Abstract
Objective:
Estrogen replacement prevents worsening body dissatisfaction with weight gain in adolescents with anorexia nervosa. However, the impact of estrogen administration on eating disorder (ED) pathology in normal-weight young women with exercise-induced amenorrhea is unknown. We hypothesized that (1) normal-weight oligo-amenorrheic athletes (OA) would show greater ED pathology than eumenorrheic athletes (EA) and non-athletes (NA), and (2) 12 months of estrogen replacement would improve those symptoms.
Trial Design:
Randomized trial
Methods:
One hundred seventeen OA, 50 EA, and 41 NA completed the Eating Disorder Inventory-2 (EDI-2) for measures of Drive for Thinness (DT) and Body Dissatisfaction (BD) and the Three-Factor Eating Questionnaire-R18 (TFEQ-R18). OA were then randomized to receive 100 mcg transdermal 17β-estradiol with cyclic progesterone (PATCH), an oral contraceptive pill (30 mcg ethinyl estradiol+0.15 mg desogestrel) (PILL), or no estrogen (E−) for 12 months. Data are reported for the subset that completed questionnaires at 0 and 12 months between 11/2009 and 10/2016.
Results:
OA showed higher EDI-2 DT and TFEQ-R18 Cognitive Restraint scores than EA and NA and higher EDI-2 BD scores than EA. Over 12 months, the E+ group (PATCH+PILL), compared to E−, showed improved trajectories for EDI-2 DT and BD scores. In 3-group comparisons, PATCH outperformed E− for decreases in EDI-2 DT and BD, and the PILL for TFEQ-R18 Uncontrolled Eating.
Conclusion:
In OA, 12 months of estrogen replacement improves ED pathology trajectories, emphasizing the broad importance of normalizing estrogen levels.
Clinical Trial Registration:
ClinicalTrials.gov identifier: NCT00946192
Keywords: Amenorrhea, Athletes, Body dissatisfaction, Drive for thinness, Eating disorders, Estrogen
1. Introduction
Disordered eating behavior and attitudes are common in conditions of functional hypothalamic amenorrhea, such as anorexia nervosa (AN) and exercise-induced amenorrhea (Beals and Hill, 2006; Quah et al., 2009), which are also associated with significant psychiatric co-morbidity, including anxiety and depression. Hypogonadism in these conditions has been implicated in psychological morbidity. Estrogen and progesterone receptors are expressed in appetite regulation centers (e.g., the hypothalamus) and regions regulating emotion and cognition (e.g., the amygdala, ventral tegmental area, insula, and hippocampus) (Campolier et al., 2016; Coyoy et al., 2016; Minervini et al., 2015). In rodent and human studies, hypogonadism has been associated with cognitive dysfunction, worsening anxiety, and dysphoric mood (Baskaran et al., 2017b; Gogos et al., 2014; Lasaite et al., 2014). For example, hypogonadal, oligo-amenorrheic athletes show impaired verbal memory and poor cognitive control, key to successful goal-directed behavior (Baskaran et al., 2017b), and both improved after 6 months of estrogen replacement (Baskaran et al., 2017a). Hypoestrogenic rodents exhibit increased anxiety-related behaviors, which improved with estrogen replacement (Diz-Chaves et al., 2012; Rachman et al., 1998). Similarly, estrogen replacement in adolescent girls with AN reduces trait anxiety (Misra et al., 2013). While these findings highlight the close link between estrogen status and cognition, emotion, and behavior, little is known regarding the impact of hypoestrogenism on eating disorder (ED) pathology.
Changes in feeding patterns across the menstrual cycle suggest an impact of gonadal hormones on eating behavior (Campolier et al., 2016; Dye and Blundell, 1997). Data from early and late follicular phases (low vs. high estrogen states) suggest that estradiol may reduce food intake by decreasing sensitivity to food cues in conditions of energy deprivation (Alonso-Alonso et al., 2011). Further, food intake is higher in the mid-luteal compared to the follicular phase (Barr et al., 1995; Dye and Blundell, 1997), indicating that unlike estrogen, progesterone (predominant in the mid-luteal phase) may increase appetite. In contrast, estradiol administration (with or without progesterone) decreased expression of pro-opiomelanocortin (POMC) mRNA in hypoestrogenic rats; increased POMC signaling is anorexigenic (Treiser and Wardlaw, 1992). Moreover, animal studies have linked female gonadal hormones to overconsumption of food coupled with a loss of control, namely binge eating behavior. Ovariectomized female adult rats display increased binge eating (Klump et al., 2011), and estradiol administration (with progesterone) reversed this effect (Yu et al., 2008). Further, in a small study of adolescents with AN, those randomized to physiologic transdermal estradiol replacement did not show the positive association of increasing body dissatisfaction (via Eating Disorder Inventory-2; EDI-2) with increasing body mass index (BMI) observed in girls not receiving estrogen (Misra et al., 2013), suggesting a beneficial role of estrogen in AN. However, AN represents an extreme state of functional hypothalamic amenorrhea; and low weight, independent of low estrogen, is likely to have an important impact on these endpoints.
The oligo-amenorrheic athlete, even when of normal weight, is also at increased risk for disordered eating behaviors and psychopathology (Cano Sokoloff et al., 2015). Data are lacking regarding the impact of estrogen administration on ED pathology in these women. Examining the link between hypoestrogenism and eating behavior/attitudes and the impact of estrogen replacement in normal-weight oligo-amenorrheic athletes permits investigation of the impact of hypoestrogenism on ED pathology without low weight as a confounder and could provide a novel strategy for improving clinical care for the female athlete triad. We hypothesized that (1) normal-weight oligo-amenorrheic athletes would show more pronounced ED pathology compared to eumenorrheic athletes and non-athletes, and (2) 12 months of estrogen replacement would improve these symptoms. We focused on primary eating attitudes and behaviors underlying ED pathology, namely body dissatisfaction, drive for thinness, cognitive restraint, uncontrolled eating, and emotional eating. Briefly, body dissatisfaction refers to a discrepancy between perceived and desired body image, while drive for thinness represents the desire to be thinner; and both body dissatisfaction and drive for thinness represent key risk factors for developing and maintaining an ED (Beals and Hill, 2006; Stice et al., 2017; Stice and Shaw, 2002). Cognitive restraint represents an individual’s effort to consciously limit caloric intake, uncontrolled eating is overconsumption of food accompanied by a perceived loss of control (binge eating), and emotional eating refers to eating in response to negative emotions. These three behaviors characterize the core behaviors of ED pathology.
