Abstract
Objectives
Allergic bronchopulmonary aspergillosis (ABPA) can accelerate lung function decline in patients with cystic fibrosis (CF). Antifungal medication can be used in addition to systemic corticosteroid treatment.
Patients and methods
We evaluated Aspergillus-specific IgE and the use of therapeutic drug monitoring of triazoles in a retrospective analysis of 32 patients.
Results
There was a significant reduction in Aspergillus IgE with posaconazole but not with other triazoles (P = 0.026). Aspergillus IgE levels were inversely correlated with the therapeutic drug level of posaconazole.
Conclusions
These data suggest that posaconazole is better than comparator azoles at decreasing serological response to Aspergillus and that this response was better with therapeutic levels of posaconazole.
Introduction
Allergic bronchopulmonary aspergillosis (ABPA) is characterized by an IgE-driven atopic response to Aspergillus species, mainly Aspergillus fumigatus. This hypersensitivity response causes bronchial inflammation and airway destruction, resulting in the formation of bronchiectasis. ABPA is recognized to be overrepresented in patients with cystic fibrosis (CF).1 Prevalence of ABPA in CF has been reported to be as high as 13% depending on the diagnostic criteria used.2 In CF patients, untreated active ABPA can hasten lung function decline.3 Although total IgE has traditionally been used to measure treatment response in ABPA,4 recent data suggest that changes in total IgE may not correlate well with clinical outcomes.5 Specific Aspergillus IgE levels effectively differentiate between patients with ABPA and sensitization,6 and Aspergillus IgE levels correspond to airflow obstruction and radiological changes in sensitized asthmatics, including those with ABPA,7 and previously have been shown to correspond with disease activity.8
Currently, the mainstay of treatment is prednisolone to reduce inflammation,9 but oral antifungal drugs, mould-active triazoles, appear to be at least equivalent to steroids and have been used as steroid-sparing agents or when steroids have proven ineffective.10–12 Triazoles inhibit lanosterol 14α-demethylase, thus inhibiting synthesis of ergosterol, which is important in fungal cell wall synthesis. Triazoles are associated with a high incidence of adverse events and as such are relatively poorly tolerated; this is exacerbated in CF patients who are prone to drug sensitivities, particularly gastrointestinal side effects.13 There are additional challenges with triazole drug interactions, for example interactions with ivacaftor for correction of CF transmembrane conductance regulator function.
Posaconazole has been approved for use in the context of prophylaxis or treatment of invasive fungal disease (IFD) in patients. The resistance profile is similar to itraconazole, but it has superior absorption and is better tolerated. There have been no trials comparing the efficacy of different antifungal agents in the treatment of ABPA. However, clinical experience at our centre is that posaconazole is more effective than other triazoles in the treatment of ABPA. We sought to assess whether there were any objective differences between triazoles.
Materials and methods
We retrospectively analysed all CF patients (a cohort of 596) at our tertiary referral centre who had been on triazoles for treatment of ABPA during 2016, examining the entire history of their ABPA treatment. We evaluated specific Aspergillus IgE levels and triazole drug levels, measured by LC-MS/MS. Aspergillus IgE slopes were derived from log10 of specific IgE levels immediately prior to commencing the specific triazole and levels at the end of treatment with that triazole. Aspergillus IgE versus triazole therapeutic dose monitoring correlation was derived from blood samples taken simultaneously, with several samples per patient, at varying timepoints during treatment. These were taken opportunistically at outpatient visits but also during inpatient admission. Triazole susceptibility was measured by standardized broth microdilution, with breakpoints determining susceptibility of 1 mg/L for itraconazole and voriconazole and 0.125 mg/L for posaconazole. Many patients had been treated sequentially with multiple triazoles, owing to either poor tolerance or progressive symptoms. During the study period, patients were treated with itraconazole, voriconazole and posaconazole.
Statistical analysis was carried out on GraphPad V7. Specific IgE change over time was compared by Mann–Whitney test. Linear regression was used to assess correlation between drug levels of azoles and specific IgE levels.
Results
A total of 32 patients were identified; the median age of patients evaluated was 24 years (IQR 20–30), 65% of whom were male. Thirty patients received itraconazole, 13 were treated with voriconazole and 18 with posaconazole. Eleven patients were treated with itraconazole alone, 12 with two different triazoles and the remaining 9 with all three triazoles. The majority of patients were commenced with itraconazole first line, and occasionally voriconazole, with posaconazole being reserved for second- or third-line treatment, with triazoles given in sequence. The median duration of treatment without a break was 39 months for itraconazole, 23 months for voriconazole and 29 months for posaconazole. The median total duration of treatment was 8 years (range from 10 months to 17 years and 5 months). The reasons noted for a switch in antifungals included drug intolerance (e.g. gastrointestinal side effects) and treatment failure, though it was not always clear how this was defined.
