Key Points
Question
What is the optimum blood pressure target in secondary stroke prevention?
Findings
In this randomized clinical trial that included 1263 patients with a history of stroke, intensive blood pressure control to less than 120/80 mm Hg tended to reduce stroke recurrence compared with standard blood pressure control (<140/90 mm Hg). When this finding was pooled with the results of prior trials of intensive blood pressure control for secondary stroke prevention in an updated meta-analysis, intensive blood pressure treatment significantly reduced stroke recurrence by 22%.
Meaning
Intensive blood pressure control to less than 130/80 mm Hg is recommended for secondary stroke prevention.
This randomized clinical trial tests the hypothesis that targeting intensive blood pressure lowering of systolic and diastolic blood pressure less than 120 mm Hg and less than 80 mm Hg, respectively, reduces the rate of stroke recurrence compared with a standard blood pressure–lowering regimen.
Abstract
Importance
The Systolic Blood Pressure Intervention Trial (SPRINT) demonstrated that a systolic blood pressure (BP) target less than 120 mm Hg was superior to less than 140 mm Hg for preventing vascular events. This trial excluded patients with prior stroke; therefore, the ideal BP target for secondary stroke prevention remains unknown.
Objective
To assess whether intensive BP control would achieve fewer recurrent strokes vs standard BP control.
Design, Setting, and Participants
Randomized clinical trial (RCT) of standard vs intensive BP control in an intent-to-treat population of patients who had a history of stroke. Patients were enrolled between October 20, 2010, and December 7, 2016. For an updated meta-analysis, PubMed and the Cochrane Central Library database were searched through September 30, 2018, using the Medical Subject Headings and relevant search terms for cerebrovascular disease and for intensive BP lowering. This was a multicenter trial that included 140 hospitals in Japan; 1514 patients who had a history of stroke within the previous 3 years were approached, but 234 refused to give informed consent.
Interventions
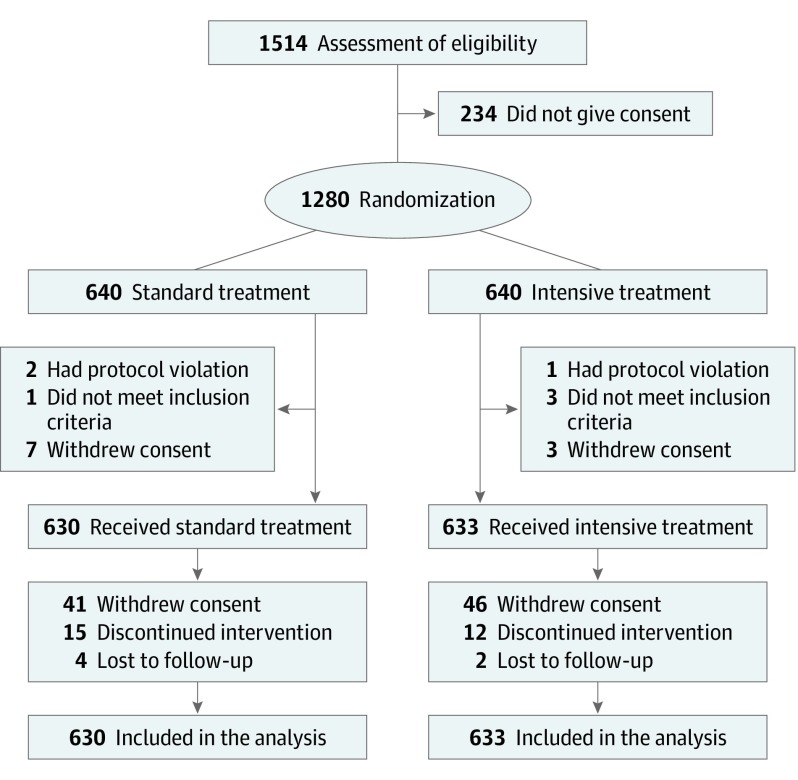
In total, 1280 patients were randomized 1:1 to BP control to less than 140/90 mm Hg (standard treatment) (n = 640) or to less than 120/80 mm Hg (intensive treatment) (n = 640). However, 17 patients never received intervention; therefore, 1263 patients assigned to standard treatment (n = 630) or intensive treatment (n = 633) were analyzed.
Main Outcomes and Measures
The primary outcome was stroke recurrence.
Results
The trial was stopped early. Among 1263 analyzed patients (mean [SD] age, 67.2 [8.8] years; 69.4% male), 1257 of 1263 (99.5%) completed a mean (SD) of 3.9 (1.5) years of follow-up. The mean BP at baseline was 145.4/83.6 mm Hg. Throughout the overall follow-up period, the mean BP was 133.2/77.7 (95% CI, 132.5-133.8/77.1-78.4) mm Hg in the standard group and 126.7/77.4 (95% CI, 125.9-127.2/73.8-75.0) mm Hg in the intensive group. Ninety-one first recurrent strokes occurred. Nonsignificant rate reductions were seen for recurrent stroke in the intensive group compared with the standard group (hazard ratio [HR], 0.73; 95% CI, 0.49-1.11; P = .15). When this finding was pooled in 3 previous relevant RCTs in a meta-analysis, the risk ratio favored intensive BP control (relative risk, 0.78; 95% CI, 0.64-0.96; P = .02; absolute risk difference, −1.5%; 95% CI, −2.6% to −0.4%; number needed to treat, 67; 95% CI, 39-250).
