Abstract
Background: The presence of glioma stem cells (GSCs) is thought to be a key factor responsible for development of the incurable glioblastoma multiforme (GBM). GSCs are often displayed during chemotherapy resistance, except for demethoxycurcumin (DMC), a component of curcumin, which has been previously confirmed to inhibit GSCs proliferation and induce apoptosis.
Purpose: The objective of this study was to identify the main mechanism underlying anti-GSCs resistance by DMC.
Patients and methods: qRT-PCR was used to determine the expression of miR-145 in glioma patients and GSCs, and GSCs were transfected with miR-145 overexpressed vectors. Then, functional analyses (in vitro and in vivo) were performed to confirm the role of miR-145 and DMC in GSCs. Finally, related proteins were tested by immunohistochemistry and Western blot.
Results: miR-145 was atypically low-expressed miRNA in GSCs, and could enhance GSC chemosensitivity to DMC both in vitro and in vivo. Upregulation of miR-145 in GSCs resulted in increased cell growth inhibition and apoptosis to DMC. Further research on the mechanism demonstrated that the combined effects of miR-145 and DMC were involved in the miR-145/SOX2-Wnt/β-catenin pathway. Overexpression of SOX2 reduced GSC resistance to growth inhibition by miR-145+ DMC treatment.
Conclusion: Our data strongly support an important role for miR-145 in enhancing GSC chemosensitivity to DMC by targeting the SOX2-Wnt/β-catenin axis.
Keywords: glioma stem cells, miR-145, demethoxycurcumin, proliferation, apoptosis
Introduction
Glioma stem cells (GSCs) are a very small subpopulation of glioma cells that have the ability to induce tumor development, maintain tumor growth, and preserve tumor heterogeneity. Under appropriate conditions, GSCs can differentiate into new tumor cells; thus, they are regarded as the origin of the occurrence, recurrence, and metastasis of tumors. They are further confirmed to be resistant to chemotherapy and radiotherapy.1 To date, even in combination with conventional treatments such as surgery and chemoradiotherapy, no treatment has demonstrated proven efficacy for this disease.
MicroRNAs are important endogenous non-coding small RNAs involved in the process of targeted gene silencing, including translation inhibition, mRNA degradation, and deadenylation. In recent years, many studies have shown that the expression levels of miRNAs in different tissues and cells are significantly different, especially in normal cells and stem cells.2,3 Because of the special ability of stem cells, many biological processes in stem cells are different from ordinary cells. Thus, it is very important to study the mechanisms of miRNAs in stem cell processes. Previous studies have shown that miR-101 and miR-152 can inhibit the malignant biological behavior of glioma stem cells by inhibiting expression of its downstream target genes.4,5 Studies by Chan et al showed that primary gliomas had a similar miRNA expression profile to neural precursor cells (NPCs).6 They found that excessive expression of miR-34a, miR-137, and miR-451 could inhibit GSC proliferation, damage GSC formation, and induce GSC differentiation; however, it did not lead to apoptosis of GSCs. At the same time, they also identified miR-138 as a molecular marker of GSCs and confirmed that it played a key role in promoting the growth and survival of GSCs. Therefore, specific inhibition of miR-138 expression could prevent the formation of GSC spheres in vitro and inhibit tumor production in vivo.
In this study, we focused on miR-145, a miRNA found with reduced expression in glioblastoma multiforme tumors (GBM) and GSCs. Recent studies demonstrated that miR-145 played an important role in the development and migration of GSCs. Lee et al examined the delivery of miR-124 and miR-145 mimics in glioma cells and GSCs and showed that GSCs expressed very low levels of these miRNAs.7 The delivered miR-145 mimics significantly decreased migration of glioma cells and self-renewal of GSCs. Morgado et al investigated the precise role of miR-145 in neural stem cell (NSC) fate decision and confirmed that miR-145 modulation was crucial for neurogenesis progression.8 In our previous research, we confirmed that miR-145 showed low expression in primary GSCs, and its overexpression could effectively inhibit cell migration and invasion of GSCs by targeting the ABCG2 signaling pathway.9 In recent years, the “Pharmaco-miR” concept has attracted great attention. Researchers have found that many miRNAs could regulate chemotherapy sensitivity. Wang et al showed that miR-381 inhibition could sensitize glioblastoma cells to the chemotherapeutic drug temozolomide (TMZ).10 Zhang et al reported that miRNA-21 inhibition sensitized human U251 stem cells to TMZ.11 In view of the chemotherapy sensitivity of miR-145, Gao et al found that miR-145 could sensitize breast cancer to doxorubicin by targeting MRP1.12 In addition, Zhan et al demonstrated that miR-145 sensitizes gallbladder cancer to cisplatin by also regulating MRP1.13
However, whether miR-145 could sensitize GSCs to chemotherapeutic drugs is still unknown. Recently, Mirgani et al found that dendrosomal curcumin significantly decreased OCT4A, OCT4B1, SOX-2, and Nanog expression along with noticeable overexpression of miR-145,14 suggesting that the function of curcuminoids on gliomas was related to miR-145 regulation.15 In our previous research, we confirmed that a curcuminoid called demethoxycurcumin (DMC) was more efficient than TMZ on anti-gliomas and GSCs.16–19 Thus, we speculated that miR-145 might regulate chemotherapy sensitivity of GSCs to DMC. Further, until now, the targeting mechanism of miR-145 on the proliferation and apoptosis of GSCs was unknown. Our present study focused on the function and potential mechanisms of miR-145 on enhancing GSC chemosensitivity to DMC.
