Introduction
Public health agencies conduct regular surveillance to prevent, investigate, and control disease outbreaks. Disease surveillance measures begin in healthcare settings because public health agencies collect disease information from healthcare providers, facilities, and clinical laboratories that are required to report certain diseases and conditions to state or local health agencies. State, tribal, local, and territorial laws determine disease and condition reporting requirements.1 Disease reports provide an understanding of disease occurrence and trends that inform planning, policy-making, and resource allocation. Traditionally, disease reports have been made manually or by telephone, mail, or fax; in these traditional formats, reports are often delayed and incomplete.2 Reporters find manual submission time-consuming and disruptive to workflow.
New technology has facilitated the transition from paper to digital for health data collection and analysis, and health systems have recently begun to transition disease reports from manual paper formats to electronic formats.3 Electronic health information sources, including electronic health records (EHRs), health information exchanges (HIEs), and syndromic surveillance systems, provide important data about population health burdens to public health practitioners and policymakers.
“Electronic disease reporting,” the transmission of clinical and laboratory findings into a designated electronic disease reporting system, can help offset the burden of reporting on healthcare and public health agencies.4 Electronic disease reporting enhances surveillance efforts by improving the quality of reports sent to public health agencies.5 Studies show improved timeliness and completeness of reports when using electronic disease reporting, compared to manual reporting, in outbreak investigations.6
Problems remain even in electronic reporting, though, because systems are largely passive, relying on reporters to identify a reportable disease and take steps to send a report with accurate disease information.7 Room for human error remains, and these errors can affect timeliness and completeness of reports.
As electronic reporting has become more widely adopted, innovative uses of this technology, such as automated reporting, or electronic case reporting (eCR), have been developed. eCR is the automated generation and transmission of case reports from EHRs to public health agencies for review and action. HIE organizations can also be involved in these automated transmissions and are, in some cases, required by law to be used.8 eCR, which entails automatic generation of provider reports based on diagnosis and laboratory result triggers, could supplement surveillance efforts and reduce data entry burden on health-care providers.9
Transforming Health through a Digital Bridge
The Digital Bridge initiative is a public-private national effort involving federal and state public health agencies, providers systems, and EHR vendors to accelerate these innovative electronic surveillance methods. Digital Bridge creates a forum for these organizations to collaborate on technical solutions for a nationally standardized, sustainable approach to exchanging and using electronic health data. The goals of the initiative include advancing greater standards-based information exchange across public health and healthcare, easing the burden and costs for all stakeholder groups through a unified approach to information exchange, and laying the foundation for greater bidirectional exchange of data so that clinicians can be more informed about population health, environmental risks, and outbreaks. The Digital Bridge initiative indicates that stakeholders across sectors can collaborate within a disciplined governance framework and achieve tangible improvements to information exchange that foster a culture of health. As its first project, Digital Bridge has designed a nationally scalable, multi-jurisdictional approach to eCR, to address disparities and complexities in the reporting of public health cases at national, state, and local levels.10
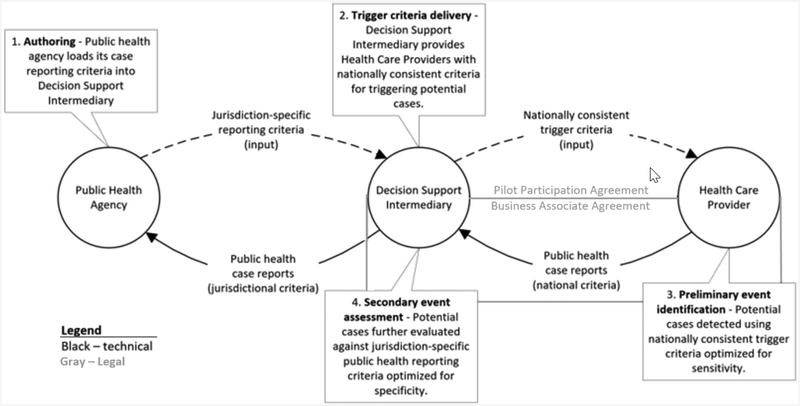
Digital Bridge collaborators have created a new approach that will automatically flag potential disease cases within existing clinical information systems, generate the reports, and digitally send them to public health agencies, in accordance with applicable healthcare privacy and public health reporting laws. This approach will lessen current manual work processes and improve outbreak management. The project’s technical approach aims to change the status quo of point-to-point data connections between healthcare organizations and their public health partners by offering a central decision support intermediary (DSI) to facilitate case reporting. The DSI operates on the Association of Public Health Laboratories (APHL) Informatics Messaging Service, a secure, cloud-based platform. Case reports are evaluated against public health reporting criteria using the Reportable Conditions Knowledge Management System, developed by the Council of State and Territorial Epidemiologists (see Figure 1).