Conclusions and Relevance
Intensive BP lowering tended to reduce stroke recurrence. The updated meta-analysis supports a target BP less than 130/80 mm Hg in secondary stroke prevention.
Trial Registration
ClinicalTrials.gov identifier: NCT01198496
Introduction
In 2010, the absolute number of people with a first stroke in the world was 16.9 million, and the number with stroke-related deaths was 5.9 million.1 Therefore, prevention of primary and secondary stroke is a priority. Elevated blood pressure (BP) is the most relevant and prevalent risk factor for stroke. Reduction in BP is the most effective intervention to prevent both primary and secondary strokes.2,3,4,5,6,7 In clinical trials for primary prevention of cardiovascular events, including stroke, the lower the better seems acceptable for stroke prevention in hypertensive patients, with less than 115 mm Hg suggested as the optimum target level of systolic BP.8 After a stroke, lowering BP in the chronic stage reduced the rates of recurrent stroke among both hypertensive and nonhypertensive patients in the Perindopril Protection Against Recurrent Stroke Study (PROGRESS).4 A post hoc analysis of the PROGRESS9 suggested that the optimum target level of systolic BP for the prevention of recurrent stroke is less than 120 mm Hg. In the Secondary Prevention of Small Subcortical Strokes (SPS3) randomized trial,10 the BP target was first evaluated in patients with recent stroke. The trial randomly assigned those with lacunar stroke to a systolic BP target of 130 to 149 mm Hg or less than 130 mm Hg, and the authors showed that the use of a systolic BP target less than 130 mm Hg is likely to be beneficial, especially for the prevention of hemorrhagic stroke. A recent meta-analysis demonstrated that strict and aggressive control of BP with achieved mean systolic and diastolic BP levels less than 130 mm Hg and less than 85 mm Hg, respectively, seemed to be beneficial for secondary prevention.7 In primary prevention, the Systolic Blood Pressure Intervention Trial (SPRINT)11 proved the benefit of aggressive BP control, demonstrating that targeting a systolic BP less than 120 mm Hg resulted in lower rates of major cardiovascular events compared with less than 140 mm Hg. Although a pooled analysis of 3 studies12,13,14 (3632 participants) comparing different systolic BP targets suggested that intensive BP lowering reduced the rate of recurrent stroke, no clinical trials to date have tested the effect of such aggressive BP lowering for secondary stroke prevention. In the Recurrent Stroke Prevention Clinical Outcome (RESPECT) Study, we herein tested the hypothesis that targeting intensive BP lowering of systolic and diastolic blood BP less than 120 mm Hg and less than 80 mm Hg, respectively, reduces the rate of stroke recurrence compared with a standard BP-lowering regimen.
Methods
Study Design and Participants
The RESPECT Study was a prospective, multicenter, open, masked–end point, randomized clinical trial (RCT) that included 140 hospitals in Japan between October 20, 2010, and December 7, 2016. In total, 1514 patients who had a history of stroke within the previous 3 years were approached, but 234 refused to give informed consent. Eligible participants had the following characteristics: age 50 to 85 years, independent ambulation, systolic BP of 130 to 180 mm Hg or diastolic BP of 80 to 110 mm Hg on a regimen of 0 to 3 antihypertensive medications, and a history of stroke within the previous 3 years (evidence of an acute disturbance of focal neurological functions, with symptoms lasting more than 24 hours, and symptomatic ischemic stroke or intracerebral hemorrhage confirmed by magnetic resonance imaging or computed tomography). Patients in whom stroke onset occurred 1 month or less previously were excluded. Participation required written informed consent, and approval was provided by all local ethics committees for human research. The trial protocol and statistical analysis plan, including clinical sites and numbers of participants, are available in Supplement 1. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Randomization
In total, 1280 patients were randomized 1:1 to 2 BP control groups with targets of either less than 140/90 mm Hg (the standard treatment group [n = 640]) or less than 120/80 mm Hg (the intensive treatment group [n = 640]) according to a parallel design. However, 17 patients never received intervention; therefore, 1263 patients assigned to standard treatment (n = 630) or intensive treatment (n = 633) were analyzed. Randomization was conducted through a password-protected, internet-based system according to a computer-generated random sequence, with patients stratified by age (<70 vs ≥70 years), presence of diabetes, chronic kidney disease, history of myocardial infarction (MI), and history of atrial fibrillation.
Intervention
The intensive treatment group received stepwise multidrug therapy with a BP target less than 120/80 mm Hg, and the standard treatment group received the same stepwise therapy with BP targets less than 140/90 mm Hg or less than 130/80 mm Hg for patients who have diabetes, chronic kidney disease, or a history of MI. The study used a combination drug of losartan potassium or other angiotensin II receptor blockers and hydrochlorothiazide, amlodipine besylate, and spironolactone to control BP. To achieve the target BP, patients received stepwise treatments orally every 4 weeks for 24 weeks at maximum during the titration period (trial protocol in Supplement 1).