Materials and methods
Primary GSCs cultures
Fresh glioblastoma tumor tissues were cut into fragments and were repeatedly blown by suckers. After 1000 r/min centrifugation for 5 min, the red cell lysate was added to gently blow for 3 min. After another 1000 r/min centrifugation for 5 min, the supernatant was removed. Next, the cells were cultured using Cyagen Neural Stem Cell Basal Medium, including B27, Penicillin-Streptomycin, Glutamine, Heparin, bFGF, and EGF (Cyagen Biosciences, Suzhou, P. R. China). A large number of suspension cell balls appeared in primary culture after 7–10 days, after which, the cell spheres were dispersed into a single cell suspension and cultured each for 7 days. Primary GSCs (pGSCs) were identified by CD133 and Nestin staining. All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Nanjing Brain Hospital Affiliated to Nanjing Medical University and Affiliated Kunshan Hospital of Jiangsu University, and written informed consent was obtained from all patients providing tissue specimens.
Luciferase targeting assay
According to bioinformatics analysis, a SOX2-3ʹUTR fragment containing predicted miR-145 binding sites was cloned into the pGL3 luciferase reporter (Promega, Madison, USA) and transfected into GSCs using FuGENE HD (Promega, Beijing, P. R. China). Predicted miR-145 binding sites of mutant sequence without putative miR-145 binding sites were used as a mutated control. The luciferase activities were assayed by the Dual-Luciferase Reporter Assay System (Promega, Beijing, P. R. China) after a 48-h transfection.
In vivo tumor model
pGSC-1 and pGSC-2 cells were directly injected subcutaneously into the flanks of nude mice. After the tumor was established and reached 75 mm3 in volume, the mice were randomized into the following groups: (a) control group: 100 μL PBS intraperitoneal injection; (b) miR-145 group: multi-point injection of miR-145 overexpression lentiviral vectors in situ; (c) 10 mg/kg DMC group: 10 mg/kg DMC intraperitoneal injection plus miR-145 negative lentiviral vector in situ injections; (d) 10 mg/kg DMC + miR-145 group: 10 mg/kg DMC intraperitoneal injection plus miR-145 overexpression lentiviral vector in situ injections; (e) 30 mg/kg DMC group: 30 mg/kg DMC intraperitoneal injection plus miR-145 negative lentiviral vector in situ injections; (f) 30 mg/kg DMC + miR-145 group: 30 mg/kg DMC intraperitoneal injection plus miR-145 overexpression lentiviral vector in situ injections. The administration dose refers to our previous report.17 Mouse body weights were recorded every 2 days. Tumor volumes were calculated using the following formula tumor volume (TV) = (length × width2) ×0.5. The relative tumor volume was calculated as (RTV) = Vt/V0 (V0, the measured tumor volume at day 0; Vt, the measured tumor volume at each time point). The relative tumor proliferation rates T/C (%) were calculated using the following formula: TV/CV (%) = (TRTV/CRTV) ×100 (TRTV: the treatment group RTV; CRTV: model control group RTV). The tumor growth inhibition rates (%) were calculated using the following formula: TW/CW (%) = (the average tumor weight of administration group/the average tumor weight of model control group)/the average tumor weight of model control group ×100%. All procedures were conducted in accordance with the animal care laws of Nanjing Medical University and Affiliated Kunshan Hospital. After 2 weeks, the mice were sacrificed, and the tumor tissues were collected for immunohistochemistry (IHC). The study was approved by the Institutional Animal Care and Use Committee of Nanjing Brain Hospital Affiliated to Nanjing Medical University and Affiliated Kunshan Hospital of Jiangsu University. All operations were performed according to international guidelines concerning the care and treatment of experimental animals.
Real-time qPCR for miR-145 detection
All miR-145 primers and probes for TaqMan miRNA assays were purchased from ThermoFisher Scientific (Shanghai, P. R. China), amplified according to the manufacturer’s instructions, and detected using the ABI 7300 HT Sequence Detection system (Applied Biosystems, Foster City, CA, USA).19 RNU6B was used as an endogenous control. Relative gene expression was calculated by the 2−∆∆Ct method.
Cell viability assay
Briefly, 1×104 GSC cells were plated in 96-well culture plates and treated with miR-145 overexpression lentiviral vectors and/or DMC for 48 h. The cell proliferation assay was determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay according to the manufacturer’s instructions. The absorbance was detected at 540 nm by a microplate reader.
TUNEL cell apoptosis detection
One-step TUNEL apoptosis detection kit was purchased from Beyotime Biotechnology (Nanjing, P. R. China). The cells were washed once with PBS and fixed with an immunostaining fixative (P0098) for 30 min. After an ice bath for 2 min in 0.1% Triton X-100, 50 μL TUNEL testing solution was added and incubated for 60 min. Following, the cells were stained using Hoechst 33258. Apoptotic cells were observed and calculated under a fluorescence microscope.
Quantification by ELISA detection
Histone-complexed DNA fragments of pGSCs after treatment were detected by the Cell Death Detection ELISA PLUS (Roche, Palo Alto, CA) according to the manufacturer’s protocol. Briefly, cells were treated with an apoptosis-inducing agent in the well of a microplate, followed by removal of the supernatant after centrifugation (200 × g). The treated cells were incubated with lysis buffer for 30 min, and an aliquot of the supernatant (lysate) was transferred to the streptavidin-coated microplate. Subsequently, the supernatant was incubated with an immunoreagent containing anti-histone and anti-DNA for 2 h. Finally, substrate solution was added to wells, incubated, and the absorbance was measured at 405 nm.
