Abstract
Objective
Electronic pharmacovigilance reporting systems are being implemented in many developing countries in an effort to improve reporting rates. This study sought to establish the factors that acted as barriers to the success of an electronic pharmacovigilance reporting system in Kenya 3 years after its implementation.
Materials and Methods
Factors that could act as barriers to using electronic reporting systems were identified in a review of literature and then used to develop a survey questionnaire that was administered to pharmacists working in government hospitals in 6 counties in Kenya.
Results
The survey was completed by 103 out of the 115 targeted pharmacists (89.5%) and included free-text comments. The key factors identified as barriers were: unavailable, unreliable, or expensive Internet access; challenges associated with a hybrid system of paper and electronic reporting tools; and system usability issues. Coordination challenges at the national pharmacovigilance center and changes in the structure of health management in the country also had an impact on the success of the electronic reporting system.
Discussion
Different personal, organizational, infrastructural, and reporting system factors affect the success of electronic reporting systems in different ways, depending on the context. Context-specific formative evaluations are useful in establishing the performance of electronic reporting systems to identify problems and ensure that they achieve the desired objectives.
Conclusion
While several factors hindered the optimal use of the electronic pharmacovigilance reporting system in Kenya, all were considered modifiable. Effort should be directed toward tackling the identified issues in order to facilitate use and improve pharmacovigilance reporting rates.
Keywords: pharmacovigilance, product surveillance, eHealth, adverse drug reactions, developing countries
BACKGROUND AND SIGNIFICANCE
Pharmacovigilance is the “science and activities relating to the detection, assessment, understanding and prevention of adverse effects or any other drug-related problems.”1 It gained prominence in the 1960s in response to the thalidomide tragedy in Europe, Australia, and Japan,2 which was blamed on marketing pressures from pharmaceutical companies and on the lack of robust pharmacovigilance systems.3
Since then, drug safety monitoring agencies have been formed at different levels to enhance patient safety when using medicines and to continuously provide balanced and reliable information on the safety of medicines. Among the first was the World Health Organization Program for International Drug Monitoring, established in 1968, which works in collaboration with the Uppsala Monitoring Centre (UMC) to maintain a global database for Individual Case Safety Reports (ICSRs) of adverse drug reactions reported from different countries.
Several developments in recent years have highlighted the need for robust pharmacovigilance systems capable of quick detection and reporting of suspected incidents. They include increased licensing and use of biologicals and biosimilars in health care,4–8 increased off-label use of drugs,9,10 growing concerns over a lack of coverage of children in drug safety monitoring during clinical trials,11,12 and an increase in health conditions arising from drug-related incidents.13
Other factors include the increased use of herbal and natural products, many of which have unknown side effects14,15; the growing use of new combination therapies for the management of chronic conditions and coinfections16,17; and the rising incidence of poor-quality medicines and medical devices, particularly in low- and middle-income countries.18 There are also growing calls for objective drug safety information to counterbalance the information published by the pharmaceutical industry, which in some cases is suspected of being biased.19 Emerging dimensions, such as safety issues arising from self-medication20 and safety concerns associated with medical applications21 and medical devices,22 further underline the importance of robust pharmacovigilance systems.
