Abstract
BACKGROUND
Left ventricular outflow tract (LVOT) obstruction is a leading cause of mortality and exclusion from transcatheter mitral valve replacement (TMVR). Intentional laceration of the anterior mitral valve leaflet to prevent LVOT obstruction (LAMPOON) is a transcatheter mimic of surgical chord-sparing leaflet resection.
OBJECTIVES
The purpose of this prospective multicenter trial was to study LAMPOON with transseptal (Edwards Lifesciences, Irvine, California) TMVR in annuloplasty rings or native mitral annular calcification (MAC).
METHODS
Subjects at high or extreme surgical risk and prohibitive risk of LVOT obstruction from TMVR were included. Eligibility was modified midtrial to exclude subjects with threatened LVOT obstruction from a Sapien 3 valve fabric skirt. The primary endpoint was procedure survival with successful LAMPOON, with successful TMVR, without reintervention, and with LVOT gradient <30 mm Hg (“optimal”) or <50 mm Hg (“acceptable”). Secondary endpoints included 30-day mortality and major adverse cardiovascular events. There was universal source-data verification and independent monitoring. All endpoints were independently adjudicated. Central laboratories analyzed echocardiogram and CT images.
RESULTS
Between June 2017 and June 2018, 30 subjects were enrolled equally between the MAC and ring arms. LAMPOON traversal and midline laceration was successful in 100%. Procedure survival was 100%, and 30-day survival was 93%. Primary success was achieved in 73%, driven by additional procedures for paravalvular leak (10%) and high-skirt neo-LVOT gradients observed before a protocol amendment. There were no strokes.
CONCLUSIONS
LAMPOON was feasible in native and annuloplasty ring anatomies in patients who were otherwise ineligible for treatment, with acceptable safety. LAMPOON was effective in preventing LVOT obstruction from TMVR. Despite LAMPOON, TMVR using Sapien 3 in annuloplasty rings and MAC still exhibits important limitations. (NHLBI DIR LAMPOON Study: Intentional Laceration of the Anterior Mitral Leaflet to Prevent Left Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Implantation; NCT03015194)
Keywords: mitral annular calcification, structural heart disease, transcatheter electrosurgery, transcatheter mitral valve replacement, transseptal interventions, valve-in-ring
Transcatheter mitral valve replacement (TMVR) is an option for patients with mitral stenosis or regurgitation who are not suitable candidates for open surgical mitral repair or replacement (1). Pending commercial development of dedicated TMVR devices, trans-catheter aortic valves have been implanted in the mitral position in bioprosthetic mitral valves (valve-in-valve), mitral annuloplasty rings or bands (valve-in-ring), and in native mitral annular calcification (MAC) (valve-in-MAC) (2,3). Left ventricular outflow tract (LVOT) obstruction is a dreaded complication of TMVR, occurring in up to 40% of valve-in-MAC, 5% of valve-in-ring, and 2% of valve-in-valve cases (2), and with 62% in-hospital mortality (3). Fear of LVOT obstruction is a leading cause for treatment exclusion, with 49% of screened patients for valve-in-MAC and 6% for valve-in-ring excluded in the MITRAL (Mitral Implantation of Transcatheter Valves) trial for predicted risk of LVOT obstruction (4,5).
TMVR-induced LVOT obstruction has 2 mechanisms. Fixed obstruction occurs when the anterior mitral valve leaflet is pushed toward the interventricular septum by the mitral valve prosthesis, creating a narrowed and elongated “neo-LVOT” (6). Predicting the neo-LVOT area on pre-procedural time-resolved computed tomography (CT) integrates multiple risk factors for LVOT obstruction, including acute angulation of the aortic and mitral annular planes, a small left ventricle, a prominent septal bulge, and ventricular deployment of the trans-catheter valve (6,7). Observational registries suggest a simulated neo-LVOT area of under 170 to 190 mm2 predicts high risk of LVOT obstruction (8,9). Dynamic obstruction occurs when the narrowed neo-LVOT generates Bernoulli forces that draw the anterior mitral leaflet toward the interventricular septum during systole (10). A long anterior mitral leaflet with redundant chordae is a risk factor (11). A long anterior mitral leaflet may also prolapse back into the trans-catheter heart valve, interfering with valve closure and causing acute valve failure (12).
Preventive strategies include either surgical transatrial leaflet resection, which involves cardio-pulmonary bypass, associated morbidity, and reported 30-day mortality of 27% (13), or prophylactic alcohol septal ablation (14,15), which causes septal infarction, sacrifices myocardium and conduction tissue, may not be anatomically feasible or effective in all patients, and delays TMVR by 4 to 6 weeks. Current expert opinion recommends that TMVR should probably be contraindicated in most of these patients who are at high risk of LVOT obstruction (16).
Contemporary surgical mitral valve replacement includes resection of the anterior mitral leaflet to prevent LVOT obstruction and sparing of the sub-valvular apparatus to preserve left ventricular function (17). Laceration of the anterior mitral valve leaflet to prevent outflow obstruction (LAMPOON) is a percutaneous transcatheter mimic of the surgical standard. Animal studies (18) and early compassionate use in humans (19) demonstrated that LAMPOON and TMVR may be feasible in these “contraindicated” patients. The split anterior mitral leaflet parts away from the LVOT and blood flow is maintained through the open cells of the transcatheter heart valve despite threatened obstruction were the anterior mitral leaflet intact.
METHODS
TRIAL DESIGN AND OVERSIGHT.
The LAMPOON IDE trial (NCT03015194) was a prospective, single-arm, multicenter study of the LAMPOON procedure with transseptal Sapien 3 valve (Edwards Lifesciences, Irvine, California) TMVR in annuloplasty ring or band, or native MAC. The U.S. Food and Drug Administration granted Investigational Device Exemption (IDE) for the study. The institutional review board at each site and at National Heart, Lung, and Blood Institute (NHLBI) approved the study protocol. The NHLBI Data Safety Monitoring Board provided study over-sight. The NHLBI was the data coordinating center. All subjects consented in writing.
The trial was designed by the investigators and sponsored by the senior author (R.J.L.) on behalf of the NHLBI. Edwards Lifesciences provided arms-length financial support for the study as part of a Collaborative Research and Development Agreement with NHLBI on transcatheter modification of the mitral valve. Edwards Lifesciences was not involved in study design or analysis, and did not review this report before submission. The study investigators have custody of all data and are responsible for the findings.
To ensure data integrity, all case report forms were independently verified with source data on-site, clinical events were independently monitored, and all echocardiography and computed tomography (CT) images were analyzed by central laboratories. An independent Clinical Event Adjudication Committee classified the primary and all clinical endpoints including death and stroke, and determined relatedness to the LAMPOON procedure and to TMVR.
SUBJECTS.
Between June 2017 and June 2018, 30 adult subjects with severe mitral stenosis or regurgitation and high or extreme risk for surgical mitral valve replacement were enrolled at 5 centers in the United States (Online Appendix). Reasons for exclusion are shown in Figure 1. No subject was excluded for excessive leaflet calcification. A central eligibility committee confirmed eligibility for transseptal Sapien 3 valve in ring or band, or native MAC, based on CT annular measurements for suitable anchoring. Only candidates with prohibitive risk of LVOT obstruction from TMVR were enrolled, based on neo-LVOT area <200 mm2, or long redundant anterior mitral leaflet. The complete selection criteria are listed in the Online Appendix.
FIGURE 1. Study Flow Chart.
