Abstract
Background
Malaria remains an important public health problem in Peru where incidence has been increasing since 2011. Of over 55,000 cases reported in 2017, Plasmodium vivax was the predominant species (76%), with P. falciparum responsible for the remaining 24%. Nyssorhynchus darlingi (previously Anopheles darlingi) is the main vector in Amazonian Peru, where hyperendemic Plasmodium transmission pockets have been found. Mazán district has pronounced spatial heterogeneity of P. vivax malaria. However, little is known about behavior, ecology or seasonal dynamics of Ny. darlingi in Mazán. This study aimed to gather baseline information about bionomics of malaria vectors and transmission risk factors in a hyperendemic malaria area of Amazonian Peru.
Methods
To assess vector biology metrics, five surveys (two in the dry and three in the rainy season), including collection of sociodemographic information, were conducted in four communities in 2016–2017 on the Napo (Urco Miraño, URC; Salvador, SAL) and Mazán Rivers (Visto Bueno, VIB; Libertad, LIB). Human-biting rate (HBR), entomological inoculation rate (EIR) and human blood index (HBI) were measured to test the hypothesis of differences in entomological indices of Ny. darlingi between watersheds. A generalized linear mixed effect model (GLMM) was constructed to model the relationship between household risk factors and the EIR.
Results
Nyssorhynchus darlingi comprised 95% of 7117 Anophelinae collected and its abundance was significantly higher along the Mazán River. The highest EIRs (3.03–4.54) were detected in March and June in URC, LIB and VIB, and significantly more Ny. darlingi were infected outdoors than indoors. Multivariate analysis indicated that the EIR was >12 times higher in URC compared with SAL. The HBI ranged from 0.42–0.75; humans were the most common blood source, followed by Galliformes and cows. There were dramatic differences in peak biting time and malaria incidence with similar bednet coverage in the villages.
Conclusions
Nyssorhynchus darlingi is the predominant contributor to malaria transmission in the Mazán District, Peru. Malaria risk in these villages is higher in the peridomestic area, with pronounced heterogeneities between and within villages on the Mazán and the Napo Rivers. Spatiotemporal identification and quantification of the prevailing malaria transmission would provide new evidence to orient specific control measures for vulnerable or at high risk populations.
Electronic supplementary material
The online version of this article (10.1186/s13071-019-3619-0) contains supplementary material, which is available to authorized users.
Keywords: Nyssorhynchus darlingi, Blood meal source, Plasmodium, Entomological inoculation rate, Human blood index, GLMM, Mazán District, Peruvian Amazon
Background
Malaria endemic riverine communities in Loreto Department, Peruvian Amazon region, are remote and understudied [1–3]. Previous research in this area found that Nyssorhynchus darlingi (previously Anopheles darlingi, [4]) is the main malaria vector, and often the only Anophelinae species that bites humans [1, 5–7]. However, despite its anthropophilic status, Ny. darlingi feeds opportunistically, i.e. we recently reported a high proportion of avian blood meals in three villages to the south and west of Iquitos (Peru) over three years even though the human blood index (HBI) ranged from 0.57 to 0.87 [8]. This finding of high proportions of avian blood meals has implications for local Plasmodium transmission. For instance, if access to a human blood meal is blocked by a screen or a net, an avian blood meal can maintain a female Ny. darlingi by increasing daily survival, even if a meal on chicken blood is not optimal (at least in the case of An. gambiae [9]). If this female were infected with Plasmodium, the overall vectorial capacity of the Ny. darlingi population could increase slightly if the infected female’s longevity was enhanced by an avian blood meal. Of equal interest, the forage ratio, calculated by a local animal census at the time of mosquito collections, demonstrates a marked preference of Ny. darlingi for Galliformes blood compared to human or other available hosts [8]. This pattern has been attributed to both host biomass and availability [8, 10], but its effect on Plasmodium transmission remains unclear.
Nyssorhynchus darlingi usually rests outdoors (exophilic) and displays heterogeneous feeding behavior throughout its distribution [11–13]. In the Iquitos region, and more broadly, the ratio of outdoor (exophagy) to indoor feeding (endophagy) varies depending on factors such as human behavior, environment and vector control methods [2, 7, 14, 15]. These behaviors influence the entomological indices, human-biting rate (HBR), infectivity rate (IR) and entomological inoculation rate (EIR), that have been a mainstay for comparing the effectiveness of different interventions and estimating malaria risk ([16] but see [17] for novel modifications). Of these indices, the annual EIR is used most commonly to estimate malaria transmission intensity, and for Ny. darlingi, the highest EIR recorded is 360.62, in eastern Brazil [18]. In Peru the EIR ranges from 0 to 144 [1, 5, 6, 19]. An annual EIR of 1 was established as a goal for national malaria control programs in sub-Saharan Africa to achieve elimination [20]; more effort needs to be made to obtain such an index in the Neotropics for comparative and monitoring purposes.
In many localities across the Amazon, Ny. darlingi is seasonal and its abundance is linked to rainfall and fluctuating river levels [5, 6, 21]. This is most likely associated with the increased numbers and availability of temporary breeding sites suitable for Ny. darlingi, such as flooded forest and rainfall pools situated near or alongside lowland rivers [5, 21, 22]. One aspect of ecology that remains unstudied is the potential influence of different river types on diversity, abundance, breeding sites, and perhaps by extension, on Plasmodium transmission of Amazonian Anophelinae. Rivers in the Amazon Basin are classified as blackwater, clearwater or whitewater [23]. Generally, blackwater rivers have lower nutrient levels, pH and conductivity compared with whitewater rivers; such physiochemical differences influence the diversity of planktonic flora and fauna. For example, blackwater rivers have been shown to have greater numbers of rotifers but fewer crustaceans, chironomids and mites [24]. Flooded forest is also affected by the nutrient regime of contiguous rivers; thus, the physicochemical attributes of anopheline larval habitats situated on floodplains of whitewater versus blackwater rivers are likely to differ. We hypothesized that one potential contributor of seasonal anopheline diversity and abundance is river type. In the present study, we chose to quantify and compare Ny. darlingi entomological indices from two villages on the whitewater Napo River and two villages on the blackwater Mazán River. We expected that the Ny. darlingi from villages along the whitewater Napo River would demonstrate higher abundance, greater diversity, and higher entomological indices compared with similar villages along the blackwater Mazán River.
Regionally, malaria cases have increased from approximately 11,000 in 2012 to over 55,000 in 2017 [25] with Loreto Department accounting for 96.2% of all recorded cases in Peru. In 2017, Plasmodium vivax contributed 76.1% (n = 40,120) of the malaria cases followed by P. falciparum, which contributed 23.8% (n = 12,563) [25]. The main objectives of this study were to gather one year of baseline vector biology data from four hyperendemic malaria communities to determine spatiotemporal local malaria transmission factors, based on river type, blood-feeding habits, Plasmodium infectivity and local risk in relation to Ny. darlingi.
Methods
Collection sites
Mosquito collections were performed in the Mazán District (Loreto Department, Peru) located 50 km northwest of the city of Iquitos, about 40 minutes by speedboat. Salvador (SAL) and Urco Miraño (URC) are located on the Napo River and Libertad (LIB) and Visto Bueno (VIB) on the Mazán River (Fig. 1). Criteria for choice of communities were based on: (i) annual parasite index (API) > 10; (ii) human population > 50 and < 500; and (iii) location on Mazán or Napo rivers (Table 1). The most common occupations of the inhabitants are fishing, farming and timber extraction. In several Mazán riverine villages, some sector of the human population is mobile [3], seeking work or tending crops away from their village, sometimes for weeks or months at a time [1]. Only Libertad has a health post: residents in Visto Bueno travel to Libertad and those from Urco Miraño and Salvador must travel to Mazán, the largest nearby town (~ 5800 inhabitants), for medical treatment. All four communities are accessible only by boat (distance to Mazán: SAL, 9 km, ~ 45 min; URC, 15 km, ~ 2 h; LIB, 16 km, ~ 3 h; VIB, 26 km ~ 5 h; time estimated traveling with a 11-horsepower motor boat).
Fig. 1.
Map showing mosquito collection sites on the Mazán and Napo Rivers, Mazán District, Loreto Department, Amazonian Peru, 2016–2017
Table 1.