Outcome Measures
The primary end point was recurrent stroke, including ischemic stroke and intracerebral hemorrhage. Recurrent stroke was clinically defined as a focal neurological deficit persisting for longer than 24 hours, as confirmed by magnetic resonance imaging or computed tomography. Stroke was deemed fatal if death occurred within 30 days. Secondary end points included the following: reductions in ischemic stroke, subtype of ischemic stroke (including atherothrombotic infarction, cardioembolic infarction, lacunar infarction, or infarction due to other and unknown etiology), intracerebral hemorrhage, subarachnoid hemorrhage, transient ischemic attack, acute MI defined by standard criteria (compatible clinical history with changes on electrocardiogram or in cardiac enzyme concentration), composite cardiovascular events (cardiovascular death, nonfatal stroke, and nonfatal MI), all-cause death, and the composite of all-cause death, nonfatal stroke, and nonfatal MI. Cardiovascular death was defined as sudden death, fatal stroke, fatal MI, fatal congestive heart failure, or death attributed to other cardiovascular disease. All reported efficacy outcomes were confirmed by a central adjudication committee that was masked to treatment assignment. Serious adverse events were defined as those that were fatal or life threatening, that resulted in clinically significant or persistent disability, that required hospitalization, or that were judged as a significant hazard or harm that required medical or surgical interventions.
Statistical Analysis
Our original plan was to recruit 5000 patients. In 2014, challenges in achieving recruitment targets prompted us to review the accumulated masked study data. In consideration of interim masked trial data showing an annual stroke recurrence rate of 4.6%, we revised our sample size to 2000 patients; we estimated that this sample size would provide 80% power (at α = .05) to detect a minimum relative reduction of 30% in the intensive treatment group, on the assumption that the cumulative recurrence rate of stroke would be 15.6% in the standard group during approximately 3.5 years of follow-up and a dropout rate of 10%.
In accordance with the intent-to-treat principle, all patients except for those who immediately withdrew their consent and those without any information after randomization were included in the analysis. Cumulative incidence was estimated using the Kaplan-Meier method and compared using a log-rank test between randomized groups. Incidences of outcomes were also estimated using a person-year approach. The effects of strict BP control on outcomes were calculated using univariable Cox proportional hazards models and are reported as hazard ratios (HRs) and 95% CIs. If multiple events of the same type occurred, the time to event was calculated as the time to first events. The effects of randomized treatment on BP during the overall follow-up period were estimated using linear mixed models. All analyses were based on the intent-to-treat principle and were performed with SAS (version 9.4; SAS Institute Inc) or R (version 3.4.1; The R Foundation for Statistical Computing). The study was registered with ClinicalTrials.gov (NCT01198496). The threshold of statistical significance was set at 2-sided P < .05.
Meta-analysis
We searched PubMed and the Cochrane Central Library database for RCTs that compared the effects of BP treatment using 2 different targets in patients with prior cerebrovascular disease through September 30, 2018. We used the Medical Subject Headings (MeSH) and relevant search terms for cerebrovascular disease (cerebrovascular disorders [MeSH] and all spellings of stroke, cerebral infarction, brain infarction, ischemic stroke, intracranial hemorrhage, intracerebral hemorrhage, cerebral hemorrhage, brain hemorrhage, hemorrhagic stroke, transient ischemic attack, and cerebral ischemia) and the MeSH and relevant search terms for intensive BP lowering (antihypertensive agents [MeSH] and all spellings of target BP, intensive BP, strict BP, and tight BP). The search was limited to RCTs. The literature search, data extraction, and quality assessment were conducted independently by 2 authors (H.A. and T.M.) using a standardized approach. All completed RCTs that compared more vs less intensive BP targets with pharmacological BP-lowering agents among patients with prior cerebrovascular disease were eligible for inclusion. Published reports were obtained for each trial, and standard information was extracted into a spreadsheet. The outcome for the meta-analysis was recurrent stroke, including ischemic stroke and intracerebral hemorrhage. For each trial, we calculated the relative risk and its variance according to the principle of intent to treat. Summary estimates of the effects and 95% CIs were calculated using a random-effects model with inverse-variance weighting. The percentage of variability across studies attributable to heterogeneity beyond chance was estimated using the I2 statistic. Meta-analysis was performed using a software program (Stata, version 15; StataCorp LP).