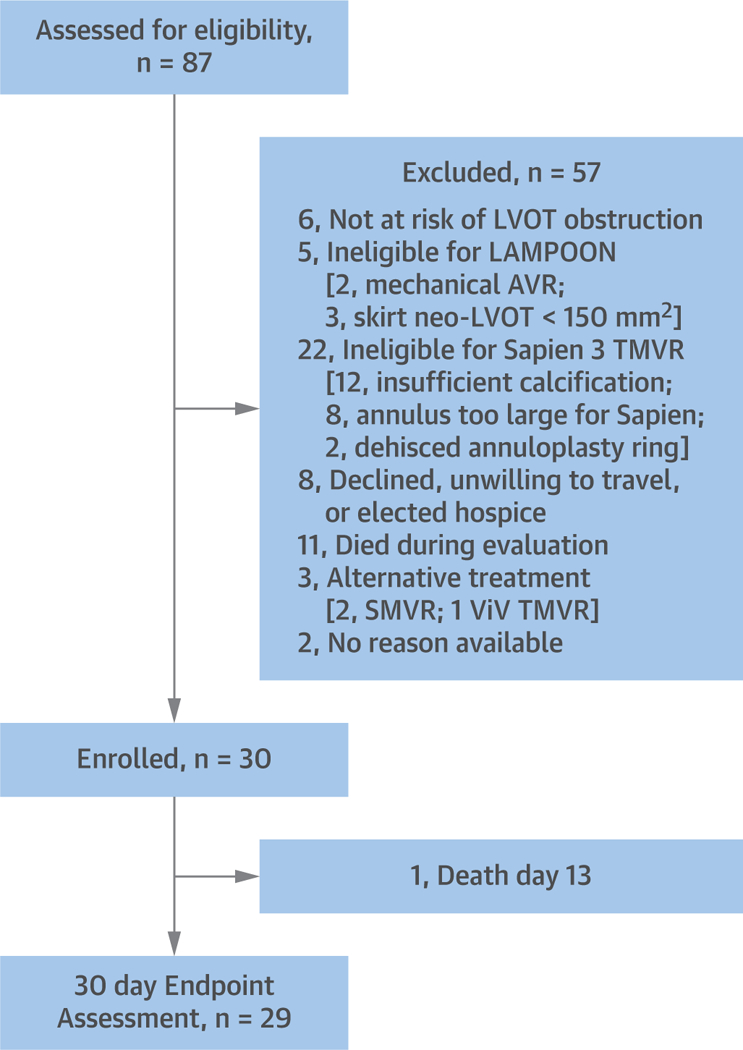
A total of 87 subjects were screened and 30 were enrolled. Reasons for exclusion are listed. AVR = aortic valve repair; LAMPOON = intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow obstruction; LVOT = left ventricular outflow tract; SMVR = surgical mitral valve replacement; TMVR = transcatheter mitral valve replacement; ViV = valve-in-valve.
The study was amended after the first 5 subjects were enrolled, after LVOT obstruction was observed despite anterior leaflet laceration requiring rescue alcohol septal ablation. These were attributed to obstruction from the covered fabric skirt at the base of the Sapien 3 valve (20). The selection criteria were therefore changed to exclude candidates with a “skirt neo-LVOT” <150 mm2 on baseline CT. All enrolled subjects were included in the final analysis.
LAMPOON TECHNIQUE.
The LAMPOON technique has been described previously (18,19). Briefly, 2 transfemoral guide catheters are advanced retrograde through the aortic valve and positioned in the LVOT and left atrium, respectively, on either side of the middle-scallop (A2) of the anterior mitral leaflet (Figure 2A). The left atrial guide catheter is stabilized using a transseptal rail. A stiff 0.014-inch guidewire (Astato XS 20, Asahi, Japan) is sheathed in an insulating polymer jacket (Piggyback Wire Convertor, Teleflex, North Carolina), and advanced from the LVOT to perforate through the center and base of the anterior mitral leaflet using a short pulse of radiofrequency energy. The guidewire tip is snare-retrieved from the left atrium to form a guidewire loop through the base of the anterior mitral leaflet, with both guidewire limbs exiting femoral arteries and insulated in catheters (Figure 2B). Tension is applied to the guidewire loop during further radio-frequency energy application, lacerating the anterior mitral leaflet from base to tip (Online Video 1). Using a retrograde guide catheter trajectory, the laceration aligns in front of the LVOT and down the midline of the anterior leaflet. The anterior mitral leaflet splays in diastole and coapts in systole (Figure 3A, Online Video 2), in part because the chordae are preserved.
FIGURE 2. LAMPOON Procedure.
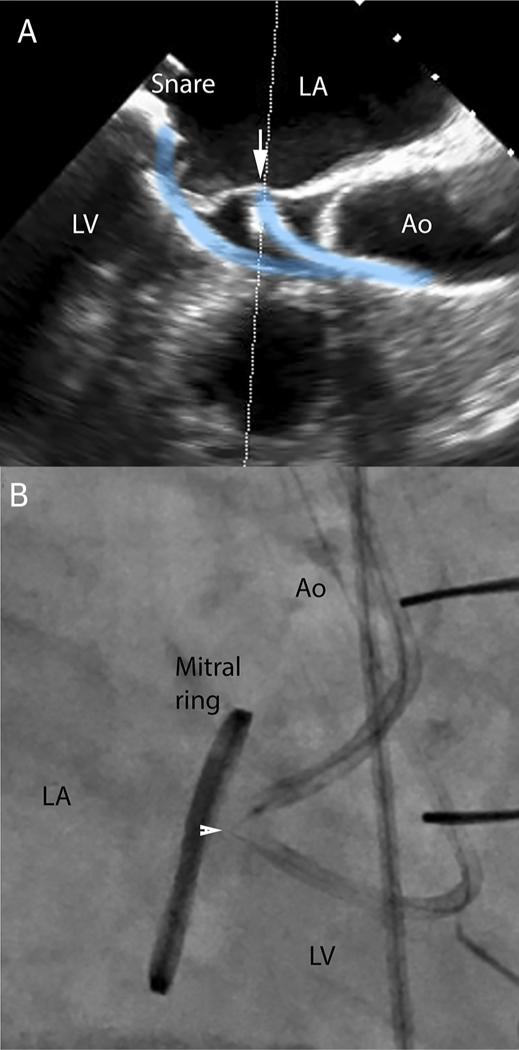
(A) Transesophageal echocardiography visualization of retro-grade LVOT catheter contacting the middle of the anterior mitral leaflet (arrow) and pointed toward a snare positioned by a second retrograde catheter in the left atrium. (B) The focally denuded lacerating guidewire straddles the anterior mitral leaflet (arrowhead) during electrification to achieve laceration (Online Video 1). Ao = Aorta; LA = left atrium; LV = left ventricle.
FIGURE 3. LAMPOON Outcomes.
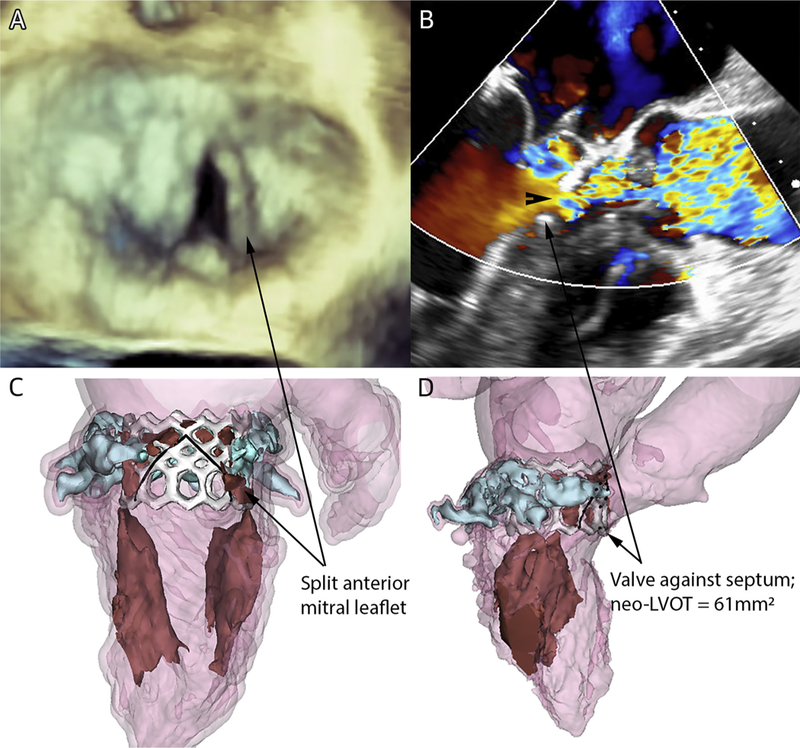
(A) Successful laceration of the anterior mitral leaflet before TMVR depicted on 3-dimensional transesophageal echocardiography (Online Video 2). (B) Color-flow across open cells of the Sapien 3 valve frame that without leaflet laceration would have completely obstructed the LVOT. The arrowhead shows the vena contracta, indicating the “leaflet lampooned” diameter; the arrow points to the edge of the valve abutting septum, indicating “leaflet intact” diameter. (C) A 3-dimensional computed tomography volume rendering after TMVR showing the split anterior mitral leaflet (black-outlined brown) splayed by the Sapien 3 valve frame (white) in the context of annular calcification (blue). (D) A 3-chamber perspective shows the valve frame contacted the interventricular septum across the LVOT (Online Video 3). Abbreviations as in Figure 1.
Immediately after laceration, transseptal TMVR is performed using a Sapien 3 balloon-expandable valve. During valve inflation, the 2 halves of the split anterior mitral leaflet parted to either side of the LVOT. Blood flows through the open cells of the Sapien 3 valve frame in the LVOT, which would have otherwise been obstructed by the anterior mitral leaflet (Figure 3). At completion, we recommended but did not require closure of the iatrogenic atrial septal defect.
