Abstract
Purpose:
Pseudocirrhosis has been demonstrated to mimic cirrhosis radiographically, but studies evaluating the pathophysiology and clinical features are lacking. To better understand the incidence, risk factors, clinical course, and etiology of pseudocirrhosis, we performed a retrospective analysis of consecutively treated patients with metastatic breast cancer (MBC).
Methods:
Of 374 patients treated for MBC from 2006-2012, 199 had imaging available for review. One radiologist evaluated computed tomography scans for evidence of pseudocirrhosis. Features of groups with and without pseudocirrhosis were compared by Kaplan-Meier product-limit survival estimates and log-rank tests. Wilcoxon Rank-Sum testing evaluated if patients more heavily treated were more likely to develop pseudocirrhosis. Univariate and multivariate Cox proportional hazard models investigated factors associated with mortality.
Results:
Pseudocirrhosis developed in 37 of 199 patients (19%). Of the patients with liver metastases, 55% developed pseudocirrhosis. Liver metastases were demonstrated in 100% of patients with pseudocirrhosis. Survival in the subset with liver metastases favored those without pseudocirrhosis, 189 versus 69 months (p = 0.01). The number of systemic regimens received were higher in patients with pseudocirrhosis (p = 0.01). Ascites was demonstrated in 68%, portal hypertension in 11%, and splenomegaly in 8% of patients with pseudocirrhosis.
Conclusions:
Pseudocirrhosis does not occur in the absence of liver metastases, can manifest as hepatic decompensation, and appears to be associated with poorer survival amongst patients with hepatic metastases. Higher cumulative exposure to systemic therapy may be causative, instead of the previously held belief of pseudocirrhosis as an adverse effect of a particular systemic agent/class.
Keywords: chemical and drug induced liver injury, breast neoplasms, liver neoplasms, cirrhosis
Introduction:
Survival from metastatic breast cancer has increased over the past several decades with the advancement of modern systemic therapy (1). The increase in survival allows time for late adverse effects of systemic therapy to manifest, along with the sequelae of a slower progressing disease. There are several hepatotoxic conditions that can result from systemic antineoplastic therapy, which include hepatic necrosis, steatosis, hepatitis, and sinusoidal obstruction syndrome. More recently, a phenomenon referred to as pseudocirrhosis has been implicated as a possible adverse effect of systemic therapy (2).
A condition referred to as hepar lobatum was first described in the early twentieth century, in which the liver develops an irregular, lobulated contour with crevices and linear depressions (3). These findings were noted in patients with tertiary syphilis, resulting from healing gummas and scar contraction (4). Over the next several decades, there were a few case reports of this phenomenon in the setting of hepatic metastases, resulting in the term hepar lobatum carcinomatosum. Honma published the first detailed pathological report in 1987. He described multifocal scars and compensatory hyperplasia of the spared liver parenchyma, accompanied by stromal retraction (5). In the modern era, this condition has been described in several case reports (6–14) and case series (15–20) and has become known as liver pseudocirrhosis.
Pseudocirrhosis mimics cirrhosis radiographically and sometimes clinically but without the corresponding histopathological characteristic findings (17, 21, 22). Only cirrhosis is characterized by the presence of regenerating nodules of hepatocytes plus fibrotic bridges between the nodules, findings that are not seen in cases of pseudocirrhosis (5). While radiographic findings are similar for cirrhosis and pseudocirrhosis, one subtle difference is that pseudocirrhosis has intervening liver tissue that is essentially intact between fibrous bands.
Early on, pseudocirrhosis was primarily described in the radiology literature, with an unclear clinical significance and natural history. More recently, pseudocirrhosis has been associated with the sequelae of portal hypertension including ascites, splenomegaly, varices and cytopenias (13, 14, 20). While a few reports have described pseudocirrhosis in patients with liver metastases from cancers other than breast (7, 9, 23, 24), multiple observations have established an association between pseudocirrhosis and breast cancer with liver metastases, in the setting of past treatment with systemic therapy (6, 11, 17, 20).
Two important case series have been published on this topic that provide insight into the clinical aspects of pseudocirrhosis (17, 18). While these studies contributed to the understanding of this phenomenon, only patients with liver metastases were included, thus limiting the ability to evaluate whether this condition occurs in the absence of liver tumors. To better understand the incidence, risk factors, clinical manifestations, etiology, and natural history of pseudocirrhosis, we performed a single-institution analysis of consecutively treated patients with metastatic breast cancer. Importantly, our study is the first to include cases of breast cancer with extrahepatic metastases in the absence of liver metastases.
Materials and Methods:
Subjects
After approval by our institutional internal review board (IRB), electronic medical records were searched for diagnosis codes of metastatic breast cancer at our university hospital between the years of 2006 and 2012 for patients treated by a single provider. Patients were included if they had stage IV breast cancer (hepatic or extrahepatic metastases allowed) without a history of cirrhosis. Patients who developed evidence of pseudocirrhosis prior to being treated at our institution were excluded. Detailed information regarding all systemic regimens received was available for all patients and data was collected that describes patient characteristics, tumor characteristics, lab values, and survival dates.
