Abstract
The object of this paper is to assess associations between serum uric acid (UA) and pulmonary arterial hypertension (PAH) risk, disease severity, and mortality in a well-characterized cohort of systemic sclerosis (SSc) patients referred for evaluation of possible PAH. Consecutive SSc patients aged >18 years with serum UA drawn within two weeks of a diagnostic right heart catheterization (RHC) were included. Associations between baseline serum UA and PAH at RHC were examined using logistic regression and receiver operating characteristic curves. Relationships between UA levels and metrics of disease severity were assessed using Pearson and Spearman correlation. Associations between UA and survival were assessed using Kaplan–Meier analysis and Cox proportional hazard modeling. A total of 162 SSc patients were included; 82 received a diagnosis of PAH at RHC. Patients found to have PAH had significantly higher UA than those without PAH. Elevated baseline UA was associated with significantly increased odds of PAH diagnosis at RHC (odds ratio [OR] = 4.07, 95% confidence interval [CI] = 2.11–7.87, P < 0.001). Each mg/dL higher UA was associated with a 14% increase in mortality (hazard ratio [HR] = 1.14, 95% CI = 1.02–1.28, P < 0.05). In multivariable models adjusting for potential confounders of the relationship between UA and survival, UA > 6.3 mg/dL remained significantly associated with increased mortality (HR = 1.84, 95% CI = 1.02–3.32, P < 0.05). Among SSc patients with suspected PAH, elevated serum UA is associated with increased risk of SSc-PAH. Among individuals diagnosed with SSc-PAH by RHC, UA is associated with disease severity and survival. These results indicate UA is a useful predictor of PAH risk and prognosis in SSc.
Keywords: diagnostic techniques and monitoring, pulmonary arterial hypertension, risk stratification and biomarkers
Pulmonary arterial hypertension (PAH) is a chronic pulmonary vascular disease characterized by pathologic pulmonary vascular remodeling leading to elevated pulmonary arterial pressures (PAP), progressive right heart failure, and death. Systemic sclerosis or scleroderma (SSc), a disease characterized by immune system dysregulation and endothelial dysfunction, is commonly complicated by PAH (SSc-PAH). Despite the advances in PAH-specific therapies over the last decades, SSc-PAH remains an incurable illness, with disproportionately high morbidity and mortality when compared with other forms of PAH.1–3 The known risk of disease development in SSc and the increased mortality rate observed in SSc-PAH make effective and reliable PAH screening particularly relevant to the SSc population. There is evidence to suggest that formal screening in SSc enables earlier detection and thereby earlier treatment of PAH; however, optimal screening strategies are poorly defined.4 Once a diagnosis of SSc-PAH is made, estimating prognosis is challenging, as SSc patients often have coexistent physical problems and functional limitations that confound traditional measures of evolving pulmonary vascular disease, such as the 6-min walk test. Further, it has been established that echocardiographic estimates of pulmonary pressures, which are often used for screening and monitoring patients, correlate poorly with invasive hemodynamic measures at right heart catheterization (RHC).5 This leaves the clinician with limited tools available for non-invasive detection of disease or determination of disease severity. In light of these challenges, biomarkers for early detection and novel assessments of disease severity in this at-risk population have the potential to improve outcomes.
A recent multicenter study, the DETECT study, sought to develop an early detection algorithm for SSc-PAH by examining associations between >100 individual clinical variables (serum markers, echocardiogram, and electrocardiogram [EKG] parameters) measured in high-risk SSc patients (a diffusing capacity for carbon monoxide [DLCO] <60% predicted was required for inclusion in the study) and a diagnosis of PAH at RHC.6 Uric acid (UA) was one of several parameters found to predict SSc-PAH in this cohort and was incorporated into the final DETECT screening algorithm. Though there are data supporting the use of UA as a prognostic biomarker in idiopathic PAH (IPAH),7,8 there were few data supporting an association between UA and SSc-PAH risk before the DETECT study, and no previous study has investigated associations between UA and disease severity or survival in SSc-PAH. We hypothesized that in addition to predicting PAH risk in SSc, UA might also serve as a marker of disease severity and survival in SSc-PAH. Thus, in this study, we sought to: (1) validate UA as a predictor of PAH in SSc in a well-characterized cohort of at-risk SSc patients; (2) assess relationships between UA levels and disease severity; and (3) investigate the relationship between UA and survival in individuals with incident, treatment-naïve SSc-PAH.
