Abstract
Background and Aim
Exercise is an effective strategy to reduce obesity-induced oxidative stress. The purpose of this study was to compare the effects of two training modalities (moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT)) on the pro/antioxidant status of different tissues in obese Zucker rats.
Methods
Eight-week-old male Zucker rats (fa/fa, n = 36) were subdivided in three groups: MICT, HIIT, and control (no exercise) groups. Trained animals ran on a treadmill (0° slope), 5 days/week for 10 weeks (MICT: 51 min at 12 m·min−1; HIIT: 6 sets of 3 min at 10 m·min−1 followed by 4 min at 18 m·min−1). Epididymal (visceral) and subcutaneous adipose tissue, gastrocnemius muscle, and plasma samples were collected to measure oxidative stress markers (advanced oxidation protein products (AOPP), oxidized low-density lipoprotein (oxLDL)), antioxidant system markers (ferric-reducing ability of plasma (FRAP), superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPx) activities), and prooxidant enzymes (NADPH oxidase and xanthine oxidase (XO) activities, myeloperoxidase content).
Results
Compared with the control, MICT increased GPx and catalase activities and the FRAP level in epididymal adipose tissue. HIIT increased the AOPP level in subcutaneous adipose tissue. In the muscle, HIIT increased both SOD and GPx activities and reduced the AOPP level, whereas MICT increased only SOD activity. Finally, plasma myeloperoxidase content was similarly decreased by both training modalities, whereas oxLDL was reduced only in the MICT group.
Conclusion
Both HIIT and MICT improved the pro/antioxidant status. However, HIIT was more efficient than MICT in the skeletal muscle, whereas MICT was more efficient in epididymal adipose tissue. This suggests that oxidative stress responses to HIIT and MICT are tissue-specific. This could result in ROS generation via different pathways in these tissues. From a practical point of view, the two training modalities should be combined to obtain a global response in people with obesity.
1. Introduction
In 2016, there were approximately 650 million people with obesity worldwide [1], making obesity a major public health problem, mainly caused by increased intake of energy-dense food and physically inactive lifestyles [2, 3]. In this context, physical activity appears as an effective strategy for obesity prevention and management [4].
For years, moderate-intensity continuous training (MICT) has been the most popular exercise modality for improving body composition, cardiorespiratory fitness, insulin resistance, and lipid profile [2, 5]. However, the long-term adherence to this type of training is low, and many people stop exercising mainly because of lack of time and/or loss of motivation and gratification [6]. Recent studies have demonstrated that high-intensity interval training (HIIT), which consists in alternating short periods of high-intensity exercise with periods of light exercise (recovery) [7], is perceived as less monotonous and more enjoyable [8], thus significantly increasing participation and adherence [9, 10]. Although not all studies are unanimous [11–13], most agree that this type of training is also a time-effective alternative to MICT, leading to higher weight loss, particularly visceral fat mass, and better improvement of the metabolic profile and cardiorespiratory fitness in people with obesity [14–16].
In 2004, Furukawa et al. were the first to demonstrate in obese mice and in humans with obesity that fat accumulation is positively associated with systemic oxidative stress, suggesting that the oxidative stress increase in people with obesity could be explained by reactive oxygen species (ROS) overproduction in adipose tissue [17]. In addition, ectopic fat accumulation in the muscle promotes ROS production in this tissue [18]. MICT can reduce oxidative stress by increasing antioxidant enzyme activity and decreasing ROS production in the skeletal muscle [19], adipose tissue [20], and vascular tissue [21]. MICT also reduces systemic oxidative stress, particularly in people with obesity [22–24].
As fat mass accumulation increases oxidative stress [17] and HIIT leads to greater fat mass loss, particularly visceral fat [15], we hypothesized that this training modality could have a greater effects on the pro/antioxidant status compared with MICT in a rat model of obesity (Zucker rats). To test this hypothesis, we compared the effects of 10 weeks of HIIT and MICT on the pro/antioxidant status (antioxidant system, prooxidant enzymes, and oxidative stress markers) in different tissues (epididymal and subcutaneous adipose tissues, muscle, and plasma) in male Zucker rats.
2. Materials and Methods
2.1. Ethical Approval
The experimental protocol was approved by the local ethics committee (CE2A-02, Auvergne, France—protocol number 3075-2015120813375547) and was in accordance with the current legislation on animal experimentation (Guide for the Care and Use of Laboratory Animals, Eighth Edition 2011). Moreover, the experiments were carried out according to the local institution's animal welfare committee.
2.2. Animal Model and Experimental Groups
Seventy-five adult (8-week-old) male Zucker (fa/fa) rats were purchased from Charles River and were housed individually in an air-conditioned room with controlled temperature (21°C) and a reverse 12/12 h light/dark cycle. Tap water and food (3% lipids, 16% proteins, 60% carbohydrates, 5% minerals, and 4% fibers—SAFE A04, France) were available ad libitum.
After 10 days of acclimatization (including 5 days of treadmill exercise), 36 rats were selected for the study after a running test. During running habituation, rats that refused to run spontaneously after the treadmill started were excluded from the study. The runners were then randomly divided into the control group (n = 12), the MICT group (n = 12), and the HIIT group (n = 12). Animals in the three groups had similar weight, body fat, and fasting glucose level.
2.3. Experimental Design
Body weight was measured weekly in all animals, and the amount of food consumed was registered daily. Body composition was measured by MRI (Echo Medical Systems, Houston, TX), and epididymal fat pads were weighted post-mortem. At the end of the protocol (week 10) and 48 h after the last exercise session, the animals were killed by dislocation before tissue harvesting. Whole blood samples were immediately centrifuged at 2000 × g at 4°C for 10 min to obtain plasma. Aliquots were stored at -80°C until analysis. Muscles (gastrocnemius) and subcutaneous/epididymal adipose tissue samples were collected, weighed, and immediately frozen in liquid nitrogen and stored at -80°C until analysis.
2.4. Training Protocol
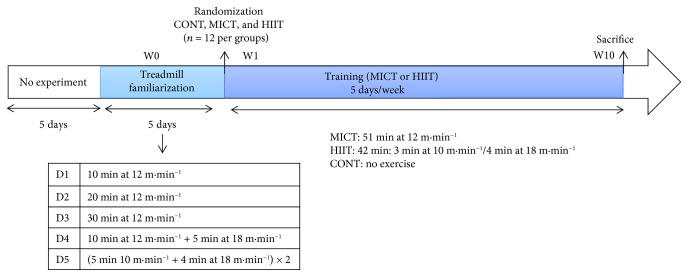
One week before the experimental period, the animals were familiarized with the training procedures using a low-intensity running protocol, as described in Figure 1. The treadmill habituation protocol finished three days before the beginning of the experimental period to avoid acute interference with the baseline measurements.
Figure 1.
Experimental design. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; W: week.
For the training protocol (10 weeks), the animals ran on a treadmill especially designed for rats (Panlab, Harvard Apparatus, LE 8710R), and all sessions were performed during the dark cycle (active period). Before each training session, animals in the MICT and HIIT groups performed a regular warm-up exercise at 10 m·min−1 for 5 min. The MICT group ran for 51 min at 12 m/min, 5 times per week for 10 weeks. The HIIT group alternated 3 min at 10 m/min and 4 min at 18 m/min (6 sets; 5 times per week for 10 weeks). The protocols were originally designed to have identical total running distance between groups, as proposed by [25–27]. Animals in the control group were managed identically as those in the MICT and HIIT groups but without exercise. Control rats were placed in the same room during the training sessions to account for the potential stress induced by environment changes.
