Abstract
Background
The American Academy of Neurology (AAN) does not recommend routine use of prophylactic antiepileptic drugs (pAEDs) in patients with newly diagnosed brain tumors. If used in the perioperative setting, discontinuation is suggested after the first postoperative week. It is unclear whether such recommendations are followed. Our objective was to compare our perioperative and long-term pAED use in glioma patients with AAN practice parameters.
Methods
Retrospective chart review was performed on 578 glioma patients from 2006 to 2013. Seizures and AED use were assessed at surgery, 3 months postoperatively and death, last visit or 16 months postoperatively. Patients were divided into three groups at surgery: seizure-free with pAED, seizure-free without pAED, and seizure patients. Long-term pAED use was defined as continued use at 3 months postsurgery without seizures. pAEDs efficacy, factors influencing its use, and survival were examined.
Results
Out of 578 patients identified, 330 (57.1%) were seizure-naïve preoperatively. There were no significant differences in age, histology, tumor location or resection status between seizure-free populations with and without prophylaxis. Of 330 seizure-naïve patients, 205 (62.1%) received pAEDs at surgery. Ninety-six (46.9%) of those patients were still on pAEDs 3 months postsurgery (median use = 58 days). Rate of long-term prophylaxis use decreased by 13.5% over 6 years (70.3% in 2006; 56.8% in 2012). Phenytoin was preferred in 2006 (98.2%) with increasing use of levetiracetam over 6 years (44.6% in 2012). The only predictive factor for pAED use was complete resection (P = .0069). First seizure prevalence was similar in both seizure-free populations (P = .91). The seizure population had more men (P = .007), younger patients (P < .0001), lower-grade gliomas (P = .0003) and survived longer (P = .001) compared with seizure-free populations.
Conclusions
In our center, long-term prophylactic AED use is high, deviating from current AAN Guidelines. Corrective measures are warranted.
Keywords: antiepileptic drug, Glioma, primary brain tumor, prophylaxis, seizures
Over 66 000 new cases of primary brain tumors, including over 18 500 cases of gliomas, are estimated to have be diagnosed in the United States in 2014.1 Approximately 40% of patients with primary brain tumors may experience seizures prior to surgical intervention, while an additional 20% of patients may have seizures later in the disease course.2,3 For these patients, the need for antiepileptic drug treatment is clear.4 While the use of prophylactic antiepileptic drugs (AEDs) was previously widely accepted and common practice,5 several studies have failed to demonstrate their efficacy in preventing a first seizure. This led the American Academy of Neurology in 2000 to issue recommendations against the use of prophylactic AEDs in seizure-naïve patients with newly diagnosed brain tumors and, if administered, to discontinue them in the first postoperative week in stable patients.4 Since their publication, these practice parameters have been supported by many other reviews,2,6,7 as well as the most recent prospective randomized controlled study on the topic.3
Despite the recommendations on evidence-based practice parameters, data leading up to 2009 show that many physicians in North America and Europe continue to prescribe prophylactic AEDs.2,8–11 More recently, a 4-year retrospective study conducted in Brazil observed a 70% rate of prophylactic AED use at two weeks postoperatively, with a minority of patients discontinuing use after a mean follow-up of five months.8 In North America, current practice patterns regarding prophylactic AED use in patients with gliomas remains unclear. Moreover, no study has reported long-term use of prophylactic AEDs and changes in patterns of practice over years.
The objective of this study was to assess our center's perioperative and long-term AED prophylaxis practice patterns in newly diagnosed patients with gliomas.
Materials and Methods
Patient Population
All patients with histologically proven gliomas evaluated in our tertiary care center between January 2006 and January 2013 were identified from our neuro-oncology database. We included low-grade gliomas (astrocytomas, oligodendrogliomas, and mixed gliomas) and high-grade gliomas (anaplastic gliomas, and glioblastoma multiformes [GBM]). Patients with spinal cord gliomas and ependymomas were excluded. Data extraction included demographics; date and extent of resection (biopsy, partial or gross complete); tumor site and histological classification (according to the World Health Organization); AED use, if applicable (type, number of AEDs, duration); seizure status at three months postoperatively; date of first seizure, if it occurred; and date of last visit or death. Long-term prophylactic AED use was defined as continued use of AED(s) at 3 months after surgery in the absence of seizures. For patients with multiple surgical interventions, only data pertaining to the first surgery were recorded. Seizures were recorded as documented by physicians in the medical chart.