While the number of ICSRs in the global database has been steadily rising over the years, the rate of reporting has been skewed toward developed countries, whose cumulative ICSRs account for >81% of the reports at the UMC.23 African countries in particular have disproportionately low reporting rates, accounting for only 0.88% of all reports despite having 15% of the global population, high disease burdens,24 and poor-quality medical products.18
Various studies have been conducted to explore the causes of the low reporting and to make recommendations on how to optimize reporting. A consistent theme in the recommendations has been calls to incorporate information and communications technology and informatics to improve signal detection, reporting, data analysis, feedback communication, and response in pharmacovigilance.25–29 Examples of such informatics interventions include integrating electronic pharmacovigilance systems into hospital information systems,30 using Internet-based reporting systems,31,32 and using desktop applications to improve access to reporting tools.33 The use of natural language processing systems and event monitoring to aid in the detection of adverse events (AEs) in clinical databases34,35 and of automated decision support systems to help detect and prevent drug-drug interactions36 have also been explored. Others include using mobile applications37,38 and social media to improve the detection of adverse drug reactions (ADRs).39,40 Text messages for pharmacovigilance reporting have also been tested as a complementary tool to other reporting systems.41–43
Many organizations have invested in electronic systems to improve pharmacovigilance reporting, but as consistently observed in recent reviews, the desired effects have not always been achieved.24,44–47 It has been argued that among the reasons for the failure of many e-Health interventions in developing countries is the fact that many are based on research performed in different contexts, usually in the developed world, where the sociocultural and organizational influences are different.48 Consequently, studies on pharmacovigilance now increasingly recommend more context-specific research on ways to improve pharmacovigilance reporting systems.24,25,45,49,50
This study examined an electronic pharmacovigilance reporting system that was introduced in Kenya in 201351 with the intention of improving the efficiency and timeliness of pharmacovigilance reporting by health workers there. The system has a web application version, a stand-alone desktop application version, and a stand-alone mobile application version.
Reports submitted through these sources are sent to servers at the National Pharmacovigilance Centre in Nairobi, where they are processed and further sent to a global database (VigiBase) at the UMC.51 However, as seen in ICSR reporting data for the country,24 the system has yet to achieve the desired objectives, and reporting rates have declined in the years following its introduction (Figure 1).
Figure 1.
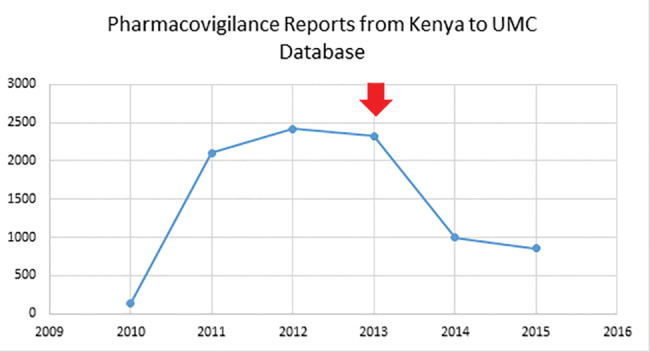
Pharmacovigilance reporting by Kenya 2010–2015 (the arrow shows point of introduction of the electronic reporting system).
OBJECTIVE
The purpose of the study was to explore users’ opinions of the potential barriers to optimal utilization of the electronic reporting system, establish the perceived modifiability of these factors, and identify other factors that may have been responsible for the decline in pharmacovigilance reporting in the country. Understanding these barriers would help in formulating recommendations for improving pharmacovigilance reporting rates in Kenya. This would have benefits for both Kenyan health care consumers and their global counterparts through ICSR data shared via the global drug safety database at UMC. Lessons from the study should also benefit other countries implementing similar electronic pharmacovigilance reporting systems.
MATERIALS AND METHODS
Two research techniques were employed in this study. The first was a literature review to explore available information on factors that could act as barriers to electronic pharmacovigilance reporting, and the second was an exploratory survey conducted among public sector pharmacists to collect complementary empirical data specific to the electronic pharmacovigilance reporting system in Kenya. Information from the 2 were then used to draw conclusions and guide the formulation of recommendations for the study.
The literature review was guided by the question: What are the barriers to using electronic systems for submission of pharmacovigilance reports to national pharmacovigilance centers? The specific search terms used in the literature search were {(barriers OR obstacles OR challenges OR hindrances OR impediments OR concerns OR hurdles) AND (electronic systems OR internet based OR online OR web based OR digital OR computerised OR computerized OR computer based OR information technology OR IT OR informatics) AND (reporting OR submission) AND (pharmacovigilance OR e-Pharmacovigilance OR adverse drug reactions OR ADR OR adverse events OR AE OR drug safety OR medicine safety OR medication safety OR post marketing surveillance)}. The review was carried out on studies from both developed and developing countries and factors that could act as barriers in the study area selected to build the questionnaire.