Radiologic technique and interpretation
Imaging studies were evaluated from the time patients developed metastatic disease to the most recent scan available. There was no requirement for the patient to have received intravenous contrast, although the vast majority were contrast studies (202/234). A designated radiologist evaluated each scan without knowledge of the official radiologic interpretation in the chart. Liver metastases were classified by the radiologist as limited (< 1/3 liver involvement) or widespread (≥ 1/3 liver involvement). Extent of pseudocirrhosis was classified by the radiologist based on capsular retraction, for which < 10% was designated as limited, 10-50% widespread, and > 50% diffuse nodularity. Presence of ascites, peritoneal carcinomatosis, portal hypertension (portosystemic varices, portal vein enlargement > 1.3 cm), and splenomegaly (spleen size > 12cm) were qualitatively reported.
Statistical analysis
Tumor and patient characteristics were collected and compared between two groups, those with pseudocirrhosis and those without pseudocirrhosis. Kaplan-Meier product-limit survival estimates were calculated and log-rank tests were performed to compare pseudocirrhosis groups against those who did not develop pseudocirrhosis in all patients and separately those with liver metastases. To examine any potential correlation between use of a particular systemic agent and development of pseudocirrhosis, the proportion of patients who received a particular systemic agent was compared between patients who developed pseudocirrhosis and those who did not. Furthermore, differences in development of pseudocirrhosis were compared between each group, based on major categories of systemic therapy (endocrine therapy, chemotherapy, both) and also by drug class (anthracyclines, taxanes, platinums, HER2-targeted therapy, and other targeted therapy). To compare groups most similar, the systemic agents received by patients with pseudocirrhosis were compared to the group of patients without pseudocirrhosis who had other visceral metastases. These comparisons were done via Chi-squared test or Fisher’s exact test. Wilcoxon Rank-Sum testing was used to evaluate if patients who were more heavily treated with systemic therapy were more likely to develop pseudocirrhosis. Univariate and multivariate Cox proportional hazard models were carried out to investigate factors associated with mortality. For all statistical investigation, tests for significance were two-tailed. A p-value less than the 0.05 significance level was considered to be statistically significant. All statistical analyses were carried out using SAS software version 9.4 (SAS Institute Inc. 2013).
Results:
Total patient sample
A total of 374 patients with metastatic breast cancer were identified at our institution during this time period, 234 of whom met all criteria for inclusion for radiology review. Following the radiology review and detailed chart review, an additional 35 patients were excluded due to having insufficient imaging of the liver, regional metastatic disease but not distant metastases, or preexisting cirrhosis. Therefore, the final analysis included 199 patients (Supplementary Fig. S1).
The median age at original diagnosis of breast cancer was 50 years (range 23-90 years). The majority (61%, 122/199) had HR+/HER2− breast cancer, 30% (59/199) had HER2-positive disease (of these, 76% (45/59) were HR+HER2+ and 24% (14/59) were HR−HER2+), and 12% had triple negative (23/199). Liver metastases were detected in 33% (67/199). The median number of systemic therapy regimens received in the metastatic setting was 3 (range 1-10). Median follow-up time from the onset of metastatic disease was 38 months (range 1-305 months). Radiologic evidence of pseudocirrhosis was detected in 19% (n = 37).
Factors associated with the development of pseudocirrhosis
Table 1 shows patient and tumor characteristics between patients who did (N = 37) and did not (N = 162) develop pseudocirrhosis. Patient and tumor characteristics between the two groups were similar in regard to age, hormone receptor and HER2 status, and initial use of chemotherapy for non-metastatic disease. Of the patients who had liver metastases (N = 67), 55% were found to have pseudocirrhosis. Liver metastases were demonstrated in 100% (N = 37) of patients with pseudocirrhosis. The median time from locoregional disease to development of metastases was 13 months earlier in the patients who developed pseudocirrhosis.
