Abstract
The pharmacological effects of tobacco products are primarily mediated by nicotine; however, research suggests that several non-nicotine tobacco constituents may alter the reinforcing effects of nicotine. This study evaluated the reinforcing effects of aqueous solutions of smoke/aerosol condensate from cigarettes, little cigars, electronic cigarettes (e-cigarettes), and waterpipe tobacco in a self-administration procedure to determine if abuse liability of these tobacco products differed. Adult male Sprague-Dawley rats (n = 64 total) were trained to self-administer intravenous nicotine (30 μg/kg/infusion) on a fixed ratio 5 schedule of reinforcement. Following nicotine dose-effect assessment (1, 7.5, 15, and 30 μg/kg/infusion), rats were given access to smoke/aerosol condensate derived from their assigned tobacco product. Rats responded for smoke/aerosol condensate containing 1, 7.5, 15, and 30 μg/kg/infusion nicotine, with the ratio of nicotine:non-nicotine constituents held constant across doses for each tobacco product. Responding for nicotine or smoke/aerosol condensate was also assessed on a progressive ratio schedule of reinforcement. Cigarette, little cigar, and e-cigarette smoke/aerosol condensates shifted the nicotine dose-effect curve leftward, whereas waterpipe tobacco smoke condensate shifted the dose-effect curve rightward. Smoke/aerosol condensate from all tobacco products produced similar levels of responding compared to nicotine alone during the progressive ratio phase. Results suggest that non-nicotine constituents in cigarettes, little cigars, and e-cigarettes differentially enhance nicotine’s reinforcing potency. In contrast, waterpipe tobacco blunted nicotine’s reinforcing potency, suggesting that it may contain unique constituents that dampen nicotine’s reinforcing effects.
Keywords: cigarette, little cigar, e-cigarette, nicotine, self-administration, waterpipe tobacco
1.0. Introduction
Tobacco products are some of the most commonly used psychoactive drugs worldwide, and their use has led to a high public health toll [1]. While nicotine is the primary constituent that promotes tobacco abuse [2], individual minor tobacco alkaloids, β-carbolines, and acetaldehyde have all been shown to increase the reinforcing efficacy of nicotine or serve as reinforcers on their own in rodent self-administration models [3–6]. Additionally, monoamine oxidase inhibition enhances nicotine self-administration in rats [7]. Furthermore, the literature is replete with demonstrations that the reinforcing effects of nicotine only account for part of the satisfaction derived from smoking [8–10]. Findings suggest that non-nicotine tobacco constituents play a role in the abuse potential of tobacco products.
The concentration of nicotine and minor tobacco alkaloids varies depending upon the genetics of the plant, environmental conditions during growth, and harvesting and processing conditions [11, 12]. Thus, the chemical constituents of tobacco can vary within the same product and across different tobacco product types. For example, in e-liquids, which typically include nicotine from tobacco extracts, and are used in electronic cigarettes (e-cigarettes), concentrations of minor tobacco alkaloids differed between manufacturers and across flavors within the same brand, differences which may be due to purification or other manufacturing processes [13]. Varying concentrations of minor tobacco alkaloids were also identified across cigarettes, cigars, snuff, chewing tobacco, and pipe tobacco [14]. Furthermore, several chemicals present in the smoke of little cigars are not present in cigarettes [15].
Studies evaluating how these differences in minor alkaloid concentration contribute to abuse liability of different tobacco products are lacking. One study that compared self-administration of nicotine alone to cigarette and roll-your-own tobacco particulate matter (TPM) in rats found that roll-your-own TPM was significantly more reinforcing than cigarette TPM and nicotine alone [16]. As such, different tobacco products may have greater reinforcing effects, irrespective of nicotine level, because of between-product differences in minor tobacco alkaloid concentrations.
The objective of the present study was to compare the reinforcing effects of the chemical mixtures contained in smoke condensate from cigarettes, little cigars, and waterpipe tobacco, and aerosol from e-cigarettes in a self-administration procedure. Results were also compared to self-administration of nicotine alone.
2.0. Materials and Methods
2.1. Subjects
Adult male Sprague-Dawley rats (Envigo, Frederick, MD), aged postnatal day 73 (250–350 g) at the start of the experiment, were singly housed in polycarbonate home cages. They were maintained in a temperature- (20–26°C) and humidity-controlled (30–70%) environment with a 12-hour light-dark cycle (lights on at 0700). Rats had free access to water in the home cage and were fed 15–17 g of rodent chow daily [5, 17], which allowed moderate weight gain throughout the study (rats weighed 340–400 g at the end of the study). Experiments complied with the principles of laboratory animal care [18], and with the Institutional Animal Care and Use Committees for RTI and the U.S. Food and Drug Administration.
2.2. Apparatus
Experimental sessions were conducted in operant conditioning chambers for rats (MED Associates, St. Albans, VT) housed inside sound-attenuating chambers (MED Associates). Each chamber contained two retractable levers, a stimulus light over each lever, and a house light. One lever was designated as the active lever and the other lever was designated as inactive. The side of the chamber associated with the active lever was counterbalanced across subjects. Fans provided ventilation and speakers provided white noise. Infusion pumps (MED Associates) were located outside the cubicle. MED-PC software (Med Associates) arranged experimental events and recorded data.
2.3. Surgery
Rats were surgically implanted with chronic indwelling jugular catheters under isoflurane anesthesia, as described previously [19, 20]. The external end of the catheter was secured by a quick connect harness (SAI Infusion Technologies, Lake Villa, IL). Rats were allowed to recover from surgery for a minimum of five days. For three days post-surgery, rats were administered 5 mg/kg ketoprofen daily. Catheter patency was confirmed before and after the study by administration of 1.0–1.5 mg of intravenous (i.v.) methohexital and observation of rapid loss of muscle tone.
2.4. Self-Administration Procedure
Rats were assigned to one of four tobacco product groups: cigarettes, little cigars, e-cigarettes, or waterpipe tobacco (n=16/group) upon arrival at the facility. The self-administration procedure consisted of five phases: 1) acquisition of nicotine self-administration, 2) nicotine dose-effect curve determination, 3) evaluation of the reinforcing efficacy of nicotine alone on a progressive ratio (PR) schedule of reinforcement, 4) tobacco smoke/aerosol dose-effect curve determination, and 5) evaluation of the reinforcing efficacy of tobacco smoke/aerosol on a PR schedule of reinforcement. Throughout the study, catheter patency was maintained by flushing catheters daily with a solution (0.96% gentamicin, 2.88% heparin, 96.2% saline) after testing.