Results
Enrollment and Baseline Characteristics
The independent data and safety monitoring committee recommended continuation of research at the first interim analysis on August 14, 2016; however, the steering committee stopped enrollment on December 31, 2016, before reaching the planned sample size of 2000 because of slow recruitment and funding cessation. In total, 1280 patients were randomized, and 17 (1.3%) were excluded from the randomized population owing to protocol violation, unmet inclusion criteria, or immediate withdrawal of consent (Figure 1); the remaining 1263 patients (mean [SD] age, 67.2 [8.8] years; 69.4% male) were enrolled in the intent-to-treat analysis. A total of 1263 participants were enrolled, including 1074 with ischemic stroke and 189 with intracerebral hemorrhage as the qualifying event, and 1257 of 1273 (99.5%) were followed up for a mean (SD) of 3.9 (1.5) years (range, 0-5.5 years) (Table 1). The mean BP at baseline was 145.4/83.6 mm Hg. Baseline characteristics did not differ substantially between the treatment groups. The median time from qualifying stroke to randomization was 4.6 months (interquartile range, 1.7-13.0 months). The frequency of permanent discontinuation of BP-lowering therapy was similar between the randomized groups (15 of 630 [2.4%] in the standard treatment group vs 12 of 633 [1.9%] in the intensive treatment group). Among 1263 patients in the cohort, 6 patients (0.5%) were lost to follow-up, and an additional 87 of 1257 patients (6.9%) withdrew their consent and ended their follow-up early.
Figure 1. Flow of Participants Included in the RESPECT Study.
RESPECT indicates Recurrent Stroke Prevention Clinical Outcome.
Table 1. Patient Characteristics by Randomized Groups.
| Characteristic | Standard Treatment (n = 630) | Intensive Treatment (n = 633) |
|---|---|---|
| Age, mean (SD), y | 67.3 (8.8) | 67.2 (8.8) |
| Male, No. (%) | 428 (67.9) | 449 (70.9) |
| Blood pressure at entry, mean (SD), mm Hg | ||
| Systolic | 145.7 (12.9) | 145.1 (12.4) |
| Diastolic | 83.7 (10.4) | 83.6 (10.7) |
| BMI, mean (SD) | 23.9 (3.3) | 23.7 (3.2) |
| History of hypertension, No. (%) | 630 (100) | 633 (100) |
| Diabetes, No. (%) | 154 (24.4) | 142 (22.4) |
| Dyslipidemia, No. (%) | 234 (37.1) | 224 (35.4) |
| Coronary heart disease, No. (%) | 17 (2.7) | 15 (2.4) |
| Chronic kidney disease, No. (%)a | 27 (4.3) | 36 (5.7) |
| Atrial fibrillation, No. (%) | 53 (8.4) | 53 (8.4) |
| Stroke/TIA before qualifying event, No. (%) | 99 (15.7) | 94 (14.8) |
| Qualifying event, No. (%) | ||
| Ischemic stroke | 542 (86.0) | 532 (84.0) |
| Intracerebral hemorrhage | 88 (14.0) | 101 (16.0) |
| Time since qualifying event, median (IQR), mo | 4.6 (1.7-12.7) | 4.7 (1.8-13.2) |
| Serum creatinine, mean (SD), mg/dL | 0.81 (0.21) | 0.83 (0.22) |
| Cholesterol, mean (SD), mg/dL | ||
| LDL | 112.0 (34.5) | 111.8 (32.2) |
| HDL | 54.1 (14.9) | 54.4 (15.5) |
| Plasma glucose, mean (SD), mg/dL | 127.6 (52.8) | 125.4 (41.6) |
| Glycated hemoglobin, % | 5.7 (0.8) | 5.7 (0.9) |
| Antihypertensive drugs at entry | ||
| No. of medications, mean | 1.4 | 1.5 |
| ARB/ACE inhibitor, No. (%) | 428 (67.9) | 421 (66.5) |
| Thiazide, No. (%) | 80 (12.7) | 88 (13.9) |
| Calcium channel blocker, No. (%) | 239 (37.9) | 257 (40.6) |
| Other, No. (%) | 56 (8.9) | 43 (6.8) |
| Concomitant drugs at entry, No. (%) | ||
| Statin | 225 (35.7) | 210 (33.2) |
| Antiplatelet drug | 455 (72.2) | 442 (69.8) |
| Anticoagulant drug | 68 (10.8) | 68 (10.7) |
Abbreviations: ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; TIA, transient ischemic attack.
SI conversion factors: To convert cholesterol levels (HDL and LDL) to millimoles per liter, multiply by 0.0259; creatinine level to micromoles per liter, multiply by 88.4; glucose level to millimoles per liter, multiply by 0.0555; and glycated hemoglobin level to proportion of total hemoglobin, multiply by 0.01.
Defined as estimated glomerular filtration rate less than 60 mL/min/1.73 m2 of body surface area.