2.5. Biochemical Analyses
Muscle and adipose tissue samples were ground in liquid nitrogen, homogenized (10%, w/v) in 1x PBS/0.5 mM EDTA on ice, and centrifuged at 12000 × g at 4°C for 10 min. Homogenates were stored in aliquots at -80°C. Total protein concentration was determined using the BCA Protein Assay Kit (Sigma-Aldrich, St Louis, USA) following the manufacturer's instructions. All the products used for oxidative stress marker measurements were from Sigma-Aldrich, and spectrophotometric measurements were performed on a TECAN Infinite 2000 plate reader (Männedorf, Switzerland). Results obtained with the skeletal muscle and adipose tissue samples were normalized to the total protein content to account for body weight variations during the experiment. Measurements were done in triplicates.
2.5.1. Oxidative Stress Markers
AOPP were determined according to the method by Witko-Sarsat et al. using a spectrophotometer and calibrated with a chloramine-T solution that absorbs at 340 nm in the presence of potassium iodide [28]. The absorbance of the reaction mixture was read at 340 nm. AOPP concentrations were expressed as μmol·L−1 of chloramine-T equivalents. The intra-assay coefficient of variation (CV) was 5.4%.
oxLDL concentration was measured in plasma using an ELISA kit (ELISA KIT, Elabscience®) according to the manufacturers' recommendations. Absorbance was read at 450 nm.
2.5.2. Antioxidant System Markers
Plasma SOD activity was determined using the method by Oberley and Spitz [29] based on the degree of SOD inhibition of the reaction between superoxide radicals, produced upon hypoxanthine oxidation by xanthine oxidase, and nitroblue tetrazolium (NTB). The blue formazan product subsequently formed was read at 560 nm for 5 min. The intra-assay CV was 5.6%.
Plasma GPx activity was determined using a modified version of the method described by Paglia and Valentine [30]. GPx activity is represented by the rate of NADPH oxidation to NADP+ after addition of glutathione reductase, reduced glutathione, and NADPH using H2O2 as the substrate. NADPH extinction was read at 340 nm for 5 min [30]. The intra-assay CV was 4.6%.
Plasma catalase activity was determined using the method described by Johansson and Borg [31] with H2O2 as the substrate and formaldehyde as the standard. Catalase activity was determined by monitoring the formaldehyde formation rate (read at 540 nm for 20 min) induced by the reaction of methanol and H2O2 using catalase as the enzyme [31]. The intra-assay CV was 3.1%.
Plasma FRAP was determined by spectrophotometry using the manual method described by Benzie and Strain [32]. FRAP concentration was calculated using an aqueous solution of a known Fe2+ concentration (FeSO4-7H2O) as the standard. Each sample was mixed at 37°C with a FRAP working solution that contains buffer acetate, 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), and ferric chloride (FeCl3-6H2O). The Fe2+-TPTZ complex formed was read at 593 nm after 4 min. The intra-assay CV was 2.9%.
2.5.3. Prooxidant Enzymes
Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) and xanthine oxidase (XO) activities were determined in plasma, as previously described [33] by the reaction of NTB with superoxide produced by hypoxanthine or NADPH with XO and NOX, respectively. NOX and XO activities were calculated by measuring spectrophotometrically the kinetic appearance of the complex formed by superoxide and NTB at 560 nm for 10 min.
Myeloperoxidase concentration was evaluated in plasma samples using a commercial ELISA kit (Myeloperoxidase DuoSet ELISA, R&D Systems, Minneapolis, MN, USA). The optical density was determined at 450 nm. The intra-assay CV was 2.9%.
2.6. Statistical Analysis
Results are expressed as the mean ± standard deviation (SD). Normality was checked using Kolmogorov-Smirnov's test. The assumption of homogeneity of variance was assessed using the Bartlett F-test. When the conditions of normality and homogeneity of variance were respected, one-way (to analyse data between the groups) or two-way mixed model ANOVAs with repeated measures (group, time, and group×time interaction) were run and a Newman-Keuls post hoc test was applied when the ANOVA reached significance level (p < 0.05). All data were analysed using the Statistica software.
3. Results
3.1. Animals' Characteristics
Measurement of whole-body mass, total fat mass (FM: g and %) and fat-free mass (FFM: g) in the three groups is presented in Table 1. The evolution of whole-body mass and FFM was comparable among groups during the study period (10 weeks). However, FM (g and %) was lower in the MICT and HIIT groups than in the CONT group at week 5 (p < 0.05) and in the HIIT group than in the CONT and MICT groups at the end of the protocol (p < 0.05).
Table 1.
Body composition pre (0 week), middle (5 weeks) and post (10 weeks) training.
| CONT | MICT | HIIT | ANOVA | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| W0 | W5 | W10 | W0 | W5 | W10 | W0 | W5 | W10 | G, T, GxT | |
| Body mass (g) | 376 ± 28 | 494 ± 41 | 505 ± 49 | 369 ± 35 | 491 ± 31 | 526 ± 37 | 380 ± 22 | 476 ± 33 | 493 ± 37 | G: 0.315 T: 0.000 GxT: 0.017 |
|
| ||||||||||
| FM (%) | 35 ± 2 | 41 ± 1 | 40 ± 2 | 35 ± 2 | 39 ± 2† | 38 ± 2 | 35 ± 3 | 38 ± 2§ | 37 ± 1∗£# | G: 0.001 (HIIT vs. CONT)∗ T: 0.02 GxT: 0.034 (MICT vs. CONT)† (HIIT vs. CONT)§ (HIIT vs. CONT)£ (HIIT vs. MICT)# |
|
| ||||||||||
| FM (g) | 129 ± 19 | 205 ± 20 | 201 ± 27 | 129 ± 17 | 186 ± 15† | 203 ± 21 | 131 ± 22 | 180 ± 11§ | 180 ± 13∗£# | G: 0.043 (HIIT vs. CONT)∗ T: 0.000 GxT: 0.000 (MICT vs. CONT)† (HIIT vs. CONT)§ (HIIT vs. CONT)£ (HIIT vs. MICT)# |
|
| ||||||||||
| FFM (g) | 209 ± 18 | 252 ± 18 | 262 ± 22 | 213 ± 18 | 268 ± 18 | 281 ± 18 | 213 ± 15 | 260 ± 22 | 273 ± 24 | G: 0.157 T: 0.000 GxT: 0.574 |
Data are presented as the mean ± SD. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; g: gram; FM (%): percentage of fat mass; FFM: fat-free mass; W0: week 0; W5: week 5; W10: week 10. ∗HIIT vs. CONT: group effect (p < 0.05); †MICT vs. CONT: group×time interaction at W5 (p < 0.05); §HIIT vs. CONT: group×time interaction at W5 (p < 0.05); £HIIT vs. CONT: group×time interaction at W10 (p < 0.05); #HIIT vs. MICT: group×time interaction at W10 (p < 0.05). These data are included in another article dedicated to the effects of HIIT and MICT on gut-adipose tissue cross-talk in obese Zucker rats [34].
Finally, cumulated food intake did not differ between groups (1699.33 ± 236.20 g for the CONT, 1852.91 ± 137.24 g for the MICT group, and 1698.33 ± 140.34 g for the HIIT group; p > 0.05).
These data are included in another article dedicated to the effects of HIIT and MICT on gut-adipose tissue cross-talk in obese Zucker rats [34].
