stimulated with IL-1β (10 ng/mL) for 24 hrs in order to simulate OA model in vitro.
Knee tissue collection
OA human cartilage tissues were collected from 20 patients with OA who underwent knee arthroplasty surgery within 4 hrs in the Jinhua Municipal Central Hospital. These patients with OA were aged 50–73 years, 65% were male. Twenty healthy tissue donors who suffered from a trauma (aged 34–50 years). Healthy articular cartilages were isolated from their knee joints (control group). Tissue collection was performed according to the terms of the Medical Ethical Committee of the Jinhua Municipal Central Hospital. The patients with OA met the American College of Rheumatology classification criteria for the diagnosis of OA.18 The informed consent was signed and provided by all participants, and this study was approved by the Institutional Ethical Committee of the Jinhua Municipal Central Hospital.
qRT-PCR
TRIzol Reagent (Takara Bio, Otsu, Japan) was employed to extract total RNA in OA tissues and CHON-001 cells according to the manufacturer’s instructions. Hairpin-itTM microRNA and U6 snRNA normalization RT-PCR quantitation kit (GenePharma, Shanghai, China) were used to detect miR-23b expression levels in an ABI Prism 7500 (Applied Biosystems, Foster City, CA, USA). The results were normalized to U6. 2−ΔΔCt method was used to quantify the relative expression of miR‐23b-3p. U6 Forward: 5′-GCTTCGGCAGCACATATACTAAAAT-3′; U6 Reverse: 5′-CGCTTCACGAATTTGCGTGTCAT-3′. MiR‐23b-3p Forward: 5′-ACACTCCAGCTGGGATCACATTGCCAGGGAT-3′; Reverse: 5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAG TTGAGGTGGTAAT-3′.
Western blotting
Total proteins were lysed using RIPA lysis buffer. Then, the concentration of protein was detected using a BCA protein kit (Thermo Fisher Scientific, Waltham, MA, USA). Proteins (40 μg per lane) were separated on 10% SDS-PAGE gel and then transferred onto polyvinylidene fluoride (Thermo Fisher Scientific) membranes. After that, the membranes were blocked with 5% non-fat milk in TBST for 1 hr at room temperature and then incubated with the primary antibodies against collagen II (Abcam Cambridge, MA, USA, 1:1,000), aggrecan (Abcam, 1:1,000), Bax (Abcam, 1:1,000), active caspase 3 (Abcam, 1:1,000), and COL11A2 (Abcam, 1:1,000) overnight at 4°C. Later on, the membranes were incubated with HRPconjugated- secondary antibodies for 1 hr a room temperature. Finally, the membranes were detected by Enhanced Chemiluminescencekit (Thermo Fisher Scientific). β-actin was used as an internal control.
Flow cytometry for detection of apoptosis
CHON-001 cells (4 × 105 cells per well) were plated into 6-well plates overnight. When the cell density reached 70% confluence, cells were stimulated with IL-1β for 24 hrs at 37°C. Annexin V/PI apoptosis-detection kit (KeyGen BioTech, Shanghai, China) was used to measure cell apoptosis according to the manufacturer’s instructions. The FASC Calibur MT flow cytometer (BD Bioscience, Franklin Lake, NJ, USA) was used to calculate the apoptosis rate.
Cell transfection
For transfection, CHON-001 cells (4 × 105 cells per well) were plated into 6-well plates overnight. When the cell density reached 70% confluence, the cells were incubated in serum-free 1640 medium for 12. After that, the cells were transfected with 100 nM miR-23b-3p inhibitor (GenePharma, Shanghai, China) for another 12 hrs using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Later on, 10 ng/ml IL-1β (Sigma-Aldrich) or PBS was added to each well for an appropriate period. miR-23b-3p inhibitor is a RNA transcript (single-stranded RNA) that was synthesized via a chemical method. miRNA inhibitor exerts their function by directly binding to their matched miRNA.19
CCK-8 assay
Cell proliferation was detected using cell counting kit-8 (CCK-8, Sigma-Aldrich) according to the manufacturer’s protocol. CHON-001 cells (5×103 cells per well) were seeded into 96-well plates and cultured for 24, 48, and 72 hrs at 37°C. Then, CCK-8 (10 μL) was added into each well for another 2 hrs. The absorbance of cells at a wavelength of 450 nm was detected using a microplate reader (BioRad, Hercules, CA, USA).
Dual-luciferase reporter assay
CHON-001 cells (4×104 cells per well) were seeded into 24-well plates overnight; wild-type COL11A2 3′-UTR (WT-COL11A2 3′-UTR) or mutant COL11A2 3′-UTR (MT-COL11A2 3′-UTR) was co-transfected with miR-23b-3p mimics or miR-control, respectively, using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions. Twenty‐four hours later, the cells were harvested, and luciferase activities were detected using a Dual-Luciferase Reporter Assay System (Promega Corporation, Fitchburg, WI, USA). The data was normalized to Renilla luciferase.
