Abstract
A common finding in human functional brain imaging studies is that damage to neural systems paradoxically results in enhanced functional connectivity between network regions, a phenomenon commonly referred to as hyperconnectivity. We describe the various ways that hyperconnectivity operates to benefit a neural network following injury while simultaneously negotiating the trade-off between metabolic cost and communication efficiency. Hyperconnectivity may be optimally expressed by increasing connections through the most central and metabolically efficient regions (i.e., hubs). While adaptive in the short-term, we propose that chronic hyperconnectivity may leave network hubs vulnerable to secondary pathological processes over the life span due to chronically elevated metabolic stress. We conclude by offering novel, testable hypotheses for advancing our understanding of the role of hyperconnectivity in systems-level brain plasticity in neurological disorders.
Trends:
Brain imaging methods coupled with network neuroscience now permit previously unavailable opportunities to examine large-scale network changes after injury.
One common response to neurological disruption of neural networks is increased brain connectivity between residual network regions, referred to as hyperconnectivity.
Network hubs are centralized nodes in brain networks and are disproportionately represented as sites of pathology and may also play an important role in reintegration of function during recovery.
There is converging evidence that increased brain metabolism, through local activity and increased network burden, is associated with neurodegeneration.
Keywords: network, connectivity, brain injury, plasticity, Alzheimer’s disease
Understanding Brain Disorders in an era of Network Neuroscience
Now firmly rooted in the era of the human connectome, novel mathematical applications are commonly paired with established functional brain imaging methods (e.g., functional MRI, magnetoencephalography) to model the consequences of brain injury and disease in humans [1–2]. In this paper, we review a literature leveraging the powerful framework of network neuroscience to understand the macro-scale, or system-level, modifications occurring in neural networks after neurological disruption. Our discussion is focused on one common finding in brain disorders: physical disruption of neural networks secondary to injury or disease may paradoxically result in a regional increase in functional connectivity (i.e., hyperconnectivity) between some parts of the disrupted neural network (see Glossary). We make the argument that the optimal expression of network change after injury, or network plasticity, is centered around network hubs, or the most highly connected and efficient network nodes. Over the course of this review, we integrate findings from several brain imaging literatures with the goal exploring the time line for expression of hyperconnectivity after neurological disruption and its role in clinical outcome in chronic brain disorders. Ultimately, we anticipate that large-scale network plasticity is guided by principles that optimize network efficiency requiring integration of principal network nodes to maintain efficient communication across a distributed, dynamic neural network.
Connections and hyperconnections
In network neuroscience, a “connection”, can be defined as the temporal covariance in the signal produced by two spatially distinct brain regions [3]. For the purposes of this discussion, hyperconnectivity, represents enhanced functional connectivity in number or strength of connections in a clinical sample compared to a control sample, and we focus our review on increased connectivity after injury as opposed to connectivity loss for two reasons. First, hyperconnectivity represents an observable brain response to neural network disruption as opposed to the absence of a response or functional deficit that has been historically a central focus in the clinical neurosciences. While this tradition of linking structural brain damage to behavioral and functional loss provides evidence of the local consequence of injury, the recent focus on hyperconnectivity provides a window into how dynamic neural systems respond to insult which is essential for understanding recovery of function and neuroplasticity. Second, we aim to reframe the conceptualization of hyperconnectivity with particular attention given to cost and efficiency as important influences on network response. Any modifications to network connectivity in response to injury are obliged to comply with the natural limits of a spatially embedded, metabolically expensive, neural network [4–5]. We propose that adaptive hyperconnectivity (not all increased connectivity will be adaptive) operates to retain a network topology that maintains communication while minimizing the costs of network connection and maximizing network efficiency (cost-efficiency). In the following, we explore the evidence for hyperconnectivity, the time line for its expression and its implications for long-term clinical outcome.
As a final introductory caveat, we focus the discussion in this paper on the coherent brain signals that are observable via functional brain imaging methods, but patterns of oscillatory brain activity are reliably constrained by brain structure [6–8]. Therefore, a functional hyperconnectivity hypothesis is best understood in the context of large-scale changes in brain structure occurring after brain injury. Importantly, there is relative consistency in the structural connectome with respect to white matter connections and network hubs across individuals and the life span [9–11] and an overlapping hub structure is evident in functional data [12] which serves as a foundation for our discussion regarding hubs as centers for neural network plasticity.
Network Science and Resilience in Brain networks
Network science draws on ideas and methods from mathematics, physics and statistics to study network representations of physical, biological and social phenomena. In abstraction, these network representations are mathematical objects called graphs, the centerpiece of graph theory, a subdomain of discrete mathematics [13]. Individual agents operating in the network are represented as vertices (or commonly, nodes), a meaningful relationship between pairs of nodes is represented by edges (links or connections) between them, which may be weighted to capture the strength of the relationship between any two nodes and the degree of any node quantifies the number of connections incident to that node. The definitions and practical usage for several key graph metrics are provided in Box 1.
Box I: The following provides a list of common graph theoretical terms relevant for the current paper. These terms are then illustrated in the Box Figure I using a simple graph example for illustration.
Clustering: measure of the local density of connections, calculated as the number of triangles over the number of connected triples (“how many of my friends are friends with each other”). For (A) clustering coefficient is 1, for (B) it is 0.
Density: The density of the graph is the ratio of number of observable connections in the network to the number of possible connections in the network
Edges: (pairwise) connections between nodes
Hub: node with high degree or high betweenness centrality. Node c (red) has high degree and betweenness, while node b (blue) has high degree within the network. Betweeness centrality of a node reflects the frequency with which it sits along the shortest path between any two other nodes in the graph.
Modules/Communities: clusters of nodes densely linked amongst themselves and much more sparsely linked to nodes outside the cluster. Three communities are circled in red.
Node: brain region of interest
Path Length: we use path length here to indicate shortest path length, or the shortest distance (in number of edges) separating two nodes, e.g., in D from a to d = 5
Weight (of an edge): relationship strength between corresponding pair of nodes (e.g., correlation). In (A) and (B) the network is unweighted, while (C) is weighted.
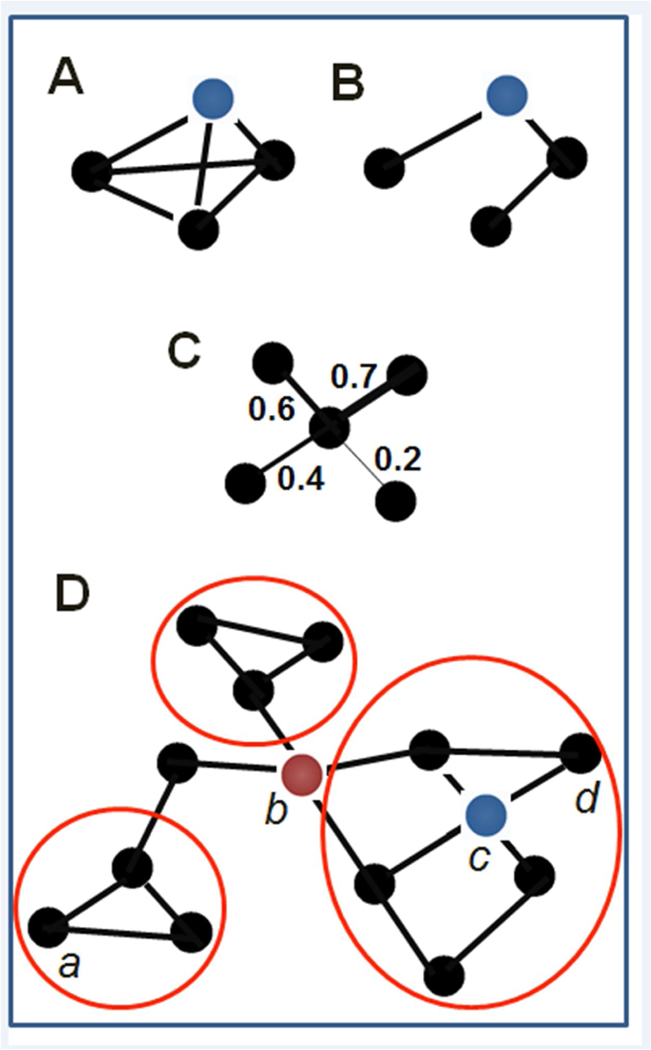
Graphs can be characterized using global metrics that describe the nature of network organization, or topology. First in the study of C. Elegans two decades ago [14] and more recently in humans [15–17] neural networks have been described by a small-world topology characterized by high density of links locally (clustering) and a few long-distance connections shortening pathways between far ends of the network (short average path length) [7]. The power of a smallworld organization is this co-presence of clustering and short average path length, which serves to maximize regional communication and retain efficient communication globally. While a smallworld topology has appeal in describing brain functioning, the exact network structure characterizing neural systems is an active area of investigation. For example, several investigators have argued that other topologies may more accurately capture the important advantages afforded by the modularity, or the presence of densely-linked subgroups of nodes, evident in neural networks (e.g., “large-world” organization [18]). What has been reliably demonstrated is that human neural networks reveal relatively high clustering and short average path length consistent with a small-world topology, but also exhibit a modular community organization with relatively few highly connected nodes [18–19] (i.e., hubs) that operate at the center of information transfer. These network characteristics likely evolved to afford efficient and sustainable encoding and communication while adhering to selection pressures of signaling time and metabolic costs [20–22]. See Box 1 for several examples of network metrics.
