Abstract
Purpose:
The aim of this article is to determine and compare the platelet activation by three main platelet activation parameters: mean platelet volume, platelet distribution width, and plateletcrit in patients with central retinal vein occlusion and control subjects.
Methods:
This study included 30 patients with nonischemic central retinal vein occlusion and 30 control subjects. The levels of mean platelet volume, platelet distribution width, and plateletcrit were measured in all groups.
Results:
The mean serum level of mean platelet volume was 10.01 ± 0.89 fl in central retinal vein occlusion group and 8.74 ± 1.45 fl in control group. The mean serum level of platelet distribution width was 14.31 ± 1.49% and 11.65 ± 1.81% in central retinal vein occlusion group and control group, respectively. Mean serum plateletcrit value was 0.27 ± 0.07% in central retinal vein occlusion group and 0.23 ± 0.07% in control group. Mean platelet volume, platelet distribution width, and plateletcrit levels were significantly higher in central retinal vein occlusion patients than controls (p < 0.05).
Conclusion:
Subclinical platelet activation reflected by mean platelet volume, platelet distribution width, and plateletcrit may have an impact on the genesis of vessel occlusion in central retinal vein occlusion. The results may be important for the clinical management of patients with central retinal vein occlusion.
Keywords: central retinal vein occlusion, mean platelet volume, platelet distribution width, platelet parameters, plateletcrit, retina
Introduction
Retinal vein occlusion (RVO) is the second common cause of retinal vascular disorder after diabetic retinopathy.1,2 Central retinal vein occlusion (CRVO) is a major cause of vision impairment and slightly more frequent in men than in women.2 The exact pathogenesis of CRVO remains unclear and controversial; it has been suggested that multiple factors including local anatomical susceptibility, degenerative changes of the vessel walls, and abnormal hematological abnormalities may contribute to the development of vascular obstruction.3 The alterations in platelet parameters may induce protrombotic status of the patient. The platelet markers have been associated with several pathological conditions, such as an increased level of mean platelet volume (MPV) has been shown as a risk factor for myocardial infarction and stroke.4 MPV is an analyzer-calculated measure of thrombocyte volume. MPV has also been shown to be increased in the diabetic patients with ocular involvement and patients with branch RVO.5,6 Platelet distribution width (PDW) shows alterations in platelet size, while plateletcrit (PCT) shows the quantitative abnormalities of platelets. MPV, PDW, and PCT are related to platelets’ morphology and proliferation kinetics.6
To the best of our knowledge, the platelet activation parameters including PDW and PCT have not been studied in patients with CRVO previously. The aim of this study is to assess the possible interrelationships of CRVO with platelet indices MPV, PDW, and PCT in order to detect subclinical platelet activation.
Patients and methods
We prospectively evaluated 30 patients with nonischemic CRVO between January 2015 and July 2017. All procedures adhered to the tenets of the Declaration of Helsinki, and local approval was received from the Ethical Committee of Ankara Diskapi Training and Research Hospital (approval number 02.04.18-48/11). The informed consents were obtained from each patient after explanation of the research purposes. All patients were Turkish Caucasians. This study is registered as Australian New Zealand Clinical Trials Registry, number ACTRN 12618000589280.
The patients were included within two study groups based on the findings of clinical ocular examination. The first group included 30 newly diagnosed acute nonischemic CRVO patients (CRVO group) and all the CRVO patients had primary hypertension. The second group included 30 adult patients serving as controls with just history of primary hypertension (control group). Patients and volunteers with history of diabetes mellitus were excluded. There were no personal or familial history of thrombotic disease in the patients and control cases. The absence of thrombotic events or a family history of thrombosis was confirmed by means of a verified questionnaire. The patients and control cases using any medication including corticosteroid, immunosuppressive therapy, and acetylsalicylic acid, and having smoking and drinking habits, or history of systemic inflammatory and ocular disease (i.e. ocular hypertension, glaucoma), were excluded from the study. All of the patients and control subjects had normal liver and renal function tests and electrolytes. All sampling procedures were performed in the morning of 12 h fasting to ensure standardization. Blood samples were taken with minimal stasis in ethylenediaminetetraacetic acid (EDTA) vacutainer tubes and studied within 60 min.
A complete ophthalmological examination including the best-corrected visual acuity, intraocular pressure measurement with Goldmann applanation tonometry, biomicroscobic examination, dilated pupil examination of the posterior segment, optic coherence tomography, and fluorescein angiography were performed in all groups. Subclinical CRVO was not detected in the control group.
