Abstract
Prostaglandins (PG) are an important class of lipid biomolecules that are essential in many biological processes, including inflammation and successful pregnancy. Despite a high bioactivity, physiological concentrations are typically low, which makes direct mass spectrometric analysis of endogenous PG species challenging. Consequently, there have not been any studies investigating PG localization to specific morphological regions in tissue sections using mass spectrometry imaging (MSI) techniques. Herein, we show that silver ions, added to the solvent used for nanospray desorption electrospray ionization (nano-DESI) MSI, enhances the ionization of PGs and enables nano-DESI MSI of several species in uterine tissue from day 4 pregnant mice. It was found that detection of [PG + Ag]+ ions increased the sensitivity by ~30 times, when compared to [PG − H]− ions. Further, the addition of isotopically labeled internal standards enabled generation of quantitative ion images for the detected PG species. Increased sensitivity and quantitative MSI enabled the first proof-of-principle results detailing PG localization in mouse uterus tissue sections. These results show that PG species primarily localized to cellular regions of the luminal epithelium and glandular epithelium in uterine tissue. Further, this study provides a unique scaffold for future studies investigating the PG distribution within biological tissue samples.
Graphical Abstract
Prostaglandins (PG) are a subgroup of eicosanoids derived from polyunsaturated fatty acids through enzymatic metabolism under the cyclooxygenase pathways, Cox1 and Cox2.1 They are important lipid mediators in inflammatory processes and play vital roles in homeostatic maintenance.1 Further, PG species are highly involved in successful pregnancies and their physiological functions have been well-reviewed from different perspectives over the years.2−4 Endogenous concentrations in tissues and plasma are low, ranging from nM to pM.5 Hence, preconcentration steps such as solid phase extraction (SPE) or liquid−liquid extraction are typically required before analysis. Analytical methods for detection of PG species include immunoassays,6,7 gas chromatography-mass spectrometry (GC/MS),8 and liquid chromatography−mass spectrometry (LC−MS).5,7−9 However, the low abundance in biological samples poses a great challenge for direct MS analysis of PG species in biological systems. This challenge is extended to the field of mass spectrometry imaging (MSI), which can provide information on the distribution and abundance of PG species in morphological regions of tissue.
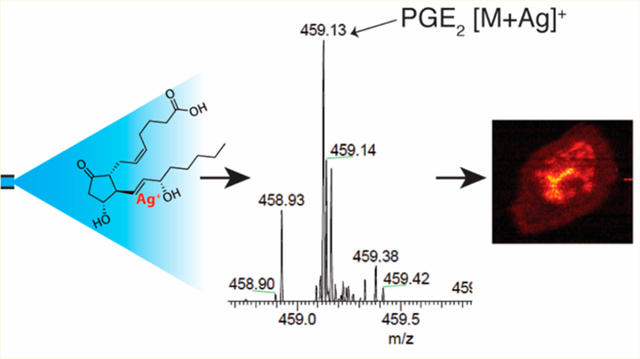
Various metal cations can be added to the electrospray solvent to effectively increase the specificity and/or sensitivity for electrospray ionization (ESI) MS of several classes of analytes, including lipids.10−13 Specifically, silver ions are known to form weak charge transfer complexes with alkenes14 and have been exploited for olefin analysis by many analytical techniques including: LC−MS,15−17 SIMS,18 and MALDIMS.19 The characteristic binding of silver ions to alkenes involves complexation between a filled alkene π orbital with an empty 5s orbital of Ag+ and an empty alkene π* orbital with a filled 4d orbital of Ag+. 20 As a result, the addition of Ag+ to a double bond can increase ionization effciency and thus detectability of a compound. Mass spectrometry of the resulting [M + Ag]+ ions offers distinct advantages including: i) the characteristic mass difference from isotope ratios between 107Ag and 109Ag, which provides two analyte peaks of equal intensity for analyte identification (Figure S1); and ii) a reduction in background noise by shifting peaks from the highly populated mass region m/z 200−300 into a less populated region of the mass spectrum (e.g., m/z 400−500). Given the inherent benefits of Ag+ complexation in mass spectrometry, relatively few studies have focused on combining liquid extraction MS techniques with silver-olefin adduct formation. Meier et al. used Ag+ adducts to enhance the sensitivity and specificity of unsaturated lipids in MS analysis of the complex chemical matrix of human serum with infrared matrix-assisted laser desorption electrospray ionization.21 Additionally, Jackson et al. showed enhanced sensitivity of olefins by DESI MS using Ag+ adducts to detect biologically relevant alkenes, triacylglycerols, and PG species.22 However, to the best of our knowledge, there has not been any studies focusing on MSI of endogenous PG species from biological systems.
Nanospray desorption electrospray ionization (nano-DESI) is a recently developed ambient sampling technique used for MSI.23,24 The nano-DESI probe is comprised of two fused silica capillaries placed at an angle to each other with a continuously flowing liquid bridge between them. When the liquid bridge comes in contact with the sample surface, endogenous compounds are desorbed into the solvent and subsequently ionized by electrospray ionization. Previously, nano-DESI has been successfully applied to quantitative MSI of small molecules25−27 and quantitative measurements of endogenous compounds from single cells.28 Additionally, a recent study highlights the use of oversampling to increase the spatial resolution for nano-DESI MSI experiments,29 enabling ion images of greater detail to be acquired. One of the main benefits of nano-DESI is flexibility of solvent composition,30 allowing for the inclusion of internal standards and other additives. Consequently, nano-DESI imaging of [M + Ag]+ species is simply realized by adding AgNO3 into the nano-DESI solvent.