The modularity evident in human neural systems and the presence of a select number of highly-connected network hubs, indicates that neural systems may be well-represented by a specific type of small-world network, namely a scale-free network. The presence of network hubs in scale-free networks is central to our discussion of network plasticity and this topological organization may have evolved as a consequence of preferential attachment dynamics during network development [23]. The principle of preferential attachment refers to the greater probability for highly connected nodes to be sites where additional connections are added during periods of network growth [23]. In practice, this means that the nodes with higher degree gain disproportionately more connections, or “rich-get-richer”, resulting in the formation of network hubs. We will return to preferential attachment in the context of neuroplasticity in greater detail later. Importantly, the scale-free topology is robust to disruption with particular resilience to random removal, e.g. injury or failure, of individual nodes or edges [24]. The resilience may be attributable to low probability selection of high degree nodes and connection redundancy [24] which is intriguing and has important implications for neural network response to injury.
Figure 1 is a plot of “random” and “targeted” attack scenarios for connection deletion in the structural connectome derived from human diffusion MRI data [25]. A targeted attack is represented as the selective deletion of high-degree nodes from the network. These data are relevant to our discussion of functional hyperconnectivity in several important ways. First, Figure 1 indicates that neural networks are robust to random insult (i.e., edge or node deletion chosen at random), which is consistent with a long history in clinical neurology and neuropsychology where not all brain lesions have observable behavioral consequences (e.g., “silent” lesions). Second, targeted attack on networks, or initiated with the deletion of highest degree nodes, has significant consequence for network efficiency and therefore behavioral functioning. We argue that a primary goal of hyperconnectivity is to sustain communication through network hubs therefore maximizing information transfer and minimizing behavioral deficit.
Figure 1. Structural connectivity and simulated response to random and targeted network attack.
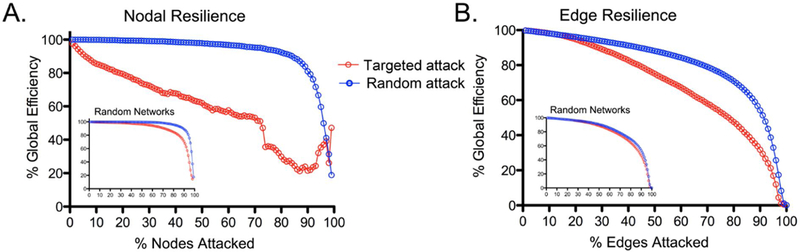
Data based upon a human structural connectome informed using diffusion tensor imaging to generate responses to simulated random and targeted deletion of nodes (A) and edges (B). Compared to random network disruption, targeted attack results in diminished global efficiency for node and degree deletion, with greatest loss of network efficiency with removal of high degree nodes.
The structural resource thresholds for network efficiency outlined in Figure 1 dovetail conceptually with cognitive reserve theory, namely that the emergence of behavioral deficit after injury can be predicted by an interaction between the magnitude of the injury and a priori intellectual status [26–27]. Enhanced cognitive reserve arising from environmental enrichment has been shown to predict connectivity in neural network hubs in healthy adults (e.g., anterior cingulate cortex [28]). Similarly, in mild cognitive impairment (MCI), considered an intermediate phase between normal aging and Alzheimer’s disease (AD), higher cognitive reserve is associated with increased connectivity in cognitive control regions [29]. Cognitive reserve may afford resilience to targeted attack in network hubs (e.g., mesial temporal and posterior regions in AD) presumably due to increased edge density [30] and ultimately set thresholds for resilience to edge deletion and degenerative network changes [31–32]. Therefore, the behavioral consequences of neurological disruption are dependent upon network organization and connection redundancy which together determine the ceiling for symptom-free edge deletion across individuals. Uncovering the interactive relationships between cognitive reserve and genetics in determining network resilience may hold important clues for the timing of hyperconnectivity and the onset and course of brain pathology. Figures 2A–B illustrates the functional connectivity in response to random (or non-hub) structural damage as well as a physical resource gradient for the expression of hyperconnectivity.
Figure 2.
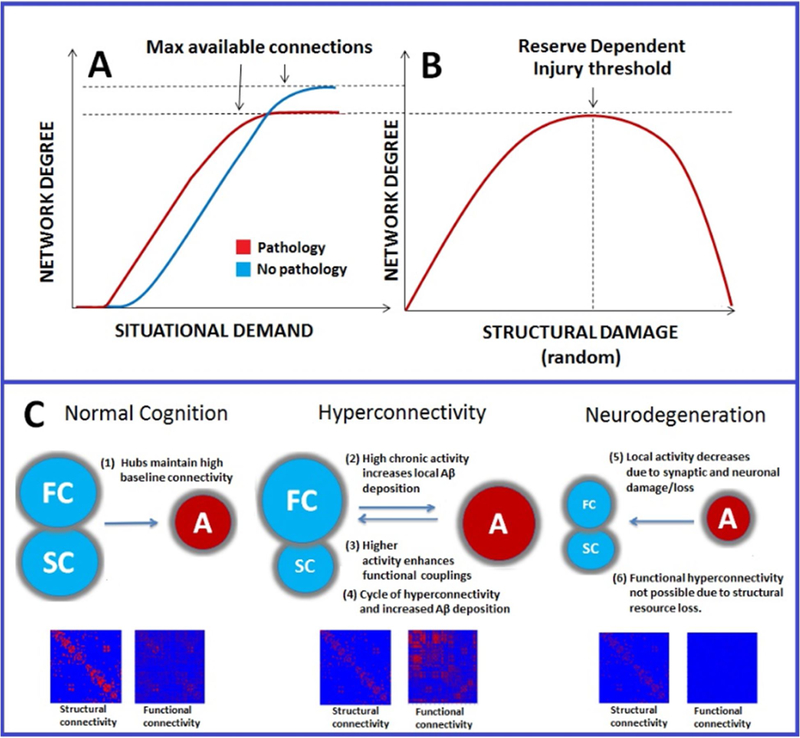
Schematic representation of interaction between structural and functional connectivity after neurological disruption
Schematic representation of proposed functional connectivity effects in neurological disorder. In panel 2A, the red slope represents earlier connectivity recruitment based upon situational demand (e.g., task load, fatigue). Increased connectivity occurs at a lower situational demand after neurological insult and reaches asymptote at a lower resource threshold compared to the noninjured system (blue). In panel 2B, increased connectivity rises to some critical injury threshold (determined by genetics and environmental enrichment) after which widespread disconnection occurs. Adapted from Hillary et al., 2015 with permission by author; Neuropsychology. Fig 2C: Proposed relationship between increased metabolism in hubs and beta-amyloid deposition (adapted from de Haan et al., 2012; PLoS Comput Biol with permission by author). Hubs have a higher intrinsic activity, making them most susceptible to pathology. In stage two hyperconnectivity results from higher local activity initiating a cycle of beta amyloid deposition and enhanced activity/connectivity. In stage three, hyperconnectivity gives way to connectivity loss at critical resource threshold resulting in neurodegeneration. Abbreviations: FC=functional connectivity, SC=structural connectivity, A=activity, PIB= 11C-PIB.
What is the evidence for the hyperconnectivity response?
The finding that physical disruption of a network following injury (i.e., local node removal) results in functional hyperconnectivity may appear counterintuitive, but this effect has been observed in multiple literatures including Parkinson’s disease [33–40], multiple sclerosis [41–51], traumatic brain injury (TBI) [52–59], cerebrovascular accident [60], and epilepsy [61–63]. We suggest that a plausible goal of hyperconnectivity is to re-establish network communication through network hubs and, if so, then the functional hyperconnectivity response should be most prominent following “random” edge deletion which (probabilistically) leaves network hubs functionally exploitable. There is an established literature demonstrating a resource/temporal gradient (i.e., loss of neural resources over time) influencing the network response with hyperconnectivity representing an early phase response to many neurodegenerative disease processes that diminishes as the network succumbs to late-stage cortical atrophy which directly affects network hubs, making connectivity sporadic or even impossible. This pattern of regionally increased functional connectivity early after disease diagnosis or after brain injury has been documented in Parkinson’s Disease [33–35], multiple sclerosis [41–42, 66–67], TBI [53,56–58] and MCI [68–69] followed by connectivity decline as symptoms and disease burden progress [70–73].
In fact, enhanced brain resource utilization may precede disease states or be indicative of risk for pathology. For example, young healthy carriers of the APOE-e4 allele, a genetic risk factor for later-life neurodegeneration including AD, demonstrate enhanced response of the DMN during rest and hyperactivation when engaged by an episodic memory task (i.e., increased local fMRI signal) [74–75] followed by diminishing connectivity during the advanced expression of the disease [72, 76–79]. Recent longitudinal work shows similar trajectories with healthy elderly Apoe4 carriers exhibiting increasing frontal to hippocampal connectivity over a 35-month time frame compared to an Apoe-2 (lower risk) group [72]. The hyperconnectivity response has become so common in the clinical neural network literature that the term “compensation” now represents an accepted, albeit non-specific, explanation of the phenomenon [see 80,81]. What will be important to determine moving forward are the factors that influence the onset and persistence of hyperconnectivity and the long-term consequences for chronic engagement of additional neural resources. For example, TBI has been increasingly recognized as a risk factor for AD [141–142] where posterior connection loss is widely observed in connectivity studies [64–65], but what remains unknown is the transition between hyper-and hypoconnected states and the vulnerabilities associated with chronically elevated resource use given evidence of hyperconnectivity in much younger brain injured samples. Figure 3, Key Figure schematically represents this graded connectivity response in network hubs (3A) highlighted by data from several important findings in the literature (3B).