The survey questionnaire had 4 sections, covering respondent characteristics, barriers to reporting, modifiability of the barriers, and a final open comment section. It consisted of a mixture of semistructured questions and structured 5-point Likert scale questions, on which respondents rated the likelihood of the identified factors to act as barriers and how modifiable the factors were. Additional factors acting as barriers were captured in the free-text section, making this a mixed-methods study. The questionnaire was pilot-tested on 17 pharmacists working in counties not included in the study area. Input was also sought from the co-authors, and amendments were made based on the feedback and recommendations from the pilot test. The questionnaire was then entered into the Bristol Online Survey tool52 and the relevant navigation and skip logic were incorporated before being retested to check for flow and clarity and to estimate the time needed for completion.
The study was conducted among pharmacists, because they are often expected to be leaders in the safety monitoring of medicines, owing to their expertise and their role as a source of critical information on medicine-related matters.53 Pharmacists working in government hospitals were chosen because they were accessible through their respective county pharmacists, and also for a clear definition of the boundaries of the study.
Six counties close to each other that had a preexisting pharmacists’ professional network (Central Kenya Region Pharmacists Network) were chosen by convenience sampling for the study. All 115 pharmacists working in the counties were invited to participate in the survey. A link to the survey, including a secure access control password, was sent to all potential participants by e-mail and through their respective professional WhatsApp™ groups. One of the authors was in Kenya to ensure rigor of the data-collection process and deal with local queries. The survey was sent out on August 12, 2016, and remained open for 60 days, with reminders sent out on the 20th (August 31, 2016) and 40th (September 20, 2016) days.
Excel 2016 and SPSS v21 were used to analyze quantitative data and generate tables, charts, and graphs. Qualitative data from the free-text sections of the questionnaire were analyzed by thematic analysis, which involved coding the data for key concepts, identifying themes based on the codes, and consolidating the resulting information into themes. The findings were then triangulated to draw conclusions for the study.
Ethical clearance to conduct the study was obtained from the University of Leeds School of Medicine Research Ethics Committee, approval number MREC15-121, and from the Kenya Pharmacy and Poisons Board (PPB). Consent was obtained from the respondents prior to their participation in the survey.
RESULTS
Databases included in the literature review were: PubMed, which generated 686 results on a 2006–2016 publication filter range; a combined search of Embase, Global Health, Health Management Information Consortium, International Pharmaceutical Abstracts, Ovid Medline, and PsycINFO databases using the same search criteria, which generated 457 results that were reduced to 374 results after deduplication; and the Cumulative Index to Nursing and Allied Health Literature database, which generated 30 results on a 10-year filter. Other complementary literature was searched in Google Scholar, the Google search engine, and textbooks, reports, and websites. From the search results, literature apposite to the study was selected based on recency, reliability, and relevance. Of the 25 factors identified, 16 factors applicable to the Kenya context were selected and used to build the questionnaire for the survey.
Of the 115 potential respondents to whom the survey link was sent, 103 returned the survey, yielding a response rate of 89.5%. The respondents comprised 62 men (60.2%) and 41 women (39.8%). A majority worked in sub-county and county hospitals (Figure 2), which are usually relatively busy, as they are the facilities most frequented by patients seeking both primary and specialist treatment services.
Figure 2.
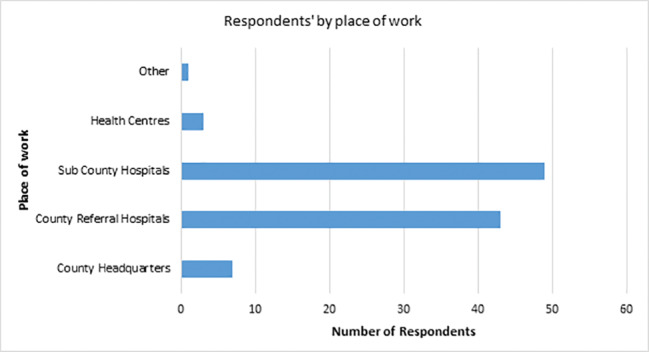
Respondents' places of work.
