Abstract
Background
Probiotics play an important role in the human and animal defense against liver damage. However, the protective mechanism of Lactobacillus plantarum C88 on chronic liver injury induced by mycotoxin remains unclear.
Results
In this study, the addition of L. plantarum C88 obviously ameliorated the increased contents of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total cholesterol and triglyceride, the diminish contents of total protein and albumin in serum of mice challenged with AFB1. Simultaneously, L. plantarum C88 attenuated the inflammatory response via significantly reducing the levels of pro-inflammatory factors, including interleukin-1β (IL-1β), IL-6, IL-8, interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) in serum. Furthermore, L. plantarum C88 remarkably down-regulated the nuclear factor kappa B (NF-κB) signaling pathways by weakening the expression of toll-like receptor 2 (TLR2) and TLR4, and inhibited NF-κB nuclear translocation through enhancing the expression of NF-κB inhibitor (IκB). Neutralization experiments confirmed that L. plantarum C88 decreased the levels of some pro-inflammatory factors due to the suppression of the NF-κB signaling pathways. Besides, L. plantarum C88 decreased the levels of Bax and Caspase-3, elevated the level of Bcl-2, and reduced mRNA expressions of Fatty acid synthetase receptor (Fas), FAS-associated death domain (FADD), TNF receptor associated death domain (TRADD) and Caspase-8 in the liver.
Conclusions
Probiotic L. plantarum C88 prevented AFB1-induced secretion of pro-inflammatory cytokines by modulating TLR2/NF-κB and TLR4/NF-κB pathways. The molecular mechanisms of L. plantarum C88 in ameliorating AFB1-induced excessive apoptosis included regulating the mitochondrial pathway and cell death receptor pathways.
Electronic supplementary material
The online version of this article (10.1186/s12866-019-1525-4) contains supplementary material, which is available to authorized users.
Keywords: Lactic acid Bacteria, Lactobacillus plantarum, Aflatoxin B1, Liver injury, NF-κB signal pathways, Anti-inflammatory, Apoptosis
Background
As an important detoxification organ, the liver is continuously exposed to certain adverse factors such as alcohol, fat, pathogens and cellular metabolites, which can cause liver injury, hepatitis and liver degradation. Long-term liver injury leads to liver fibrosis, cirrhosis and hepatocellular carcinoma (HCC) [1]. Clinically, the main causes of liver injury are viruses, alcohol, drugs, and chemicals. Liver injury can also be induced by toxins, such as AFB1. AFB1 is a metabolite of Aspergillus flavus and A. parasiticus, which is classified under Group I carcinogenic agents by International Agency for Research on Cancer (IARC) [2], and is a potent hepatotoxic and hepatic carcinogenic mycotoxin in humans. The mechanisms of AFB1-induced liver injury are complex, and previous studies have shown that inflammatory response is a critical step in the process. Mehrzad et al. [3] found that AFB1 activates TLR2 and TLR4, which trigger the NF-κB signaling pathway that leads to the synthesis and secretion of TNF-α, IL-1β, IL-6 and other pro-inflammatory cytokines in murine pure primary astrocytes. Therefore, it is necessary to find safe and effective natural anti-inflammatory agents for preventing or alleviating AFB1-induced hepatic injury.
Probiotics are used as a dietary supplement for their outstanding health benefits including immunoregulation, balancing lipid metabolism, and regulation of gene expression in diseases such as inflammatory bowel disease, alcoholic and non-alcoholic liver diseases. L. plantarum is effective in preventing chronic and acute alcohol-induced liver injury by improving intestinal barrier function [4], restoring gut microbiota [4], regulating pro-inflammatory molecules [5], reducing oxidative stress [6], and disrupting endotoxemia [7]. Meanwhile, L. plantarum mitigates non-alcoholic fatty liver disease by improving liver function, inducing adipose leptin, enhancing antioxidant capacity and decreasing lipid peroxidation [8, 9]. L. plantarum could also resist liver damage induced by endotoxins, D-galactosamine, lipopolysaccharides and cadmium by a similar pathway [10–13].
L. plantarum C88 is classified as a probiotic since it colonizes the gastrointestinal tract [14], resists bile salt or aciduricity, possesses antioxidant capacity [15], prevents chronic and acute liver injury induced by alcohol [16, 17], and modulates the metabolism of AFB1 in mice [18]. However, the effects of L. plantarum on AFB1-induced hepatic inflammation and excessive apoptosis are rarely reported. This study aimed to further reveal the molecular mechanisms of L. plantarum C88 on hepatic injury using the AFB1 challenge mouse model.
Results
Inhibition of AFB1-induced liver injury
As depicted in Table 1, Additional file 1 the levels of serum ALT, AST and ALP of the AFB1 model group were significantly increased (P < 0.05), along with similar increase in the total cholesterol and triglyceride levels (P < 0.05). As compared to the control group, the total protein and albumin levels were increased in the viable C88 and heat-killed C88 groups, while the total cholesterol and triglyceride levels were decreased, although without significant differences. The therapy group mice, treated with a combination of AFB1 and L. plantarum C88, successfully recovered the serum biochemical parameters to the levels of the control group. This recovery was significantly pronounced in the AFB1 + Viable C88 group.
Table 1.