Patients and methods
The Johns Hopkins Pulmonary Hypertension Program (JHPHP) maintains an Institutional Review Board-approved registry of patients evaluated in the clinical practice. This registry was queried for all SSc patients who were referred to our center in 2005–2017 for evaluation of possible pulmonary hypertension (PH). Reasons for referral of SSc patients to the JHPHP include unexplained dyspnea, elevated serum N-terminal pro-brain natriuretic peptide (NT-proBNP), a ratio of percent predicted forced vital capacity (FVC) to percent predicted DLCO >1.6, a decline in absolute DLCO on serial pulmonary function tests (PFTs) by ≥ 15%, or echocardiographic evidence of right ventricular (RV) dysfunction, such as RV or right atrial (RA) dilation or RV systolic pressure ≥45 mmHg.9,10 Since 2005, the JHPHP has routinely collected UA before RHC for patients with suspected PH. To ensure a tight temporal association between UA levels and hemodynamic measures of disease severity, we included individuals with a UA level measured within two weeks of diagnostic RHC. Baseline clinical data were abstracted for each participant aged >18 years with a diagnosis of SSc, including demographics, co-morbid conditions, hemodynamics, laboratory data, 6-min walk distance (6MWD), World Health Organization functional class (WHO FC), and PFTs. In accordance with 2015 ESC/ERS guidelines, PH was defined as a mean PAP (mPAP) ≥25 mmHg. Group I disease, or PAH, was defined as mPAP ≥25 mmHg, pulmonary capillary wedge pressure (PCWP) ≤15 mmHg, and pulmonary vascular resistance (PVR) >3 Wood units, in the absence of other known causes of PH.11 Group III disease, or interstitial lung disease-associated PH (ILD-PH) was defined as FVC ≤ 60% predicted with moderate fibrosis on chest computed tomography (CT), as previously described.12 Other PH classifications were designated according to the WHO classification system for PH.13 The presence of SSc was determined by expert opinion according to American College of Rheumatology (ACR) 1980 criteria for patients enrolled through 2013, after which revised ACR/European League against Rheumatism classification criteria were applied.14,15 Patients who had received PAH-specific therapy before evaluation at our center were excluded from analysis. Patients whose index RHC was performed at an outside institution were also excluded. Survival was determined by review of the electronic record and search of the Social Security Death Index.
Statistical analysis
Data are summarized using descriptive statistics. Continuous variables are reported as means with standard deviations or medians with interquartile ranges (IQR). Categorical variables are reported as frequencies and proportions. Comparisons between variables were made using the t-test or Wilcoxon rank-sum test, as appropriate. Relationships between UA and continuous clinical variables were assessed using Spearman or Pearson correlation, as appropriate. Logistic regression was performed to examine relationships between common non-invasive markers, including UA levels, and the odds of disease presence. Likelihood ratio tests were performed and receiver operating characteristic (ROC) curves were compared to characterize UA's ability to discriminate PAH compared to other non-invasive markers. UA was examined as a continuous variable and as a categorical variable dichotomized at the ROC optimal threshold. Associations between UA and survival were examined using the Kaplan–Meier product limit estimator and Cox proportional hazard modeling. UA was entered into univariable and multivariable models as a continuous variable and as a categorical variable dichotomized at the median. Factors known to impact UA levels and potential confounders of the relationship between UA and survival were incorporated into multivariable models. The proportional hazards assumption was examined for all covariates using a continuous time-varying predictor and generalized linear regression of scaled Schoenfeld residuals on functions of time.16,17 Potential confounders were limited to 1 per 10 events to avoid over-fitting of the models.18 Because of recognized sex-associated differences in UA levels and sex disparities in survival in SSc-PAH,7,19 survival analyses were also conducted in male and female subgroups. Interaction terms were used to formally test for interactions between UA levels and sex and their relationship with survival in SSc-PAH. Four patients lost to follow-up within three months of diagnostic RHC were not included in survival analyses. A P value < 0.05 was considered statistically significant for main effects and a P value < 0.10 was considered statistically significant for interactions in this modestly sized cohort.20 All analyses were performed using Stata version 14.0 (StataCorp., College Station, TX, USA).
Results
Patient characteristics
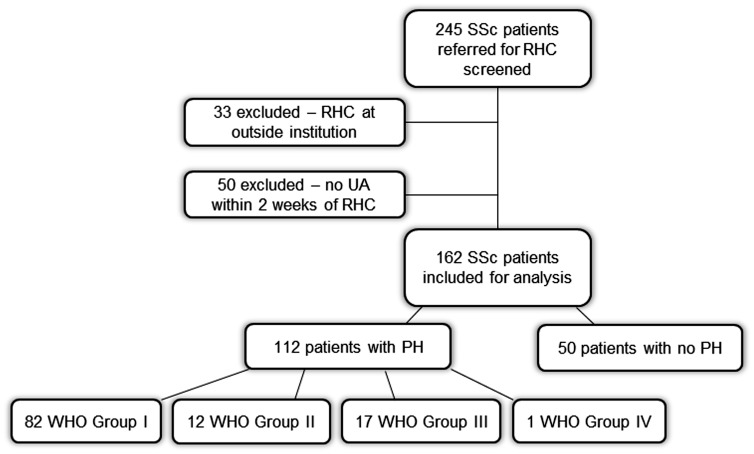
A total of 245 patients with SSc who underwent RHC between January 2005 and September 2017 were considered for inclusion (Fig. 1). Thirty-three of these patients were excluded because their index RHCs were performed at an outside institution. Fifty patients were excluded because UA was not collected within two weeks of RHC. In total, 162 individuals met the inclusion criteria and were included in the cohort for analysis; 112 patients were found to have PH, while 50 patients did not have PH. Among those 112 patients found to have PH, 82 were diagnosed with WHO group I disease, 12 with WHO group II disease, and 17 with WHO group III disease. One patient was diagnosed with chronic thromboembolic PH (CTEPH; group IV disease).
Fig. 1.