STUDY ENDPOINTS.
Primary and key secondary endpoints were independently adjudicated. The primary endpoint was technical success on exit from the catheter laboratory for both LAMPOON and TMVR, which was defined as successful LAMPOON traversal and laceration, successful deployment and correct positioning of the first intended transcatheter heart valve, final LVOT gradient <30 mm Hg, freedom from emergency surgery or reintervention, and absence of procedural mortality.
Exploratory endpoints included in-hospital or 30-day death, stroke, life-threatening bleeding, major vascular complications, major cardiac structural complications, stage 2 or 3 acute kidney injury, myocardial infarction requiring intervention, severe hypotension requiring vasopressors or unplanned mechanical assist devices, ventilation ≥48 h, repeat surgery or intervention, proper device placement, device delivery failure, device structural failure, major atrial septal defect, coronary obstruction, tamponade, valve fracture, damage to native mitral structures, LVOT gradient ≥10 mm Hg from baseline, valve thrombosis, endocarditis, hemolysis, residual mitral regurgitation, mitral valve gradient ≥5 mm Hg, and paravalvular leak (PVL). Mitral Valve Academic Research Consortium definitions (21) were used to determine endpoint success. The complete list of endpoints is provided in the Online Appendix.
IMAGING.
Baseline and post-procedure contrast-enhanced, time-resolved CT used retrospective electrocardiogram-gated acquisitions. Images were reconstructed at 5% to 10% intervals with <1.0-mm slice thickness. Baseline and follow-up neo-LVOT and skirt neo-LVOT area measurements were performed using dedicated mitral valve CT analysis software (3mensio, PIE Medical, the Netherlands). The neo-LVOT area was measured in the last systolic phase with the aortic valve fully open (typically 30% phase). This was not the phase that gave the smallest neo-LVOT area (typically 40% to 50% phase), used in some observational studies (8,9). We noted that at these later phases, the aortic valve is partially or completely closed and the earlier phase selected for this study would better represent gradient across the LVOT after TMVR. A virtual valve with dimensions of the intended Sapien 3 implant was simulated in a 70:30 ventricle to atrium position across the mitral annulus. The smallest resulting cross-sectional area along the axis of the LVOT, circumscribed by the virtual valve and the interventricular septum, was measured to predict the neo-LVOT area (Figures 4A and 4B). The same was repeated by simulating only the fabric covered portion of the Sapien 3 valve for the “skirt” neo-LVOT, and excluding the ventricular open cells as described previously (20) (Figures 4C and 4D). This simulates the neo-LVOT that would result after TMVR and complete partition, or surgical excision, of the anterior mitral leaflet. Leaflet thrombosis was defined as leaflet thickening with reduced motion on follow-up CT or echocardiography.
FIGURE 4. Neo-LVOT and Skirt Neo-LVOT Areas.
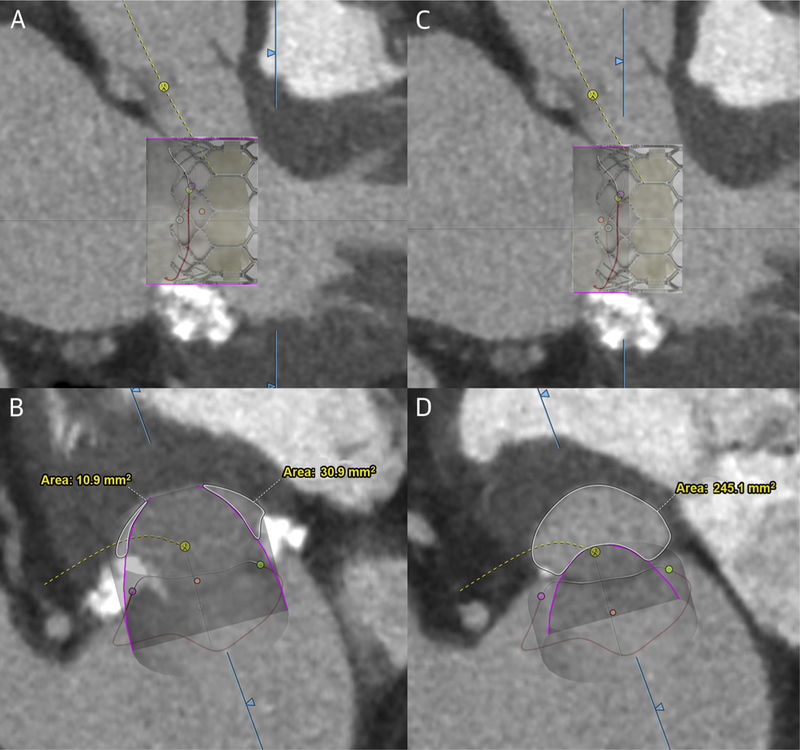
Illustration of computed tomography-based prediction of neo-LVOT (A and B) and skirt neo-LVOT (C and D) area using 3-chamber projections (top) and LVOT short-axis projections (bottom) and a simulated valve (purple outline). A Sapien 3 valve is superimposed, illustrating covered and uncovered sections of the valve frame. LVOT = left ventricular outflow tract.
Transthoracic or transesophageal echocardiograms were performed at baseline, intraprocedure, discharge, and 30 days. The neo-LVOT diameter on echocardiography was measured as the distance between the Sapien 3 stent frame and the interventricular septum in a 3-chamber view. This was called the “leaflet intact” LVOT diameter and approximated the neo-LVOT diameter assuming the anterior leaflet covered the Sapien 3 frame and ended at the valve edge. On color Doppler, the vena contracta through the neo-LVOT was measured in the same 3-chamber view (Figure 3B). This was called the “leaflet lam-pooned” LVOT diameter and approximated the physiological neo-LVOT diameter after LAMPOON. Echocardiograms and CT images were analyzed at central laboratories.
STATISTICAL ANALYSIS.
The sample size of 30 subjects was not statistically derived. Baseline subject and procedural characteristics were summarized as mean ± SD or median and interquartile range for continuous variables and counts and percentages for categorical variables. Wilcoxon signed-rank tests were used to assess the difference in the observed neo-LVOT areas before and after LAMPOON, and the differences in New York Heart Association functional class, 6-min walk distance, and Kansas City Cardiomyopathy Questionnaire quality-of-life summary scores between baseline and 30-day visits. Spearman rank tests assessed relationships between predicted neo-LVOT and skirt neo-LVOT and residual gradients.
Statistical analyses were performed using R statistical software version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
SUBJECT AND PROCEDURE CHARACTERISTICS.
Baseline subject characteristics are shown in Table 1. The majority were women and presenting late in their disease process, with end-organ failure and multiple comorbidities. All were at high or extreme risk for surgery. There were an equal number of subjects with mitral annuloplasty rings or bands and native MAC. The 29-mm Sapien 3 valves were implanted in 20 patients (67%), 26-mm in 7 (23%), and 23-mm in 3 (10%).
TABLE 1.
Baseline Subject Characteristics (n = 30)
| Age, yrs | 76 (47–89) |
| Female | 22 (73) |
| BSA, m2 | 1.8 ± 0.2 |
| End-organ failure | |
| Severe pulmonary disease | 8(27) |
| Severe pulmonary hypertension | 16 (55) |
| Home oxygen | 6 (20) |
| Severe RV dilation or dysfunction | 4(13) |
| End-stage kidney disease on dialysis | 4(13) |
| Liver cirrhosis | 4(13) |
| Comorbidities | |
| Diabetes | 17 (57) |
| Hypertension | 28 (93) |
| Peripheral artery disease | 4(13) |
| Coronary artery disease | 21 (70) |
| Prior myocardial infarction | 6 (20) |
| Prior stroke or TIA | 5(17) |
| Atrial fibrillation | 16 (53) |
| Prior endocarditis | 4(13) |
| Prior rheumatic fever | 1 (3) |
| Pacemaker or ICD | 9 (30) |
| ≥2 prior cardiac surgery | 9 (30) |
| NYHA functional class III or IV | 27 (90) |
| Frail | 14 (47) |
| STS predicted risk of mortality, % | 10.2 ± 6.2 |
| HAS-BLED score | 2.8 ± 0.9 |
| NT-proBNP, pg/ml | 515 [282–850] |
| Medication | |
| Dual antiplatelet baseline | 11 (37) |
| Dual antiplatelet discharge | 2(7) |
| Oral anticoagulant agent baseline | 10 (33) |
| Oral anticoagulant agent discharge | 26 (93) |
| TMVR setting | |
| Complete ring | 13 (43) |
| Incomplete ring/band | 2(7) |
| MAC | 15 (50) |
| Primarily mitral stenosis | 20 (67) |
| Primarily mitral regurgitation | 10 (33) |
| Mean mitral valve gradient, mm Hg | 9.6 ± 4.3 |
| Severe mitral regurgitation (ASE guideline integrated analysis) | 11 (37) |
| CT annular measurements | |
| Annular area | 551.3 ± 141.2 |
| Antero-posterior distance | 30.3 ± 3.9 |
| Intercommissural distance | 21.5 ± 3.6 |
Values are median (range), n (%), mean ± SD, or median [interquartile range].