Eleven patients had a positive fungal culture, nine with A. fumigatus and two with Aspergillusflavus. The susceptibility data were recorded for six of these samples; all but one were fully susceptible (tested against azoles and amphotericin). One patient had A. fumigatus that exhibited pan-azole resistance and had no serological response to treatment with any triazole.
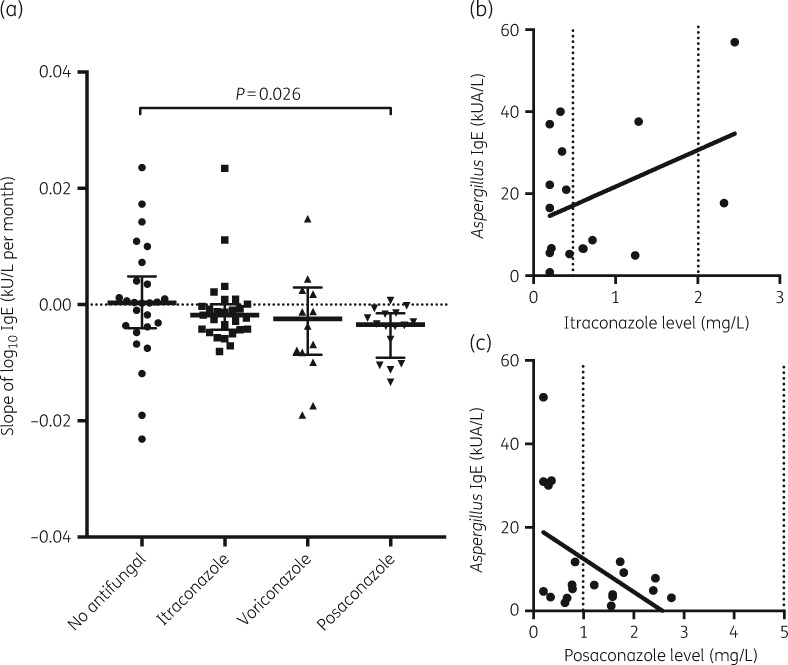
Reduction in Aspergillus IgE with treatment was significantly different between no treatment (i.e. corticosteroids only) and posaconazole (P = 0.026) (Figure 1a), but not other triazoles, as measured by the rate of Aspergillus IgE change over time. Aspergillus IgE levels were inversely correlated with therapeutic drug level of posaconazole, but not itraconazole, with insufficient data to make an effective correlation with voriconazole (Figure 1b and c). This suggests that monitoring posaconazole drug levels, and altering dose to attain therapeutic serum levels, is associated with improved serological responses in ABPA.
Figure 1.
(a) Change in Aspergillus IgE per month by treatment. These data are represented as log10 change in specific IgE in kU/L/month. Individual patients’ serological data are shown with median and IQR. Correlation between therapeutic drug monitoring and serological markers in (b) ABPA IgE level correlation with itraconazole level (R = 0.157, P = 0.116), with therapeutic range marked by dotted line, and (c) ABPA IgE level correlation with posaconazole level (R = 0.232, P = 0.031), with therapeutic range marked by dotted line.
Discussion
These are the first data that compare posaconazole with other triazoles in the treatment of ABPA in adults. They suggest that posaconazole is more effective than comparator triazoles at reducing A. fumigatus sensitization in ABPA in CF patients.
Amongst the adult CF cohort at our centre, patients have had their antifungal changed to posaconazole for a number of reasons, including poor tolerance of other triazoles, progressive symptoms and lung function decline on other triazoles. The serological response seen could be due to a number of factors including: tolerability of posaconazole; the ability to achieve therapeutic levels; and potentially increased antifungal killing in comparison with other triazoles. Not all fungal isolates were tested for triazole susceptibility, but the available results suggest no difference in susceptibility to posaconazole compared with itraconazole or voriconazole, suggesting that antifungal resistance is not a factor. These are also the first data that suggest that triazole drug level measurement has a role in the monitoring of chronic fungal disease treatment. The data suggest that altering posaconazole dose to attain greater serum levels is associated with improved serological responses in ABPA. Therapeutic dose monitoring is recommended in the management of IFD14 and chronic pulmonary aspergillosis.15 The established triazole therapeutic ranges are based on treatment of IFD; these were used to guide dose adjustment in this study. The levels of posaconazole associated with lower levels of Aspergillus IgE were within the IFD range of 1–5 mg/L. More supporting data are required before setting a specific therapeutic range for ABPA.
Further studies are required to define the impact on clinical outcome such as effect on symptoms, exacerbation frequency and lung function. Although total IgE response to treatment has correlated with clinical outcome in some studies,4 but not others,5 there have not been any published data linking specific IgE change with outcome. As such, the promising signal for the efficacy of posaconazole needs further investigation in a clinical trial, specifically looking at clinical outcomes, tolerability, the effect of therapeutic drug monitoring and relationship with fungal culture susceptibilities.