This work demonstrates nano-DESI MSI of endogenous PG species by utilizing the unique complexation between Ag+ and alkenes to form [M + Ag]+ ions. We show a clear improvement in the sensitivity for [PG + Ag]+ ions over [PG − H]− ions and report the effects of biologically relevant salts (e.g., Na+ and Cl−) on the formation of Ag+ adducts. Finally, we show ion images capturing the unique cellular localization of PG species to the cellular regions containing the luminal and glandular epithelium in mouse uterine tissue samples on day 4 of pregnancy. This is the first study detailing the localization of endogenous PG species in thin tissue sections with MSI.
EXPERIMENTAL SECTION
Reagents and Materials.
Prostaglandin standards (>99% pure), including prostaglandin E2 and prostaglandin F2α, and deuterated standards (>99% deuterated), including prostaglandin E2-d9 and prostaglandin F2α-d9, were obtained from Cayman Chemical Company (Ann Arbor, MI). Fatty acid standards (all >99% pure) including arachidonic acid, arachidonic acid-d8, and arachidic acid were purchased from Sigma-Aldrich (Darmstadt, Germany), Cayman Chemical Company, and Larodan Fine Chemicals (Solna, Sweden), respectively. Additional reagents and solvents including silver nitrate (AgNO3, ≥ 99.8%), sodium nitrate (NaNO3, ≥ 99%), formic acid (98−100%), and HPLC-grade acetonitrile and methanol were acquired from Merck. Sodium chloride (NaCl, ≥ 99.5%) was purchased from Sigma-Aldrich.
Tissue Collection and Handling.
Utilizing the LoxP-Cre system, transgenic mice with uterine specific inactivation of Wnt5ad/d (Wnt5aloxP/loxP) and Ror1d/d/Ror2d/d (Ror1loxP/loxP/Ror2loxP/loxP) were created.31 These transgenic mouse models of impaired Wnt5a-ROR noncanonical Wnt pathway signaling and impaired embryo implantation contain morphological, cellular, and molecular changes in the uterus, including disrupted luminal epithelial evaginations (crypts) at the antimesometrial domain.31 These crypts are an essential step in the receptive uterus prior to embryo attachment. Uterine tissue was collected from one Wnt5ad/d mouse and one Ror1d/d/Ror2d/d mouse on day 4 of pregnancy prior to embryo attachment. The main objective of this study was quantitative visualization of PG species in Wnt5ad/d mouse uterine tissue on day 4 of pregnancy. The Ror1d/d/Ror2d/d mouse uterine tissue was used for an initial oversampling study due to a well-defined ~500 μm hole located in the myometrium of this section, which enabled us to characterize any effects of deposited silver ions on over sampling.
All mice used in this study were housed in the Cincinnati Children’s Animal Care Facility according to NIH and institutional guidelines for the use of laboratory animals. All protocols were approved by the Institutional Animal Care and Use Committee. Uterine tissue from one Wnt5ad/d mouse and one Ror1d/d/Ror2d/d mouse was cryosectioned into 12 μm thick sections and thaw-mounted onto regular glass microscope slides. The tissue sections were kept at −80 degrees C until analysis. Ror1d/d/Ror2d/d samples were used for the over sampling validation study, and the quantitative MSI experiments were carried out using Wnt5ad/d mouse tissue sections.
Sample Analysis for Sensitivity and Fundamental Ionization Studies.
For the sensitivity comparison between [PG + Ag]+ and [PG − H]− ions, standard solutions of PGE2 and PGF2α were prepared in 9:1 methanol/water with 4 ppm Ag+ and subjected to ESI MS. For salt suppression studies, PGE2 and arachidonic acid were prepared at 1 μM in 9:1 methanol/water with 0.1% formic acid, and 10 ppm Ag+. All solutions were directly introduced to a Thermo Fisher LTQ Orbitrap Velos mass spectrometer by an Agilent 1100 Series auto sampler and pump. A 40 μL aliquot of each standard solution was injected at a flow rate of 10 μL/min, providing a minimum of 2 min of stable signal. The mass spectra were acquired with a mass resolving power of 60 000 (m/Δm at m/z 400) and a mass window of m/z 400−500 in positive mode and m/z 150−400 in negative mode. These ranges were selected to ensure comparable background total ion current for each ion mode. Other operating conditions for MS analysis were applied as follows: the source voltage was set to 3 kV for positive mode and 3.5 kV for negative mode; the inlet capillary temperature was 275 °C; the automated gain control target was 1 × 106 ions; one microscan was used; and the sheath gas flow was 10 arbitrary units for positive mode and 25 arbitrary units for negative mode.
Nano-DESI Mass Spectrometry Imaging.
An in-house constructed nano-DESI apparatus was operated as previously described28 and coupled to a Thermo Fisher Q-Exactive mass spectrometer. The setup contained a 30 μm i.d. and 90 μm o.d. primary capillary and a 50 μm i.d. and 150 μm o.d. secondary capillary, supplied from Polymicro Technologies (Arizona). Solvent was pushed through the primary capillary at 0.5 μL min−1 by a syringe pump (Legato 180, KD Scientific). The nano-DESI solvent used for imaging experiments was 9:1 acetonitrile/methanol, with 0.1% formic acid, 10 ppm Ag+ (added as AgNO3), 100 nM of PGE2-d9, 50 nM of PGF2α-d9, and 250 nM arachidonic acid-d8. Instrumental settings included a 3 kV electrospray potential applied directly to the syringe needle on the primary capillary; a heated inlet capillary temperature of 250 °C; and an AGC target of 1 × 106 ions. Spectra were acquired at a mass resolving power of 140000 (m/Δm at m/z 200) with a mass window of m/z 400−500 and with one microscan. The glass slides holding tissue sections were placed on a sample holder connected to a motorized XYZ stage (Newport, CA), controlled by in-house designed software created in LabVIEW.32 Data for the ion images were acquired by moving the sample under the nano-DESI probe at 10 μm s−1 along the x axis, and in 30 μm intervals along the y axis by stepping, producing a pixel size of approximately 10 × 30 μm.