Flowchart demonstrating selection of study cohort and classification of participants.
The demographic and clinical characteristics of the full cohort are summarized in Table 1. Overall, the majority of patients were white women aged approximately 60 years. The median UA for the cohort was 6.3 mg/dL. Patients with PH had a higher median UA than those without PH (6.7, IQR = 5.2–8.1 vs. 5.15, IQR = 4.5–6.3 mg/dL, P < 0.05). The mean estimated glomerular filtration rate (eGFR) calculated by the Modification of Diet in Renal Disease (MDRD) Study equation21 was >60 mL/min, consistent with normal renal function; this did not differ between those with and without PH. As expected, individuals with PH had higher pulmonary pressures compared to those without PH. A higher proportion of participants with PH had co-morbid hypertension (HTN) (40%), diabetes mellitus (11%), and coronary disease (7%) than those without PH; a higher proportion of participants with PH were prescribed diuretic therapy (51% vs. 32%). Patients with WHO group I disease had higher UA (6.85, IQR = 5.4–8.3 in group I vs. 6.05, IQR = 4.75–7.55 mg/dL in groups II and III, P < 0.05) and more abnormal hemodynamics (higher mPAP and PVR, lower cardiac index (CI)) than those in other WHO groups (Supplemental Table 1). Participants were followed for a median of 49 months (IQR = 28–77 months) from the time of RHC to the time of death or censor. Of the patients found to have PAH 90% were treated with pulmonary vasodilator therapy after diagnosis (58% received combination therapy and 42% received monotherapy). Of the patients diagnosed with Group II/III disease, 65% received PH therapies off label (89% of those treated received monotherapy). There was a total of 77 deaths in the full cohort over the study period, for a 48% mortality rate overall. Forty-eight deaths occurred within the PAH subgroup (48/82), six deaths within group II (6/12), and eight deaths within group III (8/17). Fifteen deaths occurred in patients without PH.
Table 1.
Demographic and clinical characteristics of full cohort.
| Overall (n=162) | All PH (n=112) | No PH (n=50) | P value | |
|---|---|---|---|---|
| Age (years) | 61 (12) | 62 (12) | 59 (13) | 0.16 |
| Sex (% female) | 80 | 79 | 88 | 0.88 |
| Race (% Caucasian) | 88 | 88 | 87.2 | 0.97 |
| BMI (kg/m2) | 27 (6) | 28 (7) | 25 (5) | <0.01 |
| WHO FC (n I/II/III/IV) | 20/61/70/1 | 10/38/60/1 | 10/23/10/0 | |
| 6MWD (m) | 358 (112) | 331 (107) | 415 (102) | <0.01 |
| eGFR (mL/min) (MDRD) | 78 (31) | 75 (28) | 84 (35) | 0.08 |
| Median UA (mg/dL) (IQR) | 6.3 (4.9–7.6) | 6.7 (5.2–8.1) | 5.2 (4.5–6.3) | <0.01 |
| Median NT-proBNP (pg/dL) (IQR) | 286 (130–796) | 389 (137–1419) | 241 (92–379) | <0.01 |
| RAP (mmHg) | 6 (4) | 7 (4) | 4 (3) | <0.01 |
| mPAP (mmHg) | 31 (12) | 36 (11) | 18 (4) | <0.01 |
| PCWP (mmHg) | 10 (4) | 11(4) | 8 (3) | <0.01 |
| CO (L/min) | 5.0 (1.6) | 5.0 (1.7) | 5.0 (1.3) | 1.00 |
| CI (L/min/m2) | 2.8 (0.8) | 2.8 (0.8) | 2.9 (0.6) | 0.50 |
| PVR (Wood units) | 4.8 (4.2) | 6.0 (4.5) | 2.1 (0.8) | <0.01 |
| FVC % predicted | 81 (19) | 78 (20) | 86 (18) | <0.05 |
| FEV1 % predicted | 77 (18) | 75 (18) | 82 (17) | <0.05 |
| FEV1/FVC % | 76 (10) | 75 (11) | 78 (8) | 0.09 |
| DLCO % predicted | 60 (22) | 54 (19) | 71 (24) | <0.01 |
| Diuretics (n (% prescribed)) | 74 (46) | 58 (52) | 16 (32) | <0.05 |
| Loop diuretic | 50 (31) | 42 (38) | 8 (16) | <0.05 |
| Thiazide diuretic | 23 (14) | 15 (13) | 8 (16) | 0.61 |
| HTN | 62 (38) | 45 (40) | 17 (34) | 0.47 |
| Diabetes mellitus | 13 (8) | 12 (11) | 1 (2) | 0.05 |
| Coronary disease | 9 (6) | 8 (7) | 1 (2) | 0.20 |
| Gout | 9 (6) | 8 (7) | 1 (2) | 0.20 |
| Urate-lowering therapy | 4 (2) | 4 (4) | 0 (0) | 0.15 |
| Deaths | 77 (48) | 62 (55) | 15 (30) | <0.01 |
Values are presented as mean (SD) or n (%) unless otherwise specified.
BMI, body mass index; WHO FC, World Health Organization functional class; 6MWD, 6-min walk distance; eGFR, estimated glomerular filtration rate; UA, uric acid; RAP, right atrial pressure; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 s; DLCO, diffusing capacity for carbon monoxide; HTN, hypertension; IQR, interquartile range.