3.2. MICT and HIIT Effects on Muscle Pro/Antioxidant Status
Analysis of the pro/antioxidant status in gastrocnemius samples from the three groups at the end of the study (week 10) showed that HIIT reduced significantly the level of advanced oxidation protein products (AOPP) compared with the control (10.17 ± 2.05 μmol · g−1 and 13.59 ± 4.14 μmol · g−1, respectively; p < 0.05) and also with MICT (13.24 ± 2.61 μmol · g−1; p < 0.05) (Figure 2(a)).
Figure 2.
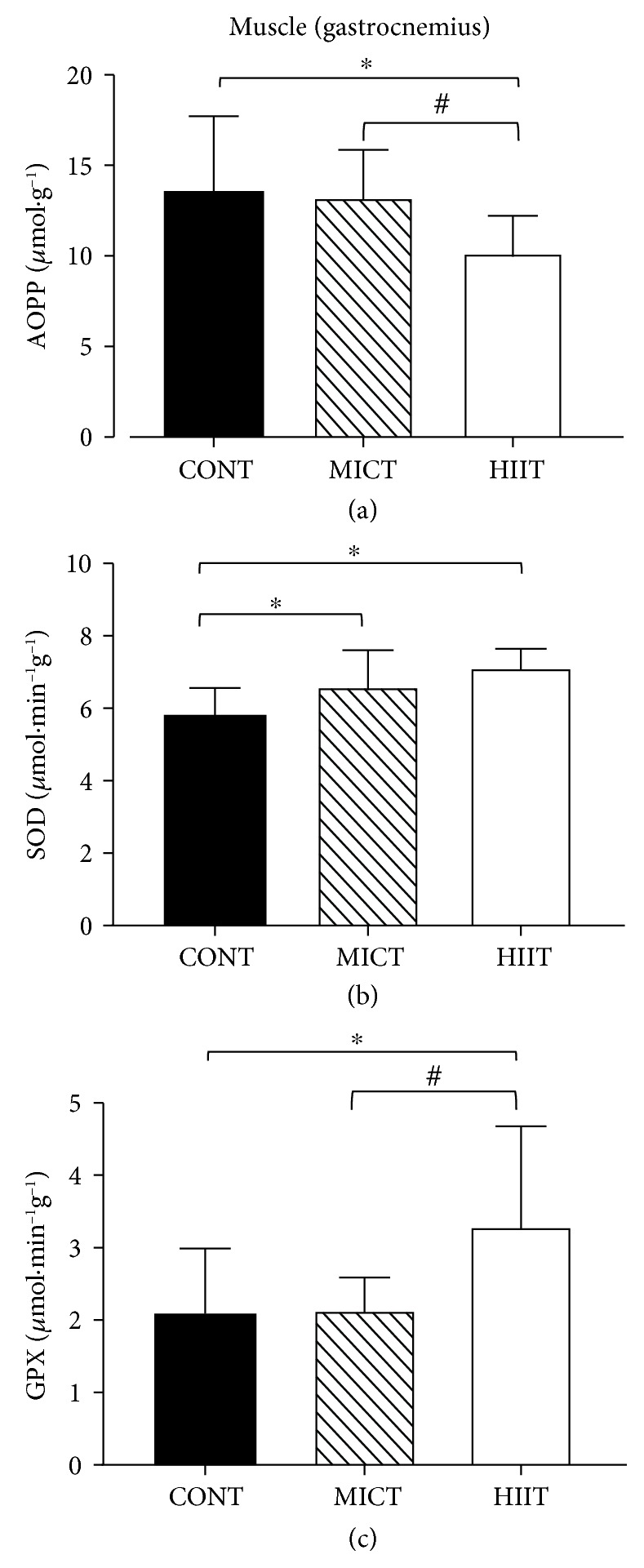
Effects of 10 weeks of exercise on (a) AOPP level (μmol·g−1), (b) SOD (μmol·min−1g−1), and (c) GPx activities (μmol·min−1g−1) in gastrocnemius samples from the three groups. Data are the mean ± SD. ∗p < 0.05, compared with CONT; #p < 0.05, compared with MICT. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; AOPP: advanced oxidation protein products; SOD: superoxide dismutase; GPx: glutathione peroxidase.
Superoxide dismutase (SOD) activity was significantly and similarly increased by both training modalities (7.13 ± 0.51 and 6.61 ± 1.00 μmol · min−1g−1 for HIIT and MICT, respectively) compared with the control (5.82 ± 0.74 μmol · min−1g−1) (Figure 2(b)).
Glutathione peroxidase (GPx) activity was significantly higher in the HIIT (3.29 ± 1.38 μmol · min−1g−1) than in the MICT (2.14 ± 0.45 μmol·min−1g−1) and control groups (2.09 ± 0.90 μmol · min−1g−1) (p < 0.05) (Figure 2(c)).
No training effect was observed for the other pro/antioxidant status markers (Table 2).
Table 2.
Gastrocnemius muscle pro/antioxidant status after 10 weeks of exercise training.
| CONT | MICT | HIIT | Group effect | |
|---|---|---|---|---|
| CAT (μmol·min−1g−1) | 2.4 ± 0.5 | 2.3 ± 0.6 | 2.2 ± 0.4 | p = 0.8 |
| FRAP (μmol·g−1) | 20 ± 8 | 24 ± 10 | 18 ± 5 | p = 0.2 |
| NADPHox (μmol·min−1g−1) | 0.48 ± 0.08 | 0.49 ± 0.08 | 0.49 ± 0.13 | p = 0.9 |
| XO (μmol·min−1g−1) | 0.29 ± 0.06 | 0.29 ± 0.06 | 0.31 ± 0.07 | p = 0.7 |
Data are presented as the mean ± SD. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; CAT: catalase; FRAP: ferric-reducing antioxidant power; NADPHox: nicotinamide adenine dinucleotide phosphate oxidase; XO: xanthine oxidase activity.
3.3. MICT and HIIT Effects on the Adipose Tissue Pro/Antioxidant Status
At the end of the study (week 10), the subcutaneous adipose tissue pro/antioxidant status was comparable in the three groups (Table 3), except for AOPP that was significantly higher in the HIIT than in the control group (655.50 ± 145.29 μmol · g−1 vs. 482.40 ± 150.27 μmol · g−1, respectively; p < 0.05) (Figure 3).
Table 3.
Adipose tissue pro/antioxidant status after 10 weeks of exercise training.
| CONT | MICT | HIIT | Group effect | |
|---|---|---|---|---|
| Subcutaneous adipose tissue | ||||
| CAT (μmol·min−1g−1) | 14 ± 5 | 14 ± 4 | 11 ± 3 | p = 0.1 |
| FRAP (μmol·g−1) | 102 ± 28 | 109 ± 39 | 115 ± 32 | p = 0.6 |
| SOD (μmol·min−1g−1) | 38 ± 15 | 29 ± 23 | 35 ± 24 | p = 0.6 |
| GPx (μmol·min−1g−1) | 19 ± 6 | 16 ± 8 | 15 ± 6 | p = 0.4 |
| NADPHox (μmol·min−1g−1) | 0.8 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.1 | p = 0.4 |
| XO (μmol·min−1g−1) | 1.2 ± 0.5 | 1.0 ± 0.4 | 1.0 ± 0.5 | p = 0.7 |
|
| ||||
| Epididymal adipose tissue | ||||
| AOPP (μmol·g−1). | 133 ± 52 | 183 ± 66 | 173 ± 46 | p = 0.08 |
| SOD (μmol·min−1g−1) | 69 ± 29 | 108 ± 40 | 95 ± 45 | p = 0.06 |
| NADPHox (μmol·min−1g−1) | 3.0 ± 0.8 | 3.7 ± 1.6 | 2.8 ± 1.0 | p = 0.2 |
| XO (μmol·min−1g−1) | 1.8 ± 1.1 | 2.8 ± 1.5 | 2.2 ± 1.4 | p = 0.3 |
Data are presented as the mean ± SD. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; CAT: catalase activity; FRAP: ferric-reducing antioxidant power; SOD: superoxide dismutase; GPx, glutathione peroxidase; NADPHox: nicotinamide adenine dinucleotide phosphate oxidase; XO: xanthine oxidase activity; AOPP: advanced oxidation protein product.