Data Processing and Analyte Identification.
All data and ion images were compiled through custom Matlab routines using centroided mzXML files converted from the original Xcalibur raw files. In this study, we present data for isomer classes of PG species, as mass spectrometry alone cannot distinguish between compounds with the same molecular formula. Previous studies investigating PG species in similar mouse uterine tissue samples have identified PGE2 and PGF2α as abundant PG species.33−35 Accordingly, we combine all possible isomers for PGE2 and PGF2α into their respective isomer class and refer to each category as equivalents of PGE2 and PGF2α. Similarly, referring to dimethyl-PGE2, PGA1, and PGJ2 includes all possible isomers. The structures of all of the PG species in this study are displayed in Figure S2.
Fragmentation patterns for both standards and endogenous [PG + Ag]+ differed from the commonly available MS/MS data in negative ion mode.36 Specifically, structurally diagnostic ions from rearrangements around the 5-membered ring were not detected. Rather, fragmentation of [PG + Ag]+ ions yielded predominantly product ions corresponding to the loss of one or two H2O molecules. A summary of detected and assigned peaks for MS/MS of endogenous and deuterated PGE2 and PGF2α [M + Ag]+ ions is displayed in Table S1. Additionally, the negative ion mode MS/MS spectra for endogenous and deuterated PGE2 are presented in Figure S3. Endogenous PGF2α was below the limit of detection in negative mode, therefore tandem mass spectrometric data for PGF2α was not possible in the negative ion mode. The combination of the exact mass isotopic pattern, positive ion mode MS/MS data, and negative mode MS/MS data for PGE2 supports the assignment of these PG species from the mouse uterine samples.
RESULTS AND DISCUSSION
Increased Sensitivity for Prostaglandins Using Silver Adducts.
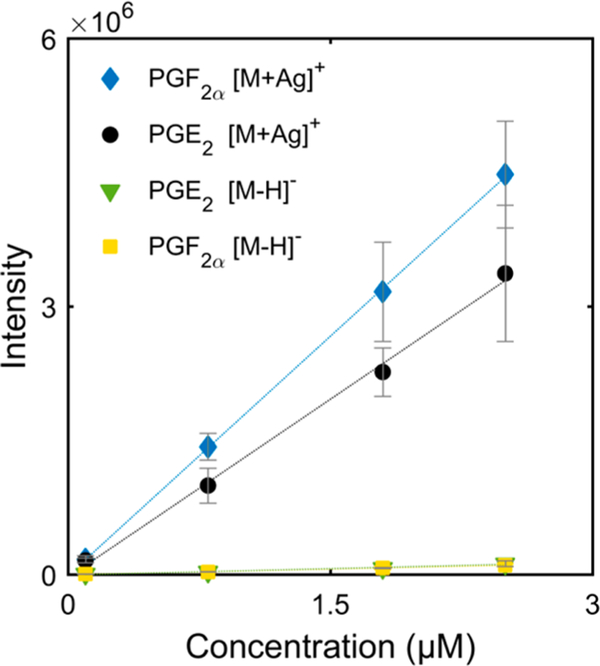
Mass spectrometry analysis of PG species typically involves monitoring deprotonated molecules in negative ion mode through loss of hydrogen from the carboxylic acid moiety. Cationization of PG species with silver offers a different ionization strategy, since silver is known to form a complex with the alkene double bond.20 To investigate any enhancement in sensitivity, a series of PGE2 and PGF2α standard solutions were analyzed in both positive mode, as [M + Ag]+ ions, and in negative mode, as [M − H]− ions. Figure 1 presents the respective results where, when using slope as a measure of analytical sensitivity, it is apparent that the addition of silver drastically improves sensitivity. Specifically, the response curve data presented in Table S2 indicates a 30 times improvement in sensitivity for [PG + Ag]+ ions, when compared to [PG − H]− ions. It should be noted that this improvement in sensitivity occurs despite the two naturally occurring silver isotopologues, 52% 107Ag and 48% 109Ag. This unique isotopic pattern is highly advantageous when corroborating the identity of silver ion analyte adducts, increasing the method specificity. Method specificity is further enhanced by the selectivity of Ag binding to the alkene functional group, rather than to the carboxylic acid functional group. Figure S4 indicates that silver ions exclusively bind to the sites of unsaturation, and not to the carboxylic acid moiety, since the fully saturated compound is not detected as [M + Ag]+. Thus, cationization with silver contributes to positively identifying molecules containing alkene functional groups and enables alkenes to be separated from other molecules with the same molecular formula, thus reducing spectral interferences. In summary, analyzing PG species as [M + Ag]+ ions provides higher sensitivity and specificity.
Figure 1.
Sensitivity evaluation for PG standards as [M + Ag]+ and [M − H]− ions. The evaluation covered a concentration range of 0.1−2.5 μM. Intensity is shown for 107Ag isotopologues of PGE2 (m/z 461.1300) and PGF2α (m/z 463.1454) in positive mode. In negative mode, intensity is shown for [M − H]− ions for PGE2 and PGF2α at m/z 351.2146 and m/z 353.2320, respectively. Each error bar indicates the standard deviation for n = 5 measurements.