Table 1:
Tumor and patient characteristics
| Pseudocirrhosis absent (n=162) | Pseudocirrhosis present (n=37) | p-value | |
|---|---|---|---|
| Median follow-up from time of first chemo* (months) | 73 (95% C.I. 66-80) | 103 (95% C.I. 76-133) | 0.03 |
| Median follow-up from time of first metastasis (months) | 45 (95% C.I. 36-54) | 73 (95% C.I. 67-81) | 0.60 |
| Mean age at initial breast cancer diagnosis | 51.9 (14.0) | 49.3 (13.5) | 0.30 |
| ILC with or without concurrent IDC | 25 (15%) | 1 (3%) | 0.11 |
| IDC | 111 (67%) | 27 (73%) | 0.92 |
| Other | 6 (4%) | 1 (3%) | 0.84 |
| Unknown | 20 (12%) | 8 (22%) | 0.32 |
| HR positive | 133 (82%) | 34 (92%) | 0.76 |
| HR+/HER2− | 98 (60%) | 24 (65%) | 0.92 |
| HR+/HER2+ | 35 (22%) | 10 (27%) | 0.72 |
| HER2 positive | 48 (30%) | 11 (30%) | 0.86 |
| HR+/HER2+ | 35 (22%) | 10 (27%) | 0.72 |
| HR−/HER2+ | 13 (8%) | 1 (3%) | 0.47 |
| Triple negative† | 21 (13%) | 2 (5%) | 0.38 |
| Liver metastasis | 30 (19%) | 37 (100%) | <0.001 |
| Bone-only metastasis | 34 (21%) | 2 (5%) | 0.03 |
| Multiple sites of metastases | 55 (34%) | 31 (84%) | <0.001 |
| Known death | 19 (12%) | 11 (30%) | 0.01 |
| Median overall survival in metastatic setting‡ | NA (95% C.I. 189-NA) | 69 (95% C.I. 41-NA) | 0.002 |
| Median overall survival in pts with liver metastases | 189 (95% C.I. 96-189) | 69 (95% C.I. 41-NA) | 0.01 |
| Mean hemoglobin | 11.6 (1.8) | 10.6 (1.8) | 0.003 |
| Mean creatinine | 0.8 (0.3) | 1.0 (0.9) | 0.004 |
| Median bilirubin | 0.9 (4.7) | 6.0 (6.7) | <0.001 |
| Mean albumin | 3.9 (0.6) | 2.7 (0.7) | <0.001 |
| Mean INR | 1.2 (0.9) | 1.3 (0.4) | 0.31 |
IDC = invasive ductal carcinoma
ILC = invasive lobular carcinoma
HR = hormone receptor positive = ER+ and/or PR+
Triple negative = ER negative, PR negative, HER2 negative
Median survival starting at date of first metastatic disease
Several antineoplastic agents were received by patients who developed pseudocirrhosis at a percentage significantly higher than patients who did not develop pseudocirrhosis. Albumin-bound paclitaxel (41% vs. 14%), capecitabine (76% vs. 38%), cisplatin (8% vs. 1%), everolimus (35% vs. 14%), exemestane (49% vs. 28%), vinorelbine (35% vs. 19%), were all shown to have been received more frequently in patients who eventually developed pseudocirrhosis, which met statistical significance. All of the agents that were received at a significantly higher rate in pseudocirrhosis patients were agents used in the metastatic setting; whereas, none of the agents received in the neoadjuvant/adjuvant setting showed significant difference. In order to minimize confounding factors, we compared patients without pseudocirrhosis who had visceral metastases (N = 55) to patients with pseudocirrhosis (N = 37). This comparison demonstrated that albumin-bound paclitaxel (p = .04) and capecitabine (p = .03) maintained statistical significance between the groups. However, cisplatin, everolimus, exemestane, and vinorelbine lost statistical significance.
In addition, we compared patients with and without pseudocirrhosis based on the category of systemic agent received. Supplementary table S1 separated therapies into category of systemic therapy. It demonstrates that patients who received platinums and other targeted therapies (palbociclib and everolimus) developed pseudocirrhosis more frequently (p = 0.05). Supplementary table S1 also compares endocrine therapy alone, chemotherapy alone, or the combination in the metastatic setting in patients with and without pseudocirrhosis. It demonstrates that patients who received regimens consisting of endocrine therapy, and who also received regimens consisting of chemotherapy at some other time point (received both over the course of their metastatic disease, but not concurrently), developed pseudocirrhosis at higher rates than patients who received either treatment modality alone over the course of their metastatic disease (p < 0.01).
In order to further explore the above findings, we performed Wilcoxon Rank-Sum testing to evaluate if patients who were more heavily treated with systemic therapy were more likely to develop pseudocirrhosis. The number of systemic regimens ever received (adjuvant/neoadjuvant and metastatic setting combined) were higher in patients who developed pseudocirrhosis (p = 0.01). The number of regimens ever received that contained chemotherapy were also higher in patients who developed pseudocirrhosis (p = 0.02).
Outcomes associated with the development of pseudocirrhosis
Mean creatinine, hemoglobin, bilirubin, and albumin were more frequently abnormal in pseudocirrhosis patients, although INR was similar between groups (table 1). Kaplan-Meier estimates of overall survival starting at the time of metastatic disease, demonstrate a median survival of 69 months in patients who developed pseudocirrhosis, while the median survival was not reached in patients who did not develop pseudocirrhosis (Fig. 1). Survival was also assessed in the subset of patients who had liver metastases, which significantly favored those without pseudocirrhosis, 189 months vs. 69 months (p = .01) (Fig. 2). Univariate analysis of all patients and multivariate analysis of patients with liver metastases was performed, assessing mortality based on pseudocirrhosis status, bone-only metastases, multiple metastases, triple negative status, and invasive lobular histology. On univariate analysis, pseudocirrhosis, metastases to multiple organs, and triple negative status were associated with greater mortality (supplementary table S2). On multivariate analysis in patients with liver metastases, pseudocirrhosis and triple negative status were associated with greater mortality (table 2).