Mice OA model
C57BL/6 male mice (8–10 weeks) were obtained from Animal Center of Chinese Academy of Sciences (Shanghai, China). The animals were provided with water and food ad libitum. The animals were housed in specific pathogen-free conditions at a temperature of 24±1°C, for a 12-hr period of light and darkness, and in a humidity-controlled (50–60%) environment. The mice were subjected to surgically induced OA by destabilization of the medial meniscus (DMM) as reported earlier.20 The mice were treated with miR-23b inhibitor via intra-articular injection. Mice in OA model group were made by DMM surgery. Mice in OA+miR-23b-3p inhibitor group were injected with 15 μL miR-23b inhibitor (5 nM) in PBS once a week after surgery. All animals were euthanized at 8 weeks after surgery. Knee joint tissues were collected for further experiments. NIH Guide for the Care and Use of Laboratory Animals was strictly followed. All animal experiment protocols were approved by the Ethics Committees of Jinhua Municipal Central Hospital Animal Center.
Immunohistochemistry (IHC)
Knee joint tissues embedded in paraffin were cut into 5-µm sections. The sections were subjected to IHC as previously described.7 Briefly, the sections were stained with Safranin O/Fast Green and hematoxylin-eosin to assess cartilage destruction. A fluorescence microscope was used to observe the stained sections. Then, we used several scoring systems for synovitis and subchondral bone thickness to determine the extent of cartilage degeneration. Osteoarthritis Research Society International (OARSI) scoring system was used to grade the destruction of articular cartilage.21 A scoring system was used to measure the severity of synovitis.22 AxioVision software (Zeiss, Oberkochen, Germany) was used to analyze the thickness of the medial subchondral bone plate according to Safranin O–stained sections.
Statistical analysis
All data were shown as the mean ± SD. SPSS 17.0 software was used for all statistical analyses. The data were conducted with GraphPad Prism version 7 (GraphPad Software, CA, USA.). The comparison between the two groups was conducted by Student’s t-test. The data were deemed to be statistically significant at P<0.05, P<0.01 in all experiments. All experiments were performed at least three independent experiments.
Results
miR-23b-3p was upregulated in OA tissues
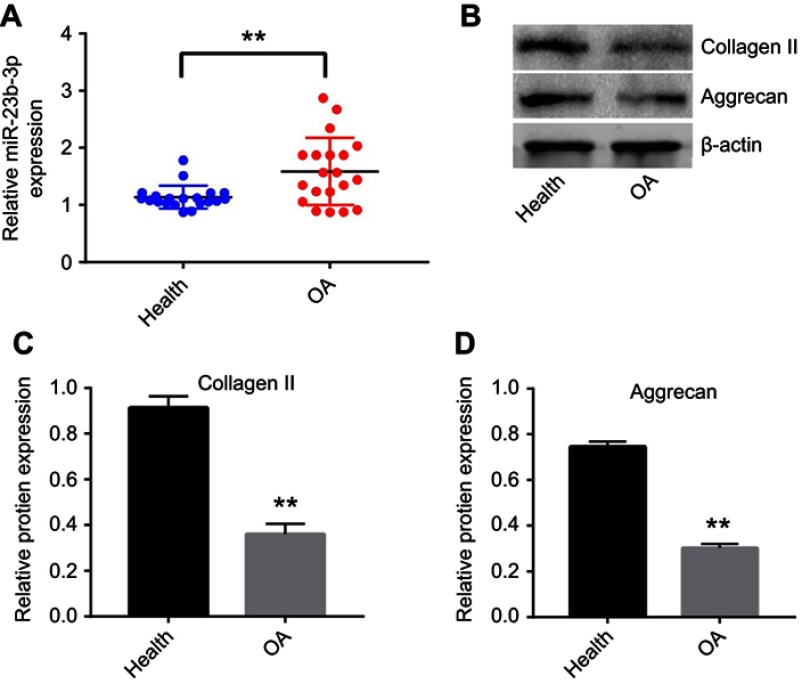
To investigate the role of miR-23b-3p in the progression of OA, the level of miR-23b-3p in OA tissues was detected by qRT-PCR. As shown in Figure 1A, the expression of miR-23b-3p was significantly upregulated in OA tissues in comparison with that in healthy tissues. In addition, the expressions of collagen II and aggrecan were markedly decreased in OA tissues compared with healthy tissues (Figure 1B–D). These data indicated that miR-23b-3p was notably upregulated in OA tissues.