Effect of L. plantarum C88 on serum biochemical parameters
| Group | ALT (U/L) | AST (U/L) | ALP (U/L) | Total cholesterol (mmol/mL) | Triglycerides (mmol/mL) | Total protein (g/L) | Albumin (g/L) |
|---|---|---|---|---|---|---|---|
| Control | 40.25 ± 2.98 c | 64.79 ± 6.79 c | 106.54 ± 7.52 c | 135.89 ± 10.59 | 87.69 ± 7.92 c | 79.68 ± 6.40 a | 50.44 ± 5.63 b |
| AFB1 | 89.78 ± 4.13 a | 120.61 ± 9.31 a | 194.39 ± 12.64 a | 283.13 ± 17.22 a | 191.83 ± 15.02 a | 45.77 ± 4.03 c | 38.85 ± 4.94 c |
| Viable C88 | 39.89 ± 2.67 c | 56.88 ± 7.95 c | 86.36 ± 7.95 c | 110.92 ± 10.23 c | 39.50 ± 4.63 d | 88.85 ± 7.41 a | 65.83 ± 6.93 a |
| Heated-killed C88 | 40.14 ± 2.92 c | 57.40 ± 6.83 c | 92.40 ± 6.71 c | 118.23 ± 12.49 c | 50.82 ± 7.21 d | 80.71 ± 4.46 a | 53.67 ± 4.62 b |
| AFB1 + Viable C88 | 69.14 ± 3.34 b | 82.92 ± 9.14 b | 154.02 ± 14.14 b | 154.28 ± 13.51 b | 111.64 ± 10.64 b | 65.16 ± 5.66 b | 45.77 ± 5.62 b |
| AFB1 + Heated-killed C88 | 76.72 ± 3.94 b | 101.53 ± 8.25 b | 181.80 ± 11.76 a | 179.91 ± 10.24 b | 118.27 ± 8.93 b | 47.32 ± 4.68 c | 42.02 ± 3.29 c |
The results are expressed as mean ± S.D.; each data point is the average of 3 repeated measurements from 10 independently replicated experiments (n = 10)
The different letters in the same rows mean significant difference (P < 0.05), the same letters in the same rows mean insignificant difference (P > 0.05)
Reduction of inflammatory response
The effects of different treatments on pro-inflammatory cytokines in serum are presented in Table 2, Additional file 2. In the AFB1 group, the levels of IL-1β, IL-6, IL-8, IFN-γ and TNF-α in serum were increased as compared to the control group (P < 0.05). However, these increases were prevented in the AFB1 + Viable C88 group or AFB1 + Heat-killed C88 group, suggesting that L. plantarum C88 supplementation could restrain the inflammatory processes in serum caused by AFB1. In addition, the levels of IL-10 showed no significant differences among the groups (P > 0.05).
Table 2.
Effect of L. plantarum C88 on the levels of IL-1β, IL-6, IL-8, IL-10, IFN-γ, and TNF-α in serum
| Group | IL-1β (pg/ml) | IL-6 (pg/ml) | IL-8 (pg/ml) | IL-10 (ng/ml) | IFN-γ (pg/ml) | TNF-α (pg/ml) |
|---|---|---|---|---|---|---|
| Control | 43.45 ± 3.90 c | 67.24 ± 8.35 c | 110.26 ± 15.02 c | 161.79 ± 9.93 a | 163.24 ± 17.90 b | 208.85 ± 33.03 c |
| AFB1 | 99.23 ± 5.52 a | 123.56 ± 12.73 a | 143.40 ± 11.80 a | 153.28 ± 10.90 a | 252.55 ± 19.65 a | 313.53 ± 44.17 a |
| Viable C88 | 41.52 ± 4.34 c | 74.83 ± 8.49 c | 91.77 ± 15.58 d | 168.72 ± 8.46 a | 169.68 ± 17.81 b | 204.39 ± 31.40 c |
| Heated-killed C88 | 42.49 ± 4.21 c | 72.35 ± 9.83 c | 98.41 ± 6.83 d | 166.97 ± 10.49 a | 166.88 ± 16.29 b | 208.37 ± 29.18 c |
| AFB1 + Viable C88 | 68.82 ± 3.72 b | 109.24 ± 13.51 b | 128.11 ± 13.21 b | 155.80 ± 7.28 a | 236.83 ± 20.53 a | 269.04 ± 24.65 b |
| AFB1 + Heated-killed C88 | 79.25 ± 4.56 b | 113.52 ± 11.69 b | 130.29 ± 11.77 b | 153.29 ± 9.84 a | 241.01 ± 21.88 a | 278.23 ± 33.42 b |
The results are expressed as mean ± S.D.; each data point is the average of 3 repeated measurements from 10 independently replicated experiments (n = 10)
The different letters in the same rows mean significant difference (P < 0.05), the same letters in the same rows mean insignificant difference (P > 0.05)
Decreased apoptosis of liver
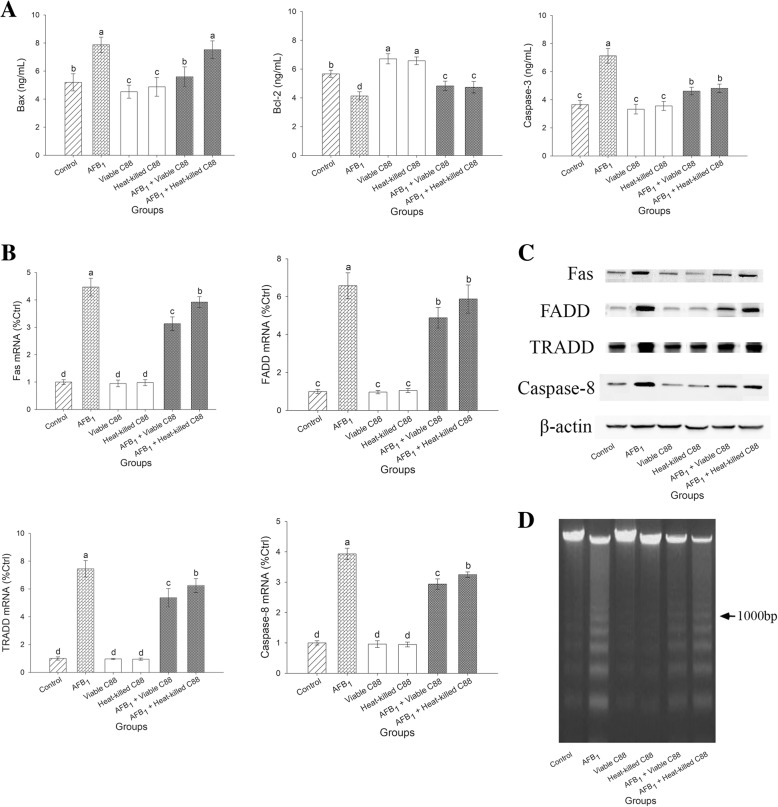
In comparison with the AFB1 model group, the levels of Bax and Caspase-3 were significantly reduced in the AFB1 + Viable C88 group or AFB1 + Heat-killed C88 group (P < 0.05), although they did not recover to the levels in the control group (Fig. 1a, Additional file 3). The addition of L. plantarum C88 to the AFB1 diet significantly restored the reduction of Bcl-2 in the liver (P < 0.05).