Figure 1. Digital Bridge Approach to Electronic Case Reporting in Public Health.
The approach leverages existing EHR systems to automatically flag potentially reportable disease cases based on nationally consistent criteria and create a case report using national electronic standards for content and format. The report is then sent digitally to the DSI to validate its format and determine whether the case is reportable to public health agencies in a particular jurisdiction. If reportable, the case is forwarded to appropriate public health agencies.11 The DSI eliminates guesswork as to which jurisdiction receives the case report and alleviates burdensome manual reporting processes for healthcare professionals. This real-time, automated process also improves the data’s timeliness, accuracy, and completeness.
A foundational component of the eCR initiative has been the development of a legal framework and data exchange agreements between partners. Based on an analysis of the project architecture and applicable privacy laws, Davis Wright Tremaine LLP developed several options for stakeholder consideration, including having the DSI serve as an agent of public health agencies, participate in state or local HIEs, or serve as a business associate of the reporting providers. The selected legal framework establishes APHL as a business associate of healthcare organizations or HIE organizations (collectively “participants”) to facilitate data exchange with public health agencies. To operationalize this approach, participants enter into a Digital Bridge pilot participation agreement (which encompasses a business associate agreement) with APHL as the platform operator.12 The business associate agreement authorizes the platform operator to disclose protected health information from participants for public health purposes. Under the agreement, APHL is subject to HIPAA privacy and security requirements. By the end of 2018, the first demonstration sites and APHL had fully executed the agreement and are expected to begin exchanging information by early 2019.
As eCR demonstration activities continue through 2019, the Digital Bridge initiative will collect data and develop evaluation findings to inform future national scalability planning. These findings might indicate needed adjustments to the legal framework, such as exploring a model where the platform operator contracts directly with the public health agencies.
eCR in Michigan
Michigan is expanding on the initial Digital Bridge approach by helping support providers that cannot participate currently with electronic exchange due to low numbers of EHR vendors capable of meeting eCR standards. Michigan Health Information Network Shared Services (MiHIN) has worked closely with the Michigan Department of Health and Human Services (MDHHS) to support a mechanism that allows providers to participate via electronic exchange of communicable disease reporting with EHRs that do not yet produce electronic initial case report (eICR) data. Michigan is leveraging the information contained in a Continuity of Care Document (CCD), a type of Consolidated-Clinical Document Architecture, that is much easier for EHRs to generate. The CCD content is used to build the eICR using an internally developed transformation tool based on specific trigger events, streamlining and automating the transmission of the eICR and Reportability Response. With this unified approach, organizations in the process of adopting the eCR standard can still participate in electronic exchange of reportable cases to MDHHS to better manage outbreaks, investigate disease trends, and provide wide-scale awareness and treatment to impacted populations. MiHIN and MDHHS anticipate that these initiatives will reduce the reporting burden for providers while improving the accuracy, effectiveness, and timeliness of disease surveillance within the state.
In accordance with Michigan’s state-level work, there is wide-spread recognition that expanding eCR nationally will continue to enhance public health.13 In the past few years, the federal government has been promoting national interoperability initiatives to move healthcare information across state lines. This national initiative would allow for comprehensive outbreak and disease surveillance on an interstate level, which would be more informative for the country’s increasingly mobile population.
Distinct from business associate agreements, the legal data sharing agreements that are required to release information from provider EHRs to Health Information Networks (HINs) are a hurdle that has hindered the exchange of healthcare information across state lines. Many times, legal agreements can delay the process of exchanging information because the legal team of each institution may have unique concerns or items they wish to amend in the standard legal documents. The result is a fragmented patchwork of legal agreements, which can take months of negotiations to achieve. The Office of the National Coordinator for Health Information Technology has recently tried to remedy this problem by releasing a draft Trusted Exchange Framework and Common Agreement (TEFCA). When the final version of TEFCA is released, all entities will be able to sign one “common [legal] agreement,” which will dictate the terms for exchanging information.14 TEFCA creates the framework and possibility for regional qualified health information networks to exchange disease surveillance and outbreak information on a national scale and implement eCR use cases15 to further these efforts. This adds value beyond what alternative solutions offer because it sets up a sustainable framework to support both existing and future initiatives for sharing this type of information, which many other entities have not been able to accomplish. Michigan and many other states have begun to position their health IT communities to accommodate this new initiative as they anxiously await potential opportunities to expand the breadth of eCR efforts.