Figure 3 (Key Figure). Centralized Hyperconnectivity Response and Resource Gradients.
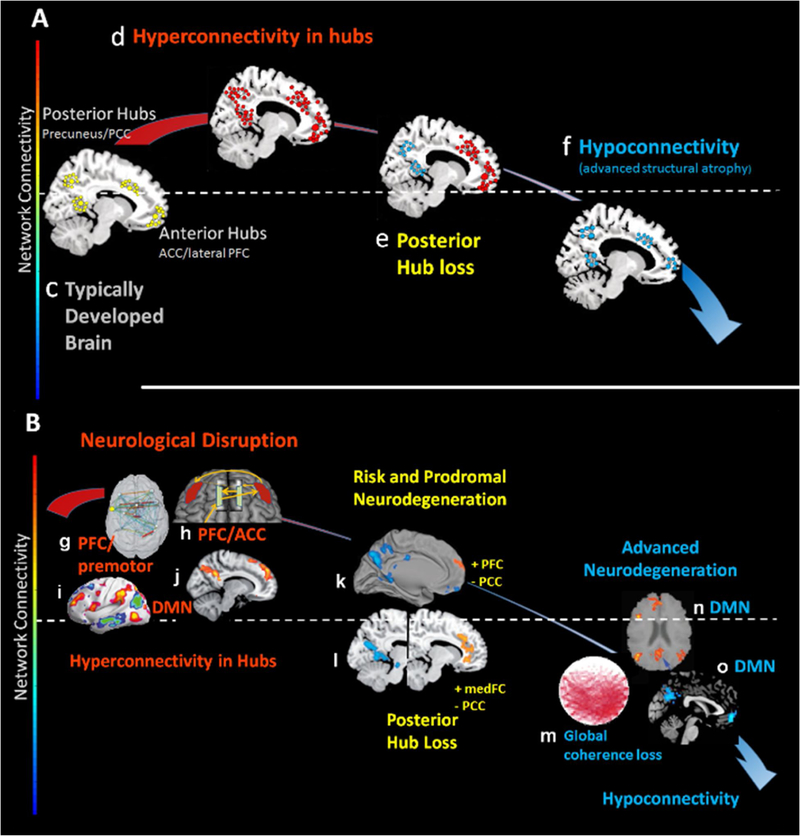
Figure 3A: Schematic representation of the hubs commonly demonstrated hyperconnectivity in injury and disease and the structural resource-dependent trajectory from hyper-to hypo-connectivity. 3B: Mirrors the schematic in 3A with evidence from seminal studies in the literature. Findings in 3B: g: brain injury results in increased prefrontal and motor connectivity[133]; h: increased frontal connectivity during task engagement in brain injury [54], i: increased connectivity in DMN hubs in multiple sclerosis [44]; j: increased connectivity in PCC and medial frontal regions of the DMN in TBI [52]; k: increased Aβ predicts increased frontal connectivity in context of posterior connectivity in healthy aging [134]; l: increased medial frontal connectivity with loss in PCC connectivity in AD [135], m: global interhemispheric connectivity loss in AD [136], n: connection loss in PCC connectivity in AD[137] and o: anterior and posterior connectivity loss in AD[138].
How is hyperconnectivity expressed?
The literature points to both regional and centralized scenarios for increased connectivity in response to injury. With regard to the former, mapping brain structure to function has provided some insight into the regional resources that could be leveraged to bolster connectivity and therefore functioning after injury. Using structural connectomics and diffusion tensor imaging, investigators have demonstrated that information transfer times are dependent upon not only the most direct path between nodes but also the utilization of available alternative or detour paths [82]. One possibility for local hyperconnectivity is that neurological disruption requires ongoing recruitment of available detour paths (see Figure 4). There is evidence for the increased use of regional resources in traditional task-based fMRI activation studies in clinical samples where a primary finding is a more spatially diffuse BOLD response in task-related regions compared to areas more circumscribed in HC samples [83–87]. Moreover, one common finding in graph theoretical studies of neurological disruption is an increased clustering coefficient [88–90], which may represent the exploitation of regional “detour” pathways to amplify signal through additional inputs and enhance alternative means of information processing. Thus, in the region of the lesion, one intuitive explanation for hyperconnectivity is the leveraging of available detour paths either transiently during recovery (Figure 4b) or more permanently as depleted networks work to maintain communication with critical network hubs (Figure 4c).
Figure 4. Regional Hyperconnectivity expressed through local detour paths.
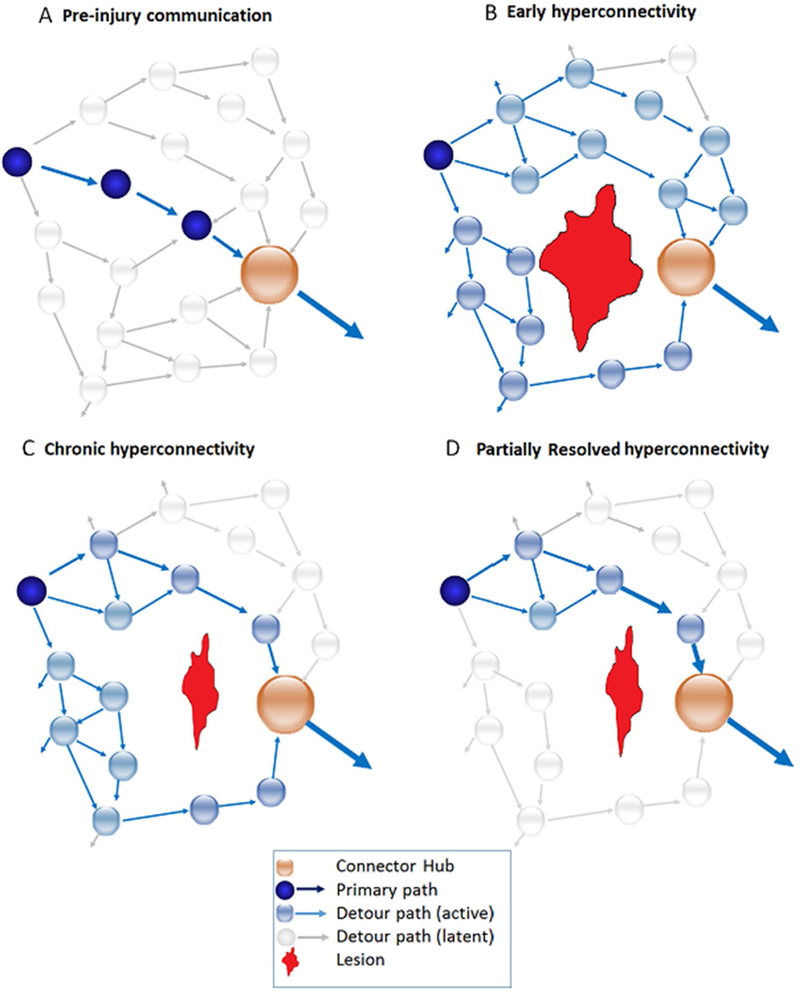
Schematic example inspired conceptually by Goni et al., (see 2014) to demonstrate the inclusion of detour paths during adaptation to the local effects of brain lesion. 3A: pre-injury communication within local network to community hub. 3B: lesion (in red) disrupts primary pathway to local hub resulting in spreading of signal via detour paths to accumulate signal necessary for signal propagation using less established inputs. Connections to hubs increase, clustering increases and path length increases locally in order to amplify signal using less established routes. 3C: Partial resolution of hyperconnectivity as signaling using detour paths becomes more permanent/refined secondary to increased use, still requiring additional input signals, 3D. Near-complete resolution of hyperconnectivity with relatively longer path length.
A separate scenario for enhanced connectivity in response to disruption is a centralized response within critical network hubs irrespective of the nature or location of pathology. There has been much recent attention given to the role of the brain’s most highly integrated network hubs (e.g., precuneus, PCC, medial/lateral frontal cortex, thalamus; commonly termed the “richclub”) in directing global brain dynamics and information transfer [91–94]. A centralized hyperconnectivity response expressed through network hubs, irrespective of the pathophysiology, may achieve two important goals for network communication following neurological disruption. First, rich club nodes demonstrate the highest stability in functional connectivity mapping [8] and are optimally positioned to coordinate novel communication between available resources as network plasticity occurs [93]. For example, recent simulations based on cortical networks in the cat demonstrate that rich-club network hubs galvanize network dynamics and facilitate synchronization moving network activity from de-centralized to centralized cortical synchronization [94]. It may not be too surprising then that these same vital network hubs are the sites of hyperconnectivity across brain disorders (e.g., multiple sclerosis [41–44,46], TBI [52–58], Parkinson’s Disease [33–35]). Functionally, thus rich club hyperconnectivity could work to amplify signal to overcome increased neural noise (as observed in aging [95]) and signal dampening due to the regional effects of injury. Second, from a neural dynamics perspective, rich club nodes may play an important role in achieving states of “criticality”, a concept from physics describing behavior in complex dynamic systems where activity at a “critical point” resides at phase transition points (e.g., water at the liquid-vapor transition point). Criticality is controversial in the neurosciences (for review see [96]), but remains an intriguing possibility to account for how complex behaviors such as cognition can emerge from the apparent cacophony of billions of time varying neural signals [97–98]. Neural systems are required to process a number of inputs on a moment-to-moment basis and, by operating at a critical point, the system maintains enough “order” to ensure coherent representations of sensory inputs, reinforcing reliable responses while simultaneously retaining a measure of “disorder” giving it flexibility to adapt. Criticality may provide neural systems with a wide dynamic range for processing various environmental inputs and maximize the opportunity for information transfer and storage [99] and hubs may play a particularly important role in maintaining these non-linear dynamics [100]. While extending these latest findings to states of injury and plasticity is speculative, the link is intriguing given the central role hubs play in guiding information transfer and the growing evidence for enhanced network involvement of centralized hubs after neurological disruption.