Sodium Salts Negatively Impact Sensitivity for Silver Adduct Ions.
The higher sensitivity and specificity for cationization with silver offers an avenue to enhance the direct analysis of PG species from thin tissue sections. With the ability to easily customize solvent composition, liquid extraction MS techniques enable the inclusion of silver into the solvent for a continuous presence at a constant concentration during analyte extraction and ionization. In a previous study, Jackson et al. found that the solvent providing the lowest limit of detection for olefin [M + Ag]+ ions with DESI was a 1:1 water/methanol solvent mix.22 A similar solvent composition should therefore be optimal for nano-DESI; however, we were unsuccessful in detecting endogenous species from tissue using the typical 9:1 methanol/water composition with the addition of Ag+. Specifically, upon initial contact of the liquid bridge with the tissue surface, a fleeting signal for [M + Ag]+ ions was observed followed by a steady signal increase for [M + Na]+ ions (Figure S5a). This effect is presumably caused by the high (mM range)37 concentration of endogenous salts such as sodium and chloride in tissue.
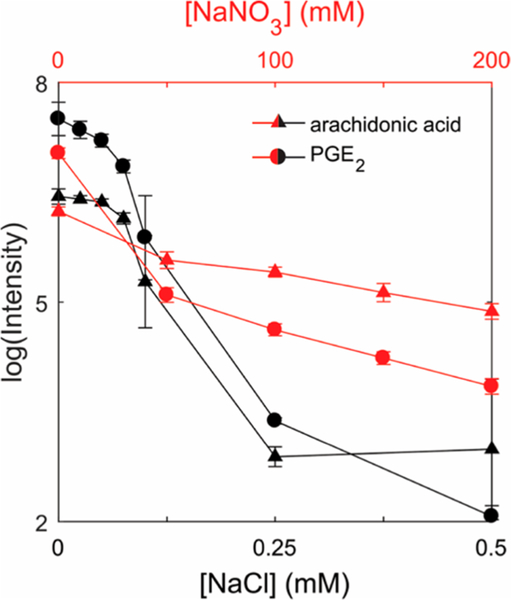
To investigate the potential impact of salts on silver cationization, silver doped (10 ppm Ag+) standard solutions of PGE2 and arachidonic acid were mixed with increasing amounts of sodium nitrate and sodium chloride and directly infused into the mass spectrometer. The results in Figure 2 show that intensities corresponding to PGE2 and arachidonic acid [M + Ag]+ ions are surprisingly resilient to increasing concentrations of sodium nitrate. Although the intensity of both analytes decreased with increasing amounts of sodium nitrate, high signals were observed even when 200 mM sodium nitrate was added. These results indicate a competition between sodium and silver ions or a negative influence on the electrospray process at these high salt concentrations. Figure 2 further shows that the addition of sodium chloride significantly reduces the signal intensity of PGE2 and arachidonic acid [M + Ag]+ ions at much lower concentrations. In fact, the first notable decrease in [M + Ag]+ signals occurs at ~0.1 mM sodium chloride. Since this is approximately the same concentration as the added silver, we conclude that free and available silver ions in the nano-DESI solvent are immediately removed by extracted chloride ions from the tissue sample, forming AgCl(s). Thus, it is essential that the concentration of chloride is kept at a minimum in the nano-DESI solvent. As the solubility of sodium chloride is four orders of magnitude less in acetonitrile than in methanol,38 acetonitrile is more suitable as experimentally shown in Figure S5a and S5b. For nano-DESI MSI of [M + Ag]+ ions from tissue, the optimal solvent was concluded to be 9:1 acetonitrile/methanol containing 10 ppm Ag+ and 0.1% formic acid, where formic acid is added to promote protonation of the PG carboxylic acid moiety.
Figure 2.
Effect of salts on the formation of silver cationization. Note that the different concentrations for each respective salt are displayed on both x axes ([NaNO3] is displayed on the top x axis and the [NaCl] is set as the bottom axis). The concentration of PGE2 (m/z 461.1300) and arachidonic acid (m/z 411.1453) was 1 μM in all solutions. Each error bar represents the standard deviation for n = 5 measurements.
Deposited Silver Does Not Affect Ion Images When Oversampling.