Postoperative Period and Follow-up
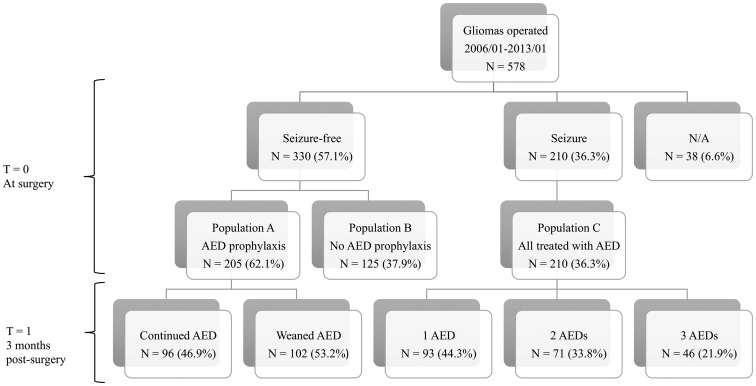
Information regarding seizures and AEDs were assessed at time of surgery (T = 0), at 3 months postoperatively (T = 1), and at death, last visit, or 16 months of follow-up (T = 2). At time of surgery, patients were divided into three groups: patients who were seizure-free and received prophylactic AEDs (population A); patients who were seizure-free and did not receive prophylactic AEDs (population B); and patients with prior seizures (population C) (Fig. 1). The local institutional ethical committee approved this study and decided that the patient's written consent was not necessary for this retrospective chart review. All collected data was anonymized.
Fig. 1.
Study design. AED, Antiepileptic Drug.
Outcomes
The primary outcome was the rate of long-term prophylactic AED use in seizure-free patients with glioma at 3 months postoperatively. Secondary outcomes were: overall rate of AED use in seizure-free patients and its trend through years; median prophylaxis duration; rates of different AEDs used and AED preference evolution over the years; efficacy of prophylactic AED use in preventing a first seizure; and overall survival estimate per group. Additionally, we aimed to characterize the seizure group and their AED therapy (preferred first-line drug, importance of polytherapy).
Statistical Analysis
Statistical analysis was performed in conjunction with our institution's Biostatistics Consultation Platform. All statistical analyses were performed with R3.0.2 computer software. A P value ≤ .05 was considered statistically significant. Hazard ratios (HR) and odd ratios (OR) are presented with 95% confidence intervals (CIs).
Descriptive statistics were used to describe the demographic and clinical characteristics of our population. Chi-square tests were performed to compare categorical variables. Logistic regression analysis was done to estimate the association between various predictors (patient's age, gender, tumor grade, location and extent of resection) and the use of AED prophylaxis. The proportion of prophylaxis use at 3 months was estimated using a Kaplan-Meier plot, adjusting for death and loss to follow-up, with the event being prophylaxis discontinuation. A time-dependent Cox proportional hazards regression model (univariate and multivariate) was used to evaluate time from surgery to first seizure by AED prophylaxis use at that time, and to compare survival of seizure-free patients on AED prophylaxis to other populations. A log-rank test was used to compare overall survival curves between seizure-naïve with or without AED prophylaxis groups. The date of diagnosis used to calculate survival was that of the first meeting with our neuro-oncology team. The minimal follow-up period for survival analysis was 16 months. A Cox proportional patient's hazards regression model was used to compare overall survival between the 3 groups.
Results
Patient Population
Between 2006 and 2013, a total of 578 patients with a histological diagnosis of glioma who underwent surgery were evaluated at our neuro-oncology clinic with a minimum follow-up of 16 months. The main clinical characteristics of the cohort are listed in Table 1. Median age was 59 years (range 49–66), and 58% were males. Four hundred sixty-two patients (79.9%) had GBM while the remainder had various gliomas (astrocytoma, oligodendroglioma, mixed oligoastrocytoma). High-grade gliomas represented 87.7% of gliomas. The majority of patients (52.4%) had biopsies. Complete tumor resection was achieved in 37.7% of patients.
Table 1.