Overview characteristics of the study sites and malaria cases in Mazán District, Loreto, Peru from 2015 to 2017
| Site | River | Coordinates | Population | No. of malaria cases (Pv/Pf) | API | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Latitude S | Longitude W | 2015 | 2016 | 2017 | 2015 | 2016 | 2017 | |||
| Salvador (SAL) | Napo | 3°26′41.6″ | 73°09′18.4″ | 390 | 48 (40/8) | 107 (79/28) | 27 (15/12) | 123 | 274.3 | 69.2 |
| Urco Miraño (URC) | Napo | 3°21′39.4″ | 73°03′52.3″ | 366 | 139 (135/4) | 29 (12/17) | 4 (4/0) | 379.7 | 79.2 | 10.9 |
| Libertad (LIB) | Mazán | 3°29′48.7″ | 73°14′03.8″ | 245 | 203 (176/27) | 107 (74/33) | 144 (112/32) | 828.5 | 436.7 | 587.7 |
| Visto Bueno (VIB) | Mazán | 3°29′48.7″ | 73°19′01.7″ | 56 | 27 (23/4) | 22 (12/10) | 34 (22/12) | 482.1 | 392.8 | 607.1 |
Abbreviations: Pv, Plasmodium vivax; Pf, Plasmodium falciparum
Sociodemographic data
A population census for each community was conducted during December 2016–January 2017, with an overall 90% coverage. Each house was identified with a unique number and georeferenced using a handheld global positioning system device (Garmin International Inc., Olathe, KS, USA). In addition, a questionnaire was administered to collect baseline demographic information, health, behavioral and socioeconomic data (i.e. daily net use and coverage household structure and characteristics, travel history, sleeping habits and malaria symptoms) and information was registered on Android tablets. These data were subsequently uploaded into a database that is maintained at the Universidad Peruana Cayetano Heredia in Lima, Peru, and at the School of Public Health, Harvard, USA.
Clinical malaria cases (Plasmodium vivax and P. falciparum) were documented from malaria episodes by means of passive case detection (with history of fever within the past 24 hours ≥ 38 °C and a positive malaria thick smear after microscopic examination) at local health facilities at the village or district level from the Direccion de Salud de Loreto (DISA-LORETO) in Mazán during the time of the survey (Table 1).
Mosquito sampling
Five cross-sectional studies were conducted in four communities in 2016–2017 during the dry (2 surveys: June and September 2016) and rainy seasons (3 surveys: March and November 2016, March 2017). Each survey consisted of two nights of human landing catch (HLC) and one night of barrier screens, with a total collection effort of 15 collection nights per village. Indoor and outdoor human landing catch collections were performed (5 m from the main doorway of each house; i.e. peridomestic) for 12 h (18:00–06:00 h), one collector indoors and one collector outdoors working simultaneously, with rotation of personnel every 3 h. Each night two houses, approximately 500 m from each other, were sampled.
To intercept and capture blood-seeking mosquitoes, two barrier screens approximately 15 m long and 2 m high were placed such that the distance from the house/breeding site/resting site was 2–7 m (as described in [8]). This method was performed for one night (18:00–06:00 h) per collection, the night following the first HLC. Resting mosquitoes were sampled by manually searching the surface of the screen with a mouth aspirator every hour, and each side of the screen (facing village/forest and village/river). For both methods, the mosquitoes collected were initially morphologically identified by trained personnel using the available taxonomic keys [26–28]. Mosquitoes were individually stored in microcentrifuge tubes with silica gel in the laboratory in Iquitos at − 20 °C.
To identify and quantify animal hosts (potential sources of blood meals) an animal census was conducted in each community in November 2016 and used to calculate the forage ratio for Ny. darlingi. All households were surveyed by consulting residents about presence and number of their domestic animals (dog, cat, chicken, turkey, pig, cow and duck) and observation of any feral animals (Additional file 1: Table S1).
Laboratory procedures
A subsample of specimens from HLC and barrier screen collections that could not be identified morphologically (cryptic Nyssorhynchus species, damaged specimens, etc.) was identified using ITS2-PCR-RFLP [29], or by sequencing the BOLD region of cox1 [30] and querying against the BOLD Identification System (http://www.boldsystems.org) or GenBank (https://www.ncbi.nlm.nih.gov/genbank). To detect the source of a blood meal, total genomic DNA of each mosquito specimen was extracted from the abdomen using the DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). Cytochrome b PCR-RFLP assays were performed to identify mammalian blood meal hosts (human, cow, pig and dog) [31]. In addition, Galliformes [32], rat and monkey hosts were included in the analysis [33, 34]. Results were visualized in agarose gel and no sequencing was performed for any sample.
Heads and thoraces from specimens were analyzed in pools of 3–6 individuals (same collection site/time) to detect P. vivax (PV247-PV210) and P. falciparum using an enzyme-linked immunosorbent assay (ELISA) [35]. ELISA kits (MR-1028K) were obtained from BEI Resources, NIAID, NIH and Plasmodium vivax Sporozoite ELISA Reagent Kit, MRA-1028K, contributed by Robert A. Wirtz. Female Ny. darlingi (colony specimens) were used as negative controls and female Ny. darlingi infected with P. vivax as positive controls. Optical density was measured at 410 nm in a Bio-Rad ELISA plate reader (Bio-Rad, Hercules, CA, USA) 60 min after addition of the substrate. The cut-off for positive samples was determined by multiplying the average OD (negative controls) by twice (2×) the negative control for each plate. Fifty-five mosquito samples, that were at or near the limit of detectability, were retested individually by real-time PCR following the protocol in Bickersmith et al. [36].
Data analysis
Linking human census and malaria case data
Clinical malaria records from the passive case detection of the Ministry of Health (MoH) were linked with the study dataset to identify malaria episodes in our study participants. The patients’ names were the only variable shared between the study database and the malaria notification system. Since names may have been misspelled and incorrectly entered, a normalized extension of the Generalized Levenshtein Distance was used [37, 38]. This is defined as the minimum cost of transforming one string into another through a sequence of weighted edit operations following the equation
An entry from the malaria notification system was associated with an individual from the census database if both have names with normalized GLD less or equal than 0.1. Moreover, to include multiple misspellings on the malaria notification system database, all entries were associated with the same individual if they have the same normalized GLD.
Entomological indicators
Human-biting rate (HBR) was calculated as the average number of Ny. darlingi bites per collector per hour. Infection rate (IR) is the proportion of Ny. darlingi that was determined to be Plasmodium positive. Parity was measured in only 40 specimens and the sample size was too small for results to be meaningfully included. These 40 individuals were used in the calculation of HBR but not tested for Plasmodium and not used to calculate IR/EIR. For calculation of entomological indices, specimens of mixed infections with P. vivax and P. falciparum were added to totals of each of these parasites [39]. The entomological inoculation rate (EIR) was calculated by multiplying the HBR (monthly) and IR. These indices were calculated for each collection (month) for each of the four communities. The human blood index (HBI) was calculated as the proportion of mosquitoes fed on a specific host divided by the number of mosquitoes analyzed (mixed blood meals were added to totals of each host) as in [8]. To test for significance between human and non-human blood meals in Ny. darlingi among seasons and localities we employed Chi-square analysis. To quantify blood meal source, host data recorded in the census were used to calculate the forage ratio (wi) [40, 41] and selection index (Bi) [42]. The weight of each blood meal source was determined following Moreno et al. [8]. We employed the R bipartite package [43] to generate a host vector quantitative interaction network for the four communities as in Moreno et al. [8].
Statistical analysis
Fisher’s exact test was used for significance testing of categorical factors for each Plasmodium spp. To assess significant differences in EIR, a generalized linear mixed effect model (GLMM) was used to handle the nested structure of sampled data: two locations (intra- and peridomestic) sampled per survey, nested within 16 households. A GLMM assuming a negative binomial distribution for the error term, and a log link function was constructed to model the relationship between household risk factors and the EIR. We estimated an incidence rate ratio (IRR) for the association between EIR and intra- and peridomestic collection, study area, number of inhabitants per household, inhabitants/room ratio, whether family was recently settled in the community, number of bednets per household, years since last bednet impregnation, and electricity supply. Statistical significance was defined as a P-value < 0.05 and 95% confidence intervals (CI) were estimated as appropriate. Factors with P-values < 0.2 for the Wald test in the univariate analysis were included in the multivariate model. Using a backward stepwise process, the final model retained all factors that were significantly associated with EIR. Interactions were systematically checked for up to order two. Likelihood ratio tests (LRTs) were used to assess statistical differences between nested models. All data analyses were conducted in STATA v.15.1 (StataCorp, College Station, TX, USA).