ASE = American Society of Echocardiography; BSA = body surface area; CT = computed tomography; ICD = implantable cardioverter-defibrillator; MAC = mitral annular calcification; NT-proBNP = N-terminal pro-B-type natriuretic peptide; NYHA = New York Heart Association; STS = Society of Thoracic Surgeons; TIA = transient ischemic attack; TMVR = transcatheter mitral valve replacement.
Table 2 details the LVOT obstruction risk for enrolled subjects. A total of 25 (83%) were at risk of fixed LVOT obstruction, defined as having a predicted neo-LVOT area <200 mm2 (mean 81 mm2). The majority (88%) had extremely small predicted neo-LVOT area (<150 mm2). A total of 5 (17%) subjects were at risk of dynamic LVOT obstruction, with anterior mitral leaflet length >24 mm (mean 28 mm). One subject had prior prophylactic alcohol septal ablation, but still had a predicted neo-LVOT area of 25 mm2 and was enrolled in the study.
TABLE 2.
LVOT Obstruction Risk
| Aorto-mitral angle,° | 117.3 ± 9.8 |
| Septal thickness, mm | 12.9 ± 3.6 |
| Fixed LVOT obstruction subset | 25 |
| Predicted neo-LVOT, mm2 | 81 ± 51 |
| Neo-LVOT <200 mm2 | 25 (100) |
| Neo-LVOT <150 mm2 | 22 (88) |
| Neo-LVOT <100 mm2 | 15 (60) |
| Neo-LVOT <50 mm2 | 7(28) |
| Predicted skirt neo-LVOT, mm2 | 230 ± 56 |
| Skirt neo-LVOT <200 mm2 | 7(28) |
| Skirt neo-LVOT <150 mm2 | 2(8) |
| Skirt neo-LVOT <100 mm2 | 0(0) |
| Dynamic LVOT obstruction subset | 5 |
| Anterior mitral leaflet length on CT, mm | 28 ± 3.1 |
| Systolic anterior motion at baseline | 1 (20) |
Values are mean ± SD, n, or n (%).
CT = computed tomography; LVOT = left ventricular outflow tract.
PROCEDURE OUTCOMES.
Table 3 shows the adjudicated primary and key secondary endpoints. The LAMPOON traversal and laceration was successful in all 30 (100%) subjects, including in subjects with heavily calcified anterior mitral leaflets, and where laceration trajectory was through a complete anterior calcium bridge. Full leaflet laceration was not achieved in 1 subject due to insufficiently basal traversal. All (100%) survived the immediate procedure and 28 (93%) survived to discharge and 30 days (100% for valve-in-ring; 87% for valve-in-MAC). The LVOT gradient immediately after TMVR was <30 mm Hg in 27 patients (90%), and on exit from the catheter laboratory was <30 mm Hg in 29 patients (97%; “optimal”) and <50 mm Hg in 30 patients (100%; “acceptable”). A total of 8 patients (27%) required further intervention before exiting the catheter laboratory: 4 had alcohol septal ablation to decrease LVOT gradients; 2 had second valve implantations for PVL and displacement during interatrial septum traversal, respectively; 1 had on-table conversion to surgery for severe PVL; and 1 had percutaneous closure for PVL through a dehisced mitral ring. The primary endpoint of success was met in the remaining 22 (73%) subjects.
TABLE 3.
Adjudicated Clinical Outcomes
| Primary endpoint (exit from catheter laboratory) | 30 |
| Successful LAMPOON traversal and laceration | 30 (100) |
| Successful access, delivery, and retrieval of LAMPOON device system | 30 (100) |
| Successful deployment of first TMVR valve | 27 (90) |
| LVOT gradient <30 mm Hg (“optimal”) | 29 (97) |
| LVOT gradient <50 mm Hg (“acceptable”) | 30 (100) |
| Freedom from emergency surgery or reintervention related to LAMPOON or TMVR | 22 (73) |
| Procedure survival | 30 (100) |
| Procedure success (all of the above) | 22 (73) |
| Secondary endpoints (30 days) | 30 |
| All death | 2(7) |
| Related to LAMPOON | 0(0) |
| Related to TMVR but not LAMPOON | 1 (3) |
| Not related to TMVR or LAMPOON | 1 (3) |
| All stroke | 0(0) |
| Life-threatening bleeding | 3(10) |
| Major vascular complication | 6 (20) |
| Major structural complication | 0(0) |
| AKI stage 2/3 | 3(10) |
| Myocardial infarction requiring PCI or CABG | 0(0) |
| Surgery or repeat intervention | 8(27) |
| Hypotension requiring increase in vasopressors or mechanical support | 12 (40) |
| Respiratory failure requiring prolonged (>48 h) intubation | 1 (3) |
| Valve position failure | 1 (3) |
| Valve structural failure | 0(0) |
| Major ASD | 0(0) |
| Coronary obstruction | 0(0) |
| Cardiac tamponade or pericardial drainage | 1 (3) |
| Damage to native mitral apparatus | 0(0) |
| LVOT ≥10 mm Hg from baseline | 2(7) |
| Aortic regurgitation 2+ or more | 0(0) |
| Valve thrombosis | 4(13) |
| Endocarditis | 0(0) |
| Hemolysis | 12 (40) |
| MR more than mild | 1 (3) |
| MVG ≥5 mm Hg | 18 (60) |
| PVL more than mild | 7(23) |
Values are n or n (%).
AKI = acute kidney injury; ASD = atrial septal defect; CABG = coronary artery bypass graft; LAMPOON = intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow obstruction; LVOT = leftventricularoutflow tract; MR = mitral regurgitation; MVG = mitral valve gradient; PCI = percutaneous coronary intervention; PVL = paravalvular leak; TMVR = transcatheter mitral valve replacement.
There were 2 post-procedural deaths. One was attributed to clostridium difficile colitis and toxic megacolon following clindamycin for dental extraction, despite a technically successful procedure. The other was a failed TMVR despite successful LAMPOON, requiring surgical conversion for PVL, with a stormy post-operative course, and death on day 38.
There were no strokes. Cerebral protection (Sentinel, Claret Medical, Santa Rosa, California) was used in 1 subject who had left atrial appendage thrombus at baseline; no debris was retrieved.
Planned intra-aortic balloon pump support was used in 43%, when the transcatheter valve was not pre-positioned in the left atrium. Table 4 shows echocardiogram and invasive hemodynamic changes with LAMPOON-TMVR. Hypotension requiring increase in vasopressor support during the procedure and recovery was common (n = 12; 40%), but only 6 subjects (20%) had hypotension during LAMPOON catheter manipulation and laceration. Unplanned mechanical support was required in 1 subject (3%) after the Sapien 3 skirt caused LVOT obstruction. Per-subject procedural hemodynamics are reported in Online Figure 1.
TABLE 4.