Conclusion
Through these innovative electronic surveillance methods, public health professionals might practically leverage federal, state, and local health data to better anticipate and plan for the needs of whole communities, including by being able to identify, plan for, and respond to disease outbreaks.
Acknowledgments
We acknowledge Jim Collins and team at MDHHS for advancing electronic case reporting thus far and contributing to the unified approach and implementation of reportable disease and syndromic surveillance initiatives in Michigan.
Footnotes
Note
This document was written in part by researchers in the Public Health Law Program (PHLP) in the Center for State, Tribal, Local, and Territorial Support at the US Centers for Disease Control and Prevention. The findings and conclusions in this article are those of the authors and do not necessarily represent the official views of CDC. For further information, please contact PHLP at phlawprogram@cdc.gov.
Dr. Elliott and Ms. Patel report 90/10 Federal State match funding - Medicaid Management Information System (MMIS) from Michigan Department of Health and Human Services, during the conduct of the study. Ms. Black, Ms. Hulkower, and Dr. Suarez have nothing to disclose.
References
- 1.Association of State and Territorial Health Officials, Infectious Disease Guiding Principles, available at <http://www.astho.org/Policy-and-Position-Statements/Policy-Statement-on-Infectious-Disease/> (last visited March 16, 2019).
- 2.Sickbert-Bennett E et al. , “Completeness of Communicable Disease Reporting, North Carolina, USA, 1995–1997 and 2000–2006,” Emerging Infectious Diseases 17, no. 1 (2011): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramanathan T et al. , “Federal Public Health Laws Supporting Data Use and Sharing,” Public Health Law, available at <https://www.cdc.gov/phlp/docs/datasharing-laws.pdf> (last visited March 16, 2019); [Google Scholar]; Schmit C et al. , “Transitioning from Paper to Digital: State Statutory and Regulatory Frameworks for Health Information Technology,” Public Health Reports 132, no. 5 (2017): 585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dixon B et al. , “Automating Provider Reporting of Communicable Disease Cases Using Health Information Technology,” Poster session presented at IUPUI Research Day (November 2014), available at <https://scholarworks.iupui.edu/handle/1805/5318> (last visited March 16, 2019); see Schmit et al., supra note 3. [Google Scholar]
- 5.Chaudhry B et al. , “Systematic Review: Impact of Health Information Technology on Quality, Efficiency, and Costs of Medical Care,” Annals of Internal Medicine 144, no. 10 (2006): 742. [DOI] [PubMed] [Google Scholar]
- 6.Effler P et al. , “Statewide System of Electronic Notifiable Disease ReportingfFrom Clinical Laboratories: Comparing Automated Reporting with Conventional Methods,” JAMA 282, no. 19 (1999): 1845, 1847. [DOI] [PubMed] [Google Scholar]
- 7.See Sickbert-Bennett, supra note 2. [Google Scholar]
- 8.“ECR Exchange Transactions,” hl7 website, available at <http://www.hl7.org/fhir/uv/ecr/2018jan/ecr-exchange.html> (last visited March 16, 2019). [Google Scholar]
- 9.Corrado R et al. , “Electronic Case Reporting Is Coming… ‘Weather’ Your PHA Is Ready or Not!” CSTE Confex. website, available at <https://cste.confex.com/cste/2018/meetingapp.cgi/Paper/9997> (last visited March 16, 2019). [Google Scholar]
- 10.MacKenzie W et al. , “The Promise of Electronic Case Reporting,” Public Health Reports 131 (2016): 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Digital Bridge, Issue Brief: eCR — Executive Summary, available at <https://digitalbridge.us/wp-content/uploads/2018/06/Final-eCR-Executive-Summary.pdf> (last visited March 16, 2019).
- 12.Id.
- 13.Council of State and Territorial Epidemiologists, “Electronic Case Reporting (eCR),” 2016, available at <https://cdn.ymaws.com/www.cste.org/resource/resmgr/2016PS/16_SI_02.pdf> (last visited March 16, 2019). [Google Scholar]
- 14.Office of the National Coordinator, “Draft Trusted Exchange Framework,” Health IT website, January 5, 2018. [Google Scholar]
- 15.Michigan Health Information Network Shared Services, “What Is a Use Case,” available at <https://mihin.org/what-is-a-use-case/> (last visited March 16, 2019).