Results
Characteristics of the study sites and association with malaria cases
From December 2016 to January 2017 a census was conducted in the four sites of the survey with a coverage of 90% across the study sites. Over 96% of the households in the communities have some kind of bednet, either purchased locally or distributed in 2016 by DIRESA-LORETO (long-lasting insecticide nets, LLINs) (Table 2). Malaria cases reported differed by study site for both P. vivax and P. falciparum, with the highest case numbers in SAL and LIB. Occupation as a housewife or student and having electricity were associated only with P. vivax malaria (P = 0.003 and P = 0.103, respectively) whilst pona (palm Iriartea deltoidea, Family Arecaceae) vs wood wall material was associated with P. falciparum (P = 0.034). Neither P. vivax nor P. falciparum infection was associated with gender or age, education level, dwelling roof material, or ownership of bednets (LLINs and non-treated) (Table 3).
Table 2.
Bednet coverage by site. Data from population census in December 2016 to January 2017
| Site | River | Only Tocuyo neta | At least one LLIN | Total | No. of households |
|---|---|---|---|---|---|
| Salvador (SAL) | Napo | 9 (13.4%) | 58 (86.6%) | 100% | 67 |
| Urco Miraño (URC) | Napo | 9 (18.7%) | 39 (81.3%) | 100% | 48 |
| Libertad (LIB) | Mazán | 10 (18.5%) | 42 (77.8%) | 96.3% | 54 |
| Visto Bueno (VIB) | Mazán | 3 (20.0%) | 12 (80.0%) | 100% | 15 |
aNon-impregnated bednets locally made of woven cotton fabric
Table 3.
Baseline characteristics of the study population and its association with P. vivax and P. falciparum malaria cases
| Characteristics | P. vivax | P. falciparum | ||||
|---|---|---|---|---|---|---|
| No. positive (%) (n = 42) |
No. negative (%) (n = 808) |
P-value | No. positive (%) (n = 35) |
No. negative (%) (n = 878) |
P-value | |
| Study area | 0.001** | 0.008** | ||||
| Salvador | 20 (47.6) | 282 (34.9) | 11 (61.1) | 291 (35.0) | ||
| Urco Miraño | 3 (7.1) | 246 (30.4) | 0 (0.0) | 249 (29.9) | ||
| Libertad | 18 (42.9) | 227 (28.1) | 6 (33.3) | 239 (28.7) | ||
| Visto Bueno | 1 (2.4) | 53 (6.6) | 1 (5.6) | 53 (6.4) | ||
| Sex | 0.636 | 0.815 | ||||
| Female | 22 (52.4) | 388 (48.0) | 8 (44.4) | 402 (48.3) | ||
| Male | 20 (47.6) | 420 (52.0) | 10 (55.6) | 430 (51.7) | ||
| Age group (years) | 0.781 | 0.456 | ||||
| <10 | 15 (35.7) | 259 (32.0) | 4 (22.2) | 270 (32.5) | ||
| 10–29.9 | 11 (26.2) | 255 (31.6) | 8 (44.4) | 258 (31.0) | ||
| ≥30 | 16 (38.1) | 294 (36.4) | 6 (33.3) | 304 (36.5) | ||
| Education | 0.343 | 0.682 | ||||
| None | 11 (26.2) | 223 (27.6) | 4 (22.2) | 230 (27.6) | ||
| Primary school | 27 (64.3) | 440 (54.5) | 12 (66.7) | 455 (54.7) | ||
| Secondary school or higher | 4 (9.5) | 145 (17.9) | 2 (11.1) | 147 (17.7) | ||
| Occupation (>18 years-old) | 0.003** | 0.254 | ||||
| Forest related | 5 (11.9) | 260 (32.2) | 9 (50.0) | 256 (30.7) | ||
| Housewife/student | 32 (76.2) | 409 (50.6) | 7 (38.9) | 434 (52.2) | ||
| Others | 5 (11.9) | 139 (17.2) | 2 (11.1) | 142 (17.1) | ||
| Dwelling wall materiala | 1.000 | 0.034** | ||||
| Wood | 35 (92.1) | 630 (92.4) | 13 (76.5) | 652 (92.7) | ||
| Pona and other related | 3 (7.9) | 52 (7.6) | 4 (23..5) | 51 (7.3) | ||
| Dwelling roof material | 0.745 | 0.321 | ||||
| Tin (calamina) | 14 (33.3) | 293 (36.3) | 4 (22.2) | 303 (36.4) | ||
| Palm leaves | 28 (66.7) | 515 (63.7) | 14 (77.8) | 529 (63.6) | ||
| Electricity | 0.103* | 0.276 | ||||
| Yes | 16 (38.1) | 207 (25.6) | 11 (61.1) | 616 (74.0) | ||
| No | 26 (61.9) | 601 (74.4) | 7 (38.9) | 216 (26.0) | ||
| Impregnated bednets | 0.381 | 0.336 | ||||
| Yes | 38 (90.5) | 680 (84.2) | 17 (94.4) | 701 (84.3) | ||
| No | 4 (9.5) | 128 (15.8) | 1 (5.6) | 131 (15.7) | ||
*P < 0.2, **P < 0.05; Fisher’s exact test
aFactor with missing values
Species composition and biting behavior
Overall, for both HLC and barrier screen methods, 7117 female Anophelinae specimens were collected in the five surveys in the four sites (Table 4). By HLC, 6365 Anophelinae were captured: VIB (n = 3530); Libertad (n = 2083); Urco Miraño (n = 679); and Salvador (n = 73). Nyssorhynchus darlingi Root, 1926, was the most abundant species in each of the four communities (99% in Visto Bueno; 96% in Libertad; 78% in Urco Miraño; and 67% in Salvador). Other Anophelinae species identified were Ny. nuneztovari Gabaldon, 1940 (s.l.), Ny. oswaldoi Peryassú, 1922 (s.l.), Ny. benarrochi Gabaldon, Cova-Garcia & Lopez, 1941 B, Ny. rangeli Gabaldon Cova-Garcia & Lopez, 1940, Ny. dunhami Causey, 1945, Ny. triannulatus Neiva & Pinto, 1922, Ny. sp. nr. konderi, An. mattogrossensis Lutz & Neiva, 1911, An. forattinii Wilkerson & Sallum, 1999, An. costai Fonseca & Silva Ramos, 1939, An. forattinii/An. costai, An. sp. nr. forattinii, and several specimens identified only to genus level (Table 4). In general, species richness was lower for HLC than barrier screen in all communities, and it was near-equal for the Mazán River communities (n = 8) vs those along the Napo River (n = 10).
Table 4.