Hemodynamics
| Echocardiography | Baseline | Pre-Discharge | 30-Day |
|---|---|---|---|
| Left ventricular end-diastolic volume, ml | 65.8 ± 29.6 | 65.0 ± 27.6 | 68.8 ± 32.4 |
| Left ventricular end-systolic volume, ml | 26.5 ± 15.9 | 28.8 ± 19.8 | 33.6 ± 25.7 |
| LVEF, % | 60.8 ± 9.7 | 56.6 ± 14.8 | 55.0 ± 15.2 |
| Left atrium volume index | 53.0 ± 16.5 | 53.4 ± 25.2 | 52.2 ± 18.1 |
| LVOT peak velocity, m/s | 1.1 ± 0.3 | 1.5 ± 0.5 | 1.4 ± 0.6 |
| LVOT peak gradient, mm Hg | 5.7 ± 4.8 | 9.8 ± 7.2 | 9.2 ± 7.3 |
| LVOT mean gradient. Mm Hg | 2.4 ± 1.4 | 4.8 ± 4.0 | 4.4 ± 3.9 |
| LVOT VTI, m | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Mean transmitral gradient, mm Hg | 9.6 ± 4.3 | 5.6 ± 2.1 | 6.6 ± 2.3 |
| Mitral regurgitation severity | |||
| None | 0(0) | 5(17) | 5(19) |
| Trace | 6(21) | 10 (34) | 8(30) |
| Mild | 6(21) | 13 (45) | 12 (44) |
| Moderate | 6(21) | 1 (3) | 0(0) |
| Severe | 11 (38) | 0 (0) | 2(7) |
| Perivalvular regurgitation | |||
| None | – | 16 (59) | 17 (63) |
| Trace | – | 3(11) | 4(15) |
| Mild | – | 8 (30) | 5(19) |
| Moderate | – | 0 (0) | 0(0) |
| Severe | – | 0 (0) | 1 (4) |
| Cath Hemodynamics | Baseline | Post-LAMPOON | Post-TMVR |
| Heart rate, beats/min | 69.6 ± 10.8 | 76.4 ± 15.5 | 73.4 ± 10.5 |
| Systolic arterial pressure, mm Hg | 123.4 ± 22.4 | 96.2 ± 20.5 | 23.3 ± 19.0 |
| Diastolic arterial pressure, mm Hg | 61.5 ± 12.0 | 51.4 ± 11.6 | 57.4 ± 15.6 |
| Mean arterial pressure, mm Hg | 85.0 ± 14.7 | 65.8 ± 14.7 | 82.7 ± 14.3 |
| Right atrial pressure, mm Hg | 13.9 ± 6.1 | – | 13.1 ± 6.3 |
| Systolic pulmonary artery pressure, mm Hg | 56.8 ± 18.5 | – | 52.8 ± 17.0 |
| Diastolic pulmonary artery pressure, mm Hg | 24.8 ± 8.3 | – | 22.6 ± 6.8 |
| Mean pulmonary artery pressure, mm Hg | 38.7 ± 11.2 | – | 34.4 ± 9.2 |
| Mean left atrial pressure, mm Hg | 26.7 ± 11.0 | – | 18.1 ± 4.7 |
| Left atrial v-wave, mm Hg | 45.2 ± 16.8 | – | 25.4 ± 8.6 |
| Mean transmitral gradient, mm Hg | 8.2 ± 4.8 | – | 2.8 ± 1.6 |
| Cardiac output, l/min | 5.1 ± 1.7 | – | 5.4 ± 1.5 |
| Left ventricular end-diastolic pressure, mm Hg | 18.3 ± 6.0 | – | 17.9 ± 5.0 |
| LVOT peak-to-peak gradient, mm Hg | 7.6 ± 6.0 | 17.7 ± 23.4 | 10.7 ± 9.3 |
| Calculated mitral valve area, cm2 | 1.5 ± 0.7 | – | 2.8 ± 0.9 |
Values are mean ± SD or n (%).
LVEF = left ventricular ejection fraction; LVOT = left ventricular outflow tract; VTI = velocity time integral
LVOT OBSTRUCTION PREDICTION AND OUTCOMES.
The predicted neo-LVOT simulated on pre-procedural CT correlated well with the observed neo-LVOT measured on post-procedural CT (Pearson r = 0.95) (Online Figure 2).
Among subjects who were at high predicted risk of LVOT obstruction with standalone TMVR, 29 (97%) exited the catheter laboratory with an LVOT gradient <30 mm Hg, and 28 (93%) had an LVOT gradient of within 10 mm Hg of their baseline on 30-day echocardiography follow-up. Both subjects who failed to meet these endpoints had a skirt neo-LVOT area <150 mm2 and were enrolled before the protocol was amended to require an adequate skirt neo-LVOT.
A total of 3 of the first 5 subjects had LVOT obstruction (gradient ≥30 mm Hg) immediately after TMVR and underwent alcohol septal ablation. Of these, 2 had a skirt neo-LVOT <150 mm2, and 1 had a heavily calcified anterior leaflet patch after prior repair for subacute endocarditis combined with a skirt neo-LVOT of 183 mm2. Thereafter, the protocol was amended to exclude subjects with a skirt neo- LVOT <150 mm2, with no further cases of LVOT obstruction. A fourth subject had an insufficiently basal anterior mitral leaflet laceration, likely limiting leaflet splay after laceration, with an LVOT gradient of 29 mm Hg; she also underwent alcohol septal ablation.
Figure 5 shows post-TMVR LVOT gradient by catheterization in relation to predicted neo-LVOT area and to predicted skirt neo-LVOT area. The majority had a predicted neo-LVOT area of <150 mm2 but did not develop LVOT obstruction after LAMPOON TMVR. There was a nonsignificant trend toward high LVOT gradient among subjects with lowest neo-LVOT and with lowest skirt neo-LVOT.
FIGURE 5. Predicted Neo-LVOT, Skirt Neo-LVOT, and Observed Gradients.
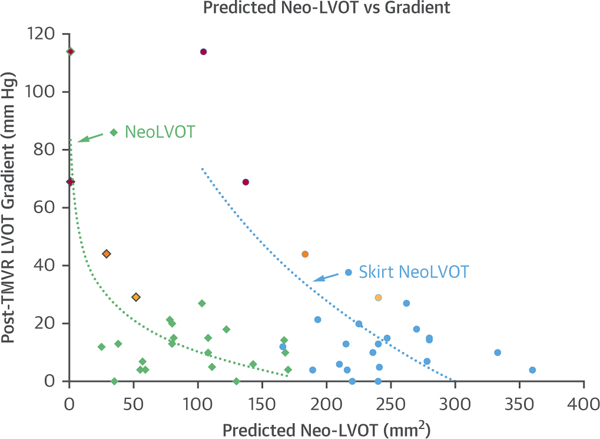
LVOT catheter gradient after TMVR is compared with predicted neo-LVOT area (diamonds) and predicted skirt neo-LVOT area (circles). Red, orange, and yellow symbols indicate subjects who underwent bailout alcohol septal ablation. Red indicates subjects with inadequate skirt neo-LVOT. Orange indicates a subject with calcific anterior mitral leaflet patch and inadequate skirt neo-LVOT. Yellow indicates a subject with insufficiently basal LAMPOON. Abbreviations as in Figure 1.
LAMPOON increased the observed neo-LVOT area. The “leaflet intact” LVOT diameter on echocardiography at discharge was 0.4 ± 0.3 cm, whereas the “leaflet lampooned” LVOT diameter was 0.8 ± 0.3 cm (p < 0.01). On post-procedural CT, the skirt neo-LVOT was 150 mm2 greater than the neo-LVOT (p < 0.001). There was no systolic anterior motion or leaflet in-folding causing TMVR dysfunction in the subjects with long anterior mitral leaflet.
PARAVALVULAR LEAK AND HEMOLYSIS.
A total of 7 subjects (23%) developed greater-than-mild para-valvular leak after first valve deployment (3 valve-in-MAC; 4 valve-in-ring). Details and treatment are provided in Table 5. One subject (4%) had greater-than-mild PVL at 30 days.
TABLE 5.
Paravalvular Leak
| PVL Mechanism | MAC | Ring or Band |
|---|---|---|
| High deployment | 1 (severe), converted to surgery | 2, repeat TMVR-in-TMVR (1 immediate, 1 day 3) |
| Ring dehiscence | – | 2, exacerbated from baseline, treated percutaneously with occluders |
| Malapposition | 2 (moderate), treated conservatively | 0 |
In total, 12 subjects (40%) developed hemolysis, 7 (23%) clinical requiring blood transfusion and 5 (17%) subclinical. Hemolysis was associated with trace (n = 4), mild (n = 7), and moderate (n = 1) para-valvular leak. No subject developed hemolysis in the absence of paravalvular leak.
OTHER CLINICAL ENDPOINTS.
Subjects improved in New York Heart Association functional class and Kansas City Cardiomyopathy Questionnaire (22) Clinical and Overall Summary scores, but not 6-min walking distance (Figure 6). N-terminal pro-B-type natriuretic peptide did not improve from baseline to 30 days (515 pg/ml [first and third quartiles: 282 to 850 pg/ml] to 736 pg/ml [first and third quartiles: 318 to 1,427 pg/ml]). A total of 18 subjects (60%) had mean mitral valve gradient ≥5 mm Hg, of whom 1 (4%) had a mitral gradient ≥10 mm Hg on 30-day echocardiography. Leaflet thrombosis was seen in 4 subjects (13%) and resolved with anticoagulation. A total of 6 subjects (20%) had major vascular complications: 5 with groin hematomas and 1 with retroperitoneal hematoma. Iatrogenic atrial septal defects were electively closed during the index procedure in 25 (83%). All subjects had general anesthesia, and 22 (73%) were extubated in the catheter laboratory. Total procedure-to-hemostasis time was 195 ± 77 min; LAMPOON-to-TMVR time was 22 ± 27 min; fluoroscopy time was 120 ± 53 min; and contrast volume used was 47 ± 48 ml. Baseline and procedure characteristics and outcomes are stratified by subjects with mitral stenosis and mitral regurgitation in Online Tables 1 and 2.