Patient demographics, tumor characteristics, and surgical management
| Parameters | General Population | Population A No Seizure (Prophylactic AED) | Population B No Seizure (No Prophylactic AED) | Population C Seizure (AED Therapy) | N/A* | P Valuea |
|---|---|---|---|---|---|---|
| Total number | 578* | 205 (35.5%) | 125 (21.6%) | 210 (36.3%) | 38 (6.6%) | |
| Median age, y (range) | 59 (49–66) | 60 (50–67) | 62 (53–67) | 55 (45–64) | <.0001 | |
| Age at diagnosis, y | ||||||
| <40 | 52 (9%) | 15 (7.3%) | 5 (4.0%) | 37 (17.6%) | ||
| 40–60 | 265 (46%) | 93 (45.4%) | 50 (40.0%) | 101 (48.1%) | ||
| ≥60 | 261 (45%) | 97 (47.3%) | 70 (56.0%) | 72 (34.3%) | ||
| Men, No. | 337 (58.3%) | 107 (52.2%) | 66 (52.8%) | 139 (66.2%) | 25 (65.8%) | .007 |
| Tumor location, lobe | .53 | |||||
| Frontal | 232 (40.1%) | 83 (40.5%) | 42 (33.6%) | 95 (45.2%) | 12 (31.6%) | |
| Temporal | 184 (31.8%) | 65 (31.7%) | 47 (37.6%) | 65 (31.0%) | 7 (18.4%) | |
| Parietal | 117 (20.2%) | 45 (22.0%) | 27 (21.6%) | 40 (19.0%) | 5 (13.2%) | |
| Occipital | 26 (4.5%) | 9 (4.4%) | 8 (6.4%) | 9 (4.3%) | 0 | |
| Imprecise | 19 (3.3%) | 3 (1.5%) | 1 (0.1%) | 1 (0.5%) | 24 (63.1%) | |
| Histology | <.0001 | |||||
| GBM | 462 (79.9%) | 117 (86.3%) | 112 (89.6%) | 139 (66.2%) | ||
| LGGb | 71 (12.3%) | 16 (7.8%) | 7 (5.6%) | 47 (22.4%) | 1 (2.6%) | .0003 |
| Astrocytoma | 20 (3.5%) | 3 (1.5%) | 3 (2.4%) | 14 (6.7%) | ||
| Oligodendroglioma | 13 (2.2%) | 3 (1.5%) | 0 (0%) | 9 (4.3%) | ||
| Mixed gliomac | 38 (6.6%) | 10 (4.9%) | 4 (3.2%) | 24 (11.4%) | ||
| HGGd | 507 (87.7%) | 189 (92.2%) | 118 (94.4%) | 163 (77.6%) | 37 (97.4%) | |
| Anaplastic astrocytoma | 30 (5.2%) | 7 (3.4%) | 5 (4.0%) | 17 (8.1%) | ||
| Anaplastic oligodendroglioma | 15 (2.6%) | 5 (2.4%) | 1 (0.8%) | 7 (3.3%) | ||
| Extent of resection | .09 | |||||
| Biopsy | 303 (52.4%) | 95 (46.3%) | 75 (60.0%) | 114 (54.3%) | 19 (50.0%) | |
| Subtotal | 57 (9.9%) | 19 (9.3%) | 11 (8.8%) | 24 (11.4%) | 3 (7.9%) | |
| Gross total | 218 (37.7%) | 91 (44.4%) | 39 (31.2%) | 72 (34.3%) | 16 (42.1%) | |
| Died at 3 months | 72 (12.5%) | 36 (17.6%) | 20 (16.0%) | 16 (7.6%) |
Categorial variables: N (%), Values (%); Continued variables: Median value (range).
Patients were only categorized for their first surgical intervention.
Abbreviations: AED, antiepileptic drug; GBM, glioblastoma multiforme; LGG, low-grade glioma; HGG, high-grade glioma.
a P values for comparison of seizure vs no-seizure populations (3 populations).
bLow-grade glioma: astrocytoma; oligodendroglioma; oligoastrocytoma.
cMixed glioma: oligoastrocytoma.
dHigh-grade glioma: anaplastic astrocytoma; anaplastic oligodendroglioma; GBM (there was no anaplastic oligoastrocytoma).
*First seizure could not be determined with certainty from records in 38 patients (N/A).