The statistical analysis was performed by a t test; any p value < 0.05 was considered statistically significant. Differences between two groups for ages and sex were evaluated using Mann–Whitney U test. All statistics in this study were analyzed using SPSS software version 18.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
A total of 60 patients were included into this clinical research. There were 42 men (70%) and 18 women (30%). The mean age was (mean ± SD) 63.40 ± 8.14 years in the CRVO group and 65.2 ± 9.51 years in the control group. There was no difference regarding the age and sex of the patients in the two studied groups (p = 0.30 and p = 0.27) (Table 1).
Table 1.
Demographic data of the groups and the comparison of platelet parameters (mean ± SD) for two groups.
| CRVO group (n = 30) | Control group (n = 30) | p | |
|---|---|---|---|
| Age (years) | 63.40 ± 8.14 | 65.26 ± 9.51 | 0.30 |
| Sex (men/women) | 22/8 | 20/10 | 0.27 |
| MPV (fl) | 10.01 ± 0.89 | 8.74 ± 1.45 | 0.002 |
| PDW (%) | 14.31 ± 1.49 | 11.65 ± 1.81 | 0.001 |
| PCT (%) | 0.27 ± 0.07 | 0.23 ± 0.07 | 0.03 |
CRVO, central retinal vein occlusion; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width.
The mean Snellen best-corrected visual acuity was 20/200 (range = 20/800–20/60) in the CRVO group. Biochemistry parameters including glucose, lipid, and homocysteine values; clotting; plasma viscosity; and inflammatory markers were normal.
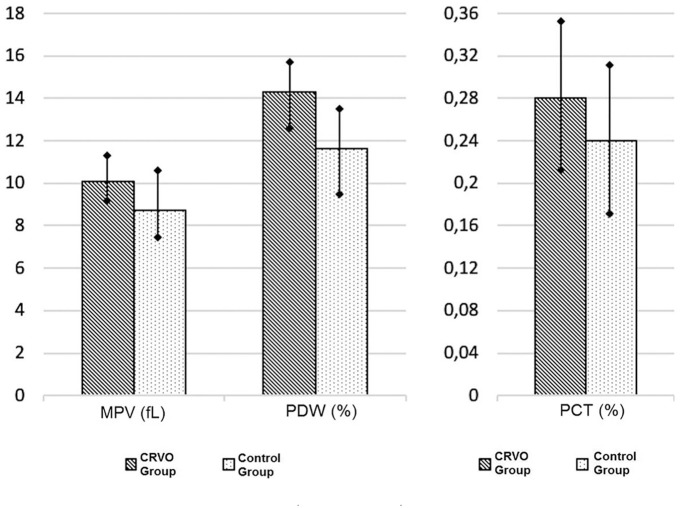
The blood samples showed a significant elevation in MPV levels in CRVO patients (p = 0.002), compared with controls. The mean serum level of MPV was 10.01 ± 0.89 fl in the CRVO group and 8.74 ± 1.45 fl in the control group (Table 1 and Figure 1).
Figure 1.
The comparison of platelet parameters (mean ± SD) for two groups.
CRVO, central retinal vein occlusion; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width.
Increments in the serum level of PDW were observed in CRVO patients (p = 0.001) when compared with the control group. The mean serum level of PDW was 14.31 ± 1.49% in the CRVO group and 11.65 ± 1.81% in the control group (Table 1 and Figure 1).
The mean PCT level was 0.27 ± 0.07% in CRVO patients, and PCT level was significantly higher than those in control subjects (0.23 ± 0.07%; p = 0.03) (Table 1 and Figure 1).
Discussion
CRVO is a result of multifactorial pathogenesis. Four basic pathological processes have been described in CRVO including abnormalities in the vessel wall (endothelial dysfunction or damage), abnormal hematological factors, abnormal blood flow (abnormal rheology), and abnormal perivascular status.7
Increased MPV might take a role in the vessel occlusion process of RVO. Platelet activation also correlated with increased MPV levels. The high value of MPV is an indicator of increased thrombocyte size. Large platelets are more active, more functional, and denser than smaller ones. Thus, higher MPV levels may increase the possibility of vascular complications.8 Previous studies showed that MPV was associated with disorders such as coronary and peripheral artery disease, myocardial infarction, and cerebral ischemia.9
PDW is also related to platelet activation and may be a more specific marker than MPV because blood values of PDW do not increase during simple platelet swelling.10 PDW shows variations in platelet size that may be an indicator of active platelet release.11 PDW levels may vary in several conditions, and increased levels are observed in sickle cell patients with the vaso-occlusive crisis.12 PDW may be a more specific marker than MPV to demonstrate platelet activation, and increased levels of PDW might show impaired deformability of thrombocytes and be related with microvascular resistance.10–12 It has been suggested that PDW is a specific and simple marker for coagulation activation.10–12 Thus, increased PDW levels could be a risk factor and play a role in the pathogenesis of CRVO.