2.4.1. Response lever training.
Rats were trained to respond on the active lever for food through autoshaping [21, 22] for three sessions. During autoshaping, active lever extension was paired with delivery of a sucrose pellet (45 mg; Bioserv Inc., Frenchtown, NJ) on a random time 60 s schedule. Following 15 s of lever extension, or immediately after a lever press, a pellet was delivered. Pellet delivery was followed by a 20 s timeout signaled by illumination of both stimulus lights. Autoshaping sessions delivered approximately 60 pellets within the first 90 min. Rats then remained in the chamber for 30 min with only the inactive lever present. Throughout all training and self-administration sessions, the inactive lever was extended, and presses on this lever were recorded, but had no programmed consequence.
2.4.2. Nicotine self-administration.
Following autoshaping, nicotine (30 μg/kg/infusion) was available for i.v. self-administration on a fixed ratio (FR) 1 schedule of reinforcement for five days, on an FR2 for three days, and on an FR5 for a minimum of 12 days [23–25]. This training dose was chosen because it reliably led to self-administration in previous research [16, 25, 26]. Infusions were 0.1 ml, occurred over the course of 3.8 s, and were followed by a 20 s timeout signaled by illumination of both stimulus lights [27–31]. Meeting the ratio requirement on the active lever produced an infusion of nicotine. Self-administration sessions lasted two hrs [16, 32, 33]. The criteria for acquisition of self-administration were: 1) ≥ 10 active responses, and 2) ≥ 2:1 ratio of active to inactive responses, both of which needed to be met for five consecutive sessions [24, 25]. Rats that did not meet these criteria after 18 days on FR5 were removed from the study.
In the next phase, rats had access to 1.0, 7.5, 15.0, and 30.0 μg/kg/infusion nicotine on an FR5 schedule of reinforcement. Doses were presented in ascending order, and each dose was tested for three consecutive sessions [23, 26]. Then, rats had access to saline for five sessions to provide a vehicle comparison in which significant increases in infusions earned compared to saline indicated that a dose was reinforcing. Subsequently, responding was reestablished on the training dose for a minimum of five sessions.
After dose-effect curve completion, rats were given access to 30 μg/kg/infusion nicotine on a PR schedule of reinforcement for four sessions. The PR schedule increased the response requirement following each infusion according to an exponential scale (3, 6, 10, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, etc.) [24, 26, 34, 35]. PR sessions ended after two hrs or 20 min without a response, whichever occurred first [26, 28, 29, 33, 36]. All rats then had access to saline on a PR schedule for five sessions for comparison.
2.4.3. Smoke/aerosol self-administration.
In the next phase, responding was reestablished on the nicotine training dose. Re-acquisition criteria were identical to those for acquisition. Rats then had access to an aqueous solution derived from the smoke/aerosol of their assigned tobacco product (cigarettes, little cigars, e-cigarettes, or waterpipe tobacco) containing 1.0, 7.5, 15.0, and 30.0 μg/kg/infusion nicotine on an FR5 schedule of reinforcement. Doses were presented in ascending order to avoid potential aversive effects of higher doses impacting responding for doses evaluated later [23]. Each dose was tested for three sessions, then saline was evaluated for five sessions for comparison. Subsequently, responding was reestablished on smoke/aerosol condensate containing 30 μg/kg/infusion nicotine.
In the final phase, rats were given access to smoke/aerosol condensate containing 30 μg/kg/infusion nicotine on a PR schedule of reinforcement for four sessions. Session parameters were identical to those used with nicotine alone. Finally, all rats were exposed to saline on a PR schedule for five sessions for comparison.
2.5. Chemicals/Drugs
Smoking and formulation methods for smoke/aerosol condensate are described in the supplementary information. Concentrations of nicotine, nornicotine, anabasine, anatabine, cotinine, myosmine, harmane, norharmane, and acetaldehyde were measured for each tobacco product. (−)-Nicotine tartrate salt (Sigma-Aldrich, St. Louis, MO) and smoke/aerosol condensate were dissolved in physiological saline (Patterson Veterinary Supply, Columbus, OH). Due to mild acidity, the pH of the nicotine and waterpipe tobacco smoke condensate solutions were adjusted to approximately neutral (initial nicotine pH ≈ 3; initial waterpipe pH ≈ 4–5; adjusted pH ≈ 7). The pH of the cigarette, little cigar, and e-cigarette smoke/aerosol condensates was not adjusted (pH ≈ 5.5–8.0). Smoke/aerosol condensates were diluted such that the nicotine concentrations were equal to those used throughout the study (1.0, 7.5, 15.0, and 30.0 μg/kg/infusion). Non-nicotine tobacco constituents were diluted proportional to their concentration in the smoke/aerosol condensate. Hence, the ratio of nicotine:non-nicotine constituent concentrations was held constant across smoke/aerosol condensate and self-administration solution (see supplementary information). Nicotine doses are expressed as μg/kg of the base. Gentamicin and heparin were purchased from Patterson Veterinary Supply.
Tobacco products were chosen based on market popularity, and tobacco constituent profiles. Tobacco products were purchased between December 2016 and January 2018. Camel unfiltered cigarettes (Muslehs Tobacco Shop, Durham, NC; Sam’s Club, Morrisville, NC; Kroger, Cary, NC) and Cheyenne filtered full flavored little cigars (Muslehs Tobacco Shop, Moory Tobacco Outlet, Sam’s Club, Durham, NC) were chosen due to notable differences in the nicotine:non-nicotine constituent ratios of the respective smoke condensates from these two combustible tobacco types. Furthermore, previous experiments have examined self-administration of Camel unfiltered cigarette smoke condensate in rats [34, 37]. Blu Classic Tobacco e-cigarettes (Fontem US Inc., Charlotte, NC) were chosen because of differences in some tobacco constituent levels compared to Camel unfiltered cigarettes. Additionally, cigalike style e-cigarettes, such as Blu, are used in other research because they cannot be easily manipulated by the user. Nakhla Two Apples waterpipe tobacco (Smoking-Hookah, Houston, TX) was chosen for analysis to obviate the potential interacting pharmacological effects of menthol in mint-flavored products. Nakhla Two Apples had the highest nicotine:non-nicotine constituent ratio of the tobacco products examined in the present study, and has been used in previous clinical studies [38, 39].
2.6. Data Analysis
Six rats did not complete both dose-effect assessments (e.g., catheter malfunctions, inability to meet acquisition criteria). Thus, their data were excluded from all analyses. One additional rat from the cigarette group completed both dose-effect assessments, but only one PR phase. Therefore, his data were included for dose-effect assessments, but excluded from PR analyses.