Immunohistochemistry
Immunohistochemistry assay was performed to analyze cell proliferation as described previously.17 Briefly, paraffin sections were routinely dewaxed and antigen-repaired, and then, endogenous peroxidase was blocked with hydrogen peroxide for 10 min. Next, 50–100 μL of goat serum was added in a dropwise manner for 20 min. PCNA antibody (1:100 dilution) was added to the 50–100 μL and incubated at 37 °C for 2 h in a wet box. After immersing 3 times with PBS, 50 μL of IgG antibody-Fab fragment-HRP polymer was added, and incubation was carried out for 30 min at 20~30 °C. After adding DAB solution for color development, the sections were placed in hematoxylin staining solution, stained for 10 min, and finally dehydrated and sealed. Protein expression in the tissue cells was observed under a microscope, and three high-expression areas were photographed and stored. PCNA-positive staining was mainly located in the nucleus, which had a brownish-yellow, granular appearance.
Western blot analysis
Total proteins were isolated using RIPA Lysis and Extraction Buffer (Thermo Scientific, Shanghai, P. R. China). Protein electrophoresis using Tris-MOPS-SDS Running Buffer Powder (Genscript, Nanjing, P. R. China) and PAGE Gel transfer using Transfer Buffer Powder (Genscript, Nanjing, P. R. China) were conducted according to the manufacturer’s protocol. After PVDF membranes were incubated with the primary (SOX2, cyclin D1, c-myc, β-catenin, and GAPDH) and secondary antibodies, BeyoECL Plus (Beyotime Biotechnology, Nanjing, P. R. China) was added, and staining was detected using a fluoro imager.
Statistical analysis
Each independent experiment was repeated three times, and data were analyzed using the SPSS Graduate Pack 19.0 statistical software package (SPSS, Chicago, IL). Descriptive statistics, including mean, SE, and one-way ANOVAs, were used to determine significant differences. P<0.05 was considered significant.
Results
Extremely low expression of miR-145 in primary glioma tissues and pGSCs
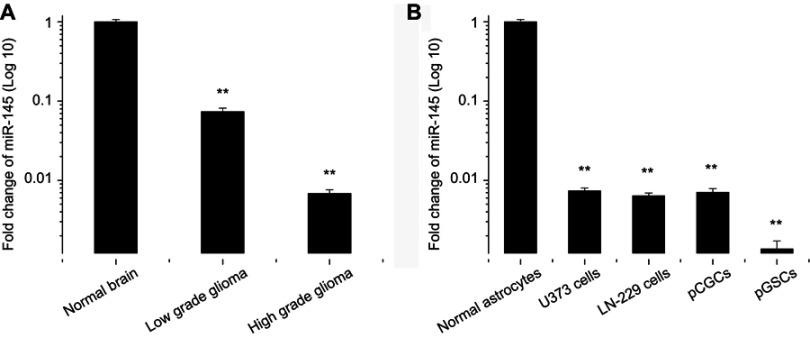
In this study, brain tissue samples, including 23 normal brain tissues, 17 cases of grade I-II glioma tissues, and 36 cases of grade III-IV glioma tissues, were collected to analyze miR-145 expression levels. Grade I-II gliomas were defined as low-grade gliomas, while grade III-IV gliomas were defined as high-grade glioma tissues. Among them, seven cell lines of pGSCs from primary glioma tissues were successfully cultured and established. Normal brain tissue samples were obtained from patients with severe traumatic brain injury who needed internal decompression. The clinical characteristics of the subjects are shown in Table 1. Subsequently, the relative expression of miR-145 in primary glioma tissues and pGSCs compared to normal brain tissues was detected by RT-qPCR. As shown in Figure 1A, miR-145 was significantly downregulated in glioma tissues compared to normal brain tissues, especially in high-grade gliomas (P<0.05). Further, we compared expression in U373 cells, LN-229 cells, primary cultured glioma cells (pCGCs), and their corresponding GSCs (pGSCs) to normal astrocytes and showed the lowest expression in GSCs (Figure 1B). We selected the lowest expression of miR-145 in pGSCs (pGSC-1 and pGSC-2) for the following studies.
Table 1.
Clinical characteristics of the subjects
| Observation indicator | Gliomas | Normal brain tissues |
|---|---|---|
| N | 53 | 23 |
| Age, years (mean ± SD) | 46.7±13.0 | 33.3±5.5 |
| Gender, n | ||
| Male | 21 | 17 |
| Female | 32 | 6 |
| Specimen location, n | ||
| Supratentorial | 51 | 23 |
| Infratentorial | 2 | 0 |
| Tumor grade, n | ||
| I-II | 17 | NA |
| III-IV | 36 | NA |
| Medical History, n | ||
| Hypertension | 16 | 7 |
| Diabetes | 3 | 0 |
Figure 1.
miR-145 expression in glioma tissues and cell lines. (A) RT-qPCR assay for miR-145 expression in low- and high-grade glioma tissues, compared with normal brain tissues. (B) RT-qPCR assay for miR-145 expression in U373 cells, LN-229 cells, pCGCs, and pGSCs, compared with normal astrocytes.
miR-145 regulates proliferation, but not apoptosis, of pGSCs
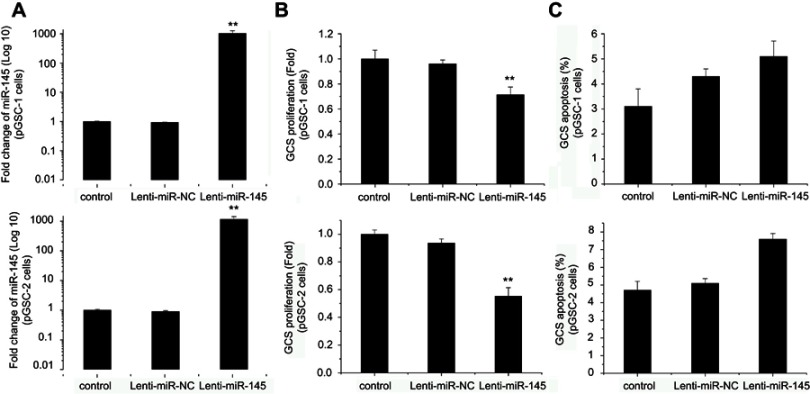
Our previous research showed that miR-145 upregulation could inhibit cell migration and invasion of GSCs.9 Rani et al found that miR-145 overexpression induced growth arrest in glioma cells.20 However, until now, no studies have shown any data regarding the effect of miR-145 on cell proliferation and apoptosis of GSCs. Here, we upregulated miR-145 in pGSCs through Lenti-GFP-miR-145 transfection. As shown in Figure 2A, miR-145 increased more than 1000-fold in pGSC-1 and pGSC-2 cell lines after Lenti-GFP-miR-145 transfection. Next, the cells were cultured for 48 h after transfection, and proliferation and apoptosis were analyzed by the MTT assay and TUNEL cell apoptosis detection, respectively. As shown in Figure 2B, the MTT assay displayed remarkable proliferation inhibition after Lenti-GFP-miR-145 transfection compared to the control group (P<0.05). However, no significant differences in cell apoptosis between groups were observed by TUNEL cell apoptosis detection after miR-145 overexpression (Figure 2C, P>0.05).