BP Control
Two treatments resulted in a rapid and sustained between-group difference in systolic and diastolic BP (eFigure 1 in Supplement 2). At 1 year of follow-up, the achieved BP was 132.0/77.5 (95% CI, 130.9-133.0/76.6-78.3) mm Hg in the standard target group and 123.7/72.8 (95% CI, 122.6-124.8/72.0-73.7) mm Hg in the intensive target group, for a mean difference of 8.3 mm Hg in systolic BP. Target BP levels were achieved by 61.7% (374 of 606) in the standard group and 32.0% (197 of 615) in the intensive group. Throughout the overall follow-up period, the mean BP was 133.2/77.7 (95% CI, 132.5-133.8/77.1-78.4) mm Hg in the standard group and 126.7/74.4 (95% CI, 125.9-127.2/73.8-75.0) mm Hg in the intensive group, for a mean difference of 6.5/3.3 (95% CI, 5.7-7.5/2.5-4.2) mm Hg. The mean number of antihypertensive drugs was 1.4 at baseline. At 1 year, patients in the intensive treatment group had received more antihypertensive drugs than those in the standard treatment group, and the mean numbers of antihypertensive drugs were 1.6 and 2.8 during the overall follow-up period in the standard and intensive groups, respectively. The relative distribution of antihypertensive classes used was almost similar in the 2 groups, although the use of diuretics was greater in the intensive treatment group.
Primary Outcome
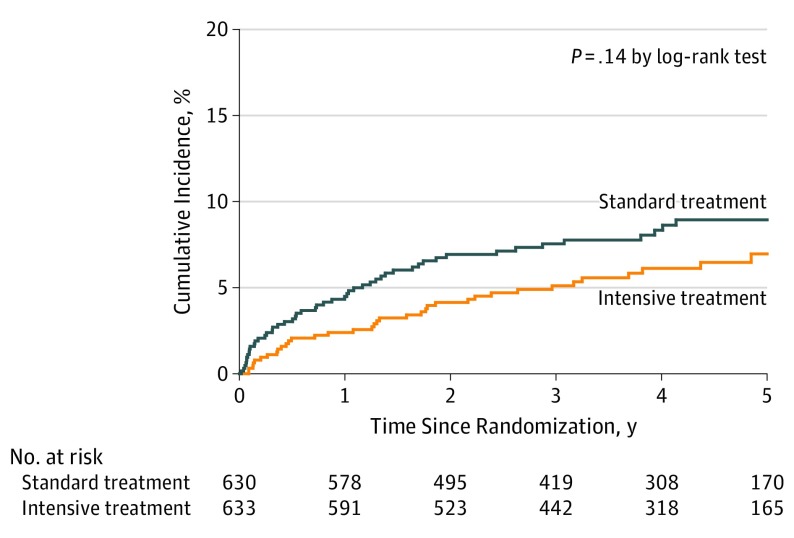
The primary outcome was stroke recurrence. During the follow-up period, 91 first recurrent strokes occurred. Of these, 79 (86.8%) were ischemic strokes, and 12 (13.2%) were intracerebral hemorrhages. The annualized rate of recurrent stroke in the standard treatment group was 2.26% compared with 1.65% in the intensive treatment group (Table 2), with an HR of 0.73; 95% CI, 0.49-1.11 (P = .15). Although the cumulative incidence of recurrent stroke seemed to separate over time between the randomized groups, the difference was not statistically significant (Figure 2). There was no heterogeneity in treatment effect on the primary outcome in any of the clinical subgroups (eFigure 2 in Supplement 2).
Table 2. Effects of Intensive Blood Pressure Treatment on Primary and Secondary Outcomes.
| Outcome | No. of Events (Annual Rate) | Hazard Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Standard Treatment (n = 630) | Intensive Treatment (n = 633) | |||
| Primary Outcome | ||||
| Strokea | 52 (2.26) | 39 (1.65) | 0.73 (0.49-1.11) | .15 |
| Secondary Outcomes | ||||
| Ischemic stroke | 41 (1.76) | 38 (1.60) | 0.91 (0.59-1.42) | .69 |
| Lacunar infarction | 12 (0.50) | 14 (0.58) | 1.16 (0.54-2.52) | .70 |
| Atherothrombotic infarction | 9 (0.37) | 4 (0.16) | 0.44 (0.14-1.42) | .17 |
| Cardiogenic embolism | 5 (0.21) | 4 (0.16) | 0.79 (0.21-2.96) | .73 |
| Other | 16 (0.67) | 19 (0.78) | 1.17 (0.60-2.27) | .65 |
| Intracerebral hemorrhage | 11 (0.46) | 1 (0.04) | 0.09 (0.01-0.70)b | .02b |
| Subarachnoid hemorrhage | 0 (0) | 1 (0.04) | Not calculable | |
| Transient ischemic attack | 3 (0.12) | 8 (0.33) | 2.66 (0.71-10.02) | .15 |
| Myocardial infarction | 4 (0.17) | 5 (0.20) | 1.23 (0.33-4.59) | .75 |
| Major vascular eventc | 59 (2.57) | 46 (1.95) | 0.76 (0.52-1.12) | .17 |
| All-cause death | 37 (1.52) | 30 (1.22) | 0.80 (0.49-1.29) | .36 |
| Composite outcomed | 86 (3.75) | 68 (2.88) | 0.77 (0.56-1.06) | .11 |
Ischemic stroke and intracerebral hemorrhage.
When the bootstrap percentile method was used for intracerebral hemorrhage, the 95% CI was 0.00 to 0.29, and the P value was less than .001.
Cardiovascular death, nonfatal stroke, and nonfatal myocardial infarction.