Figure 1.
miR-23b-3p was upregulated in OA tissues. (A) The level of miR-23b-3p in joint tissues from patients of OA and healthy ones was detected with qRT-PCR. (B) Expressions of collagen II and aggrecan in OA and healthy tissues were detected with Western blotting. β-actin was used as an internal control. (C, D) The relative expressions of collagen II and aggrecan were quantified via normalizing to β-actin. **P<0.01 compared with the health group.
miR-23b-3p was upregulated in IL-1β-treated CHON-001 cells
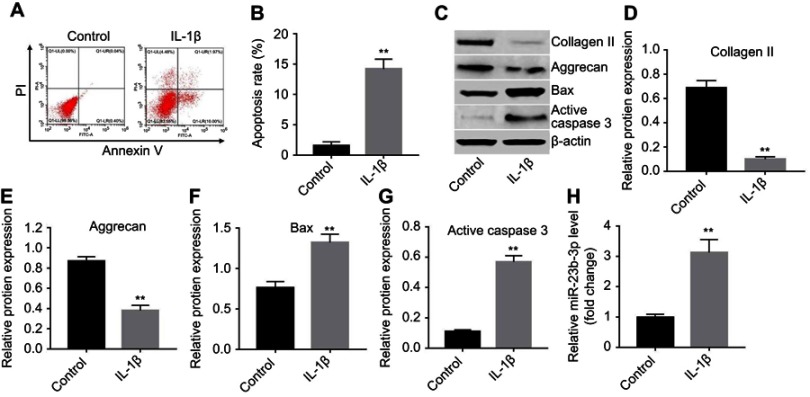
A previous study indicated that IL-1β contribute to the destruction of articular cartilage.23 CHON-001 cells were stimulated with IL-1β (10 ng/mL) for 24 hrs in order to simulate OA model in vitro. As shown in Figure 2A and B, the apoptosis rate in IL-1β-treated CHON-001 cells was significantly increased. In addition, the expressions of collagen II and aggrecan were markedly decreased, while the levels of apoptosis-related proteins Bax and active caspase 3 were significantly increased in IL-1β-induced CHON-001 cells compared with the control group (Figure 2C–G). Meanwhile, the level of miR-23b-3p was markedly upregulated in IL-1β-treated CHON-001 cells (Figure 2H). These results suggested that the in vitro OA cell model was established successfully.
Figure 2.
miR-23b-3p was upregulated in IL-1β-treated CHON-001 cells. CHON-001 cells were stimulated with IL-1β for 24 hrs for in vitro OA model establishment. (A, B) Apoptotic cells were detected with Annexin V and PI double staining. (C) Expressions of collagen II, aggrecan, Bax, and active caspase 3 in IL-1β-treated CHON-001 cells were detected with Western blotting. β-actin was used as an internal control. (D–G) The relative expressions of collagen II, aggrecan, Bax, and active caspase 3 were quantified via normalizing to β-actin. (H) The level of miR-23b-3p in IL-1β-treated CHON-001 cells was detected using qRT-PCR. **P<0.01 compared with the control group.
Downregulation of miR-23b-3p inhibited IL-1β-induced cell apoptosis
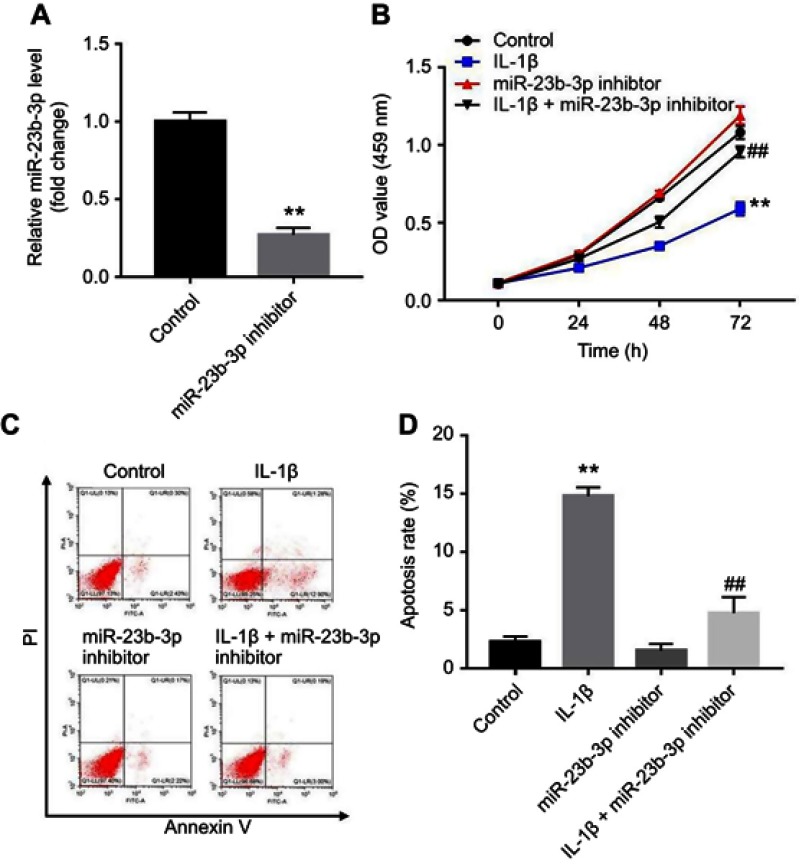
In order to further investigate the role of miR-23b-3p during the progress of OA, CHON-001 cells were transfected with miR-23b-3p inhibitor. As shown in Figure 3A, the level of miR-23b-3p was significantly decreased following transfection with miR-23b-3p inhibitor. In addition, CCK-8 assay indicated that IL-1β significantly inhibited the proliferation of CHON-001 cells, which was markedly reversed by a miR-23b-3p inhibitor (Figure 3B). Moreover, IL-1β-induced apoptosis in CHON-001 cells was notably inhibited by following transfection with miR-23b-3p inhibitor (Figure 3C and D). These data suggested that downregulation of miR-23b-3p could significantly reverse IL-1β-induced cell apoptosis.