Figure 2.
Effects of miR-145 on pGSCs. (A) The miR-145 level was significantly upregulated after Lenti-GFP-miR-145 transfection. (B) Effects of miR-145 overexpression on proliferation of pGSCs measured by MTT assay. (C) Effects of miR-145 overexpression on apoptosis of pGSCs measured by TUNEL cell apoptosis detection.
miR-145 enhances DMC effects on anti-pGSCs in vitro
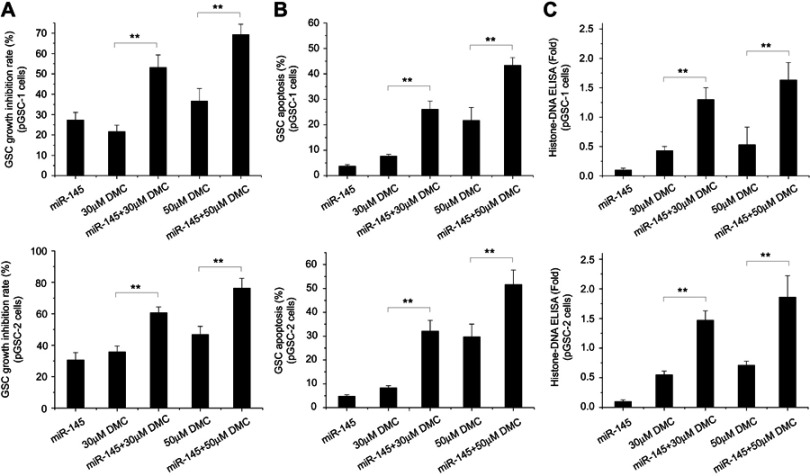
Our previous research showed that DMC could inhibit GSC proliferation and induce apoptosis.16 Liu et al reported that miR-145 overexpression increased the cytotoxic effect of Sunitinib in GBM.21 However, whether miR-145 could enhance the effects of DMC on pGSCs was still unknown. To confirm the effect of miR-145 to enhance GSC chemosensitivity to DMC, pGSCs were transfected with Lenti-GFP-miR-145 and then treated with 30 or 50 μM DMC for 48 h. Cell growth inhibition rate was calculated by MTT, which showed that DMC combined with Lenti-GFP-miR-145 pretreatment significantly increased the cell growth inhibition rate in pGSCs compared to DMC or Lenti-GFP-miR-145 treatment alone (Figure 3A). TUNEL cell apoptosis detection also confirmed increased apoptosis in pGSCs after Lenti-GFP-miR-145 and DMC combined treatment (Figure 3B). Next, cell apoptosis was quantified by Histone-DNA ELISA to detect cytoplasmic histone-DNA fragments. A similar trend was observed in Figure 3C that Lenti-GFP-miR-145 pretreatment increased cell apoptosis of pGSCs induced by DMC, which was also accompanied by an increased drug concentration of DMC.
Figure 3.
Effects of miR-145 and DMC on pGSCs in vitro. (A) Effects of miR-145 and DMC on proliferation of pGSCs measured by MTT assay. (B) Effects of miR-145 and DMC on apoptosis of pGSCs measured by TUNEL cell apoptosis detection. (C) Effects of miR-145 and DMC on apoptosis of pGSCs measured by Histone-DNA ELISA.
miR-145 enhances DMC effects on tumor growth inhibition of GSCs in vivo
We further evaluated the in vivo effects of Lenti-GFP-miR-145 and DMC treatment on the cell growth of pGSCs. Briefly, 5×106 pGSCs were implanted into immunodeficient nude mice. After the tumor reached 75 mm3 in volume, nude mice were treated with i.p. injections of DMC (10 and 30 mg/kg) plus multi-point injections of Lenti-GFP-miR-145. The relative tumor proliferation rate TV/CV (%) and tumor growth inhibition rate TW/CW (%) were used to evaluate the antitumor activity. A lower TV/CV (%) and higher TW/CW (%) indicated higher antitumor activity. As shown in Figure 4A, TV/CV (%) in Lenti-GFP-miR-145 treatment was about 61.82%, while TV/CV (%) in the 10 mg/kg and 30 mg/kg DMC groups was about 45.2% and 32.64%, respectively, which demonstrated that treatment with either Lenti-GFP-miR-145 or DMC alone had anti-tumor activity on pGSCs xenograft tumors (P<0.05). Further, combined treatment of Lenti-GFP-miR-145 and DMC showed lower TV/CV (%) (about 31.17% in miR-145+10 mg/kg DMC, 23.26% in miR-145+30 mg/kg DMC) than either Lenti-GFP-miR-145 or DMC alone treatment (P<0.05). In addition, we analyzed the tumor growth inhibition rate TW/CW (%) and showed that TW/CW(%) in the Lenti-GFP-miR-145 treatment group was 34.09%, while the rate (%) in the 10 mg/kg and 30 mg/kg DMC groups was about 47.14% and 52.79%, respectively. Further, the rate (%) in Lenti-GFP-miR-145 combined with 10 mg/kg or 30 mg/kg DMC groups was about 62.63% and 66.27%, respectively. Statistical analysis showed combined treatment of Lenti-GFP-miR-145 and DMC had higher TW/CW (%) than either Lenti-GFP-miR-145 or DMC alone treatment (P<0.05) (Figure 4B). Additionally, immunohistochemistry demonstrated that the positive ratios of proliferating cell nuclear antigen (PCNA) in Lenti-GFP-miR-145 combined with the 10 mg/kg or 30 mg/kg DMC groups was also significantly lower than the other groups (P<0.05) (Figure 4C and D).