2. Materials and Method
2.1. Cross-sectional study
The cross-sectional study included 117 oligo-amenorrheic athletes, 50 EA, and 41 NA, 14-25 years old. All were normal weight; 10th-90th percentiles for age and >85% of median BMI. None met criteria for AN at enrollment. However, many athletes, particularly oligo-amenorrheic athletes, had a history of disordered eating (Table 1). Specifically, of the 35 participants with a lifetime history of an ED, 18 had a history of AN, one had a history of bulimia nervosa (BN), 5 had an ED not otherwise specified (EDNOS) in the past, one had a history of both BN and EDNOS, and the specific ED was not recorded for 8 participants. Importantly, none of the participants had a current diagnosis of AN. The initial interview by a study physician included assessment of lifetime ED symptoms and diagnosis. Participants endorsing ongoing ED symptoms were interviewed by the study psychologist to confer diagnosis. Further exclusion criteria included conditions other than excessive activity that may cause oligo-menorrhea, including pregnancy, thyroid dysfunction, primary ovarian insufficiency, hyperprolactinemia, and polycystic ovarian disease.
TABLE 1:
Baseline clinical characteristics and Eating Disorder Inventory-2 (EDI-2) and Three-Factor Eating Questionnaire-R18 (TFEQ-R18) scores in oligo-amenorrheic athletes (OA), eumenorrheic athletes (EA), and non-athletes (NA)
| Characteristics | OA (n = 117) |
EA (n = 50) |
NA (n = 41) |
Overall comparison (ANOVA) | Tukey-Kramer HSD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F/χ2 | df | p | ηp2 | OA vs. EA | OA vs. NA | EA vs. NA | |||||||
| p | d | p | d | p | d | ||||||||
| Age (years) | 19.8±0.2 | 19.0±0.4 | 19.8±0.4 | 1.61 | 2, 205 | .202 | 0.02 | .193 | 0.31 | .998 | 0.00 | .373 | −0.32 |
| Height (cm) | 164.6±0.6 | 165.2±1.1 | 163.1±1.2 | 1.03 | 2, 194 | .358 | 0.01 | .868 | −0.09 | .474 | 0.23 | .349 | 0.28 |
| Weight (kg) | 55.9±0.7 | 61.4±1.5 | 57.9±1.4 | 7.26 | 2, 194 | .0009 | 0.07 | .0006 | −0.66 | .401 | −0.26 | .126 | 0.37 |
| BMI (kg/m2) | 20.6±0.2 | 22.4±0.4 | 21.7±0.4 | 10.66 | 2, 194 | <.0001 | 0.10 | <.0001 | −0.75 | .034 | −0.48 | .338 | 0.29 |
| BMI Z-score | −0.09±0.08 | 0.61±0.11 | 0.43±0.13 | 15.00 | 2, 190 | <.0001 | 0.14 | <.0001 | −0.83 | .001 | −0.62 | .581 | 0.24 |
| Percent body fat | 24.5±0.4 | 27.4±0.6 | 31.2±0.8 | 31.70 | 2, 194 | <.0001 | 0.25 | .002 | −0.63 | <.0001 | −1.20 | .0007 | −0.78 |
| Total daily caloric intake (kcal) | 2,156±70 | 2,001±104 | 1,995±88 | 1.25 | 2, 149 | .291 | 0.02 | .445 | 0.24 | .402 | 0.26 | .999 | 0.01 |
| Activity (hours/week) | 10.6±0.5 | 11.6±0.9 | 0.7±0.2 | 63.99 | 2, 189 | <.0001 | 0.40 | .484 | −0.18 | <.0001 | 1.57 | <.0001 | 1.58 |
| Resting energy expenditure (kcal) | 1,206±16 | 1,359±32 | 1,222±31 | 11.17 | 2, 186 | <.0001 | 0.11 | <.0001 | −0.78 | .894 | −0.09 | .003 | 0.65 |
| Eating disorder (ED) status: No history Past history Ongoing restrictive eating behavior Ongoing diagnosed ED |
86 (73.5) 17 (14.5) 3 (2.6) 11 (9.4) |
46 (92.0) 4 (8.0) 0 (0.0) 0 (0.0) |
41 (100.0) 0 (0.0) 0 (0.0) 0 (0.0) |
29.76 | 6 | <.0001 | |||||||
| Menarchal age (years) | 13.6±0.2 | 12.5±0.2 | 12.4±0.2 | 14.84 | 2, 201 | <.0001 | 0.13 | <.0001 | 0.61 | <.0001 | 0.67 | .955 | 0.08 |
| Estradiol (pg/mL) | 44.6±5.2 | 70.2±12.6 | 83.6±16.1 | 10.32 | 2, 167 | <.0001 | 0.11 | .005 | −0.59 | .0004 | −0.75 | .725 | −0.21 |
| EDI-2 | n = 116 | n = 50 | n = 41 | ||||||||||
| Drive for Thinness | 4.21±0.52 | 1.66±0.42 | 1.61±0.48 | 7.80 | 2, 204 | .0005 | 0.07 | .004 | 0.50 | .007 | 0.50 | .999 | 0.02 |
| Body Dissatisfaction | 5.06±0.58 | 2.60±0.53 | 3.00±0.62 | 4.65 | 2, 204 | .011 | 0.04 | .020 | 0.43 | .091 | 0.35 | .934 | −0.10 |
| TFEQ-R18 | n = 105 | n = 47 | n = 37 | ||||||||||
| Cognitive Restraint | 46.05±2.20 | 33.52±2.88 | 34.57±3.22 | 7.43 | 2, 179 | .0008 | 0.08 | .003 | 0.57 | .015 | 0.52 | .973 | −0.05 |
| Uncontrolled Eating | 33.55±1.78 | 31.76±2.10 | 29.63±2.77 | 0.76 | 2, 186 | .471 | 0.01 | .822 | 0.10 | .455 | 0.22 | .838 | 0.14 |
| Emotional Eating | 26.51±2.20 | 26.95±3.46 | 30.63±4.21 | 0.44 | 2, 186 | .648 | 0.01 | .994 | −0.02 | .629 | −0.18 | .756 | −0.15 |
Mean±SEM or n (%); significant p-values are bolded. ANOVA, analysis of variance; df, degrees of freedom; HSD, honestly significant difference.
Athletes were endurance athletes engaged in ≥4 hours of weight-bearing activity or ≥20 miles of running per week for ≥6 months in the preceding year. Non-athletes were defined as individuals engaged in <2 hours of weight-bearing activity per week and not involved in any team sports. Oligo-amenorrhea was defined as ≥3 months of amenorrhea within ≥6 preceding months of oligomenorrhea (cycle length >6 weeks) or absence of menarche at ≥15 years. Eumenorrhea was defined as ≥9 menses in the preceding 12 months (cycle length 21-35 days).
