Abstract
Maternal care is critical for the survival, development and long-term success of offspring. Despite our current understanding of the role of endogenous estrogen in both maternal behavior and the maternal brain, the potential effects of exogenous estrogens on these endpoints remain poorly understood. Here, pregnant CD-1 mice were exposed to low doses of 17α-ethinyl estradiol (EE2), commonly used as a positive control in studies of other xenoestrogens, from day 9 of pregnancy until weaning. Using traditional maternal behavior assays, we document no significant changes in maternal behavior throughout the lactational period. However, EE2 induced increases in repetitive tail retrieval, which may indicate a stereotypy or obsessive compulsive (OCD)-like behavior. We also observed a significant reduction in tyrosine hydroxylase (TH) immunoreactivity in the ventral tegmental area (VTA), a region important for maternal motivation. These results suggest that pregnant adult females are not immune to the effects of this compound.
Keywords: endocrine disruptor, low dose effects, maternal behavior, obsessive compulsive, open field, pup retrieval, stereotypy, xenoestrogen
Introduction
Maternal responsiveness is critical for the development and long-term success of offspring, and represents a complex and multimodal integration of physiological, endocrine and neural systems [1-4]. In rodents, the quality of maternal care has lasting effects on offspring behaviors into adulthood, including multigenerational effects on maternal behavior itself [5-7]. In humans, mistreatment and neglect during childhood can have long-term neurobiological and behavioral consequences [8-11]. Childhood maltreatment is also associated with increased vulnerability to a number of diseases including heart disease and cancer [12].
Research investigating the hormonal underpinnings of maternal behavior has demonstrated that in the rat, the onset of maternal behavior at parturition is induced by withdrawal of progesterone followed by elevation of 17β-estradiol (E2) and is maintained by prolactin, oxytocin and thereafter through tactile interactions with offspring [13-19]. In the mouse, the role of estrogen in the establishment of maternal behavior is less well understood [20]. Nulliparous female laboratory mice spontaneously demonstrate maternal behavior after brief exposure to pups, in contrast to wild caught virgin females [21] and nulliparous rats, the latter of which require an exposure of six to eight days [22-26]. Due to the spontaneous display of maternal care in laboratory mice, the onset of maternal behavior in these females has long been considered to be relatively free from hormonal control [26, 27]. However, when ovariectomized prior to the onset of puberty, fewer mice will display maternal behavior in adulthood, suggesting a role for estrogen during development for the later display of maternal behavior [28]. Additionally, ovarian hormones have been demonstrated to influence maternal responsiveness in parturient females [29] and ERα knockout females display poor maternal behavior when tested with surrogate pups [30, 31].
There is evidence that dopaminergic neural circuits act on the medial preoptic area (MPOA) of the forebrain, a brain region required for the onset of maternal behavior [32, 33]; conditional silencing of ERα in MPOA neurons abolishes maternal behavior [34]. It has been proposed that E2 and dopamine may interact or act via similar intracellular mechanisms to mediate MPOA output for maternal behavior [35, 36]. The ventral tegmental area (VTA) is also critical for maternal behavior; lesion of the VTA interferes with the display of maternal behavior [37-39] and can lead to infanticide [37]. More specifically, 6-Hydroxydopamine lesion of dopaminergic neurons in the VTA reduces pup retrieval [39]. Numan et al. propose that MPOA projections to the VTA activate dopaminergic input to the nucleus accumbens to regulate “motivational” aspects of maternal behavior [35, 38] and there is additional evidence that the neurons that project from the MPOA to the VTA are sensitive to estrogen [40, 41].
A number of studies have demonstrated that endocrine disrupting chemicals (EDCs) with estrogenic properties can disrupt maternal behavior in both mice and rats (reviewed in [42, 43]). Our recent work demonstrated that the ER agonist bisphenol S (BPS) alters maternal behaviors in female mice exposed during pregnancy and lactation, as well as in their daughters exposed during gestational and perinatal development [44]. However, it is not yet clear if the effects of EDCs, including BPS, are due to their estrogenic activity, and if these effects can be expected from other compounds with similar modes of action.
17α-ethinyl estradiol (EE2) is the active synthetic estrogen in female contraceptive pills, used by an estimated 100 million women worldwide [45-47]. EE2 is excreted into wastewater after its use, is found in surface waters and can induce reproductive changes in aquatic animals [48]. EE2 is widely used as a positive control for estrogenicity in both endocrinology and toxicology studies and was recently included in a NIEHS-US FDA collaboration exploring the effects of bisphenol A (BPA) on a range of endpoints [49-51]. However, the usefulness of EE2 as an estrogenic control for BPA and other EDCs remains to be resolved, as dissimilar effects have been found when comparing BPA with either estradiol or EE2. For example, BPA has been shown to have divergent effects on ER compared to estradiol [52] and to have different effects on behavior [53] and neural endpoints [54] compared to EE2. Additionally, in spite of its acknowledged estrogenic properties, there are relatively few studies examining the effects of EE2 at low doses; many studies that use it as a positive control examine high doses, including doses that induce overt signs of toxicity [55, 56]. For this reason, additional evaluations to determine the suitability of EE2 as a positive control for all estrogenic chemicals are needed, and these evaluations should assess the effects of low doses.
A small number of studies have examined the effects of EE2 on maternal behavior [57-59]. In two of these studies, conducted by the same research group, female rats were exposed to 15 μg EE2/kg/day from pregnancy days 9-14, which induced significant reproductive toxicity. In the first study, EE2-treated dams retrieved fewer pups and the latency to retrieve was longer when compared to controls [57]. In the follow-up study, surprisingly, the authors observed a reduced latency to retrieve pups in EE2-treated dams relative to controls and increased pup directed behaviors, which the authors characterized as improved maternal behavior [59]. The authors hypothesized that differences in lighting in the first study may have induced a stress response leading to maternal anxiety-like behavior that could explain the differing effects they observed between their two studies [59]. Neither of these studies examined potential effects of EE2 on the maternal brain.
Here, we investigated the effects of low doses of EE2 on maternal behavior. Because maternal behavior changes across the postpartum period as the needs of the pups shift during development [4, 60-63], we chose three time points representative of early, mid and late postpartum to test potential effects of EE2 on well-characterized maternal behaviors. We also examined ERα expression in the MPOA due to its critical importance for the display of maternal behavior in mice, and tyrosine hydroxylase (TH) immunoreactivity in the VTA, given its importance for maternal motivation and its connectivity with the MPOA.
Methods
Animals
Timed pregnant female CD-1 mice (Charles River Laboratories, Raleigh, NC) were acclimated for at least two days and individually housed in polysulfone cages with one cotton nestlet. Food (ProLab IsoDiet) and tap water (in glass bottles) were provided ad libitum. The animals were maintained in temperature (23 ± 2°C), humidity (45 ± 15%) and light controlled (12h light, 12h dark, lights on at 0800 h) conditions at the University of Massachusetts, Amherst Central Animal Facility. All experimental procedures were approved by the University of Massachusetts Institutional Animal Care and Use Committee.
Beginning on pregnancy day eight, female mice were weighed daily and randomly allocated to treatment groups using statistical software designed to normally distribute mice in treatment groups based on body weight. EE2 dosage was adjusted daily for body weight. From pregnancy day 9 – lactational day [LD] 20, dams were provided a small wafer (Nabisco, East Hanover, NJ) treated with EE2 (Sigma Aldrich, St. Louis, MO; >98% purity) or vehicle alone (70% ethanol, allowed to dry to completion prior to feeding). Wafers were dosed with solutions designed to deliver 0.01 or 1 μg EE2/kg/day (n=12-16 for each dose). The 0.01 μg/kg/day dose was selected because it has previously been shown to disrupt estrogen-sensitive endpoints in exposed offspring [64]. The higher 1 μg/kg/day dose was selected because it can induce uterotrophic responses in pubertal females and is ∼2× higher than the concentrations in prescription birth control pills [65].