Fig. 1.
Effect of L. plantarum C88 on decreased apoptosis of liver. a The levels of Bax, Bcl-2 and Caspase-3 in liver were measured. b FAS, FADD, TRADD and Caspase-8 gene expression was measured by RT-PCR. c Expression of FAS, FADD, TRADD, Caspase-8 and β-actin was detected by Western blot analysis. β-actin was used as a housekeeping control. d DNA was extracted and analyzed by agarose gel electrophoresis to analyze the DNA fragmentation. The different letters in the same rows mean significant difference (P < 0.05), the same letters in the same rows mean insignificant difference (P > 0.05).
The mRNA and protein expression levels of relative genes in liver involved in death receptor pathway, including FAS, FADD, TRADD and Caspase-8, are presented in Fig. 1b and c. AFB1 significantly up-regulated the mRNA expression levels of FAS, FADD, TRADD and Caspase-8 in the liver (P < 0.05). However, addition of L. plantarum C88 to AFB1 diet significantly reversed the increase in mRNA expression of above genes caused by AFB1 treatment (P < 0.05). The mRNA expression of FADD was down-regulated, although not significantly, in the AFB1 + Heat-killed C88 group (P > 0.05). Western blotting also revealed that AFB1 exposure significantly up-regulated the death receptor pathway as a consequence of 21 days of AFB1 exposure, while L. plantarum C88 pretreatment inhibited the expression of FAS, FADD, TRADD and Caspase-8 (Fig. 1c).
Genomic DNA fragmentation was assayed to confirm the occurrence of hepatocyte apoptosis. An inconspicuous DNA fragmentation occurred in the control group, while no DNA fragmentation was found in viable and heat-killed L. plantarum C88 groups (Fig. 1d). Maximum DNA fragmentation was observed in AFB1 group, while co-treated with AFB1 and L. plantarum C88 was able to inhibit the charac-teristic apoptotic DNA fragmentation.
Regulation of NF-κB signaling pathway
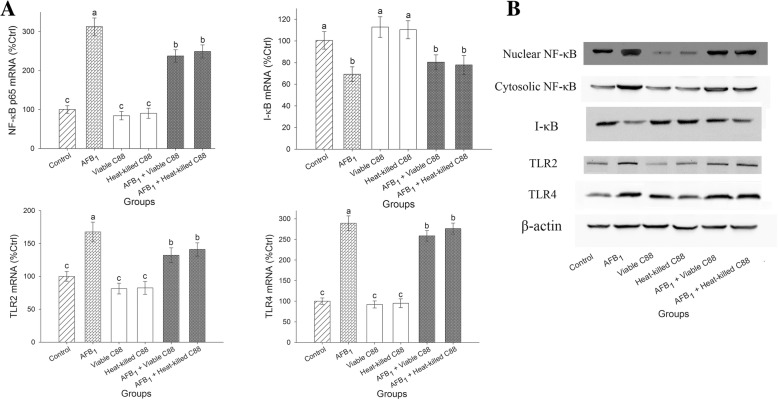
The NF-κB signaling pathways are involved in the inflammatory response and cell injury. The mRNA expression of NF-κB was significantly upregulated in the liver of mice fed with AFB1 diet (P < 0.05). The increased mRNA expression of NF-κB was highly inhibited in the therapy group of AFB1 + L. plantarum C88. Since NF-κB is activated after its nuclear translocation, Western blot analysis was performed using antibodies to the p65 subunit of NF-κB to assess the distribution of NF-κB in liver tissue. A significant increase in NF-κB protein expression was observed in the nucleus of the mice fed with AFB1, in conjunction with a decrease in IκB level, indicating a nuclear translocation from the cytoplasm. The addition of L. plantarum C88 to the AFB1 diet prompted a considerable down-regulation in protein expression of NF-κB.
TLR2 and TLR4 are important signaling receptors for activating NF-κB signaling pathway. In mice exposed to the AFB1 diet, the mRNA expression levels of TLR2 and TLR4 were up-regulated in the liver, and were nearly 1.67-fold and 2.89-fold those of the control group, respectively. However, in the therapy groups, the mRNA expression levels of TLR2 and TLR4 were significantly decreased (P < 0.05), and the decrease in protein expression levels were also confirmed by Western blot (Fig. 2).
Fig. 2.
Effect of L. plantarum C88 on NF-κB signaling in liver of mice. a NF-κB, I-κB, TLR2, and TLR4 gene expression was measured by RT-PCR. b Expression of Nuclear NF-κB and Cytoplasmic NF-κB, I-κB, TLR2,TLR4 and β-actin was detected by Western blot analysis. β-actin was used as a housekeeping control. The results are expressed as mean ± S.D.; each data point is the average of 3 repeated measurements from 10 independently replicated experiments (n = 10). The different letters in the same rows mean significant difference (P < 0.05), the same letters in the same rows mean insignificant difference (P > 0.05)
NF-κB pathway stimulates the synthesis of pro-inflammatory cytokines
The purpose of the neutralization trial was to investigate whether down-regulation of pro-inflammatory cytokines by L. plantarum C88 is specifically related to inhibition of NF-κB signaling pathways. Increased expressions of pro-inflammatory cytokines were observed in the AFB1 model group, while addition of L. plantarum C88 reversed this trend. Addition of Pyrrolidinedithiocarbamic acid (PDTC) led to decrease in the expression of IL-1β, IL-6, and TNF-α, indicating that NF-κB was strongly associated with the synthesis of pro-inflammatory cytokines (Fig. 3).
Fig. 3.