UA as a predictor of risk of PAH
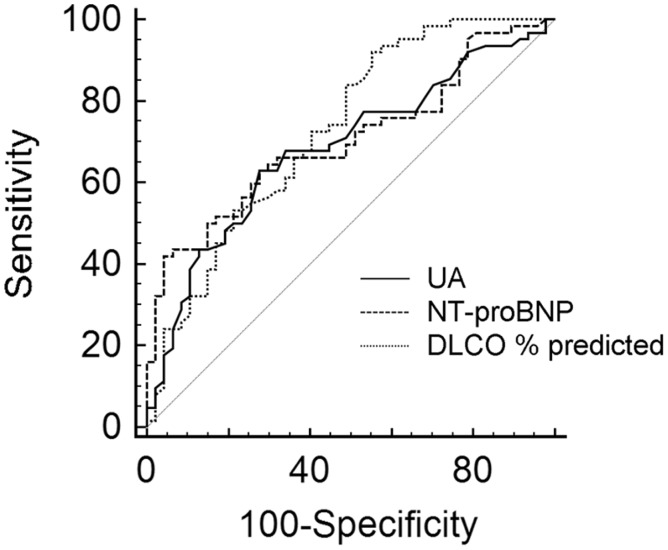
UA levels were significantly higher in patients found to have PAH (6.9 mg/dL, IQR = 5.4–8.3 mg/dL) compared with those with no PH (median UA = 5.2 mg/dL, IQR =4.5–6.3 mg/dL) (Supplemental Figure 1). For every mg/dL higher UA at the time of RHC, the odds of PAH increased by 51% (odds ratio [OR] = 1.51, 95% confidence interval [CI] = 1.24–1.84, P < 0.001). The odds of PAH at RHC for SSc patients with UA > 6.2 mg/dL (the ROC optimal threshold for disease detection) were four times higher than for those with UA ≤ 6.2 mg/dL (OR = 4.07, 95% CI =2.11–7.87, P < 0.001). ROC analysis showed UA discriminated PAH from no PH with area under the curve (AUC) 0.725, P < 0.01, and sensitivity and specificity were 68% and 74%, respectively. The AUC for NT-proBNP (0.721, P < 0.001) and for DLCO % predicted (0.721, P < 0.001), two other commonly used screening biomarkers, were not statistically different from the AUC for UA, demonstrating non-inferiority of UA relative to NT-proBNP or DLCO % predicted for detection of PAH (Fig. 2). Adding UA to a logistic regression model with NT-proBNP and DLCO % predicted significantly improved prediction of PAH (likelihood ratio χ2 5.08, P < 0.05). The AUC for a multivariable logistic regression model incorporating all three markers (NT-proBNP, DLCO % predicted, and UA) was significantly higher than the AUC for any one marker (0.844, P < 0.05, Supplemental Table 2), demonstrating the value of adding UA to other common noninvasive predictors of PAH.
Fig. 2.
ROC curves showing UA (AUC 0.725, P < 0.01), NT-proBNP (0.721, P < 0.001), and DLCO % predicted (0.721, P < 0.001) as discriminants of PAH. There are no significant differences in AUC among these predictors.
UA as a marker of disease severity
Among patients with PAH, baseline UA correlated positively with mPAP (r = 0.44, P < 0.01), right atrial pressure (RAP) (r = 0.42, P < 0.01), and PVR (r = 0.37, P < 0.01) and negatively with cardiac output (r = –0.24, P < 0.05), and cardiac index (r = –0.32, P < 0.05). UA correlated positively with WHO FC (r = 0.27, P < 0.05), NT-proBNP (r = 0.34, P < 0.01), and creatinine (Cr) (r = 0.51, P < 0.01). Interestingly, there was no relationship between baseline serum UA and 6MWD in men, but there was a modest correlation in women (r = –0.3, P < 0.01).
When disease severity metrics of SSc-PAH patients with UA levels higher than the median were compared with severity metrics of patients with UA levels lower than the median, significant differences were observed in 6MWD, WHO FC, RAP, mPAP, and PVR (Table 2).
Table 2.
Comparison of variables in SSc-PAH patiens with UA below vs. above the median value for the cohort (6.3 mg/dL).
| UA < 6.3 mg/dL (n=32) | UA > 6.3 mg/dL (n = 50) | P value | |
|---|---|---|---|
| Age, years | 63 (14) | 62 (12) | NS |
| WHO FC (n III/IV (%)) | 25 (32) | 46 (62) | <0.01 |
| 6MWD (m) | 362 (102) | 320 (95) | 0.06 |
| RAP (mmHg) | 6 (3) | 8 (4) | <0.05 |
| mPAP (mmHg) | 33 (8) | 41 (12) | <0.01 |
| PVR (Wood units) | 5.4 (3.5) | 7.8 (5.4) | <0.05 |
| CO (L/min) | 5.1 (1.4) | 4.6 (1.7) | 0.17 |
| CI (L/min/m2) | 2.9 (0.9) | 2.6 (0.8) | 0.12 |
Values are given as mean (SD) unless otherwise specified.
