Abstract
OBJECTIVE.
To determine the feasibility and value of developing a regional antibiogram for community hospitals.
DESIGN.
Multicenter retrospective analysis of antibiograms.
SETTING AND PARTICIPANTS.
A total of 20 community hospitals in central and eastern North Carolina and south central Virginia participated in this study.
METHODS.
We combined antibiogram data from participating hospitals for 13 clinically relevant gram-negative pathogen–antibiotic combinations. From this combined antibiogram, we developed a regional antibiogram based on the mean susceptibilities of the combined data.
RESULTS.
We combined a total of 69,778 bacterial isolates across 13 clinically relevant gram-negative pathogen–antibiotic combinations (median for each combination, 1100; range, 174–27,428). Across all pathogen–antibiotic combinations, 69% of local susceptibility rates fell within 1 SD of the regional mean susceptibility rate, and 97% of local susceptibilities fell within 2 SD of the regional mean susceptibility rate. No individual hospital had > 1 pathogen–antibiotic combination with a local susceptibility rate > 2 SD of the regional mean susceptibility rate. All hospitals’ local susceptibility rates were within 2 SD of the regional mean susceptibility rate for low-prevalence pathogens (<500 isolates cumulative for the region).
CONCLUSIONS.
Small community hospitals frequently cannot develop an accurate antibiogram due to a paucity of local data. A regional antibiogram is likely to provide clinically useful information to community hospitals for low-prevalence pathogens.
Antibiograms provide relevant clinical information for providers, infection preventionists, and antimicrobial stewardship programs (ASPs),1 most notably providing information about the prevalence of resistant pathogens at an institution. To provide accurate information about pathogen susceptibilities, however, antibiograms must have sufficient numbers of isolates for individual pathogens. In fact, the Clinical and Laboratory Standards Institute (CLSI) M39-A2 guidelines require that only pathogens with at least 30 isolates be included in an antibiogram to achieve an accuracy level of ߡ 5% for empiric susceptibilities.2
Unfortunately, community hospitals typically do not follow CLSI guidelines for antibiogram development.3 A key reason is that microbiology laboratories in most community hospitals do not isolate the required number of individual pathogens to develop a reliable annual antibiogram, which can leave providers without adequate information to make appropriate empiric therapy decisions.
In small facilities where the number of clinical isolates is inadequate to prepare an antibiogram, aggregating data from multiple hospitals into a single regional antibiogram may be a practical alternative.4 Previous studies have compiled “geographically focused” antibiograms including data from both tertiary-care and community hospitals. Unfortunately, the results of such efforts typically involve more isolates from tertiary-care centers, skewing susceptibility patterns toward facilities with more intensive care unit (ICU) beds and higher local prevalence of antimicrobial-resistant pathogens.5,6 As a result, physicians in community hospitals using this type of regional antibiogram may overestimate the prevalence of multidrug-resistant organisms (MDROs).
We aggregated antimicrobial susceptibility data from multiple community hospitals within a single geographic region to provide physicians working in small community hospitals with clinically useful data on regional susceptibility patterns. The objectives of this study were (1) to develop a regional antibiogram and (2) to assess its utility by comparing rates reported at individual hospitals to the regional mean.
METHODS
The Duke Infection Control Outreach Network (DICON) is a collaborative network of community hospitals in the southeastern United States that shares surveillance data.7 We combined antibiogram data for 13 clinically relevant combinations of bacterial pathogens and antibiotics (Table 1) collected during calendar year 2012 from 20 DICON hospitals located in central and eastern North Carolina and south-central Virginia. Each hospital has its own microbiology lab and developed its own antibiogram. Among 20 local hospitals, 6 stratified isolates by location (inpatient vs outpatient vs ICU) or source (urine vs non-urine) when preparing their local antibiogram; the remainder did not. For the purposes of this study, we included all isolates from all locations and sources in the aggregate regional antibiogram, even if there were fewer than 30 isolates from an individual facility.
TABLE 1.