Nyssorhynchus and Anopheles species identification and composition in SAL, URC, LIB, and VIB communities, Mazán District, Peru in 2016–2017 by HLC and barrier screen mosquito collection methods
| Site | Year | Month | Method | |
|---|---|---|---|---|
| HLC (n) | Barrier screen (n) | |||
| SAL | 2016 | March | Ny. darlingi (2) | |
| Nyssorhynchus spp. (2) | ||||
| An. forattinii/An. costai (1) | ||||
| Anopheles spp. (1) | ||||
| June | Ny. darlingi (60) | Ny. darlingi (10) | ||
| Ny. nuneztovari (s.l.) (1) | ||||
| Nyssorhynchus spp. (4) | Nyssorhynchus spp. (14) | |||
| forattinii/An. costai (1) | ||||
| Anopheles spp. (7) | ||||
| Ny. benarrochi B (2) | ||||
| September | Ny. darlingi (2) | |||
| Nyssorhynchus spp. (3) | ||||
| Total (n) | 73 | 37 | ||
| URC | 2016 | March | Ny. darlingi (15) | |
| Ny. oswaldoi (s.l.) (1) | ||||
| An. forattinii/An. costai (1) | ||||
| June | Ny. darlingi (487) | Ny. darlingi (133) | ||
| An. forattinii/An. costai (3) | ||||
| An. sp. nr. forattinii (1) | An. sp. nr. forattinii (1) | |||
| An. mattogrossensis (1) | ||||
| Nyssorhynchus spp. (133) | Nyssorhynchus spp. (24) | |||
| Anopheles spp. (8) | ||||
| Ny. triannulatus (s.l.) (1) | ||||
| Ny. rangeli (1) | ||||
| Ny. dunhami (2) | ||||
| Ny. sp. nr. konderi (1) | ||||
| September | Ny. darlingi (18) | |||
| Nyssorhynchus spp. (1) | ||||
| 2017 | February | Ny. darlingi (15) | Ny. darlingi (1) | |
| Nyssorhynchus spp. (1) | Nyssorhynchus spp. (1) | |||
| Anopheles spp. (2) | ||||
| Total (n) | 679 | 173 | ||
| LIB | 2016 | March | Ny. darlingi (188) | Ny. darlingi (8) |
| Ny. dunhami (1) | ||||
| Ny. oswaldoi (s.l.) (1) | ||||
| An. sp. nr. forattinii (4) | ||||
| An. forattinii/An. costai (1) | ||||
| Nyssorhynchus spp. (74) | ||||
| June | Ny. darlingi (1439) | Ny. darlingi (101) | ||
| An. forattinii/An. costai (2) | ||||
| An. mattogrossensis (1) | ||||
| Anopheles spp. (1) | ||||
| Nyssorhynchus spp. (4) | ||||
| September | Ny. darlingi (72) | Ny. darlingi (3) | ||
| Nyssorhynchus spp. (1) | ||||
| November | Ny. darlingi (4) | |||
| 2017 | February | Ny. darlingi (294) | ||
| Nyssorhynchus spp. (1) | ||||
| Total (n) | 2083 | 117 | ||
| VIB | 2016 | March | Ny. darlingi (370) | Ny. darlingi (4) |
| Ny. oswaldoi (s.l.) (2) | ||||
| Ny. benarrochi B (1) | ||||
| Nyssorhynchus spp. (13) | ||||
| forattinii/An. costai (2) | ||||
| An. sp. nr. forattini (1) | ||||
| Anopheles spp. (1) | ||||
| June | Ny. darlingi (2730) | Ny. darlingi (406) | ||
| September | Ny. darlingi (141) | Ny. darlingi (12) | ||
| Nyssorhynchus spp. (1) | Nyssorhynchus spp. (1) | |||
| November | Ny. darlingi (3) | |||
| forattinii (1) | ||||
| 2017 | February | Ny. darlingi (264) | Ny. darlingi (1) | |
| Nyssorhynchus spp. (1) | ||||
| Total (n) | 3530 | 425 | ||
There were more Anophelinae collected consistently in the peridomestic area (n = 5024; 78.9%) compared with inside houses (n = 1341; 21.1%) in all communities, and more biting occurred prior to midnight (Fig. 2 and Additional file 1: Table S2), with 56% of all mosquitoes collected from 18:00 to 22:00 h. In SAL there were two outdoor peaks and one indoors before 23:00 h. In URC, the peak biting time was earlier outdoors (21:00 h) than indoors (23:00 h). In LIB, there were no real biting peaks and in VIB most outdoor biting occurred between 21:00 and 23:00 h and indoor biting was uninterrupted until 3:00 h.
Fig. 2.
Mean indoor (intradomestic) and outdoor (peridomestic) human-biting rate at four communities in the Mazán District 2016–2017. a Salvador (SAL). b Urco Miraño (URC). c Libertad (LIB). d Visto Bueno (VIB). No specimens were collected in November and March 2017 in Salvador or in November 2016 in Urco Miraño. Right Y-axis is the human-biting rate. Grey background shading represents the proportion of the human population under bednets (left Y-axis) each hour of the night (X-axis)
Plasmodium detection in mosquitoes and entomological indices
All Ny. darlingi samples collected by HLC were tested in pools of 3–5 specimens using ELISA, and calculations were based on the conservative assumption of n = 1 infected specimen per pool. The overall infection rate (IR) was 1.6% (91/5870 specimens of Ny. darlingi tested) and this varied among communities: Libertad, 27/1895 (1.4% IR); Visto Bueno, 50/3394 (1.5% IR), Urco Miraño, 13/519 (2.5% IR); and Salvador, 1/62 (1.6% IR) were positive (Table 5). Plasmodium vivax V210 was the most frequent variant in 62 pools (SAL = 0, URC = 6, LIB = 12, VIB = 44), followed by P. falciparum in 19 pools (SAL = 0, URC = 2, LIB = 12, VIB = 5) and P. vivax V247 in 10 pools (SAL = 1, URC = 5, LIB = 3, VIB = 1). Seven pools included specimens with mixed infections.
Table 5.
Infection rate (IR), human-biting rate (HBR) and entomological inoculation rate (EIR) by month in four Mazán District communities between 2016 and 2017; Napo River (SAL and URC) and Mazán River (LIB and VIB)
| Collection date | IR | HBR | EIR | IR | HBR | EIR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| In | Out | In ± SE | Out ± SE | In | Out | In | Out | In ± SE | Out ± SE | In | Out | |
| SAL | URC | |||||||||||
| Mar 2016 | 0 | 0 | 0.3 ± 0.3 | 1.0 ± 1.0 | 0 | 0 | 0 | 0 | 1.0 ± 1.3 | 3.0 ± 2.0 | 0 | 0 |
| Jun 2016 | 0.03 | 0 | 8.3 ± 5.8 | 8.3 ± 3.8 | 0.3 | 0 | 0.01 | 0.03 | 37.3 ± 24.3 | 119 ± 37.5 | 0.3 | 3.60 |
| Sep 2016 | 0 | 0 | 0.5 ± 0.5 | 0 | 0 | 0 | 0 | 0 | 0.8 ± 0.3 | 4.0 ± 0 | 0 | 0 |
| Nov 2016 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mar 2017 | 0 | 0 | 0 | 0 | 0 | 0 | 0.14 | 0 | 1.8 ± 0.8 | 2.8 ± 0.3 | 0.3 | 0 |
| LIB | VIB | |||||||||||
| Mar 2016 | 0.02 | 0.02 | 34.0 ± 26.0 | 33.3 ± 4.7 | 0.8 | 0.72 | 0.02 | 0.07 | 29.8 ± 3.3 | 67.5 ± 6.0 | 0.58 | 4.54 |
| Jun 2016 | 0.03 | 0.01 | 65.8 ± 9.25 | 294.8 ± 107.8 | 2.3 | 3.52 | 0.03 | 0.01 | 114.3 ± 14.8 | 568.3 ± 12.8 | 3.05 | 3.03 |
| Sep 2016 | 0.00 | 0.02 | 4.3 ± 1.3 | 14.0 ± 2.0 | 0 | 0.34 | 0 | 0.04 | 4.5 ± 1 | 31.0 ± 14.5 | 0 | 1.19 |
| Nov 2016 | 0.00 | 0.25 | 0 | 1.0 ± 1.0 | 0 | 0.25 | 0 | 0 | 0.3 ± 0.3 | 0.8 ± 0.7 | 0 | 0 |
| Mar 2017 | 0.00 | 0 | 19.3 ± 11.3 | 54.5 ± 14.0 | 0 | 0.00 | 0.02 | 0.01 | 13.3 ± 4.8 | 53 ± 8.5 | 0.28 | 0.29 |
Abbreviation: SE, standard error
HBR, calculated only for Ny. darlingi, presented a similar trend in all communities and peaked in June 2016 (Table 5). In Visto Bueno, HBR ranged from 0.3 to 568.3 b/p/n; in Libertad, from 0 to 294.8 b/p/n; in Urco Miraño, from 0 to 119 b/p/n; and in Salvador, from 0 to 8.3 b/p/n. The distribution of infected mosquitoes across the 12 h collections varied by month and locality. More were captured before 24:00 h, i.e. 55/91. The highest EIRs were detected in March and June, mostly outdoors (Table 5). According to the 2016 census, bednet use was ~100% and most community inhabitants were virtually covered between 19:00 and 4:00 h (with either LLINs or untreated bednets). Bednet coverage was very high and similar in all communities (Table 2, Fig. 2).
Risk factors for malaria transmission
Among the study sites, the multivariate analysis indicated that the EIR was >12 times higher in URC (IRR: 12.49; 95% CI: 0.65–238.1; P = 0.093), > 26 in LIB (IRR: 26.61; 95% CI: 1.47–480.6, P = 0.026) and > 56 times higher in VIB (IRR: 56.46; 95% CI: 3.19–997.8; P = 0.006) compared with SAL (Table 6). The EIR was >2-fold higher in the peridomestic vs intradomestic area in both uni- (IRR: 2.42; 95% CI: 1.23–4.76; P = 0.010) and multivariate models (IRR: 2.46; 95% CI: 1.23–4.89; P = 0.010). Recent settlement in the community, electricity and time of the last bednet insecticide impregnation were associated with a higher risk of EIR but these factors were not retained in the multivariate model (Table 6).
Table 6.