Figure 3.
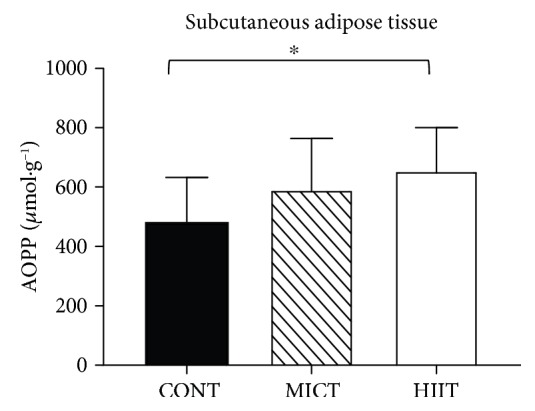
Effects of 10 weeks of exercise on the AOPP level (μmol·g−1) in subcutaneous adipose tissue samples from the three groups. Data are the mean ± SD. ∗p < 0.05, compared with the CONT group. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; AOPP: advanced oxidation protein products.
In epididymal (visceral) adipose tissue, only MICT significantly increased the activity of catalase (59.47 ± 28.74 μmol · min−1g−1 in the MICT group and 38.31 ± 11.01 μmol · min−1g−1 in the control group; p < 0.05). The activity of GPx (57.31 ± 51.62 μmol · min−1g−1 in the MICT and 20.52 ± 12.24 μmol · min−1g−1 in the control group; p < 0.05) and ferric-reducing ability of plasma (FRAP) (77.79 ± 33.17 in the MICT group and 53.08 ± 13.64 μmol · g−1 in the control group; p < 0.05) is also increased only by MICT (Figure 4).
Figure 4.
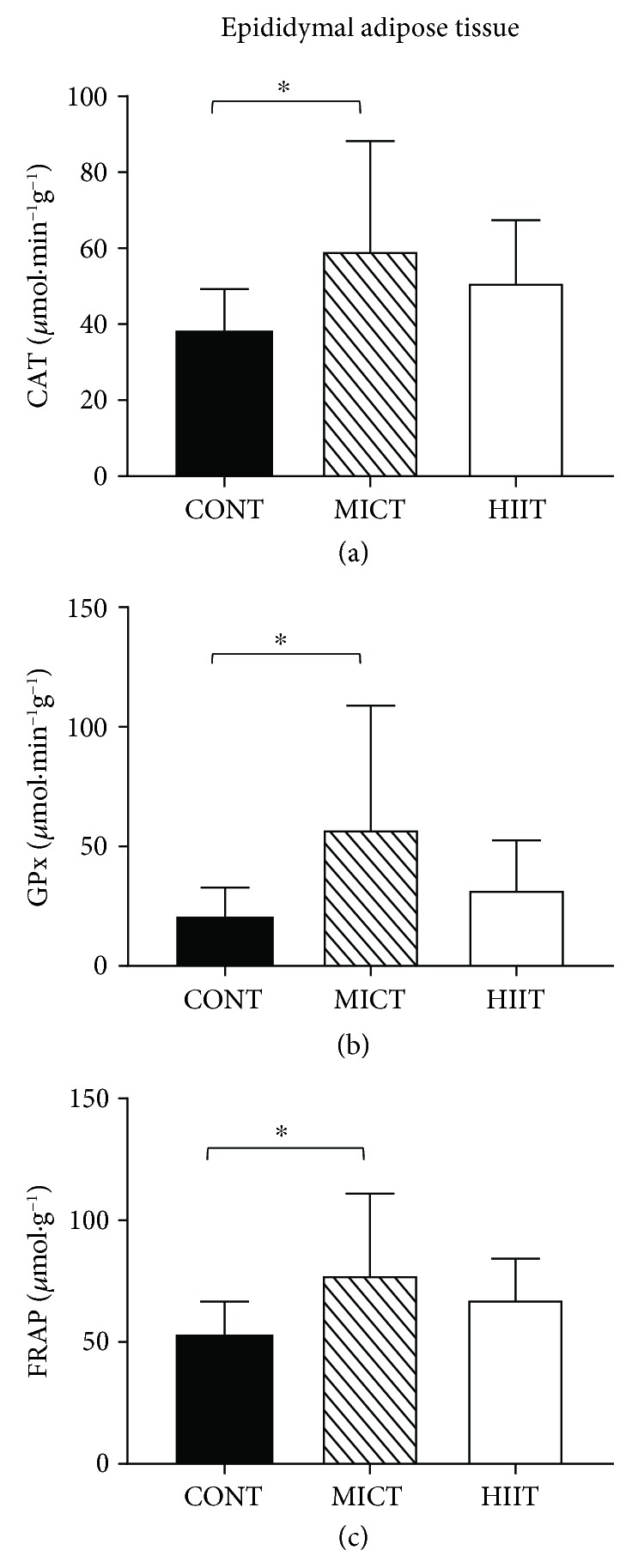
Effects of 10 weeks of exercise on (a) CAT (μmol·min−1g−1) and (b) GPx (μmol·min−1g−1) activities, and (c) FRAP (μmol·g−1) in the epididymal adipose tissue. Data are mean ± SD. ∗p < 0.05, compared with the CONT group. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; CAT: catalase; GPx, glutathione peroxidase; FRAP: ferric-reducing antioxidant power.
No training effect was observed for the other tested markers (Table 3).
3.4. MICT and HIIT Effects on the Systemic Pro/Antioxidant Status
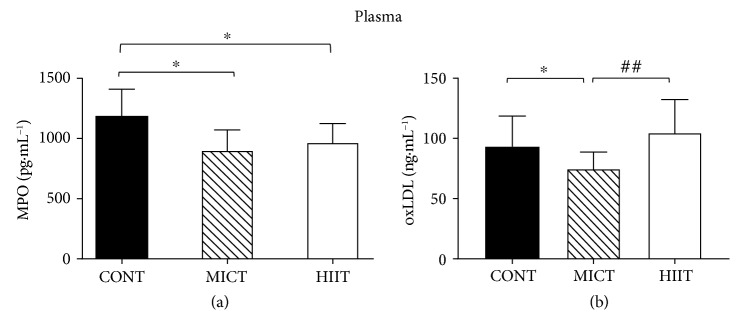
At week 10, myeloperoxidase content was similarly reduced by both training modalities (975.03 ± 158.02 for the HIIT group and 909.12 ± 170.84 pg · mL−1 for the MICT group) compared with the control (1196.63 ± 224.33 pg · mL−1) (p < 0.05) (Figure 5(a)).
Figure 5.