We have argued that a common response post injury is increased connectivity and that this enhancement in neural networks is resource-dependent, maintains a resource gradient based upon disease progression and leverages network hubs to restore network communication. However, alternative explanations are possible. From a physiological perspective, hyperconnectivity could be explained as a “release” of neural signaling from inhibitory control. From a mathematical perspective, one might argue that hyperconnectivity is an inevitable outcome of node removal (e.g., network failures in the US power grid [101]). If accurate, these alternative explanations pose two distinct problems for the current argument. First, hyperconnectivity is not purposeful. Second, hyperconnectivity is an artifactual outcome of its measurement. In response, we turn to three sources of evidence that hyperconnectivity is purposeful and neurologically meaningful. First, support for the hypothesis that hyperconnectivity largely occurs in network hubs is not consistent with the possibility that connection changes are random, represent widespread disinhibition, or are the result of mathematical artifact. The sites for hyperconnectivity occurring in across multiple literatures show much higher consistency than one would expect if the network response to node removal was random. Second, in a majority of the studies cited, the magnitude of the altered connectivity has been consistently linked to behavioral outcome (i.e., task performance, clinical outcome) indicating a purposeful network response. Third, hyperconnectivity has been observed across a number of distinct pathophysiological disorders and, in a recent meta-analysis, local lesions were not strong predictors of network response unless they were isolated to hubs [102]. This evidence reduces the likelihood that hyperconnectivity is aberrant signaling due to pathology-induced disinhibition.
The trouble with increasing connectivity
So far, we have presented a literature where complex neural networks adapt to significant disruption through adjustments in connectivity with the primary goal of enhancing network communication in order to maintain meaningful responses to environmental demands. But communication has metabolic cost in neural systems, so any adjustment to network connectivity after injury must be balanced against the natural tensions between network cost and efficiency. Neural networks maintain an intricate balance between metabolic and structural cost and communication efficiency[19–21,103] and therefore functional recovery is likely optimized by network modification that negotiates these constraints. As connection density increases, so does routing efficiency [19], so the greatest efficiency would be achieved if all possible neural connections were expressed. However, in order to adhere to the brain’s budgetary constraints in space (physical material), energy (metabolism) and time (signaling latencies over long distances), the evolutionary solution is a sparse network of strategically placed connections [21–22].
One important assumption in network neuroscience is that the relationships between nodes modeled statistically actually translates to scalable metabolically demanding brain connections. That is, if degree established via correlational analysis is capturing meaningful biological communication between any two network nodes, it should also be a reliable indicator of metabolic cost. In seminal work examining these relationships, nodal degree established via rsfMRI and PET methods has been shown to maintain a strong association with glucose uptake (see [21]; Figure 5). The coupling between network links established using resting-state functional connectivity and neurometabolism has been observed elsewhere [104–105] and, importantly, these relationships appear to hold even in cases of catastrophic brain injury resulting in persistent vegetative state [106]. Examining the number and Euclidean distance of network connections, we described evidence for this cost-efficiency negotiation in TBI where network cost is greatest at 3-months post injury and this response partially equilibrates by 6 to 12-months post injury as the network arguably accommodates cost-efficiency demands [see 132]. This time line maps nicely onto clinical recovery trajectories reported in the TBI literature which has traditionally documented the largest clinical gains during in the first year after injury [107]. If we can assume that connections established via fMRI methods have real metabolic cost, then re-establishing network communication after disruption via available but commonly dormant pathways (depicted in Figure 4), must be accompanied by a parallel increase in metabolic cost and possibly even cost in transmission time and wiring. We therefore propose that any response to injury must negotiate the same cost/efficiency trade-offs and one way for doing so organize plasticity through hubs. Thus, adjustments post injury may be a recapitulation of the principle of preferential attachment, where those centralized nodes with the greatest connectivity are the regional focus of network plasticity.
Figure 5. Relationship between connectivity results using fMRI and glucose metabolism.
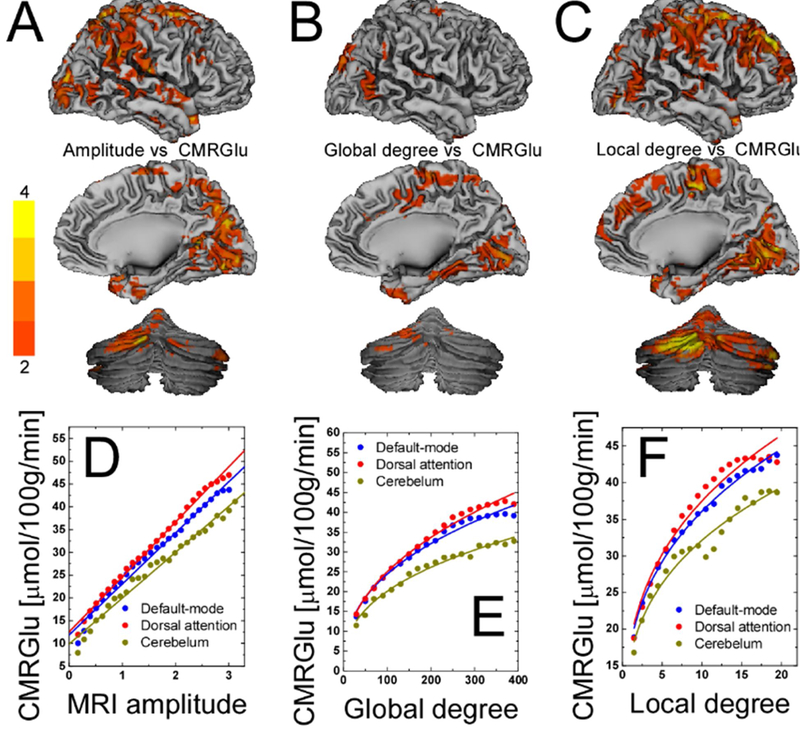
Statistical maps of the voxelwise correlations between (A) CMRGlu and R-fMRI signal amplitude and between CMRGlu and (C) global and (D) local degree across 54 healthy subjects, superimposed on surface views of the cerebral cortex (medial and lateral) and the posterior cerebellum. The color bars indicate t-score values. Scatter plots exemplifying the linear association between CMRGlu and R-fMRI signal amplitudes (D) and the power scaling of CMRGlu and degree (E and F) across 54 healthy subjects for three different networks. The color lines are reduced-major axis regression fits to the data (0.96 < R2 < 0.99). Figure adapted with permission from Tomasi et al., 2013; PNAS.
Reconfiguration through hubs to balance network cost-efficiency
Network modeling based upon mouse data demonstrates that the scale-free nature of neural networks in sensory and auditory cortex is achieved by preferential attachment [108] and this is consistent with in vitro work examining “hub neurons” in mouse hippocampus during the first month of development [109]. The appearance of network hubs has been identified as an important developmental trajectory point in pre-term human infants [110], so the formation of hub connectivity may be an essential goal of early neurodevelopment. Again, given that network hubs are common sites of enhanced connectivity after neurological disruption, a mechanism like preferential attachment focused on network hub plasticity may serve to preserve global information transfer and network stability. Until critical thresholds are reached and functioning within network hubs is lost due to injury or disease process, these centralized nodes may remain the focal point of network plasticity and, in fact, may become more centralized operating as a magnet for adaptive collateral connections during recovery [44]. By the same reasoning, when hubs are lost to injury or disease, the consequences are devastating (e.g., advanced AD, cases of catastrophic TBI).
If hyperconnectivity after disruption poses a potential metabolic crisis, neural systems require a strategic response for connectivity enhancements. Investigators have demonstrated that the same regions commonly identified as network hubs, are also the most metabolically efficient regions in the brain with respect to glucose uptake [21] and this design has been shown to be highly heritable in twin studies, with a small group of hubs showing the highest cost-efficiency [111]. Consistent with this, demonstrations using network control theory reveal that rich-club regions facilitate low-energy dynamics and when hubs are removed, network cost increases during state transitions [see 112].
Therefore, capitalizing upon hub efficiency may mitigate cost-efficiency problems associated with hyperconnectivity. One possibility is that preferential attachment or related neurodevelopmental processes that evolved to segregate the brain into distinct modules may also guide neuroplasticity throughout the life span and could play a crucial role during recovery after neurological insult (see Box 2). Through enhanced connectivity via established network hubs either in prodromal phases of disease (e.g., MCI) or after formal disruption of the system (e.g., TBI, multiple sclerosis), neural networks can flexibly restore encoding and communication while maximizing metabolic efficiency. One possible concern for this blue print for network plasticity is that it disproportionately shifts network load onto hubs.