Fixed effects of univariate and multivariate multilevel negative binomial models of the entomological inoculation rate (EIR). Data analysis is based on census in December 2016–January 2017 and entomological data incorporated from the 5 surveys starting March 2016 (12 month period)
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| IRR | 95% CI | P-value | IRR | 95% CI | P-value | |
| Null model | <0.001 | |||||
| Constant | 0.08 | 0.02–0.33 | <0.001 | 0.003 | 0.0001–0.06 | |
| Study area (ref = Salvador) | ||||||
| Libertad | 26.50 | 1.46–479.4 | 0.027** | 26.61 | 1.47–480.6 | 0.026** |
| Urco Miraño | 12.62 | 0.66–240.8 | 0.092* | 12.49 | 0.65–238.1 | 0.093* |
| Visto Bueno | 56.32 | 3.18–997.1 | 0.006** | 56.46 | 3.19–997.8 | 0.006** |
| Location (ref = intradomestic) | ||||||
| Peridomestic | 2.42 | 1.23–4.76 | 0.010** | 2.46 | 1.23–4.89 | 0.010** |
| No. of inhabitants per household | 0.91 | 0.72–1.16 | 0.455 | |||
| Inhabitants/room ratio | 0.89 | 0.67–1.17 | 0.398 | |||
| Recent settlement in community (ref = no) | ||||||
| Yes | 0.26 | 0.04–1.81 | 0.172* | |||
| No. of bednets | 0.95 | 0.64–1.39 | 0.785 | |||
| Years since last bednet impregnation (ref = never) | ||||||
| One year | 0.32 | 0.10–1.10 | 0.07* | |||
| Two years | 0.45 | 0.12–1.68 | 0.233 | |||
| Electricity (ref = no) | ||||||
| Yes | 4.66 | 1.93–11.24 | 0.001** | |||
Mixed-effects negative binomial models, with random intercepts, Wald Test P-value, *P < 0.2, **P < 0.05
Abbreviations: IRR, incidence rate ratio; CI, confidence interval
Blood meal source identification
Eight different species of Anophelinae were collected on the barrier screens (n = 752) (Table 4). Nyssorhynchus darlingi (n = 679) was the most abundant, followed by Ny. dunhami (n = 2), Ny. benarrochi B (n = 2), Ny. rangeli (n = 1), An. sp. nr. forattinii (n = 1), Ny. triannulatus (s.l.) (n = 1), Ny. konderi (n = 1), Anopheles (Anopheles) spp. (n = 18) and several damaged specimens that could not be identified (n = 47) (Table 4). Nearly all mosquitoes (95%) examined during this study were captured in June 2016 (716/752); the lowest monthly capture was November 2016. More Anophelinae (n = 415) were captured on the village-forest barrier screen compared with the village-breeding barrier screen (n = 337). Because mosquito abundance in June accounted for > 95% of all the mosquitoes collected, data analyses comparing abundance among months or seasons were not performed. Regarding screen side, 41.2% of the mosquitoes were captured on the village side, 29.7% on the forest side and 28.9% on the breeding/river side (Additional file 1: Figure S1). Abundance was highest from 21:00 to 24:00 h (n = 344), and then evenly distributed among the remaining three time periods: 18:00–21:00 h (n = 184); 24:00–3:00 h (n = 128) and 3:00–6:00 h (n = 96). Only 6.8% of the Anophelinae were determined by visual inspection to be blood-fed.
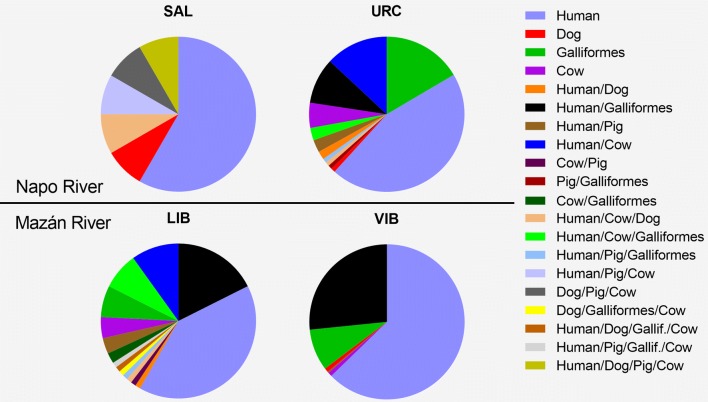
Blood meal source was determined for 699 Ny. darlingi as follows: VIB (n = 425); URC (n = 143); LIB (n = 107); and SAL (n = 24) (Table 7). Single-host blood meals accounted for the highest percentage of those identified (68.5%; 394/575) and humans were the most common source of blood (81%; 320/394). This was followed by Galliformes (14.2%; 56/394), cows (3.3%; 13/394) and dogs (1.2%; 5/394); blood meal source could not be identified for 18% of the samples. Multiple blood meals were found in 181 mosquitoes (31.5%). Double feeds (n = 160) were distributed among three communities: (VIB = 96, LIB = 32 and URC = 32). Triple feeds were found in LIB (n = 10), URC (n = 5) and SAL (n = 3), and quadruple feeds (n = 3) only in LIB and SAL (Table 7). There were significant differences in the proportion of human blood meals among the four sites (χ2 = 23.9, df = 3, P < 0.0001), with a higher proportion of human blood meals in VIB than in the other three sites (0.75 in VIB, 0.72 in LIB, 0.62 in URC and 0.42 in SAL). Forage ratio A (based on number of animals) was highest for humans only in VIB. In URC and LIB, it was highest for cows and in SAL for pigs. In contrast, forage ratio B (based on biomass calculated by multiplying the estimated weight of each animal by its abundance according to the animal census) was highest for Galliformes in URC, LIB and VIB, and for cows in SAL (Additional file 1: Table S3). The quantitative interaction network of blood meal source by locality (Fig. 3) supported patterns of organization based on the trophic preference (human, chicken, cow, pig and dog) from the four mosquito populations (SAL, URC, LIB and VIB). Figure 4 shows the proportion of all single and multiple blood meals in each community.
Table 7.
Summary of blood meal sources identified in the abdomen of Nyssorhynchus darlingi collected by barrier screens
| Host blood meal | Site | |||
|---|---|---|---|---|
| SAL | URC | LIB | VIB | |
| Single blood meals | ||||
| Human | 7 | 52 | 37 | 224 |
| Dog | 1 | 1 | – | 3 |
| Galliformes | – | 19 | 6 | 31 |
| Cow | – | 6 | 4 | 3 |
| Total | 8 | 78 | 47 | 261 |
| Mixed blood meals | ||||
| Human/dog | – | 2 | 1 | – |
| Human/Galliformes | – | 11 | 16 | 95 |
| Human/pig | – | 3 | 3 | 1 |
| Human/cow | – | 15 | 9 | – |
| Cow/pig | – | – | 1 | – |
| Pig/Galliformes | – | 1 | – | – |
| Cow/Galliformes | – | – | 2 | – |
| Human/cow/dog | 1 | 1 | 1 | – |
| Human/cow/Galliformes | – | 3 | 7 | – |
| Human/cow/pig | 1 | – | – | – |
| Human/pig/Galliformes | – | 1 | 1 | – |
| Dog/pig/cow | 1 | – | – | – |
| Dog/Galliformes/cow | – | – | 1 | – |
| Human/dog/Galliformes/cow | – | – | 1 | – |
| Human/pig/Galliformes/cow | – | – | 1 | – |
| Human/dog/pig/cow | 1 | – | – | – |
| Total | 4 | 37 | 44 | 96 |
| Not identified | 12 | 28 | 16 | 68 |
| Total | 24 | 143 | 107 | 425 |
Fig. 3.

Quantitative interaction network of Ny. darlingi blood meal sources in SAL, URC, LIB and VIB, 2016–2017. Network is constructed based on blood meal source analysis for 622 Ny. darlingi females
Fig. 4.
Proportion of blood meal sources based on Ny. darlingi collected by barrier screens in Mazán District between 2016 and 2017
Based on the present study, we found no support for the hypothesis that there would be greater abundance, higher species richness, and higher entomological indices in the communities along the whitewater Napo River (SAL, URC) compared with the blackwater Mazán (LIB, VIB). In fact, the trends were nearly opposite: abundance and entomological indices were higher in the Mazán River communities, and species richness was nearly identical for Anophelinae along the two river systems. There are many environmental variables that influence these aspects of the vector biology of Ny. darlingi, but either river type is not one of these, or we did not sample an adequate number of populations, or not for long enough, to determine any effect.