Wafers were administered in the afternoon, after any planned behavioral assays were completed. During the administration of wafers, dams were transferred to clean cages for a brief period until the dam consumed the entire wafer. After the pups were born, time away from the home cage was closely monitored in order to avoid separation effects [66]. Pups were not handled during wafer administration to avoid potential handling effects. Dams were allowed to deliver naturally (birth designated LD0). Litters were culled to 10 pups on LD1.
Maternal behavior assays
Maternal behavior was assessed on LD 2, 7, and 14 by independent observers. First, spontaneous maternal behavior was observed for a period of 90-minutes at the beginning of the light phase. Without disturbing the dams or litters, observations were recorded every three minutes for the following measures: dam position on/off nest, nursing posture, pup licking and grooming, nest repair and non-pup directed behaviors designated “self-care” (self grooming, eating, and drinking) by observers blinded to treatment groups.
At the end of the observational period, nest size and quality were measured. The dam and pups were carefully removed from the cage and the nest dimensions were measured. The internal volume of the nest was calculated from the average inner nest diameter (measured using the internal walls constructed from a single cotton nestlet) and nest depth. External volume of the nest was calculated from the average outer nest diameter (measured as the outer extremes of the walls constructed from a single cotton nestlet) and nest depth. Two independent observers scored the nest quality using a 5-point scale adapted from the Hess Scale (further described in [43]).
Following nest measurement, dams were assessed using a pup retrieval assay. The pups and dams were separated during nest assessment, after which pups were scattered in the cage on the opposite side from the nest and the dam was returned to the nest. The latency to touch the first pup and return pups to the nest was recorded for a period of ten minutes. Data reported for time to retrieve pups is only reported for dams that successfully retrieved at least one pup.
Open Field Behavioral Assay
The open field test is a standard behavioral measure of anxiety and locomotion. Dams were tested on pregnancy day 16 and LD 10 or 11 using a standard open field apparatus 40 cm × 40 cm × 40 cm [67]. Testing was recorded with a camera positioned above the arena, connected to a computer using EthoVision ×T10 software (Noldus, Wageningen, Netherlands). The software quantified distance traveled, mean velocity, and time spent in the center 5 cm × 5 cm. Additional measures were scored by independent observers blind to treatment group including rearing against the walls, rearing away from the walls, freeze/stops, and grooming events.
Immunohistochemistry
On LD 21, brains were collected from dams and fixed in Neutral Buffered Formalin (10%) (Fisher Scientific, Pittsburgh, PA) using methods previously optimized in our laboratory [44, 68]. Briefly, brains were cut in 40 μm transverse sections and the MPOA and VTA were identified using a mouse brain atlas [69, 70]. Free-floating sections were processed for ERα or tyrosine hydroxylase (TH, a marker for catecholamine biosynthesis, found in dopamine neurons) immunoreactivity. Antigen retrieval was performed using 0.01 Citric Acid Buffer (pH 6.0) and endogenous peroxidases were quenched using 3% hydrogen peroxide in methanol. Sections were washed, blocked with normal goat serum in 1.5% milk, and incubated overnight at 4°C with rabbit anti-ERα antibody directed against the C-terminus of the rat ERα (1:20,000 anti-ERalpha C1355, Fisher Scientific) or a polyclonal antibody for TH (1:2000, Abcam, Cambridge, MA, ab112). Sections were then washed and incubated with biotin labeled secondary antibody (goat anti-rabbit Ab 64256, Abcam) followed by streptavidin peroxidase complex (Ab64269, Abcam). A colorimetric detection method was performed using diaminobenzidene (DAB) chromogen and substrate (ab64238, Abcam). Sections were rinsed in tap water, stored in PBS with 0.1% Tween-20 until mounted on slides, dehydrated and coverslipped.
One image per section was collected using a Zeiss AxioImager microscope (120× magnification for the MPOA; 100× for the VTA) and Zeiss high-resolution color camera. Using ImageJ software (National Institutes of Health), the image was converted from RGB color to 8 bit, background was subtracted followed by automatic thresholding, as well as masking and watershed functions,. Cells expressing ERα in the MPOA and TH in the VTA were counted on anatomically matched sections. While we observed differences in staining intensity across sections, due to theoretical limitations in the quantification of DAB signal we did not assess staining intensity; coloration is not linearly related to amount of protein [71, 72]. For feasibility reasons, ERα positive cells were quantified in two MPOA sections per animal; one from the rostral MPOA (∼0.14 mm from Bregma), one from the caudal MPOA (∼0.02 mm from Bregma), by an observer blind to treatment. The central MPOA was identified using neuroanatomical landmarks as described in [73]. The VTA was identified using neuroanatomical landmarks [69, 70] and TH immunoreactivity as demonstrated in [74]. TH-positive cells were counted on two anatomically matched sections (-2.92 and ∼3.08 from Bregma).
Statistical Analysis
Both behavioral and immunohistochemical analyses were conducted by experimenters blind to treatment groups. Data were analyzed using SPSS Version 22. For assessments of maternal behavior, continuous variable data were analyzed using 2-way ANOVA General Linear Model analyses with lactational day and treatment group as independent variables, followed by Bonferroni posthoc tests. Open field data were analyzed using 1-way ANOVA with treatment group as the independent variable. Categorical data were analyzed using Chi Square. Data were considered statistically significant at p<0.05. Graphs illustrate means ± standard error unless otherwise stated. Sample sizes for behavioral analyses and MPOA counts were: control (n=16), 0.01 μg EE2/kg/day (n=16) and 1 μg EE2/kg/day (n=12). Samples sizes for VTA counts were: control (n=5), 0.01 μg EE2/kg/day (n=7) and 1 μg EE2/kg/day (n=6).
Results
Low dose EE2 exposure does not induce developmental toxicity
Pups were counted, sexed and weighed on postnatal day (PND1), prior to litter culling. There were no treatment-related effects on litter size, sex ratio, litter weight, or average pup weight (Table 1). Poorly cleaned pups were observed in one litter from a dam treated with 0.01 μg EE2/kg/day.
Table 1. Litter outcomes indicate no developmental toxicity in F1 pups associated with low dose EE2 treatment.
| Control | 0.01 μg EE2/kg/day | 1 μg EE2/kg/day | |
|---|---|---|---|
| Litter size (live pups on PND1)a | 12.50 ± 0.78 | 12.63 ± 0.54 | 12.17 ± 0.55 |
| Total litter weight (g) on PND1 a | 23.84 ± 1.35 | 24.32 ± 1.03 | 24.06 ± 1.13 |
| Average pup weight (g) on PND1 a | 1.93 ± 0.038 | 1.94 ± 0.061 | 1.98 ± 0.040 |
| Sex ratio (% males) a | 49.3 ± 4.2 | 50.2 ± 3.2 | 45.4 ± 2.9 |
| Mortality rate b | 0 (0/200 pups) | 1% (2/202 pups) | 0 (0/146 pups) |
No significant differences were noted using 1-way ANOVA.
No significant differences were noted using Chi Square.
Mortality was also very low in the F1 generation. Only two pups died after culling and prior to weaning; both were from litters treated with 0.01 μg EE2/kg/day (Table 1). Furthermore, overt birth defects were not observed in any pups in any treatment group although one female treated with 1 μg EE2/kg/day developed severe hydroencephaly in adulthood and had to be euthanized.
EE2 exposure does not alter time spent on the nest, nursing, grooming pups, or non-pup directed self-care
To assess the effects of EE2 exposure on maternal behavior, dams were observed in their home cage without any experimental interventions. On LD2, dams in both EE2 treatment groups as well as the control group spent >70% of observed time on the nest, with no significant differences between groups (Figure 1A). Throughout the lactational period assessed, the time dams spent on the nest decreased (ANOVA p<0.001), but no effects of EE2 were observed. Similar patterns were seen for time spent grooming pups (Figure 1B) and time spent on self-care (Figure 1C), which were affected by lactational period but not EE2.