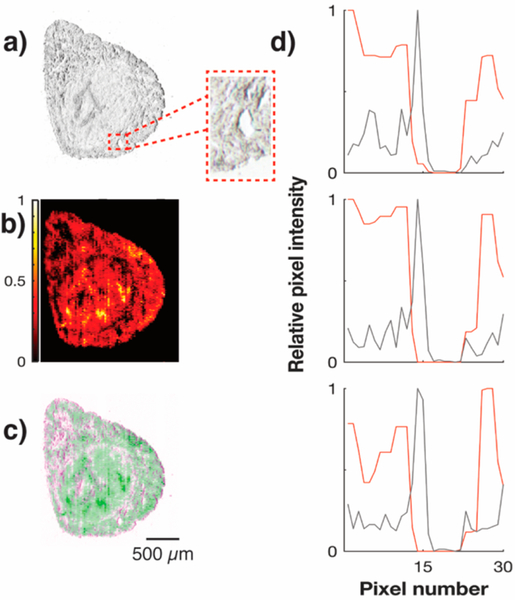
Recently, we reported that the spatial resolution of nano-DESI MSI can be successfully increased by oversampling with negligible analyte redistribution.29 In oversampling, the step between each acquired line is smaller than the diameter of the nano-DESI probe, causing the tissue to be repeatedly analyzed. Despite being a successful strategy for regular nano-DESI, silver in the solvent deposits on the tissue and surrounding edges of the glass slide during analysis which could negatively impact resulting quantitative ion images of [M + Ag]+ ions. To evaluate the impact of silver deposition on quantitative nano-DESI MSI, a mouse uterus tissue section was oversampled three times by stepping at 30 μm intervals between each sequential line on the y axis using a 90 μm OD primary capillary. Figure 3 (panels a, b, and c) displays the optical image, the ion image for arachidonic acid normalized to arachidonic acid-d8, and concomitant false color fused image, respectively. Arachidonic acid was selected because of the high abundance in all regions of the mouse uterine tissue. The ion image has a pixel size of 10 × 30 μm, as a result of oversampling, depicting details of the tissue outline and excellent agreement between the surrounding border of the tissue section in the optical and ion image. Visually, the ion image in Figure 3b shows no impact of silver deposition. If silver deposition would impact the resulting ion image it would be especially apparent along the borders of the tissue where an expanded liquid bridge, due to the more polar glass surface, would push solvent residues toward the boundary generating higher signal intensity. To evaluate this effect, three line scans were generated in silico perpendicular to the sampling direction and over the small hole enlarged in Figure 3a. The corresponding line scan data in Figure 3d show excellent correlation between the optical image (gray trace) and the ion image (red trace), suggesting that silver is not significantly impacting the acquired data. Note that the spike in the line scan of the optical signal near pixel number 15 represents an artifact of the optical shadow at the left edge of the hole. The three in silico generated line scans further tells us that there is no significant deposition of the internal standard on the tissue from repeated sampling events. If significant internal standard was deposited during nano-DESI sampling, the hole in Figure 3d would appear larger than it actually is. This would be an effect of a lower ratio of endogenous to internal standard intensities. However, Figure 3d clearly displays that this is not the case. These results therefore prove that neither deposited silver nor internal standard have a noticeable effect on the observed signal intensity for endogenous silver adduct species. Hence, quantitative ion images can be obtained also for [M + Ag]+ ions using nano-DESI MSI.
Figure 3.
Evaluation of silver deposition effects on oversampling. (a) Optical image of an Ror1d/d/Ror2d/d mouse uterine tissue section, (b) ion image for the 109Ag+ adduct of arachidonic acid, and (c) false color fusion of the optical and ion image. Part (d) illustrates the agreement between the ion image (red) and inverted optical image (gray) for sections of data sampled along the x axis for the hole presented in the optical image (a).
Quantitative Mass Spectrometry Imaging of Prostaglandins.
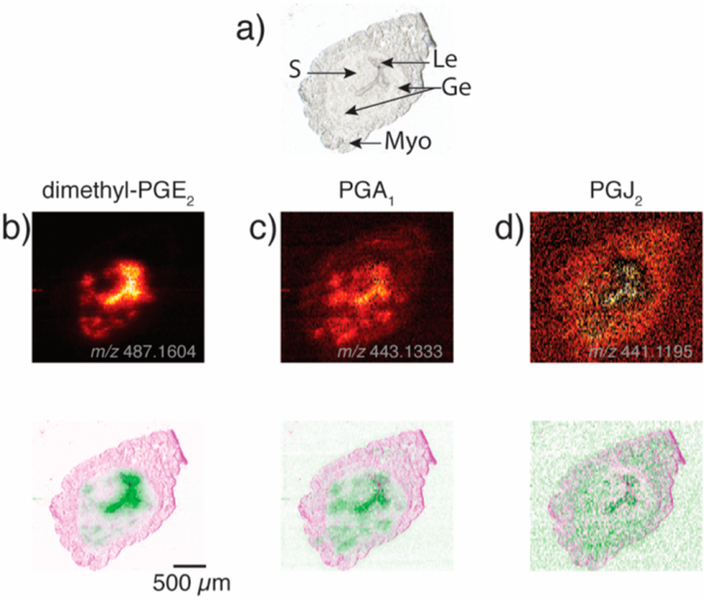
Prostaglandins are important regulators for inflammation and are critical in all stages of pregnancy, ranging from embryo implantation to birth. However, little is known about the abundance and localization of PG species in uterine tissue since these compounds cannot be selectively stained and are typically below the limit of detection for MSI studies. Here, we use mouse uterine tissue sections for the first proof-of-principle study to image PG species in tissue regions using nano-DESI MSI with silver-doped solvent. While PGE2 and PGF2α have been detected in similar mouse uterine tissue sections using LC−MS,33−35 the characteristic isotopic pattern of silver allowed us to tentatively identify three additional PG isomer classes previously not reported in mouse uterine tissue: dimethyl-PGE2 (m/z 487.1604, [C22H36O 1075Ag]+), PGA1 (m/z 443.1333, [C20H32O 1074Ag]+), and PGJ2 (m/z 441.1195, [C20H30O 1074Ag]+). Dimethyl-PGE2, a known analog to PGE2, is regarded as pro-inflammatory, while both PGA1 and PGJ2 increase later in the inflammatory process and are considered to be anti-inflammatory.39,40 The assignments of these PG species are based on the accurate mass and the isotopic signature of [M + Ag]+ ions. The ion images of dimethyl-PGE2, PGA1, and PGJ2 are displayed in Figure 4. All ion images are normalized to the internal standard PGE2-d9 to minimize ion suppression effects over the tissue section as discussed in the Supporting Information and Figure S9. While the ion image of dimethyl-PGE2 in Figure 4 is over 10 times more intense than the other two PG species, they all share localization to both the luminal epithelium and the glandular epithelium. This specific distribution of dimethyl-PGE2, PGA1, and PGJ2 is shared with the more commonly studied PGE2 and PGF2α (Figure 5), further supporting the assignment of the additional PG species found in this study.