Study participants were recruited through advertisements on local college campuses and websites and flyers posted in offices of pediatricians, adolescent medicine physicians, and internists. The study was approved by the Partners Healthcare Institutional Review Board (IRB). Informed consent was obtained from participants ≥18 years and parents of participants <18 years. Assent was obtained from those <18 years old. The study was registered at ClinicalTrials.gov (identifier: NCT00946192).
After confirming eligibility, participants underwent a baseline visit. Weight was measured on an electronic scale to 0.01 kg and height in triplicate against a wall-mounted stadiometer. Participants completed the EDI-2 (Garner, 1991) measures of Drive for Thinness (DT) and Body Dissatisfaction (BD) and the Three-Factor Eating Questionnaire-R18 (TFEQ-R18) (de Lauzon et al., 2004) as self-report assessments of eating attitudes and behaviors. Baseline data for a subset have been previously reported (Cano Sokoloff et al., 2015).
2.2. Randomized controlled trial (RCT)
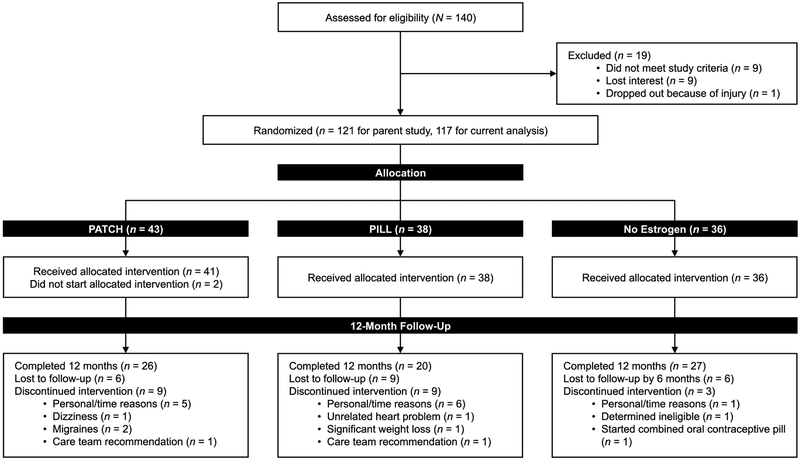
Participants included in this analysis were a subset of participants (n = 117) from a larger pool of oligo-amenorrheic athletes (N = 121) enrolled in a study examining the impact of estrogen administration on bone endpoints (primary outcomes for the parent study). Only those participants in the parent study that also completed the EDI-2 and/or the TFEQ-R18 (secondary analyses of the parent study) were included in the current analysis (Figure 1). Oligo-amenorrheic athletes were randomized to either the 100 mcg transdermal 17β-estradiol patch with cyclic progesterone (PATCH), a combined oral contraceptive pill (OCP) with 30 mcg ethinyl estradiol and 0.15 mg desogestrel (PILL), or no estrogen (E−) for 12 months (1:1:1). The study was not blinded given regulatory body concerns (NIH and IRB) for a blinded randomization design that included an OCP as a possible intervention in an adolescent and young adult sample. The concern was that with a blinded design, some participants in the PATCH and E− arms may assume that they were randomized to an OCP based on symptomatology and not use adequate contraceptive measures when necessary. However, the primary endpoints for the parent study were bone density and structure. As participants were informed that the aim of the study was to investigate the impact of PATCH and PILL on bone health, we believe that our participants were unlikely to have been biased in perceiving and/or reporting (changes in) ED symptoms based on allocation to a specific randomization arm. The randomization sequence was computer-generated and maintained and allocated by the research pharmacy. Participants were enrolled by study investigators and coordinators.
FIGURE 1:
CONSORT flow diagram
Participants in the PATCH group used the 100 mcg 17β-estradiol patch continuously (applied twice weekly) and took micronized progesterone for 12 days of every month to avoid unopposed estrogenic stimulation of the uterine endometrium. 17β-estradiol is the physiologic form of estrogen (also referred to simply as estradiol) and micronized progesterone the physiologic form of progesterone; this group thus received physiologic forms of hormone replacement. However, unlike the normal physiologic state where estradiol and progesterone levels vary across the menstrual cycle, estradiol levels were overall constant throughout the study duration. Participants in the PILL group took the combined ethinyl estradiol and desogestrel pill continuously for three weeks followed by a week of placebo pills (28-day pill pack) and restarted a fresh pack immediately after completing the previous pack. This pill (30 mcg ethinyl estradiol and 0.15 mg desogestrel) is one of the most commonly used OCP formulations. Participants were followed every 3 months. Interviews at subsequent visits, medication calendars, and counts of leftover medications in medication containers at subsequent visits were used to assess compliance. Compliance was also assessed at study completion using measurement of estradiol levels in the PATCH group (which delivers 17β-estradiol, the physiologic form of estrogen, and is thus measurable in the standard estradiol assay), and sex hormone binding globulin (SHBG) levels in the PILL group (which delivers ethinyl estradiol, which is not measurable in the standard estradiol assay). However, ethinyl estradiol (but not 17β-estradiol) causes an increase in SHBG, thus an increase in SHBG indicates overall compliance with the PILL. Participants in the E− group did not use systemic hormonal contraception for the study duration.
A subset of 117 oligo-amenorrheic participants (43, 38, and 36 oligo-amenorrheic athletes in the PATCH, PILL, and E− arms respectively) completed the EDI-2 and/or TFEQ-R18 at baseline. Twenty-six, 20, and 27 young women in the PATCH, PILL, and E− groups, respectively completed the EDI-2, and 22, 16, and 23, respectively completed the TFEQ-R18 at both baseline and 12-month follow-up visits. Thus, 62.4% of participants completed one or both questionnaires at both time points. Completers did not differ from non-completers for baseline characteristics except for a slightly lower weight (but similar BMI; data not shown). Data were collected between 11/2009 and 10/2016. The study was conducted at the Clinical Research Center of our institution. Stopping rules included pregnancy or occurrence of study-related severe adverse events.
2.3. Assessment of ED pathology
The EDI-2 (Garner, 1991) is a 91-item 11-subscale self-report measure assessing eating attitudes and behaviors and related psychopathology on a 6-point scale ranging from “Always” to “Never” with responses rated 0-3. For the current study, responses were added up to scale scores for the DT and BD subscales (primary outcomes) with higher values indicating more pronounced presentation of the captured construct. The TFEQ-R18 (de Lauzon et al., 2004) is an 18-item 3-scale self-report assessment of eating behavior, shortened and revised from the original 51-item TFEQ (Stunkard and Messick, 1985). Participants rated dietary practices on a 4-point response scale ranging from “Definitely true” to “Definitely false.” Scores were then added up to a total score and scale score for three domains, namely Cognitive Restraint, Uncontrolled Eating, and Emotional Eating (secondary outcomes). Scale raw scores were transformed to a percentage scale with higher scores indicating greater ED pathology (Karlsson et al., 2000). The EDI-2 and TFEQ-R18 were administered at baseline and after 12 months of the study. The questionnaires were reviewed by the study psychologist.