PCT is a marker of blood-circulating platelets in a unit volume. PCT detects quantitative abnormalities of platelets and is calculated by the Platelet × MPV/107 formula.13 Akpinar and colleagues14 reported that PCT has a significant predictive value for saphenous vein graft disease. They proposed that it can be used as a marker for antiplatelet therapy to prevent graft atherosclerosis in patients undergoing bypass surgery. PCT was also correlated with the C-reactive protein (CRP) in chronic inflammatory diseases.15
Several studies have reported that MPV levels are higher in hypertensive patients with no RVO diagnosis.5,6,16 In addition, there is also one publication reporting that MPV is significantly higher in hypertensive patients with CRVO than the hypertensive control group.17 In this study, control subjects were randomly selected from hypertensive patients instead of normal healthy individuals in order to eliminate the effect of hypertension alone on platelet parameters.
In this study, CRVO patients showed significantly higher levels of MPV, PDW, and PCT that may play role in pathogenesis of CRVO. Increment in these platelet parameters may increase activation and aggregation of platelets and secretion of vasoactive mediators such as thromboxane A2 causing vasoconstriction, endothelial dysfunction, and impaired blood flow, which results in microvascular occlusion. Some studies have suggested that thrombophilic parameters are altered in the patients with RVO.16,18 However, to the best of our knowledge, MPV, PDW, and PCT have not been evaluated previously in the patients with CRVO. Kuhli-Hattenbach and colleagues investigated whether platelet hyperaggregability caused by adenosine diphosphate (ADP) was related to nonarterial anterior ischemic optic neuropathy (NAION) or RVO. They found significantly greater platelet aggregation of ADP among NAION and RVO patients compared with healthy controls.19 MPV values were evaluated in RVO patients and retinal artery occlusion patients and were found to be high in both diseases. Therefore, the authors stated that larger platelets may contribute to the pathogenesis of retinal vascular occlusion.20,21 Meyer and colleagues22 emphasized that COX-2 inhibitors may cause prothrombotic effects and patients with predisposed thrombosis may be at risk for cardiovascular and ocular thrombotic events.
Multiple factors come to the fore in the pathogenesis of RVO. The mechanical pressure in the lamina cribrosa, hemodynamic disturbances, vessel wall alterations, and the changes within the blood constituents are possible factors for CRVO.2 Systemic hypercoagulable factors including platelet activity may also impact as a triggering activity. Hayreh and colleagues23 investigated routine hematological tests and some specific hematological parameters, such as platelet aggregation, antithrombin III, and α2 globulin in patients with RVO, to investigate hematological abnormalities in RVO patients. They showed that various hematological abnormalities can be seen in relation to different types of RVOs. They also noted that it is not necessary to undergo extensive, expensive, specific hematological examinations of all patients with RVO, and that routine hematological evaluation was generally sufficient for RVO patients. They also emphasized that treatment with anticoagulants or platelet anti-aggregating agents did not have any evidence of protective or beneficial effects. In another study, Hayreh and colleagues24 showed that the use of aspirin, other antiplatelet aggregating agents, or anticoagulants had no apparent benefit in patients with CRVO and hemi-CRVO. However, clinicians should be aware of the ongoing subclinical thrombocyte activation during the clinical management of the patients with CRVO. Another important point is that the absence of a personal or familial history of thrombotic disease in patients and controls does not assure that any prothrombotic mutation or abnormality is present (i.e. sporadic mutations).
There are several limitations in this study. Limited number of patients is the most important one. Second, this study is based on baseline values that cannot reflect a patient’s long-term status.
Based on the results of this study, CRVO patients have significantly higher serum levels of MPV, PDW, and PCT than control subjects. MPV, PDW, and PCT seem to play an important role in the genesis of that unique form of CRVO. The platelet parameters may be helpful in the diagnosis and prevention of CRVO. The measurements of PDW, MPV, and PCT may be useful because the increase in these markers indicates platelet activation. The results may be important for the clinical management of the patients with CRVO in everyday clinical practice as those platelet activation parameters can be routinely detected in the complete blood count analyses. Further large-scale and comprehensive studies are needed to support these results.
Footnotes
Conflict of interest statement: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethics Committee Approval Number: 02.04.18-48/11.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
Informed consent: A written informed consent was obtained from the patient to publish medical data and images.