Figure 1:
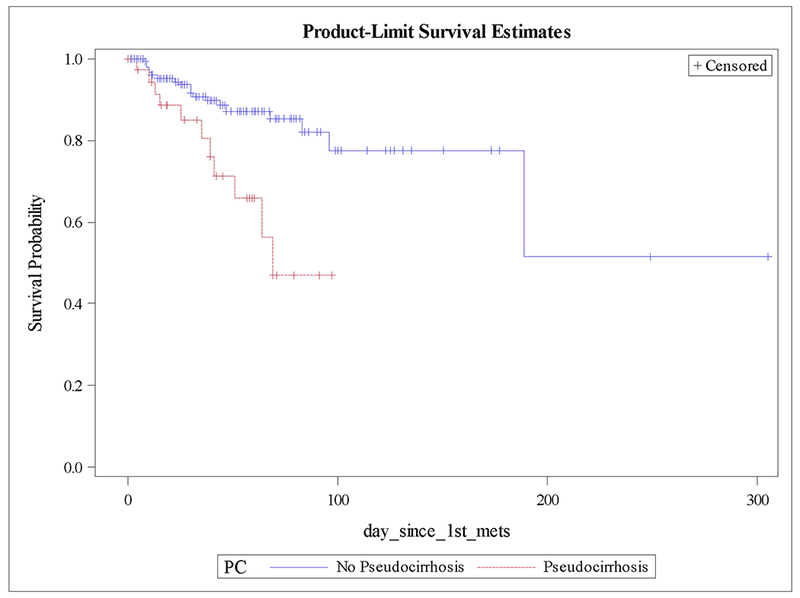
Kaplan-Meier plot of overall survival by pseudocirrhosis status.
Figure 2:
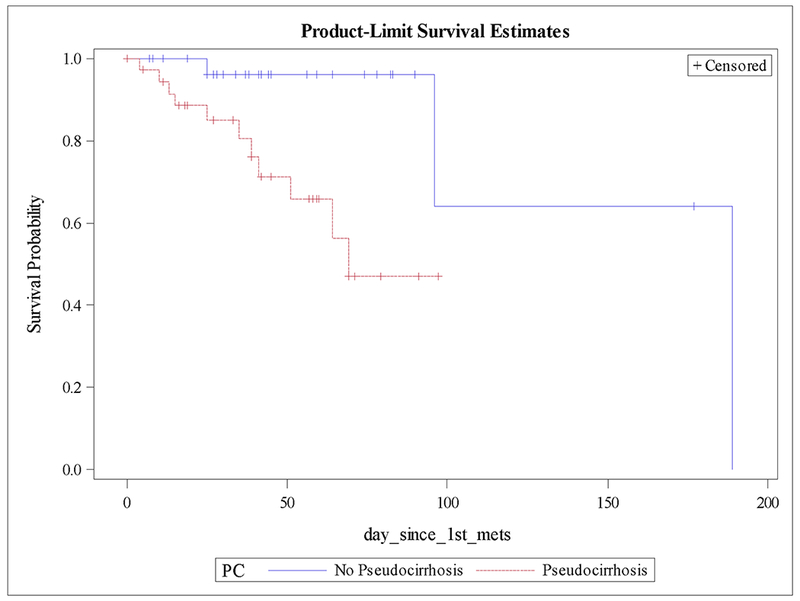
Kaplan-Meier plot of overall survival by pseudocirrhosis status in pts with liver metastases.
Table 2:
Multivariate Cox proportional hazards model investigating factors associated with mortality
| Effect | Hazard Ratio (RR) | lower 95% Confidence Interval for HR | upper 95% Confidence Interval for HR | p-value | |
|---|---|---|---|---|---|
| Pseudocirrhosis Status | PC vs Non-PC | 3.87 | 1.00 | 14.95 | 0.050 |
| Bone only metastases Status | Bone only metastases vs Not | 0.43 | 0.08 | 2.21 | 0.313 |
| Multiple metastases Status | Multiple metastases vs Not | 2.44 | 0.91 | 6.54 | 0.075 |
| ER,PR, HER2 Status | Triple negative vs Others | 4.91 | 1.83 | 13.18 | 0.002 |
| ILC | ILC vs Others | 1.70 | 0.55 | 5.28 | 0.360 |
| Liver metastases Status | Liver metastases vs Not | 0.49 | 0.12 | 1.98 | 0.317 |
PC = Pseudocirrhosis
ILC = Invasive lobular carcinoma
Triple negative = ER negative, PR negative, and HER2 negative
Pseudocirrhosis characteristics
The time to the development of pseudocirrhosis was evaluated in patients who had liver metastases on initial CT scan (49%, 18/37 of patients). The median time to the development of pseudocirrhosis from first identified liver metastasis was 18 months (range 3 - 77). The pattern of pseudocirrhosis was recorded at the most severe level seen on any CT reviewed. Limited pattern was seen in 60% (22/37), widespread pattern in 11% (4/37), and diffuse nodularity in 30% (11/37). The extent of liver metastases in patients with pseudocirrhosis was classified as limited in 24% (9/37) and widespread in 73% (27/37). Widespread liver metastases were demonstrated in 100%, 68%, and 72% of patients with pseudocirrhosis patterns classified as widespread, limited, and diffuse nodularity, respectively.
Scans were evaluated for radiologic signs of portal hypertension. Ascites was demonstrated in 68% (25/37), portal hypertension in 11% (4/37), and splenomegaly in 8% (3/37) of patients with pseudocirrhosis. Diffuse nodularity was the pattern most often seen when signs of portal hypertension were demonstrated. Figures 3 and 4 include CT and MRI of patients included in this study that demonstrate the typical findings of pseudocirrhosis, as well as advancing pseudocirrhosis over time.