FIGURE 6. Quality of Life and Function.
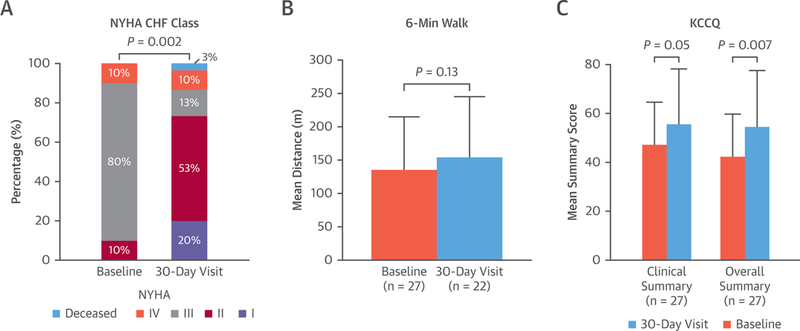
Changes before and 30 days after LAMPOON TMVR in New York Heart Association (NYHA) functional class (p = 0.004), 6-min walk test (nonsignificant p = 0.15 based on n = 20 nonmissing pairs), and Kansas City Cardiomyopathy Questionnaire (Kansas City Cardiomyopathy Questionnaire)-15 Overall (p = 0.007) and Clinical Summary (p = 0.050) scores. Abbreviations as in Figure 1.
DISCUSSION
The LAMPOON IDE study enrolled 30 subjects at prohibitive risk of LVOT obstruction equally between valve-in-ring and valve-in-MAC arms. None were eligible for standalone TMVR and most were deemed ineligible for any other therapy. Most would have been excluded from contemporaneous cohort and registry reports of TMVR for valve-in-ring and valve-in-MAC because of the baseline risk of LVOT obstruction (3,4,23,24). The main findings were that LAMPOON was feasible in 100% of subjects across a variety of native and annuloplasty ring anatomies and calcium patterns. There was 100% procedure survival and 93% in-hospital and 30-day survival (100% valve-in-ring, 87% valve-in-MAC) in a very sick cohort, many presenting with end-organ failure, in whom outcomes have historically been very poor. There were no neurological events (Central Illustration).
CENTRAL ILLUSTRATION. Anterior Leaflet Laceration to Prevent Ventricular Outflow Tract Obstruction During Transcatheter Mitral Valve Replacement.
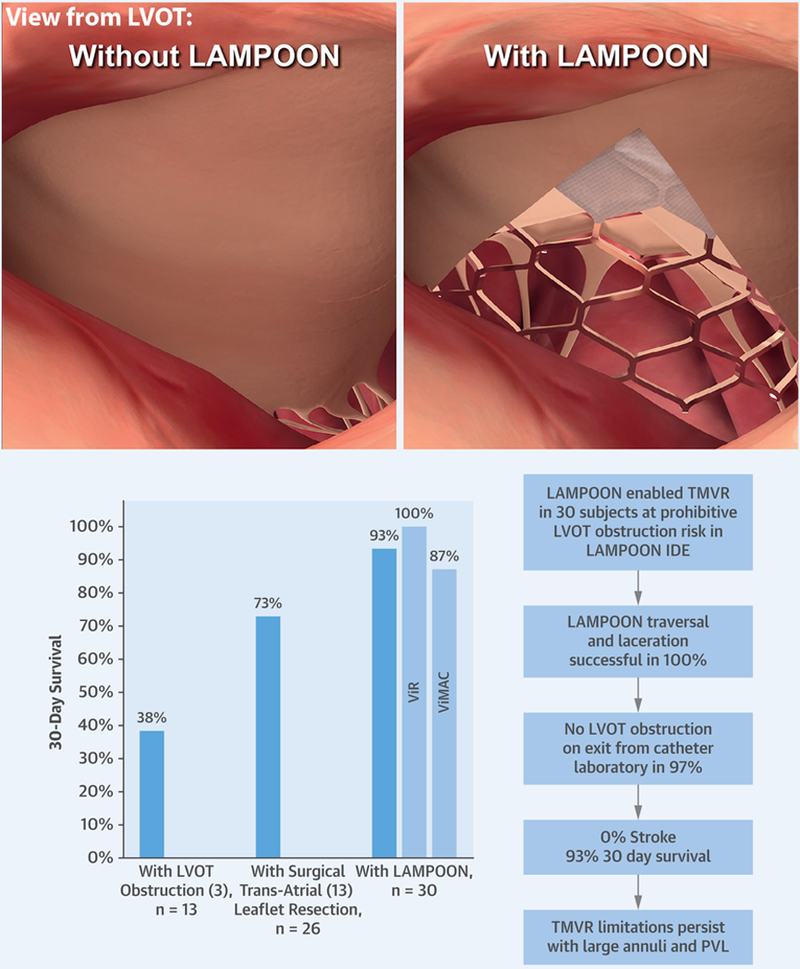
(Top) View from left ventricular outflow tract with and without intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow obstruction. (Bottom) Summary trial results. LAMPOON = intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow obstruction; LVOT = left ventricular outflow tract; PVL = paravalvular leak; TMVR = transcatheter mitral valve replacement.
THE LAMPOON TECHNIQUE.
The LAMPOON technique, although technically challenging, was successfully performed in all sites with proctorship and without roll-in procedures for new sites. The technique cannot be used with the transfemoral approach in subjects with mechanical aortic valves. Furthermore, LAMPOON, or other anterior leaflet modification or resection strategies, is unlikely to prevent LVOT obstruction when there is a small predicted skirt neo-LVOT area. Extent and pattern of anterior mitral leaflet calcification was not a contra-indication to LAMPOON. There was no collateral damage to adjacent structures, particularly the aortic valve, from LAMPOON.
One safety consideration raised by this study is LVOT obstruction despite LAMPOON. The 3 cases of high LVOT gradients after TMVR highlight the value of appropriate screening, especially for skirt neo-LVOT area in extremely small ventricles.
A second safety consideration is transient procedural hypotension. Leaflet manipulation and laceration was associated with increased vasopressor support in 20% of subjects. There was a high rate of planned intra-aortic balloon pump use as, with increasing comfort with the technique, operators stopped pre-positioning the Sapien 3 valve before laceration, increasing time from laceration to valve deployment. Emergency mechanical support was required in only 1 subject to relieve severe LVOT obstruction. Reasons for the relative hemodynamic stability observed with LAMPOON procedures may be that the lacerated anterior leaflet still coapts in systole, and that patients with chronic mitral disease may tolerate a further acute exacerbation in the short period between LAMPOON and TMVR.
TRANSCATHETER MITRAL VALVE REPLACEMENT.
TMVR in the setting of annuloplasty rings and in native MAC has previously been demonstrated to be feasible but with problems, particularly LVOT obstruction and PVL. The 30-day survival in carefully selected patients screened out for LVOT obstruction in the MITRAL trial was 93% for valve-in-ring and 81% for valve-in-MAC (4,5). The 30-day survival in the TMVR Registry was 90% for valve-in-ring and 65% for valve-in-MAC (2). In patients who developed LVOT obstruction, who likely represent the cohort enrolled in this study, 30-day survival was only 48% and 1-year survival was 15% (3).
Although LAMPOON was largely technically successful, there were important safety considerations for TMVR not related to LAMPOON, namely clinically significant PVL and hemolysis, and high residual transmitral gradients. Large annuli (9% screen failure rate) and insufficient mitral calcification (14% screen failure rate) remain important exclusion criteria for TMVR using aortic valves.
Moderate or severe PVL occurred in 23% after first valve deployment, and the majority required further intervention. Even trace PVL was associated with hemolysis. It is possible that blood flow through the open valve cells in the LVOT caused additional hemolysis, but no patient developed hemolysis in the absence of PVL, and so we were unable to test this hypothesis.
High transmitral valve gradients were seen in this study. Other groups reporting similar rates (82.4% [24]) have used a threshold of mean mitral valve gradient <10 mm Hg when assessing success of mitral valve-in-valve, valve-in-ring, and valve-in-MAC (23,24) based on American College of Echocardiography guidelines (25) and guidelines for transcatheter aortic valve failure (26). In a study comparing surgical mitral valve replacement with TMVR, mean mitral valve gradients at 30 days were similar (6.5 ± 2.5 mm Hg surgery vs. 7.1 ± 2.5 mm Hg TMVR; p = 0.42) (27), and compares with mean gradients in this study (6.6 ± 2.3 mm Hg). However, studies with dedicated TMVR devices have reported lower transmitral gradients (28,29), reflecting a shortcoming of using transcatheter aortic valve devices in the mitral position.