Reducing the production of pro-inflammatory cytokines through modulating the NF-κB signaling pathway. IL-1β (a), IL-6 (b) and TNF-α (c) gene expression was measured by RT-PCR. Expression of IL-1β, IL-6 and TNF-α (d) and β-actin was detected by Western blot analysis. β-actin was used as a housekeeping control. The results are expressed as mean ± S.D.; each data point is the average of 3 repeated measurements from 10 independently replicated experiments (n = 10). The different letters in the same rows mean significant difference (P < 0.05), the same letters in the same rows mean insignificant difference (P > 0.05)
Discussion
L. plantarum C88 exerts various probiotic properties, including ameliorating oxidative stress, improving intestinal barrier dysfunction, inhibiting intestinal endotoxin-mediated inflammation, blocking alcohol-induced chronic and acute liver damage. Hence, the underlying mechanisms of L. plantarum C88 on AFB1-induced chronic liver injury in mice were investigated in this study.
Cumulative evidence suggested that inflammatory response could facilitate structural and functional liver injury [19]. In fact, the increase of inflammatory cytokines was an important factor for causing liver cancer [20]. In this study, the levels of pro-inflammatory cytokines increased in the AFB1 model group, but the increase in the levels of inflammatory cytokines could be significantly prevented by L. plantarum C88. Our results are consistent with previous finding that L. plantarum supplementation could reduce TNF-α in the small intestines of AFB1-treated mice [21].
As a central regulator of cellular stress in hepatocytes, NF-κB regulates the processes of inflammation, apoptosis and cell injury [22]. Among the identified Toll-like receptors (TLRs), TLR2 and TLR4 are the major receptors for AFB1 recognition [23, 24]. The activation of TLR2 and TLR4 signaling led to degradation of IκB, NF-κB translocation to the nucleus, followed by NF-κB binding to the promoters of inflammatory genes and initiation of transcription in Kupffer cells [25]. In the present study, under AFB1 challenge, the mRNA expressions of TLR2, TLR4, and NF-κB were obviously up-regulated. However, the diet supplemented with L. plantarum C88 down-regulated the expression of NF-κB, reduced the level of NF-κB in the nucleus and up-regulated the expression of IκB in the liver. Another study indicated that L. casei weakened the lipopolysaccharide-induced overexpression of TLR4 and NF-κB mRNAs [26]. Ma et al. [27] proposed that AFB1-induced elevation of inflammatory cytokines was attributed to up-regulation of the NF-κB signaling pathway. Therefore, we hypothesized that L. plantarum C88 could diminish pro-inflammatory cytokines in the serum of mice, which might be related to the down-regulation of NF-κB expression. To confirm this hypothesis, neutralization test was performed. Pre-treatment with PDTC, an inhibitor of NF-κB, led to decline in the mRNA levels of IL-1β and TNF-α, indicating that the change of pro-inflammatory cytokine levels was positively correlated with the level of NF-κB, which was also verified by Western-blot. Similar results were obtained in the AFB1 + C88 group. Thus, L. plantarum C88 exhibited anti-inflammatory effects by down-regulating the NF-κB signaling pathway. Sun et al. [28] found that L. paracasei restrained the production of pro-inflammatory cytokines via inhibition of the TLR2/NF-κB signaling pathway. MiRNAs were shown to play a role in the regulation of the TLR2/NF-κB pathway in probiotic-mediated innate immune responses [29]. Probiotic genomic DNA was also found to have similar inhibitory functions [30]. Further studies are required to identify the exact structures of L. plantarum C88 that are responsible for inhibiting AFB1-induced inflammatory responses through NF-κB signaling pathways. Interestingly, the expression of TNF-α is regulated by the NF-κB signaling pathway, which, in turn, is activated by the combination of TNF-α and tumor necrosis factor receptor 1 (TNFR1) [22]. TNFR1, as the primary mediator of TNF-α, can be activated to trigger NF-κB and apoptosis signaling pathways [31]. Petrof et al. [32] showed that conditioned media from L. plantarum inhibited NF-κB activation from TNFR pathways. The present study focused on the mechanisms of TNF-α expression regulated by NF-κB in AFB1-induced mice, but the regulation of NF-κB by TNF-α is unclear. NF-κB regulates various apoptosis-related genes, including Bcl-2 families, TNFR-associated factors, JNK, etc. NF-κB could inhibit Caspase-8 expression and promote JNK activation by inducing the expression of IAP family, and thus play a role in anti-apoptosis [33]. In the early stage of viral hepatitis, NF-κB was activated, and subsequently induced hepatocyte apoptosis and acute liver failure by up-regulating the expression of Fas receptor [34]. Hence, the activation of the pro-apoptotic pathway or anti-apoptotic pathway by NF-κB was perhaps associated with differences in cell types and stimuli.
Apoptosis is related to inflammatory response. Previous researches have demonstrated that the mitochondrial pathways or the cell death receptor pathways were associated with excessive apoptosis of AFB1-induced hepatocytes. In this study, the proteins related to mitochondria-mediated apoptosis, such as Bax, Bcl-2 and caspase-3, were investigated. The pro-apoptotic protein Bax antagonizes the anti-apoptotic protein Bcl-2, which regulates the mitochondrial membrane permeability, and facilitates mitochondrial apoptotic factors, such as cytochrome C, into the cytoplasmic matrix to promote apoptosis. The protein kinase C (PKC), which is activated by hydrolysis of Caspase-3, executes the apoptotic process. In the present study, the levels of Bax and Caspase-3 were elevated, while the level of Bcl-2 was decreased in the liver of mice after oral administration of AFB1. Furthermore, the therapy groups reversed the above trend, indicating that L. plantarum C88 protected against excessive apoptosis induced by AFB1 through impeding the mitochondrial pathway. Zhang et al. [35] found that L. rhamnosus GG culture supernatant attenuated alcohol-induced hepatic apoptosis in mice. We also explored the cell death receptor pathways. Death receptors, such as Fas and TNF-α, bind to the associated death domains FADD and TRADD, respectively, and subsequently activate caspase-8 to induce apoptosis [36, 37]. Josse et al. [38] observed that the expression of Fas was increased after treatment of human hepatocytes with 0.05 μM AFB1. L. paracasei GMNL-32 protected cardiomyocytes from systemic lupus erythematosus mice via reducing the signaling pathway of Fas death receptor [39]. However, the changes in TRADD expression level in the liver after treatment with AFB1 have not been reported. In the present study, L. plantarum C88 restrained the increase in the mRNA expression levels of Fas, FADD, TRADD and Caspase-8 induced by AFB1, which demonstrated that L. plantarum C88 could protect liver tissue and restore liver cells from AFB1-induced hepatocyte apoptosis.