The number of infusions and responses on the active and inactive lever were recorded for each session. Additionally, the last completed ratio for each PR session was noted. Only the final two sessions of exposure to each dose in the dose-effect curves and PR phases were used in graphical and statistical analyses [40–43]. The mean number of infusions or responses across these two sessions served as the individual subject data. Graphed values reflect the mean [± standard error of the mean (SEM)] of these data.
2.6.1. Dose-effect assessments.
Separate within-subject factorial (dose × lever) Analyses of Variance (ANOVAs) were used to analyze active and inactive responses across dose for nicotine-alone dose-effect curves and for smoke/aerosol condensate dose-effect curves. Separate within-subject factorial (nicotine dose × drug formulation) ANOVAs were used to compare infusions for smoke/aerosol condensate and nicotine-alone dose-effect curves within tobacco groups.
2.6.2. PR assessments.
Due to the heteroscedasticity of breakpoint data, infusions were analyzed in place of breakpoints to avoid violating the assumption of homogeneity of variance [24, 44]. Vehicle infusions for both PR phases were compared with a split-plot drug (nicotine vehicle vs smoke condensate vehicle) × tobacco type ANOVA. Due to group differences in vehicle responding, infusion data were normalized as percentage of vehicle for further analysis. Average percent of vehicle infusions earned on the PR schedule were compared with a split-plot drug (nicotine vs smoke condensate) × tobacco type ANOVA.
All significant ANOVAs were followed by Tukey-Kramer post hoc tests (α = 0.05) to specify differences among means for the main effects and/or interactions. Number Cruncher Statistical Systems software (NCSS Statistical Software, Kaysville, UT) was used for all analyses. Data were graphed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
3.0. Results
3.1. Acquisition
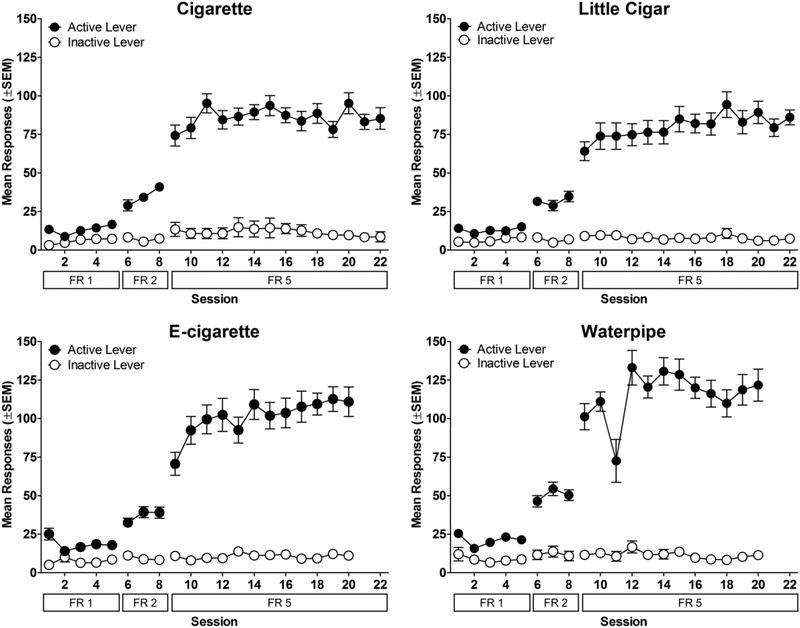
Rats in all groups learned to self-administer nicotine alone (Figure 1). While responding on both levers was similar during FR1, during FR2, rats pressed primarily the active lever. As the FR requirement continued to increase, rats increased their active lever presses, whereas inactive lever presses remained low. All rats met the acquisition criteria after 12–18 days on FR5, except one rat which was dropped from the study.
Figure 1.
Acquisition of nicotine self-administration (30 μg/kg/infusion). Mean number of active and inactive lever presses as a function of session and FR value. Data for each tobacco group are shown in separate panels. Note that tobacco group names refer to the tobacco product that rats were exposed to in the subsequent phase; all groups were exposed to nicotine alone in this phase. n=13 for cigarette; n=16 for little cigar; n=15 for e-cigarette; n=14 for waterpipe.
3.2. Dose-effect assessments
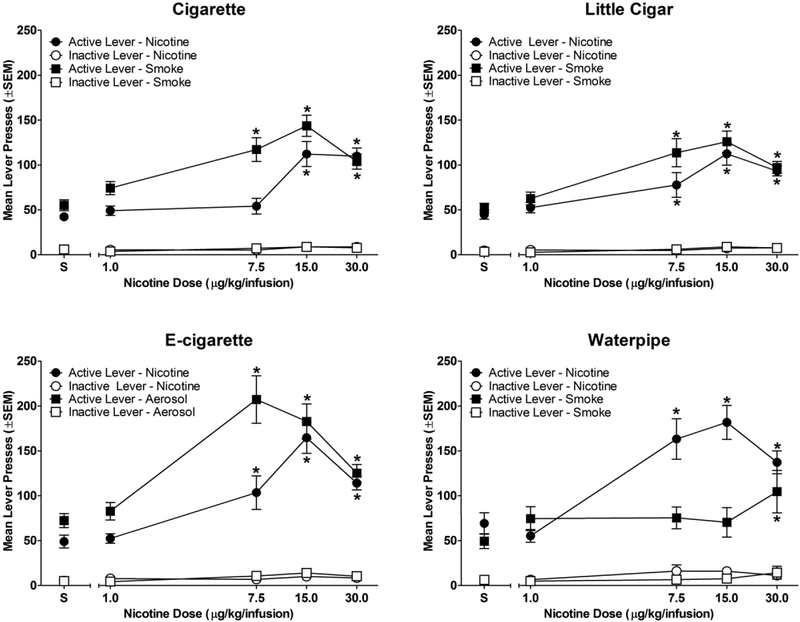
Figure 2 shows the number of active and inactive responses as a function of nicotine dose for each group. As expected, nicotine alone served as a reinforcer at two or more doses in all four groups, as indicated by greater active lever responses for nicotine than for vehicle (dose × lever interaction [cigarette: F(4,48) = 30.22; p<0.05; little cigar: F(4,60) = 20.31; p<0.05; e-cigarette: F(4,56) = 34.28; p<0.05; waterpipe: F(4,52) = 41.93; p<0.05;]). Nicotine-containing smoke condensate/aerosol also served as a reinforcer at one or more doses in each of the four groups (Figure 2). Rats in the cigarette, little cigar, and e-cigarette groups responded more on the active lever for smoke/aerosol solutions containing 7.5, 15.0, and 30.0 μg/kg/infusion nicotine than for vehicle. In contrast, rats in the waterpipe group only responded more on the active lever for the smoke/aerosol solution containing 30.0 μg/kg/infusion nicotine than for vehicle (dose × lever interaction [cigarette: F(4,48) = 31.15; p<0.05; little cigar: F(4,60) = 35.30; p<0.05; e-cigarette: F(4,56) = 31.15; p<0.05; waterpipe: F(4,52) = 22.62; p<0.05]). Additionally, responding on the active lever was greater than responding on the inactive lever for all nicotine and all smoke/aerosol doses, including vehicle, for all groups.