Regional Susceptibilities Among 20 DICON Hospitals
| Pathogen | Test | No. of Isolates Tested (No. of Hospitals Reporting) | Hospitals Reporting <30 Isolates, No. (%) | Regional Mean % Susceptible (95% CI) | Hospitals Within 1 SD, No. (%) | Hospitals Within 2 SD, No. (%) | Hospitals > 1 SD Below Mean Regional Susceptibility, No. (%) | Hospitals > 2 SD Below Mean Regional Susceptibility, No. (%) |
|---|---|---|---|---|---|---|---|---|
| Escherichia coli | FQ | 27,428 (20) | 0 (0) | 71 (62–81) | 14 (70) | 19 (95) | 3 (15) | 1 (5) |
| E. coli | TMP/SMX | 27,425 (20) | 0 (0) | 70 (61–80) | 15 (75) | 19 (95) | 3 (15) | 0 (0) |
| Proteus mirabilis | FQ | 4,607 (20) | 1 (5) | 66 (48–84) | 13 (65) | 19 (95) | 4 (20) | 1 (5) |
| Pseudomonas aeruginosa | FQ | 3,509 (19) | 1 (5) | 70 (57–83) | 13 (68) | 18 (95) | 1 (5) | 1 (5) |
| P. aeruginosa | Pip/Tazo | 2,878 (17) | 1 (6) | 89 (74–100) | 13 (76) | 16 (94) | 2 (12) | 1 (6) |
| Enterobacter cloacae | FQ | 1,213 (18) | 4 (22) | 88 (74–100) | 11 (61) | 17 (94) | 4 (22) | 1 (6) |
| E. cloacae | Carb | 1,100 (17) | 4 (24) | 98 (94–100) | 14 (82) | 17 (100) | 3 (18) | 0 (0) |
| Citroboacter freundii | FQ | 437 (12) | 7 (58) | 85 (72–98) | 8 (67) | 12 (100) | 1 (8) | 0 (0) |
| Acinetobacter baumannii | Amp/Sul | 296 (7) | 3 (43) | 80 (58–100) | 4 (57) | 7 (100) | 2 (29) | 0 (0) |
| A. baumannii | Carb | 311 (8) | 4 (50) | 69 (30–100) | 4 (50) | 8 (100) | 1 (13) | 0 (0) |
| Morganella morganii | FQ | 226 (6) | 3 (50) | 61 (44–79) | 4 (67) | 6 (100) | 1 (17) | 0 (0) |
| Stenotrophomonas maltophilia | TMP/SMX | 174 (7) | 5 (71) | 93 (83–100) | 4 (57) | 7 (100) | 1 (14) | 0 (0) |
| S. maltophilia | Levo | 174 (7) | 5 (71) | 81 (66–96) | 5 (71) | 7 (100) | 1 (14) | 0 (0) |
NOTE. DICON, Duke Infection Control Outreach Network; FQ, fluoroquinolone; Pip/Tazo, piperacillin/tazobactam; TMP/SMX, trimethoprim/sulfamethoxazole; Levo, levofloxacin; Amp/Sul, ampicillin/sulbactam; Carb, carbapenem.
We calculated regional mean susceptibility (standard deviation, SD) by dividing the total number of susceptible organisms for each pathogen–antibiotic combination across all 20 hospitals by the total number of tested organisms for that pathogen–antibiotic combination. We then created modified box-and-whisker plots for each pathogen–antibiotic combination to demonstrate each hospital-specific susceptibility in comparison to the regional mean susceptibility (Figure 1).
FIGURE 1.
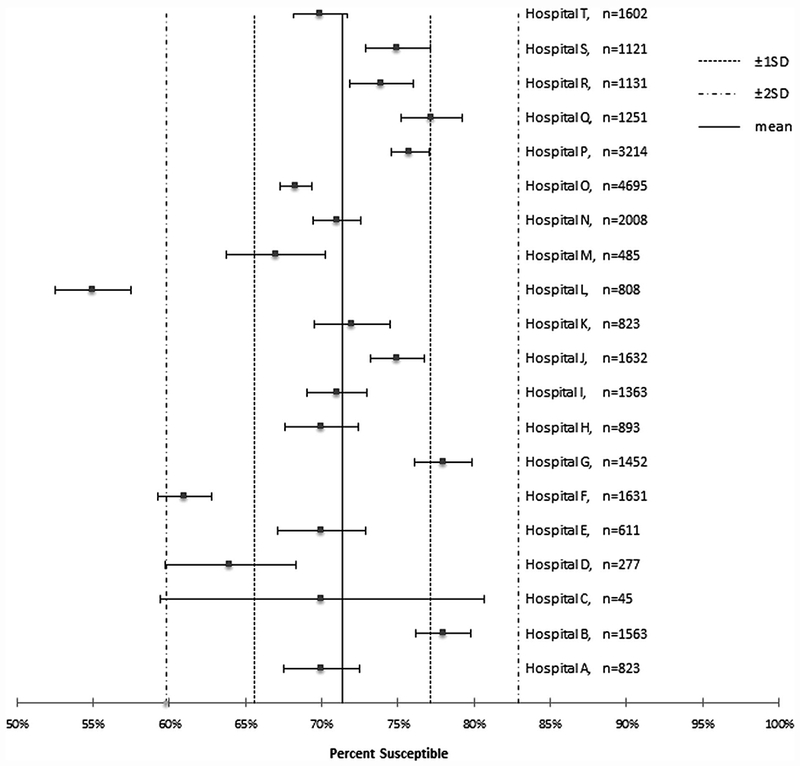
Hospital versus regional susceptibilities for Escherichia coli and fluoroquinolones.