Figure 3:
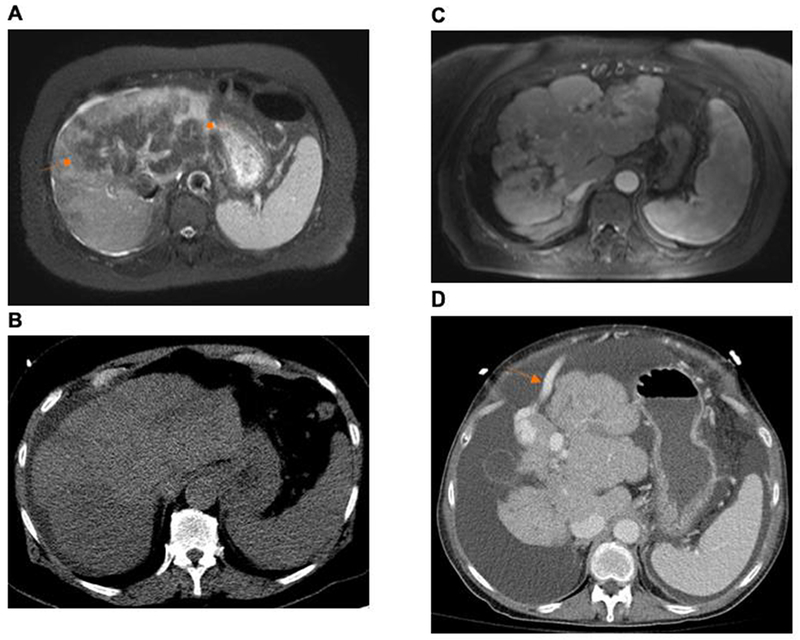
Liver images of a single patient over time. From liver metastases to the development of pseudocirrhosis, with subsequent progression of pseudocirrhosis characterized as diffuse nodularity.
A: 4/23/12: Axial Fat-Suppressed T2W MR images, demonstrating diffuse tumor infiltration, predominating in the left lobe of the liver (hypointense regions, arrows). B: 5/3/12: Non-contrast CT showing diffusely heterogeneous liver, compatible with diffuse tumor infiltration. C: 7/22/14: Contrast-enhanced T1W MR demonstrating smaller liver with macronodular change. Increasing splenic size. D: 3/18/16: CT with contrast. Progressive decrease in size and increase in nodularity of the liver. Increasing ascites and enlarging portosystemic varices (arrow).
Figure 4:
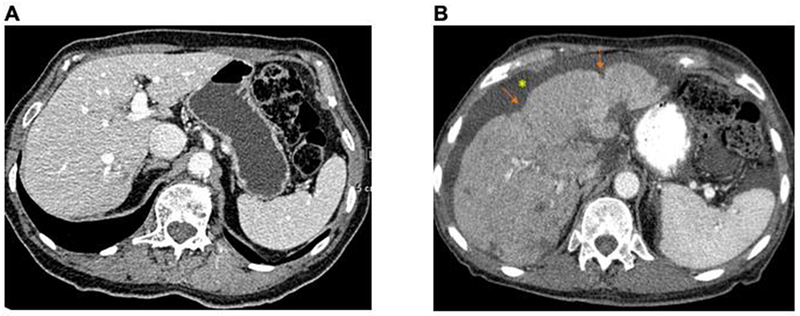
Liver images of a single patient over time. Normal liver with subsequent progression to pseudocirrhosis
A: 8/14/09; smooth surface contour. B: 1/3/11 areas of capsular retraction (arrows) and generalized more fine nodularity of liver surface contour. New ascites*
Median time from first chemotherapy ever received (adjuvant, neoadjuvant, or regimen for de novo metastases) to the date of pseudocirrhosis was 67 months. Median time from first metastatic regimen to the date of pseudocirrhosis was 40 months. Of the agents that were shown to have a significantly higher rate of use in pseudocirrhosis patients, the median time from first receiving the agent to development of pseudocirrhosis was 19.5 months for albumin-bound paclitaxel, 17 months for capecitabine, 2 months for cisplatin, 13.5 months for everolimus, 24 months for exemestane, and 14 months for vinorelbine.
Discussion:
Our results indicate that pseudocirrhosis does not occur in the absence of liver metastases, is not associated with a particular breast tumor biology or receptor phenotype, can manifest as portal hypertension and hepatic decompensation, and appears to be associated with poorer survival in patients with hepatic metastases. Factors that may be causative include presence of liver metastases and exposure to multiple past lines of systemic therapy. Interestingly, it has been previously described that the cirrhotic liver lacks the milieu to support the development of metastases (25). As we have shown, the local hepatic environment in the setting of pseudocirrhosis is capable of supporting metastases, which we consider another important distinction between cirrhosis and pseudocirrhosis. Our findings provide additional insight into this disease process and further support the findings of some past studies.