All-cause death, nonfatal stroke, and nonfatal myocardial infarction.
Figure 2. Cumulative Incidence of Stroke by Randomized Groups.
Stroke is a composite of ischemic stroke and intracerebral hemorrhage.
Secondary Outcomes
With regard to secondary end points, the rate of intracerebral hemorrhage (0.04% per patient-year) was reduced in the intensive treatment group compared with that in the standard group (0.46% per patient-year) (HR, 0.09; 95% CI, 0.01-0.70; P = .02). However, the rates of ischemic stroke (1.60% per patient-year in the intensive group vs 1.76% per patient-year in the standard group), subtypes of ischemic stroke, and mortality were similar (Table 2).
Adverse Events
Serious adverse events were similar between the 2 groups (eTable in Supplement 2). Adverse events related to hypotension, such as syncope and dizziness, were also infrequent and similar between the groups. The most frequent serious adverse event was malignant neoplasm in both groups (5.08% in the standard treatment group and 3.63% in the intensive treatment group).
Updated Meta-analysis
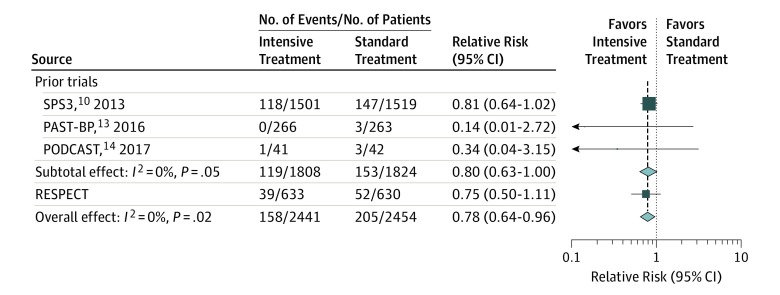
Three RCTs (the SPS3 trial,10 the Prevention After Stroke–Blood Pressure [PAST-BP] trial,13 and the Prevention of Decline in Cognition After Stroke Trial [PODCAST]14) investigated the effects of intensive BP lowering on recurrent stroke (cerebral infarction and intracerebral hemorrhage for the SPS3 trial10 and any stroke for the others) among patients with prior cerebrovascular disease (target systolic BP <125 mm Hg or <130 mm Hg). A meta-analysis of the 3 prior trials showed that no statistically significant reduction in recurrent stroke was seen in intensive BP lowering than in control treatment (relative risk, 0.80; 95% CI, 0.63-1.00; P = .05) (Figure 3). When an updated meta-analysis was conducted for the present trial with the 3 prior trials of intensive BP treatment for secondary prevention of stroke, the pooled risk ratio of this study and that of the 3 prior trials of intensive BP lowering for secondary stroke prevention favored intensive BP control (relative risk, 0.78; 95% CI, 0.64-0.96; P = .02), with no evidence of heterogeneity (I2 = 0%) (Figure 3). When the absolute risk difference was pooled for all 4 trials, the estimated risk difference was −1.5% (95% CI, −2.6% to −0.4%), and the estimated number needed to treat to avoid 1 recurrent stroke was 67 (95% CI, 39-250). In a meta-analysis performed for ischemic and hemorrhagic stroke separately, the pooled risk ratio favored intensive BP control only for hemorrhagic stroke (relative risk, 0.25; 95% CI, 0.07-0.90) and not for ischemic stroke (relative risk, 0.88; 95% CI, 0.71-1.08) (eFigure 3 in Supplement 2).
Figure 3. Effects of Intensive Blood Pressure Lowering on Recurrent Stroke in a Meta-analysis of Randomized Clinical Trials.
Boxes and horizontal lines represent relative risks and 95% CIs for each trial. The size of boxes is proportional to the inverse variance. Diamonds show the 95% CIs for pooled estimates of effect and are centered on the pooled relative risk. PAST-BP indicates Prevention After Stroke–Blood Pressure13; PODCAST, Prevention of Decline in Cognition After Stroke Trial14; RESPECT, Recurrent Stroke Prevention Clinical Outcome; and SPS3, Secondary Prevention of Small Subcortical Strokes.10
Discussion
Among adults with a history of stroke, the RESPECT Study showed that lowering BP to a target goal less than 120/80 mm Hg compared with the standard goal of less than 140/90 mm Hg resulted in nonsignificant reductions in all strokes. These effects were consistent across major subgroups, including patients 70 years or older and patients with diabetes. When combined in a meta-analysis of 3 other intensive BP treatment trials, there was a significant reduction in stroke recurrence in patients randomized to intensive BP treatment.