Figure 2.
Nicotine and smoke/aerosol condensate dose-effect functions. Mean number of active and inactive lever presses as a function of dose. * indicates significant difference from saline vehicle (S) within dose-effect curve. n=13 for cigarette; n=16 for little cigar; n=15 for e-cigarette; n=14 for waterpipe.
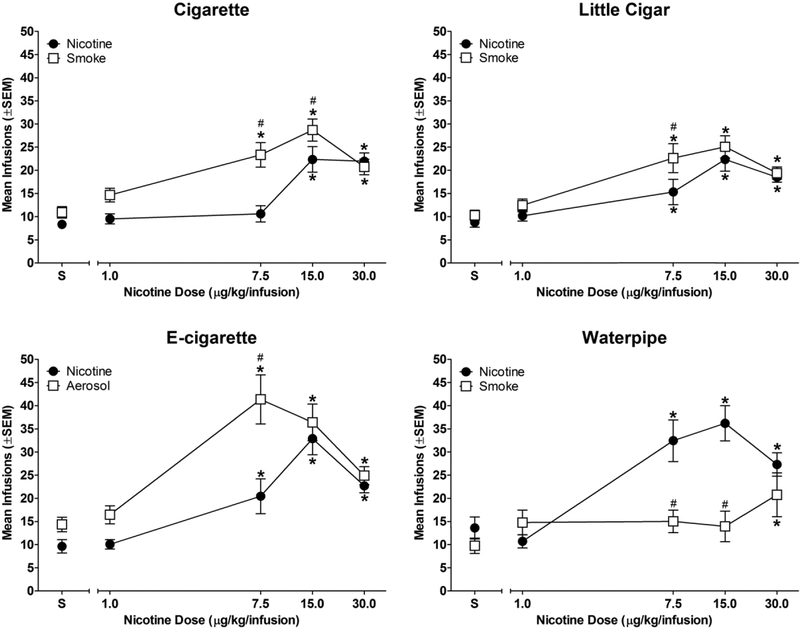
Figure 3 shows infusions earned as a function of nicotine dose in each group. As expected, nicotine alone served as a reinforcer. Rats in all groups earned more infusions of two or more doses of nicotine than vehicle (dose × drug interaction [cigarette: F(4,48) = 9.43; p<0.05; cigar: F(4,60) = 4.54; p<0.05; e-cigarette: F(4,56) = 12.64; p<0.05; waterpipe: F(4,52) = 22.62; p<0.05]). Nicotine-containing smoke/aerosol condensate also served as a reinforcer in all groups. Rats in the cigarette, little cigar, and e-cigarette groups earned more infusions of three condensate doses (7.5, 15.0, and 30.0 μg/kg nicotine/infusion) than vehicle, whereas rats in the waterpipe group only earned more 30.0 μg/kg nicotine/infusion condensate infusions than vehicle.
Figure 3.
Nicotine and smoke/aerosol condensate dose-effect functions. Mean number of infusions as a function of dose. * indicates significant difference from saline vehicle (S) within dose-effect curve (p < 0.05); # indicates significant difference from nicotine-alone dose-effect curve for smoke/aerosol condensate at same nicotine dose (p < 0.05). n=13 for cigarette; n=16 for little cigar; n=15 for e-cigarette; n=14 for waterpipe.
When nicotine and smoke/aerosol condensate were compared within group, results showed that the cigarette group earned more 7.5 and 15.0 μg/kg nicotine/infusion condensate infusions than nicotine alone. Similarly, the little cigar and e-cigarette groups earned more 7.5 μg/kg nicotine/infusion condensate infusions than nicotine alone. In contrast, the waterpipe tobacco group earned fewer 7.5 and 15.0 μg/kg nicotine/infusion condensate infusions than nicotine alone (Figure 3).
3.3. PR assessments
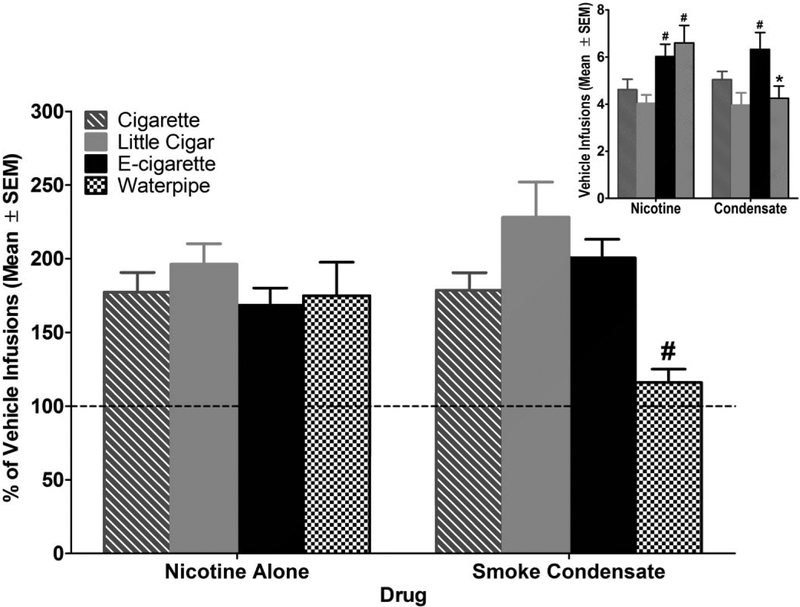
Vehicle infusions earned during PR assessments are shown in Figure 4 Inset. Rats in the e-cigarette and waterpipe groups earned more infusions of nicotine vehicle (both groups) and smoke/aerosol vehicle (e-cigarette only) than the cigarette group [drug × tobacco type interaction: F(3,53) = 10.69; p<0.05]. Furthermore, rats in the waterpipe group earned significantly fewer smoke condensate vehicle infusions than nicotine vehicle infusions.