Figure 1. EE2 exposure does not alter time spent on the nest, grooming pups, or non-pup directed self-care.
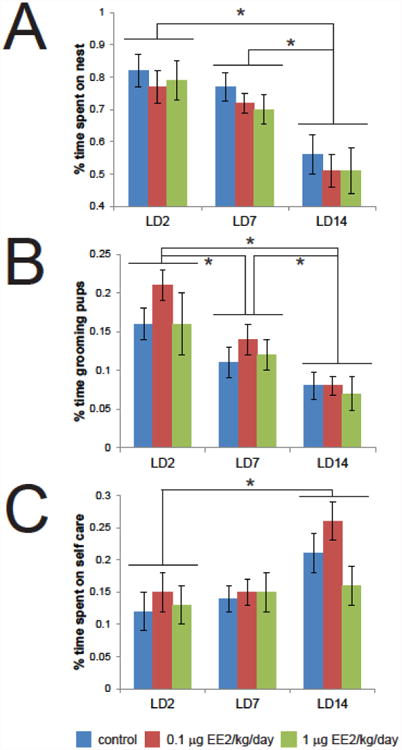
(A) On LD2, dams in both EE2 treatment groups as well as the control group spent >70% of observed time on the nest, with no significant differences between treatment groups. The time dams spent on the nest decreased significantly as the lactational period progressed, but no effects of EE2 were observed. (B) On LD2, dams exposed to 0.01 μg EE2/kg/day spent approximately 30% more observed time grooming their pups compared to controls, however, this difference was not statistically significant. The observed time spent grooming pups decreased significantly across the lactational period, with no differences observed between treatment groups. (C) Although there were significant differences in non-pup directed behaviors in early and late lactation, there were no significant differences between treatment groups. In all panels, blue = vehicle control, red = 0.01 μg EE2/kg/day, green = 1 μg EE2/kg/day. * indicates significant effects of lactational age, 2-way ANOVA with Bonferroni posthoc tests. [figure can be prepared in B&W for print version]
EE2-treated dams spend more time retrieving their own tails to the nest during home cage observations
During home cage observations, we quantified a behavior that is not well documented in rodents, repetitive tail retrieval. In the display of this behavior, occurring usually while the dam was in the home cage and off the nest, a female would repeatedly circle, grasp her own tail with her mouth, and retrieve it to the nest (Supplemental video). This behavior was observed in all treatment groups at LD2 and LD7, but in only one dam at LD14 (Table 2); more treated dams displayed tail retrieval compared to control dams, but these increases were not statistically significant. Examining the frequency of this behavior in females that displayed this behavior, dams from both EE2 treatment groups spent significantly more time (ANOVA, p=0.021) engaged in this behavior compared to controls (Figure 2).
Table 2. Frequency of tail retrieval in the home cage and during pup retrievals.
| LD 2 | LD 7 | LD 14 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.01 μg EE2 | 1 μg EE2 | Control | 0.01 μg EE2 | 1 μg EE2 | Control | 0.01 μg EE2 | 1 μg EE2 | |
| Home cage: | 6/16 38% | 10/16 63% | 5/12 42% | 6/16 38% | 8/16 50% | 5/12 42% | 0/16 0% | 1/16 6% | 0/12 0% |
| Pup retrieval: | 2/16 13% | 4/16 25% | 2/12 17% | 1/16 6% | 2/16 13% | 2/12 17% | 0/16 0% | 1/16 6% | 0/12 0% |
| Either assay: | 7/16 44% | 12/16 75% | 6/12 50% | 7/16 44% | 10/16 63% | 5/12 42% | 0/16 0% | 1/16 6% | 0/12 0% |
Repetitive tail retrieval was observed in all treatment groups at LD2 and LD7, and in only one dam exposed to 0.01 μg EE2/kg/day at LD14
Figure 2. EE2-treated dams spend more time retrieving their own tails to the nest in the home cage.
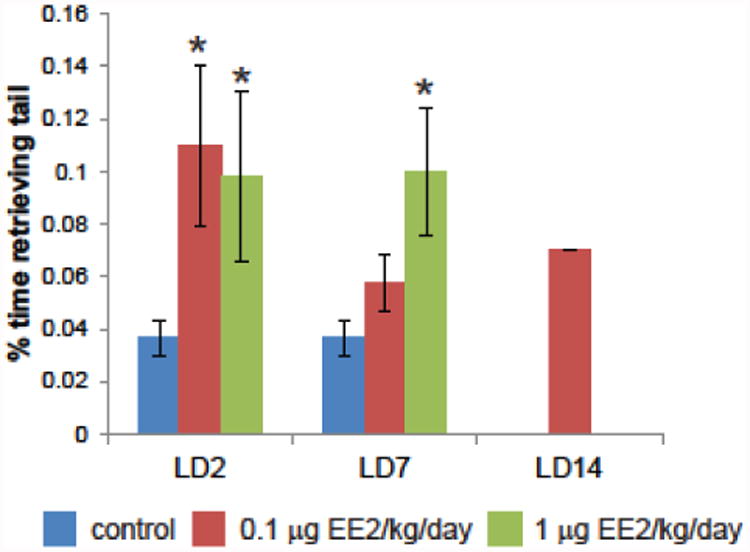
Time spent on tail retrieval was quantified at all three periods of lactation at three minute intervals throughout the 90 minute observational period. Dams from both EE2 treatment groups spent more observed time engaged in this behavior, which was not extinguished in one dam from the low EE2 group by LD14. In all panels, blue = vehicle control, red = 0.01 μg EE2/kg/day, green = 1 μg EE2/kg/day. * indicates p<0.05 compared to controls of the same lactational age, Bonferroni posthoc test, after significant 2-way ANOVA. [figure can be prepared in B&W for print version]
EE2 treatment does not influence nest size or quality
To determine whether EE2 treatment altered nesting parameters, quantitative and qualitative measures were collected from nests built from one cotton nestlet. Although lactational day affected inner nest diameter and nest quality (ANOVA, p<0.01, data not shown), EE2 treatment did not affect either parameter at LD2, LD7 or LD14 (data not shown). Neither lactation day nor EE2 treatment affected external nest volume (data not shown).
EE2 treatment did not alter pup retrieval across the early to mid-lactational period
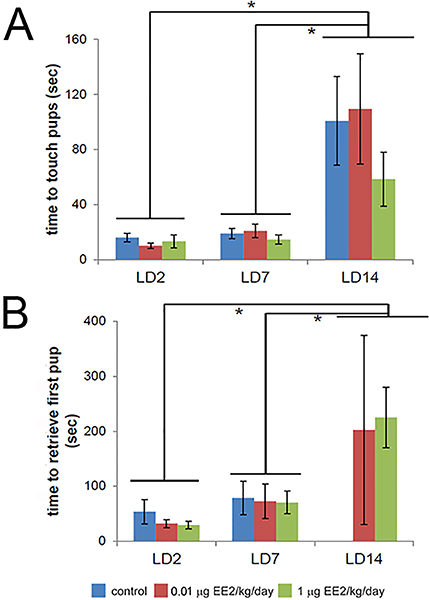
To determine the influence of EE2 on mouse dams' abilities to retrieve their pups to the nest, standard pup retrieval assays were conducted at LD2, LD7, and LD14. The number of dams that successfully retrieved at least one pup is indicated in Table 3. Although lactational stage affected time to retrieve (ANOVA, p<0.001), no significant differences were found based on EE2 treatment in the time to first touch the pups (Figure 3A) or retrieve the first pup (Figure 3B). Notably, only EE2-treated dams retrieved pups on LD14; retrieval at this age was striking due to the size and overall mobility of the pups.