In our study, 55% of patients with liver metastases developed a pseudocirrhosis pattern that manifested with significant capsular retraction. Estimates of survival starting at the time of metastatic disease demonstrate that patients with pseudocirrhosis have a higher mortality than those without. It was anticipated that this difference may have been confounded by a multitude of factors. For instance, the presence of visceral metastases in pseudocirrhosis would portend a worse survival compared with patients who had osseous metastases alone. To account of this, we compared survival in patients with liver metastases and pseudocirrhosis to patients with liver metastases without pseudocirrhosis. Survival remained significantly longer for patients with liver metastases who did not have pseudocirrhosis (Fig. 2).
Several theories have been proposed regarding the pathogenesis of pseudocirrhosis, which fit into two main categories: a toxicity of systemic therapy, or from processes related to hepatic metastases. Some authors have speculated that pseudocirrhosis may be tissue collapse after tumor regression from chemotherapy, followed by an organizing phase of healing and scar contraction (26). While others acknowledge the possibility of contributions by toxicity from a systemic agent and tumor, but seem to favor the initiating factor to be the general insult of chemotherapy on the liver (5, 27). Alternatively, it has been described as architectural changes in response to the infiltrating tumor, resulting in extensive fibrosis unrelated to adverse effects of chemotherapy and unrelated to tumor response to treatment (14).
The vast majority of cases of pseudocirrhosis in the literature have been reported in patients who had liver metastases and had a history of receiving chemotherapy. However, there have been several cases reported in patients with liver metastases who had not received chemotherapy. In each case, there was diffuse hepatic metastases with widespread distortion of the hepatic architecture (28–31). We included patients without liver metastases in order to indirectly test the likelihood of pseudocirrhosis occurring as a toxicity from systemic therapy. We demonstrate that pseudocirrhosis did not occur in the absence of liver metastases even in patients who received multiple lines of systemic therapy. This finding draws doubt to pseudocirrhosis being a toxicity of systemic therapy as the sole pathophysiological mechanism. Comparison of patients with and without pseudocirrhosis in the subset of our patients with hepatic metastases demonstrates that albumin-bound paclitaxel and capecitabine were received more frequently in patients with pseudocirrhosis than those without pseudocirrhosis. However, instead of a culpable systemic agent, this is more representative of the concept that pseudocirrhosis develops in patients who have been more heavily pretreated. This concept is further supported in our finding that more lines of systemic therapy received at any point (adjuvant, neoadjuvant, metastatic), despite the category of therapy (chemotherapy, hormone therapy, targeted therapy), increases the likelihood of pseudocirrhosis developing in patients with preexisting liver metastases.
There have been case reports of pseudocirrhosis in other primary cancers that have a propensity for liver metastases. Pseudocirrhosis in patients with primary rectal, pancreatic, thyroid, esophageal and gastric cancers have been published (9, 21, 23, 24, 32). These reports suggest that a particular tumor subtype of breast cancer is unlikely to be behind the development of pseudocirrhosis. This is further supported by our results, in which there was no correlation found amongst specific tumor biology or receptor phenotype and the development of pseudocirrhosis.
The first series that examined the pathological correlation with pseudocirrhosis appeared in 1994. Young et al. reviewed 65 CT scans of 22 patients who received chemotherapy for treatment of metastatic breast cancer and later developed pseudocirrhosis, and examined the pathological findings from liver biopsy. In all patients, there was retraction of the capsular surface resulting in a lobular margin, a finding that they report to mimic cirrhosis. Interestingly, in all patients with solitary or focal liver metastases, the changes were limited to the lobe containing malignant cells. This finding supports the notion of pseudocirrhosis being a response to local tumor size fluctuations, as opposed to a toxicity of a particular therapy (20).
The publication most similar to our current series examined the association of hepatic capsule retraction with number of metastatic liver lesions, size of lesions, change in size of lesions over time, tumor biology, and chemotherapeutic agent (18). There were 58 patients with breast cancer metastatic to the liver, of whom, 50% manifested capsule retraction on CT. The size of the largest metastatic lesion in the patients with capsular retraction was significantly greater than the size of the largest metastatic lesion in patients without capsular retraction (p < 0.05). There were no correlations between the presence of capsular retraction and the quantity of liver metastatic lesions; between breast cancer receptor status or the type of chemotherapy in patients with capsular retraction, which is consistent with our results. Interestingly, greater change in size of the metastasis was correlated with the development of capsule retraction (p < 0.05). When considered with the findings in our current study, this supports the notion that changes in liver metastases in more heavily pretreated livers are associated with the development of pseudocirrhosis.
Pseudocirrhosis, while a potential cause of portal hypertension and liver failure, does not show the full spectrum of clinical features of cirrhosis. For example, it does not affect synthetic function (2, 21). Our results are consistent with the literature up to the present time, as the median total bilirubin was significantly higher in patients with pseudocirrhosis (6.0 vs 0.9, p < .001), while there was no difference in mean INR. Qayyum et al. also reported the frequency of hepatic impairment, which was seen in 6 of 16 patients with diffuse nodularity, but in only 1 of 52 patients with limited or widespread retraction, and only in 1 of 23 patients with no contour abnormalities (p < 0.01) (17). In our study, portal hypertension (when applying the definition set forth by Qayyum et al.) occurred in 4 of 26 (15%) and 3 of 13 (23%) of the combined group of limited plus widespread pattern and diffuse nodularity pattern, respectively.