Possible Consequences of Chronic Hyperconnectivity
Findings revealing that elevated brain metabolism is associated with unwanted consequences has been intensifying across separate literatures for some time. There was strong circumstantial evidence a decade ago that the most highly connected networks (e.g., posterior DMN) contained nodes with disproportionately higher deposition of amyloid beta (Aβ) [113], a marker for AD. Research in animals [114–116] and humans [117–119] has more directly demonstrated that regions with the highest metabolism during normal brain functioning show enhanced Aβ deposition. A meta-analysis of brain lesion studies implicated hubs as the common site for pathology irrespective of disease [60] and recent network simulations using a neural mass model reveal that transient increases in activity preceded gradual declines and these effects are disproportionately evident in hub regions [120]. Emerging from these data is the perspective that elevated metabolic demand in rich-club nodes places these hubs at risk for the development of pathophysiology in abnormal aging [see 121] and that hub “overload” may be a marker for pathology [1].
In the context of this growing awareness of the relationship between elevated metabolism and neurodegeneration, chronic hyperconnectivity should not be viewed solely as an adaptive “compensatory” mechanism [81]. While the causal link between elevated activity and Aβ deposition remains a point of debate, one clear possibility is that the relationship is reciprocal. That is, increased connectivity and genetic risk for Aβ deposition results in continued neural recruitment and chronic elevations in metabolism [119]. According to this “acceleration hypothesis”, once initiated, Aβ deposition may be hastened by abnormally increased brain metabolism particularly in vulnerable regions (e.g., posterior regions within the default mode network). There is compelling evidence using metabolic imaging in humans that elevated metabolism is not solely a reaction to Aβ deposition [68] and this finding is consistent with interstitial fluid measurements of Aβ and metabolism, including diminished Aβ in regions of depressed neuronal function, providing support to the proposition that that persistently and abnormally elevated neuronal activity modulates Aβ concentration [122]. It has also been demonstrated that communities of neurons enduring greater oxidative stress are susceptible to degenerative processes [123–124] and, again, this distress exacerbates Aβ deposition [124].
If it is the case that increased brain metabolism secondary to local hyperconnectivity is a predictor of Aβ deposition and eventually sites of neurodegeneration, then the long-term influences of hyperconnectivity in all neurological disorders must be examined with a critical eye as a possible indicator of risk for degeneration later in life (see Figure 3C). This risk is perhaps accentuated by genetic vulnerability and less developed cognitive reserve. What remains uncertain is if the metabolic vulnerability that has been repeatedly linked to posterior network hubs such as the PCC and hippocampus is reserved to those systems, or if hyperconnectivity following injury and disease could drive similar metabolic crisis in other hubs as well. Important, recent work has begun to answer this question by examining a large sample of individuals (n=128) representing distinct stages of the AD disease process. Connectivity data demonstrate that hub overload may be initiated in posterior regions but then spread across network hubs resulting a “cascade” of network failure [81]. These findings implicate frontal system failure later in the AD process, so it remains to be determined if the timeline and hub chronology for these cascading affects is mirrored in other injury/disease processes (e.g., TBI, multiple sclerosis). Research efforts are needed to examine connectivity changes in late-life brain disorders decades after diagnosis to determine the possibility for disorder-specific markers of hub-overload.
For now, the link between hyperconnectivity, elevated metabolism and neurodegenerative risk requires additional clarification, in particular given the rather complicated relationship between Aβ and tauopathy, where the latter has been demonstrated to be more clearly linked with neurobehavioral impairment [125–126]. As one example, persistent challenges to cognitive reserve may occur during repetitive closed TBI which shows a stronger link with eventual tauopathy rather than Aβ pathology [127]. With the advent of PET-tau tracers, [128–130] recent work has begun to map these relationships in the healthy elderly brain [131]. In Outstanding Questions we offer several direct approaches and testable hypotheses for advancing our understanding of hyperconnectivity in the context of cost/efficiency and risk and resilience.
Concluding Remarks:
We have made the argument that hyperconnectivity is a fundamental response to neuropathology and may be an important signal for system-level plasticity. We have focused on regional hubs as the most likely candidates for its expression but the role and permanence of enhanced network signaling may be contextually dependent. For example, we anticipate that increased hub connectivity may precede disease onset, may be directly linked to the disorder (regional), or may be a non-specific response designed to enhance whole-brain signaling (centralized). The specificity of the hyperconnectivity response remains an open question and work is needed to determine if resources galvanized to adapt to insidious disease processes differ mechanistically from what is observed in acute injury and chronic disease states (see Outstanding Questions). While hyperconnectivity may play a role in system-level dynamics, there is also evidence of specific lesion-connection adaptations and not all responses may be equally efficacious. Because of the implications hyperconnectivity has for network cost-efficiency and risk of neurodegeneration, future detailed analyses determining its spatio-temporal dynamics are essential to understand the robustness of any heightened connectivity response over time (for example see[12]). In sum, future models of neurological disruption should conceptualize the effects of injury beyond the tradition of lesion-to-connection loss pairing and formally integrate the proactive network response observed as hyperconnectivity.
Outstanding questions:
What is the chronicity for hyperconnectivity after neurological disruption and determinants for resolution? The transience of hyperconnectivity and its consequences for behavior require examination over longer windows of time including early after recovery and decades after diagnosis.
What are the strategic network regions and optimal physical distances for increased connectivity post injury? What is the consequence for strategic increases in network response with regard to network efficiency and cost?
Is hyperconnectivity differentially effective (or indicative of different processes) at distinct oscillatory frequencies? The underlying coherent oscillatory behavior in large neuronal assemblies observable as hyperconnectivity in network modeling will be important to uncover.
What are the relationships between sustained hyperconnectivity, cognitive reserve and structural resource loss? Hyperconnectivity must be examined in the context of resource availability while considering factors of risk (genetics) and resilience (cognitive reserve).
Is hyperconnectivity a driver for abnormal neurometabolism? While there is strong evidence that network hubs are sites for Aβ deposition, what is less clear is if hyperconnectivity contributes to these effects both within and outside hubs.
Acknowledgments:
We thank Drs. Sarah Rajtmajer, Reka Albert and Olaf Sporns for helpful suggestions and valuable feedback during previous versions of this manuscript. This work was supported by the National Center for Advancing Translational Sciences, NIHUL Tr000127 through the Clinical Translational Science Institute
Glossary:
- Alzheimer’s disease:
a neurodegenerative disease occurring in older age characterized behaviorally by dense memory impairments in new learning and progressive global cognitive decline.
- APOE, Apoe:
APOE indicates the gene and ApoE indicates the protein. APOE is polymorphic, with three forms ε2, ε3, and ε4, and has been linked to cholesterol and lipid transfer, as well as a broader role in neural tissue repair and synaptogenesis. The Apo-ε4 allele is a predictor of increased risk for neurodegeneration later in life.[139]
- Beta Amyloid (Aβ):
a peptide found in the brain that, when elevated, forms amyloid plaques which are a marker for Alzheimer’s disease although it remains unclear if this relationship is causal.
- Cost-efficiency:
Connections in biological networks have metabolic, time, and wiring costs. With regard to metabolism, cost-efficiency can be defined as the trade-off between the metabolic demand of a network and the effectiveness of information transfer.
- Default mode network:
Brain network associated originally observed as anti-correlated with task-related functioning and has been linked to non-goal directed cognition, memory, and semantic processing. Plays a critical role in the brain’s ongoing or intrinsic activity [140]
- Functional MRI:
Non-invasive technique using strong magnetic fields to examine blood flow changes in the brain to infer brain functioning.
- Hyperconnectivity:
enhanced network connectivity observed in clinical samples as compared to control sample; measured as an increase in the number or strength of connections in the network.
- Mild cognitive impairment:
considered an intermediate stage between normal aging and the onset of a formal neurodegenerative process such as Alzheimer’s disease.
- Network neuroscience:
positioned at the intersection of brain and network science, a group of methods that capitalizes upon mathematical and computational modeling to study brain network organization and function
- Preferential attachment:
a hypothesized principle for network development, whereby those nodes having more connections are disproportionately more likely to gain new connections as the network grows, i.e., a “rich get richer” principle.
- Tau/tauopathy:
brain protein primarily distributed in axons and plays a role in microtubule functioning, and aggregation of tau results in intracellular fibrillary deposits in neurons and glia (tauopathy). Tauopathy has been linked to neurodegeneration such as Alzheimer’s disease.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Frank G. Hillary, Pennsylvania State University.
Jordan H. Grafman, Feinberg School of Medicine, Northwestern University.