Discussion
Entomological surveillance is imperative to characterize malaria transmission patterns that will lead to planning adequate vector control measures in a specific context. Overall, this study reflects the behavioral heterogeneity of Ny. darlingi for biting pattern, location and feeding choices at a microgeographical scale. Remarkably, our findings show that EIR is significantly higher in the peridomestic area (exophagic mosquitoes) in all study sites, but particularly elevated in the Mazán River micro-basin. Contrary to our hypothesis, our study shows that the present study sites (villages) located on the bank of blackwater rivers have higher mosquito abundance and EIR than those on whitewater rivers. Characterization of the biotic and abiotic factors that determine the distribution of Anophelinae larval habitats in both watersheds will provide a more comprehensive malaria transmission scenario. For instance, Prussing et al. [44] have outlined some components correlated with aquatic larval habitats of Ny. darlingi in the peri-Iquitos area, such as low forest coverage, low sunlight exposure and emergent vegetation.
Our findings support those of a prior study that revealed Ny. darlingi to be the predominant Anophelinae species in the Mazán and Napo river communities [1]. The abundance of this species was higher along the Mazán River compared with the Napo River regardless of seasonality. More specifically, the HBR in Visto Bueno was two-fold higher than in Libertad (same river), and 4-fold and 68-fold higher than in Urco Miraño and Salvador, respectively, both on the Napo River. The HBR varied by season, with the highest value detected in June in all localities. This contrasts with the Ny. darlingi peak in other communities in peri-Iquitos, for example in Lupuna, Cahuide and Villa del Buen Pastor, where the highest HBR was reported in March-April for the same mosquito species [6]. Because mosquito abundance in this ecological scenario is more related to river levels than to precipitation [5–7], differences may be explained due to the different watersheds under observation [i.e. Mazán and Napo rivers in the present study compared to the Itaya and Nanay rivers (closer to Iquitos) in the above-mentioned studies].
In general, Ny. darlingi prefers to bite outdoors independently of the site, although the outdoor:indoor ratio varies slightly and it decreases in March, probably when mosquitoes are seeking shelter during the rainy season [7, 13] or due to differences in microclimatic conditions inside the houses [45–47]. Outdoor feeding behavior has been described commonly in Ny. darlingi in the region [5, 6, 48], but a recent trend of increased feeding inside houses has been reported [7] possibly because of a combination of efficacy-loss of LLINs distributed by the government and a reduction of IRS for the last five years in the region. In the present study sites, bednet coverage was high (over 77% of the population covered by LLINs) and a high proportion of individuals would be protected when using them during the night. Salvador was the only site with a similar proportion of mosquitoes biting inside and outside the houses, although mosquito sample size was very low. We acknowledge that bednet ownership and population coverage data variables are not sufficient to estimate the accessibility of mosquitoes to humans during the night time and other sociodemographic characteristics such as bednet use, vulnerable population sleeping under a bednet (children under five years-old) and malaria knowledge should be integrated in a more comprehensive study [49]. For instance, in an observational study in the rural communities of the Peruvian Amazon [50], nets were lifted a mean of 6.1 times per night. The authors conclude that this bednet use pattern may contribute to residual transmission.
The mosquito biting pattern was variable among river basins, communities and biting location. Most of the outdoor bites occurred before 23:00 h, with a peak around 9:00 h, except SAL that had a bimodal pattern (19:00 and 22:00 h). Indoor biting also differed among sites with unimodal (SAL and LIB) and bimodal (VIB and URC) patterns. The Plasmodium infection rate was higher in mosquito populations from the Mazán River (VIB, LIB) compared to the Napo River in specimens collected inside and outside. The EIR was also higher in VIB and LIB, with higher values outdoors than indoors and within a range comparable to previous studies in the area [6, 48]. This reinforces the idea that, at least in these two communities, malaria transmission occurs also inside the houses.
The combination of a low HBR and almost zero EIR in Salvador with a considerable number of malaria cases, raises the question of where and when the actual transmission is occurring. This study did not explicitly record all the population activities or collect mosquitoes other than in the peridomestic area or inside the houses. We believe that infective mosquito bites may take place in areas where people work or perform other activities such as bathing in creeks or social activities such as playing soccer or watching TV. Our statistical multivariate model supports this premise, estimating up to 56 times higher EIR in the peridomestic area than indoors; therefore, individuals who expend more time outdoors during malaria vector peak activity constitute a high-risk group and specific mosquito feeding deterrent measures might be considered. In line with this, a multivariate analysis detected higher P. vivax prevalence related to occupation (loggers, fishermen and farmers) from URC and Gamitanacocha (Mazán River), reinforcing the idea that some malaria transmission might occur at some distance from the village sites [3]. Spatiotemporal identification and quantification of the prevailing malaria transmission would provide new evidence to orient specific control measures for vulnerable or at high risk populations [51, 52].
Some heterogeneities in biting behavior might be explained by intrinsic characteristics of the mosquito populations, such as genetic differentiation as a result of changed biting behavior. Two genetically distinct subpopulations of Ny. darlingi have been identified within the Iquitos area associated with habitat ecological characteristics (riverine vs highway) [48]. These subpopulations also presented variation in biting activity time and HBR estimates. In contrast, regarding biting behavior (exo-endophagic and biting time), Ny. darlingi appears to constitute a genetically homogeneous population [7]. Genetic characterization of the sampled mosquito populations was beyond the aim of our study and was not performed but further analysis might provide insightful information.
Our findings support the efficacy of the barrier screen methodology to intercept mosquito specimens suitable for blood meal identification analysis in this region [8]. The higher species richness composition detected with barrier screens compared with HLC suggests that other Anophelinae aside from Ny. darlingi are present at the time of the survey that were not collected using human baits. In summary, HBI was higher along the Mazán River than the Napo River, with VIB demonstrating the highest and SAL the lowest, with values similar to those described previously in the Peruvian Amazon and for Ny. darlingi [8]. In agreement with Moreno et al. [8], Galliformes, of non-human hosts, were the most common mosquito choice except in SAL, where cows were preferred (although in SAL, a few mosquitoes were collected by HCL or barrier screens). Additionally, blood meal sources were more diverse in URC and LIB than in the other two communities. In a longitudinal study in the area, bivariate models for P. vivax parasitemia identified a higher P. vivax prevalence associated with livestock in dwellings in URC [3]. In our study, the forage ratio estimation (after biomass adjustment) in this site detected cows as the second most common host, after Galliformes followed by humans. Therefore, heterogeneities in blood meal mosquito preferences and/or host availability may have an impact on the complexity of malaria transmission patterns in the area.
An association between housing structures (wall material) was detected for P. falciparum cases but not for P. vivax. In contrast, higher P. vivax parasite prevalence was recorded in houses with walls made of palm leaf or straw in Gamitanacocha (Mazán River) [3]. Even though most of the mosquitoes bit outdoors, we detected infected mosquitoes collected inside the dwellings in the Mazán River sites; therefore, housing improvements might be recommended in addition to the current vector control strategies [53, 54].
Conclusions
Our research revealed heterogeneity in malaria transmission and vector bionomics at a microgeographical scale and that the peridomestic area in these communities contributes substantially to maintain Plasmodium transmission in the Peruvian Amazon. Even with elevated coverage and use of LLINs there is a gap in malaria control that needs to be addressed in the current elimination era. Specific strategies based on behavioral aspects of Neotropical malaria vectors and the human population should be developed and tested for their efficacy in these settings.
Additional file
Additional file 1: Table S1. Animal host census in SAL, URC, LIB and VIB, Mazan district, Peru, performed in November 2016. Table S2. Summary of Anophelinae specimens collected indoors/outdoors (peridomestic). Table S3. Forage ratio (wi) and host selection index (Bi) of Ny. darlingi in Mazán District sites between 2016 and 2017. Figure S1. Number of Anophelinae mosquitoes collected with barrier screen by position in Salvador (SAL), Urcomiraño (URC), Libertad (LIB) and Visto Bueno (VIB). Abbreviations: V-F, village-forest; V-BS, village-breeding site.
Acknowledgements
We thank all study participants and the local authorities of Salvador, Urco Miraño, Libertad and Visto Bueno for their enthusiastic support and all field workers (David Arimuya, Abrahan Vilchez, Mario Florez, Delfin Mamerto, Gloria Rodriguez, Santiago Ruiz, Papa Segundo, Papa Wilson and Victor Aquituari) for their dedication during the surveys. We also thank all people involved in the TDR-Peru project in Lima and Iquitos. We are grateful to Dirección Regional de Salud (DIRESA, Iquitos, Loreto) for collaboration and facilitating logistics in Loreto Department. This publication has been possible thanks to the authorization and permits (no. 0424-2012-AG-DGFFS-DGEFFS) from Direccion de Gestión Forestal y de Fauna Silvestre y la Dirección General Forestal y de Fauna Silvestre del Ministerio de Agricultura de la República del Perú.