WHO FC, World Health Organization functional class; 6MWD, 6-min walk distance; RAP, right atrial pressure; mPAP, mean pulmonary arterial pressure; PCWP, pulmonary capillary wedge pressure; CO, cardiac output; CI, cardiac index; PVR, pulmonary vascular resistance.
UA as a predictor of mortality
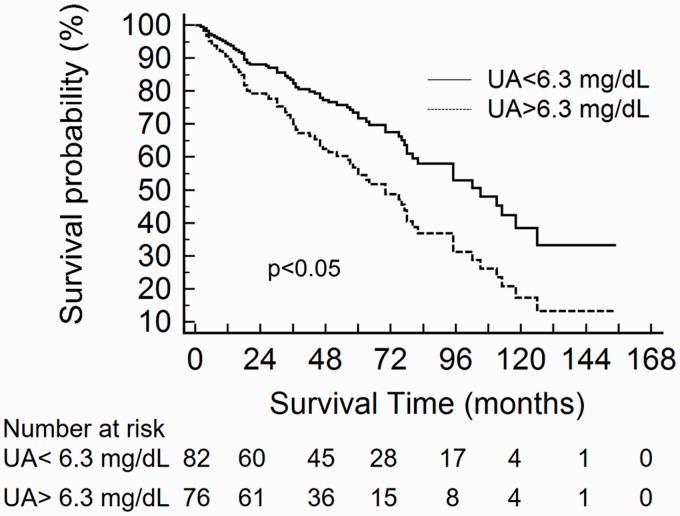
In the full cohort, comparison of baseline serum UA between survivors and non-survivors showed a significantly higher median UA in non-survivors (6.6, IQR = 5.2–8.1 vs. 5.6, IQR = 4.5–7.1 mg/dL, P < 0.01). When Kaplan–Meier survival curves were plotted according to the cohort median UA (6.3 mg/dL), elevated UA was associated with higher mortality (log-rank χ2 = 6.12, P < 0.05).
Univariable Cox proportional HR) for clinical variables, including UA and other common prognostic biomarkers, are presented for both the overall cohort and for the PAH subgroup in Supplemental Table 3. In the overall cohort, UA greater than the median (6.3 mg/dL) was associated with a significantly increased risk of death, with a HR of 1.77 (95% CI 1.12-2.80, p<0.05). When examined as a continuous variable, each mg/dL higher UA was associated with a 14% increase in mortality (hazard ratio [HR] = 1.14, 95% CI = 1.02–1.28, P < 0.05). The relationship between elevated UA and mortality persisted in a multivariable model adjusting for age, sex, BMI, eGFR, presence of PAH, presence of systemic HTN, and treatment status (no PH-specific therapy, monotherapy, or combination therapy) (HR = 1.84, 95% CI = 1.02–3.32, P < 0.05) (Fig. 3).
Fig. 3.
Cox proportional hazard curves for individuals with UA levels above vs. below the cohort median (6.3 mg/dL) in the full SSc cohort adjusted for age, sex, BMI, eGFR, presence of PAH, presence of systemic HTN, and treatment status (no PH-specific therapy, monotherapy, or combination therapy).
In multivariable analyses of individuals with SSc-PAH, the relationship between UA and mortality persisted with adjustment for age, sex, BMI, and: treatment status; urate-lowering therapy; HTN; diabetes; and coronary disease. The relationship was attenuated with adjustment for alternative prognostic markers, including WHO FC, 6MWD, DLCO % predicted, and NT-proBNP (Table 3). Interaction models demonstrated quantitative differences in the estimated hazard of elevated UA by sex with a P value of 0.06. In a stratified analysis of women with SSc-PAH, the association between UA and survival was stronger and persisted with adjustment for potential confounders of the relationship between UA and survival (Table 3). There was an insufficient sample size to conduct multivariable modeling in men with SSc-PAH.
Table 3.
Multivariable Cox proportional hazard ratios (HR) for survival.
| All SSc-PAH HR (95% CI, P value) (n = 82) | Female SSc-PAH HR* (95% CI, P value) (n = 65) | |
|---|---|---|
| Uric acid > 6.3 mg/dL | 1.68 (0.89–3.15, 0.11) | 2.40 (1.16–4.98, < 0.05) |
| Adjusted for age, sex, BMI, and: | Adjusted for age, BMI, and: | |
| eGFR (mL/min) | 1.84 (0.93–3.67, 0.08) | 2.61 (1.20–5.71, < 0.05) |
| PAH-specific therapy | 1.98 (1.01–3.87, < 0.05) | 2.76 (1.29–5.92, < 0.01) |
| Monotherapy vs. combo | 2.12 (1.06–4.26, < 0.05) | 2.91 (1.33–6.40, < 0.01) |
| Diuretic therapy | 1.79 (0.90–3.57, 0.09) | 2.52 (1.15–5.51, < 0.05) |
| HTN | 2.20 (1.11–4.37, < 0.05) | 3.30 (1.52–7.17, < 0.01) |
| Diabetes mellitus | 2.00 (1.03–3.91, < 0.05) | 2.84 (1.33–6.07, < 0.01) |
| Coronary disease | 2.30 (1.13–4.68, < 0.05) | 3.34 (1.48–7.53, < 0.01) |
| WHO FC (III, IV vs. I, II) | 1.72 (0.86–3.46, 0.13) | 2.45 (1.11–5.39, < 0.05) |
| 6MWD (m) | 1.89 (0.89–4.03, 0.10) | 2.49 (1.09–5.73, < 0.05) |
| mPAP (mmHg) | 1.59 (0.77–3.29, 0.21) | 2.34 (1.03–5.30, < 0.05) |
| CO (L/min) | 1.85 (0.93–3.67, 0.08) | 2.54 (1.16–5.56, < 0.05) |
| PVR (Wood units) | 1.70 (0.84–3.43, 0.14) | 2.42 (1.09–5.37, < 0.05) |
| DLCO % predicted | 2.01 (0.96–4.19, 0.06) | 2.72 (1.20–6.15, < 0.05) |
| NT-proBNP (pg/dL) | 1.77 (0.76–4.10, 0.19) | 2.63 (1.01–6.85, < 0.05) |
Sex is not included as a covariate in multivariable models in the female SSc-PAH subgroup.