Based on our findings, the diagnosis of pseudocirrhosis can be considered when there is radiological evidence of progressive liver disease without or without clinical evidence of hepatic decompensation in a patient with liver metastases who has received multiple past lines of systemic therapy. The clinical importance of identifying pseudocirrhosis can impact crucial management decisions in patients with liver metastases. If pseudocirrhosis is identified, then temporarily holding systemic therapy while pursuing aggressive supportive management (diuresis, paracentesis, etc) may lead to clinical improvement, followed by resuming the same systemic therapy. If hepatic decompensation is incorrectly attributed to progressing malignancy or toxicity from systemic therapy, then unnecessary changes to the oncologic treatment strategy may occur. Additionally, it may be prudent to monitor liver metastases with MRI instead of CT, as the former is superior in characterizing tissue component in the setting of scattered fibrous tissue which may obscure such visualization on CT (7).
Our study has several limitations. Most remarkable, there was no pathological analysis of our patients with pseudocirrhosis. Nevertheless, we can speculate that the more advanced and nodular the pattern of pseudocirrhosis, the possibility of developing clinical sequelae of portal hypertension may increase. Prospective studies that evaluate biopsy samples are necessary to support this conjecture.
Pseudocirrhosis resembles cirrhosis radiographically and can result in hepatic decompensation occasionally. Far from a rare entity, we demonstrate that pseudocirrhosis is a common finding in patients with metastatic breast cancer with liver metastases who have been heavily treated over the course of their disease, regardless of specific tumor biology. Furthermore, there appears to be an increased association of pseudocirrhosis in patients with metastatic breast cancer with liver metastases who have received agents that are given as third-line and beyond, representing the concept that pseudocirrhosis develops in heavily treated patients. Pseudocirrhosis should be considered in patients with liver metastases without prior intrinsic liver disease who present with hepatic decompensation. When present, incorrectly attributing such clinical findings with advancing malignancy or toxicity from systemic therapy could be potentially counterproductive to subsequent management decisions. Future prospective studies will seek to further validate these findings.
Supplementary Material
Acknowledgments
Financial support: Dr. Hurvitz receives funding from NCI/NIH CA016042 as well as the Marni Levine Memorial Research Award.
Compliance with ethical standards:
This is a declaration that all aspects of this study comply with the current laws of the United States. There was no sponsor that funded this study. The authors declare that they have no conflicts of interest. This retrospective analysis was approved by the Institutional Review Board of the University of California, Los Angeles, which determined that informed consent was exempt for a retrospective study.
Footnotes
The authors have no conflicts of interest to report.
References:
- 1.Chia SK, Speers CH, D’yachkova Y, et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer. 2007. September 1;110(5):973–9. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Houshyar R, Bhosale P, et al. Chemotherapy induced liver abnormalities: an imaging perspective. Clin Mol Hepatol. 2014. September;20(3):317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Busni NA: Hepar lobatum carcinomatosum. Virchows Arch A Pathol Anat Histopathol 1924;252:727–733. [DOI] [PubMed] [Google Scholar]
- 4.Symmers D, Spain DM: Hepar lobatum: clinical significance of the anatomic changes. Arch Pathol 1946;42:64–68. [PubMed] [Google Scholar]
- 5.Honma K Hepar lobatum carcinomatosum due to metastatic breast carcinoma. Virchows Arch A Pathol Anat Histopathol. 1987;410(6):465–9. [DOI] [PubMed] [Google Scholar]
- 6.Lee SL, Chang ED, Na SJ, et al. Pseudocirrhosis of breast cancer metastases to the liver treated by chemotherapy. Cancer Res Treat 2014. January;46(1):98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Battisti S, Guida FM, Pagliara E, et al. Pseudocirrhosis after anti-EGFR-based neoadjuvant therapy for hepatic metastasis from colon cancer: a different point of view. Clin Colorectal Cancer. 2014. September;13(3):e13–5. [DOI] [PubMed] [Google Scholar]
- 8.Jeong WK, Choi SY, Kim J. Pseudocirrhosis as a complication after chemotherapy for hepatic metastasis from breast cancer. Clin Mol Hepatol. 2013. June;19(2):190–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teke Z, Nessar G, Kiremitci S, et al. Hepar lobatum carcinomatosum associated with metastatic rectal carcinoma: an unusual cause of liver dysmorphy. Med Princ Pract 2011;20(1):93–6. [DOI] [PubMed] [Google Scholar]