2.4. Caloric intake and biochemical analysis
Total caloric intake was assessed using a four-day food diary validated for use in young women (3 weekdays and 1 weekend day; exploratory outcome) (Chinnock, 2006; Sawaya et al., 1996). Only a subset of 16, 15, and 22 participants in the PATCH, PILL, and E− groups submitted food diaries at both the baseline and 12-month follow-up visits. Estradiol was assessed using the LabCorp Esoteric Testing, Burlington, NC, USA with a sensitivity of 25.0 pg/mL and intra-assay coefficient of variation of 1.2-6.7%.
2.5. Statistical analysis
JMP Pro (version 13; SAS Institute, Cary, NC, USA) was used for analysis, except for the Mann-Whitney U-test and Wilcoxon signed-rank test, which were performed in STATA (version 14.2, StataCorp, College Station, TX, USA). Data are reported as mean±SEM. The initial power calculation was based on the primary outcome measures of the parent study and is not reported here. A power calculation was also performed at the time of secondary data analysis for EDI-2 and TFEQ-R18 outcomes. Primary study outcomes were changes in DT and BD (EDI-2); secondary outcomes included changes in TFEQ-R18 measures. For the RCT, the study had at least 80% power to detect an effect size of 0.5×SD at a 2-sided alpha level of .05 for EDI-2 outcomes for the 2-group comparison.
For the cross-sectional study, oligo-amenorrheic athletes, eumenorrheic athletes, and non-athletes were compared using analysis of variance (ANOVA) followed by the Tukey-Kramer honestly significant difference test to control for multiple comparisons. Cohen’s d and ηp2 are reported as estimates of effect size for between-group differences. Estradiol data were log-transformed prior to the analysis. This procedure was also applied to compare baseline characteristics across randomization groups in the RCT. For the RCT, E+ (PATCH+PILL) and E− groups were compared using the Mann-Whitney U-test. This was followed by a comparison Of the PATCH, PILL, and E− groups using the Kruskal-Wallis test followed by the Steel-Dwass test to control for multiple comparisons. While rank-based tests were conducted, mean and SEM are reported instead of median and IQR. In addition to the p-value for 2 or 3-group comparisons, r = Z/sqrt(N) and ε2 are reported as estimates of effect size for between-group differences between change scores. For changes in EDI-2 and TFEQ-R18 scores over 12 months, we also controlled for (i) baseline values, and (ii) baseline values, age, and weight changes over 12 months (as these variables can independently affect changes in EDI-2 and TFEQ-R18 scores). For EDI-2 and TFEQ-R18 scores, to determine whether observed changes represented an improvement or absence of worsening of the measure over time, we performed Wilcoxon signed-rank tests within E+ and E− groups and within PATCH, PILL, and E− groups, which are complemented by r as effect size estimate. As exploratory analyses, we also examined the effects of study interventions on total caloric intake over the 12-month period. Finally, the number of individuals reporting adverse events was contrasted across randomization groups using Fisher’s exact test. A p-value of <.05 was considered statistically significant and between .05-0.10 a trend for all analyses.
3. Results
Participant enrollment, study visits, and data management occurred between 11/2009 to 10/2016. The trial was concluded after the required number of participants (per power calculations) had been enrolled in the randomized trial.
3.1. Cross-sectional study
3.1.1. Baseline clinical characteristics:
Oligo-amenorrheic athletes, eumenorrheic athletes, and non-athletes did not differ for age and height. As expected, although in the normal range, BMI and BMI Z-scores were lower in oligo-amenorrheic athletes than eumenorrheic athletes and non-athletes and percent body fat lowest in oligo-amenorrheic athletes, intermediate in eumenorrheic athletes, and highest in non-athletes. As per study design, the number of hours of activity per week was higher in oligo-amenorrheic athletes and eumenorrheic athletes than non-athletes with no difference between the two athlete groups. Resting energy expenditure was higher in eumenorrheic athletes than oligo-amenorrheic athletes and non-athletes, menarchal age higher, and estradiol levels lower in oligo-amenorrheic athletes than eumenorrheic athletes and non-athletes. Groups did not differ in total daily caloric intake (Table 1).
3.1.2. EDI-2 and TFEQ-R18 scores:
Oligo-amenorrheic athletes showed higher EDI-2 DT and TFEQ-R18 Cognitive Restraint scores than eumenorrheic athletes and non-athletes and displayed a higher EDI-2 BD score than eumenorrheic athletes (Table 1).
3.2. RCT
3.2.1. Baseline characteristics:
Randomization groups (PATCH, PILL, and E−) did not differ for clinical characteristics or EDI-2 and TFEQ-R18 scores at baseline (Supplemental Table 1). Figure 1 depicts study enrollment and subsequent attrition, which did not significantly differ between intervention groups.
3.2.2. Changes in EDI-2 and TFEQ-R18 scores over 12 months in groups that did or did not receive estrogen (E+ and E− groups):
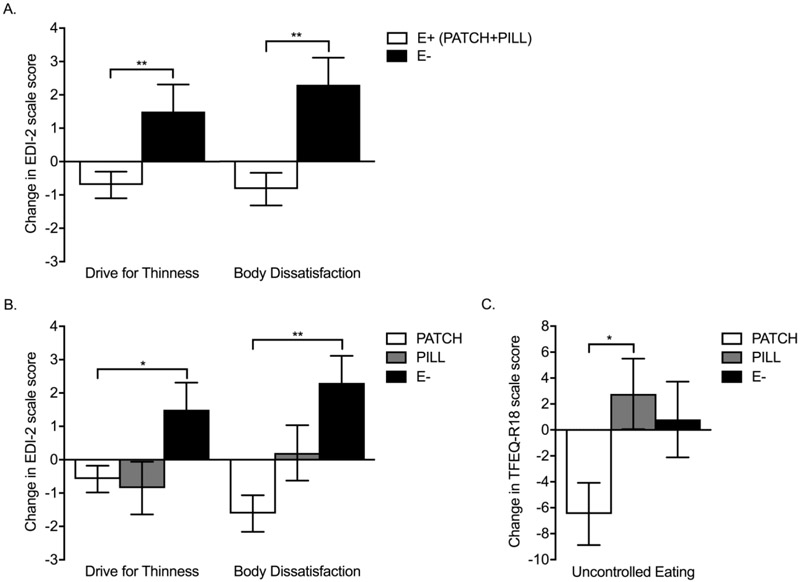
Over 12 months, the E+ group (PATCH+PILL), compared to E−, showed improved trajectories for EDI-2 DT and BD scores (Table 2, Figure 2A). These differences remained significant after controlling for baseline values, and for baseline values, age, and weight changes over 12 months. Based on Wilcoxon signed-rank tests, the difference between E+ vs. E− groups for BD was driven by an increase in BD in the E− group (p =.016, r = 0.33) that was not evident in the E+ group (p = .172, r = −0.14). The difference in DT resulted from an increase in DT scores in the E− group (p = .042, r = 0.28) versus a trend for a decrease in DT in the E+ group (p = .086, r = −0.18). Changes in TFEQ-R18 scores did not differ between groups, neither did weight, BMI, or BMI Z-score (Table 2).