BMI, body mass index; eGFR, estimated glomerular filtration rate; WHO FC, World Health Organization functional class; 6MWD, 6-min walk distance; DLCO, diffusing capacity for carbon monoxide; BNP, brain natriuretic peptide.
Discussion
To our knowledge, this is the first study to directly examine the utility of serum UA as a predictor of disease severity and survival in patients with SSc-PAH. We found significant differences in metrics of disease severity in patients with high versus low UA; UA levels were associated with survival in both the full at-risk SSc cohort and in patients with SSc-PAH.
Our results also confirm the association between elevated UA and PAH risk observed in the DETECT study. Serum UA obtained near the time of diagnostic RHC was significantly higher in SSc patients found to have PAH compared with SSc patients without PAH. The presence of a UA level > 6.2 mg/dL at the time of RHC conferred a fourfold increased odds of diagnosing PAH in our cohort. Importantly, combining UA with other commonly used non-invasive screening biomarkers significantly improved prediction of PAH at RHC.
Previous studies have shown the diagnostic and prognostic utility of serum UA in other forms of PAH. In IPAH, UA correlates with disease severity and declines with initiation of vasodilator therapy.7 Decreases in serial UA levels in IPAH patients on pulmonary vasodilator therapy have been associated with longer survival and delayed clinical worsening.8 UA has also been implicated in a variety of other cardiovascular and metabolic diseases, such as coronary atherosclerosis, diabetes, and obesity.22 Interestingly, UA risk thresholds for poor outcomes across different cardiovascular disease states have been in the range of 5–6 mg/dL, falling within what has historically been considered the “normal” range for UA.23,24 In the DETECT study, the mean UA among SSc-PAH patients was 5.9 mg/dL. Thus, a top-normal UA level of 6.2 mg/dL, the optimal threshold for disease detection in our cohort, aligns with these prior findings.
The potential for serum UA to serve as a biomarker in SSc-PAH may reflect biologically relevant metabolic alterations in PAH and in scleroderma. UA is the final product of purine catabolism in humans and is generated by the oxidative action of the enzyme xanthine oxidoreductase (XOR) on xanthine, which also results in the generation of reactive oxygen species (ROS).25 Purine degradation products metabolized by XOR were among several plasma metabolites identified as closely linked with RV-pulmonary vascular dysfunction in a recently published metabolic profile of human patients with PH.26 In SSc, increased ROS production and deficient antioxidant defense mechanisms are thought to contribute to disease pathogenesis,27,28 and scleroderma is regarded as a disease of increased oxidative stress. Studies have demonstrated that ROS cause endothelial cell damage, intimal thickening, and fibroblast production,29–31 factors which may contribute to vascular injury in PAH.
Whether UA plays a causal role in PAH pathogenesis or serves only as an easily measurable surrogate for XOR activity is unclear. Preclinical models support contributory roles for both XOR and UA in the pathobiology of PAH. In a rat model of hypoxia-induced PH, XOR activity significantly increased during hypoxic exposure, and pulmonary pressures, RV hypertrophy, and pulmonary vascular remodeling were significantly attenuated when animals were treated with the XOR inhibitor allopurinol.32 In vitro, UA stimulates platelet-derived growth factor and vasoconstrictors such as thromboxane, angiotensin II, and endothelin 1.33–37 In a porcine model, UA stimulated the enzyme arginase, thereby reducing levels of the pulmonary vasodilators nitric oxide (NO) and cyclic guanine monophosphate (cGMP).38
Additional studies are needed to investigate the roles of UA and XOR in the pathophysiology of SSc-PAH, as XOR may represent a plausible therapeutic target. In systemic hypertension, XOR inhibition with allopurinol has been associated with reductions in blood pressure.39 In patients with left heart failure, administration of allopurinol has been associated with improvements in myocardial efficiency, endothelial function, and 6MWD, as well as reductions in NT-proBNP.40–42 In one notable heart failure study, endothelial function improved in participants given allopurinol compared with those given placebo; however improved endothelial function was not observed in participants given probenecid, a uricosuric drug that has no effect on XOR activity.42 Proportionate reductions in UA were seen with both drugs, suggesting improved endothelial function was brought about by blocking the oxidative action of XOR, rather than by simply lowering UA. Future studies are needed to investigate the effects of XOR inhibitors and other urate-lowering therapies in PAH.