Blood pressure lowering has been strongly recommended for secondary prevention of chronic management after stroke since the results of the PROGRESS4 were published, but the optimum BP level and significance of intensive BP lowering needed to be examined. The SPS3 trial10 is the only large-scale study to date that has examined the effect of intensive BP lowering in stroke survivors. In patients with lacunar infarction, the researchers showed that within 180 days (median, 62 days) intensive BP lowering to a target less than 130 mm Hg (mean, 127 mm Hg) resulted in a nonsignificant reduction in all strokes (HR, 0.81; 95% CI, 0.64-1.03) and a significant reduction in intracerebral hemorrhages (HR, 0.37; 95% CI, 0.15-0.95) compared with standard BP lowering to a target of 130 to 149 mm Hg. Although the target goal of BP in the RESPECT Study was 120 mm Hg, the results are in line with those of the SPS3 trial,10 and our meta-analysis showed that intensive BP lowering is more beneficial for the prevention of recurrent stroke, especially intracerebral hemorrhage. Furthermore, risk reduction and BP difference may be consistent across trials: risk reduction and systolic BP difference, respectively, were 0.72 and 9.0 mm Hg in the PROGRESS,4 0.81 and 11.0 mm Hg in the SPS3 trial,10 0.90 and 4.0 mm Hg in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial,15 0.73 and 6.5 mm Hg in the RESPECT Study, and 0.73 and 7.0 mm Hg in a systematic review of all BP-lowering trials.16 Another RCT testing the effect of 3 different BP targets (135 to <145, 125 to <135, and <125 mm Hg) on stroke recurrence is ongoing.17
The post hoc analysis of the PROGRESS9 showed that lower BP is better and demonstrated that the target systolic BP level should be less than 120 mm Hg. Our RESPECT Study supported that the proposed target level of BP in the PROGRESS post hoc analysis is valid for stroke prevention. However, the consequences of BP lowering were more evident for intracerebral hemorrhage than for cerebral infarction. Asian epidemiological data18 also have shown that the association between BP and hemorrhagic stroke is greater than that between BP and ischemic stroke. In the PROGRESS,9 the rate of intracerebral hemorrhage was 0.04% per patient-year in patients with achieved a systolic BP of less than 120 mm Hg, which is consistent with the rate in the intensive treatment group of the present study (0.04% per patient-year). In the standard treatment group of the RESPECT Study and the group with achieved BP of 130 to 139 mm Hg in the PROGRESS,9 the rate of intracerebral hemorrhage was also similar (0.10% vs 0.10% per patient-year). In contrast, the benefit of intensive BP lowering for the prevention of recurrent cerebral infarction is unclear compared with standard treatment. In the post hoc analysis of the PROGRESS,9 the risk of cerebral infarction was similar between the achieved BP levels of 130 to 139 mm Hg and less than 130 mm Hg. In the SPS3 trial,10 the rate of ischemic stroke was similar between the standard and intensive treatment groups (HR, 0.84; 95% CI, 0.66-1.09). Our results also showed that the rate of cerebral infarction was similar between the standard and intensive treatment groups (HR, 0.91; 95% CI, 0.59-1.42). These results suggested that intensive BP lowering did not show clear benefit in preventing recurrent ischemic stroke, but this point needs further investigation.
Limitations
The RESPECT Study had several limitations. First, the trial did not have sufficient power to draw statistical significance for all strokes, which was the primary end point. However, our meta-analysis that combined the results of 3 other trials found a significant benefit of intensive BP lowering compared with standard treatment for stroke recurrence. Second, the assignment of either treatment group herein was not masked, which potentially introduced bias. However, the end points and adverse events were confirmed by an adjudication committee who were unaware of the allocation, as in previous larger hypertension trials.10,11 Third, we excluded patients older than 85 years because Japanese guidelines19 recommended a target BP less than 150/90 mm Hg in elderly hypertensive patients when the RESPECT Study started enrollment. It remains unclear whether intensive BP lowering is beneficial for very elderly patients with a history of stroke. Fourth, none of the individual studies had significant results for secondary stroke prevention, although the meta-analysis showed clear benefit.
Conclusions
Rate reductions seen for recurrent stroke in the intensive group compared with the standard group were not significant in this study. However, a meta-analysis of our results together with those of 3 previous trials support that the use of a BP target less than 130/80 mm Hg is likely to be beneficial in patients with a history of stroke.