Conclusions
The present study showed that L. plantarum C88 prevented AFB1-induced liver injury. L. plantarum C88 significantly decreased the levels of ALT and AST and ALP in serum, inhibited the secretion of pro-inflammatory cytokines, impeded the NF-κB nuclear translocations via enhancing the expression of I-κB, and ameliorated the excessive apoptosis in liver.
Materials and methods
Bacterial strain
L. plantarum C88 was cultured in Man–Rogosa–Sharpe (MRS) medium for 18 h at 37 °C. Thereafter, the bacterial density was adjusted to 1010 CFU/mL using phosphate-buffered saline (pH 7.2). Heat-killed L. plantarum C88 sample (1010 CFU/mL) was obtained by autoclaving the viable bacterial samples.
Animals and experimental design
Male ICR mice, 6-week-old, body weight approximately 18–22 g, were supplied by by the Institutional Animal Care and Use Committee of Jilin University (SCXK 2015–0001). The mice were maintained in artificial illuminated (12 h light/dark cycles), constant temperature (25 °C) and environment humidity (50 ± 5%) room, with free access to food and water during the 1-week acclimation period. All mice received humane care. Ninety healthy ICR mice were randomly assigned to the six groups (n = 15, per group). The control mice received normal saline. The viable or heat-killed L. plantarum C88 group respectively received 4.0 × 1010 CFU/kg bw (body weight) viable or heat-killed L. plantarum C88 by gavage. The AFB1 group mice received 300 μg AFB1/kg bw, because of moderate toxicity symptoms was shown in the mice at this dose in the pre-experiment. In the therapy groups, the same dose of the viable or heat-killed L. plantarum C88 was administered after 300 μg AFB1/kg bw exposure (AFB1 + Viable or Heat-killed C88) for 21 days continuously. Twenty-four hours after the final treatments (i.e. day 21), the mice were anesthetized by inhalation of diethyl ether, blood samples were collected from retro-orbital venous plexus for the determination. After blood samples were collected, all mice were killed by cervical dislocation. The extracted blood samples were clotted and centrifuged (2000×g, 10 min, 4 °C) to obtain serum for subsequent analysis. The liver samples were divided into two parts. One half of the liver tissue was homogenized with cold physiological saline to prepare a 10% liver homogenate. The other half of the liver tissue was stored at − 80 °C for analysis of mRNA and protein expression levels (Additional file 4).
Measurement of biochemical parameters in serum
The levels of ALT, AST, ALP, total cholesterol, triglycerides, total protein and albumin in serum were analyzed to evaluate liver damage. Alanine, aspartate and phenyl phosphate were used as substrates, respectively, to determinate the enzyme activities of ALT, AST and ALP in accordance with the manufacturer’s recommended procedure of assay kits (Nanjing Jiancheng, China). The absorbance was determined at 510.0 nm (ALT and AST) or 520.0 nm (ALP) using a microplate reader (Bio-Rad model 680, USA). The levels of total cholesterol, triglycerides, total protein and albumin in serum were measured as per the methods provided with the assay kits (Nanjing Jiancheng, China), using a microplate reader (Bio-Rad model 680, USA).
Measurement of inflammatory cytokines in serum
IL-1β, IL-6, IL-8, IL-10, IFN-γ, and TNF-α levels in serum were determined by enzyme-linked immunosorbent assay (ELISA) as per the manufacturer’s recommended procedure of the ELISA kits (R&D Systems, USA).
Measurement of Bax, Bcl-2 and Caspase-3 in liver
The ELISA kits (Cusabio, China) were used to detect the levels of Bax, Bcl-2, and Caspase-3 in liver. The absorbance was determined at 450.0 nm using a microplate reader (Bio-Rad model 680, USA).
Analysis of DNA fragmentation in liver
The collected liver samples were homogenized in cold physiological saline. The DNA of liver tissue was extracted using the apoptosis DNA ladder detection kit (Dingguo Changsheng, China) as per the manufacturer’s instructions. The DNA fragmentation was assayed by electrophoresis in a 1.5% agarose gel and visualized by UV fluorescence after staining by ethidium bromide.
Neutralization
As a potent inhibitor of NF-κB, pyrrolidine dithiocarbamate (PDTC) was used in the neutralization experiments. The isolation and culture of splenocytes were performed as previously described [18]. Splenocytes were divided into the control, C88, AFB1, AFB1 + PDTC and AFB1 + C88 groups. 0.1 μmol/ml PDTC (MedChemExpress, USA) and 1010 CFU/ml L. plantarum C88 was added to the AFB1 + PDTC and AFB1+ C88 groups, respectively, for 1 h. Then, normal saline or L. plantarum C88 were added to the control group and C88 group, respectively, while the other groups were exposed to 2 μg/ml AFB1 for 2d. The cells were collected and used to test the expression of IL-1β, IL-6 and TNF-α using real-time PCR analysis and Western blot analysis.
Quantitative real-time PCR analysis
The procedure was consistent with a previous study [17]. Total RNAs were collected from frozen samples and reverse-transcribed into cDNA. Real-time polymerase chain reaction (RT-PCR) analysis was performed to determine the mRNA levels of specific genes on a LightCycler 96 Real-Time PCR system (Roche Diagnostics GmbH, Germany). The PCR conditions were 95.0 °C for 3.0 min, 40 cycles at 95.0 °C for 15.0 s, and 60.0 °C for 1.0 min. The primer sequences for RT-PCR are listed in Table 3. β-actin, a housekeeping gene, was used as an endogenous normalization control.
Table 3.