Our study offers several advantages over prior studies of UA in SSc. All patients included were diagnosed with PH by the gold standard, RHC. UA levels were measured within two weeks of RHC, limiting the effect of fluctuations in serum UA levels that may occur with time. Additionally, factors known to affect UA levels, such as sex, renal function, diuretic use, urate-lowering therapies, pulmonary vasodilator therapies, and the presence of co-morbidities such as HTN, diabetes mellitus, and coronary disease were incorporated into multivariable models.
This study has some limitations. While we did not require high-risk features to be present for inclusion in our cohort, the majority of patients were referred to our center for evaluation of suspected PH. The DETECT study required that patients have a measured DLCO < 60% predicted and SSc for at least three years in order to be included. Thus, neither our cohort nor the DETECT cohort represents a truly unselected, asymptomatic SSc population, which is the population to which screening strategies must be calibrated. It is notable, however, that 42% of participants in our SSc cohort had a DLCO > 60% predicted; moreover, 27% of the patients ultimately found to have PAH in our cohort had a DLCO > 60% predicted, implicating greater generalizability of these results than suggested by DETECT. Additionally, there are multiple factors aside from XOR activity that impact the generation and excretion of UA. We did not measure XOR activity directly in this study and it is was not feasible to account for all possible factors impacting UA levels in our analyses. This multiplicity of factors affecting UA generation and excretion may represent a real-world limitation to the use of UA as a biomarker. Finally, this is a single-center study with a limited number of male participants, and our results suggest associations between UA levels and survival may differ according to sex. Additional investigation in multi-center cohorts with larger numbers of male participants is warranted.
In conclusion, refining methods for screening and accomplishing early detection of PAH in the SSc population is essential. This study demonstrates that elevated UA is associated with fourfold increased odds of PAH in SSc patients and that combining UA with other non-invasive markers of disease improves prediction of PAH. Further, UA correlates with disease severity; increased UA is associated with increased risk of mortality in both SSc and in SSc-PAH. Taken together, our results suggest that serum UA is a valuable biomarker in the non-invasive assessment of disease risk, severity, and outcomes in SSc-PAH. Future studies are needed to confirm these results in larger populations of unselected, asymptomatic SSc patients. Additional investigation is needed to elucidate mechanisms underlying increased UA production and to assess the effects of XOR inhibition in SSc-PAH.
Supplemental Material
Supplemental Material for Serum uric acid as a marker of disease risk, severity, and survival in systemic sclerosis-related pulmonary arterial hypertension by Catherine E. Simpson, Rachel L. Damico, Laura Hummers, Rubina M. Khair, Todd M. Kolb, Paul M. Hassoun and Stephen C. Mathai in Pulmonary Circulation
Conflict of interest
SCM has served as a consultant to Actelion, Arena, and Bayer.
Funding
This study was supported, in part, by National Institutes of Health T32 (NHLBI T32HL007534) (CES), National Institutes of Health K23 (NHLBI K23 HL093387) (SCM), Pulmonary Hypertension Association K23 Supplemental Award (SCM), and Scleroderma Foundation Grant (SCM).
References
- 1.Fisher MR, Mathai SC, Champion HC, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum 2006; 54: 3043–3050. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfire M, Huffman MD, Krishnan S, et al. Survival in systemic sclerosis with pulmonary arterial hypertension has not improved in the modern era. Chest 2013; 144: 1282–1290. [DOI] [PubMed] [Google Scholar]
- 3.Chung L, Domsic RT, Lingala B, et al. Survival and predictors of mortality in systemic sclerosis-associated pulmonary arterial hypertension: outcomes from the pulmonary hypertension assessment and recognition of outcomes in scleroderma registry. Arthritis Care Res (Hoboken) 2014; 66: 489–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Yaici A, de Groote P, et al. Screening for pulmonary arterial hypertension in patients with systemic sclerosis: clinical characteristics at diagnosis and long-term survival. Arthritis Rheum 2011; 63: 3522–3530. [DOI] [PubMed] [Google Scholar]
- 5.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med 2009; 179: 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coghlan JG, Denton CP, Grunig E, et al. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 2014; 73: 1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagaya N, Uematsu M, Satoh T, et al. Serum uric acid levels correlate with the severity and the mortality of primary pulmonary hypertension. Am J Respir Crit Care Med 1999; 160: 487–492. [DOI] [PubMed] [Google Scholar]
- 8.Dhaun N, Vachiery JL, Benza RL, et al. Endothelin antagonism and uric acid levels in pulmonary arterial hypertension: clinical associations. J Heart Lung Transplant 2014; 33: 521–527. [DOI] [PubMed] [Google Scholar]
- 9.Drummond MB, Schwartz PF, Duggan WT, et al. Intersession variability in single-breath diffusing capacity in diabetics without overt lung disease. Am J Respir Crit Care Med 2008; 178: 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steen V, Medsger TA., Jr Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum 2003; 48: 516–522. [DOI] [PubMed] [Google Scholar]