TABLE 2:
Changes in Eating Disorder Inventory-2 (EDI-2) and Three-Factor Eating Questionnaire-R18 (TFEQ-R18) scores, weight, body mass index (BMI), and BMI Z-score in oligo-amenorrheic athletes randomized to estrogen (E+, PATCH+PILL) or no estrogen (E−)
| Changes over 12 months |
E+ (PATCH+PILL) |
E− | E+ vs. E− (Mann-Whitney U-test) |
E+ vs. E− controlled for baseline |
E+ vs. E− controlled for baseline, age, and weight changes over 12 months |
|||
|---|---|---|---|---|---|---|---|---|
| U | df | p | r | p | p | |||
| EDI-2 | n = 46 | n = 27 | ||||||
| Drive for Thinness | −0.70±0.40 | 1.50±0.81 | 375.0 | 1 | .007 | −0.32 | .014 | .018 |
| Body Dissatisfaction | −0.83±0.49 | 2.30±0.81 | 377.0 | 1 | .004 | −0.33 | .001 | .0002 |
| TFEQ-R18 | n = 38 | n = 23 | ||||||
| Cognitive Restraint | −4.94±3.10 | −4.04±4.27 | 395.5 | 1 | .994 | 0.001 | .925 | .835 |
| Uncontrolled Eating | −2.58±1.93 | 0.81±2.92 | 372.5 | 1 | .336 | −0.12 | .208 | .119 |
| Emotional Eating | 2.63±2.95 | 4.35±3.87 | 434.0 | 1 | .964 | −0.01 | .438 | .123 |
| n = 48 | n = 27 | |||||||
| Weight (kg) | 1.71±0.67 | 1.94±0.52 | 636.5 | 1 | .899 | −0.02 | N/A | N/A |
| BMI (kg/m2) | 0.59±0.24 | 0.66±0.19 | 644.0 | 1 | .965 | −0.005 | N/A | N/A |
| BMI Z-score | 0.02±0.12 | 0.10±0.12 | 589.0 | 1 | .953 | −0.01 | N/A | N/A |
| Total daily caloric intake (kcal) | 11±99 | −243±126 | 258.0 | 1 | .134 | 0.21 | N/A | N/A |
Mean±SEM; significant p-values are bolded. df, degrees of freedom.
FIGURE 2:
A. Changes in Eating Disorder Inventory-2 (EDI-2) Drive for Thinness and Body Dissatisfaction scores in oligo-amenorrheic athletes randomized to estrogen (E+) or no estrogen (E−). B. Changes in EDI-2 Drive for Thinness and Body Dissatisfaction scores and C. Three-Factor Eating Questionnaire-R18 (TFEQ-R18) Uncontrolled Eating scores in oligo-amenorrheic athletes randomized to transdermal 17β-estradiol with cyclic progesterone (PATCH), oral ethinyl estradiol and desogestrel (PILL), or no estrogen (E−). *p < .05, **p < .01.
3.2.3. Changes in EDI-2 and TFEQ-R18 scores over 12 months in groups receiving transdermal vs. oral vs. no estrogen:
In 3-group comparisons (PATCH, PILL, and E−), the overall comparison revealed a significant impact of the factor group for EDI-2 DT and BD and TFEQ-R18 Uncontrolled Eating (UE) scores. Using posthoc pairwise comparisons, improved trajectories were observed for changes in EDI-2 DT and BD scores in the PATCH versus the E− group, with trends for improvements in TFEQ-R18 Uncontrolled Eating (UE) scores (Table 3, Figures 2B and C). In addition, TFEQ-R18 UE in the PATCH group showed an improved trajectory compared to the PILL group. After controlling for baseline scores, and for baseline scores, age, and weight changes over 12 months, differences among groups persisted at a significant level for EDI-2 BD and TFEQ-R18 UE scores, and at a trend level for changes in EDI-2 DT scores (Table 3). Based on Wilcoxon signed-rank tests, differences among groups were primarily driven by an increase over 12 months in the E− group in EDI-2 DT and BD scores (p = .042, r = 0.28 and p = .016 and r = 0.33, respectively) versus a trend for a decrease in DT scores (p = .098, r = −0.23) and a significant decrease in BD scores (p = .013, r = −0.35) in the PATCH group. For TFEQ-R18 UE scores, the PATCH group demonstrated reductions in scores over 12 months (p = .015, r = −0.37) versus no changes in the E− group (p = .783, r = 0.04). The PILL group showed a similar numerical pattern as the PATCH group of a decrease in EDI-2 DT but increases in EDI-2 BD and TFEQ-R18 UE scores over 12 months. However, none of the changes were significant.