Figure 4.
Percent vehicle infusions earned on a progressive ratio schedule of reinforcement. Bars show the average percent vehicle of the last 2 sessions of each condition. Nicotine and condensate data were normalized to the corresponding vehicle data because responding for vehicle was different across groups (see inset). # indicates significant difference compared to smoke condensate for e-cigarettes and little cigars (p < 0.05). Inset: Vehicle infusions earned on a PR schedule of reinforcement during both vehicle phases. * indicates significant difference from nicotine alone (p < 0.05) ; # indicates significant difference from cigarette group (p < 0.05). n=12 for cigarette; n=16 for little cigar; n=15 for e-cigarette; n=14 for waterpipe.
The percent of vehicle infusions earned during PR assessments are shown in Figure 4. Rats in all groups earned a similar number of infusions of 30.0 μg/kg/infusion nicotine (expressed as a percentage of vehicle infusions). The smoke/aerosol condensate from all tobacco types engendered a similar number of infusions as nicotine alone (expressed as a percentage of vehicle infusions), suggesting that both formulation types were similar in reinforcing efficacy for all tobacco products at this nicotine dose. Although rats in the waterpipe group earned fewer infusions of tobacco smoke condensate than nicotine alone, this effect was not statistically significant. Comparison across groups showed that the waterpipe tobacco group earned significantly fewer smoke/aerosol condensate infusions than the little cigar and e-cigarette groups [drug × tobacco type interaction: F(3,53) = 3.51; p<0.05].
3.4. Smoke/aerosol condensates
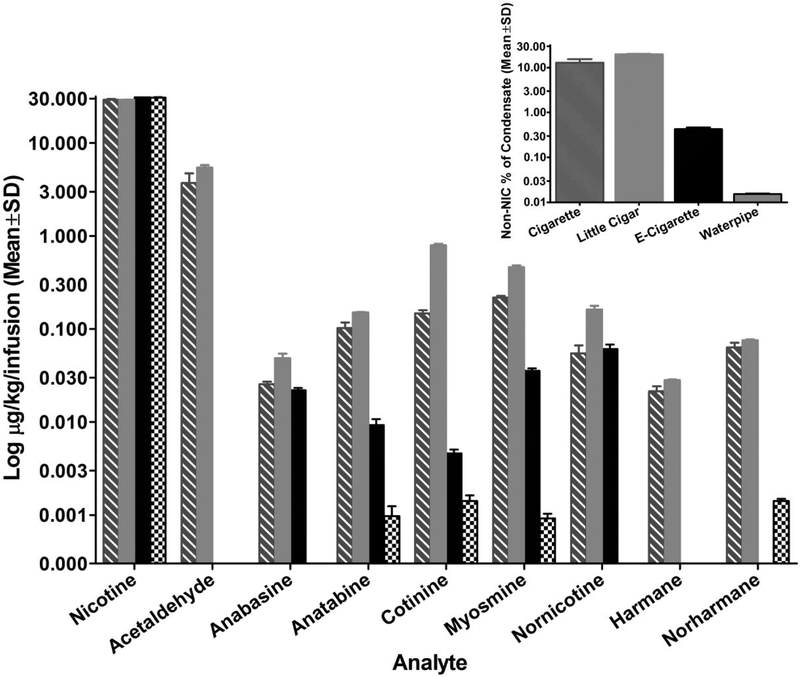
The concentrations of nicotine, nornicotine, anabasine, anatabine, cotinine, myosmine, harmane, norharmane, and acetaldehyde in each infusion of smoke/aerosol condensate are shown in Figure 5. Nicotine concentrations were held constant across tobacco products, whereas concentrations of non-nicotine tobacco constituents varied across products. While concentrations for many of the analytes were comparable in cigarette and little cigar formulations, concentrations in the little cigar formulation were consistently higher when there was a difference. In contrast, e-cigarette aerosol contained fewer and less concentrated amounts of the measured tobacco constituents, and a considerably lower percent of non-nicotine tobacco constituents than cigarette and little cigar smoke condensates (Figure 5, inset). Non-nicotine tobacco constituent concentrations were even less for the waterpipe smoke condensate, with only four of the eight analytes showing measurable levels. Anatabine, cotinine, and myosmine were the only measured non-nicotine analytes that were detected in smoke/aerosol condensate from all products.
Figure 5.
Concentration of tobacco constituents in each infusion of smoke/aerosol condensate for each tobacco product. Two batches of formulations were created for each tobacco type, and bars show the mean (± SD) of both batches on a log scale. Absence of a bar indicates analyte concentration was below the level of quantitation. Inset: Log concentration of non-nicotine tobacco constituents expressed as a percentage of all analytes (including nicotine) for each smoke/aerosol condensate. NIC=nicotine.
4.0. Discussion
Consistent with past studies, rats in all groups acquired self-administration of 30 μg/kg/infusion nicotine [16, 23–26]. Nicotine produced similarly shaped dose-effect curves in all groups, comparable to past studies of nicotine alone [3, 45]. Further, the reinforcing effects of nicotine alone (30 μg/kg/infusion) were similar across all groups when nicotine was available on a PR schedule of reinforcement.
For the cigarette, little cigar, and e-cigarette groups, comparison of the nicotine alone and smoke/aerosol condensate dose-effect curves revealed an apparent leftward shift for condensate. Each group self-administered significantly more infusions of one or more doses of the nicotine-containing condensate compared to the comparable dose of nicotine alone, suggesting that non-nicotine constituents in the smoke/aerosol condensate of these three tobacco products increased the reinforcing potency of nicotine. In contrast, waterpipe tobacco smoke condensate produced an apparent rightward shift in the dose-effect curve, suggesting that the non-nicotine constituents in waterpipe tobacco smoke condensate decreased the reinforcing potency of nicotine. These results support the theory that unique additives that differ across tobacco products, while not measured in the present study, likely contribute to nicotine’s reinforcing effects and the product’s abuse potential.
While nicotine doses below 30 μg/kg/infusion shifted the dose-effect curves for smoke/aerosol condensate relative to nicotine alone when evaluated under an FR schedule of reinforcement, smoke/aerosol condensates containing 30 μg/kg/infusion nicotine produced similar levels of responding compared to nicotine alone during the FR and PR phases of the study. Given that lower doses were not tested during the PR phase, it is unclear whether non-nicotine tobacco constituents alter the reinforcing efficacy of lower doses of nicotine.