Figure 3.
Downregulation of miR-23b-3p inhibited IL-1β-induced cell apoptosis. (A) CHON-001 cells were transfected with miR-23b-3p inhibitor for 24 hrs. The level of miR-23b-3p in CHON-001 cells were detected using qRT-PCR following transfection with miR-23b-3p inhibitor. (B) CHON-001 cells were transfected with miR-23b-3p inhibitor for 12 hrs using Lipofectamine 2000. Then, IL-1β or PBS was added to each well and incubated for 24, 48, and 72 hrs. CCK-8 assay was used to detect the viability of CHON-001 cells. (C, D) Apoptotic cells were detected with Annexin V and PI double staining. **P<0.01 compared with the control group, ##P<0.01 compared with IL-1β treatment group.
Downregulation of miR-23b-3p-inhibited IL-1β-induced collagen II and aggrecan degradation in CHON-001 cells
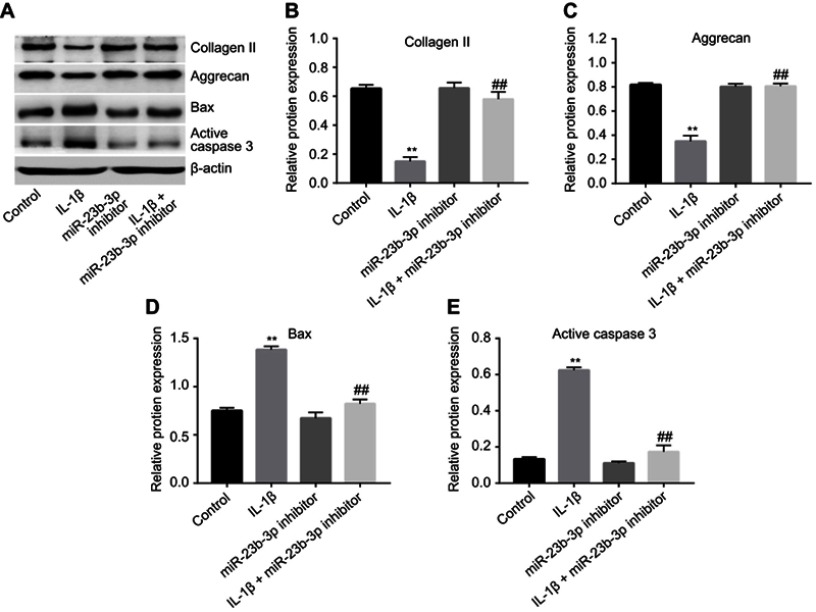
Next, to further assess the function of miR-23b-3p in IL-1β-treated CHON-001 cells, Western blot was used. As indicated in Figure 4A–C, downregulation of miR-23b-3p significantly inhibited IL-1β-induced collagen II and aggrecan degradation in CHON-001 cells. In addition, IL-1β-induced Bax and active caspase 3 protein increases in CHON-001 cells were significantly reduced by following transfection with miR-23b-3p inhibitor (Figure 4A, D and E). All these results suggested that downregulation of miR-23b-3p could inhibit IL-1β-induced collagen II and aggrecan degradation in CHON-001 cells.
Figure 4.
Downregulation of miR-23b-3p inhibited IL-1β-induced collagen II and aggrecan degradation in CHON-001 cells. CHON-001 cells were transfected with miR-23b-3p inhibitor for 12 hrs using Lipofectamine 2000. Then, IL-1β or PBS was added to each well and the cells were incubated for another 72 hrs. (A) Expressions of collagen II, aggrecan, Bax, and active caspase 3 in IL-1β-treated CHON-001 cells were detected with Western blotting. β-actin was used as an internal control. (B–E) The relative expressions of collagen II, aggrecan, Bax, and active caspase 3 were quantified by normalizing to β-actin. **P<0.01 compared with the control group, ##P<0.01 compared with IL-1β treatment group.