Abbreviations
- EIR
entomological inoculation rate
- GLMM
generalized linear mixed effect model
- HBI
human blood index
- HBR
human-biting rate
- HLC
human landing catch
- LIB
Libertad village
- SAL
Salvador village
- URC
Urco Miraño village
- VIB
Visto Bueno village
Authors’ contributions
MPS, FA, JEC, GEC, DG and MM designed the study and oversaw field collections. MPS, FA, CV, GEC and MM collected field data. SAB and CP conducted molecular identifications. JS conducted blood meal identification assays. MPS and CF performed ELISA assays. MG compiled malaria case data, CT helped with the morphological identification of specimens. GCE, CP and MPS conducted data analyses. MPS, JEC, GCE, CP and MM wrote the manuscript with contributions from DG, SAB and JMV. All authors read and approved the final manuscript.
Funding
This project was funded by TDR/WHO (201460655) to Dionicia Gamboa and supported in part by NIH-NIAID (U19AI089681) to JMV and NIH-NIAID (R01AI110112) to JEC. The Biodefense and Emerging Infectious Disease training fellowship Grant T32AI05532901 provided partial support for CP.
Availability of data and materials
The data supporting the conclusions of this article are included within the article. The raw data used and/or analyzed in this study are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Study protocols were approved by the Ethics Review Board of the Regional Health Direction of Loreto (477-2016), Universidad Peruana Cayetano Heredia in Lima (184-09-16) and WHO Ethics Review Committee (0002669).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Marlon P. Saavedra, Email: msaavedraromero75@gmail.com
Jan E. Conn, Email: jan.conn@health.ny.gov
Freddy Alava, Email: ffalare@hotmail.com.
Gabriel Carrasco-Escobar, Email: gabriel.carrasco@upch.pe.
Catharine Prussing, Email: cprussing@albany.edu.
Sara A. Bickersmith, Email: sara.bickersmith@health.ny.gov
Jorge L. Sangama, Email: jlsangamad@gmail.com
Carlos Fernandez-Miñope, Email: carlos.fernandez.m@upch.pe.
Mitchel Guzman, Email: guzman.mitch@gmail.com.
Carlos Tong, Email: ctong32@gmail.com.
Carlos Valderrama, Email: carlosvalderramarioja@gmail.com.
Joseph M. Vinetz, Email: joseph.vinetz@yale.edu
Dionicia Gamboa, Email: dionicia.gamboa@upch.pe.
Marta Moreno, Email: Marta.moreno@lshtm.ac.uk.
References
- 1.Parker BS, Paredes Olortegui M, Penataro Yori P, Escobedo K, Florin D, Rengifo Pinedo S, et al. Hyperendemic malaria transmission in areas of occupation-related travel in the Peruvian Amazon. Malar J. 2013;12:178. doi: 10.1186/1475-2875-12-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosas-Aguirre A, Gamboa D, Manrique P, Conn JE, Moreno M, Lescano AG, et al. Epidemiology of Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2016;95(Suppl. 6):133–144. doi: 10.4269/ajtmh.16-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrasco-Escobar G, Gamboa D, Castro MC, Bangdiwala SI, Rodriguez H, Contreras-Mancilla J, et al. Micro-epidemiology and spatial heterogeneity of P. vivax parasitaemia in riverine communities of the Peruvian Amazon: a multilevel analysis. Sci Rep. 2017;7:8082. doi: 10.1038/s41598-017-07818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foster PG, de Oliveira TMP, Bergo ES, Conn JE, Sant’Ana DC, Nagaki SS, et al. Phylogeny of Anophelinae using mitochondrial protein coding genes. R Soc Open Sci. 2017;4:170758. doi: 10.1098/rsos.170758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reinbold-Wasson DD, Sardelis MR, Jones JW, Watts DM, Fernandez R, Carbajal F, et al. Determinants of Anopheles seasonal distribution patterns across a forest to periurban gradient near Iquitos, Peru. Am J Trop Med Hyg. 2012;86:459–463. doi: 10.4269/ajtmh.2012.11-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14:290. doi: 10.1186/s12936-015-0804-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prussing C, Moreno M, Saavedra MP, Bickersmith SA, Gamboa D, Alava F, et al. Decreasing proportion of Anopheles darlingi biting outdoors between long-lasting insecticidal net distributions in peri-Iquitos, Amazonian Peru. Malar J. 2018;17:86. doi: 10.1186/s12936-018-2234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreno M, Saavedra MP, Bickersmith SA, Prussing C, Michalski A, Tong Rios C, et al. Intensive trapping of blood-fed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Negl Trop Dis. 2017;11:e0005337. doi: 10.1371/journal.pntd.0005337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyimo IN, Ferguson HM. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends Parasitol. 2009;25:189–196. doi: 10.1016/j.pt.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Navia-Gine WG, Loaiza JR, Miller MJ. Mosquito–host interactions during and after an outbreak of equine viral encephalitis in Eastern Panama. PLoS ONE. 2013;8:e81788. doi: 10.1371/journal.pone.0081788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiwat H, Bretas G. Ecology of Anopheles darlingi root with respect to vector importance: a review. Parasites Vectors. 2011;4:177. doi: 10.1186/1756-3305-4-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vezenegho SB, Adde A, Pommier de Santi V, Issaly J, Carinci R, Gaborit P, et al. High malaria transmission in a forested malaria focus in French Guiana: how can exophagic Anopheles darlingi thwart vector control and prevention measures? Mem Inst Oswaldo Cruz. 2016;111:561–569. doi: 10.1590/0074-02760160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174. doi: 10.1186/1475-2875-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahumada ML, Pareja PX, Buitrago LS, Quinones ML. Biting behavior of Anopheles darlingi Root, 1926 (Diptera: Culicidae) and its association with malaria transmission in Villavicencio (Meta, Colombia) Biomedica. 2013;33:241–250. [PubMed] [Google Scholar]
- 15.Rosas-Aguirre A, Speybroeck N, Llanos-Cuentas A, Rosanas-Urgell A, Carrasco-Escobar G, Rodriguez H, et al. Hotspots of malaria transmission in the Peruvian Amazon: rapid assessment through a parasitological and serological survey. PLoS ONE. 2015;10:e0137458. doi: 10.1371/journal.pone.0137458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni MA, Desrochers RE, Kajeguka DC, Kaaya RD, Tomayer A, Kweka EJ, et al. 10 Years of environmental change on the slopes of Mount Kilimanjaro and its associated shift in malaria vector distributions. Front Public Health. 2016;4:281. doi: 10.3389/fpubh.2016.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dash JP, Watt MS, Pearse GD, Heaphy M, Dungey HS. Assessing very high resolution UAV imagery for monitoring forest health during a simulated disease outbreak. ISPRS J Photogramm Remote Sens. 2017;131:1–14. doi: 10.1016/j.isprsjprs.2017.07.007. [DOI] [Google Scholar]
- 18.Galardo AK, Arruda M, D’Almeida Couto AA, Wirtz R, Lounibos LP, Zimmerman RH. Malaria vector incrimination in three rural riverine villages in the Brazilian Amazon. Am J Trop Med Hyg. 2007;76:461–469. doi: 10.4269/ajtmh.2007.76.461. [DOI] [PubMed] [Google Scholar]
- 19.Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11. doi: 10.4269/ajtmh.2006.74.3. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich JN, Naranjo DP, Alimi TO, Muller GC, Beier JC. How much vector control is needed to achieve malaria elimination? Trends Parasitol. 2013;29:104–109. doi: 10.1016/j.pt.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angella AF, Salgueiro P, Gil LH, Vicente JL, Pinto J, Ribolla PE. Seasonal genetic partitioning in the neotropical malaria vector, Anopheles darlingi. Malar J. 2014;13:203. doi: 10.1186/1475-2875-13-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanchez-Ribas J, Oliveira-Ferreira J, Gimnig JE, Pereira-Ribeiro C, Santos-Neves MSA, Silva-do-Nascimento TF. Environmental variables associated with anopheline larvae distribution and abundance in Yanomami villages within unaltered areas of the Brazilian Amazon. Parasites Vectors. 2017;10:571. doi: 10.1186/s13071-017-2517-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan W, Fernandes M. Physicochemical characterization of the white, black and clearwater rivers of the Amazon Basin and its implications on the distribution of freshwater stingrays (Chondrichthyes, Potamotrygonidae) Panam JAS. 2010;5:454–464. [Google Scholar]
- 24.Ribeiro JS, Darwich AJ. Phytoplanktonic primary production of a fluvial island lake in the Central Amazon (Lago do Rei, Ilha do Careiro) Amazoniana. 1993;12:365–383. [Google Scholar]
- 25.Direccion General de Epidemiologia, Ministry of Health Peru. 2017. www.dge.gob.pe/portal/docs/vigilancia/sala/2017/SE20/malaria.pdf.