Table 3. Number of dams that retrieved at least one pup during pup retrieval assays.
| Control | 0.01 μg EE2/kg/day | 1 μg EE2/kg/day | |
|---|---|---|---|
| LD2 | 14/16 88% | 14/16 88% | 10/12 83% |
| LD7 | 12/16 75% | 12/16 75% | 10/12 83% |
| LD14 | 0/16 0% | 2/16 13% | 2/12 17% |
Retrieval of at least one pup was observed in all treatment groups on LD2 and LD7, and declined sharply by LD14.
Figure 3. EE2 treatment has no effect on time to touch or retrieve pups.
(A) Time to retrieve pups was affected by postpartum stage but not EE2 at any stage examined. (B) Time to retrieve pups was calculated only in dams with litter sizes of 9-10 pups that retrieved at least one pup, i.e. “active” mothers. There were no significant differences in the time to retrieve the first pup based on EE2 treatment, although notably, only treated dams retrieved pups on LD14. In all panels, blue = vehicle control, red = 0.01 μg EE2/kg/day, green = 1 μg EE2/kg/day. * indicates significant effects of lactational age, 2-way ANOVA with Bonferroni posthoc tests. [figure can be prepared in B&W for print version]
Tail retrieval was again observed during the pup retrieval assay (Table 2). At LD2, tail retrieval was observed in all treatment groups during pup retrieval assays, and although it was more common in both of the EE2 treatments, these increases were not statistically significant. By LD7, tail retrieval was only observed in the two EE2 groups, and by LD14, it was observed in a single dam exposed to 0.01 μg EE2/kg/day. Importantly, although there was some overlap, dams that retrieved their tails in the pup retrieval assay were not necessarily the same dams that retrieved their tails in the home cage.
Results from open field assays are not consistent with EE2-induced anxiety-like behavior or effects on locomotion
In order to evaluate potential effects of exposure on anxiety-like behaviors or locomotion, we conducted open field tests on dams at pregnancy day 16 and LD 10-11. Overall, no significant differences were noted for average velocity or total distance traveled, although there was a statistical trend (p<0.1) for an increase in the % time spent in the center of the open field in the dams treated with 0.01 μg EE2/kg/day at pregnancy day 16 (Figure 4). Increased time spent in the center of the open field is typically associated with lower measures of anxiety [75-80]. No significant differences were observed for rears, grooming events, or number of freeze/stops (data not shown).
Figure 4. Results from open field assays are not consistent with EE2-induced anxiety-like behavior or alterations to activity levels.
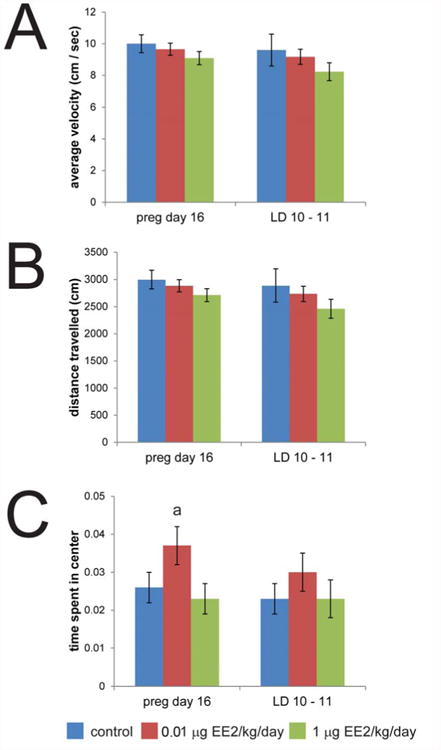
Open field tests were conducted on gestational day 16 and lactational day 10 – 11. (A) No effect of treatment was observed for average velocity. (B) Total distance travelled was similarly not affected by EE2. (C) No significant differences were observed for any of the automated measures collected during open field tests (average velocity, total distance traveled, time spent in the center) although there was a non-significant increase in the % time spent in the center of the open field in dams treated with 0.01 μg EE2/kg/day at pregnancy day 16 (a indicates a trend, p < 0.1 compared to controls, ANOVA with Bonferroni posthoc test). In all panels, blue = vehicle control, red = 0.01 μg EE2/kg/day, green = 1 μg EE2/kg/day. [figure can be prepared in B&W for print version]
ERα expression in the MPOA is not affected by EE2 exposures in dams
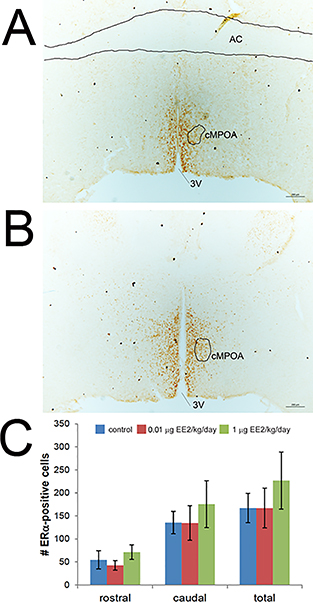
To determine whether expression of ERα in neurons in the MPOA is altered by EE2 exposure, brains were collected from dams on LD21, approximately 20 hours after the last administered EE2 dose. Following immunohistochemical analyses, cells expressing ERα were quantified in two matched sections of the central MPOA, a specific sub-region in the MPOA found to be essential for pup retrieval in the mouse using excitotoxic lesion studies [73]. One section was selected from the rostral central MPOA and the second was selected from the caudal central MPOA. No significant differences were observed in ERα expression in either region based on treatment (Figure 5).
Figure 5. EE2 does not alter expression of ERα in the central MPOA at lactational day 21.
ERα-immunoreactive cells were quantified in two matched sections, a rostral (A) and caudal (B) section of the central MPOA. AC = anterior commissure, 3V = third ventricle. Inset shows higher magnification view of ERα-positive neurons. C) No effects of EE2 were observed in ERα-positive neurons in either region of the MPOA. Blue = vehicle control, red = 0.01 μg EE2/kg/day, green = 1 μg EE2/kg/day. [figure can be prepared in B&W for print version]
TH-immunoreactivity in the VTA is reduced after EE2 exposure in dams
Because the VTA is important for maternal behavior and dopamine in the MPOA may interact with E2 in output circuits to this region [35], we quantified TH-immunoreactive cells in the VTA in two anatomically matched sections. These evaluations were also conducted in brains were collected from dams on LD21. There was a statistically significant decrease in TH-immunoreactivity in the VTA in the 1 μg EE2/kg/day group (Figure 6). No effect was seen in dams exposed to the lower EE2 dose.
Figure 6. EE2 treatment reduced TH-immunoreactivity in the VTA.
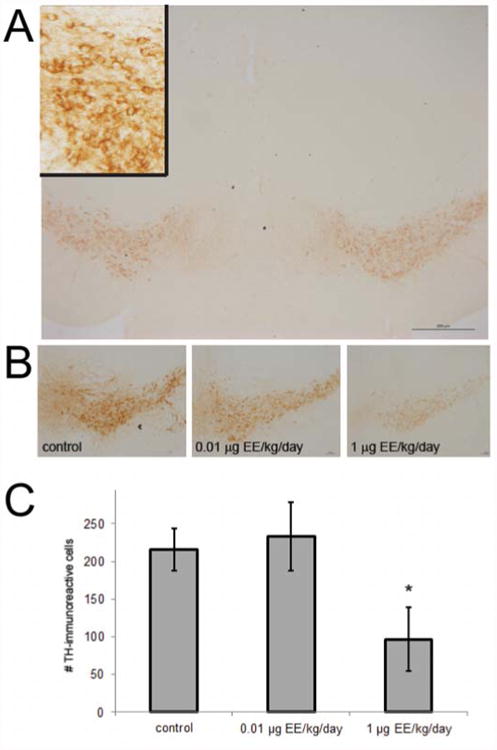
(A) TH-positive cells were counted in two matched sections of the VTA. Inset shows higher magnification view of TH-positive neurons. (B) Shown are representative examples of VTA samples from dams of all three treatment groups. (C) Quantification of TH-immunoreactive cells in two VTA sections revealed significant decreases in dams exposed to 1 μg EE2/kg/day. * indicates significant effects of treatment, 1-way ANOVA with Bonferroni posthoc test p<0.05. [figure can be prepared in B&W for print version]
Discussion
Here, we have demonstrated that low doses of EE2 do not appear to induce disruptions to maternal behavior in mice exposed during pregnancy and lactation. Two prior studies examining the effects of EE2 on pregnant females injected 15μg EE2/kg/day, i.p., inducing significant reproductive and developmental toxicity; ∼50% of dams aborted and ∼50% of neonates did not survive [57, 59]. This high level of toxicity as well as the small sample sizes (3-8 dams/treatment) likely confounded the analysis of maternal responses.