TABLE 3:
Changes in Eating Disorder Inventory-2 (EDI-2) and Three-Factor Eating Questionnaire-R18 (TFEQ-R18) scores, weight, body mass index (BMI), and BMI Z-score in oligo-amenorrheic athletes randomized to transdermal 17β-estradiol with cyclic progesterone (PATCH), oral ethinyl estradiol and desogestrel (PILL), or no estrogen (E−)
| Changes over 12 months |
PATCH | PILL | E− | Overall comparison (Kruskal-Wallis test) |
Overall comparison adjusted for baseline |
Overall comparison adjusted for baseline age, and weight changes over 12 months |
Steel-Dwass pairwise comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ε2 | df | p | ε2 | p | p | PATCH vs. PILL |
PATCH vs. E− |
PILL vs. E− | |||||||
| p | r | p | r | p | r | ||||||||||
| EDI-2 | n = 26 | n = 20 | n = 27 | ||||||||||||
| Drive for Thinness | −0.58±0.40 | −0.85±0.79 | 1.50±0.81 | 7.29 | 2 | .026 | 0.10 | .049 | .063 | .982 | −0.03 | .026 | −0.36 | .131 | −0.28 |
| Body Dissatisfaction | −1.62±0.55 | 0.20±0.83 | 2.30±0.81 | 11.94 | 2 | .003 | 0.17 | .001a | .0003a | .109 | −0.30 | .002 | −0.46 | .398 | −0.19 |
| TFEQ-R18 | n = 22 | n = 16 | n = 23 | ||||||||||||
| Cognitive Restraint | −3.89±2.88 | −6.25±6.10 | −4.04±4.27 | 0.02 | 2 | .990 | 0.002 | .992 | .973 | .999 | 0.01 | .989 | 0.02 | .992 | −0.02 |
| Uncontrolled Eating | −6.48±2.40 | 2.78±2.72 | 0.81±2.92 | 7.39 | 2 | .025 | 0.12 | .047 | .043 | .037 | −0.40 | .098 | −0.31 | .737 | 0.12 |
| Emotional Eating | 2.53±3.79 | 2.78±4.81 | 4.35±3.87 | 0.005 | 2 | .998 | 0.001 | .740 | .305 | 0.9999 | −0.002 | .999 | 0.01 | .991 | −0.02 |
| n = 26 | n = 22 | n = 27 | |||||||||||||
| Weight (kg) | 1.70±0.81 | 1.72±1.12 | 1.94±0.52 | 0.44 | 2 | .803 | 0.01 | N/A | N/A | .826 | 0.09 | .969 | 0.03 | .875 | −0.07 |
| BMI (kg/m2) | 0.53±0.26 | 0.66±0.43 | 0.66±0.19 | 0.41 | 2 | .814 | 0.01 | N/A | N/A | .858 | 0.08 | .936 | 0.05 | .884 | −0.07 |
| BMI Z-score | 0.11±0.16 | −0.09±0.17 | 0.10±0.12 | 0.51 | 2 | .773 | 0.01 | N/A | N/A | .709 | 0.12 | .974 | 0.03 | .933 | −0.05 |
| Total daily caloric intake (kcal) | 192±135 | −181±132 | −243±126 | 5.99 | 2 | .050 | 0.12 | N/A | N/A | .095 | 0.37 | .081 | 0.35 | .954 | 0.05 |
Mean±SEM; significant p-values are bolded.
p < 0.05 PATCH vs. E−.
3.2.4. Changes in weight measures and caloric intake:
Groups did not significantly differ for weight changes over 12 months (Tables 2 and 3). However, in a subset of 16, 15 and 22 participants who submitted food diaries at both baseline and 12-month follow-up visits, the total caloric intake trended higher in the PATCH versus PILL and E− groups (Table 3).
3.2.5. Adverse events:
Groups did not differ for reported adverse events (Supplemental Table 2).
4. Discussion
Investigating ED pathology in young normal-weight oligo-amenorrheic athletes and the impact of 12 months of estrogen replacement on these symptoms for the first time, our study reveals two key findings. First, and consistent with the literature, compared to eumenorrheic athletes and non-athletes, normal-weight oligo-amenorrheic athletes show more pronounced ED pathology. Specifically, oligo-amenorrheic athletes reported more DT than eumenorrheic athletes and non-athletes (d = 0.50 for both), more cognitive restraint than eumenorrheic athletes and non-athletes (d = 0.57 and 0.52, respectively), and more BD than eumenorrheic athletes (d = 0.43). Second, within oligo-amenorrheic athletes, those who received estrogen (E+: PATCH+PILL) did not demonstrate the increase in DT or BD observed in oligo-amenorrheic athletes not receiving estrogen over 12 months, suggesting that estrogen administration has a beneficial effect with medium effect size on these endpoints in young oligo-amenorrheic athletes (r = −0.32 and −0.33, respectively). The observed pattern, including prevention of a worsening trajectory, is consistent with the impact of estrogen replacement reported in a previous study of adolescent girls with AN, in whom estrogen replacement prevented increases in BD with increasing BMI observed in girls who did not receive estrogen replacement (Misra et al., 2013). The results are also consistent with findings of Racine et al (Racine et al., 2012), who demonstrated a negative association between DT and BD and estradiol levels across the menstrual cycle. However, our study critically extends both investigations by providing causal evidence for a positive impact of estrogen replacement on ED pathology in female oligo-amenorrheic athletes within the normal weight range, allowing for an investigation of the impact of estrogen on the endpoints independent of weight.
Comparing the routes of administration, effect sizes indicate that the observed beneficial effects of estrogen replacement on ED pathology were primarily driven by transdermal estradiol. In a three-group comparison (PATCH vs. PILL vs. E−) differences amongst groups for DT and BD were driven by the PATCH group with medium to large effect sizes in comparing changes in EDI-2 DT and BD scores between PATCH and E− groups (r = −0.36 and −0.46, respectively). Furthermore, the PATCH group showed a significantly improved trajectory in TFEQ-R18 UE scores than the PILL group (r = −0.40) and demonstrated a significant reduction in EDI-2 BD and TFEQ-R18 UE scores from baseline to 12-month follow-up (r = −0.35 and −0.37, respectively) and a trend for a reduction in DT (r = −0.23) versus an increase in DT and BD scores in the E− group (r = 0.28 and 0.33, respectively).
Effects of transdermal 17β-estradiol were thus most marked for BD, mirroring effects in previously investigated populations and contexts, namely for transdermal estradiol in girls with AN (Misra et al., 2013), and with menstrual cycle data indicating a negative association between estradiol levels and the degree of BD (Racine et al., 2012). Like in the AN study, in which participants of the E+ and E− groups gained weight in a similar fashion over the 18-month study period (3.8 vs. 3.3 kg; p = .73), numerical weight changes across all randomization groups also occurred in this study, although to a lesser extent (1.70, 1.72, and 1.94 kg for PATCH, PILL, and E-, respectively), over the 12-month intervention period. For the sample of the present study, whose mean age was 19.8 years, weight gain of ≤ 1 kg is to be expected per CDC growth charts (https://www.cdc.gov/growthcharts/data/set2clinical/cj41c072.pdf). Such weight gain may, in part, help to explain worsening of ED pathology, particularly BD, in individuals randomized to no estrogen.
The observed improvement in UE in the PATCH group is consistent with menstrual phase data suggesting an inhibitory effect of estradiol on appetite (Alonso-Alonso et al., 2011) and data from female rats demonstrating an increase in food-seeking behavior and hyperphagia after oophorectomy and a reduction in food reward behavior with estradiol administration (Richard et al., 2017). It also aligns with animal studies demonstrating increased binge eating following adult ovariectomy that was reversed with estradiol (with progesterone) treatment (Klump et al., 2011; Yu et al., 2008). This further supports a causal role of female gonadal hormones in binge eating.