References
- 1.Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci (2014) 15(10):683–95. doi: 10.1038/nrn3801. Review. [DOI] [PubMed] [Google Scholar]
- 2.Fornito A, Bullmore ET, Zalesky A, (2017) Opportunities and challenges for psychiatry in the connectomic eraPsychiatry in the connectomic era, Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 10.1016/j.bpsc.2016.08.003 [DOI] [PubMed]
- 3.Friston KJ,Frith CD,Liddle PF,andFrackowiak RS(1993) Functional connectivity: the principal-component analysis of large (PET) data sets. J.Cereb.BloodFlowMetab 13,5–14.doi: 10.1038/jcbfm.1993.4 [DOI] [PubMed] [Google Scholar]
- 4.Bullmore E & Sporns O (2012) The economy of brain network organization. Nat. Rev. Neurosci 13, 336–349. doi: 10.1038/nrn3214 [DOI] [PubMed] [Google Scholar]
- 5.Raichle ME & Gusnard D (2002) Appraising the brain’s energy budget. Proc. Natl. Acad. Sci. U. S. A 99, 10237–10239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Heuvel MP, Mandl RC, Kahn RS, Hulshoff Pol HE. (2009) Functionally linked resting-state networks reflect the underlying structural connectivity architecture of the human brain. Hum Brain Mapp 30:3127–3141. doi: 10.1002/hbm.20737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, Sporns O (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen K, Hutchison RM, Bezgin G, Everling S, McIntosh AR. (2015) Network structure shapes spontaneous functional connectivity dynamics. J Neurosci 8;35(14):5579–88. doi: 10.1523/JNEUROSCI.4903-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betzel RF, et al. , (2014) Changes in structural and functional connectivity among resting-state networks across the human lifespan. Neuroimage 102P2: p. 345–357. doi: 10.1016/j.neuroimage.2014.07.067. [DOI] [PubMed] [Google Scholar]
- 10.Perry A et al. (2015) The organisation of the elderly connectome. Neuroimage 114:414–26. doi: 10.1016/j.neuroimage.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Contreras JA, Goñi J, Risacher SL, Sporns O, Saykin AJ. (2015) The Structural and Functional Connectome and Prediction of Risk for Cognitive Impairment in Older Adults. Curr Behav Neurosci Rep 2(4):234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shine JM, Koyejo O, Poldrack RA. (2016) Temporal metastates are associated with differential patterns of time-resolved connectivity, network topology, and attention. Proc Natl Acad Sci U S A 113(35):9888–91. doi: 10.1073/pnas.1604898113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman MEJ (2003) The structure and function of complex networks. SIAM Review, 45(2):167–256 [Google Scholar]
- 14.Watts DJ,Strogatz SH (1998) Collective dynamics of ‘small-world’ networks. Nature 393, 440–2. [DOI] [PubMed] [Google Scholar]
- 15.Salvador R et al. (2005) Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb. Cortex 15, 1332–1342. [DOI] [PubMed] [Google Scholar]
- 16.Stam CJ (2004) Functional connectivity patterns of human magnetoencephalographic recordings: a ‘small-world’ network? Neurosci. Lett 355, 25–28. [DOI] [PubMed] [Google Scholar]
- 17.Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. (2006) Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci U S A 103(51):19518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilgetag CC, Goulas A. (2016) Is the brain really a small-world network? Brain Struct Funct 221(4):2361–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallos LK, Makse HA, Sigman M. (2012) A small world of weak ties provides optimal global integration of self-similar modules in functional brain networks. Proc Natl Acad Sci U S A 109(8):2825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bassett DS et al. (2009) Cognitive fitness of cost-efficient brain functional networks. Proc. Natl. Acad. Sci. U. S. A 106, 11747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomasi D, Wang G-J & Volkow ND (2013) Energetic cost of brain functional connectivity. Proc. Natl. Acad. Sci 110, 13642–13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Y, Wang S, Hilgetag CC, Zhou C. (2013) Trade-off between multiple constraints enables simultaneous formation of modules and hubs in neural systems. PLoS Comput Biol 9(3):e1002937. doi: 10.1371/journal.pcbi.1002937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barabási A-L & Albert R (1999) Emergence of scaling in random networks. Science 286, 509–512. [DOI] [PubMed] [Google Scholar]
- 24.Albert R, Jeong H & Barabasi (2000) A. Error and attack tolerance of complex networks. Nature 406, 378–82. [DOI] [PubMed] [Google Scholar]
- 25.Crossley NA et al. (2014) The hubs of the human connectome are generally implicated in the anatomy of brain disorders. Brain 137, 2382–2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barulli D, Stern Y. (2013) Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci 17(10):502–9. doi: 10.1016/j.tics.2013.08.012. Epub 2013 Sep 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stern Y (2012) Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol 11(11):1006–12. doi: 10.1016/S1474-4422(12)70191-6. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arenaza-Urquijo EM et al. (2013) Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage 83, 450–7 (2013) [DOI] [PubMed] [Google Scholar]
- 29.Franzmeier N, Caballero MÁ, Taylor AN, Simon-Vermot L, Buerger K, Ertl-Wagner B, Mueller C, Catak C, Janowitz D, Baykara E, Gesierich B, Duering M, Ewers M. (2016) Alzheimer’s Disease Neuroimaging Initiative.. Resting-state global functional connectivity as a biomarker of cognitive reserve in mild cognitive impairment. Brain Imaging Behav Epub ahead of print]. [DOI] [PubMed]
- 30.Santarnecchi E, Rossi S & Rossi A (2014) The smarter, the stronger: Intelligence level correlates with brain resilience to systematic insults. Cortex 64C, 293–309. [DOI] [PubMed] [Google Scholar]
- 31.Premi E et al. (2013) Cognitive Reserve in Granulin-Related Frontotemporal Dementia: From Preclinical to Clinical Stages. PLoS One 8, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumowski JF, Wylie GR, Deluca J & Chiaravalloti N (2010) Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: Functional magnetic resonance imaging evidence for cognitive reserve. Brain 133, 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorges M et al. (2014) To rise and to fall: functional connectivity in cognitively normal and cognitively impaired patients with Parkinson’s disease. Neurobiol. Aging 1–9) doi: 10.1016/j.neurobiolaging.2014.12.026 [DOI] [PubMed]
- 34.Fernández-Seara MA et al. (2015) Resting state functional connectivity of the subthalamic nucleus in Parkinson’s disease assessed using arterial spin-labeled perfusion fMRI. Hum. Brain Mapp 36, 1937–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Stam CJ, Berendse HW. (2008) Increased cortico-cortical functional connectivity in early-stage Parkinson’s. Neuroimage 41:212–222. [DOI] [PubMed] [Google Scholar]
- 36.Yu R, Liu B, Wang L, Chen J, Liu X. (2013) Enhanced func-tional connectivity between putamen and supplemen-tary motor area in Parkinson’s disease patients. PLoSONE 8: e59717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Helmich RC, Derikx LC, Bakker M, Scheeringa R, Bloem BR, Toni I. (2010) Spatial remapping of cortico-striatal connectivity in Parkinson’s disease. Cereb Cortex 20(5):1175–86. [DOI] [PubMed] [Google Scholar]
- 38.Simioni AC, Dagher A, Fellows LK. (2015) Compensatory striatal-cerebellar connectivity in mild-moderate Parkinson’s disease. Neuroimage Clin 11;10:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwak Y, Peltier S, Bohnen NI, Müller ML, Dayalu P, Seidler RD. (2010) Altered resting state cortico-striatal connectivity in mild to moderate stage Parkinson’s disease. Front Syst Neurosci 2010 September 15;4:143. doi: 10.3389/fnsys.2010.00143. eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vervoort G, Alaerts K, Bengevoord A, Nackaerts E, Heremans E, Vandenberghe W, Nieuwboer A. (2016) Functional connectivity alterations in the motor and fronto-parietal network relate to behavioral heterogeneity in Parkinson’s disease. Parkinsonism Relat Disord ;24:48–55. [DOI] [PubMed] [Google Scholar]
- 41.Roosendaal SD, Schoonheim MM, Hulst HE, et al. (2010) Resting state networks change in clinically isolated syndrome. Brain 133(Pt 6):1612–1621 [DOI] [PubMed] [Google Scholar]
- 42.Faivre A et al. (2012) Assessing brain connectivity at rest is clinically relevant in early multiple sclerosis. Mult. Scler. J 18, 1251–1258. [DOI] [PubMed] [Google Scholar]
- 43.Bonavita S et al. (2011) Distributed changes in default-mode resting-state connectivity in multiple sclerosis. Mult. Scler. J 17, 411–422. [DOI] [PubMed] [Google Scholar]
- 44.Hawellek DJ, Hipp JF, Lewis CM, Corbetta M & Engel AK (2011) Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc. Natl. Acad. Sci 108, 19066–19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leavitt VM, Sumowski JF, Chiaravalloti N & DeLuca J (2012) Warmer outdoor temperature is associated with worse cognitive status in multiple sclerosis. Neurology 78, 964–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Faivre et al. (2015) Brain functional plasticity at rest and during action in multiple sclerosis patients. J Clin. Neurosci [DOI] [PubMed]
- 47.Schoonheim MM, Hulst HE, Brandt RB, Strik M, Wink AM, Uitdehaag BM, Barkhof F, Geurts JJ. (2015) Thalamus structure and function determine severity of cognitive impairment in multiple sclerosis. Neurology 24;84(8):776–83. [DOI] [PubMed] [Google Scholar]
- 48.Rocca MA, Absinta M, Moiola L, Ghezzi A, Colombo B, Martinelli V, Filippi M (2010) Functional and structural connectivity of the motor network in pediatric and adultonset relapsing-remitting multiple sclerosis. Radiology, 254, 541–550. doi: 10.1148/radiol.09090463 [DOI] [PubMed] [Google Scholar]
- 49.Dogonowski AM, Siebner HR, Sørensen PS, Wu X, Biswal B, Paulson OB, Madsen KH (2013) Expanded functional coupling of subcortical nuclei with the motor resting-state network in multiple sclerosis. Multiple Sclerosis, 19, 559–566. [DOI] [PubMed] [Google Scholar]