We then determined the number of hospitals with susceptibilities within 1 or 2 SD of the regional mean to determine how well the regional mean represented the hospitals. We plotted the distance between hospital-specific susceptibilities and the regional mean susceptibilities by percentage of isolates contributed to the regional antibiogram for each pathogen–antibiotic combination to demonstrate the effect of number of pathogens isolated on the difference between local and regional mean susceptibilities. We examined the number of isolates contributed by hospitals based on their bed capacity as well. Hospitals with ≥250 beds were considered “large” community hospitals; hospitals with <250 beds were considered “small” community hospitals.
For instance, to develop the regional mean susceptibility of Escherichia coli to trimethoprim-sulfamethoxazole, we calculated the total number of E. coli cultures susceptible to trimethoprim-sulfamethoxazole across all 20 hospitals and divided by the total number of E. coli cultures. We then compared each hospital-specific susceptibility to the regional mean susceptibility to determine whether the hospital-specific susceptibility was within 1 and/or 2 SD of the regional susceptibility. Finally, we compared the difference between each hospital-specific and regional mean susceptibilities based on how many E. coli isolates and corresponding trimethoprim-sulfamethoxazole susceptibilities they contributed to the regional antibiogram.
RESULTS
We combined a total of 69,778 bacterial isolates across the 13 clinically relevant gram-negative pathogen–antibiotic combinations (median for each combination, 1,100; range, 174–27,428) to develop our regional antibiogram. For each pathogen–antibiotic combination, we report the number of isolates, number of hospitals reporting, regional mean susceptibility, and number of hospitals with susceptibility within 1 and 2 SD of the regional mean susceptibility (Table 1).
Escherichia coli (range, 45–4,695 isolates) was the most prevalent pathogen included in the analysis; all 20 hospitals reported > 30 isolates in accordance with CLSI guidelines. Stenotrophomonas maltophilia was the least frequently reported pathogen, with only 7 hospitals reporting susceptibility data for this pathogen, and only 2 hospitals had > 30 isolates (range, 14–35). Across all pathogen–antibiotic combinations, 69% of hospital-specific susceptibilities fell within 1 SD of the regional mean susceptibility rate, and 97% of hospital-specific susceptibilities fell within 2 SD of the regional mean susceptibility rate. No individual hospital had > 1 pathogen–antibiotic combination susceptibility > 2 SD from the regional mean susceptibility rate. All hospital-specific susceptibilities were within 2 SD of the regional mean susceptibility rate for low prevalence pathogens (<500 isolates cumulative for the region).
Our network has a significant degree of variation in bed sizes, but the contribution of each hospital was distributed evenly. The 7 large community hospitals contributed 49% of the isolates for this regional antibiogram. The remaining 13 small community hospitals contributed 51% of the isolates.
In total, we analyzed 178 hospital-specific pathogen–antibiotic susceptibility combinations for this study. Overall, 138 (77.5%) hospital-specific combinations comprised <10% of the isolates for their respective pathogen–antibiotic combinations, and 40 (22.5%) hospital-specific combinations represented ≥10% of the total isolates for each respective pathogen–antibiotic combination.
Hospitals contributing fewer isolates were not more likely to have hospital-specific susceptibility rates > 1 SD from the regional mean. Of the 178 hospital-specific pathogen–antibiotic susceptibility combinations analyzed in this study, 56 (31.5%) were > 1 SD from the regional mean susceptibility for their respective pathogen–antibiotic combinations. Of the 138 susceptibility combinations that comprised <10% of the isolates contributed to the regional mean, 44 (31.8%) were > 1 SD from the regional mean susceptibility. Of the 40 susceptibility combinations that comprised ≥ 10% of the isolates contributed to the regional mean, 12 (30.0%) were > 1 SD from the regional mean susceptibility. The difference between hospital-specific susceptibilities and the regional means was unchanged regardless of the percentage of isolates contributed by that hospital (Figure 2).