Figure 4.
Effects of miR-145 and DMC on pGSCs in vivo. (A) Effects of miR-145 and DMC on TV/CV (%) of pGSCs. (B) Effects of miR-145 and DMC on TW/CW (%) of pGSCs. (C) Effects of miR-145 and DMC on proliferation of pGSCs were measured by PCNA immunohistochemistry. (D) The positive ratios of PCNA were calculated in each group.
miR-145 enhances DMC effects on GSCs by targeting SOX2-Wnt/β-catenin axis
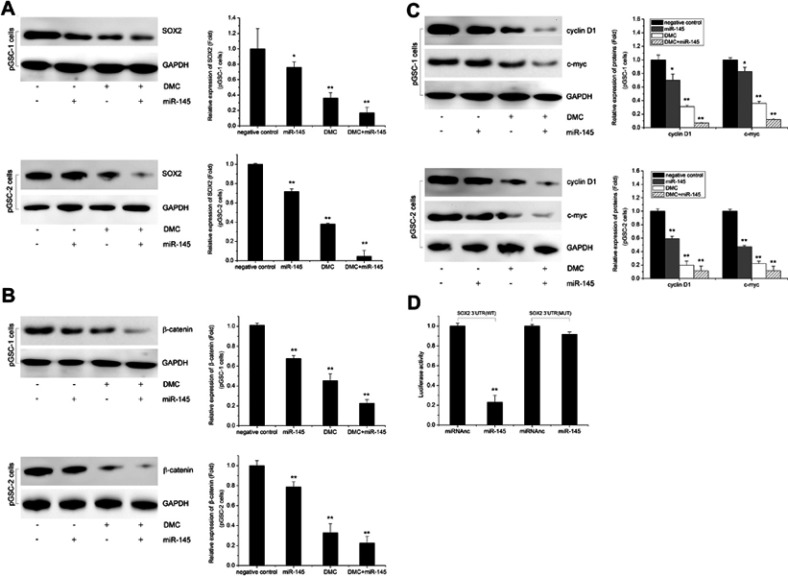
The above data showed the synergistic effects of Lenti-GFP-miR-145 and DMC on GSCs; however, the potential mechanism remained unclear. SOX2, a pluripotent stem cell marker, plays a key role in maintaining the undifferentiated state and proliferation of stem cells. Mirgani et al found that miR-145 could regulate SOX2 in U87 cells.14 In this study, we found that combined treatment of Lenti-GFP-miR-145 and DMC had potent inhibition effects on SOX2 and its downstream Wnt/β-catenin signaling pathway. As shown in Figure 5A, SOX2 expression was reduced in GSCs after Lenti-GFP-miR-145 transfection or DMC alone treatment. Moreover, its expression was further decreased after Lenti-GFP-miR-145 and DMC combined treatment, compared to Lenti-GFP-miR-145 or DMC alone treatment (P<0.05). As shown in Figure 5B, β-catenin, the key protein of the Wnt/β-catenin downstream signaling pathway, was significantly decreased in the combined treatment compared to the other groups (P<0.05). Increased levels of β-catenin can initiate transcriptional activation of proteins such as cyclin D1 and c-myc, which control the G1-to-S phase transition in the cell cycle and are responsible for cell proliferation. As shown in Figure 5C, expression levels of cyclin D1 and c-myc were also significantly inhibited after combined treatment (P<0.05). These data implied that SOX2 and its downstream Wnt/β-catenin signaling pathway were involved in anti-GSCs of Lenti-GFP-miR-145 and DMC.
Figure 5.
Effects of miR-145 and DMC on SOX2-Wnt/β-catenin axis. (A) Effects of miR-145 and DMC on SOX2 expression. (B) Effects of miR-145 and DMC on β-catenin expression. (C) Effects of miR-145 and DMC on cyclin D1 and c-myc expression. (D) SOX2 is a direct target miR-145 in pGSCs confirmed by luciferase reporter.
Further, we confirmed that SOX2 was the direct target of miR-145 in GSCs. To determine whether SOX2 was a direct target gene of miR-145, a luciferase reporter vector with the putative SOX2 3ʹUTR target site for miR-145 was constructed. Luciferase reporter vector together with Lenti-GFP-miR-145 or Lenti-GFP-scramble was transfected into pGSCs. As shown in Figure 5D, a significant decrease in relative luciferase activity was observed after SOX2 3ʹUTR co-transfection with Lenti-GFP-miR-145 compared to the control (P<0.05). This result suggested that SOX2 was the direct target of miR-145 in pGSCs.