Previous studies that examined the reinforcing effects of tobacco smoke condensate show conflicting findings. For example, nicotine plus a minor tobacco alkaloid cocktail or aqueous solutions of cigarette smoke condensate led to greater acquisition and maintenance of self-administration on an FR schedule of reinforcement than nicotine alone in some studies [16, 23, 34], but not in others [7, 37]. Similarly, some studies found that nicotine plus a minor tobacco alkaloid cocktail or tobacco smoke condensate produced similar levels of responding on a PR schedule of reinforcement compared to nicotine alone [7, 34], but there were exceptions [23]. Additionally, other studies found no difference in acquisition of self-administration or demand elasticity for nicotine alone versus nicotine-containing, flavored e-liquids [46, 47].
Differences in methodologies complicate direct comparisons between results of these past studies and the present one. First, the ratio of nicotine:non-nicotine constituents in the formulations differed across studies. The present study utilized condensate formulations, which maintained the nicotine:non-nicotine constituent ratios found in smoke/aerosol. In contrast, some previous studies used self-administration cocktails with considerably lower nicotine:non-nicotine constituent ratios than measured in tobacco smoke [7, 23]. Differences in minor alkaloid concentrations between tobacco brands within the same class (e.g., different cigarette brands) could also contribute to variant findings across studies. For example, cigarette smoke condensate from Holiday brand cigarettes (Australia) had similar reinforcing effects as nicotine alone [16]. Second, doses and schedules of reinforcement varied across studies, which may have yielded different results. Third, the present study compared self-administration of nicotine alone and smoke/aerosol condensate within subject, whereas many previous studies used between-subject designs wherein one group of rats received nicotine and other groups received smoke extract or cocktail [23, 34, 37]. Different baseline levels of nicotine self-administration across groups in the present study (Figure 1) highlight a potential limitation of such between-subject designs. Finally, the present study was relatively comprehensive by systematically replicating the effects of four types of tobacco products evaluated under identical conditions.
The present findings suggest that Nakhla Two Apples waterpipe tobacco smoke condensate contains tobacco constituents or additives that blunt the reinforcing effects of nicotine. Indeed, waterpipe tobacco smoke condensate only increased infusions above vehicle when it contained the highest dose of nicotine evaluated in this study, and only when infusions were available on an FR schedule of reinforcement. Interestingly, when the work required to earn this dose of condensate was increased under the PR schedule of reinforcement, the number of infusions earned decreased to just slightly above vehicle levels.
The identity of constituents/additives contained in waterpipe tobacco that might blunt nicotine’s reinforcing effects is unknown. Nicotine content was roughly equivalent for all tobacco product formulations, and all contained anatabine, cotinine, and myosmine. Further, waterpipe tobacco smoke condensate contained the lowest concentration of anabasine, anatabine, cotinine, myosmine, and nornicotine, and the lowest percentage of non-nicotine constituents of the tobacco products analyzed in the present study. Given the observed increases in nicotine’s reinforcing potency for the other three tobacco products, it is unlikely that one of these measured constituents blunted the reinforcing potency of nicotine in waterpipe tobacco. Rather, a direct or indirect blunting effect of unique constituents/additives is the most parsimonious explanation for the differences seen with waterpipe tobacco. Previous research has identified numerous non-nicotine chemicals and substances contained in waterpipe tobacco, including volatile organic compounds, polycyclic aromatic hydrocarbons, carbonylic compounds, and heavy metals [48–50]. Additionally, the waterpipe tobacco examined in this study was flavored because the vast majority of waterpipe tobacco users use flavored products, and fruit flavors in particular [51–53]. Nicotine is present in low abundance in flavored waterpipe tobacco, while oxygenated monoterpenes and esters are the most abundant volatile compounds in apple flavored waterpipe tobacco [54]. Thus, chemicals used for the apple flavoring also could have modulated nicotine’s effects [55].
A limitation of this study is that only a single product (i.e., brand, flavor) of each tobacco type was examined. Nonetheless, the products selected were based on market popularity, contained a range of analyte levels (allowing for examination of between-product differences), and have been used in previous human and rodent behavioral studies [34, 37–39]. Another limitation is that rats were trained to respond for nicotine alone, and then tested with tobacco smoke/aerosol condensate, but responding for nicotine alone was not reassessed. Thus, we cannot rule out the potential that prior experience with nicotine alone altered later responding for smoke/aerosol condensate; however, the relatively stable level of responding for the training dose of nicotine throughout the study provides confidence that this training history did not produce sensitization to overall effects of nicotine.
In March 2018, the Food and Drug Administration issued an Advanced Notice of Proposed Rulemaking that sought information for consideration in the development of a product standard that would reduce the addictiveness of combustible cigarettes by lowering nicotine levels in these products to minimally addictive or non-addictive levels [56]. Although nicotine is the primary addictive constituent in tobacco products [2], the present data suggest that non-nicotine tobacco constituents may contribute to the reinforcing effects of nicotine in some tobacco products. These effects were most apparent at lower nicotine doses (e.g., 7.5 μg/kg/infusion), as smoke condensate from cigarettes and little cigars, and aerosol condensate from e-cigarettes significantly increased FR responding relative to nicotine alone.
Several conclusions may be drawn from this study. First, nicotine alone and all nicotine-containing tobacco products served as effective reinforcers, emphasizing that nicotine is a robust reinforcer that contributes to tobacco product use. Second, condensate from cigarettes, little cigars, and e-cigarettes engendered more infusions than equal doses of nicotine alone, suggesting that non-nicotine constituents of these tobacco products enhance nicotine’s reinforcing potency without affecting the reinforcing efficacy of the training dose. This enhancement was especially prominent for cigarettes. Finally, results suggest the presence of unique constituents/additives that differ across tobacco products and have varying effects on nicotine reinforcement. Although condensate from waterpipe tobacco was reinforcing at 30 μg/kg/infusion nicotine, it blunted the reinforcing potency of nicotine at lower doses. Interestingly, this tobacco product also contained the lowest percentage of non-nicotine tobacco constituents, suggesting that unique, but unidentified constituents/additives may have contributed to this suppressive effect.
Supplementary Material
Highlights.
Non-nicotine tobacco constituents play a role in abuse of tobacco products.
Cigarette, little cigar, and e-cigarette tobacco enhanced nicotine’s reinforcing potency.
Waterpipe tobacco blunted nicotine’s reinforcing potency.
Tobacco constituents modulate nicotine’s reinforcing effects and alter drug intake.