Effects of 10 weeks of exercise on (a) MPO content (pg·mL−1) and (b) oxLDL (ng·mL−1) level in plasma. Data are the mean ± SD. ∗p < 0.05, compared with CONT. ##p < 0.01, compared with MICT. CONT: control (no exercise); MICT: moderate-intensity continuous training; HIIT: high-intensity interval training; MPO: myeloperoxidase; oxLDL: oxidized low-density lipoprotein.
However, only MICT significantly reduced oxidized low-density lipoprotein (oxLDL) level in plasma compared with the control (74.93 ± 13.82 ng · mL−1 and 93.12 ± 25.49 ng · mL−1, respectively; p < 0.05) and also with HIIT (74.93 ± 3.99 ng · mL−1 and 104.82 ± 27.44 ng · mL−1, respectively; p < 0.05) (Figure 5(b)).
HIIT and MICT did not modulate plasma SOD activity (13.14 ± 3.95 for the HIIT group and 13.71 ± 4.47 μmol · min · L−1 for the MICT group) compared with the control (12.65 ± 4.15 μmol · min · L−1).
4. Discussion
This study is the first to compare the effects of two exercise training modalities (MICT vs. HIIT) on the pro/antioxidant status in obese rats focusing especially on three main tissues: (1) adipose tissue which is the main tissue involved in ROS production in the case of obesity, (2) the muscle which is involved in exercise and where ROS are mainly produced, and (3) the blood which reflects the systemic aspect. We hypothesized that HIIT, by decreasing total and visceral fat mass to a greater extent than MICT, would lead to greater benefits on the pro/antioxidant status. As expected, HIIT led to higher reduction of the FM percentage than MICT (p < 0.05) at the end of the protocol; however, our results indicate that HIIT improved the pro/antioxidant status more efficiently than MICT only in the muscle, where ROS are mainly produced during exercise. Conversely, MICT was more efficient in epididymal adipose tissue.
In this study, we analyzed the effects of MICT and HIIT in Zucker rats (fa/fa). This animal model of obesity is the best known and most widely used model of genetic obesity and displays the whole biochemical and metabolic pathological outcomes of the disease (hyperinsulinemia, hyperlipidaemia and insulin resistance, and adipocyte hypertrophy/hyperplasia) [35, 36]. Zucker rats are significantly hyperphagic and quickly develop obesity (at around 4 weeks of age) due to a mutation in the leptin receptor (fa gene), like in human obesity [37]. Moreover, obese Zucker rats are a good model to test the beneficial effects of therapeutic intervention programs based on physical activity [38, 39].
We focused our attention on the pro/antioxidant status because oxidative stress has been proposed to be the unifying mechanism in the development of major obesity-related comorbidities, particularly cardiovascular disease and diabetes [40]. Several mechanisms may explain the oxidative stress increase in people with overweight/obesity, particularly (i) a decrease of antioxidant defenses, (ii) an increase in mitochondrial ROS production due to excessive energy expenditure, (iii) an increase in plasma lipids that are oxidation targets, (iv) an increase of the leptin level that may stimulate intracellular ROS production, and (v) ROS overproduction by adipose tissue due to cytokine release [41]. In this context, interventions that can protect against oxidative stress are essential to prevent obesity-related complications and should be encouraged [42]. In addition, physical training is likely to be the only known method that reduces oxidative stress independently of body adiposity [42]. In our study, we demonstrated that each training modality induced tissue-specific modulations of oxidative stress.
Our results on the differential adipose tissue pro/antioxidant status modulation by the two training modalities bring new knowledge to the existing literature. In epididymal adipose tissue, only MICT induced beneficial effects by increasing GPx and catalase activities and the FRAP level. Conversely and surprisingly, in subcutaneous adipose tissue, HIIT increased the AOPP level. It is known that basal oxidative stress increases in the white adipose tissue of obese animals [17, 43, 44] due to upregulation of NADPH oxidase subunits and downregulation of antioxidant enzymes (SOD and GPx), leading to higher H2O2 production and lipid peroxidation [17]. Very few studies investigated the adipose tissue pro/antioxidant status in response to training, and to our knowledge, no study compared HIIT and MICT in obese animals. Like others [45, 46], we found that the activity of antioxidant enzymes (catalase and GPx) was increased in epididymal adipose tissue after MICT, confirming that exercise training acts as an antioxidant also in this tissue. Conversely, oxidative stress markers were not decreased in adipose tissue after MICT. This is different from a previous study [47] showing that after 10 weeks of aerobic training, the protein carbonyl group level was reduced in epididymal fat depots. This discrepancy could be explained by the different exercise intensity of the training protocols (20 m·min−1 vs. 12 m·min−1 in our study).
In our study, MICT specifically modulated the pro/antioxidant status in visceral adipose tissue, but not in subcutaneous adipose tissue, as previously reported by Sakurai et al. [20]. These authors showed that exercise training increases manganese-dependent SOD (Mn-SOD) activity and decreases lipid peroxidation in epididymal adipose tissue, but not in subcutaneous adipose tissue [20]. The underlying mechanisms remain to be elucidated. The sensitivity of adipocytes to exercise-induced changes could play a role because the catecholamine-induced lipolytic response is higher in omental (visceral adipose tissue) than in subcutaneous adipocytes [48]. Finally, the absence of HIIT protective effect in the two adipose tissues suggests that MICT and HIIT act on different redox mechanisms in function of the tissue.
In the muscle, both training modalities increased SOD activity; however, only HIIT promoted significantly GPx activity and decreased AOPP, suggesting that in obese rats, the muscle pro/antioxidant status is improved more efficiently by HIIT than by MICT. The significant AOPP reduction in the HIIT group might be explained by the induction of GPx activity observed with this training modality. Therefore, it is likely that by improving the muscle antioxidant defenses, particularly GPx activity, HIIT reduces the muscle oxidative damage. In normal-weight rats, high-intensity training, but not continuous training, increases GPx activity in the muscle [49]. The underlying mechanisms are not fully understood, but HIIT could induce the activation of redox-sensitive protein signalling pathways [50] via higher ROS production in the muscle. Indeed, as muscle ROS production is related to the exercise intensity [51], during HIIT, each bout of intense exercise might induce an important increase in H2O2 production, thus outmatching the muscle antioxidant defenses. Conversely, during MICT, the hydroxide peroxide (H2O2) content can be more easily regulated. This hypothesis is supported by the finding that compared with continuous exercise, intermittent exercise induces a greater activation of redox-dependent signalling pathways due to the higher metabolic demand [52]. Another hypothesis can be formulated to explain the greater increase in antioxidant enzyme activities in the muscle in response to HIIT. We cannot exclude decrease in ROS production by mitochondria as previously demonstrated in healthy people, in response to aerobic training [53] and/or a decrease in ROS production due to a greater loss of intramuscular triglycerides (IMTG) in response to HIIT. Indeed, although in healthy people IMTG is used as an energy source and may be increased by endurance training exercise [54, 55], Ko et al. [56] demonstrated in an obese mouse model that 8 weeks of treadmill exercise contributes to decreased IMTG volume by activating lipolysis factors. To our knowledge, no study investigated the effect of different modalities of training (HIIT vs. MICT) on intramuscular triglycerides and especially in relation to the oxidative stress status. It may be hypothesized that HIIT decreases IMTG more than MICT because of the higher IMGT lipolysis resulting from higher lactate production. In turn, ROS production (which is related to this percentage in obese subjects) should decrease [57].