- 26.Faran MELK. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae) Mosq Syst. 1981;13:1–81. [Google Scholar]
- 27.Consoli RA, Lourenço de Oliveira R. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro: Fundação Oswaldo Cruz; 1994. [Google Scholar]
- 28.Forattini P. Entomologia medica. São Paulo: Faculdade de Higiene e Sáude Publica; 1962. [Google Scholar]
- 29.Matson R, Rios CT, Chavez CB, Gilman RH, Florin D, Sifuentes VL, et al. Improved molecular technique for the differentiation of neotropical anopheline species. Am J Trop Med Hyg. 2008;78:492–498. doi: 10.4269/ajtmh.2008.78.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 31.Fornadel CM, Norris DE. Increased endophily by the malaria vector Anopheles arabiensis in southern Zambia and identification of digested blood meals. Am J Trop Med Hyg. 2008;79:876–880. doi: 10.4269/ajtmh.2008.79.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ngo KA, Kramer LD. Identification of mosquito bloodmeals using polymerase chain reaction (PCR) with order-specific primers. J Med Entomol. 2003;40:215–222. doi: 10.1603/0022-2585-40.2.215. [DOI] [PubMed] [Google Scholar]
- 33.de Carvalho GC, dos Santos Malafronte R, Miti Izumisawa C, Souza Teixeira R, Natal L, Marrelli MT. Blood meal sources of mosquitoes captured in municipal parks in São Paulo, Brazil. J Vector Ecol. 2014;39:146–152. doi: 10.1111/j.1948-7134.2014.12081.x. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro G, Jr, Gurgel-Goncalves R, Reis RB, Santos CG, Amorim A, Andrade SG, et al. Frequent house invasion of Trypanosoma cruzi-infected triatomines in a suburban area of Brazil. PLoS Negl Trop Dis. 2015;9:e0003678. doi: 10.1371/journal.pntd.0003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wirtz RA, Zavala F, Charoenvit Y, Campbell GH, Burkot TR, Schneider I, et al. Comparative testing of monoclonal antibodies against Plasmodium falciparum sporozoites for ELISA development. Bull World Health Organ. 1987;65:39–45. [PMC free article] [PubMed] [Google Scholar]
- 36.Bickersmith SA, Lainhart W, Moreno M, Chu VM, Vinetz JM, Conn JE. A sensitive, specific and reproducible real-time polymerase chain reaction method for detection of Plasmodium vivax and Plasmodium falciparum infection in field-collected anophelines. Mem Inst Oswaldo Cruz. 2015;110:573–576. doi: 10.1590/0074-02760150031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner RA, Fischer MJ. The string-to-string correction problem. J Assoc Comput Mach. 1974;21:10. doi: 10.1145/321796.321811. [DOI] [Google Scholar]
- 38.Yujian L, Bo L. A normalized Levenshtein distance metric. IEEE Trans Pattern Anal Mach Intell. 2007;29:1091–1095. doi: 10.1109/TPAMI.2007.1078. [DOI] [PubMed] [Google Scholar]
- 39.Povoa MM, Conn JE, Schlichting CD, Amaral JC, Segura MN, Da Silva AN, et al. Malaria vectors, epidemiology, and the re-emergence of Anopheles darlingi in Belem, Para, Brazil. J Med Entomol. 2003;40:379–386. doi: 10.1603/0022-2585-40.4.379. [DOI] [PubMed] [Google Scholar]
- 40.Hess AH, Haves RO, Tempelis CH. The use of forage ratio technique in mosquito host preference studies. Mosq News. 1968;28:386–389. [Google Scholar]
- 41.Savage RE. The relation between the feeding of the herring off the east coast of England and the plankton of the surrounding waters: fishery investigations. London: Ministry of Agriculture, Food and Fisheries; 1931. [Google Scholar]
- 42.Manly B, McDonald L, Thomas D. Resource selection by animals. Statistical design and analysis for field studies. London: Champan and Hall; 1993. [Google Scholar]
- 43.Dormann CF, Fründ J, Blüthgen N, Gruber B. Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol J. 2009;2:7–24. doi: 10.2174/1874213000902010007. [DOI] [Google Scholar]
- 44.Prussing C, Saavedra MP, Bickersmith SA, Alava F, Guzman M, Manrique E, et al. Malaria vector species in Amazonian Peru co-occur in larval habitats but have distinct larval microbial communities. PLoS Negl Trop Dis. 2019;13:e0007412. doi: 10.1371/journal.pntd.0007412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirby MJ, Lindsay SW. Responses of adult mosquitoes of two sibling species, Anopheles arabiensis and A. gambiae s.s. (Diptera: Culicidae), to high temperatures. Bull Entomol Res. 2004;94:441–448. doi: 10.1079/BER2004316. [DOI] [PubMed] [Google Scholar]
- 46.Koenraadt CJ, Githeko AK, Takken W. The effects of rainfall and evapotranspiration on the temporal dynamics of Anopheles gambiae s.s. and Anopheles arabiensis in a Kenyan village. Acta Trop. 2004;90:141–153. doi: 10.1016/j.actatropica.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 47.Ngowo HS, Kaindoa EW, Matthiopoulos J, Ferguson HM, Okumu FO. Variations in household microclimate affect outdoor-biting behaviour of malaria vectors. Wellcome Open Res. 2017;2:102. doi: 10.12688/wellcomeopenres.12928.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lainhart W, Bickersmith SA, Nadler KJ, Moreno M, Saavedra MP, Chu VM, et al. Evidence for temporal population replacement and the signature of ecological adaptation in a major neotropical malaria vector in Amazonian Peru. Malar J. 2015;14:375. doi: 10.1186/s12936-015-0863-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanyangarara M, Hamapumbu H, Mamini E, Lupiya J, Stevenson JC, Mharakurwa S, et al. Malaria knowledge and bed net use in three transmission settings in southern Africa. Malar J. 2018;17:41. doi: 10.1186/s12936-018-2178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harvey SA, Lam Y, Martin NA, Olortegui MP. Multiple entries and exits and other complex human patterns of insecticide-treated net use: a possible contributor to residual malaria transmission? Malar J. 2017;16:265. doi: 10.1186/s12936-017-1918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tangena JA, Thammavong P, Lindsay SW, Brey PT. Risk of exposure to potential vector mosquitoes for rural workers in northern Lao PDR. PLoS Negl Trop Dis. 2017;11:e0005802. doi: 10.1371/journal.pntd.0005802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crawshaw AF, Maung TM, Shafique M, Sint N, Nicholas S, Li MS, et al. Acceptability of insecticide-treated clothing for malaria prevention among migrant rubber tappers in Myanmar: a cluster-randomized non-inferiority crossover trial. Malar J. 2017;16:92. doi: 10.1186/s12936-017-1737-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rek JC, Alegana V, Arinaitwe E, Cameron E, Kamya MR, Katureebe A, et al. Rapid improvements to rural Ugandan housing and their association with malaria from intense to reduced transmission: a cohort study. Lancet Planet Health. 2018;2:e83–e94. doi: 10.1016/S2542-5196(18)30010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ippolito MM, Searle KM, Hamapumbu H, Shields TM, Stevenson JC, Thuma PE, et al. House structure is associated with Plasmodium falciparum infection in a low-transmission setting in Southern Zambia. Am J Trop Med Hyg. 2017;97:1561–1567. doi: 10.4269/ajtmh.17-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Animal host census in SAL, URC, LIB and VIB, Mazan district, Peru, performed in November 2016. Table S2. Summary of Anophelinae specimens collected indoors/outdoors (peridomestic). Table S3. Forage ratio (wi) and host selection index (Bi) of Ny. darlingi in Mazán District sites between 2016 and 2017. Figure S1. Number of Anophelinae mosquitoes collected with barrier screen by position in Salvador (SAL), Urcomiraño (URC), Libertad (LIB) and Visto Bueno (VIB). Abbreviations: V-F, village-forest; V-BS, village-breeding site.
Data Availability Statement
The data supporting the conclusions of this article are included within the article. The raw data used and/or analyzed in this study are available from the corresponding author upon reasonable request.