Acknowledgements
The authors thank Daniel Barrus, James Blake, Michael Boyd, Donna Browning, Randi Carter, Donna Coleman, Ricardo Cortes, Kim Custer, Alex Kovach, Timothy Lefever, Randy Price, Nikita Pulley, Shanequa Taylor, and Teruyo Uenoyama for technical assistance, and Jeannie Jeong-Im and Chad Reissig for technical review of the study design. The authors have no conflicts of interest. The views and opinions expressed in this manuscript are those of the authors only, and do not represent the views, official policy, or position of the Food and Drug Administration Center for Tobacco Products. Research was generously supported by the U.S. Food and Drug Administration [contract HHSF223201310034I]. The funding sponsor played a role in study design, interpretation of data, writing of the report; and in the decision to submit the article for publication.
References
- [1].Centers for Disease Control and Prevention, The health consequences of smoking – 50 years of progress: a report of the Surgeon General, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA, 2014. [Google Scholar]
- [2].Benowitz NL, Nicotine addiction, N Engl J Med 362 (2010) 2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bardo M, Green T, Crooks P, Dwoskin L, Nornicotine is self-administered intravenously by rats, Psychopharmacology 146 (1999) 290–296. [DOI] [PubMed] [Google Scholar]
- [4].Belluzzi JD, Wang R, Leslie FM, Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats, Neuropsychopharmacology 30 (2005) 705–12. [DOI] [PubMed] [Google Scholar]
- [5].Caine SB, Collins GT, Thomsen M, Wright C, Lanier RK, Mello NK, Nicotine-like behavioral effects of the minor tobacco alkaloids nornicotine, anabasine, and anatabine in male rodents, Exp Clin Psychopharmacol 22 (2014) 9–22. [DOI] [PubMed] [Google Scholar]
- [6].Hall BJ, Wells C, Allenby C, Lin MY, Hao I, Marshall L, Rose JE, Levin ED, Differential effects of non-nicotine tobacco constituent compounds on nicotine self-administration in rats, Pharmacol Biochem Behav 120 (2014) 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Smith TT, Schaff MB, Rupprecht LE, Schassburger RL, Buffalari DM, Murphy SE, Sved AF, Donny EC, Effects of MAO inhibition and a combination of minor alkaloids, beta-carbolines, and acetaldehyde on nicotine self-administration in adult male rats, Drug Alcohol Depend 155 (2015) 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alpert HR, Agaku IT, Connolly GN, A study of pyrazines in cigarettes and how additives might be used to enhance tobacco addiction, Tob Control 25 (2016) 444–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kroemer NB, Veldhuizen MG, Delvy R, Patel BP, O’Malley SS, Small DM, Sweet taste potentiates the reinforcing effects of e-cigarettes, Eur Neuropsychopharmacol 28 (2018) 1089–1102. [DOI] [PubMed] [Google Scholar]
- [10].Rabinoff M, Caskey N, Rissling A, Park C, Pharmacological and chemical effects of cigarette additives, Am J Public Health 97 (2007) 1981–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lisko JG, Stanfill SB, Duncan BW, Watson CH, Application of GC-MS/MS for the analysis of tobacco alkaloids in cigarette filler and various tobacco species, Anal Chem 85 (2013) 3380–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Djordjevic MV, Doran KA, Nicotine content and delivery across tobacco products, Handb Exp Pharmacol (2009) 61–82. [DOI] [PubMed] [Google Scholar]
- [13].Lisko JG, Tran H, Stanfill SB, Blount BC, Watson CH, Chemical Composition and Evaluation of Nicotine, Tobacco Alkaloids, pH, and Selected Flavors in E-Cigarette Cartridges and Refill Solutions, Nicotine and Tobacco Research 17 (2015) 1270–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jacob P 3rd, Yu L, Shulgin AT, Benowitz NL, Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes, Am J Public Health 89 (1999) 731–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Klupinski TP, Strozier ED, Friedenberg DA, Brinkman MC, Gordon SM, Clark PI, Identification of new and distinctive exposures from little cigars, Chem Res Toxicol 29 (2016) 162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Brennan KA, Crowther A, Putt F, Roper V, Waterhouse U, Truman P, Tobacco particulate matter self-administration in rats: differential effects of tobacco type, Addict Biol 20 (2015) 227–35. [DOI] [PubMed] [Google Scholar]
- [17].Palmatier MI, Smith AL, Odineal EM, Williams EA, Sheppard AB, Bradley CA, Nicotine Self-Administration With Tobacco Flavor Additives in Male Rats, Nicotine & Tobacco Research (2019) [Epub ahead of print], doi: 10.1093/ntr/ntz053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].National Research Council, Guide for the Care and Use of Laboratory Animals, 8th ed., National Academies Press (US), Washington, D.C., 2011. [Google Scholar]
- [19].Lefever TW, Marusich JA, Antonazzo KR, Wiley JL, Evaluation of WIN 55,212–2 self-administration in rats as a potential cannabinoid abuse liability model, Pharmacol Biochem Behav 118 (2014) 30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Marusich JA, Beckmann JS, Gipson CD, Bardo MT, Cue effects on methylphenidate self-administration in rats, Behav Pharmacol 22 (2011) 714–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Brown PL, Jenkins HM, Auto-shaping of the pigeon’s key-peck, J Exp Anal Behav 11 (1968) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Beckmann JS, Marusich JA, Gipson CD, Bardo MT, Novelty seeking, incentive salience and acquisition of cocaine self-administration in the rat, Behav Brain Res 216 (2011) 159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Clemens KJ, Caille S, Stinus L, Cador M, The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats, International Joural Neuropsychopharmacology 12 (2009) 1355–66. [DOI] [PubMed] [Google Scholar]
- [24].Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE, Nicotine self-administration in rats on a progressive ratio schedule of reinforcement, Psychopharmacology 147 (1999) 135–142. [DOI] [PubMed] [Google Scholar]
- [25].Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF, Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency, Psychopharmacology 136 (1998) 83–90. [DOI] [PubMed] [Google Scholar]
- [26].Shram MJ, Funk D, Li Z, Le AD, Nicotine self-administration, extinction responding and reinstatement in adolescent and adult male rats: evidence against a biological vulnerability to nicotine addiction during adolescence, Neuropsychopharmacology 33 (2008) 739–48. [DOI] [PubMed] [Google Scholar]