SOX2 upregulation induces GSC resistance to synergistic effects of Lenti-GFP-miR-145 and DMC
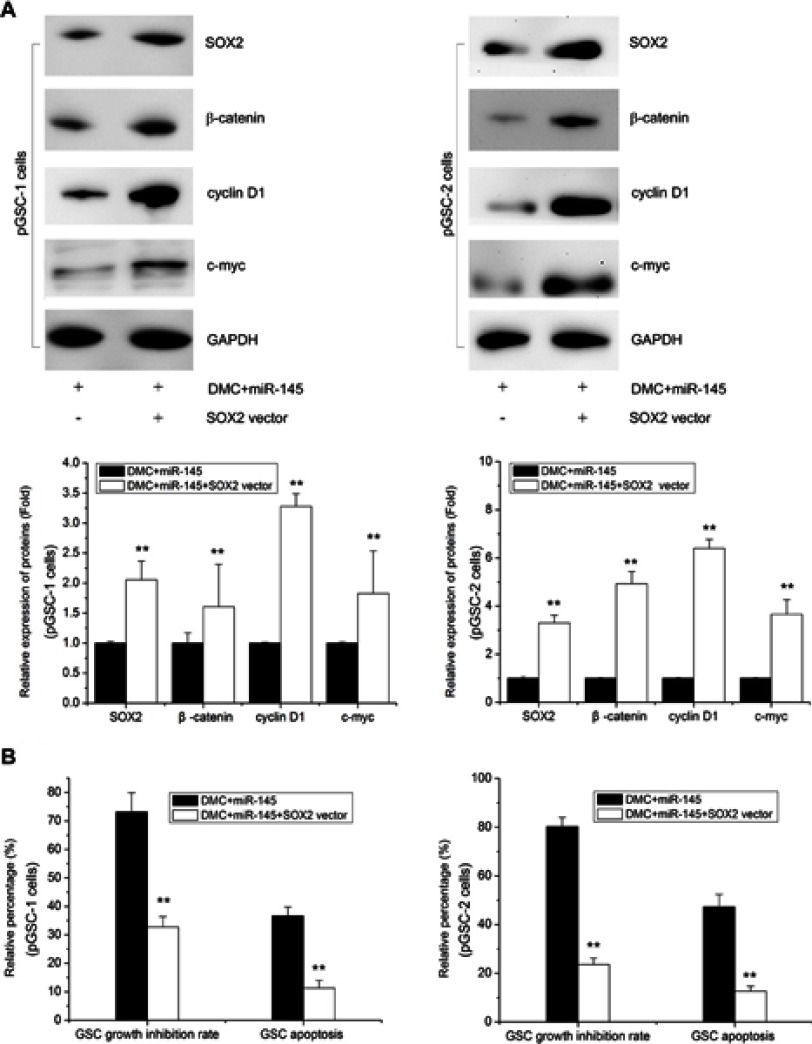
To further determine the importance of the SOX2-Wnt/β-catenin axis on the synergistic effects of Lenti-GFP-miR-145 and DMC in GSCs, we constructed SOX2 overexpression vectors named SOX2 vector to counteract the reduced expression of SOX2 by Lenti-GFP-miR-145 and DMC combined treatment. As shown in Figure 6A, GSCs treated with Lenti-GFP-miR-145 and DMC were cotransfected with SOX2 vector for 48 h, which resulted in a major increase in SOX2 expression. Further, expression of its downstream Wnt/β-catenin proteins, ie β-catenin, cyclin D1 and c-myc, was restored (Figure 6A). Functional analysis revealed that SOX2 overexpression reversed the synergistic inhibition effects of Lenti-GFP-miR-145 and DMC on pGSCs, including cell proliferation and apoptosis (Figure 6B). These results demonstrated that the SOX2-Wnt/β-catenin axis contributed to the synergistic effects of Lenti-GFP-miR-145 and DMC on anti-GSCs.
Figure 6.
SOX2 overexpression reverses the anti-tumor effects of miR-145 and DMC on GSCs. (A) SOX2, β-catenin, cyclin D1, and c-myc expression in pGSCs after SOX2 vector transfection and/or miR-145+ DMC treatment measured by Western blot. (B) The cell proliferation and apoptosis of pGSCs after SOX2 vector transfection and/or miR-145+ DMC treatment measured by MTT assay and TUNEL cell apoptosis detection.
Discussion
GSCs are confirmed to be critical for tumor cell progression and relapse and are also resistant to chemoradiotherapy.9 Previous studies by Luthra et al22 and our group15 showed that DMC had potent anti-glioma effects, and we further confirmed that DMC had significant anti-GSCs effects.17 However, the potential mechanism of DMC on anti-GSCs was still unclear. In this study, we confirmed miR-145 as a key factor for regulating the antitumor effect of DMC on GSCs.
Currently, miRNAs are reported to regulate cell growth, migration, and invasion of in multidrug-resistant cancer stem cells (CSCs).23,24 miR-145 is an important miRNA in glioma cell progression, which is decreased in human glioma samples and cell lines.25 Overexpression of miR-145 increased glioma cell apoptosis by inhibiting BNIP3 and Notch signaling,25 and inhibited cell proliferation, adhesion, and invasion by targeting Sox9 and adducin 3.20 These data showed that miR-145 functioned in a tumor-suppressive role in gliomas. Our recent work showed that miR-145 also functioned in an important role in GSCs, which could inhibit migration and invasion of GSCs by targeting ABCG2.9 However, its function and mechanism in the antitumor effect of DMC on GSCs were not well established. In this study, we constructed a miR-145 overexpression vector to upregulate its levels in GSCs. Our results showed that miR-145 overexpression increased cell proliferation inhibition rate and cell apoptosis rate in vitro, which implied that the anti-GSCs effects of DMC was significantly enhanced after upregulation of miR-145 expression. Further in vivo studies also confirmed that raising miR-145 expression levels improved DMC control of glioma growth.