Rates of leaflet thrombosis were similar to reported case series (30), suggesting the value of anti-coagulation in these patients and the heightened risk of thrombosis in transcatheter mitral valve replacement (31).
STUDY LIMITATIONS.
CT-predicted neo-LVOT area measurements are not standardized, and there is variation between centers in choice of cardiac phase, virtual valve depth, and orientation. Observational registries support a neo-LVOT cut-off of 170 mm2 when a later cardiac phase is used (9) and 190 mm2 when a more ventricular implantation depth is used (8). The confidence intervals around these measurements remain broad. Therefore, using the methodology in this study, 200 mm2 appears to be an acceptable cut-off for high risk of LVOT obstruction. The CT-predicted neo-LVOT area, although an advance over previous linear measurements, remains a 2-dimensional assessment of a 3-dimensional structure that varies during the cardiac cycle. Computational fluid dynamics models accounting for this time-varying structure as well as variations in flow and behavior of the anterior mitral leaflet will help better predict risk of LVOT obstruction (32–34). The 5 subjects enrolled were deemed at high risk of LVOT obstruction from an excessively long anterior mitral leaflet, but it is possible that these subjects may not have developed systolic anterior motion of the mitral leaflet after TMVR. The problem of the long anterior mitral leaflet needs to be further studied, both in causing LVOT obstruction as well as in-folding interfering with transcatheter heart valve function (10,12).
Given the small sample size, no meaningful comparisons could be made between several procedure parameters and their effect on LVOT gradient, particularly nonbasal or eccentric lacerations, surgically implanted neo-chordae, anterior mitral leaflet calcium volume and pattern, and degree of antero-posterior valve oversizing. The merits of closing the atrial septal defect were not tested in this study.
There was no control group in this study because the subjects enrolled were not eligible for standalone TMVR due to risk of life-threatening LVOT obstruction. No subject had severe left ventricular dysfunction, perhaps reflecting the low likelihood of these patients developing LVOT obstruction from TMVR due to increased ventricular size, and so the safety of LAMPOON in this cohort was not tested.
We await 1-year outcomes to assess long-term safety. Successful patient outcomes depended on both successful LAMPOON and successful transseptal TMVR using an aortic transcatheter valve off-label. The results of this study should be applied with caution when combining LAMPOON with other transcatheter valves. The lessons learned on cautions and contraindications to LAMPOON and to TMVR are summarized in Online Table 3.
FUTURE DIRECTIONS.
Dedicated TMVR devices have attempted several valve designs to reduce the risk of LVOT obstruction (1). However, LVOT obstruction remains a leading reason to exclude candidates for investigational TMVR devices to treat severe native mitral regurgitation (22% excluded for LVOT obstruction in the Intrepid TMVR device early feasibility study [29]; 21% excluded for anatomical reasons in general in the Tendyne TMVR device early feasibility study [35]). LAMPOON has been successfully used with a Tendyne valve (Abbott, Chicago, Illinois) (11) at risk of dynamic LVOT obstruction from a long anterior leaflet and acute aortomitral angulation. The utility of LAMPOON with other novel TMVR devices remains to be tested. LAMPOON has also been used to treat LVOT obstruction from systolic anterior motion of the native mitral valve leaflet after TMVR (10). Dedicated devices for LAMPOON, TMVR, and increased experience should elevate this procedure to an acceptable therapy for high-risk patients with mitral valve dysfunction.
LAMPOON is an entirely percutaneous technique and is the first endovascular procedure to our knowledge to create a controlled cut in cardiac tissue. The technique can be adapted and has been used in other settings, such as to free the anterior mitral leaflet from an Alfieri stitch prior to TMVR (36) and to prevent coronary obstruction from transcatheter aortic valve replacement (bioprosthetic or native aortic scallop intentional laceration to prevent iatrogenic coronary artery obstruction) (37).
The LAMPOON IDE study corroborates findings from the first-in-human series (19), and provides further support for percutaneous leaflet laceration enabling TMVR in very sick patients at risk of LVOT obstruction. Longer-term follow-up and future larger studies will be further helpful in assessing safety and efficacy in the long term.
CONCLUSIONS
In selected cases, LAMPOON overcomes the most common contraindication to TMVR for valve-in-ring or valve-in-MAC, which is risk of LVOT obstruction. This cohort study demonstrates that LAMPOON is technically feasible in a variety of native and annuloplasty ring morphologies. LAMPOON enabled TMVR in patients deemed otherwise ineligible for therapy. Candidates with a low skirt neo-LVOT do not benefit from LAMPOON and remain contraindicated. LAMPOON exhibits an acceptable safety profile and did not cause death or stroke.
TMVR in annuloplasty rings and MAC remains challenging, with high complication rates, especially related to large mitral annuli and residual para-valvular leak. Efficacy and functional improvement with TMVR in these subgroups needs to be assessed with longer-term follow-up, in larger studies, and in less critically ill subjects.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: LVOT obstruction is a common complication of mitral valve replacement that is associated with a high risk of fatality. Similar to chord-sparing leaflet resection in patients undergoing mitral valve surgery, deliberate laceration of the anterior mitral leaflet achieved with the LAMPOON technique can prevent LVOT obstruction and improve clinical outcomes in patients undergoing TMVR.
TRANSLATIONAL OUTLOOK: Larger studies with longer-term follow-up are necessary to confirm the safety and efficacy of the LAMPOON approach in patients undergoing TMVR.
Acknowledgments
This work was supported by the Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health (Z01-HL006040–7), and by the intramural programs of the participating centers. The National Heart, Lung, and Blood Institute and Edwards Lifesciences have a collaborative research and development agreement on transcatheter modification of mitral valve leaflets, including joint financial support of this study. Drs. Khan, Rogers, and Lederman are co-inventors on patents, assigned to the National Institutes of Health, on catheter devices to lacerate valve leaflets. Dr. Babaliaros has served as a consultant for Edwards Lifesciences and Abbott Vascular; and his employer has research contracts for clinical investigation of transcatheter aortic and mitral devices from Edwards Lifesciences, Abbott Vascular, Medtronic, St. Jude Medical, and Boston Scientific. Dr. Greenbaum has served as a proctor for Edwards Lifesciences, Medtronic, and Abbott Vascular; and has served as a consultant and a scientific advisor for and is an equity holder in Transmural Systems. Drs. Foerst and Rogers have served as consultants/proctors for Edwards Lifesciences and Medtronic. Dr. Yazdani, Paone, and Eng have served as proctors for Edwards Lifesciences. Dr. Leshnower has served on the Speakers Bureau for Medtronic. Dr. Wang has served as a consultant for Edwards Lifesciences, Boston Scientific, and Materialise. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose
ABBREVIAT IONS AND ACRONYMS
- LAMPOON
intentional laceration of the anterior mitral leaflet to prevent left ventricular outflow obstruction
- LVOT
left ventricular outflow tract
- MAC
mitral annular calcification
- NHLBI
National Heart Lung and Blood Institute
- PVL
paravalvular leak
- TMVR
transcatheter mitral valve replacement
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
APPENDIX For an expanded Methods section as well as supplemental tables, figures, and videos, please see the online version of this paper.
 Listen to this manuscript’s audio summary by Editor-in-Chief Dr. Valentin Fuster on JACC.org.
Listen to this manuscript’s audio summary by Editor-in-Chief Dr. Valentin Fuster on JACC.org.
REFERENCES
- 1.Regueiro A, Granada JF, Dagenais F, Rodes-Cabau J. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol 2017;69:2175–92. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SH, Whisenant BK, Bleiziffer S, et al. Outcomes of transcatheter mitral valve replacement for degenerated bioprostheses, failed annuloplasty rings, and mitral annular calcification. Eur Heart J 2019;40:441–51. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero M, Urena M, Himbert D, et al. 1-year outcomes of transcatheter mitral valve replacement in patients with severe mitral annular calcification. J Am Coll Cardiol 2018;71:1841–53. [DOI] [PubMed] [Google Scholar]
- 4.Guerrero ME. MITRAL (Mitral Implantation of Transcatheter Valves): 30-day outcomes of transcatheter mitral valve replacement in patients with severe mitral valve disease secondary to mitral annular calcification or failed annuloplasty rings. Paper presented at: Transcatheter Therapeutics; Denver, Colorado; November 1, 2017. [Google Scholar]
- 5.Guerrero ME. Mitral Implantation of TRAnscatheter vaLves (MITRAL). 2015. Available at: https://clinicaltrials.gov/ct2/show/NCT02370511. Accessed April 13, 2019.