We specifically examined low doses of EE2, which were selected to avoid reproductive and developmental toxicity. Our high dose (1μg EE2/kg/day) is similar to those used to induce estrogenic effects in adult females (i.e. a uterotrophic response, see [81]) and approximately twice as high as the dose found in typical oral contraceptives. The low dose (0.01 μg EE2/kg/day) has been shown to induce effects on estrogen-sensitive endpoints in mice exposed during prenatal development [64]. Not only did we anticipate that these low doses would avoid overt signs of toxicity, but we also selected these doses because they are more appropriate to evaluate whether relatively minor alterations to estrogen levels could affect maternal outcomes [82].
Counter to our predictions based on our prior studies of other xenoestrogens [44], we did not observe any effects of EE2 on traditional measures of pup-retrieval, pup grooming, time spent on the nest or on maternal self-care. We also did not observe effects of EE2 on quantitative measures of nest size or nest quality, considered sensitive to hormones during pregnancy [43]. We were surprised by these findings, as EE2 is considered a positive control for estrogenicity, and many other studies of estrogenic EDCs have demonstrated effects on a range of maternal behaviors (see [42, 43]). Additional evaluations are needed to determine how different estrogenic compounds, all known to bind to ER, could induce such different effects.
It is also plausible that EE2 affects subtle aspects of maternal behavior that we did not evaluate, or affects maternal behavior at more active times of day (e.g., during the dark phase). We did observe effects of BPS during the light phase [44], and thus found it surprising that EE2 did not have effects during this less-active period. However, prior work revealed differences in maternal behavior in response to the estrogenic pesticide methoxychlor in the dark compared to light phases [83]. Thus, future studies are necessary to investigate potential effects of EE2 on maternal behavior during the dark phase.
We did identify significant increases in a repetitive behavior, tail retrieval, in EE2-exposed dams during lactation (Figure 2). Importantly, this behavior was observed during the home cage observations when the dam was undisturbed and the pup retrieval assay, suggesting that this behavior can manifest during both stressful and relatively stress-free periods. Tail retrieval is not well documented in the literature, and its interpretation remains unresolved; anecdotally, researchers report that mice and rats will retrieve odd objects during pup retrieval assays, but this behavior has rarely been quantified. Further, these behaviors are typically evaluated when foreign objects are added to the cage, whereas we observed these effects without disrupting the dams. One group posits that tail retrieval behaviors may indicate preparation for maternal behavior in dams during late pregnancy but this hypothesis has not yet been tested [84].
It is possible that tail retrieval represents a form of perseverative or obsessive compulsive disorder (OCD)-like behavior or stereotypy. This hypothesis should be assessed in future work using tests for repetitive behaviors, insistence on sameness modeling or a signal attenuation test [85-89]. We found that tail retrieval behaviors first manifested late in pregnancy or shortly after parturition and was extinguished in most dams by LD14. In humans, postpartum depression is a focus of major research efforts, and there is mounting evidence that postpartum OCD develops early in parenthood [90-93]. The late pregnancy and early postpartum period is thought to be associated with increased preoccupations in both mothers and fathers [94]. Elevated vigilance to protect a newborn from harm is thought to drive repeated behaviors such as constant, unnecessary checking on the baby; neural regions that respond to the cry of one's own baby were shown to change across the postpartum period and may be similar to circuits involved in OCD [94-97]. Initially considered relatively uncommon, a recent study demonstrated that about 11% of women who became new mothers presented with symptoms of OCD at two weeks postpartum, and half of these women's symptoms persisted 6 months post-partum [98].
EE2 treatment did not influence ERα expression in the MPOA, which is critical for maternal behavior in both rats and mice [30, 34, 99-101]. Our prior study demonstrated that BPS increased expression of ERα in the MPOA in females after the same exposure period used in this study [44]. Future studies in both rats and mice will need to examine the effects of EE2 on the MPOA at earlier lactational time points. It is also possible that ERα gene expression rather than ERα immunoreactivity is affected by EE2 in the MPOA, or that EE2 acts on other hormone receptors in this region. However, because no effects of EE2 were observed on maternal behaviors even in the early postpartum period, it is plausible and perhaps even likely that no effects on the MPOA occurred, even at earlier time points.
We observed a reduction in TH-immunoreactivity in the VTA in the 1 μg EE2/kg/day group. Dopaminergic neurons in the VTA play a role in motivation and reward and the VTA is involved in a number of neural circuits with connections to the prefrontal cortex in the mesocortical system, and the anteromedial caudate-putamen, nucleus accumbens, as well as other ventral striatal areas in the mesostriatal system [102-106]. The observed reduction in TH-immunoreactivity may be due to decreased TH gene expression or may reflect decreases of TH–immunoreactivity in cell bodies falling below the limit of antibody detection. Future studies are necessary to distinguish between these possibilities as well as to investigate if reduction in TH-immunoreactivity is due to cell loss [107, 108]. It is also possible that the observed reduction in TH-immunoreactivity in the VTA in the 1 μg EE2/kg/day group is due to a treatment related suppression of an upregulation of the TH phenotype that may naturally occur during this postpartum period.
Conclusions
Contrary to our expectations, we did not observe statistically significant differences in traditional measures of maternal behavior such as pup retrieval or pup-directed behaviors after low dose EE2 treatment. Numerous other studies have demonstrated effects of EDCs and synthetic estrogens on traditional measures of maternal behavior [43]. Our failure to observe effects of EE2 may be a result of differences in study design and doses selected, however, because EE2 is frequently used as an estrogenic control in EDC studies, these discrepancies may instead suggest that estrogenic EDCs disrupt maternal behaviors via unique interactions with ER, or via non-ER-mediated mechanisms.
Our study focused on the effects of EE2 on maternal behavior and brain in the parental generation, e.g., in females exposed in adulthood only. Many studies of estrogenic EDCs have shown more potent effects after developmental exposures compared to the effects observed on exposed mothers [109-111]. Thus, future studies should examine estrogen-sensitive endpoints, including those relevant to maternal behaviors, in the F1 generation. Although many studies focus on effects of maternal EDC exposures on offspring outcomes, evaluating the effect of treatment on the maternal brain and behavior of the mother is important, as alterations in maternal care may contribute to effects that are observed in offspring [112-114].
Understanding the neuroendocrine basis for maternal behavior, as well as how it can be disrupted by hormonally active compounds, is an important area of scientific inquiry as deficient parental care has important implications for physiological and psychological development of offspring, with deleterious effects lasting into adulthood [8-11, 115]. Our experiments indicate that low doses of a synthetic estrogen are sufficient to alter the maternal brain in exposed adult females and exacerbate stereotypy or repetitive behavior, suggesting that adults are not immune to the disruptive effects of these types of compounds.
Highlights.