- 50.Loitfelder M, Filippi M, Rocca M, Valsasina P, Ropele S, Jehna M, Enzinger C (2012) Abnormalities of resting state functional connectivity are related to sustained attention deficits in MS. PLoS ONE, 7, e42862. doi: 10.1371/journal.pone.0042862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valsasina P, Rocca MA, Absinta M, Sormani MP, Mancini L, De Stefano N, Filippi M (2011) A multicentre study of motor functional connectivity changes in patients with multiple sclerosis. European Journal of Neuroscience, 33, 1256–1263. doi: 10.1111/j.1460-9568.2011.07623. [DOI] [PubMed] [Google Scholar]
- 52.Sharp DJ et al. (2011) Default mode network functional and structural connectivity after traumatic brain injury. Brain 134, 2233–47. [DOI] [PubMed] [Google Scholar]
- 53.Hillary FG et al. (2014) The Rich Get Richer: Brain Injury Elicits Hyperconnectivity in Core Subnetworks. PLoS One 9, e104021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hillary FG, Medaglia JD, Gates K, Molenaar PC, Slocomb J, Peechatka A, & Good DC (2011) Examining working memory task acquisition in a disrupted neural network. Brain, 134, 1555–1570. doi: 10.1093/brain/awr043 [DOI] [PubMed] [Google Scholar]
- 55.Hillary FG, Slocomb J, Hills EC, Fitzpatrick NM, Medaglia JD, Wang J,. Wylie GR (2011) Changes in resting connectivity during recovery from severe traumatic brain injury. International Journal of Psychophysiology, 82, 115–123. [DOI] [PubMed] [Google Scholar]
- 56.Iraji A, Benson RR, Welch RD, O’Neil BJ, Woddard JL, Ayaz SI & Kou Z (2016) Resting state functional connectivity in mild traumatic brain injury at the acute stage: Independent component and seed-based analyses. Journal of Neurotrauma, 32, 1031–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bharath RD, Munivenkatappa A, Gohel S, Panda R, Saini J, Rajeswaran J & Biswal BB (2015) Recovery of resting brain connectivity ensuing mild traumatic brain injury. Frontiers in Human Neuroscience, 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson B Zhang K, Gay M, Horovitz S, Hallett M, Sebastianelli W, & Slobounov S (2012) Alteration of brain default network in subacute phase of injury in concussed individuals: Resting-state fMRI study. Neuroimage, 59, 511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayer AR, Mannell MV, Ling J, Gasparovic C, & Yeo RA (2011) Functional connectivity in mild traumatic brain injury. Human Brain Mapping, 32, 1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nicolo P, Rizk S, Magnin C, Pietro MD, Schnider A, Guggisberg AG.(2015) Coherent neural oscillations predict future motor and language improvement after stroke. Brain October;138(Pt 10):3048–60. [DOI] [PubMed] [Google Scholar]
- 61.Caeyenberghs K, Powell HW, Thomas RH, Brindley L, Church C, Evans J, Muthukumaraswamy SD, Jones DK, Hamandi K. (2014) Hyperconnectivity in juvenile myoclonic epilepsy: a network analysis. Neuroimage Clin 7:98–104. doi: 10.1016/j.nicl.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.O’Muircheartaigh J, Vollmar C, Barker GJ, Kumari V, Symms MR, Thompson P,Duncan JS, Koepp MJ, Richardson MP. (2012) Abnormal thalamocortical structural and functional connectivity in juvenile myoclonic epilepsy. Brain 135(Pt 12):3635–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vollmar C, O’Muircheartaigh J, Barker GJ, Symms MR, Thompson P, Kumari V, Duncan JS, Janz D, Richardson MP, Koepp MJ. (2011) Motor system hyperconnectivity in juvenile myoclonic epilepsy: a cognitive functional magnetic resonance imaging study. Brain 134(Pt 6):1710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheline YI, & Raichle ME (2013) Resting state functional connectivity in preclinical Alzheimer’s disease. Biological Psychiatry, 74, 340–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tijms BM, Wink AM, de Haan W, van der Flier WM, Stam CJ, Scheltens P, & Barkhof F (2013) Alzheimer’s disease: Connecting findings from graph theoretical studies of brain networks. Neurobiology of Aging, 34, 2023–2036. [DOI] [PubMed] [Google Scholar]
- 66.Tona F et al. (2014) Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology 271, 814–21. [DOI] [PubMed] [Google Scholar]
- 67.Duong MVA et al. (2005) Modulation of effective connectivity inside the working memory network in patients at the earliest stage of multiple sclerosis. Neuroimage 24, 533–538. [DOI] [PubMed] [Google Scholar]
- 68.Cohen AD Price JC, Weissfeld LA, James J, Rosario BL, Bi W, Nebes RD, Saxton JA, Snitz BE, Aizenstein HA, Wolk DA, DeKosky ST, Mathis CA, Klunk WE (2009) Basal cerebral metabolism may modulate the cognitive effects of Aβ in mild cognitive impairment: an example of brain reserve. J. Neurosci 29, 14770–14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gour N et al. (2011) Basal functional connectivity within the anterior temporal network is associated with performance on declarative memory tasks. Neuroimage 58, 687–697. [DOI] [PubMed] [Google Scholar]
- 70.Olde Dubbelink KT, Schoonheim MM, Deijen JB, Twisk JW, Barkhof F, Berendse HW. (2014) Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology 83(22):2046–53. [DOI] [PubMed] [Google Scholar]
- 71.Hacker CD, Perlmutter JS, Criswell SR, Ances BM, Snyder AZ. (2012) Resting state functional connectivity of the striatum in Parkinson’s disease. Brain 135(Pt 12):3699–3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ye Q, Su F, Shu H, Gong L, Xie C, Zhang Z, Bai F. (2016) The apolipoprotein E gene affects the three-year trajectories of compensatory neural processes in the leftlateralized hippocampal network. Brain Imaging Behav [Epub ahead of print] [DOI] [PubMed]
- 73.Tewarie P, Schoonheim MM, Schouten DI, Polman CH, Balk LJ, Uitdehaag BM, Geurts JJ, Hillebrand A, Barkhof F, Stam CJ. (2015) Functional brain networks: linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG study. Hum Brain Mapp 36(2):603–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bookheimer SY et al. (2000) Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med 343, 450–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Filippini N et al. Distinct patterns of brain activity in young carriers of the APOEepsilon4 allele. Proc. Natl. Acad. Sci. U. S. A 106, 7209–7214 (2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bondi MW, Houston WS, Eyler LT & Brown GG (2005) fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64, 501–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Brien JL et al. (2010. Longitudinal fMRI in elderly reveals loss of hippocampal activation with clinical decline. Neurology 74, 1969–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown J. a. et al. (2011) Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc. Natl. Acad. Sci 108, 20760–20765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Machulda MM et al. (2011) Effect of APOE ε4 status on intrinsic network connectivity in cognitively normal elderly subjects. Arch. Neurol 68, 1131–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hillary FG et al. (2015) Hyperconnectivity is a fundamental response to neurological disruption. Neuropsychology 29, 59–75 [DOI] [PubMed] [Google Scholar]
- 81.Jones DT, Knopman DS, Gunter JL, Graff-Radford J, Vemuri P, Boeve BF, Petersen RC, Weiner MW, Jack CR Jr. (2016) Alzheimer’s Disease Neuroimaging Initiative. Cascading network failure across the Alzheimer’s disease spectrum. Brain 139(Pt 2):547–62. doi: 10.1093/brain/awv338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Goñi J et al. (2014) Resting-brain functional connectivity predicted by analytic measures of network communication. Proc. Natl. Acad. Sci 111, 833–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christodoulou C et al. (2001) Functional magnetic resonance imaging of working memory impairment after traumatic brain injury. J. Neurol. Neurosurg. Psychiatry 71, 161–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ernst T, Chang L, Jovicich J, Ames N & Arnold S (2002) Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59, 1343–1349 [DOI] [PubMed] [Google Scholar]
- 85.Forn C et al. (2012) Functional magnetic resonance imaging correlates of cognitive performance in patients with a clinically isolated syndrome suggestive of multiple sclerosis at presentation: an activation and connectivity study. Mult. Scler. J 18, 153–163 [DOI] [PubMed] [Google Scholar]
- 86.McAllister TW et al. (2001) Differential working memory load effects after mild traumatic brain injury. Neuroimage 14, 1004–12 [DOI] [PubMed] [Google Scholar]
- 87.Chiaravalloti N et al. (2005) Cerebral activation patterns during working memory performance in multiple sclerosis using FMRI. J. Clin. Exp. Neuropsychol 27, 33–54 [DOI] [PubMed] [Google Scholar]
- 88.Nakamura T, Hillary FG & Biswal BB (2009) Resting network plasticity following brain injury. PLoS ONE 4,. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gamboa OL et al. (2014) Working memory performance of early MS patients correlates inversely with modularity increases in resting state functional connectivity networks. Neuroimage 94, 385–395. [DOI] [PubMed] [Google Scholar]
- 90.Pederson M, Omidvarnia A, Walz J & Jackson G (2015) Increased segregation of brain networks in focal epilepsy: An fMRI graph theory finding. Neuroimage Clin [DOI] [PMC free article] [PubMed]
- 91.Zamora-López G et al. , (2010) Cortical hubs form a module for multisensory integration on top of the hierarchy of cortical networks. Front Neuroinform 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van den Heuvel MP, Sporns O. (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.van den Heuvel MP, Kahn RS, Goñi J, Sporns O (2012) High-cost, highcapacity backbone for global brain communication. Proc Natl Acad Sci U S A 109:11372–11377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gómez-Gardeñes J, Zamora-López G, Moreno Y, Arenas A (2010) From modular to centralized organization of synchronization in functional areas of the cat cerebral cortex. PLoS ONE 5:e12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hong SL, Rebec GV. (2012) A new perspective on behavioral inconsistency and neural noise in aging: compensatory speeding of neural communication. Front Aging Neurosci 25;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beggs JM, Timme N. (2012) Being critical of criticality in the brain. Front Physiol. 7;3:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chialvo DR (2010) Emergent complex neural dynamics. Nat. Phys 6, 744–750 [Google Scholar]