Seizure-free Populations
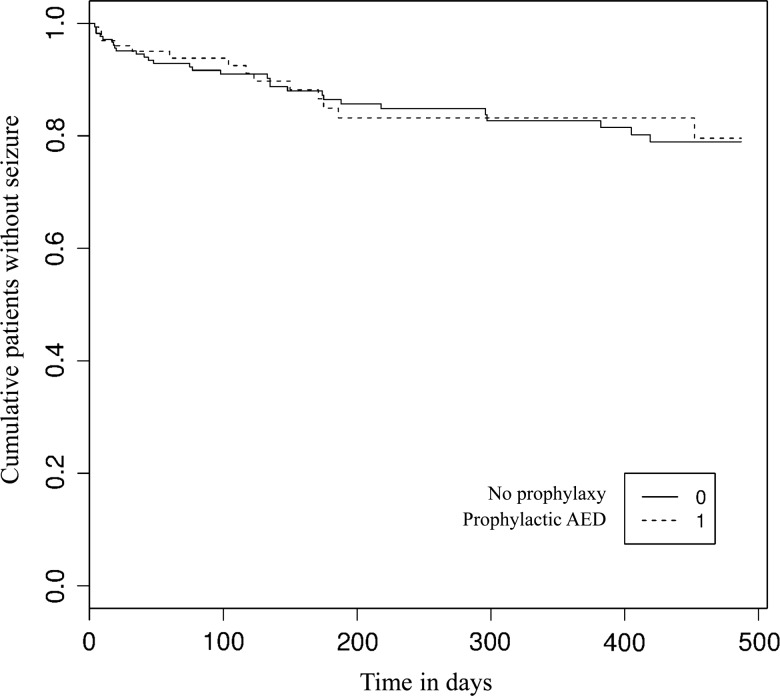
In this cohort, 330 of 578 patients (57.1%) were seizure-naïve preoperatively. Prophylactic AEDs were given to 205 (62.1%) patients at the time of surgery. At 3 months after surgery, 96 out of 205 (46.9%; 95% CI 40.2–54.7) were still on AEDs with a median use of 58 days (95% CI 31–152) (Fig. 1). Prevalence of long-term prophylaxis use slightly decreased over a period of six years (70.3% in 2006 vs 56.8% in 2012) (Supplementary Fig. S1). Phenytoin was the preferred agent in 2006 (98.2%) with increasing use of levetiracetam over the years (44.6% in 2012) (Supplementary Fig. S2). There were no significant differences in age; tumor histology, grade, and location; and resected status between the seizure-free populations with and without prophylaxis (Table 1). Logistic regression revealed that the only predictive factor associated with prophylactic AED use was extent of tumor resection for patients with seizures compared with patients without seizures (Table 2). Patients with complete resection received 2.03 times more prophylaxis than patients with biopsy only (P = .007, OR = 2.0292, 95% CI 1.2202–3.4177). The use of prophylactic AED was not associated with a reduction in the prevalence of a first seizure in perioperative and long-term settings (P = .91, HR = 0.9627, 95% CI 0.5153–1.806) (Table 3). At 2 weeks, 3 months, and 16 months postoperatively, 97.2%, 91.6% and 78.9% of patients on AED prophylaxis were still seizure-free, respectively, compared with 96.9%, 93.8% and 79.6% of patients without prophylaxis (Fig. 2).
Table 2.
Logistic regression for predictive factors of prophylactic AED use (population A compared to population B)
| Parameters | OR (odds ratio) | 95% CI | P Value |
|---|---|---|---|
| Age at diagnosis | 0.9878 | 0.9671-1.0080 | .23 |
| Sex (male) | 0.9244 | 0.5810-1.4668 | .74 |
| Occipital lobe | 0.5572 | 0.1945-1.6151 | .27 |
| Parietal lobe | 0.8954 | 0.4845-1.6663 | .73 |
| Temporal lobe | 0.6101 | 0.3488-1.0586 | .08 |
| Gross complete resectiona | 2.0292 | 1.2206-3.4177 | .007 |
| Partial resectiona | 1.4587 | 0.6405-3.4620 | .38 |
| LGG vs HGG | 0.7703 | 0.2801-1.9421 | .59 |
aCompared with biopsy. Abbreviations: LGG, low-grade glioma; HGG, high-grade glioma.
Population A, seizure-free patients without AED prophylaxis; Population B, seizure-free with AED prophylaxis.
Table 3.