ORCID iD: Mehmet Citirik  https://orcid.org/0000-0002-0558-5576
https://orcid.org/0000-0002-0558-5576
References
- 1. Laouri M, Chen E, Looman M, et al. The burden of disease of retinal vein occlusion: review of the literature. Eye 2011; 25: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Citirik M, Haznedaroglu I. Clinical risk factors underlying the occurrence of retinal vein occlusion. Int J Ophthalmic Res 2016; 2: 91–95. [Google Scholar]
- 3. Ugurlu N, Taslipınar-Uzel AG, Sengun A. Pathogenesis of retinal vein occlusion. Int J Ophthalmic Res 2016; 2: 130–132. [Google Scholar]
- 4. Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol 2005; 46: 284–290. [DOI] [PubMed] [Google Scholar]
- 5. Citirik M, Beyazyildiz E, Simsek M, et al. MPV may reflect subcinical platelet activation in diabetic patients with and without diabetic retinopathy. Eye 2015; 29: 376–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Beyazyıldız E, Çıtırık M, Şimşek M, et al. Branch retinal vein occlusion associated with platelet activation. Turk J Med Sci 2019; 49: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kadayifçilar S, Ozatli D, Ozcebe O, et al. Is activated factor VII associated with retinal vein occlusion? Br J Ophthalmol 2001; 85: 1174–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ates O, Kiki I, Bilen H, et al. Association of mean platelet volume with the degree of retinopathy in patients with diabetes mellitus. Eur J Gen Med 2009; 6: 99–102. [Google Scholar]
- 9. Kodiatte TA, Manikyam UK, Rao SB, et al. Mean platelet volume in type 2 diabetes mellitus. J Lab Physicians 2012; 4: 5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vagdatli E, Gounari E, Lazaridou E, et al. Platelet distribution width: a simple, practical and specific marker of activation of coagulation. Hippokratia 2010; 14: 28–32. [PMC free article] [PubMed] [Google Scholar]
- 11. Osselaer JC, Jamart J, Scheiff JM. Platelet distribution width for differential diagnosis of thrombocytosis. Clin Chem 1997; 43: 1072–1076. [PubMed] [Google Scholar]
- 12. Amin MA, Amin AP, Kulkarni HR. Platelet distribution width (PDW) is increased in vaso-occlusive crisis in sickle cell disease. Ann Hematol 2004; 83: 331–335. [DOI] [PubMed] [Google Scholar]
- 13. Muxel S, Fasola F, Radmacher MC, et al. Endothelial functions: translating theory into clinical application. Clin Hemorheol Microcirc 2010; 45: 109–115. [DOI] [PubMed] [Google Scholar]
- 14. Akpinar I, Sayin MR, Gursoy YC, et al. Plateletcrit. A platelet marker associated with saphenous vein graft disease. Herz 2014; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 15. Sahin F, Yazar E, Yıldız P. Prominent features of platelet count, plateletcrit, mean platelet volume and platelet distribution width in pulmonary tuberculosis. Multidiscip Respir Med 2012; 7: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coban E, Adanir H, Bilgin D. The association of mean platelet volume levels with hypertensive retinopathy. Platelets 2008; 19: 115–118. [DOI] [PubMed] [Google Scholar]
- 17. Bawankar P, Samant P, Lahane T, et al. Mean platelet volume and central retinal vein occlusion in hypertensive patients. Can J Ophthalmol 2019; 54: 275–279. [DOI] [PubMed] [Google Scholar]
- 18. Yilmaz T, Yilmaz A. Altered platelet morphological parameters in patients with retinal vein occlusion. Eur Rev Med Pharmacol Sci 2016; 20: 1934–1939. [PubMed] [Google Scholar]
- 19. Kuhli-Hattenbach C, Hellstern P, Kohnen T, et al. Platelet activation by ADP is increased in selected patients with anterior ischemic optic neuropathy or retinal vein occlusion. Platelets 2017; 28: 720–723. [DOI] [PubMed] [Google Scholar]
- 20. Sahin A, Sahin M, Yüksel H, et al. The mean platelet volume in patients with retinal vein occlusion. J Ophthalmol 2013; 2013: 236371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sahin M, Sahin A, Yüksel H, et al. Mean platelet volume in patients with retinal artery occlusion. Arq Bras Oftalmol 2016; 79: 12–14. [DOI] [PubMed] [Google Scholar]
- 22. Meyer CH, Schmidt JC, Rodrigues EB, et al. Risk of retinal vein occlusions in patients treated with rofecoxib (vioxx). Ophthalmologica 2005; 219: 243–247. [DOI] [PubMed] [Google Scholar]
- 23. Hayreh SS, Zimmerman MB, Podhajsky P. Hematologic abnormalities associated with various types of retinal vein occlusion. Graefes Arch Clin Exp Ophthalmol 2002; 240: 180–196. [DOI] [PubMed] [Google Scholar]
- 24. Hayreh SS, Podhajsky PA, Zimmerman MB. Central and hemicentral retinal vein occlusion: role of anti-platelet aggregation agents and anticoagulants. Ophthalmology 2011; 118: 1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]