Trial Protocol and Statistical Analysis Plan
eFigure 1. Systolic and Diastolic Blood Pressure by Randomized Groups
eFigure 2. Effects of Intensive Blood Pressure Treatment on Recurrent Stroke by Subgroups
eFigure 3. Effects of Intensive Blood Pressure Lowering on Recurrent Ischemic Stroke and Intracerebral Hemorrhage: Meta-analysis of Randomized Controlled Trials
eTable. Serious Adverse Events by Randomized Groups
Data Sharing Statement
References
- 1.Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. ; Global Burden of Diseases, Injuries, and Risk Factors Study 2010 (GBD 2010) and the GBD Stroke Experts Group . Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2014;383(9913):218.]. Lancet. 2014;383(9913):245-254. doi: 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Staessen JA, Wang JG, Thijs L. Cardiovascular protection and blood pressure reduction: a meta-analysis. Lancet. 2001;358(9290):1305-1315. doi: 10.1016/S0140-6736(01)06411-X [DOI] [PubMed] [Google Scholar]
- 3.Gueyffier F, Boissel JP, Boutitie F, et al. ; INDANA (Individual Data Analysis of Antihypertensive Intervention Trials) Project Collabora tors. Effect of antihypertensive treatment in patients having already suffered from stroke: gathering the evidence. Stroke. 1997;28(12):2557-2562. doi: 10.1161/01.STR.28.12.2557 [DOI] [PubMed] [Google Scholar]
- 4.PROGRESS Collaborative Group Randomised trial of a perindopril-based blood-pressure–lowering regimen among 6,105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358(9287):1033-1041. doi: 10.1016/S0140-6736(01)06178-5 [DOI] [PubMed] [Google Scholar]
- 5.Liu L, Wang Z, Gong L, et al. Blood pressure reduction for the secondary prevention of stroke: a Chinese trial and a systematic review of the literature. Hypertens Res. 2009;32(11):1032-1040. doi: 10.1038/hr.2009.139 [DOI] [PubMed] [Google Scholar]
- 6.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34(11):2741-2748. doi: 10.1161/01.STR.0000092488.40085.15 [DOI] [PubMed] [Google Scholar]
- 7.Katsanos AH, Filippatou A, Manios E, et al. Blood pressure reduction and secondary stroke prevention: a systematic review and metaregression analysis of randomized clinical trials. Hypertension. 2017;69(1):171-179. doi: 10.1161/HYPERTENSIONAHA.116.08485 [DOI] [PubMed] [Google Scholar]
- 8.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903-1913. doi: 10.1016/S0140-6736(02)11911-8 [DOI] [PubMed] [Google Scholar]
- 9.Arima H, Chalmers J, Woodward M, et al. ; PROGRESS Collaborative Group . Lower target blood pressures are safe and effective for the prevention of recurrent stroke: the PROGRESS trial. J Hypertens. 2006;24(6):1201-1208. doi: 10.1097/01.hjh.0000226212.34055.86 [DOI] [PubMed] [Google Scholar]
- 10.Benavente OR, Coffey CS, Conwit R, et al. ; SPS3 Study Group . Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382(9891):507-515. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright JT Jr, Williamson JD, Whelton PK, et al. ; SPRINT Research Group . A randomized trial of intensive versus standard blood-pressure control [published correction appears at N Engl J Med. 2017;377(25):2506]. N Engl J Med. 2015;373(22):2103-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zonneveld TP, Richard E, Vergouwen MD, et al. Blood pressure–lowering treatment for preventing recurrent stroke, major vascular events, and dementia in patients with a history of stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2018;7:CD007858. doi: 10.1002/14651858.CD007858.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mant J, McManus RJ, Roalfe A, et al. Different systolic blood pressure targets for people with history of stroke or transient ischaemic attack: PAST-BP (Prevention After Stroke–Blood Pressure) randomised controlled trial. BMJ. 2016;352:i708. doi: 10.1136/bmj.i708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bath PM, Scutt P, Blackburn DJ, et al. ; PODCAST Trial Investigators . Intensive versus guideline blood pressure and lipid lowering in patients with previous stroke: main results from the pilot “Prevention of Decline in Cognition After Stroke Trial” (PODCAST) randomised controlled trial. PLoS One. 2017;12(1):e0164608. doi: 10.1371/journal.pone.0164608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yusuf S, Diener HC, Sacco RL, et al. ; PRoFESS Study Group . Telmisartan to prevent recurrent stroke and cardiovascular events. N Engl J Med. 2008;359(12):1225-1237. doi: 10.1056/NEJMoa0804593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salam A, Atkins E, Sundström J, et al. ; Blood Pressure Lowering Treatment Trialists’ Collaboration . Effects of blood pressure lowering on cardiovascular events, in the context of regression to the mean: a systematic review of randomized trials. J Hypertens. 2019;37(1):16-23. doi: 10.1097/HJH.0000000000001994 [DOI] [PubMed] [Google Scholar]
- 17.Zanchetti A, Liu L, Mancia G, et al. Blood pressure and LDL-cholesterol targets for prevention of recurrent strokes and cognitive decline in the hypertensive patient: design of the European Society of Hypertension–Chinese Hypertension League Stroke in Hypertension Optimal Treatment randomized trial. J Hypertens. 2014;32(9):1888-1897. doi: 10.1097/HJH.0000000000000254 [DOI] [PubMed] [Google Scholar]
- 18.Lawes CM, Rodgers A, Bennett DA, et al. ; Asia Pacific Cohort Studies Collaboration . Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21(4):707-716. doi: 10.1097/00004872-200304000-00013 [DOI] [PubMed] [Google Scholar]
- 19.Shimamoto K, Ando K, Fujita T, et al. ; Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension . The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37(4):253-390. doi: 10.1038/hr.2014.20 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure 1. Systolic and Diastolic Blood Pressure by Randomized Groups
eFigure 2. Effects of Intensive Blood Pressure Treatment on Recurrent Stroke by Subgroups
eFigure 3. Effects of Intensive Blood Pressure Lowering on Recurrent Ischemic Stroke and Intracerebral Hemorrhage: Meta-analysis of Randomized Controlled Trials
eTable. Serious Adverse Events by Randomized Groups
Data Sharing Statement