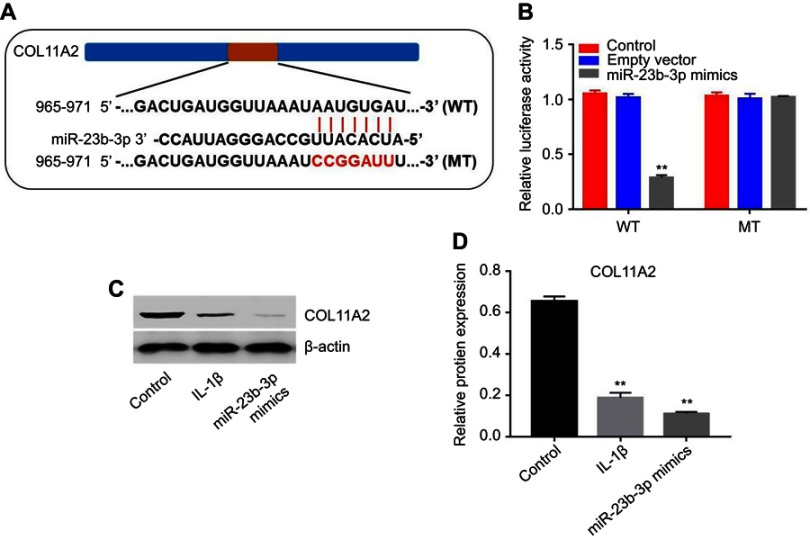
COL11A2 was a direct target of miR-23b-3p
To explore the potential mechanism by which miR‐23b-3p affects the progress of OA, three online bioinformatics databases (TargetScan, miRDB, or microRNA) were used to predict the potential targets of miR‐23b-3p. The results showed that COL11A2 might be a potential target (Figure 5A). In addition, the luciferase assay data indicated that reduced luciferase activity was observed in CHON-001 cells following transfection with psiCHECK-2-COL11A2-WT and miR-23b-3p mimics (Figure 5B). Moreover, the expression of COL11A2 was markedly decreased in IL-1β-stimulated CHON-001 cells (Figure 5C and D). Meanwhile, overexpression of miR-23b-3p significantly decreased the level of COL11A2 in CHON-001 cells (Figure 5C and D). In conclusion, COL11A2 was a direct target of miR-23b-3p.
Figure 5.
COL11A2 was a direct target of miR-23b-3p. (A) Gene structure of COL11A2 at the position of 965–971 indicates the predicted target site of miR-23b-3p in its 3ʹUTR, with a sequence of AAUGUGAU. (B) The luciferase activity was measured in CHON-001 cells following co-transfecting with WT/MT COL11A2 3′-UTR plasmid and miR-23b-3p with the dual-luciferase reporter assay. (C) CHON-001 cells were stimulated with IL-1β for 24 hrs. In addition, CHON-001 cells were transfected with miR-23b-3p mimics for 72 hrs. Expression level of COL11A2 in CHON-001 cells were detected with Western blotting. β-actin was used as an internal control. (D) The relative expression of COL11A2 was quantified via normalizing to β-actin. **P<0.01 compared with the control group.
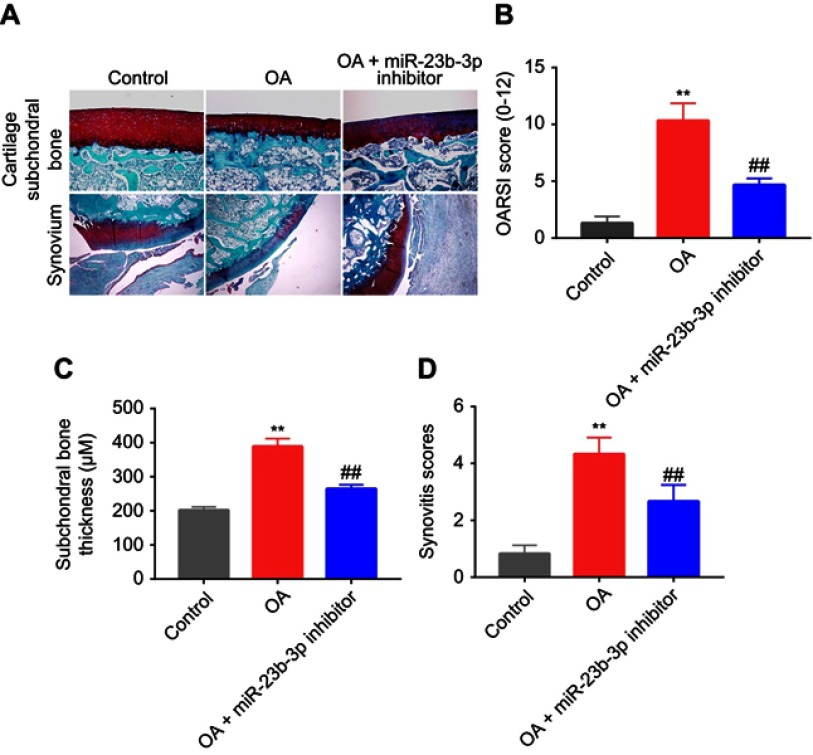
Downregulation of miR-23b-3p delayed the progression of OA in mice
To confirm the function of miR-23b-3p during the progress of OA in vivo, mouse model of OA was established. The Safranin O/Fast Green staining and hematoxylin-eosin staining revealed that the surface of the cartilage was unbroken in the control group. Cartilage superficial destruction, cartilage erosion, and obvious hypocellularity were observed in the OA group (Figure 6A). However, less cartilage erosion and cartilage destruction were observed in OA+miR-23b-3p inhibitor group compared to OA group (Figure 6A). Similarly, the OARSI scores of OA group were evidently higher than the control group. As expected, downregulation of miR-23b-3p markedly decreased OARSI scores of mice (Figure 6B). In addition, miR-23b-3p inhibitor treatment obviously decreased subchondral bone thickness and synovitis scores compared to OA group (Figure 6C and D). Taken together, these data indicated that downregulation of miR-23b-3p could attenuate the progression of OA in mice.