Univariate & multivariate Cox proportional hazards analysis of first seizure prevalence by use of antiepileptic drug (AED) prophylaxis (or not), controlling for factors influencing seizure rate (population A compared with population B)c using a time-dependent Cox proportional hazards regression model (see also Figure 2)
| AED | HR (hazard ratio) | 95% CI | P Value |
|---|---|---|---|
| On first AED | 0.9647 | 0.5153-1.8060 | .91 |
| Controlled Factors | HR (hazard ratio) | 95% CI | P Value |
| On first AED | 1.0225 | 0.5410-1.9324 | .95 |
| Age at diagnosis | 1.0038 | 0.9772-1.0311 | .78 |
| Sex (male) | 1.3897 | 0.7526-2.5660 | .29 |
| Gross complete resectiona | 0.3719 | 0.1839-0.7523 | .006 |
| Partial resectionb | 1.4357 | 0.6133-3.3606 | .40 |
| LGG vs HGG | 1.5263 | 0.3605-6.4625 | .57 |
aGross complete resection, resection of ≥ 95% of the preoperatively defined contrast-enhancing tumor portions on first MRI < 48 hours postsurgery.
bPartial resection, resection of < 95% of the preoperatively defined contrast-enhancing tumor portions on first MRI < 48 hours postsurgery.
cPopulation A, seizure-free patients without AED prophylaxis; Population B, seizure-free with AED prophylaxis.
Abbreviations: LGG, low-grade glioma; HGG, high-grade glioma.
Fig. 2.
Efficacy of prophylactic AED to prevent a first seizure in populations A and B. When a patient from population A was discontinuing his or her prophylactic AED, he or she was categorized in population B.
Seizure Population
Of the 578 glioma patients, 210 (36.3%) had at least one seizure preoperatively. Our seizure population was younger (17.6% <40 years) (P < .0001) and had a higher proportion of males (66.2%) (P = .007) and low-grade gliomas (22.5%) (P = .0003) compared with the seizure-free populations. There was no difference in the tumor location or resected status between seizure and seizure-free populations (Table 1). All 210 patients were treated with AEDs: 93 (44.3%) were on monotherapy, 71 (33.8%) were on duo therapy and 46 (21.9%) were on a combination of three AEDs (Fig. 1). Overall, the most commonly administered first-line AED was phenytoin, (81.8%) followed by levetiracetam (14.1%) and carbamazepine (2.4%). Oxcarbazepine, topiramate, and clobazam each represented less than 0.5% of prescribed AEDs.
Survival
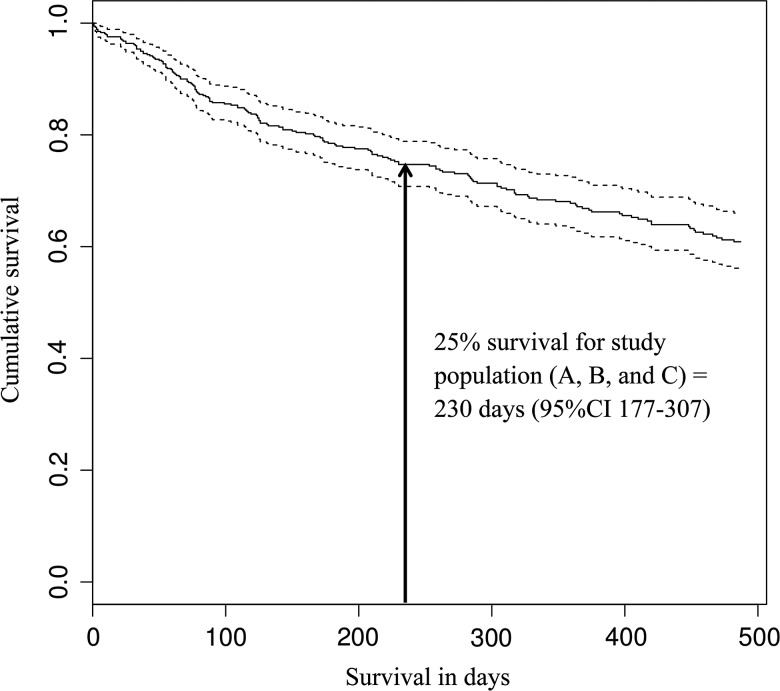
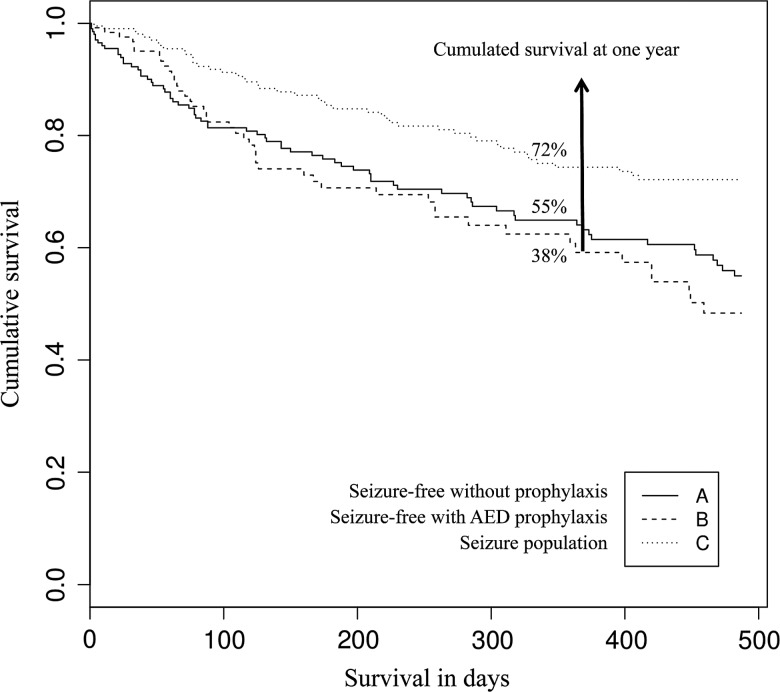