Conclusions
In summary, we believe these are the first data to suggest the possibility that posaconazole has better therapeutic efficacy in comparison with itraconazole and voriconazole in ABPA amongst CF patients. These data also indicate that achieving therapeutic serum levels of posaconazole is associated with serological response in ABPA.
Funding
This work was supported by Astellas who provided funding that supported J. P.’s post. The Wellcome Trust (grant 204566/Z/16/Z), Medical Research Council (grant MR/R014590/1) and Natural Environment Research Council (grant NE/P001165/1) support D. A.-J. The data in this paper were gathered during routine work at Royal Brompton & Harefield NHS Foundation Trust.
Transparency declarations
S. S. has received personal fees from Gilead Sciences; D. A.-J. has received grants and personal fees from Pulmocide Ltd and Gilead Sciences. All other authors: none to declare.
Disclaimer
The funders had no role in the study design, data collection, analysis, interpretation or decision to submit the work for publication.
References
- 1. Nelson LA, Callerame ML, Schwartz RH.. Aspergillosis and atopy in cystic fibrosis. Am Rev Respir Dis 1979; 120: 863–73. [DOI] [PubMed] [Google Scholar]
- 2. Chotirmall SH, Branagan P, Gunaratnam C. et al. Aspergillus/allergic bronchopulmonary aspergillosis in an Irish cystic fibrosis population: a diagnostically challenging entity. Respir Care 2008; 53: 1035–41. [PubMed] [Google Scholar]
- 3. Kraemer R, Deloséa N, Ballinari P. et al. Effect of allergic bronchopulmonary aspergillosis on lung function in children with cystic fibrosis. Am J Respir Crit Care Med 2006; 174: 1211–20. [DOI] [PubMed] [Google Scholar]
- 4. Ricketti AJ, Greenberger PA, Patterson R.. Serum IgE as an important aid in management of allergic bronchopulmonary aspergillosis. J Allergy Clin Immunol 1984; 74: 68–71. [DOI] [PubMed] [Google Scholar]
- 5. Agarwal R, Gupta D, Aggarwal AN. et al. Clinical significance of decline in serum IgE levels in allergic bronchopulmonary aspergillosis. Respir Med 2010; 104: 204–10. [DOI] [PubMed] [Google Scholar]
- 6. Baxter CG, Denning DW, Jones AM. et al. Performance of two Aspergillus IgG EIA assays compared with the precipitin test in chronic and allergic aspergillosis. Clin Microbiol Infect 2013; 19: E197–204. [DOI] [PubMed] [Google Scholar]
- 7. Woolnough KF, Richardson M, Newby C. et al. The relationship between biomarkers of fungal allergy and lung damage in asthma. Clin Exp Allergy 2017; 47: 48–56. [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg M, Patterson R, Roberts M.. Immunologic responses to therapy in allergic bronchopulmonary aspergillosis: serum IgE value as an indicator and predictor of disease activity. J Pediatr 1977; 91: 914–7. [DOI] [PubMed] [Google Scholar]
- 9. Stevens DA, Moss RB, Kurup VP. et al. Allergic bronchopulmonary aspergillosis in cystic fibrosis—state of the art: Cystic Fibrosis Foundation Consensus Conference. Clin Infect Dis 2003; 37: S225–64. [DOI] [PubMed] [Google Scholar]
- 10. Glackin L, Leen G, Elnazir B. et al. Voriconazole in the treatment of allergic bronchopulmonary aspergillosis in cystic fibrosis. Ir Med J 2009; 102: 29.. [PubMed] [Google Scholar]
- 11. Agarwal R, Dhooria S, Sehgal IS. et al. A randomized trial of itraconazole vs prednisolone in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Chest 2018; 153: 656–64. [DOI] [PubMed] [Google Scholar]
- 12. Agarwal R, Dhooria S, Sehgal IS. et al. A randomized trial of voriconazole and prednisolone monotherapy in acute-stage allergic bronchopulmonary aspergillosis complicating asthma. Eur Respir J 2018; 52: 1801159. [DOI] [PubMed] [Google Scholar]
- 13. Burgel PR, Paugam A, Hubert D. et al. Aspergillus fumigatus in the cystic fibrosis lung: pros and cons of azole therapy. Infect Drug Resist 2016; 9: 229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Walsh TJ, Raad I, Patterson TF. et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin Infect Dis 2007; 44: 2–12. [DOI] [PubMed] [Google Scholar]
- 15. Denning DW, Cadranel J, Beigelman-Aubry C. et al. Chronic pulmonary aspergillosis: rationale and clinical guidelines for diagnosis and management. Eur Respir J 2016; 47: 45–68. [DOI] [PubMed] [Google Scholar]