Concerning plasma markers of the pro/antioxidant status, both training modalities similarly reduced the myeloperoxidase level, but only MICT could decrease the oxLDL level. The decrease of myeloperoxidase, a marker of neutrophil activation/degranulation and superoxide radical production [58], by both training modalities is relevant because recent studies suggest that elevated circulating myeloperoxidase levels could predict the appearance of coronary events [59]. Previous works reported a decreased plasma myeloperoxidase level in response to continuous training in healthy animals [60, 61]. The only study on the effects of MICT and HIIT in sedentary healthy men in hypoxic conditions [62] found that only HIIT could reduce plasma myeloperoxidase. Plasmatic myeloperoxidase can interact with polyunsaturated fatty acids in the cell membrane, enhancing lipid peroxidation [63]. Moreover, we found that oxLDL, a marker of lipid peroxidation and a major candidate in the pathogenesis of atherosclerosis [64], was decreased only in the MICT group. This effect could contribute to reduce the cardiovascular risk. In humans, the decrease of plasma oxLDL after exercise training programs is well documented in lean older people [65] and also in people with obesity [66]. By comparing the high and low volume of intense exercise training (90% of the maximal heart rate) in healthy overweight men (BMI: 25-30), Tjonna et al. found that oxLDL was reduced only after high-volume training [16]. This difference may suggest that the total volume of HIIT in our study was too low to affect oxLDL in obese Zucker rats.
Delwing-de Lima et al. [67] evaluated the effects of MICT and HIIT on the pro/antioxidant status of obese rats induced by a high-fat diet (HFD) and compared the responses in two different tissues which are the blood and the liver. As in our study, the authors observe tissue-specific responses to MICT and HIIT. In the blood, both training protocols prevented the increase in TBARS and protein carbonyl content induced by HFD and prevented a reduction in erythrocyte CAT activity. HIIT protocol is the sole enhancing erythrocyte SOD activity. In the liver, both training protocols prevented the increase in protein carbonyl content and only MICT prevented an alteration in CAT activity. In our study, the muscle compared to adipose tissue is the most sensitive tissue to favour the upregulation of AO enzyme activities (SOD and GPX) and decreased level of AOPP, especially after the HIIT protocol. It is well established that the muscle is the tissue that produces the most ROS during exercise [68]. Since ROS produced during exercise are known to cause an activation of MAP kinases which in turn activated the NF-κB pathway and consequently the expression of important enzymes associated with defense against ROS [69], it is not surprising that the muscle is the tissue where the effects of training were more pronounced regarding AO enzyme activity leading to lower oxidative damages.
Even if we demonstrated that both HIIT and MICT improved the pro/antioxidant status in a different way according to the tissues considered, our study presents limitations. The first is the use of the gastrocnemius which has the advantage of being large and has allowed us to perform all our measurements on the same tissue but has the disadvantage of being a mixed type fiber. HIIT and MICT effects may have been different on a slow muscle like soleus, especially for MICT. Only male rodents were examined and given the antioxidant effects of oestrogen; the use of a group of females would probably have revealed gender-specific responses. We also have to acknowledge that we did not evaluate the aerobic capacity of rats. However, the speed that we used for the HIIT group is close to the maximal aerobic speed (16-24 m·min−1) and above the speed at the lactate threshold (12.5 m·min−1) measured in Zucker rats in previous studies [38, 70]. In addition, the speed used for the MICT group is below the speed at the lactate threshold (10 vs. 12.5 m·min−1) for Zucker rats. Finally, in the beneficial effects we observed, it is impossible to distinguish what is due to the fat mass reduction or to the antioxidant effects of chronic exercise.
5. Conclusions
MICT and HIIT exert beneficial, but tissue-specific, effects on the pro/antioxidant status. Both training modalities can increase SOD activity in the muscle and reduce the plasma concentration of myeloperoxidase, a major cardiovascular risk indicator in plasma. As hypothesized, HIIT was more beneficial than MICT for improving the pro/antioxidant status in the muscle, where ROS are mainly produced during exercise. Conversely, MICT was more effective in epididymal adipose tissue suggesting that the two training modalities could be combined to obtain a global response in people with obesity.
Acknowledgments
This study was supported by (i) the “Région Auvergne-Rhône-Alpes” (PREVAMIC project), (ii) the French government IDEX-ISITE initiative 16-IDEX-0001 (CAP 20-25) and I-SITE project (CAP 2025) of the University of Clermont Auvergne, (iii) INSERM (U1071) and INRA (USC-2018), and (iv) grants from the Association F. Aupetit (AFA).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
No conflicts of interest, financial or otherwise, are declared by the authors.
Authors' Contributions
Vincent Pialoux and Nathalie Boisseau contributed equally to the study.
References
- 1.WHO. World Health Organisation; 2016. Fact sheet: obesity and overweight. [Google Scholar]
- 2.Donnelly J. E., Blair S. N., Jakicic J. M., Manore M. M., Rankin J. W., Smith B. K. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and Science in Sports and Exercise. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 3.Kopelman P. G. Obesity as a medical problem. Nature. 2000;404(6778):635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 4.Haskell W. L., Lee I. M., Pate R. R., et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Medicine and Science in Sports and Exercise. 2007;39(8):1423–1434. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 5.Duggan G. E., Hittel D. S., Sensen C. W., Weljie A. M., Vogel H. J., Shearer J. Metabolomic response to exercise training in lean and diet-induced obese mice. Journal of Applied Physiology. 2011;110(5):1311–1318. doi: 10.1152/japplphysiol.00701.2010. [DOI] [PubMed] [Google Scholar]
- 6.Bartlett J. D., Close G. L., MacLaren D. P. M., Gregson W., Drust B., Morton J. P. High-intensity interval running is perceived to be more enjoyable than moderate-intensity continuous exercise: implications for exercise adherence. Journal of Sports Sciences. 2011;29(6):547–553. doi: 10.1080/02640414.2010.545427. [DOI] [PubMed] [Google Scholar]
- 7.Weston M., Taylor K. L., Batterham A. M., Hopkins W. G. Effects of low-volume high-intensity interval training (HIT) on fitness in adults: a meta-analysis of controlled and non-controlled trials. Sports Medicine. 2014;44(7):1005–1017. doi: 10.1007/s40279-014-0180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira B. R. R., Santos T. M., Kilpatrick M., Pires F. O., Deslandes A. C. Affective and enjoyment responses in high intensity interval training and continuous training: a systematic review and meta-analysis. PLoS One. 2018;13(6, article e0197124) doi: 10.1371/journal.pone.0197124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gaesser G. A., Angadi S. S. High-intensity interval training for health and fitness: can less be more? Journal of Applied Physiology. 2011;111(6):1540–1541. doi: 10.1152/japplphysiol.01237.2011. [DOI] [PubMed] [Google Scholar]