FIGURE 2.
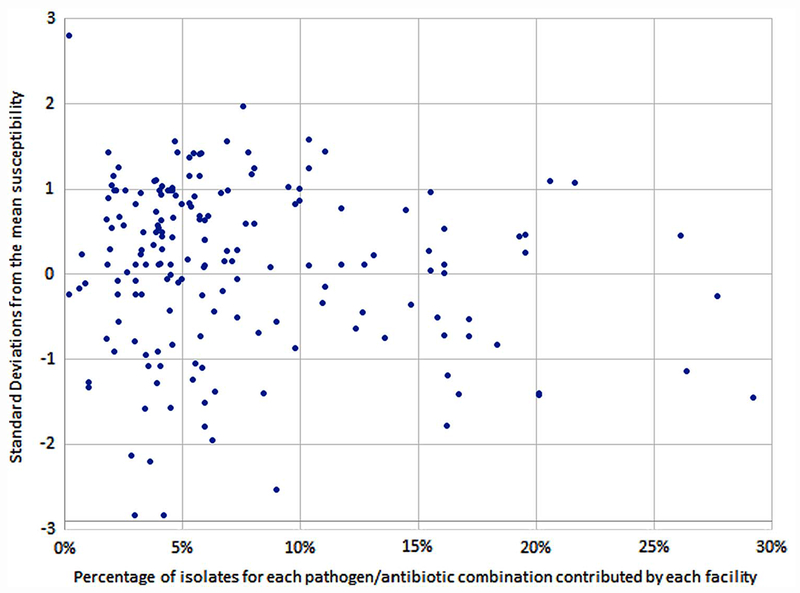
Distribution of hospital-specific difference from regional mean susceptibility compared to percentage of isolates contributed to the regional antibiogram.
Hospital-specific susceptibility combinations that appeared to be outliers could not be predicted by whether a hospital followed CLSI reporting guidelines. Of the 56 hospital-specific pathogen–antibiotic susceptibility combinations that were > 1 SD from the regional mean susceptibility, only 9 (16%) came from hospitals reporting <30 isolates for that pathogen/antibiotic combination.
DISCUSSION
Our study showed that susceptibility data from a large number of geographically co-located community hospitals can be combined to form a meaningful regional antibiogram. Our regional antibiogram for high-prevalence pathogens such as E. coli demonstrated that susceptibility results in 95% of hospitals are clustered around the regional mean (within 2 SD), indicating that susceptibility rates for this common pathogen did not vary widely across the geographic region. A regional approach may be most advantageous, however, for infrequently isolated pathogens. All hospital-specific susceptibilities were clustered around the regional means for these pathogens with fewer than 30 local isolates.
Hospitals that contributed few isolates to the regional antibiogram did not have significantly greater differences between hospital-specific susceptibilities and the regional mean susceptibility compared to hospitals that contributed most isolates. This finding is important because it reflects the relative homogeneity of the data by hospital contribution.
While underestimating a pathogen’s susceptibility to an antibiotic may change empiric therapy choices, overestimating a pathogen’s susceptibility to an antibiotic has the potential to harm patients. The overall low percentage of outliers indicates that the likelihood of overestimating the susceptibility for a pathogen–antibiotic combination in a clinically significant way when using regional susceptibility is low. However, hospitals may choose to use their local susceptibility rates in place of the regional susceptibility rates if their local susceptibility rates appear significantly lower than the regional mean for frequently isolated pathogens.
Importantly, no hospital-specific susceptibility for a pathogen–antibiotic combination was > 2 SD of the regional mean susceptibility. Indeed, resistance patterns among such low-prevalence pathogens did not vary significantly across our geographic region.
Small community hospitals that have too few isolates of important pathogens to allow them to prepare a meaningful antibiogram are most likely to benefit from a regional antibiogram. With a regional antibiogram, providers will have useful adjunctive information to assist in choosing appropriate empiric therapy.
This study has several limitations. We used pooled mean susceptibility rates; thus, susceptibility data from larger hospitals may have had a disproportionately greater influence on the regional mean susceptibility rates. Although susceptibility testing was standardized in accordance with CLSI guidelines, antibiogram reporting methods were not standardized across hospitals. However, failure to observe CLSI reporting guidelines is well documented in the literature and is not unique to our cohort of community hospitals.3,8,9
Our future directions include disseminating this regional antibiogram to hospitals in our network and then assessing how it is used by community hospitals. We also plan to emphasize that regional susceptibility rates should not replace local data when such information is available. Finally, the likelihood of receiving regionally appropriate empiric therapy can be calculated before and after dissemination of the antibiogram in hospitals with an absence of locally useful data for specific pathogen–antibiotic combinations.