There are conflicting results on effects of ethinyl estradiol on maternal behavior
Maternal exposures to EE2 did not affect pup survival, care, or retrieval
EE2 induced increased repetitive tail retrieval, a stereotypy or OCD-like effect
The VTA, but not the MPOA, was affected by EE2 in the mother
Acknowledgments
The authors gratefully acknowledge input from Drs. R Thomas Zoeller, Jerrold Meyer, and Mariana Pereira. We also thank members of the Vandenberg lab who helped with behavioral data collection including Corinne Hill, Durga Kolla, Charlotte LaPlante, Sarah Sapouckey, and Alfred Kimani.
Funding: MCC was supported by a fellowship from the University of Massachusetts – Amherst Center for Research on Families. LNV was supported by start-up funds from the University of Massachusetts and Award Number K22ES025811 from the National Institute of Environmental Health Sciences of the National Institutes of Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Massachusetts.
Footnotes
Disclosures: LNV has received travel reimbursement from Universities, Governments, NGOs and Industry, to speak about endocrine-disrupting chemicals. MCC has nothing to disclose.
Author Contributions: MCC and LNV designed and conducted experiments, collected and analyzed data. MCC wrote the first draft of the manuscript. LNV edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Bowlby J. Maternal care and mental health. Bull World Health Organ. 1951;3:355–533. [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenblatt JS. Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatr Suppl. 1994;397:3–8. doi: 10.1111/j.1651-2227.1994.tb13259.x. [DOI] [PubMed] [Google Scholar]
- 3.Barrett J, Fleming AS. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–97. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- 4.Pereira M, Ferreira A. Neuroanatomical and neurochemical basis of parenting: Dynamic coordination of motivational, affective and cognitive processes. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Ann N Y Acad Sci. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- 6.Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–41. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champagne FA, Curley JP. Maternal regulation of estrogen receptor alpha methylation. Curr Opin Pharmacol. 2008;8:735–9. doi: 10.1016/j.coph.2008.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bifulco A, Moran PM, Baines R, Bunn A, Stanford K. Exploring psychological abuse in childhood: II. Association with other abuse and adult clinical depression. Bull Menninger Clin. 2002;66:241–58. doi: 10.1521/bumc.66.3.241.23366. [DOI] [PubMed] [Google Scholar]
- 9.Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J Clin Psychiatry. 2004;65:249–54. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- 10.Gunnar MR, Fisher PA, Early Experience S, Prevention N. Bringing basic research on early experience and stress neurobiology to bear on preventive interventions for neglected and maltreated children. Dev Psychopathol. 2006;18:651–77. [PubMed] [Google Scholar]
- 11.Gilbert R, Widom CS, Browne K, Fergusson D, Webb E, Janson S. Burden and consequences of child maltreatment in high-income countries. Lancet. 2009;373:68–81. doi: 10.1016/S0140-6736(08)61706-7. [DOI] [PubMed] [Google Scholar]
- 12.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14:245–58. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 13.Bridges RS, DiBiase R, Loundes DD, Doherty PC. Prolactin stimulation of maternal behavior in female rats. Science. 1985;227:782–4. doi: 10.1126/science.3969568. [DOI] [PubMed] [Google Scholar]
- 14.Morishige WK, Pepe GJ, Rothchild I. Serum luteinizing hormone, prolactin and progesterone levels during pregnancy in the rat. Endocrinology. 1973;92:1527–30. doi: 10.1210/endo-92-5-1527. [DOI] [PubMed] [Google Scholar]
- 15.Siegel HI, Rosenblatt JS. Duration of estrogen stimulation and progesterone inhibition of maternal behavior in pregnancy-terminated rats. Horm Behav. 1978;11:12–9. doi: 10.1016/0018-506x(78)90054-5. [DOI] [PubMed] [Google Scholar]
- 16.Rosenblatt JS, Mayer AD, Giordano AL. Hormonal basis during pregnancy for the onset of maternal behavior in the rat. Psychoneuroendocrinology. 1988;13:29–46. doi: 10.1016/0306-4530(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 17.Shaikh AA. Estrone and estradiol levels in the ovarian venous blood from rats during the estrous cycle and pregnancy. Biol Reprod. 1971;5:297–307. doi: 10.1093/biolreprod/5.3.297. [DOI] [PubMed] [Google Scholar]
- 18.Numan M, Insel Thomas R. The Neurobiology of Parental Behavior. 1. Springer-Verlag; New York: 2003. [Google Scholar]
- 19.Lonstein JS, Morrell JI. Neuropharmacology and neuroendocrinology of maternal motivation and behavior. In: Blaustein JD, editor. Handbook of Neurochemistry and Molecular Biology. Vol. 18. Behavioral Neurobiology: Springer Press; 2007. pp. 195–245. [Google Scholar]
- 20.Stolzenberg DS, Champagne FA. Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Horm Behav. 2016;77:204–10. doi: 10.1016/j.yhbeh.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy MM, Bare JE, vom Saal FS. Infanticide and parental behavior in wild female house mice: effects of ovariectomy, adrenalectomy and administration of oxytocin and prostaglandin F2 alpha. Physiol Behav. 1986;36:17–23. doi: 10.1016/0031-9384(86)90066-1. [DOI] [PubMed] [Google Scholar]
- 22.Wiesner BP, Sheard Norah M. Maternal Behaviour in the Rat Oliver and Boyd. 1933 [Google Scholar]
- 23.Leblond C. Extrahormonal factors in maternal behavior. Proceedings of the Society for Experimental Biology and Medicine. 1938:66–70. [Google Scholar]
- 24.Leblond C. Nervous and Hormonal Factors in the Maternal Behavior of the Mouse. Journal of Genetic Psychology. 1940;57:327–44. [Google Scholar]
- 25.Rosenblatt JS. Nonhormonal basis of maternal behavior in the rat. Science. 1967;156:1512–4. doi: 10.1126/science.156.3781.1512. [DOI] [PubMed] [Google Scholar]
- 26.Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011;23:345–54. doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gandelman R. Maternal behavior in the mouse: effect of estrogen and progesterone. Physiol Behav. 1973;10:153–5. doi: 10.1016/0031-9384(73)90101-7. [DOI] [PubMed] [Google Scholar]
- 28.Gandelman R. The ontogeny of maternal responsiveness in female Rockland-Swiss albino mice. Horm Behav. 1973;4:257–68. doi: 10.1016/0018-506x(73)90010-x. [DOI] [PubMed] [Google Scholar]
- 29.Hauser H, Gandelman R. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Horm Behav. 1985;19:454–68. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- 30.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139:5070–81. doi: 10.1210/endo.139.12.6357. [DOI] [PubMed] [Google Scholar]
- 31.Couse JF, Curtis Hewitt S, Korach KS. Receptor null mice reveal contrasting roles for estrogen receptor alpha and beta in reproductive tissues. J Steroid Biochem Mol Biol. 2000;74:287–96. doi: 10.1016/s0960-0760(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 32.Miller SM, Lonstein JS. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav Neurosci. 2005;119:1072–83. doi: 10.1037/0735-7044.119.4.1072. [DOI] [PubMed] [Google Scholar]
- 33.Miller SM, Lonstein JS. Dopaminergic projections to the medial preoptic area of postpartum rats. Neuroscience. 2009;159:1384–96. doi: 10.1016/j.neuroscience.2009.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro AC, Musatov S, Shteyler A, Simanduyev S, Arrieta-Cruz I, Ogawa S, et al. siRNA silencing of estrogen receptor-alpha expression specifically in medial preoptic area neurons abolishes maternal care in female mice. Proc Natl Acad Sci U S A. 2012;109:16324–9. doi: 10.1073/pnas.1214094109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Stolzenberg DS, McKenna JB, Keough S, Hancock R, Numan MJ, Numan M. Dopamine D1 receptor stimulation of the nucleus accumbens or the medial preoptic area promotes the onset of maternal behavior in pregnancy-terminated rats. Behav Neurosci. 2007;121:907–19. doi: 10.1037/0735-7044.121.5.907. [DOI] [PubMed] [Google Scholar]