- 98.Moretti P, Muñoz MA (2013) Griffiths phases and the stretching of criticality in brain networks. Nat Commun 4:2521. [DOI] [PubMed] [Google Scholar]
- 99.Petermann T, Thiagarajan TC, Lebedev MA, Nicolelis MAL, Chialvo DR, and Plenz D (2009) Spontaneous cortical activity in awake monkeys composed of neuronal avalanches. Proc. Natl. Acad. Sci. U.S.A 106, 15921–15926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Minati L, Chiesa P, Tabarelli D, D’Incerti L, Jovicich J. Synchronization, non-linear dynamics and low-frequency fluctuations: analogy between spontaneous brain activity and networked single-transistor chaotic oscillators. Chaos 2015. March;25(3):033107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crucitti P, Latora V & Marchiori M Model for cascading failures in complex networks. Phys. Rev. E 69, 045104 (2004) [DOI] [PubMed] [Google Scholar]
- 102.Aerts H, Fias W, Caeyenberghs K, Marinazzo D. (2016) Brain networks under attack: robustness properties and the impact of lesions. Brain [DOI] [PubMed]
- 103.Achard S & Bullmore E (2007) Efficiency and cost of economical brain functional networks. PLoS Comput. Biol 3, 0174–0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wagner G, Gussew A, Köhler S, de la Cruz F, Smesny S, Reichenbach JR, Bär KJ. (2016) Resting state functional connectivity of the hippocampus along the anteriorposterior axis and its association with glutamatergic metabolism. Cortex 81:104–17. doi: 10.1016/j.cortex.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 105.Passow S et al. (2015) Default-mode network functional connectivity is closely related to metabolic activity. Hum. Brain Mapp 2038, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soddu A, Gómez F, Heine L, Di Perri C, Bahri MA, Voss HU, Bruno MA, Vanhaudenhuyse A, Phillips C, Demertzi A, Chatelle C, Schrouff J, Thibaut A, CharlandVerville V, Noirhomme Q, Salmon E, Tshibanda JF, Schiff ND, Laureys S. Correlation between resting state fMRI total neuronal activity and PET metabolism in healthy controls and patients with disorders of consciousness. Brain Behav 2015. December 29;6(1):e00424. doi: 10.1002/brb3.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Novack TA, Bush BA, Meythaler JM, Canupp K. Outcome after traumatic brain injury: pathway analysis of contributions from premorbid, injury severity, and recovery variables. Arch Phys Med Rehabil 2001. March;82(3):300–5. [DOI] [PubMed] [Google Scholar]
- 108.Sadovsky AJ & MacLean JN (2013) Scaling of topologically similar functional modules defines mouse primary auditory and somatosensory microcircuitry. J. Neurosci 33, 14048–14060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schroeter MS, Charlesworth P, Kitzbichler MG, Paulsen O & Bullmore ET (2015) Emergence of rich-club topology and coordinated dynamics in development of hippocampal functional networks in vitro. J Neurosci 35, 5459–5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cao M, He Y, Dai Z, Liao X, Jeon T, Ouyang M, Chalak L, Bi Y, Rollins N, Dong Q, Huang H. (2016) Early Development of Functional Network Segregation Revealed by Connectomic Analysis of the Preterm Human Brain. Cereb Cortex pii: bhw038. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- 111.Fornito A et al. (2011) Genetic influences on cost-efficient organization of human cortical functional networks. J. Neurosci 31, 3261–3270. doi: 10.1523/JNEUROSCI.4858-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Betzel RF, Gu S, Medaglia JD, Pasqualetti F, Bassett DS. (2016) Optimally controlling the human connectome: the role of network topology. Sci Rep 6:30770.doi: 10.1038/srep30770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Buckner RL et al. (2005) Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 25, 7709–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Busche MA, et al. , (2012) Critical role of soluble amyloid-beta for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc Natl Acad Sci U S A, 109(22): p. 8740–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cirrito JR, et al. , (2005) Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron 48(6): p. 913–22. [DOI] [PubMed] [Google Scholar]
- 116.Bero AW, Bauer AQ, Stewart FR, White BR, Cirrito JR, Raichle ME, et al. (2012) Bidirectional relationship between functional connectivity and amyloidb deposition in mouse brain. J Neurosci 32: 4334–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. (2009) Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J Neurosci 11;29(6):1860–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA (2010) Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ ) deposition. Proc Natl Acad Sci U S A 107(41):17763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Myers N, Pasquini L, Göttler J, Grimmer T, Koch K, Ortner M, Neitzel J, Mühlau M, Förster S, Kurz A, Förstl H, Zimmer C, Wohlschläger AM, Riedl V, Drzezga A, Sorg C. (2012) Within-patient correspondence of amyloid-β and intrinsic network connectivity in Alzheimer’s disease. Brain 137(Pt 7):2052–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Haan W, Mott K, van Straaten EC, Scheltens P & Stam CJ (2012) Activity dependent degeneration explains hub vulnerability in Alzheimer’s disease. PLoS Comput Biol 8, e1002582. doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fornito A, Zalesky A, Breakspear M. (2015) The connectomics of brain disorders. Nat Rev Neurosci 16(3):159–72. doi: 10.1038/nrn3901 [DOI] [PubMed] [Google Scholar]
- 122.Brody DL et al. (2008) Amyloid-beta dynamics correlate with neurological status in the injured human brain. Science 321, 1221–4 doi: 10.1126/science.1161591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dumont M, Wille E, Stack C, Calingasan NY, Beal MF, Lin MT. (2009) Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer’s disease. FASEB J 23(8):2459–66. doi: 10.1096/fj.09-132928. Epub 2009 Apr 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin MT, Beal MF. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–95. [DOI] [PubMed] [Google Scholar]
- 125.Arriagada PV, Growdon JH, Hedley-Whyte ET & Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42, 631–639. [DOI] [PubMed] [Google Scholar]
- 126.Nelson PT et al. (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: a review of the literature. J Neuropathol Exp Neurol 71, 362–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McKee AC et al. (2009) Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol 68, 709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Maruyama M et al. (2013) Imaging of tau pathology in a tauopathy mouse model and in Alzheimer patients compared to normal controls. Neuron 79, 1094–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harada R et al. (2013) Comparison of the binding characteristics of [18F]THK-523 and other amyloid imaging tracers to Alzheimer’s disease pathology. Eur. J. Nucl. Med. Mol. Imaging 40, 125–32. [DOI] [PubMed] [Google Scholar]
- 130.Okamura N et al. (2013) Novel 18F-labeled arylquinoline derivatives for noninvasive imaging of tau pathology in Alzheimer disease. J. Nucl. Med 54, 1420–7. [DOI] [PubMed] [Google Scholar]
- 131.Sepulcre J, Schultz AP, Sabuncu M, Gomez-Isla T, Chhatwal J, Becker A, Sperling R, Johnson KA. (2016) In Vivo Tau, Amyloid, and Gray Matter Profiles in the Aging Brain. J Neurosci 36(28):7364–74. doi: 10.1523/JNEUROSCI.0639-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Roy A, Bernier RA, Wang JL, Benson M, French J, Good DC, Hillary FG (in press) The evolution of cost-efficiency in neural networks during recovery from traumatic brain injury. PLoS One [DOI] [PMC free article] [PubMed]
- 133.Caeyenberghs K, Leemans A, Heitger MH, Leunissen I, Dhollander T, Sunaert S, Dupont P, Swinnen SP. (2012) Graph analysis of functional brain networks for cognitive control of action in traumatic brain injury. Brain 135(Pt 4):1293–307. doi: 10.1093/brain/aws048. [DOI] [PubMed] [Google Scholar]
- 134.Mormino EC, Smiljic A, Hayenga AO, Onami SH, Greicius MD, Rabinovici GD, Janabi M, Baker SL, Yen IV, Madison CM, Miller BL, Jagust WJ. (2011) Relationships between β-amyloid and functional connectivity in different components of the default mode network in aging. Cereb Cortex (10):2399–407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed]
- 135.Zhou J, Greicius MD, Gennatas ED, Growdon ME, Jang JY, Rabinovici GD, Kramer JH, Weiner M, Miller BL, Seeley WW. (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133(Pt 5):1352–67. doi: 10.1093/brain/awq075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stam CJ, de Haan W, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM, Montez T, Verbunt JP, de Munck JC, van Dijk BW, Berendse HW, Scheltens P (2009) Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain 132(Pt 1):213–24. doi: 10.1093/brain/awn262. [DOI] [PubMed] [Google Scholar]
- 137.Greicius MD, Srivastava G, Reiss AL, Menon V. (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A 101(13):4637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Agosta F, Pievani M, Geroldi C, Copetti M, Frisoni GB, Filippi M. (2012) Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol Aging 33(8):1564–78. doi: 10.1016/j.neurobiolaging.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 139.Corder EH et al. (1993) Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 261, 921–923. [DOI] [PubMed] [Google Scholar]
- 140.Raichle ME, Snyder AZ. (2007) A default mode of brain function: a brief history of an evolving idea. Neuroimage 37(4):1083–90; discussion 1097–9. Review. [DOI] [PubMed] [Google Scholar]
- 141.Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, et al. Head trauma as a risk factor for Alzheimer’s disease: a collaborative reanalysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol 1991; 20 Suppl 2:S28–S35. [DOI] [PubMed] [Google Scholar]
- 142.Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer’s disease: a population-based study. Am J Epidemiol 1999; 149:32–40. [DOI] [PubMed] [Google Scholar]