- 10.Kong Z., Fan X., Sun S., Song L., Shi Q., Nie J. Comparison of high-intensity interval training and moderate-to-vigorous continuous training for cardiometabolic health and exercise enjoyment in obese young women: a randomized controlled trial. PLoS One. 2016;11(7, article e0158589) doi: 10.1371/journal.pone.0158589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbertson N. M., Eichner N. Z. M., Francois M., et al. Glucose tolerance is linked to postprandial fuel use independent of exercise dose. Medicine and Science in Sports and Exercise. 2018;50(10):2058–2066. doi: 10.1249/MSS.0000000000001667. [DOI] [PubMed] [Google Scholar]
- 12.Rowan C. P., Riddell M. C., Gledhill N., Jamnik V. K. Aerobic exercise training modalities and prediabetes risk reduction. Medicine and Science in Sports and Exercise. 2017;49(3):403–412. doi: 10.1249/MSS.0000000000001135. [DOI] [PubMed] [Google Scholar]
- 13.Nybo L., Sundstrup E., Jakobsen M. D., et al. High-intensity training versus traditional exercise interventions for promoting health. Medicine and Science in Sports and Exercise. 2010;42(10):1951–1958. doi: 10.1249/MSS.0b013e3181d99203. [DOI] [PubMed] [Google Scholar]
- 14.Dias K. A., Ingul C. B., Tjonna A. E., et al. Effect of high-intensity interval training on fitness, fat mass and cardiometabolic biomarkers in children with obesity: a randomised controlled trial. Sports Medicine. 2018;48(3):733–746. doi: 10.1007/s40279-017-0777-0. [DOI] [PubMed] [Google Scholar]
- 15.Maillard F., Pereira B., Boisseau N. Effect of high-intensity interval training on total, abdominal and visceral fat mass: a meta-analysis. Sports Medicine. 2018;48(2):269–288. doi: 10.1007/s40279-017-0807-y. [DOI] [PubMed] [Google Scholar]
- 16.Tjonna A. E., Leinan I. M., Bartnes A. T., et al. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PLoS One. 2013;8(5, article e65382) doi: 10.1371/journal.pone.0065382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furukawa S., Fujita T., Shimabukuro M., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi J. K., Vidal-Puig A. J. Targeting fat to prevent diabetes. Cell Metabolism. 2007;5(5):323–325. doi: 10.1016/j.cmet.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Moal E., Pialoux V., Juban G., et al. Redox control of skeletal muscle regeneration. Antioxidants & Redox Signaling. 2017;27(5):276–310. doi: 10.1089/ars.2016.6782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai T., Izawa T., Kizaki T., et al. Exercise training decreases expression of inflammation-related adipokines through reduction of oxidative stress in rat white adipose tissue. Biochemical and Biophysical Research Communications. 2009;379(2):605–609. doi: 10.1016/j.bbrc.2008.12.127. [DOI] [PubMed] [Google Scholar]
- 21.Chirico E. N., Faes C., Connes P., Canet-Soulas E., Martin C., Pialoux V. Role of exercise-induced oxidative stress in sickle cell trait and disease. Sports Medicine. 2016;46(5):629–639. doi: 10.1007/s40279-015-0447-z. [DOI] [PubMed] [Google Scholar]
- 22.Emami S. R., Jafari M., Haghshenas R., Ravasi A. Impact of eight weeks endurance training on biochemical parameters and obesity-induced oxidative stress in high fat diet-fed rats. Journal of Exercise Nutrition & Biochemistry. 2016;20(1):30–36. doi: 10.20463/jenb.2016.03.20.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samjoo I. A., Safdar A., Hamadeh M. J., Raha S., Tarnopolsky M. A. The effect of endurance exercise on both skeletal muscle and systemic oxidative stress in previously sedentary obese men. Nutrition & Diabetes. 2013;3(9, article e88) doi: 10.1038/nutd.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youssef H., Groussard C., Lemoine-Morel S., et al. Aerobic training suppresses exercise-induced lipid peroxidation and inflammation in overweight/obese adolescent girls. Pediatric Exercise Science. 2015;27(1):67–76. doi: 10.1123/pes.2014-0008. [DOI] [PubMed] [Google Scholar]
- 25.Haram P. M., Kemi O. J., Lee S. J., et al. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovascular Research. 2009;81(4):723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapravelou G., Martinez R., Andrade A. M., et al. Aerobic interval exercise improves parameters of nonalcoholic fatty liver disease (NAFLD) and other alterations of metabolic syndrome in obese Zucker rats. Applied Physiology, Nutrition, and Metabolism. 2015;40(12):1242–1252. doi: 10.1139/apnm-2015-0141. [DOI] [PubMed] [Google Scholar]
- 27.Metz L., Vermaelen M., Lambert K., et al. Endurance training increases lactate transport in male Zucker fa/fa rats. Biochemical and Biophysical Research Communications. 2005;331(4):1338–1345. doi: 10.1016/j.bbrc.2005.04.054. [DOI] [PubMed] [Google Scholar]
- 28.Witko-Sarsat V., Friedlander M., Capeillere-Blandin C., et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney International. 1996;49(5):1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 29.Oberley L. W., Spitz D. R. Assay of superoxide dismutase activity in tumor tissue. Methods in Enzymology. 1984;105:457–464. doi: 10.1016/S0076-6879(84)05064-3. [DOI] [PubMed] [Google Scholar]
- 30.Paglia D. E., Valentine W. N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 31.Johansson L. H., Håkan Borg L. A. A spectrophotometric method for determination of catalase activity in small tissue samples. Analytical Biochemistry. 1988;174(1):331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- 32.Benzie I. F. F., Strain J. J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Analytical Biochemistry. 1996;239(1):70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 33.Laouafa S., Ribon-Demars A., Marcouiller F., et al. Estradiol protects against cardiorespiratory dysfunctions and oxidative stress in intermittent hypoxia. Sleep. 2017;40(8) doi: 10.1093/sleep/zsx104. [DOI] [PubMed] [Google Scholar]
- 34.Maillard F., Vazeille E., Sauvanet P., et al. High intensity interval training promotes total and visceral fat mass loss in obese Zucker rats without modulating gut microbiota. PLoS One. 2019;14(4, article e0214660) doi: 10.1371/journal.pone.0214660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teixeira de Lemos E., Reis F., Baptista S., et al. Exercise training decreases proinflammatory profile in Zucker diabetic (type 2) fatty rats. Nutrition. 2009;25(3):330–339. doi: 10.1016/j.nut.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Durham H. A., Truett G. E. Development of insulin resistance and hyperphagia in Zucker fatty rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2006;290(3):R652–R658. doi: 10.1152/ajpregu.00428.2004. [DOI] [PubMed] [Google Scholar]
- 37.Phillips M. S., Liu Q., Hammond H. A., et al. Leptin receptor missense mutation in the fatty Zucker rat. Nature Genetics. 1996;13(1):18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 38.Almeida J. A., Petriz B. A., Gomes C. P. C., Rocha L. A., Pereira R. W., Franco O. L. Determination of the maximal lactate steady state in obese Zucker rats. International Journal of Sports Medicine. 2013;34(3):214–217. doi: 10.1055/s-0032-1316360. [DOI] [PubMed] [Google Scholar]
- 39.Almeida J. A., Petriz B. A., Gomes C. P. C., Araujo R. C., Pereira R. W., Franco O. L. Exercise training at MLSS decreases weight gain and increases aerobic capacity in obese Zucker rats. International Journal of Sports Medicine. 2014;35(3):199–202. doi: 10.1055/s-0033-1349872. [DOI] [PubMed] [Google Scholar]
- 40.Higdon J. V., Frei B. Obesity and oxidative stress: a direct link to CVD? Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(3):365–367. doi: 10.1161/01.ATV.0000063608.43095.E2. [DOI] [PubMed] [Google Scholar]
- 41.Vincent H. K., Taylor A. G. Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans. International Journal of Obesity. 2006;30(3):400–418. doi: 10.1038/sj.ijo.0803177. [DOI] [PubMed] [Google Scholar]