- 6.Blanke P, Naoum C, Dvir D, et al. Predicting LVOT obstruction in transcatheter mitral valve implantation: concept of the neo-LVOT. J Am Coll Cardiol Img 2017;10:482–5. [DOI] [PubMed] [Google Scholar]
- 7.Bapat V, Pirone F, Kapetanakis S, Rajani R, Niederer S. Factors influencing left ventricular outflow tract obstruction following a mitral valve-in-valve or valve-in-ring procedure, part 1. Catheter Cardiovasc Interv 2015;86:747–60. [DOI] [PubMed] [Google Scholar]
- 8.Wang DD, Eng MH, Greenbaum AB, et al. Validating a prediction modeling tool for left ventricular outflow tract (LVOT) obstruction after transcatheter mitral valve replacement (TMVR). Catheter Cardiovasc Interv 2018;92:379–87. [DOI] [PubMed] [Google Scholar]
- 9.Yoon SH, Bleiziffer S, Latib A, et al. Predictors of left ventricular outflow tract obstruction after transcatheter mitral valve replacement. J Am Coll Cardiol Intv 2019;12:182–93. [DOI] [PubMed] [Google Scholar]
- 10.Khan JM, Trivedi U, Gomes A, Lederman RJ, Hildick-Smith D. ‘Rescue’ LAMPOON to treat transcatheter mitral valve replacement-associated left ventricular outflow tract obstruction. J Am Coll Cardiol Intv 2019. February 7 [E-pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan JM, Lederman RJ, Devireddy CM, et al. LAMPOON to facilitate Tendyne transcatheter mitral valve replacement. J Am Coll Cardiol Intv 2018;11:2014–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenbaum AB, Condado JF, Eng M, et al. Long or redundant leaflet complicating transcatheter mitral valve replacement: case vignettes that advocate for removal or reduction of the anterior mitral leaflet. Catheter Cardiovasc Interv 2018;92: 627–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Praz F, Khalique OK, Lee R, et al. Transatrial implantation of a transcatheter heart valve for severe mitral annular calcification. J Thorac Cardiovasc Surg 2018;156:132–42. [DOI] [PubMed] [Google Scholar]
- 14.Deharo P, Urena M, Himbert D, et al. Bail-out alcohol septal ablation for left ventricular outflow tract obstruction after transcatheter mitral valve replacement. J Am Coll Cardiol Intv 2016;9:e73–6. [DOI] [PubMed] [Google Scholar]
- 15.Guerrero M, Wang DD, O’Neill W. Percutaneous alcohol septal ablation to acutely reduce left ventricular outflow tract obstruction induced by transcatheter mitral valve replacement. Catheter Cardiovasc Interv 2016;88:E191–7. [DOI] [PubMed] [Google Scholar]
- 16.Urena M, Himbert D, Brochet E, et al. Transseptal transcatheter mitral valve replacement using balloon-expandable transcatheter heart valves: a step-by-step approach. J Am Coll Cardiol Intv 2017;10:1905–19. [DOI] [PubMed] [Google Scholar]
- 17.David TE. Mitral valve replacement with preservation of chordae tendinae: rationale and technical considerations. Ann Thorac Surg 1986;41: 680–2. [DOI] [PubMed] [Google Scholar]
- 18.Khan JM, Rogers T, Schenke WH, et al. Intentional laceration of the anterior mitral valve leaflet to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: pre-clinical findings. J Am Coll Cardiol Intv 2016;9:1835–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babaliaros VC, Greenbaum AB, Khan JM, et al. Intentional percutaneous laceration of the anterior mitral leaflet to prevent outflow obstruction during transcatheter mitral valve replacement: first-in-human experience. J Am Coll Cardiol Intv 2017;10:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan JM, Rogers T, Babaliaros VC, Fusari M, Greenbaum AB, Lederman RJ. Predicting left ventricular outflow tract obstruction despite anterior mitral leaflet resection: the “Skirt Neo- LVOT”. J Am Coll Cardiol Img 2018;11:1356–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone GW, Adams DH, Abraham WT, et al. Clinical trial design principles and endpoint definitions for transcatheter mitral valve repair and replacement: part 2: endpoint definitions: a consensus document from the Mitral Valve Academic Research Consortium. J Am Coll Cardiol 2015;66:308–21. [DOI] [PubMed] [Google Scholar]
- 22.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol 2000;35:1245–55. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SH, Whisenant BK, Bleiziffer S, et al. Transcatheter mitral valve replacement for degenerated bioprosthetic valves and failed annuloplasty rings. J Am Coll Cardiol 2017;70: 1121–31. [DOI] [PubMed] [Google Scholar]
- 24.Urena M, Brochet E, Lecomte M, et al. Clinical and haemodynamic outcomes of balloon-expandable transcatheter mitral valve implantation: a 7-year experience. Eur Heart J 2018;39:2679–89. [DOI] [PubMed] [Google Scholar]
- 25.Zoghbi WA, Chambers JB, Dumesnil JG, et al. Recommendations for evaluation of prosthetic valves with echocardiography and doppler ultrasound: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Task Force on Prosthetic Valves, developed in conjunction with the American College of Cardiology Cardiovascular Imaging Committee, Cardiac Imaging Committee of the American Heart Association, the European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography and the Canadian Society of Echocardiography, endorsed by the American College of Cardiology Foundation, American Heart Association, European Association of Echocardiography, a registered branch of the European Society of Cardiology, the Japanese Society of Echocardiography, and Canadian Society of Echocardiography. J Am Soc Echocardiogr 2009;22:975–1014, quiz 1082–4. [DOI] [PubMed] [Google Scholar]
- 26.Capodanno D, Petronio AS, Prendergast B, et al. Standardized definitions of structural deterioration and valve failure in assessing long-term durability of transcatheter and surgical aortic bioprosthetic valves: a consensus statement from the European Association of Percutaneous Cardiovascular Interventions (EAPCI) endorsed by the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2017;38:3382–90. [DOI] [PubMed] [Google Scholar]
- 27.Kamioka N, Babaliaros V, Morse MA, et al. Comparison of clinical and echocardiographic outcomes after surgical redo mitral valve replacement and transcatheter mitral valve-in- valve therapy. J Am Coll Cardiol Intv 2018;11: 1131–8. [DOI] [PubMed] [Google Scholar]
- 28.Muller DWM, Farivar RS, Jansz P, et al. Transcatheter mitral valve replacement for patients with symptomatic mitral regurgitation: a global feasibility trial. J Am Coll Cardiol 2017;69: 381–91. [DOI] [PubMed] [Google Scholar]
- 29.Bapat V, Rajagopal V, Meduri C, et al. Early experience with new transcatheter mitral valve replacement. J Am Coll Cardiol 2018;71:12–21. [DOI] [PubMed] [Google Scholar]
- 30.Eng MH, Greenbaum A, Wang DD, et al. Thrombotic valvular dysfunction with transcatheter mitral interventions for postsurgical failures. Catheter Cardiovasc Interv 2017;90:321–8. [DOI] [PubMed] [Google Scholar]
- 31.Khan JM, Lederman RJ. Unnatural milieu: thrombus after transcatheter mitral valve replacement. Catheter Cardiovasc Interv 2017;90: 329–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan JM, Lederman RJ. Adventures across the second dimension: predicting left ventricular outflow tract obstruction following transcatheter mitral valve replacement. Catheter Cardiovasc Interv 2018;92:388–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohli K, Wei ZA, Yoganathan AP, Oshinski JN, Leipsic J, Blanke P. Transcatheter mitral valve planning and the neo-LVOT: utilization of virtual simulation models and 3D printing. Curr Treat Options Cardiovasc Med 2018;20:99. [DOI] [PubMed] [Google Scholar]
- 34.De Vecchi A, Marlevi D, Nordsletten DA, et al. Left ventricular outflow obstruction predicts increase in systolic pressure gradients and blood residence time after transcatheter mitral valve replacement. Sci Rep 2018;8:15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Urena M, Vahanian A, Sondergaard L. Patient selection for transcatheter mitral valve implantation: why is it so hard to find patients? Euro-Intervention 2018;14:AB83–90. [DOI] [PubMed] [Google Scholar]
- 36.Khan JM, Lederman RJ, Sanon S, et al. Transcatheter mitral valve replacement after transcatheter electrosurgical laceration of Alfieri STItCh (ELASTIC): first-in-human report. J Am Coll Cardiol Intv 2018;11:808–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan JM, Dvir D, Greenbaum AB, et al. Transcatheter laceration of aortic leaflets to prevent coronary obstruction during transcatheter aortic valve replacement: concept to first-in-human. J Am Coll Cardiol Intv 2018;11:677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