The PILL group neither demonstrated the beneficial effect of estrogen on eating attitudes and behavior observed in the PATCH group nor a worsening in the assessed scores over 12 months, as observed in the E− group. This deviating pattern may be related to the longer duration of progesterone received by oligo-amenorrheic athletes randomized to the PILL versus PATCH (21 days of desogestrel vs. 12 days of micronized progesterone) or the formulation of progesterone and/or estrogen. Specifically, the PATCH group received a physiologic dose and formulation of estrogen (17β-estradiol), whereas the PILL group received ethinyl estradiol, the form of estrogen available in OCPs, which is not the physiologic form of estrogen. Further, the bioequivalence of various estrogen preparations is not completely clear, and the dose of ethinyl estradiol in the PILL may have greater bioactivity than the physiologic estrogen dosing with the PATCH, which might impact eating behaviors adversely. Further, the PATCH group received a physiologic form of progesterone for 12 days of every month, consistent with progesterone levels being higher in the luteal phase of the menstrual cycle, which lasts about 12- 14 days. In contrast, the PILL group received desogestrel, a synthetic progesterone, for 21 days of the month, which is not physiological. Of note, a positive association has been reported between progesterone levels and DT and BD over the menstrual cycle (vs. the negative association noted with estradiol) (Racine et al., 2012). As the ratio of estrogen and progesterone may impact eating behavior, this ratio is likely more physiologic with the PATCH than the PILL. Studies are necessary to differentiate these effects based on formulation and dose of estrogen/progesterone.
Our data show greater ED pathology, including DT and BD, in hypoestrogenemic young female athletes compared to normo-estrogenemic eumenorrheic athletes and non-athletes. Together with the observed worsening of ED pathology in oligo-amenorrheic athletes who did not receive estrogen over 12 months, these data indicate a need for intervention. DT and BD are key factors that promote disordered eating behavior and negative affect and typically precede the development of an ED (Beals and Hill, 2006; Stice et al., 2017; Stice and Shaw, 2002). Our finding that estrogen replacement may protect against worsening of DT and BD suggests that estrogen replacement may be a candidate preventative intervention for ED, often observed in the athlete triad. Further, estrogen replacement may prove to be an important clinical tool in managing ED pathology in hypogonadal young women. By preventing a worsening of ED pathology during treatment and weight regain, estradiol may facilitate long-term recovery.
Study limitations include the small sample size, the high attrition, and self-report assessment of ED pathology. In terms of generalizability, future studies are required to replicate our findings in a larger cohort. Attrition was consistent with that reported in other RCTs in similar populations (Misra et al., 2013), and participants who dropped out did not differ from study completers with regard to baseline characteristics (data not shown). In addition, future studies should combine self-report assessments with interview-based and behavioral measures of ED pathology. Furthermore, the non-blinded nature of the intervention and lack of a placebo control to protect individuals from false assumptions about contraceptive status could potentially have biased study outcomes. A randomized, double-blind, placebo-controlled design would provide the most stringent test of the outlined hypothesis. However, as the primary study outcomes highlighted in the study consent form were bone density and structure, while ED pathology was assessed as one of many secondary measures only, it is unlikely that participants formed specific expectations about treatment effects on eating attitudes and behaviors that could account for the observed findings. Finally, improvements in trajectories were observed in the PATCH but not PILL group, both of which represent intervention groups with comparable contact between participants and investigators. Thus, more extensive study involvement cannot account for the results.
Future studies are needed to investigate potential mediators of the observed beneficial effects of estrogen replacement on ED pathology in oligo-amenorrheic athletes. For example, the negative association between estradiol levels and DT and BD across the menstrual cycle may be mediated by changes in anxiety levels across the cycle, as both can be conceptualized as anxiety-driven concerns about body size (Racine et al., 2012). We have reported a reduction in trait anxiety following 18 months of estradiol administration as the 100 mcg 17β-estradiol patch (with cyclic progesterone) in girls with AN (Misra et al., 2013). That study also showed a non-significant reduction in DT following estradiol administration. It is possible that a larger sample size would have shown a significant improvement in eating behaviors, concurrent with improvements in trait anxiety. Further, the differential role of specific forms of estrogen needs to be clarified in a larger sample.
5. Conclusions
We have shown that normal-weight oligo-amenorrheic young athletes show more pronounced ED pathology than eumenorrheic athletes and non-athletes, and that 12 months of estrogen replacement improves symptom trajectories. Our findings emphasize the importance of normalizing estrogen levels in this population, and future studies are required to further investigate this relationship and its potential as a management tool of ED pathology in other populations of hypogonadal adolescent girls and young adult women Beyond the potential clinical implications, the results highlight the key role of estrogen deficiency in ED pathology, particularly attitudes guiding calorie consumption. Focusing on normal-weight oligo-amenorrheic athletes allowed us, for the first time, to investigate the impact of estrogen on eating attitudes and behaviors independent of low weight as a confounder. Furthermore, while our assessment of eating behavior and attitudes targeted the whole range from healthy to clinical eating behaviors, the majority of the individuals in the study did not fulfill criteria for an ED. Therefore, our finding of a potential endocrine-behavioral link in the context of eating behaviors warrants further investigation to better understand the complex interplay of hormonal and cognitive processes at play in regulating eating behavior.
Supplementary Material
Highlights.
Oligo-amenorrrheic athletes have greater eating disorder pathology than controls
Estrogen replacement improves drive for thinness and body dissatisfaction scores
Administration of transdermal estradiol (vs. the pill) reduces uncontrolled eating
Acknowledgments
Funding/support: This research was supported by grants R01HD060827, K24HD071843, K23DK110419-01, and UL1TR001102 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Role of the sponsor: The sponsor had no role in the design, analysis, interpretation, or publication of this study.
Conflicts of Interest
The authors have no conflicts to disclose in the context of this manuscript.