The first quartile (25%) survival estimate for our entire study population was 230 days (95% CI 177–307) (Fig. 3). Of the 578 patients, 72 patients (12.5%) were dead at three months (56 [32.7%] in populations A and B vs 16 [7.6%] in population C). Seventy percent of patients (95% CI 63–75) survived one year or longer. By the end of the study period, 40% of patients had died (45% in population A, 62% in population B and 28% in population C). Population C survived significantly longer than populations A and B (P = .004) (Fig. 4). The HR estimated with a Cox proportional hazards regression model and adjusted for sex, age, tumor location and resection status was 0.56 (95% CI 0.3805–0.8298) (Table 4), with age at diagnosis (P < .0001) and complete resection (P < .0001) influencing survival the most. Survival time for seizure-free patients was 1.78 times shorter than survival time for seizure patients, whether or not seizure-free patients were on AED prophylaxis.
Fig. 3.
Overall survival in study population. Population A, seizure-free patients without AED prophylaxis; Population B, seizure-free with AED prophylaxis; Population C, seizure population.
Fig 4.
Overall survival in 3 groups (Kaplan-Meier). The seizure population (C) survived longer than the seizure-free populations (A and B) (P = .04).
Table 4.
Univariatea & multivariatea Cox proportional hazards analysis for survival, controlling for factors influencing survival
| Populationa | HR (hazard ratio) | 95% CI | P Value |
|---|---|---|---|
| Population B | 1.1319 | 0.7770-1.6488 | .52 |
| Population C | 0.5423 | 0.3742, 0.7859 | .001 |
| Controlled Factors | HR (hazard ratio) | 95%CI | P Value |
| Population B | 0.8574 | 0.5758-1.2471 | .40 |
| Population C | 0.5619 | 0.3805-0.8298 | .004 |
| Sex (male) | 1.0429 | 0.7516-1.4384 | .80 |
| Age at diagnosis | 1.0599 | 1.0434-1.0768 | <.0001 |
| Occipital lobe | 0.5931 | 0.2694-1.3059 | .19 |
| Parietal lobe | 1.1238 | 0.7320-1.7255 | .59 |
| Temporal lobe | 1.0810 | 0.7487-1.5608 | .68 |
| LGG vs HGG | 1.9961 | 0.9520-4.1854 | .07 |
| Gross complete resection | 0.3835 | 0.2658-0.5534 | <.0001 |
| Partial resection | 0.6005 | 0.3333-1.0821 | .09 |
aCompared with population A. Abbreviations: LGG, low-grade glioma; HGG, high-grade glioma.
Population A, seizure-free without AED prophylaxis; Population B, seizure-free with AED prophylaxis; Population C, seizure population.