- 42.Vincent H. K., Innes K. E., Vincent K. R. Oxidative stress and potential interventions to reduce oxidative stress in overweight and obesity. Diabetes, Obesity & Metabolism. 2007;9(6):813–839. doi: 10.1111/j.1463-1326.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 43.Curtis J. M., Grimsrud P. A., Wright W. S., et al. Downregulation of adipose glutathione S-transferase A4 leads to increased protein carbonylation, oxidative stress, and mitochondrial dysfunction. Diabetes. 2010;59(5):1132–1142. doi: 10.2337/db09-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houstis N., Rosen E. D., Lander E. S. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440(7086):944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 45.de Farias J. M., Bom K. F., Tromm C. B., et al. Effect of physical training on the adipose tissue of diet-induced obesity mice: interaction between reactive oxygen species and lipolysis. Hormone and Metabolic Research. 2013;45(3):190–196. doi: 10.1055/s-0032-1323740. [DOI] [PubMed] [Google Scholar]
- 46.Ko J., Kim K. Effects of exercise and diet composition on expression of MCP-1 and oxidative stress-related mRNA of adipose tissue in diet-induced obese mice. The Journal of Exercise Nutrition & Biochemistry. 2013;17(4):181–188. doi: 10.5717/jenb.2013.17.4.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krskova K., Eckertova M., Kukan M., et al. Aerobic training lasting for 10 weeks elevates the adipose tissue FABP4, Giα, and adiponectin expression associated by a reduced protein oxidation. Endocrine Regulations. 2012;46(03):137–146. doi: 10.4149/endo_2012_03_137. [DOI] [PubMed] [Google Scholar]
- 48.Wajchenberg B. L. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocrine Reviews. 2000;21(6):697–738. doi: 10.1210/edrv.21.6.0415. [DOI] [PubMed] [Google Scholar]
- 49.Criswell D., Powers S., Dodd S., et al. High intensity training-induced changes in skeletal muscle antioxidant enzyme activity. Medicine & Science in Sports & Exercise. 1993;25(10):1135–1140. doi: 10.1249/00005768-199310000-00009. [DOI] [PubMed] [Google Scholar]
- 50.Gomez-Cabrera M. C., Vina J., Ji L. L. Role of redox signaling and inflammation in skeletal muscle adaptations to training. Antioxidants. 2016;5(4):p. 48. doi: 10.3390/antiox5040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powers S. K., Radak Z., Ji L. L. Exercise-induced oxidative stress: past, present and future. The Journal of Physiology. 2016;594(18):5081–5092. doi: 10.1113/JP270646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Combes A., Dekerle J., Webborn N., Watt P., Bougault V., Daussin F. N. Exercise-induced metabolic fluctuations influence AMPK, p38-MAPK and CaMKII phosphorylation in human skeletal muscle. Physiological Reports. 2015;3(9):p. e12462. doi: 10.14814/phy2.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mrakic-Sposta S., Gussoni M., Porcelli S., et al. Training effects on ROS production determined by electron paramagnetic resonance in master swimmers. Oxidative Medicine and Cellular Longevity. 2015;2015:8. doi: 10.1155/2015/804794.804794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Loon L. J. C., Koopman R., Manders R., van der Weegen W., van Kranenburg G. P., Keizer H. A. Intramyocellular lipid content in type 2 diabetes patients compared with overweight sedentary men and highly trained endurance athletes. American Journal of Physiology-Endocrinology and Metabolism. 2004;287(3):E558–E565. doi: 10.1152/ajpendo.00464.2003. [DOI] [PubMed] [Google Scholar]
- 55.Goodpaster B. H., He J., Watkins S., Kelley D. E. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. The Journal of Clinical Endocrinology and Metabolism. 2001;86(12):5755–5761. doi: 10.1210/jcem.86.12.8075. [DOI] [PubMed] [Google Scholar]
- 56.Ko K., Woo J., Bae J. Y., Roh H. T., Lee Y. H., Shin K. O. Exercise training improves intramuscular triglyceride lipolysis sensitivity in high-fat diet induced obese mice. Lipids in Health and Disease. 2018;17(1):p. 81. doi: 10.1186/s12944-018-0730-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nikooie R., Samaneh S. Exercise-induced lactate accumulation regulates intramuscular triglyceride metabolism via transforming growth factor-β1 mediated pathways. Molecular and Cellular Endocrinology. 2016;419:244–251. doi: 10.1016/j.mce.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 58.Kehrer J. P. Free radicals as mediators of tissue injury and disease. Critical Reviews in Toxicology. 1993;23(1):21–48. doi: 10.3109/10408449309104073. [DOI] [PubMed] [Google Scholar]
- 59.Nicholls S. J., Hazen S. L. Myeloperoxidase and cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(6):1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 60.Barcelos R. P., Souza M. A., Amaral G. P., et al. Caffeine intake may modulate inflammation markers in trained rats. Nutrients. 2014;6(4):1678–1690. doi: 10.3390/nu6041678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matsumoto Y., Adams V., Jacob S., Mangner N., Schuler G., Linke A. Regular exercise training prevents aortic valve disease in low-density lipoprotein-receptor-deficient mice. Circulation. 2010;121(6):759–767. doi: 10.1161/CIRCULATIONAHA.109.892224. [DOI] [PubMed] [Google Scholar]
- 62.Weng T. P., Huang S. C., Chuang Y. F., Wang J. S. Effects of interval and continuous exercise training on CD4 lymphocyte apoptotic and autophagic responses to hypoxic stress in sedentary men. PLoS One. 2013;8(11, article e80248) doi: 10.1371/journal.pone.0080248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brennan M. L., Hazen S. L. Emerging role of myeloperoxidase and oxidant stress markers in cardiovascular risk assessment. Current Opinion in Lipidology. 2003;14(4):353–359. doi: 10.1097/00041433-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 64.Gao S., Liu J. Association between circulating oxidized low-density lipoprotein and atherosclerotic cardiovascular disease. Chronic Diseases and Translational Medicine. 2017;3(2):89–94. doi: 10.1016/j.cdtm.2017.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cornelissen V. A., Arnout J., Holvoet P., Fagard R. H. Influence of exercise at lower and higher intensity on blood pressure and cardiovascular risk factors at older age. Journal of Hypertension. 2009;27(4):753–762. doi: 10.1097/HJH.0b013e328322cf60. [DOI] [PubMed] [Google Scholar]
- 66.Park J. H., Park H., Lim S. T., Park J. K. Effects of a 12-week healthy-life exercise program on oxidized low-density lipoprotein cholesterol and carotid intima-media thickness in obese elderly women. Journal of Physical Therapy Science. 2015;27(5):1435–1439. doi: 10.1589/jpts.27.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delwing-de Lima D., Ulbricht A. S. S. F., Werlang-Coelho C., et al. Effects of two aerobic exercise training protocols on parameters of oxidative stress in the blood and liver of obese rats. The Journal of Physiological Sciences. 2018;68(5):699–706. doi: 10.1007/s12576-017-0584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bailey D. M., Lawrenson L., McEneny J., et al. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free Radical Research. 2007;41(2):182–190. doi: 10.1080/10715760601028867. [DOI] [PubMed] [Google Scholar]
- 69.Gomez-Cabrera M. C., Domenech E., Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radical Biology & Medicine. 2008;44(2):126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 70.Rosa T. S., Simões H. G., Rogero M. M., et al. Severe obesity shifts metabolic thresholds but does not attenuate aerobic training adaptations in Zucker rats. Frontiers in Physiology. 2016;7:p. 122. doi: 10.3389/fphys.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.