Figure 4.
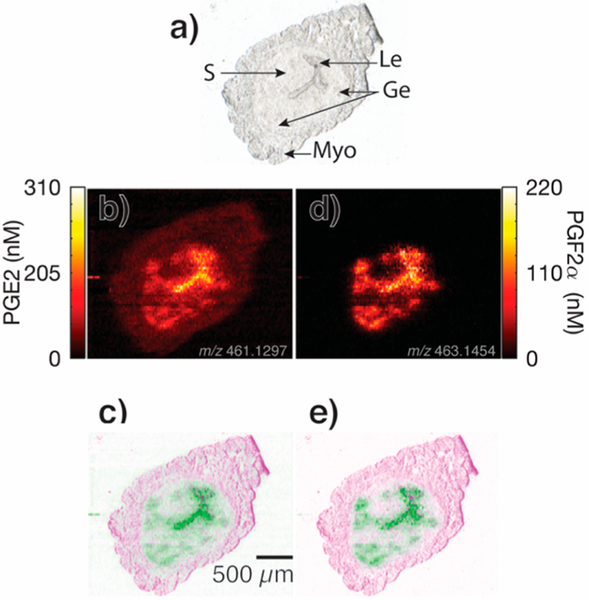
Ion images for tentatively assigned PG species in Wnt5ad/d mouse uterine tissue on day 4 of pregnancy, (a) optical image, (b, c, and d) ion images and false color fused image for dimethyl-PGE2 (m/z 487.1604, [C22H36O5Ag]), PGA1 (m/z 443.1333, [C20H30O4Ag]+), and PGJ2 (m/z 441.1195, [C20 H32O4Ag]+), respectively. All ion images are for 109Ag adducts and have been normalized to the internal standard PGE2-d9 as discussed in the Supporting Information and Figure S9. Brighter colors correspond to higher signal intensities. Le, luminal epithelium; Ge, glandular epithelium; S, stroma; and Myo, myometrium.46
Figure 5.
Quantitative ion images for PGE2 and PGF2α from Wnt5ad/d mouse uterine tissue on day 4 of pregnancy, (a) optical image, (b) ion image for PGE2 (109Ag+ adduct), and (c) resulting false color fused image detailing PGE2 localization. The ion image and false color image for PGF2α are shown in (d and e, respectively). Le, luminal epithelium;Ge, glandular epithelium; S, stroma; and Myo, myometrium.46
The implications of PGE2 and PGF2α in early pregnancy and birth have been previously established.31,41−44 Embryo implantation is considered a proinflammatory reaction, with the cyclooxygenase (Cox)-derived prostaglandins increasing endometrial vascular permeability at the embryo-uterus attachment site. In mice, Cox2 (Ptgs2) is induced at the attachment site, but Cox1 (Ptgs1) and cPLA2α (Pla2g4a) are expressed in the luminal and glandular epithelium early on day 4 of pregnancy in the receptive uterus prior to embryo attachment and implantation.45 For simultaneous quantification and imaging of PGE2 and PGF2α in Wnt5ad/d uterine sections, collected on day 4 of pregnancy prior to embryo attachment, the nano-DESI solvent was doped with deuterated internal standards for both PGE2 and PGF2α, in addition to 10 ppm Ag+. This enabled subsequent generation of the quantitative ion images in Figure 5, where the concentration of endogenous PG is calculated in every pixel of the ion image: the intensity of the endogenous PG is normalized to the intensity of the corresponding internal standard and multiplied with the concentration of the internal standard, as previously reported.26,27 No signal averaging or pixel smoothing was employed when generating the ion images. Note that the quantitative ion images include a color scale representing the nanomolar concentration of endogenous PG instead of the typically used relative abundance scaling. Further, Figure S6 suggests that the PG species are immediately and fully extracted during sampling, indicating that these quantitative ion images can provide insight into the absolute quantities of endogenous PGE2 and PGF2α. On the basis of the quantitative scale, it is clear from Figure 5 (panels b and c) that PGE2 is detected at ~100 nM over the entire tissue section, with higher concentrations localizing primarily to the luminal epithelium and secondarily to the glandular epithelium. At specific locations in the luminal epithelium, PGE2 is detected up to 310 nM, while PGF2α, also localizing to the luminal and glandular epithelium as shown in Figure 5 (panels d and e), has an overall lower abundance of about two-thirds of the concentration of PGE2. These concentration ranges are also true for the replicate images shown in Figures S7 and S8. The increased image quality afforded by oversampling allows the visualization of the glandular epithelium, which are ~100−300 μm in diameter in the ion images and comparable to previously published fluorescent images.46 Thus, doping the nano-DESI solvent with both silver ions and internal standards enables, for the first time, the quantitative visualization of several PG species in Wnt5ad/d mouse uterine tissue sections. Since successful implantation involves close interaction between the embryo and the maternal luminal epithelium cells lining the uterus, we hypothesize that this localization of PG species can be caused by PG species acting as molecular links in mediating embryo-uterine interactions.47
Although several studies have observed PG species directly from tissue,19,48 this is the first example demonstrating quantitative MSI of PG species from tissue samples. Future in situ nano-DESI MSI PG studies will extend upon these initial proof-of-principle experiments to quantitatively compare the distribution of PG species in Wnt5ad/d to wild-type mice and other mouse models of compromised embryo implantation. It is anticipated that future results will provide unique insights into PG species acting as key molecular regulators and enabling cross-talk between the embryo and the receptive uterus resulting in successful implantation.45,49