- 37.Gaffori O, Le Moal M. Disruption of maternal behavior and appearance of cannibalism after ventral mesencephalic tegmentum lesions. Physiol Behav. 1979;23:317–23. doi: 10.1016/0031-9384(79)90373-1. [DOI] [PubMed] [Google Scholar]
- 38.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98:712–27. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 39.Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- 40.Morrell JI, Schwanzel-Fukuda M, Fahrbach SE, Pfaff DW. Axonal projections and peptide content of steroid hormone concentrating neurons. Peptides. 1984;5(1):227–39. doi: 10.1016/0196-9781(84)90281-x. [DOI] [PubMed] [Google Scholar]
- 41.Fahrbach SE, Morrell JI, Pfaff DW. Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. J Comp Neurol. 1986;247:364–82. doi: 10.1002/cne.902470307. [DOI] [PubMed] [Google Scholar]
- 42.Palanza P, Nagel SC, Parmigiani S, Vom Saal FS. Perinatal exposure to endocrine disruptors: sex, timing and behavioral endpoints. Current opinion in behavioral sciences. 2016;7:69–75. doi: 10.1016/j.cobeha.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catanese MC, Suvorov A, Vandenberg LN. Beyond a means of exposure: a new view of the mother in toxicology research. Toxicol Res. 2015;4:592–612. [Google Scholar]
- 44.Catanese MC, Vandenberg LN. Bisphenol S (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology. 2017;158:516–30. doi: 10.1210/en.2016-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pletzer BA, Kerschbaum HH. 50 years of hormonal contraception-time to find out, what it does to our brain. Front Neurosci. 2014;8:256. doi: 10.3389/fnins.2014.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petitti DB. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med. 2003;349:1443–50. doi: 10.1056/NEJMcp030751. [DOI] [PubMed] [Google Scholar]
- 47.Daniels K, Daugherty J, Jones J, Mosher W. Current Contraceptive Use and Variation by Selected Characteristics Among Women Aged 15-44: United States, 2011-2013. Natl Health Stat Report. 2015:1–14. [PubMed] [Google Scholar]
- 48.Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, et al. Effects of the environmental estrogenic contaminants bisphenol A and 17alpha-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen Comp Endocrinol. 2015;214:195–219. doi: 10.1016/j.ygcen.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Kostich M, Flick R, Martinson J. Comparing predicted estrogen concentrations with measurements in US waters. Environ Pollut. 2013;178:271–7. doi: 10.1016/j.envpol.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 50.Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, et al. Effects of the environmental estrogenic contaminants bisphenol A and 17alpha-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. Gen Comp Endocrinol. 2015 doi: 10.1016/j.ygcen.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Hinteman T, Schneider C, Scholer HF, Schneider RJ. Field study using two immunoassays for the determination of estradiol and ethinylestradiol in the aquatic environment. Water Res. 2006;40:2287–94. doi: 10.1016/j.watres.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 52.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, et al. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–14. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 53.Ryan BC, Vandenbergh JG. Developmental exposure to environmental estrogens alters anxiety and spatial memory in female mice. Horm Behav. 2006;50:85–93. doi: 10.1016/j.yhbeh.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Ceccarelli I, Della Seta D, Fiorenzani P, Farabollini F, Aloisi AM. Estrogenic chemicals at puberty change ERalpha in the hypothalamus of male and female rats. Neurotoxicol Teratol. 2007;29:108–15. doi: 10.1016/j.ntt.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 55.vom Saal FS, Akingbemi BT, Belcher SM, Crain DA, Crews D, Guidice LC, et al. Flawed experimental design reveals the need for guidelines requiring appropriate positive controls in endocrine disruption research. Toxicol Sci. 2010;115:612–3. doi: 10.1093/toxsci/kfq048. [DOI] [PubMed] [Google Scholar]
- 56.vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research. 2006;100:50–76. doi: 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 57.Dugard ML, Tremblay-Leveau H, Mellier D, Caston J. Prenatal exposure to ethinylestradiol elicits behavioral abnormalities in the rat. Brain Res Dev Brain Res. 2001;129:189–99. doi: 10.1016/s0165-3806(01)00205-x. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson SA, Delclos KB, Newbold RR, Flynn KM. Dietary ethinyl estradiol exposure during development causes increased voluntary sodium intake and mild maternal and offspring toxicity in rats. Neurotoxicol Teratol. 2003;25:491–501. doi: 10.1016/s0892-0362(03)00015-1. [DOI] [PubMed] [Google Scholar]
- 59.Arabo A, Lefebvre M, Fermanel M, Caston J. Administration of 17alpha-ethinylestradiol during pregnancy elicits modifications of maternal behavior and emotional alteration of the offspring in the rat. Brain Res Dev Brain Res. 2005;156:93–103. doi: 10.1016/j.devbrainres.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Reisbick S, Rosenblatt JS, Mayer AD. Decline of maternal behavior in the virgin and lactating rat. J Comp Physiol Psychol. 1975;89:722–32. doi: 10.1037/h0077059. [DOI] [PubMed] [Google Scholar]
- 61.Pereira M, Ferreira A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav Brain Res. 2006;175:139–48. doi: 10.1016/j.bbr.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 62.Pereira M, Morrell JI. The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: facilitation followed by inhibition. Behav Brain Res. 2009;205:238–48. doi: 10.1016/j.bbr.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoji H, Kato K. Maternal behavior of primiparous females in inbred strains of mice: a detailed descriptive analysis. Physiol Behav. 2006;89:320–8. doi: 10.1016/j.physbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 64.Wadia PR, Cabaton NJ, Borrero MD, Rubin BS, Sonnenschein C, Shioda T, et al. Low-Dose BPA Exposure Alters the Mesenchymal and Epithelial Transcriptomes of the Mouse Fetal Mammary Gland. PLoS ONE. 2013;8:e63902. doi: 10.1371/journal.pone.0063902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanno J, Onyon L, Haseman J, Fenner-Crisp P, Ashby J, Owens W. The OECD program to validate the rat uterotrophic bioassay to screen compounds for in vivo estrogenic responses: phase 1. Environ Health Perspect. 2001;109:785–94. doi: 10.1289/ehp.01109785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmes A, le Guisquet AM, Vogel E, Millstein RA, Leman S, Belzung C. Early life genetic, epigenetic and environmental factors shaping emotionality in rodents. Neurosci Biobehav Rev. 2005;29:1335–46. doi: 10.1016/j.neubiorev.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Kim B, Colon E, Chawla S, Vandenberg LN, Suvorov A. Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ Health. 2015;14:64. doi: 10.1186/s12940-015-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Catanese MC, Suvorov A, Vandenberg LN. Beyond a means of exposure: a new view of the mother in toxicology research. Toxicol Res. 2015;4:592–612. [Google Scholar]
- 69.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 70.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. 4th. Academic Press; 2012. [Google Scholar]
- 71.van der Loos CM. Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J Histochem Cytochem. 2008;56:313–28. doi: 10.1369/jhc.2007.950170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rudbeck L. Adding quality to your qualitative IHC. MLO Med Lab Obs. 2015;47:18–9. 21. [PubMed] [Google Scholar]
- 73.Tsuneoka Y, Maruyama T, Yoshida S, Nishimori K, Kato T, Numan M, et al. Functional, anatomical, and neurochemical differentiation of medial preoptic area subregions in relation to maternal behavior in the mouse. J Comp Neurol. 2013;521:1633–63. doi: 10.1002/cne.23251. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi T, Qi J, Wang HL, Zhang S, Morales M. Glutamatergic and dopaminergic neurons in the mouse ventral tegmental area. Eur J Neurosci. 2015;41:760–72. doi: 10.1111/ejn.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hall C. Emotional behavior in the rat. I. Defecation and urination as meas-ures of individual differences in emotionality. J Comp Psychol. 1934;18:385–403. [Google Scholar]