- 10.Graber I, Dumortier J, Poncet G, et al. Hepar lobatum carcinomatosum revealing an occult metastatic lobular carcinoma of the breast. Ann Diagn Pathol 2010. December;14(6):438–42. [DOI] [PubMed] [Google Scholar]
- 11.Sass DA, Clark K, Grzybicki D, et al. Diffuse desmoplastic metastatic breast cancer simulating cirrhosis with severe portal hypertension: a case of “pseudocirrhosis”. Dig Dis Sci 2007. March;52(3):749–52. [DOI] [PubMed] [Google Scholar]
- 12.Nakajima T, Sekoguchi S, Nishikawa T, et al. Multifocal intraportal invasion of breast carcinoma diagnosed by laparoscopy-assisted liver biopsy. World J Gastroenterol 2005. April 21;11(15):2360–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chandrakar V, Isaacs C. Breast cancer-related pseudocirrhosis and esophageal varices. Breast J. 2005. Jul-Aug;2(4):301–2. [DOI] [PubMed] [Google Scholar]
- 14.Nascimento AB, Mitchell DG, Rubin R, et al. Diffuse desmoplastic breast carcinoma metastases to the liver simulating cirrhosis at MR imaging: report of two cases. Radiology. 2001. October;221(1): 117–21. [DOI] [PubMed] [Google Scholar]
- 15.Alberti N, Bechade D, Dupuis F, et al. Hepar lobatum carcinomatosum associated with liver metastases from breast cancer: report of five cases. Diagn Interv Imaging. 2015. January;96(1):73–8. [DOI] [PubMed] [Google Scholar]
- 16.Sonnenblick A, Appelbaum L, Peretz T. Liver failure on the background of pseudocirrhosis in patients with liver metastasis from breast cancer, who responded to treatment. Onkologie. 2011. ;34(4): 199–201. [DOI] [PubMed] [Google Scholar]
- 17.Qayyum A, Lee GK, Yeh BM, et al. Frequency of hepatic contour abnormalities and signs of portal hypertension at CT in patients receiving chemotherapy for breast cancer metastatic to the liver. Clin Imaging. 2007. Jan-Feb;31(l):6–10. [DOI] [PubMed] [Google Scholar]
- 18.Fennessy FM, Mortele KJ, Kluckert T, et al. Hepatic capsular retraction in metastatic carcinoma of the breast occurring with increase or decrease in size of subjacent metastasis. AJR Am J Roentgenol 2004. March;182(3):651–5. [DOI] [PubMed] [Google Scholar]
- 19.Soyer P, Bluemke DA, Vissuzaine C, et al. CT of hepatic tumors: prevalence and specificity of retraction of the adjacent liver capsule. AJR Am J Roentgenol 1994. May;162(5):1119–22. [DOI] [PubMed] [Google Scholar]
- 20.Young ST, Paulson EK, Washington K, et al. CT of the liver in patients with metastatic breast carcinoma treated by chemotherapy: findings simulating cirrhosis. AJR Am J Roentgenol 1994. December;163(6):1385–8. [DOI] [PubMed] [Google Scholar]
- 21.Jha P, Poder L, Wang ZJ, et al. Radiologic mimics of cirrhosis. AJR Am J Roentgenol 2010. April;194(4):993–9. [DOI] [PubMed] [Google Scholar]
- 22.Lipson JA, Qayyum A, Avrin DE, et al. CT and MRI of hepatic contour abnormalities. AJR Am J Roentgenol 2005. January;184(1):75–81.Review. Erratum in: AJR Am J Roentgenol. 2005 Mar;184(3):1028. [DOI] [PubMed] [Google Scholar]
- 23.Harry BL, Smith ML, Burton JR Jr, et al. Medullary thyroid cancer and pseudocirrhosis: case report and literature review. Curr Oncol 2012. February;19(1):e36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang SP, Taddei T, McLennan B, et al. Pseudocirrhosis in a pancreatic cancer patient with liver metastases: a case report of complete resolution of pseudocirrhosis with an early recognition and management. World J Gastroenterol 2008. March 14;14(10):1622–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zotti S, Piccigallo E, Rampinelli L, et al. Primary and metastatic tumors of the liver associated with cirrhosis. A study based on laparoscopy and autopsy. Gastrointest Endosc 1986. April;32(2):91–5. [DOI] [PubMed] [Google Scholar]
- 26.Uhlmann F, Martin H, Ringk H, et al. Hepar lobatum carcinomatosum due to chemotherapy of a metastatic breast carcinoma. Gen Diagn Pathol 1996. March;141(3-4):279–84. Review. [PubMed] [Google Scholar]
- 27.Shirkhoda A, Baird S. Morphologic changes of the liver following chemotherapy for metastatic breast carcinoma: CT findings. Abdom Imaging. 1994. Jan-Feb;19(1):39–42. [DOI] [PubMed] [Google Scholar]
- 28.Micolonghi T, PPineda E, Stanley MM. Metastatic carcinomatous cirrhosis of the liver; report of a case in which death followed hemorrhage from esophageal varices and hepatic coma. AMA Arch Pathol 1958. January;65(1):56–62. [PubMed] [Google Scholar]
- 29.Amtrup F Metastatic carcinomatous liver cirrhosis. Dan Med Bull 1971May;18(2):46–8. [PubMed] [Google Scholar]
- 30.Cracium EC, Aslan A, Caffe L. Cirrhose atrophique neoplastique secondaire. Ann Anat Pathol 1931; 8:1089–1112. [Google Scholar]
- 31.Wegener F Metastatisch-krebsige leberzirrhose. Acta Hepatosplenol 1961; 8:14–24. [Google Scholar]
- 32.Chin NW, Chapman I, Jimenez FA. Complete chemotherapeutic regression of hepatic metastases with resultant hepar lobatum. Am J Gastroenterol 1987. February;82(2):149–51. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