- 11.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D42–50. [DOI] [PubMed] [Google Scholar]
- 12.Mathai SC, Hummers LK, Champion HC, et al. Survival in pulmonary hypertension associated with the scleroderma spectrum of diseases: impact of interstitial lung disease. Arthritis Rheum 2009; 60: 569–577. [DOI] [PubMed] [Google Scholar]
- 13.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 14.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 2013; 65: 2737–2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preliminary criteria for the classification of systemic sclerosis (scleroderma) Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum 1980; 23: 581–590. [DOI] [PubMed] [Google Scholar]
- 16.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med 1995; 14: 1707–1723. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld DA. Sample-size formula for the proportional-hazards regression model. Biometrics 1983; 39: 499–503. [PubMed] [Google Scholar]
- 18.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996; 15: 361–387. [DOI] [PubMed] [Google Scholar]
- 19.Pasarikovski CR, Granton JT, Roos AM, et al. Sex disparities in systemic sclerosis-associated pulmonary arterial hypertension: a cohort study. Arthritis Res Ther 2016; 18: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selvin S. Statistical analysis of epidemiologic data, 3rd ed Oxford; New York: Oxford University Press, 2004. [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254. [DOI] [PubMed] [Google Scholar]
- 22.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008; 359: 1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiba A, Vinker S, Dinour D, et al. Uric acid levels within the normal range predict increased risk of hypertension: a cohort study. J Am Soc Hypertens 2015; 9: 600–609. [DOI] [PubMed] [Google Scholar]
- 24.Prasad M, Matteson EL, Herrmann J, et al. Uric acid is associated with inflammation, coronary microvascular dysfunction, and adverse outcomes in postmenopausal women. Hypertension 2017; 69: 236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maiuolo J, Oppedisano F, Gratteri S, et al. Regulation of uric acid metabolism and excretion. Int J Cardiol 2016; 213: 8–14. [DOI] [PubMed] [Google Scholar]
- 26.Lewis GD, Ngo D, Hemnes AR, et al. Metabolic profiling of right ventricular-pulmonary vascular function reveals circulating biomarkers of pulmonary hypertension. J Am Coll Cardiol 2016; 67: 174–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sfrent-Cornateanu R, Mihai C, Stoian I, et al. Antioxidant defense capacity in scleroderma patients. Clin Chem Lab Med 2008; 46: 836–841. [DOI] [PubMed] [Google Scholar]
- 28.Stein CM, Tanner SB, Awad JA, et al. Evidence of free radical-mediated injury (isoprostane overproduction) in scleroderma. Arthritis Rheum 1996; 39: 1146–1150. [DOI] [PubMed] [Google Scholar]
- 29.Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J 1990; 265: 659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Maestro R, Thaw HH, Bjork J, et al. Free radicals as mediators of tissue injury. Acta Physiol Scand Suppl 1980; 492: 43–57. [PubMed] [Google Scholar]
- 31.Blann AD, Illingworth K, Jayson MI. Mechanisms of endothelial cell damage in systemic sclerosis and Raynaud's phenomenon. J Rheumatol 1993; 20: 1325–1330. [PubMed] [Google Scholar]
- 32.Hoshikawa Y, Ono S, Suzuki S, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. J Appl Physiol (1985) 2001; 90: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 33.Cheng TH, Lin JW, Chao HH, et al. Uric acid activates extracellular signal-regulated kinases and thereafter endothelin-1 expression in rat cardiac fibroblasts. Int J Cardiol 2010; 139: 42–49. [DOI] [PubMed] [Google Scholar]
- 34.Corry DB, Eslami P, Yamamoto K, et al. Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens 2008; 26: 269–275. [DOI] [PubMed] [Google Scholar]
- 35.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 2002; 13: 2888–2897. [DOI] [PubMed] [Google Scholar]
- 36.Kang DH, Park SK, Lee IK, et al. Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J Am Soc Nephrol 2005; 16: 3553–3562. [DOI] [PubMed] [Google Scholar]
- 37.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001; 38: 1101–1106. [DOI] [PubMed] [Google Scholar]
- 38.Zharikov S, Krotova K, Hu H, et al. Uric acid decreases NO production and increases arginase activity in cultured pulmonary artery endothelial cells. Am J Physiol Cell Physiol 2008; 295: C1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feig DI, Soletsky B, Johnson RJ. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: a randomized trial. JAMA 2008; 300: 924–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cappola TP, Kass DA, Nelson GS, et al. Allopurinol improves myocardial efficiency in patients with idiopathic dilated cardiomyopathy. Circulation 2001; 104: 2407–2411. [DOI] [PubMed] [Google Scholar]
- 41.Ansari-Ramandi MM, Maleki M, Alizadehasl A, et al. Safety and effect of high dose allopurinol in patients with severe left ventricular systolic dysfunction. J Cardiovasc Thorac Res 2017; 9: 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.George J, Carr E, Davies J, et al. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006; 114: 2508–2516. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Serum uric acid as a marker of disease risk, severity, and survival in systemic sclerosis-related pulmonary arterial hypertension by Catherine E. Simpson, Rachel L. Damico, Laura Hummers, Rubina M. Khair, Todd M. Kolb, Paul M. Hassoun and Stephen C. Mathai in Pulmonary Circulation