CONCLUSIONS
Nano-DESI MSI with silver-doped solvent was employed to image PG species directly from mouse uterine tissue sections. Analysis of [PG + Ag]+ ions provided an increase in sensitivity of ~ 30 times, when compared to [PG − H]− ions. However, successful detection of [PG + Ag]+ ions from tissue required chloride concentrations in the nano-DESI solvent to be kept to a minimum, thus acetonitrile was employed over methanol. Oversampling resulted in high quality quantitative ion images of PG species cationized with silver. In particular, three PG isomer classes, dimethyl-PGE2, PGA1, and PGJ2, were found to localize to the same cellular regions as the well-characterized PGE2 and PGF2α in the relatively small (~2 mm) Wnt5ad/d mouse uterine sections. Specifically, all detected PG species localized to the luminal epithelium and glandular epithelium of Wnt5ad/d mouse uterine sections at day 4 of pregnancy prior to embryo attachment. The presented results successfully demonstrate quantitative nano-DESI MSI of [PG + Ag]+ species directly from biological tissue. The described approach is anticipated to be highly valuable for future studies investigating the role of PG species in biological systems.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this work was provided by the Swedish Foundation for Strategic Research (SSF ICA-6) (IL) and the Swedish Research Council (VR 621-2013-4231) (IL). Research at Pacific Northwest National Laboratory (PNNL) was supported by the NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R21 HD084788 (KEBJ). PNNL is a multiprogram national laboratory operated by Battelle for the DOE under Contract DE-AC05–76RLO 1830. Work in Dey’s lab was supported in part by NIH grants (R01HD068524, DA006668, and P01CA77839) and grants from the March of Dimes (S.K. Dey).
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.analchem.8b00350.
Mass spectrum for PGE2-d9, structures of PG species, tandem MS data for endogenous and deuterated [PG + Ag]+ ions, tandem mass spectrometry spectra for endogenous PGE2 sampled from mouse uterine tissue and PGE2-d9 from a standard solution, response curve equations for PGE2 and PGF2α, evidence for the specificity of Ag+ binding to the unsaturated FA 20:4, chronograms for line scans across a mouse uterine tissue section with nano-DESI MS, relative PGE2 signal intensity for five consecutive line scans across the same area of mouse uternine tissue, replicate quantitative PGE2 ion images for three mouse uterine tissue sections, replicate quantitative PGF2α ion images for three mouse uterine tissue sections, and ion images of internal standards for PGE2 and PGF2α (PDF)
REFERENCES
- (1).Ricciotti E; FitzGerald GA Arterioscler., Thromb., Vasc. Biol 2011, 31 (5), 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Yagami T; Koma H; Yamamoto Y Mol. Neurobiol 2016, 53 (7), 4754–4771. [DOI] [PubMed] [Google Scholar]
- (3).Alhouayek M; Muccioli GG Trends Pharmacol. Sci 2014, 35 (6), 284–292. [DOI] [PubMed] [Google Scholar]
- (4).Harizi H; Corcuff J-B; Gualde N Trends Mol. Med 2008, 14 (10), 461–469. [DOI] [PubMed] [Google Scholar]
- (5).Lubin A; Geerinckx S; Bajic S; Cabooter D; Augustijns P; Cuyckens F; Vreeken RJ J. Chromatogr. A 2016, 1440, 260–265. [DOI] [PubMed] [Google Scholar]
- (6).Gao Y; Hou R; Liu F; Cai R; Fang L; Peng C; Qi Y Bioanalysis 2015, 7 (19), 2597–2607. [DOI] [PubMed] [Google Scholar]
- (7).Izumi Y; Aritake K; Urade Y; Fukusaki EJ Biosci. Bioeng 2014, 118 (1), 116–118. [DOI] [PubMed] [Google Scholar]
- (8).Tsikas D; Zoerner AAJ Chromatogr. B: Anal. Technol. Biomed. Life Sci 2014, 964, 79–88. [DOI] [PubMed] [Google Scholar]
- (9).Willenberg I; Ostermann AI; Schebb NH Anal. Bioanal. Chem 2015, 407 (10), 2675–2683. [DOI] [PubMed] [Google Scholar]
- (10).Takino M; Daishima S; Yamaguchi K; Nakahara TJ Chromatogr. A 2001, 928 (1), 53–61. [DOI] [PubMed] [Google Scholar]
- (11).Zehethofer N; Pinto DM; Volmer DA Rapid Commun. Mass Spectrom 2008, 22 (13), 2125–2133. [DOI] [PubMed] [Google Scholar]
- (12).Ho Y-P; Huang P-C; Deng K-H Rapid Commun. Mass Spectrom 2003, 17 (2), 114–121. [DOI] [PubMed] [Google Scholar]