Primer sequences for real-time PCR
| Gene | Primer sequence | NCBI Reference Sequence: | References |
|---|---|---|---|
| NF-κB p65 |
Forward 5′ - GGACAGCACCACCTACGATG - 3′ Reverse 5′ - CTGGATCACTTCAATGGCCTC - 3’ |
NM_009045.4 | Present study |
| I-κB |
Forward 5’ - CAGGAGCCAAAACCGACAAC - 3′ Reverse 5′ - TGGTTGTCAGGTCTGCAATTTT - 3’ |
NM_001306222.1 | Present study |
| Fas |
Forward 5’ - CCAAACGGAAATTGCAGGGG - 3′ Reverse 5′ - AAGCACCAGTTCACAGATGGA - 3’ |
NM_001146708.1 | Present study |
| FADD |
Forward 5’ - TGCTCCACCTATCCACCAGA - 3′ Reverse 5′ - CAATGCGGAAGGCGATTGAG - 3’ |
NM_010175.6 | Present study |
| TRADD |
Forward 5’ - GAGCTGCTGGAGTGCAACTA - 3′ Reverse 5′ - GGTCCGGGTACTTAGAGGGT - 3’ |
NM_001033161.2 | Present study |
| Caspase-8 |
Forward 5’ - CCAGACAGAGAAGGGGCTTG - 3′ Reverse 5′ - TCACTGCCCAGTTCTTCAGC - 3’ |
NM_001080126.1 | Present study |
| IL-1β |
Forward 5’ - TCGTGCTGTCGGACCCATAT - 3′ Reverse 5′ - GTCGTTGCTTGGTTCTCCTTGT - 3’ |
NM_008361.4 | Present study |
| IL-6 |
Forward 5’ - GACAAAGCCAGAGTCCTTCAGA - 3′ Reverse 5′ - TGTGACTCCAGCTTATCTCTTGG - 3’ |
NM_001314054.1 | Present study |
| TNF-α |
Forward 5’ - GCGGAGTCCGGGCAGGTCTA - 3′ Reverse 5′ - GGGGGCTGGCTCTGTGAGGA - 3’ |
NM_001278601.1 | Present study |
| β-actin |
Forward 5’ - TGCTGTCCCTGTATGCCTCTG - 3′ Reverse 5′ - TTGATGTCACGCACGATTTCC - 3’ |
NM_007393.4 | Present study |
Western blot analysis
The extraction and Western blotting analysis of total protein and nuclear protein from liver tissue were accomplished as previously described [17]. Protein was separated by gel electrophoresis and then transferred to nitrocellulose membranes (Merck Millipore, USA), which were probed using primary antibodies, including anti-TLR2, anti-TLR4, anti-NF-κB p65, anti-IκB, anti-Fas, anti-FADD, anti-TRADD, anti- Caspase-8 and anti-β-actin (Biosynthesis Biotech Co., China), for 12 h at 4 °C. Then the membranes were incubated with horseradish peroxidase-conjugated antibody for 1 h. ChemiScope 5600 image analyzer (Clinx Science Instruments, China) was used to quantify the antibody-bound proteins.
Statistical analyses
Experimental results were numerically represented as means ± standard error. Data differences among the groups were analyzed using t-test. P < 0.05 was considered to be statistically significant difference.
Additional files
The raw datas of biochemical parameters in serum, include ALT, AST, ALP, total cholesterol, triglycerides, total protein and albumin. (XLSX 14 kb)
The raw datas of IL-1β, IL-6, IL-8, IL-10, IFN-γ, and TNF-α in serum. (XLSX 15 kb)
The raw datas of Bax, Bcl-2 and Caspase-3 in liver. (XLSX 13 kb)
The ARRIVE Guidelines Checklist. (PDF 1067 kb)
Acknowledgements
We thank Zhanqing Yang, and Bin Guo of Jilin University for technical assistance.
Abbreviations
- AFB1
Aflatoxin B1
- ALP
Alkaline phosphatase
- ALT
Alanine aminotransferase
- AST
Aspartate aminotransferase
- FADD
FAS-associated death domain
- Fas
Fatty acid synthetase receptor
- HCC
Hepatocellular carcinoma
- IARC
International Agency for Research on Cancer
- IFN-γ
Interferon-γ
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- IL-8
Interleukin-8
- IκB
Nuclear factor kappa B inhibitor
- NF-κB
Nuclear factor kappa B
- PDTC
Pyrrolidinedithiocarbamic acid
- PKC
Protein kinase C
- TLR2
Toll-like receptor 2
- TLR4
Toll-like receptor
- TLRs
Toll-like receptors
- TNFR1
Tumor necrosis factor receptor 1
- TNF-α
Tumor necrosis factor-α
- TRADD
TNF receptor associated death domain
Authors’ contributions
LH, JX and SL designed the project and revised the manuscript. LH performed the experiment, analyzed the data and wrote the manuscript. LH, ZZ, CD and YZ analyzed the data. LH, CW, GY, LG and CN collected the serum and liver samples. All authors read and approved the final manuscript.
Funding
This work was financially supported by Agricultural Science and Technology Innovation Program of Jilin Province (CXGC2017ZD011), National Natural Science Foundation of China (31570507), Modern Agroindustrial Technology Research Systems in China (CARS-36), and Changchun Industry-University-Research & Innovation demonstration site construction project (16CX20). The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing this manuscript.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its supplementary information files.
Ethics approval and consent to participate
All animal procedures were approved by the Institutional Animal Care and Use Committee of Jilin University (SCXK 2015–0001).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Li Huang, Email: huangl332@nenu.edu.cn.
Zijian Zhao, Email: zhaojaas@163.com.
Cuicui Duan, Email: duancui19841111@163.com.
Chao Wang, Email: applechao@163.com.
Yujuan Zhao, Email: zhaoyujuan2046@yahoo.com.cn.
Ge Yang, Email: yangge1900@163.com.
Lei Gao, Email: narcc501@126.com.
Chunhua Niu, Email: narcc318@126.com.
Jingbo Xu, Phone: +86 431 89165610, Email: xujb515@nenu.edu.cn.
Shengyu Li, Phone: +86 431 87063289, Email: lisy720@126.com.