- 76.Hall C. Emotional behavior in the rat. II. The relationship between need and emotionality. J Comp Psychol. 1936;22:61–8. [Google Scholar]
- 77.Archer J. Tests for emotionality in rats and mice: a review. Anim Behav. 1973;21:205–35. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- 78.Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–9. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- 79.Prut L, Belzung C. The open field as a paradigm to measure the effects of drugs on anxietylike behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 80.Spruijt BM, Peters SM, de Heer RC, Pothuizen HH, van der Harst JE. Reproducibility and relevance of future behavioral sciences should benefit from a cross fertilization of past recommendations and today's technology: “Back to the future”. J Neurosci Methods. 2014;234:2–12. doi: 10.1016/j.jneumeth.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Ohta R, Takagi A, Ohmukai H, Marumo H, Ono A, Matsushima Y, et al. Ovariectomized mouse uterotrophic assay of 36 chemicals. J Toxicol Sci. 2012;37:879–89. doi: 10.2131/jts.37.879. [DOI] [PubMed] [Google Scholar]
- 82.Vandenberg LN. Low-dose effects of hormones and endocrine disruptors. Vitam Horm. 2014;94:129–65. doi: 10.1016/B978-0-12-800095-3.00005-5. [DOI] [PubMed] [Google Scholar]
- 83.Palanza P, Morellini F, Parmigiani S, vom Saal FS. Ethological methods to study the effects of maternal exposure to estrogenic endocrine disrupters: a study with methoxychlor. Neurotoxicol Teratol. 2002;24:55–69. doi: 10.1016/s0892-0362(01)00191-x. [DOI] [PubMed] [Google Scholar]
- 84.Zucchi FC, Yao Y, Ward ID, Ilnytskyy Y, Olson DM, Benzies K, et al. Maternal stress induces epigenetic signatures of psychiatric and neurological diseases in the offspring. PLoS One. 2013;8:e56967. doi: 10.1371/journal.pone.0056967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Garner JP, Mason GJ. Evidence for a relationship between cage stereotypies and behavioural disinhibition in laboratory rodents. Behav Brain Res. 2002;136:83–92. doi: 10.1016/s0166-4328(02)00111-0. [DOI] [PubMed] [Google Scholar]
- 86.Low M. Stereotypies and behavioural medicine: confusions in current thinking. Aust Vet J. 2003;81:192–8. doi: 10.1111/j.1751-0813.2003.tb11468.x. [DOI] [PubMed] [Google Scholar]
- 87.Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007;17:448–59. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gross AN, Richter SH, Engel AK, Wurbel H. Cage-induced stereotypies, perseveration and the effects of environmental enrichment in laboratory mice. Behav Brain Res. 2012;234:61–8. doi: 10.1016/j.bbr.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 89.Joel D. The signal attenuation rat model of obsessive-compulsive disorder: a review. Psychopharmacology (Berl) 2006;186:487–503. doi: 10.1007/s00213-006-0387-2. [DOI] [PubMed] [Google Scholar]
- 90.Maina G, Albert U, Bogetto F, Vaschetto P, Ravizza L. Recent life events and obsessive-compulsive disorder (OCD): the role of pregnancy/delivery. Psychiatry Res. 1999;89:49–58. doi: 10.1016/s0165-1781(99)00090-6. [DOI] [PubMed] [Google Scholar]
- 91.Abramowitz JS, Schwartz SA, Moore KM, Luenzmann KR. Obsessive-compulsive symptoms in pregnancy and the puerperium: a review of the literature. J Anxiety Disord. 2003;17:461–78. doi: 10.1016/s0887-6185(02)00206-2. [DOI] [PubMed] [Google Scholar]
- 92.Fairbrother N, Abramowitz JS. New parenthood as a risk factor for the development of obsessional problems. Behav Res Ther. 2007;45:2155–63. doi: 10.1016/j.brat.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 93.Forray A, Focseneanu M, Pittman B, McDougle CJ, Epperson CN. Onset and exacerbation of obsessive-compulsive disorder in pregnancy and the postpartum period. J Clin Psychiatry. 2010;71:1061–8. doi: 10.4088/JCP.09m05381blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive-compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- 95.Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. J Neural Transm (Vienna) 2004;111:753–71. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- 96.Swain JE. Baby stimuli and the parent brain: functional neuroimaging of the neural substrates of parent-infant attachment. Psychiatry (Edgmont) 2008;5:28–36. [PMC free article] [PubMed] [Google Scholar]
- 97.Swain JE, Kim P, Spicer J, Ho SS, Dayton CJ, Elmadih A, et al. Approaching the biology of human parental attachment: brain imaging, oxytocin and coordinated assessments of mothers and fathers. Brain Res. 2014;1580:78–101. doi: 10.1016/j.brainres.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller ES, Hoxha D, Wisner KL, Gossett DR. Obsessions and Compulsions in Postpartum Women Without Obsessive Compulsive Disorder. J Womens Health (Larchmt) 2015;24:825–30. doi: 10.1089/jwh.2014.5063. [DOI] [PubMed] [Google Scholar]
- 99.Giordano AL, Ahdieh HB, Mayer AD, Siegel HI, Rosenblatt JS. Cytosol and nuclear estrogen receptor binding in the preoptic area and hypothalamus of female rats during pregnancy and ovariectomized, nulliparous rats after steroid priming: correlation with maternal behavior. Horm Behav. 1990;24:232–55. doi: 10.1016/0018-506x(90)90007-k. [DOI] [PubMed] [Google Scholar]
- 100.Giordano AL, Siegel HI, Rosenblatt JS. Nuclear estrogen receptor binding in the preoptic area and hypothalamus of pregnancy-terminated rats: correlation with the onset of maternal behavior. Neuroendocrinology. 1989;50:248–58. doi: 10.1159/000125230. [DOI] [PubMed] [Google Scholar]
- 101.Giordano AL, Siegel HI, Rosenblatt JS. Nuclear estrogen receptor binding in microdissected brain regions of female rats during pregnancy: implications for maternal and sexual behavior. Physiol Behav. 1991;50:1263–7. doi: 10.1016/0031-9384(91)90594-e. [DOI] [PubMed] [Google Scholar]
- 102.Dahlstroem A, Fuxe K. Evidence for the Existence of Monoamine-Containing Neurons in the Central Nervous System. I. Demonstration of Monoamines in the Cell Bodies of Brain Stem Neurons. Acta Physiol Scand Suppl. 1964;SUPPL 232:1–55. [PubMed] [Google Scholar]
- 103.Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–53. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- 104.Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–65. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- 105.Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends Neurosci. 2007;30:188–93. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 106.Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 107.Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–69. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 108.Johnson ML, Ho CC, Day AE, Walker QD, Francis R, Kuhn CM. Oestrogen receptors enhance dopamine neurone survival in rat midbrain. J Neuroendocrinol. 2010;22:226–37. doi: 10.1111/j.1365-2826.2010.01964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gore AC, Heindel JJ, Zoeller RT. Endocrine disruption for endocrinologists (and others) Endocrinology. 2006;147:S1–3. doi: 10.1210/en.2005-1367. [DOI] [PubMed] [Google Scholar]
- 111.Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Jr, Lee DH, et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol. 2013;38:1–15. doi: 10.1016/j.reprotox.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cummings JA, Nunez AA, Clemens LG. A cross-fostering analysis of the effects of PCB 77 on the maternal behavior of rats. Physiol Behav. 2005;85:83–91. doi: 10.1016/j.physbeh.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 113.Cummings JA, Clemens LG, Nunez AA. Mother counts: how effects of environmental contaminants on maternal care could affect the offspring and future generations. Front Neuroendocrinol. 2010;31:440–51. doi: 10.1016/j.yfrne.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 114.Tomihara K, Zoshiki T, Kukita SY, Nakamura K, Isogawa A, Ishibashi S, et al. Effects of diethylstilbestrol exposure during gestation on both maternal and offspring behavior. Front Neurosci. 2015;9:79. doi: 10.3389/fnins.2015.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]