Antibiograms remain an important tool for aiding clinical decisions related to empiric antimicrobial treatment, but they have limitations. Many small community hospitals struggle to develop antibiograms due to the low prevalence of some pathogens.3 We believe a regional antibiogram can overcome such limitations and thus provide clinically useful susceptibility data on low-prevalence pathogens to clinicians practicing in these settings.
ACKNOWLEDGMENTS
Financial support: Funded in part by grant support from the CDC Prevention Epicenters Program (grant no. U54CK000483 to D.J.A.). C.J.H. received a trainee travel grant from IDSA to present this work at IDWeek 2016.
Potential conflicts of interest: C.J.H. reports having consulted for GLG Consulting and LDA Research. R.W.M., E.D.A, M.J., S.S.L., D.J.S., and D.J.A. report receiving royalties from UpToDate, Inc. R.W.M receives grant support from the CDC, the CDC Foundation, and the AHRQ. E.D.A. reports having consulted for the HANYS, the NYSCHP, and the SHEA and receiving grant support from the CDC. M.J. reports consulting for Health and Wellness Partners, receiving grant support from Merck and Charles River Laboratories, and receiving payment for lectures from Society of Infectious Disease Pharmacists, Campbell University, ProCE, and the Potomac Center for Medical Education. D.J.S. reports consulting and providing expert testimony for J&J, receiving payment for development of educational presentations from the National Football League, and holding stock with Magnolia Medical Technologies and Sterilis Medical. D.J.A. receives additional grant support from the NIH/NIAID and is an ARLG Board Member.
Footnotes
PREVIOUS PRESENTATION. This work was presented in the Posters in the Parkand HAI: Epidemiologic Methods Poster sessions during IDWeek 2016 on October 26 and October 28, respectively, in New Orleans, Louisiana (poster #1359).
REFERENCES
- 1.Pakyz AL. The utility of hospital antibiograms as tools for guiding empiric therapy and tracking resistance. Insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 2007;27:1306–1312. [DOI] [PubMed] [Google Scholar]
- 2.Hindler JF, Stelling J. Analysis and presentation of cumulative antibiograms: a new consensus guideline from the Clinical and Laboratory Standards Institute. Clin Infect Dis 2007;44: 867–873. [DOI] [PubMed] [Google Scholar]
- 3.Moehring RW, Hazen KC, Hawkins MR, Drew RH, Sexton DJ, Anderson DJ. Challenges in preparation of cumulative antibiogram reports for community hospitals. J Clin Microbiol 2015; 53:2977–2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delisle G, Quach C, Domingo MC, et al. Escherichia coli anti-microbial susceptibility profile and cumulative antibiogram to guide empirical treatment of uncomplicated urinary tract infections in women in the province of Quebec, 2010–2015. J Antimicrob Chemother 2016;71:3562–3567. [DOI] [PubMed] [Google Scholar]
- 5.Var SK, Hadi R, Khardori NM. Evaluation of regional antibiograms to monitor antimicrobial resistance in Hampton Roads, Virginia. Ann Clin Microbiol Antimicrob 2015;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fridkin SK. Increasing prevalence of antimicrobial resistance in intensive care units. Crit Care Med 2001;29(4 Suppl):N64–N68. [DOI] [PubMed] [Google Scholar]
- 7.Anderson DJ, Miller BA, Chen LF, et al. The network approach for prevention of healthcare-associated infections: long-term effect of participation in the Duke Infection Control Outreach Network. Infect Control Hosp Epidemiol 2011;32:315–322. [DOI] [PubMed] [Google Scholar]
- 8.Lautenbach E, Nachamkin I. Analysis and presentation of cumulative antimicrobial susceptibility data (antibiograms): substantial variability across medical centers in the United States. Infect Control Hosp Epidemiol 2006;27:409–412. [DOI] [PubMed] [Google Scholar]
- 9.Xu R, Polk RE, Stencel L, et al. Antibiogram compliance in University HealthSystem Consortium participating hospitals with Clinical and Laboratory Standards Institute guidelines. Am J Health Syst Pharm 2012;69:598–606. [DOI] [PubMed] [Google Scholar]