Discussion
Our study showed that in our glioma cohort, spanning 6 years (2006-2013), over 60% of seizure-naïve patients were on prophylactic AEDs at the time of surgery and close to a third were still receiving AEDs at 3 months after surgery. The high use of AED prophylaxis suggests that the AAN practice parameters for anticonvulsant prophylaxis in patients with newly diagnosed brain tumors are not followed. Indeed, the AAN quality standard subcommittee recommended in 2000 that prophylactic anticonvulsants not be used routinely in patients with newly diagnosed brain tumors. Furthermore, in patients with brain tumors who had not had a seizure, tapering and discontinuing anticonvulsants after the first postoperative week was deemed appropriate, particularly for those who are medically stable and who are experiencing anticonvulsant-related side effects. In addition to their inability to prevent a first seizure in this context, the risk of adverse events is yet another reason to avoid unnecessary use of these drugs. Potential side effects include nausea, dizziness, tremor, blurred vision, gait unsteadiness, cognitive impairment, dermatologic reactions, liver dysfunction and myelosuppression. Furthermore, enzyme inducers such as phenytoin, phenobarbital, and carbamazepine may reduce corticosteroids' efficacy, and result in markedly accelerated metabolism of some chemotherapeutic agents used in gliomas such as nitrosoureas, irinotecan, and methotrexate. Corticosteroids and chemotherapeutic agents may in turn alter AED metabolism, resulting in underdosing or overdosing.4
Our current observations corroborate studies performed both prior to and following the publication of the 2000 AAN practice parameters. A prospective longitudinal database of malignant glioma patients seen at 55 centers between 1997 and 2000 showed that 57% of North American patients were receiving prophylactic AEDs.11 In a retrospective study from Brazil (2008–2012), a 70.2% rate of AED prophylaxis was reported at 1 and 2 weeks after surgery.8 Finally, two practice surveys on AED prophylaxis for glioma patients in Canada (2003) and the United States (2005) showed that 55% and 70% of responders, respectively, either recommended or reported the routine use of AED prophylaxis.10,12 It is noteworthy that only 9% of Canadian physician responders were aware of existing guidelines in the 2003 survey,10 suggesting that physicians prescribing prophylactic AEDs continue to do so because it is common practice rather than because they disagree with the AAN practice parameters. Findings from our study show that since 2006, use of prophylactic AEDs has remained common practice, even several years after the publication of the practice parameters. Only minimal reduction of AED prophylaxis use was seen over the 6-year period of our study. The fact that the only predictive factor associated with AED prophylaxis use was the extent of resection may imply that neurosurgeons fear seizures in craniotomies and gross total resections to a greater extent than with biopsies.
While the rate of AED prophylaxis has remained relatively static over the years, our data indicate an important change in the choice of anticonvulsant. The use of levetiracetam rose from 2% before 2008 to 44.6% in 2012. The shift from phenytoin to levetiracetam as the anticonvulsant of choice is most likely attributable to its favorable pharmacokinetic profile, rapid titration, lack of significant interactions with antineoplastic agents and steroids,13 and better safety profile. In three recent studies, levetiracetam was found to be at least as efficacious but with fewer side effects than phenytoin for the prevention of postoperative seizures in glioma patients.12,14,15 Nonetheless, until more data are available, anticonvulsant prophylaxis (even with newer and possibly better tolerated AEDs) is not supported due to an absence of short-term and long-term efficacy.3 Not surprisingly, the prophylactic use of AEDs in our own cohort did not influence the prevalence of first seizures, as previously reported.
The use of AED prophylaxis did not affect survival in seizure-naïve patients. Our seizure group, which was younger, predominantly male, and with more low-grade gliomas, showed significant increases in survival compared with seizure-naïve patients at the time of surgery. These results are consistent with previous studies16,17 and can be attributable to confounding effects of a younger age, associated with a lower grade pathology, and to the fact that tumors associated with seizures are recognized earlier, thereby conferring a better prognosis,18 rather than representing an independent risk factor for seizures.
Although this study is, to our knowledge, the largest and the longest retrospective cohort exploring anticonvulsant use in glioma patients, we acknowledge the limitations inherent to all retrospective studies. For example, some seizures may have gone unrecognized (especially if subtle or minor) or not mentioned at all in medical charts. The justifications for prescribing prophylactic AED to each individual patient, as well as side effects and toxicities of prophylactic AED use, could not be reliably determined by reviewing medical records. Postoperative follow-up visits were not scheduled in a systematic fashion at regular intervals or with standardized questionnaires. Type and dosage of anticonvulsant varied between patients and there was no formal assessment of compliance or serum drug level assessments.
Despite these limitations, we feel that our findings are an accurate reflection of our experiences in the neuro-oncology clinics and in agreement with previous studies. This report may reflect the need for further education regarding the use of AED prophylaxis for brain tumors and increased communication between treating services. Efforts have been initiated at our institution to correct this behaviour. A committee composed of neurosurgeons, neuro-oncologists, radiation oncologists, neurologists, medical oncologists, and oncology pivot nurse was created to standardize our interdisciplinary practice regarding our use of prophylactic AEDs in newly diagnosed gliomas; guidelines were posted on our institution's intranet; and oral presentations were given in each of the involved departments to raise awareness towards this topic. We plan to assess the impact of these interventions in a follow-up study. Looking forward, our study may be used as a new baseline comparison in future works to assess the efficacy of corrective measures in dissemination of guidelines.