The miR-145/SOX2/cyclin D1 axis was found to be critical in the isorhapontigenin-mediated inhibition of gliomas.26 SOX2 is a member of the SOX family and marker of pluripotent stem cells, which plays a key role in maintaining the undifferentiated state and proliferation of stem cells. Further, it promotes cell proliferation and tumor formation by regulating the transcriptional regulation of target genes. Recent studies showed that high expression of the SOX2 gene could be found in many tumors, including breast cancer, signet ring cell carcinoma, mucous adenocarcinoma, and glioma.27 In gliomas, it was reported that miR-21 promoted cell migration and invasion of gliomas through activation of Sox2 and β-catenin signaling.28 Stoltz et al showed that the Sox2 reporter system modeled the cellular heterogeneity in glioma.29 Garros-Regulez et al found that SOX2 and SOX9 overexpression in glioblastoma was responsible for temozolomide resistance.30 In this study, we showed that overexpressed miR-145 could inhibit SOX2 expression, and we further confirmed SOX2 as a direct target of miR-145 in GSCs using the Luciferase targeting assay. Moreover, DMC exhibited more potent effects on inhibiting SOX2 expression after miR-145 overexpression in GSCs, which indicated that miR-145 could enhance DMC by inhibiting SOX2 signaling. SOX2 is an important regulatory factor in the Wnt/β-catenin signaling pathway, which can activate the TCF/LEF/β-catenin cascade through the Wnt/β-catenin signaling pathway, while the occurrence of glioblastoma is often accompanied by abnormal activation of the Wnt/β-catenin pathway. When Wnt signaling is activated, receptor complexes of the Wnt protein, FZ receptor, and LRP5/6 (FZ/LRP) form, cytoplasmic Dsh is recruited to the cell membrane, and GSK-3β is phosphorylated, which falls off from the β-catenin degradation complex, resulting in a large number of free β-catenin aggregation in the cytoplasm to enter the nucleus. When β-catenin enters the nucleus, it combines with LEF/TCF to activate downstream target genes, such as c-myc and cyclin D1, and these genes play an important role in the occurrence and metastasis of tumors.31 Here, we also confirmed that downstream of the SOX2-Wnt/β-catenin axis, including β-catenin, cyclin D1, and c-myc, was inhibited by DMC, and this effect could be further enhanced by increased expression of miR-145 in GSCs.
Conclusion
This study confirmed that the miR-145/SOX2-Wnt/β-catenin axis was critical in the DMC-mediated inhibition of GSCs, and upregulation of miR-145 could effectively enhance DMC effects on anti-GSCs, which might become new therapeutic targets for GSC resistance.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81772691, 81000963, and 81370062) and China Postdoctoral Science Foundation Grant (2017M620196 and 2018T110467). The funders had no role in the study design, data collection and analysis, decision to publish, or in the preparation of the manuscript.
Abbreviation list
GSCs, glioma stem cells; GBM, glioblastoma multiforme; DMC, demethoxycurcumin; NPCs, neural precursor cells; PCNA, proliferating cell nuclear antigen; CSCs, cancer stem cells.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Auffinger B, Spencer D, Pytel P, Ahmed AU, Lesniak MS. The role of glioma stem cells in chemotherapy resistance and glioblastoma multiforme recurrence. Expert Rev Neurother. 2015;15(7):741–752. doi: 10.1586/14737175.2015.1051968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty C, Chin KY, Das S. miRNA-regulated cancer stem cells: understanding the property and the role of miRNA in carcinogenesis. Tumour Biol. 2016;37(10):13039–13048. doi: 10.1007/s13277-016-5156-1 [DOI] [PubMed] [Google Scholar]
- 3.Li N, Long B, Han W, Yuan S, Wang K. microRNAs: important regulators of stem cells. Stem Cell Res Ther. 2017;8(1):110. doi: 10.1186/s13287-017-0601-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao YL, Ma J, Wang P, et al. miR-101 acts as a tumor suppressor by targeting Kruppel-like factor 6 in glioblastoma stem cells. CNS Neurosci Ther. 2015;21(1):40–51. doi: 10.1111/cns.12321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yao Y, Ma J, Xue Y, et al. Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett. 2015;359(1):75–86. doi: 10.1016/j.canlet.2014.12.051 [DOI] [PubMed] [Google Scholar]
- 6.Chan XH, Nama S, Gopal F, et al. Targeting glioma stem cells by functional inhibition of a prosurvival oncomiR-138 in malignant gliomas. Cell Rep. 2012;2(3):591–602. doi: 10.1016/j.celrep.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 7.Lee HK, Finniss S, Cazacu S, et al. Mesenchymal stem cells deliver synthetic microRNA mimics to glioma cells and glioma stem cells and inhibit their cell migration and self-renewal. Oncotarget. 2013;4(2):346–361. doi: 10.18632/oncotarget.868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morgado AL, Rodrigues CM, Solá S. MicroRNA-145 regulates neural stem cell differentiation through the SOX2-Lin28/let-7 signaling pathway. Stem Cells. 2016;34(5):1386–1395. doi: 10.1002/stem.2309 [DOI] [PubMed] [Google Scholar]