References
- 1.Chatterjee R, Mitra A. An overview of effective therapies and recent advances in biomarkers for chronic liver diseases and associated liver cancer. Int Immunopharmacol. 2015;24(2):335–345. doi: 10.1016/j.intimp.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 2.IARC . Monographs on the evaluation of carcinogenic risks in humans. Lyon: International Agency for Research on Cancer (IARC). IARC Press; 2002. [Google Scholar]
- 3.Mehrzad J, Malvandi AM, Alipour M, Hosseinkhani S. Environmentally relevant level of aflatoxin B1 elicits toxic pro-inflammatory response in murine CNS-derived cells. Toxicol Lett. 2017;279:96–106. doi: 10.1016/j.toxlet.2017.07.902. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, Wang Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Microbiol. 2018;9:1953. doi: 10.3389/fmicb.2018.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim WG, Kim HI, Kwon EK, Han MJ, Kim DH. Lactobacillus plantarum LC27 and Bifidobacterium longum LC67 mitigate alcoholic steatosis in mice by inhibiting LPS-mediated NF-κB activation through restoration of the disturbed gut microbiota. Food Funct. 2018;9(8):4255–4265. doi: 10.1039/C8FO00252E. [DOI] [PubMed] [Google Scholar]
- 6.Lin X, Xia Y, Wang G, Xiong Z, Zhang H, Lai F, Ai L. Lactobacillus plantarum AR501 alleviates the oxidative stress of D-galactose-induced aging mice liver by upregulation of Nrf2-mediated antioxidant enzyme expression. J Food Sci. 2018;83(7):1990–1998. doi: 10.1111/1750-3841.14200. [DOI] [PubMed] [Google Scholar]
- 7.Shukla Pradeep K., Meena Avtar S., Manda Bhargavi, Gomes-Solecki Maria, Dietrich Paula, Dragatsis Ioannis, Rao RadhaKrishna. Lactobacillus plantarum prevents and mitigates alcohol-induced disruption of colonic epithelial tight junctions, endotoxemia, and liver damage by an EGF receptor–dependent mechanism. The FASEB Journal. 2018;32(11):6274–6292. doi: 10.1096/fj.201800351R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li C, Nie SP, Zhu KX, Ding Q, Li C, Xiong T, Xie MY. Lactobacillus plantarum NCU116 improves liver function, oxidative stress and lipid metabolism in rats with high fat diet induced non-alcoholic fatty liver disease. Food Funct. 2014;5(12):3216–3223. doi: 10.1039/C4FO00549J. [DOI] [PubMed] [Google Scholar]
- 9.Zhang X, Wua Y, Wang Y, Wang X, Piao C, Liu H, Liu Y, Wang Y. The protective effects of probiotic-fermented soymilk on high-fat diet-induced hyperlipidemia and liver injury. J Funct Foods. 2017;30:220–227. doi: 10.1016/j.jff.2017.01.002. [DOI] [Google Scholar]
- 10.Rishi P, Bharrhan S, Singh G, Kaur IP. Effect of Lactobacillus plantarum and L-arginine against endotoxin-induced liver injury in a rat model. Life Sci. 2011;89(23–24):847–853. doi: 10.1016/j.lfs.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 11.Osman N, Adawi D, Ahrné S, Jeppsson B, Molin G. Endotoxin- and D-galactosamine-induced liver injury improved by the administration of Lactobacillus, Bifidobacterium and blueberry. Dig Liver Dis. 2007;39(9):849–856. doi: 10.1016/j.dld.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Peng X, Jiang Y. Protective effects of Lactobacillus plantarum NDC 75017 against lipopolysaccharide-induced liver injury in mice. Inflammation. 2014;37(5):1599–1607. doi: 10.1007/s10753-014-9886-1. [DOI] [PubMed] [Google Scholar]
- 13.Zhai Q, Wang G, Zhao J, Liu X, Tian F, Zhang H, Chen W. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl Environ Microbiol. 2013;79(5):1508–1515. doi: 10.1128/AEM.03417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Y, Duan C, Gao L, Yu X, Niu C, Li S. Genome shuffling of Lactobacillus plantarum C88 improves adhesion. Biosci Biotechnol Biochem. 2017;81(1):184–193. doi: 10.1080/09168451.2016.1224637. [DOI] [PubMed] [Google Scholar]
- 15.Li S, Zhao Y, Zhang L, Zhang X, Huang L, Li D, Niu C, Yang Z, Wang Q. Antioxidant activity of Lactobacillus plantarum strains isolated from traditional Chinese fermented foods. Food Chem. 2012;135(3):1914–1919. doi: 10.1016/j.foodchem.2012.06.048. [DOI] [PubMed] [Google Scholar]
- 16.Duan C, Zhao Y, Huang C, Zhao Z, Gao L, Niu C, Wang C, Liu X, Zhang C, Li S. Hepatoprotective effects of Lactobacillus plantarum C88 on LPS/DGalN-induced acute liver injury in mice. J Funct Foods. 2018;43:146–153. doi: 10.1016/j.jff.2018.02.005. [DOI] [Google Scholar]
- 17.Zhao L, Jiang Y, Ni Y, Zhang T, Duan C, Huang C, Zhao Y, Gao L, Li S. Protective effects of Lactobacillus plantarum C88 on chronic ethanolinduce liver injury in mice. J Funct Foods. 2017;35:97–104. doi: 10.1016/j.jff.2017.05.017. [DOI] [Google Scholar]
- 18.Huang L, Duan C, Zhao Y, Gao L, Niu C, Xu J, Li S. Reduction of aflatoxin B1 toxicity by Lactobacillus plantarum C88: a potential probiotic strain isolated from Chinese traditional fermented food “tofu”. PLoS One. 2017;12(1):e0170109. doi: 10.1371/journal.pone.0170109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinton DM, Myers MJ, Raybourne RA, Francke-Carroll S, Sotomayor RE, Shaddock J, Warbritton A, Chou MW. Immunotoxicity of aflatoxin B1 in rats: effects on lymphocytes and the inflammatory response in a chronic intermittent dosing study. Toxicol Sci. 2003;73(2):362–377. doi: 10.1093/toxsci/kfg074. [DOI] [PubMed] [Google Scholar]
- 20.Qin H, Li H, Zhou X, Peng C, Tan H, Wang M. Effect of superoxide and inflammatory factor on aflatoxin B1 triggered hepatocellular carcinoma. Am J Transl Res. 2016;8(9):4003–4008. [PMC free article] [PubMed] [Google Scholar]