Conclusion
We present patterns of care for a large group of patients with newly diagnosed glioma treated in the modern era. In our tertiary care center, perioperative and long-term prophylactic AED use is high, deviating from current AAN practice parameters and more recent literature. Corrective measures are hence necessary. An increase in new generation AED use for seizure prophylaxis was noted, which may warrant a critical appraisal of recent evidence with these newer AEDs to update AAN guidelines.
Funding
None.
Conflict of interest statement. The authors declare no conflict of interest.
Supplementary Material
References
- 1. Ostrom QT, Gittleman H, Farah P et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tremont-Lukats IW, Ratilal BO, Armstrong T, Gilbert MR. Antiepileptic drugs for preventing seizures in people with brain tumors. Cochrane Database Syst Rev. 2008(2):CD004424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wu AS, Trinh VT, Suki D et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013;118(4):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glantz MJ, Cole BF, Forsyth PA et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;54(10):1886–1893. [DOI] [PubMed] [Google Scholar]
- 5. Yin AA, Cai S, Dong Y et al. A meta-analysis of temozolomide versus radiotherapy in elderly glioblastoma patients. J Neurooncol. 2014;116(2):315–324. [DOI] [PubMed] [Google Scholar]
- 6. Vecht CJ, van Breemen M. Optimizing therapy of seizures in patients with brain tumors. Neurology. 2006;67(12 Suppl 4):S10–S13. [DOI] [PubMed] [Google Scholar]
- 7. Wen PY, Marks PW. Medical management of patients with brain tumors. Curr Opin Oncol. 2002;14(3):299–307. [DOI] [PubMed] [Google Scholar]
- 8. de Oliveira JA, Santana IA, Caires IQ et al. Antiepileptic drug prophylaxis in primary brain tumor patients: is current practice in agreement to the consensus? J Neurooncol. 2014;120(2):399–403. [DOI] [PubMed] [Google Scholar]
- 9. Bruna J, Miro J, Velasco R. Epilepsy in glioblastoma patients: basic mechanisms and current problems in treatment. Expert Rev Clin Pharmacol. 2013;6(3):333–344. [DOI] [PubMed] [Google Scholar]
- 10. Brouwers MC, Chambers A, Perry J, Neuro-oncology Disease Site G. Can surveying practitioners about their practices help identify priority clinical practice guideline topics? BMC Health Serv Res. 2003;3(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lwu S, Hamilton MG, Forsyth PA, Cairncross JG, Parney IF. Use of peri-operative anti-epileptic drugs in patients with newly diagnosed high grade malignant glioma: a single center experience. J Neurooncol. 2010;96(3):403–408. [DOI] [PubMed] [Google Scholar]
- 12. Siomin V, Angelov L, Li L, Vogelbaum MA. Results of a survey of neurosurgical practice patterns regarding the prophylactic use of anti-epilepsy drugs in patients with brain tumors. J Neurooncol. 2005;74(2):211–215. [DOI] [PubMed] [Google Scholar]
- 13. Crepeau AZ, Treiman DM. Levetiracetam: a comprehensive review. Expert Rev Neurother. 2010;10(2):159–171. [DOI] [PubMed] [Google Scholar]
- 14. Lim DA, Tarapore P, Chang E et al. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: a randomized phase II pilot study. J Neurooncol. 2009;93(3):349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kargiotis O, Markoula S, Kyritsis AP. Epilepsy in the cancer patient. Cancer Chemother Pharmacol. 2011;67(3):489–501. [DOI] [PubMed] [Google Scholar]
- 16. Ostrom QT, Bauchet L, Davis FG et al. The epidemiology of glioma in adults: a "state of the science" review. Neuro Oncol. 2014;16(7):896–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hildebrand J, Lecaille C, Perennes J, Delattre JY. Epileptic seizures during follow-up of patients treated for primary brain tumors. Neurology. 2005;65(2):212–215. [DOI] [PubMed] [Google Scholar]
- 18. Maschio M, Dinapoli L, Sperati F et al. Effect of pregabalin add-on treatment on seizure control, quality of life, and anxiety in patients with brain tumour-related epilepsy: A pilot study. Epileptic Disord. 2012;14(4):388–397. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.