Figure 6.
Downregulation of miR-23b-3p delayed the progression of OA in mice. Mice were randomly divided into three groups: control group, OA group, and OA+miR-23b-3p inhibitor group. (A) Histological analysis of OA was evaluated by Safranin O staining and hematoxylin-eosin staining. (B) Osteoarthritis Research Society International scores, (C) subchondral bone plate thickness, and (D) synovitis scores were used to measure the progression of OA. **P<0.01 compared with the control group, ##P<0.01 compared with OA group.
Discussion
It has been shown that miRNAs play a vital role in the development of OA.24 In this study, we confirmed that the level of miR-23b-3p was significantly upregulated in both OA tissues and IL-1β-treated CHON-001 cells. Guo et al indicated that the expression of miR-23b-3p was increased in TNF-α-treated OA cells, which was consistent with our results.14 A previous study indicated that inflammation response plays an important role in the development and progression of OA.25 Dodge et al indicated that IL-1β was upregulated in OA articular cartilage.26 In addition, TNF-α and IL-1β both could promote damage in arthritic cartilage and increase the levels of matrix-degradation proteins.27 These data preliminarily showed that a high level of miR-23b-3p was closely related with the progression of OA. However, miR-23b-3p was down regulated by LPS treatment and downregulation of miR-23b-3p further increased LPS-induced apoptosis in OA cells via regulation NF-κB/Notch pathways.13 This situation might because that the different stimulants could trigger different pathways, then leading to oppositely results. The connection between miRNAs and proinflammatory factors is complicated in the progression of OA, and the underlying mechanism needs further studying.
Here, to further explore the biological function of miR-23b-3p in the development of OA, in vitro and in vivo experiments were performed. Collagen II and aggrecan are the special matrix ingredients in cartilage.28 Loss of collagen-II and aggrecan could lead to accelerating the progress of OA.29 In this study, 10 ng/mL IL-1β significantly induced apoptosis of CHON-001 cells. Meanwhile, the levels of collagen II and aggrecan were markedly decreased in patients with OA and in IL-1β-treated CHON-001 cells, which was consistent with previous results.30 It has been demonstrated that DMM was an effective way for in vivo OA model establishment.31 In this study, cartilage superficial destruction, cartilage erosion and obvious hypocellularity were observed in the mice treated with DMM. Therefore, in vitro and in vivo experiment OA models were successfully established.
Our subsequent experiments showed that downregulation of miR-23b-3p significantly inhibited IL-1β-induced apoptosis in CHON-001 cells via decreasing the levels of Bax and active caspase 3. Meanwhile, IL-1β-induced collagen II and aggrecan proteins increases in CHON-001 cells were reversed by following transfection with miR-23b-3p inhibitor. Similarly, schisandrin A inhibited the IL-1β-induced cartilage degradation via upregulating the expressions of collagen II and aggrecan.32 In addition, the level of PVT1 was upregulated in IL-1β-simulated OA chondrocytes; downregulation of PVT1 could ameliorate the progression of OA by inflammatory response. These data were consistent with our results.33 These data indicated that downregulation of miR-23b-3p could alleviate the progression of OA in vitro. In addition, we indicated that inhibition of miR-23b-3p obviously alleviated the progression of OA in mice. Considering these data, it can be deduced that downregulation of miR-23b-3p may ameliorate the progression of OA.
It has been shown that miRNAs exhibit their function mainly based on binding their target genes.34 To demonstrate the potential mechanism of miR-23b-3p in the progression of OA, online bioinformatics databases were applied to identify targets gene of miR-23b-3p. We found that COL11A2 was selected as a potential target of miR-23b-3p. COL11A2 is a member of the fibrillar collagen subgroup, which plays an important role in cartilage collagen fibril formation and ECM organization.35 In addition, COL11A2 is a differentially methylated site in OA.36 Next, we confirmed that COL11A2 was a direct target gene of miR-23b-3p using a luciferase reporter assay. Overexpression of miR-23b-3p significantly inhibited the level of COL11A2. Meanwhile, IL-1β markedly decreased the expression of COL11A2 while increased the level of miR-23b-3p. Collectively, the alleviation of IL-1β-induced cell damage was proven to be realized via upregulating the expression of COL11A2. In addition, more validations should be investigated in vivo in future experiments.