- (13).Duncan KD; Volmer DA; Gill CG; Krogh ET J. Am. Soc. Mass Spectrom 2016, 27 (3), 443–450. [DOI] [PubMed] [Google Scholar]
- (14).Frenking G; Fröhlich N Chem. Rev 2000, 100 (2), 717–774. [DOI] [PubMed] [Google Scholar]
- (15).Nikolova-Damyanova BJ Chromatogr. A 2009, 1216 (10), 1815–1824. [DOI] [PubMed] [Google Scholar]
- (16).Momchilova S; Nikolova-Damyanova BJ Sep. Sci 2003, 26 (3−4), 261–270. [Google Scholar]
- (17).Nikolova-Damyanova B; Momchilova SJ Liq. Chromatogr. Relat. Technol 2002, 25 (13–15), 1947−1965. [Google Scholar]
- (18).Hand OW; Winger BE; Cooks RG Biomed. Environ. Mass Spectrom 1989, 18 (1), 83–85. [Google Scholar]
- (19).Dufresne M; Thomas A; Breault-Turcot J; Masson J-F; Chaurand P Anal. Chem 2013, 85 (6), 3318–3324. [DOI] [PubMed] [Google Scholar]
- (20).Morris LJ J. Lipid Res 1966, 7 (6), 717–732. [PubMed] [Google Scholar]
- (21).Meier F; Garrard KP; Muddiman DC Rapid Commun. Mass Spectrom 2014, 28 (22), 2461–2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Jackson AU; Shum T; Sokol E; Dill A; Cooks RG Anal. Bioanal. Chem 2011, 399 (1), 367–376. [DOI] [PubMed] [Google Scholar]
- (23).Roach PJ; Laskin J; Laskin A Analyst 2010, 135 (9), 2233–2236. [DOI] [PubMed] [Google Scholar]
- (24).Laskin J; Heath BS; Roach PJ; Cazares L; Semmes OJ Anal. Chem 2012, 84 (1), 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Lanekoff I; Thomas M; Laskin J Anal. Chem 2014, 86 (3), 1872–1880. [DOI] [PubMed] [Google Scholar]
- (26).Bergman H-M; Lundin E; Andersson M; Lanekoff I Analyst 2016, 141 (12), 3686–3695. [DOI] [PubMed] [Google Scholar]
- (27).Lanekoff I; Thomas M; Carson JP; Smith JN; Timchalk C; Laskin J Anal. Chem 2013, 85 (2), 882–889. [DOI] [PubMed] [Google Scholar]
- (28).Bergman H-M; Lanekoff I Analyst 2017, 142 (19), 3639–3647. [DOI] [PubMed] [Google Scholar]
- (29).Duncan KD; Lanekoff I Anal. Chem 2018, 90 (4), 2451–2455. [DOI] [PubMed] [Google Scholar]
- (30).Duncan KD; Bergman H-M; Lanekoff I Analyst 2017, 142 (18), 3424–3431. [DOI] [PubMed] [Google Scholar]
- (31).Cha J; Bartos A; Park C; Sun X; Li Y; Cha S-W; Ajima R; Ho H-YH; Yamaguchi TP; Dey SK Cell Rep.2014, 8 (2), 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Lanekoff I; Heath BS; Liyu A; Thomas M; Carson JP; Laskin J Anal. Chem 2012, 84 (19), 8351–8356. [DOI] [PubMed] [Google Scholar]
- (33).Reese J; Paria BC; Brown N; Zhao X; Morrow JD; Dey SK Proc. Natl. Acad. Sci. U. S. A 2000, 97 (17), 9759–9764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Hirota Y; Daikoku T; Tranguch S; Xie H; Bradshaw HB; Dey SK J. Clin. Invest 2010, 120 (3), 803–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Cha J; Bartos A; Egashira M; Haraguchi H; Saito-Fujita T; Leishman E; Bradshaw H; Dey SK; Hirota YJ Clin. Invest 2013, 123 (9), 4063–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Murphy RC Tandem Mass Spectrometry of Lipids; New Developments in Mass Spectrometry; The Royal Society of Chemistry, 2015.
- (37).Forbes GB; Lewis AM J. Clin. Invest 1956, 35 (6), 596–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Burgess JD Metal Ions in Solution; Halsted Press: Chichester, New York, 1978. [Google Scholar]
- (39).Gilroy DW; Colville-Nash PR; Willis D; Chivers J; Paul-Clark MJ; Willoughby DA Nat. Med 1999, 5, 698. [DOI] [PubMed] [Google Scholar]
- (40).Poligone B; Baldwin AS J. Biol. Chem 2001, 276 (42), 38658–38664. [DOI] [PubMed] [Google Scholar]
- (41).Lanekoff I; Cha J; Kyle JE; Dey SK; Laskin J; Burnum-Johnson KE Sci. Rep 2016, 6, 33023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Lim H; Paria BC; Das SK; Dinchuk JE; Langenbach R; Trzaskos JM; Dey SK Cell 1997, 91 (2), 197–208. [DOI] [PubMed] [Google Scholar]
- (43).Chakraborty I; Das SK; Wang J; Dey SK J. Mol. Endocrinol 1996, 16 (2), 107–122. [DOI] [PubMed] [Google Scholar]
- (44).Song H; Lim H; Paria BC; Matsumoto H; Swift LL; Morrow J; Bonventre JV; Dey SK Development 2002, 129 (12), 2879–2889. [DOI] [PubMed] [Google Scholar]
- (45).Cha J; Sun X; Dey SK Nat. Med 2012, 18, 1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Sun X; Bartos A; Whitsett JA; Dey SK Mol. Endocrinol 2013, 27 (9), 1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Wang H; Dey SK Prostaglandins Other Lipid Mediators 2005, 77 (1), 84–102. [DOI] [PubMed] [Google Scholar]
- (48).Girod M; Shi Y; Cheng J-X; Cooks RG Anal. Chem 2011, 83 (1), 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Wang H; Dey SK Nat. Rev. Genet 2006, 7, 185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.