References
- Alonso-Alonso M, Ziemke F, Magkos F, Barrios FA, Brinkoetter M, Boyd I, Rifkin-Graboi A, Yannakoulia M, Rojas R, Pascual-Leone A, Mantzoros CS, 2011. Brain responses to food images during the early and late follicular phase of the menstrual cycle in healthy young women: Relation to fasting and feeding. Am J Clin Nutr 94, 377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr SI, Janelle KC, Prior JC, 1995. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr 61, 39–43. [DOI] [PubMed] [Google Scholar]
- Baskaran C, Cunningham B, Plessow F, Singhal V, Woolley R, Ackerman KE, Slattery M, Lee H, Lawson EA, Eddy K, Misra M, 2017a. Estrogen replacement improves verbal memory and executive control in oligomenorrheic/amenorrheic athletes in a randomized controlled trial. J Clin Psychiatry 78, e490–e497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskaran C, Plessow F, Ackerman KE, Singhal V, Eddy KT, Misra M, 2017b. A cross-sectional analysis of verbal memory and executive control across athletes with varying menstrual status and non-athletes. Psychiatry Res 258, 605–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals KA, Hill AK, 2006. The prevalence of disordered eating, menstrual dysfunction, and low bone mineral density among US collegiate athletes. Int J Sport Nutr Exerc Metab 16, 1–23. [DOI] [PubMed] [Google Scholar]
- Campolier M, Thondre SP, Clegg M, Shafat A, McIntosh A, Lightowler H, 2016. Changes in PYY and gastric emptying across the phases of the menstrual cycle and the influence of the ovarian hormones. Appetite 107, 106–115. [DOI] [PubMed] [Google Scholar]
- Cano Sokoloff N, Eguiguren ML, Wargo K, Ackerman KE, Baskaran C, Singhal V, Clarke H, Slattery M, Lee H, Eddy KT, Misra M, 2015. Bone parameters in relation to attitudes and feelings associated with disordered eating in oligo-amenorrheic athletes, eumenorrheic athletes, and nonathletes. Int J Eat Disord 48, 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnock A, 2006. Validation of an estimated food record. Public Health Nutr 9, 934–941. [DOI] [PubMed] [Google Scholar]
- Coyoy A, Guerra-Araiza C, Camacho-Arroyo I, 2016. Metabolism regulation by estrogens and their receptors in the central nervous system before and after menopause. Horm Metab Res 48, 489–496. [DOI] [PubMed] [Google Scholar]
- de Lauzon B, Romon M, Deschamps V, Lafay L, Borys JM, Karlsson J, Ducimetiere P, Charles MA, Fleurbaix Laventie Ville Sante Study, G., 2004. The Three-Factor Eating Questionnaire-R18 is able to distinguish among different eating patterns in a general population. J Nutr 134, 2372–2380. [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Kwiatkowska-Naqvi A, Von Hulst H, Pernia O, Carrero P, Garcia-Segura LM, 2012. Behavioral effects of estradiol therapy in ovariectomized rats depend on the age when the treatment is initiated. Exp Gerontol 47, 93–99. [DOI] [PubMed] [Google Scholar]
- Dye L, Blundell JE, 1997. Menstrual cycle and appetite control: Implications for weight regulation. Hum Reprod 12, 1142–1151. [DOI] [PubMed] [Google Scholar]
- Garner DM, 1991. Eating Disorder Inventory-2 professional manual. Psychological Assessment Resources, USA, Odessa, FL. [Google Scholar]
- Gogos A, Wu YC, Williams AS, Byrne LK, 2014. The effects of ethinylestradiol and progestins ('the pill') on cognitive function in pre-menopausal women. Neurochem Res 39, 2288–2300. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Persson LO, Sjostrom L, Sullivan M, 2000. Psychometric properties and factor structure of the Three-Factor Eating Questionnaire (TFEQ) in obese men and women. Results from the Swedish Obese Subjects (SOS) study. Int J Obes Relat Metab Disord 24, 1715–1725. [DOI] [PubMed] [Google Scholar]
- Klump KL, Suisman JL, Culbert KM, Kashy DA, Keel PK, Sisk CL, 2011. The effects of ovariectomy on binge eating proneness in adult female rats. Horm Behav 59, 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaite L, Ceponis J, Preiksa RT, Zilaitiene B, 2014. Impaired emotional state, quality of life and cognitive functions in young hypogonadal men. Andrologia 46, 1107–1112. [DOI] [PubMed] [Google Scholar]
- Minervini V, Rowland NE, Robertson KL, Foster TC, 2015. Role of estrogen receptor-alpha on food demand elasticity. J Exp Anal Behav 103, 553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra M, Katzman DK, Estella NM, Eddy KT, Weigel T, Goldstein MA, Miller KK, Klibanski A, 2013. Impact of physiologic estrogen replacement on anxiety symptoms, body shape perception, and eating attitudes in adolescent girls with anorexia nervosa: Data from a randomized controlled trial. J Clin Psychiatry 74, e765–e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quah YV, Poh BK, Ng LO, Noor MI, 2009. The female athlete triad among elite Malaysian athletes: Prevalence and associated factors. Asia Pac J Clin Nutr 18, 200–208. [PubMed] [Google Scholar]
- Rachman IM, Unnerstall JR, Pfaff DW, Cohen RS, 1998. Estrogen alters behavior and forebrain c-fos expression in ovariectomized rats subjected to the forced swim test. Proc Natl Acad Sci U S A 95, 13941–13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine SE, Culbert KM, Keel PK, Sisk CL, Burt SA, Klump KL, 2012. Differential associations between ovarian hormones and disordered eating symptoms across the menstrual cycle in women. Int J Eat Disord 45, 333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JE, Lopez-Ferreras L, Anderberg RH, Olandersson K, Skibicka KP, 2017. Estradiol is a critical regulator of food-reward behavior. Psychoneuroendocrinology 78, 193–202. [DOI] [PubMed] [Google Scholar]
- Sawaya AL, Tucker K, Tsay R, Willett W, Saltzman E, Dallal GE, Roberts SB, 1996. Evaluation of four methods for determining energy intake in young and older women: comparison with doubly labeled water measurements of total energy expenditure. Am J Clin Nutr 63, 491–499. [DOI] [PubMed] [Google Scholar]
- Stice E, Gau JM, Rohde P, Shaw H, 2017. Risk factors that predict future onset of each DSM-5 eating disorder: Predictive specificity in high-risk adolescent females. J Abnorm Psychol 126, 38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Shaw HE, 2002. Role of body dissatisfaction in the onset and maintenance of eating pathology: A synthesis of research findings. J Psychosom Res 53, 985–993. [DOI] [PubMed] [Google Scholar]
- Stunkard AJ, Messick S, 1985. The three-factor eating questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29, 71–83. [DOI] [PubMed] [Google Scholar]
- Treiser SL, Wardlaw SL, 1992. Estradiol regulation of proopiomelanocortin gene expression and peptide content in the hypothalamus. Neuroendocrinology 55, 167–173. [DOI] [PubMed] [Google Scholar]
- Yu Z, Geary N, Corwin RL, 2008. Ovarian hormones inhibit fat intake under binge-type conditions in ovariectomized rats. Physiol Behav 95, 501–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.