Conclusion
Taken together, miR-23b-3p was upregulated in IL-1β-treated OA cells and OA tissues. In addition, downregulation of miR-23b-3p could alleviate IL-1β-induced cell injury. Thus, downregulation of miR-23b-3p might be a potential therapeutic strategy for the treatment of OA.
Acknowledgment
This research was supported by Zhejiang Basic Public Welfare Research Project (LGF19H060005).
Disclosure
The authors declare no competing financial interests in this work.
References
- 1.Li Y, Li Z, Li C, Zeng Y, Liu Y. Long noncoding RNA TM1P3 is involved in osteoarthritis by mediating chondrocyte extracellular matrix degradation. J Cell Biochem. 2019. [DOI] [PubMed] [Google Scholar]
- 2.Samuel AJ, Kanimozhi D. Outcome measures used in patient with knee osteoarthritis: with special importance on functional outcome measures. Int J Health Sci (Qassim). 2019;13(1):52–60. [PMC free article] [PubMed] [Google Scholar]
- 3.Ragni E, Perucca Orfei C, De Luca P, et al. Identification of miRNA reference genes in extracellular vesicles from adipose derived mesenchymal stem cells for studying osteoarthritis. Int J Mol Sci. 2019;20(5):1108. doi: 10.3390/ijms20051108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turkiewicz A, Petersson IF, Bjork J, et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthritis Cartilage. 2014;22(11):1826–1832. doi: 10.1016/j.joca.2014.07.015 [DOI] [PubMed] [Google Scholar]
- 5.Cavalli E, Levinson C, Hertl M, et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci Rep. 2019;9(1):4275. doi: 10.1038/s41598-019-40575-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang Y, Wang P, Xia L, et al. Aberrantly hydroxymethylated differentially expressed genes and the associated protein pathways in osteoarthritis. Peer J. 2019;7:e6425. doi: 10.7717/peerj.6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng W, Feng Z, You S, et al. Fisetin inhibits IL-1beta-induced inflammatory response in human osteoarthritis chondrocytes through activating SIRT1 and attenuates the progression of osteoarthritis in mice. Int Immunopharmacol. 2017;45:135–147. doi: 10.1016/j.intimp.2017.02.009 [DOI] [PubMed] [Google Scholar]
- 8.Peterson L, Vasiliadis HS, Brittberg M, Lindahl A. Autologous chondrocyte implantation: a long-term follow-up. Am J Sports Med. 2010;38(6):1117–1124. doi: 10.1177/0363546509357915 [DOI] [PubMed] [Google Scholar]
- 9.Katopodi T, Tew SR, Clegg PD, Hardingham TE. The influence of donor and hypoxic conditions on the assembly of cartilage matrix by osteoarthritic human articular chondrocytes on Hyalograft matrices. Biomaterials. 2009;30(4):535–540. doi: 10.1016/j.biomaterials.2008.09.064 [DOI] [PubMed] [Google Scholar]
- 10.Lawrence EA, Kague E, Aggleton JA, et al. The mechanical impact of col11a2 loss on joints; col11a2 mutant zebrafish show changes to joint development and function, which leads to early-onset osteoarthritis. Philos Trans R Soc Lond B Biol Sci. 2018;373:1759. doi: 10.1098/rstb.2017.0072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Jia H, Xing W, et al. Genetic variants in COL11A2 of lumbar disk degeneration among Chinese Han population. Mol Genet Genomic Med. 2019;7(2):e00524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho KWD, Wallace MR, Sibille KT, et al. Single nucleotide polymorphism in the COL11A2 gene associated with heat pain sensitivity in knee osteoarthritis. Mol Pain. 2017;13:1744806917724259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Z, Tang Y, Zhao Q, Lu H, Xu G. Down-regulation of microRNA-23b aggravates LPS-induced inflammatory injury in chondrogenic ATDC5 cells by targeting PDCD4. Iran J Basic Med Sci. 2018;21(5):529–535. doi: 10.22038/IJBMS.2018.25856.6364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Min Z, Jiang C, et al. Downregulation of HS6ST2 by miR-23b-3p enhances matrix degradation through p38 MAPK pathway in osteoarthritis. Cell Death Dis. 2018;9(6):699. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidl CI, Martinez-Sanchez A, Murphy CL. Derepression of microRNA-138 contributes to loss of the human articular chondrocyte phenotype. Arthritis Rheumatol. 2016;68(2):398–409. doi: 10.1002/art.39428 [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Ning Y, Zhou B, et al. Integrated bioinformatics analysis of the osteoarthritisassociated microRNA expression signature. Mol Med Rep. 2018;17(1):1833–1838. doi: 10.3892/mmr.2017.8057 [DOI] [PubMed] [Google Scholar]