- 9.Shi L, Wang Z, Sun G, Wan Y, Guo J, Fu X. miR-145 inhibits migration and invasion of glioma stem cells by targeting ABCG2. Neuromolecular Med. 2014;16(2):517–528. doi: 10.1007/s12017-014-8305-y [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Yang J, Xu G, et al. Targeting miR-381-NEFL axis sensitizes glioblastoma cells to temozolomide by regulating stemness factors and multidrug resistance factors. Oncotarget. 2015;6(5):3147–3164. doi: 10.18632/oncotarget.3061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang S, Wan Y, Pan T, et al. MicroRNA-21 inhibitor sensitizes human glioblastoma U251 stem cells to chemotherapeutic drug temozolomide. J Mol Neurosci. 2012;47(2):346–356. doi: 10.1007/s12031-012-9759-8 [DOI] [PubMed] [Google Scholar]
- 12.Gao M, Miao L, Liu M, et al. miR-145 sensitizes breast cancer to doxorubicin by targeting multidrug resistance-associated protein-1. Oncotarget. 2016;7(37):59714–59726. doi: 10.18632/oncotarget.10845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan M, Zhao X, Wang H, et al. miR-145 sensitizes gallbladder cancer to cisplatin by regulating multidrug resistance associated protein 1. Tumour Biol. 2016;37(8):10553–10562. doi: 10.1007/s13277-016-4957-6 [DOI] [PubMed] [Google Scholar]
- 14.Tahmasebi Mirgani M, Isacchi B, Sadeghizadeh M, et al. Dendrosomal curcumin nanoformulation downregulates pluripotency genes via miR-145 activation in U87MG glioblastoma cells. Int J Nanomedicine. 2014;9:403–417. doi: 10.2147/IJN.S48136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi L, Sun G. Low-dose DMC significantly enhances the effect of TMZ on glioma cells by targeting multiple signaling pathways both in vivo and in vitro. Neuromolecular Med. 2015;17(4):431–442. doi: 10.1007/s12017-015-8372-8 [DOI] [PubMed] [Google Scholar]
- 16.Shi L, Fei X, Wang Z. Demethoxycurcumin was prior to temozolomide on inhibiting proliferation and induced apoptosis of glioblastoma stem cells. Tumour Biol. 2015;36(9):7107–7119. doi: 10.1007/s13277-015-3427-x [DOI] [PubMed] [Google Scholar]
- 17.Leng L, Zhong X, Sun G, Qiu W, Shi L. Demethoxycurcumin was superior to temozolomide in the inhibition of the growth of glioblastoma stem cells in vivo. Tumour Biol. 2016;37(12):15847–15857. doi: 10.1007/s13277-016-5399-x [DOI] [PubMed] [Google Scholar]
- 18.Darling JL. The in vitro biology of human brain tumors In: Thomas DGT, editor. Neuro-Oncology: Primary Malignant Brain Tumors. Baltimore (MD): Johns Hopkins University Press; 1990:1–25. [Google Scholar]
- 19.Chen C, Ridzon DA, Broomer AJ, et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005;33:e179. doi: 10.1093/nar/gni178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rani SB, Rathod SS, Karthik S, Kaur N, Muzumdar D, Shiras AS. MiR-145 functions as a tumor-suppressive RNA by targeting SOX9 and adducin 3 in human glioma cells. Neuro Oncol. 2013;15(10):1302–1316. doi: 10.1093/neuonc/not090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu H, Liu Z, Jiang B, Huo L, Liu J, Lu J. Synthetic miR-145 mimic enhances the cytotoxic effect of the antiangiogenic drug sunitinib in glioblastoma. Cell Biochem Biophys. 2015;72(2):551–557. doi: 10.1007/s12013-014-0501-8 [DOI] [PubMed] [Google Scholar]
- 22.Luthra PM, Kumar R, Prakash A. Demethoxycurcumin induces Bcl-2 mediated G2/M arrest and apoptosis in human glioma U87 cells. Biochem Biophys Res Commun. 2009;384(4):420–425. doi: 10.1016/j.bbrc.2009.04.149 [DOI] [PubMed] [Google Scholar]
- 23.Kwon T, Chandimali N, Huynh DL, et al. BRM270 inhibits cancer stem cell maintenance via microRNA regulation in chemoresistant A549 lung adenocarcinoma cells. Cell Death Dis. 2018;9(2):244. doi: 10.1038/s41419-018-1111-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tan W, Liu B, Qu S, Liang G, Luo W, Gong C. MicroRNAs and cancer: key paradigms in molecular therapy. Oncol Lett. 2018;15(3):2735–2742. doi: 10.3892/ol.2017.7638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du Y, Li J, Xu T, Zhou DD, Zhang L, Wang X. MicroRNA-145 induces apoptosis of glioma cells by targeting BNIP3 and Notch signaling. Oncotarget. 2017;8(37):61510–61527. doi: 10.18632/oncotarget.18604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu Z, Zeng X, Xu J, et al. Isorhapontigenin suppresses growth of patient-derived glioblastoma spheres through regulating miR-145/SOX2/cyclin D1 axis. Neuro Oncol. 2016;18(6):830–839. doi: 10.1093/neuonc/nov298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Schaijik B, Davis PF, Wickremesekera AC, Tan ST, Itinteang T. Subcellular localisation of the stem cell markers OCT4, SOX2, NANOG, KLF4 and c-MYC in cancer: a review. J Clin Pathol. 2018;71(1):88–91. doi: 10.1136/jclinpath-2017-204815 [DOI] [PubMed] [Google Scholar]
- 28.Luo G, Luo W, Sun X, et al. MicroRNA-21 promotes migration and invasion of glioma cells via activation of SOX2 and β-catenin signaling. Mol Med Rep. 2017;15(1):187–193. doi: 10.3892/mmr.2016.5971 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Stoltz K, Sinyuk M, Hale JS, et al. Development of a SOX2 reporter system modeling cellular heterogeneity in glioma. Neuro Oncol. 2015;17(3):361–371. doi: 10.1093/neuonc/nou320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garros-Regulez L, Aldaz P, Arrizabalaga O, et al. mTOR inhibition decreases SOX2-SOX9 mediated glioma stem cell activity and temozolomide resistance. Expert Opin Ther Targets. 2016;20(4):393–405. doi: 10.1517/14728222.2016.1151002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelleher FC, O’Sullivan H. FOXM1 in sarcoma: role in cell cycle, pluripotency genes and stem cell pathways. Oncotarget. 2016;7(27):42792–42804. doi: 10.18632/oncotarget.8669 [DOI] [PMC free article] [PubMed] [Google Scholar]