- [27].Gomez AM, Sun WL, Midde NM, Harrod SB, Zhu J, Effects of environmental enrichment on ERK1/2 phosphorylation in the rat prefrontal cortex following nicotine-induced sensitization or nicotine self-administration, Eur J Neurosci 41 (2015) 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Karatayev O, Lukatskaya O, Moon SH, Guo WR, Chen D, Algava D, Abedi S, Leibowitz SF, Nicotine and ethanol co-use in Long-Evans rats: Stimulatory effects of perinatal exposure to a fat-rich diet, Alcohol 49 (2015) 479–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Morganstern I, Lukatskaya O, Moon SH, Guo WR, Shaji J, Karatayev O, Leibowitz SF, Stimulation of nicotine reward and central cholinergic activity in Sprague-Dawley rats exposed perinatally to a fat-rich diet, Psychopharmacology 230 (2013) 509–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fowler CD, Kenny PJ, Intravenous nicotine self-administration and cue-induced reinstatement in mice: effects of nicotine dose, rate of drug infusion and prior instrumental training, Neuropharmacology 61 (2011) 687–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Sorge RE, Clarke PB, Rats self-administer intravenous nicotine delivered in a novel smoking-relevant procedure: effects of dopamine antagonists, J Pharmacol Exp Ther 330 (2009) 633–40. [DOI] [PubMed] [Google Scholar]
- [32].Arnold MM, Loughlin SE, Belluzzi JD, Leslie FM, Reinforcing and neural activating effects of norharmane, a non-nicotine tobacco constituent, alone and in combination with nicotine, Neuropharmacology 85 (2014) 293–304. [DOI] [PubMed] [Google Scholar]
- [33].Brunzell DH, Boschen KE, Hendrick ES, Beardsley PM, McIntosh JM, Alpha-conotoxin MII-sensitive nicotinic acetylcholine receptors in the nucleus accumbens shell regulate progressive ratio responding maintained by nicotine, Neuropsychopharmacology 35 (2010) 665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Costello MR, Reynaga DD, Mojica CY, Zaveri NT, Belluzzi JD, Leslie FM, Comparison of the reinforcing properties of nicotine and cigarette smoke extract in rats, Neuropsychopharmacology 39 (2014) 1843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Depoortere R, Li D, Lane J, Emmett-Oglesby M, Parameters of self-administration of cocaine in rats under a progressive-ratio schedule, Pharmacology, Biochemistry, and Behavior 45(1993)539–548. [DOI] [PubMed] [Google Scholar]
- [36].Garcia KL, Le AD, Tyndale RF, Effect of food training and training dose on nicotine self-administration in rats, Behav Brain Res 274 (2014) 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gellner CA, Belluzzi JD, Leslie FM, Self-administration of nicotine and cigarette smoke extract in adolescent and adult rats, Neuropharmacology 109 (2016) 247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cobb CO, Blank MD, Morlett A, Shihadeh A, Jaroudi E, Karaoghlanian N, Kilgalen B, Austin J, Weaver MF, Eissenberg T, Comparison of puff topography, toxicant exposure, and subjective effects in low- and high-frequency waterpipe users: a double-blind, placebo-control study, Nicotine and Tobacco Research 17 (2015) 667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Blank MD, Cobb CO, Kilgalen B, Austin J, Weaver MF, Shihadeh A, Eissenberg T, Acute effects of waterpipe tobacco smoking: a double-blind, placebo-control study, Drug Alcohol Depend 116 (2011) 102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KA, Sex differences in the contribution of nicotine and nonpharmacological stimuli to nicotine self-administration in rats, Psychopharmacology 180 (2005) 258–66. [DOI] [PubMed] [Google Scholar]
- [41].Grebenstein P, Burroughs D, Zhang Y, LeSage MG, Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy, Pharmacology, Biochemistry, and Behavior 114–115 (2013) 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Grebenstein PE, Burroughs D, Roiko SA, Pentel PR, LeSage MG, Predictors of the nicotine reinforcement threshold, compensation, and elasticity of demand in a rodent model of nicotine reduction policy, Drug Alcohol Depend 151 (2015) 181–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF, Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement, Psychopharmacology 169 (2003) 68–76. [DOI] [PubMed] [Google Scholar]
- [44].Richardson NR, Roberts DC, Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy, J Neurosci Methods 66 (1996) 1–11. [DOI] [PubMed] [Google Scholar]
- [45].Caille S, Clemens K, Stinus L, Cador M, Modeling nicotine addiction in rats, Methods Mol Biol 829 (2012) 243–56. [DOI] [PubMed] [Google Scholar]
- [46].LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, Harris AC, Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats, Drug Alcohol Depend 168 (2016) 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Smethells JR, Harris AC, Burroughs D, Hursh SR, LeSage MG, Substitutability of nicotine alone and an electronic cigarette liquid using a concurrent choice assay in rats: A behavioral economic analysis, Drug Alcohol Depend 185 (2018) 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Schubert J, Muller FD, Schmidt R, Luch A, Schulz TG, Waterpipe smoke: source of toxic and carcinogenic VOCs, phenols and heavy metals?, Arch Toxicol 89 (2015) 2129–39. [DOI] [PubMed] [Google Scholar]
- [49].Shihadeh A, Schubert J, Klaiany J, El Sabban M, Luch A, Saliba NA, Toxicant content, physical properties and biological activity of waterpipe tobacco smoke and its tobacco-free alternatives, Tob Control 24 Suppl 1 (2015) i22–i30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Al-Kazwini AT, Said AJ, Sdepanian S, Compartmental analysis of metals in waterpipe smoking technique, BMC Public Health 15 (2015) 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Bonhomme MG, Holder-Hayes E, Ambrose BK, Tworek C, Feirman SP, King BA, Apelberg BJ, Flavoured non-cigarette tobacco product use among US adults: 2013–2014, Tob Control 25 (2016) ii4–ii13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Smith-Simone S, Maziak W, Ward KD, Eissenberg T, Waterpipe tobacco smoking: knowledge, attitudes, beliefs, and behavior in two U.S. samples, Nicotine Tob Res 10 (2008) 393–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Sutfin EL, Song EY, Reboussin BA, Wolfson M, What are young adults smoking in their hookahs? A latent class analysis of substances smoked, Addict Behav 39 (2014) 1191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Farag MA, Elmassry MM, El-Ahmady SH, The characterization of flavored hookahs aroma profile and in response to heating as analyzed via headspace solid-phase microextraction (SPME) and chemometrics, Sci Rep 8 (2018) 17028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Schubert J, Luch A, Schulz TG, Waterpipe smoking: analysis of the aroma profile of flavored waterpipe tobaccos, Talanta 115 (2013) 665–74. [DOI] [PubMed] [Google Scholar]
- [56].U.S. Food and Drug Administration, Tobacco product standard for nicotine level of combusted cigarettes, Fed Regist 83 (2018) 11818–11843. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.