- 21.Jebali R, Ben Salah-Abbès J, Abbès S, Hassan AM, Abdel-Aziem SH, El-Nekeety AA, Oueslati R, Abdel-Wahhab MA. Lactobacillus plantarum alleviate aflatoxins (B1 and M1) induced disturbances in the intestinal genes expression and DNA fragmentation in mice. Toxicon. 2018;146:13–23. doi: 10.1016/j.toxicon.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 22.Luedde T, Schwabe RF. NF-κB in the liver--linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011;8(2):108–118. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrzad J, Bahari A, Bassami MR, Mahmoudi M, Dehghani H. Immunobiologically relevant level of aflatoxin B1 alters transcription of key functional immune genes, phagocytosis and survival of human dendritic cells. Immunol Lett. 2018;197:44–52. doi: 10.1016/j.imlet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 24.Mohammadi A, Mehrzad J, Mahmoudi M, Schneider M. Environmentally relevant level of aflatoxin B1 dysregulates human dendritic cells through signaling on key toll-like receptors. Int J Toxicol. 2014;33(3):175–186. doi: 10.1177/1091581814526890. [DOI] [PubMed] [Google Scholar]
- 25.Ben Ari Z, Avlas O, Pappo O, Zilbermints V, Cheporko Y, Bachmetov L, Zemel R, Shainberg A, Sharon E, Grief F, Hochhauser E. Reduced hepatic injury in toll-like receptor 4-deficient mice following D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure. Cell Physiol Biochem. 2012;29(1–2):41–50. doi: 10.1159/000337585. [DOI] [PubMed] [Google Scholar]
- 26.Zhao D, Wu T, Yi D, Wang L, Li P, Zhang J, Hou Y, Wu G. Dietary supplementation with Lactobacillus casei alleviates lipopolysaccharide-induced liver injury in a porcine model. Int J Mol Sci. 2017;18(12):2535. doi: 10.3390/ijms18122535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma Q, Li Y, Fan Y, Zhao L, Wei H, Ji C, Zhang J. Molecular mechanisms of lipoic acid protection against aflatoxin B1-induced liver oxidative damage and inflammatory responses in broilers. Toxins (Basel) 2015;7(12):5435–5447. doi: 10.3390/toxins7124879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun KY, Xu DH, Xie C, Plummer S, Tang J, Yang XF, Ji XH. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine. 2017;92:1–11. doi: 10.1016/j.cyto.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 29.González-Rascón A, Mata-Haro V. MicroRNAs: regulators of TLR2-mediated probiotic immune response. MicroRNA. 2015;4(3):168–174. doi: 10.2174/2211536605666151208123209. [DOI] [PubMed] [Google Scholar]
- 30.Kim CH, Kim HG, Kim JY, Kim NR, Jung BJ, Jeong JH, Chung DK. Probiotic genomic DNA reduces the production of pro-inflammatory cytokine tumor necrosis factor-alpha. FEMS Microbiol Lett. 2012;328(1):13–19. doi: 10.1111/j.1574-6968.2011.02470.x. [DOI] [PubMed] [Google Scholar]
- 31.Berghe TV, Linkermann A, Jouan-Lanhouet S, Walczak H, Vandenabeele P. Regulated necrosis: the expanding network of non-apoptotic cell death pathways. Nat Rev Mol Cell Biol. 2014;15:135–147. doi: 10.1038/nrm3737. [DOI] [PubMed] [Google Scholar]
- 32.Petrof EO, Claud EC, Sun J, Abramova T, Guo Y, Waypa TS, He SM, Nakagawa Y, Chang EB. Bacteria-free solution derived from Lactobacillus plantarum inhibits multiple NF-kappaB pathways and inhibits proteasome function. Inflamm Bowel Dis. 2009;15(10):1537–1547. doi: 10.1002/ibd.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Papa S, Zazzeroni F, Pham CG, Bubici C, Franzoso G. Linking JNK signaling to NF-kappaB: a key to survival. J Cell Sci. 2004;117(Pt 22):5197–5208. doi: 10.1242/jcs.01483. [DOI] [PubMed] [Google Scholar]
- 34.Kühnel F, Zender L, Paul Y, Tietze MK, Trautwein C, Manns M, Kubicka S. NF-kappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275(9):6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Wang C, Wang C, Zhao H, Zhao C, Chen Y, Wang Y, McClain C, Feng W. Enhanced AMPK phosphorylation contributes to the beneficial effects of Lactobacillus rhamnosus GG supernatant on chronic-alcohol-induced fatty liver disease. J Nutr Biochem. 2015;26(4):337–344. doi: 10.1016/j.jnutbio.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hirsova P, Gores GJ. Death receptor-mediated cell death and proinflammatory signaling in nonalcoholic steatohepatitis. Cell Mol Gastroenterol Hepatol. 2015;1(1):17–27. doi: 10.1016/j.jcmgh.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou X, Jiang W, Liu Z, Liu S, Liang X. Virus infection and death receptor-mediated apoptosis. Viruses. 2017;9(11):316. doi: 10.3390/v9110316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Josse R, Dumont J, Fautrel A, Robin MA, Guillouzo A. Identification of early target genes of aflatoxin B1 in human hepatocytes, inter-individualvariability and comparison with other genotoxic compounds. Toxicol Appl Pharmacol. 2012;258(2):176–187. doi: 10.1016/j.taap.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Hu WS, Rajendran P, Tzang BS, Yeh YL, Shen CY, Chen RJ, Ho TJ, Vijaya Padma V, Chen YH, Huang CY. Lactobacillus paracasei GMNL-32 exerts a therapeutic effect on cardiac abnormalities in NZB/W F1 mice. PLoS One. 2017;12(9):e0185098. doi: 10.1371/journal.pone.0185098. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The raw datas of biochemical parameters in serum, include ALT, AST, ALP, total cholesterol, triglycerides, total protein and albumin. (XLSX 14 kb)
The raw datas of IL-1β, IL-6, IL-8, IL-10, IFN-γ, and TNF-α in serum. (XLSX 15 kb)
The raw datas of Bax, Bcl-2 and Caspase-3 in liver. (XLSX 13 kb)
The ARRIVE Guidelines Checklist. (PDF 1067 kb)
Data Availability Statement
All data generated or analysed during this study are included in this published article and its supplementary information files.