- 17.Ham O, Lee CY, Song BW, et al. Upregulation of miR-23b enhances the autologous therapeutic potential for degenerative arthritis by targeting PRKACB in synovial fluid-derived mesenchymal stem cells from patients. Mol Cells. 2014;37(6):449–456. doi: 10.14348/molcells.2014.0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmieri B, Lodi D, Capone S. Osteoarthritis and degenerative joint disease: local treatment options update. Acta Biomed. 2010;81(2):94–100. [PubMed] [Google Scholar]
- 19.Tang L, Chen HY, Hao NB, et al. microRNA inhibitors: natural and artificial sequestration of microRNA. Cancer Lett. 2017;407:139–147. doi: 10.1016/j.canlet.2017.05.025 [DOI] [PubMed] [Google Scholar]
- 20.Vasheghani F, Zhang Y, Li YH, et al. PPARgamma deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann Rheum Dis. 2015;74(3):569–578. doi: 10.1136/annrheumdis-2014-205743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritzker KP, Gay S, Jimenez SA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014 [DOI] [PubMed] [Google Scholar]
- 22.Lewis JS, Hembree WC, Furman BD, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis Cartilage. 2011;19(7):864–873. doi: 10.1016/j.joca.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li M, Zhao J, Jia L. USP14-mediated IkappaBalpha degradation exacerbates NF-kappaB activation and IL-1beta-stimulated chondrocyte dedifferentiation. Life Sci. 2019;218:147–152. doi: 10.1016/j.lfs.2018.12.014 [DOI] [PubMed] [Google Scholar]
- 24.Soyocak A, Kurt H, Ozgen M, Turgut Cosan D, Colak E, Gunes HV. miRNA-146a, miRNA-155 and JNK expression levels in peripheral blood mononuclear cells according to grade of knee osteoarthritis. Gene. 2017;627:207–211. doi: 10.1016/j.gene.2017.06.027 [DOI] [PubMed] [Google Scholar]
- 25.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford). 2005;44(1):7–16. doi: 10.1093/rheumatology/keh344 [DOI] [PubMed] [Google Scholar]
- 26.Dodge GR, Poole AR. Immunohistochemical detection and immunochemical analysis of type II collagen degradation in human normal, rheumatoid, and osteoarthritic articular cartilages and in explants of bovine articular cartilage cultured with interleukin 1. J Clin Invest. 1989;83(2):647–661. doi: 10.1172/JCI113929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi: 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Liu S, Guo W, et al. Coculture of hWJMSCs and pACs in oriented scaffold enhances hyaline cartilage regeneration in vitro. Stem Cells Int. 2019;2019:5130152. doi: 10.1155/2019/5130152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubey NK, Ningrum DNA, Dubey R, et al. Correlation between diabetes mellitus and knee osteoarthritis: a dry-to-wet lab approach. Int J Mol Sci. 2018;19(10):3021. doi: 10.3390/ijms19103021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speichert S, Molotkov N, El Bagdadi K, Meurer A, Zaucke F, Jenei-Lanzl Z. Role of norepinephrine in IL-1beta-induced chondrocyte dedifferentiation under physioxia. Int J Mol Sci. 2019;20(5):1212. doi: 10.3390/ijms20051212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei Y, Bai L. Recent advances in the understanding of molecular mechanisms of cartilage degeneration, synovitis and subchondral bone changes in osteoarthritis. Connect Tissue Res. 2016;57(4):245–261. doi: 10.1080/03008207.2016.1177036 [DOI] [PubMed] [Google Scholar]
- 32.Tu C, Huang X, Xiao Y, et al. Schisandrin A inhibits the IL-1beta-induced inflammation and cartilage degradation via suppression of MAPK and NF-kappaB signal pathways in rat chondrocytes. Front Pharmacol. 2019;10:41. doi: 10.3389/fphar.2019.00041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Zhao J, Guo X, She J, Liu Y. Long non-coding RNA PVT1, a molecular sponge for miR-149, contributes aberrant metabolic dysfunction and inflammation in IL-1beta-simulated osteoarthritic chondrocytes. Biosci Rep. 2018;38(5):BSR20180576. doi: 10.1042/BSR20180576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo H, Ingolia NT, Weissman JS, Bartel DP. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466(7308):835–840. doi: 10.1038/nature09267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275(14):10370–10378. doi: 10.1074/jbc.275.14.10370 [DOI] [PubMed] [Google Scholar]
- 36.Jeffries MA, Donica M, Baker LW, et al. Genome-wide DNA methylation study identifies significant epigenomic changes in osteoarthritic cartilage. Arthritis Rheumatol. 2014;66(10):2804–2815. doi: 10.1002/art.38762 [DOI] [PubMed] [Google Scholar]