All respondents were familiar with the pharmacovigilance reporting process, with 84 (81.5%) reporting that they had submitted a report before, through either the paper reporting system (34, 40.5%), the electronic reporting system (9, 10.7%), or both (41, 48.8%). The numbers of male and female pharmacists who submitted reports were not significantly different. Most of them were comfortable or very comfortable with sending text messages, using smartphones, and using computers. This was regardless of the number of years worked as a pharmacist and not significantly different for male and female pharmacists.
Table 1 shows the factors that acted as barriers and the distribution of the responses in percentages. For each factor, a chi-squared test was carried out to test the hypothesis that responses were from a uniform distribution, that is, that respondents chose their response category at random. For all but 2 of the factors, respondents showed significant preferences for particular categories.
Table 1.
Likelihood of factors to act as barriers (N = 103)
| Factors affecting electronic reporting | Respondent ratings in % |
||||||
|---|---|---|---|---|---|---|---|
| VeryLikely |
VeryUnlikely |
Chi-squared statisticon 4 degrees offreedom; P-value | |||||
| 1 | 2 | 3 | 4 | 5 | |||
| Most Likely | Lack of Internet access provision at the workplace | 36.9 | 37.9 | 4.9 | 13.6 | 6.8 | 54.04; <.001 |
 |
Unreliable Internet coverage at the workplace | 37.9 | 31.1 | 10.7 | 13.6 | 6.8 | 38.31; <.001 |
| Existence of a paper-based system as an alternative for reporting | 27.2 | 32.0 | 17.5 | 12.6 | 10.7 | 17.73; .001 | |
| Lack of a culture of pharmacovigilance reporting | 34.0 | 21.4 | 17.5 | 23.3 | 3.9 | 24.43; <.001 | |
| Lack of support/incentives from management to use the system for reporting | 22.3 | 37.9 | 19.4 | 11.7 | 8.7 | 26.85; <.001 | |
| Extra cost of electronic reporting (Internet data costs) | 24.3 | 35.9 | 8.7 | 16.5 | 14.6 | 22.68; <.001 | |
| Extra time involved in using the system to submit reports | 15.5 | 41.7 | 19.4 | 13.6 | 9.7 | 32.97; <.001 | |
| Difficulty downloading and installing the app versions of the system | 21.4 | 30.1 | 15.5 | 17.5 | 15.5 | 7.73; .102 | |
| Difficulty accessing the system online | 13.6 | 31.1 | 22.3 | 20.4 | 12.6 | 11.52; .021 | |
| Lack of awareness of existence of the electronic reporting system | 25.2 | 15.5 | 13.6 | 26.2 | 19.4 | 6.56; .161 | |
| Difficulty navigating the system when reporting | 10.7 | 32.0 | 21.4 | 15.5 | 20.4 | 13.07; .011 | |
| Limited access to computers at the workplace | 18.4 | 23.3 | 7.8 | 25.2 | 25.2 | 11.22; .024 | |
| Lack of an option for anonymous reporting in the system | 11.7 | 20.4 | 15.5 | 23.3 | 29.1 | 9.48; .050 | |
| No practical benefits of using the electronic system | 6.8 | 14.6 | 19.4 | 25.2 | 34.0 | 22.00; <.001 | |
| Unreliable electricity at the workplace | 5.8 | 17.5 | 10.7 | 28.2 | 37.9 | 35.01; <.001 | |
| Least Likely | Dislike of computer technology | 3.9 | 8.7 | 11.7 | 31.1 | 44.7 | 61.13; <.001 |
Table 2 shows the distribution of responses to the perceived modifiability of the same factors as Table 1, allowing a link to the perceived importance. Factors perceived as most modifiable included difficulty in navigating the electronic system when reporting, difficulty downloading and installing the application versions of the reporting system, and lack of awareness of the existence of the electronic reporting system. Conversely, factors deemed to be least modifiable included the perception that the electronic reporting system had no practical benefits, unreliable electricity supply at the workplace, and a dislike of computer technology. As with the previous table, for each factor, a chi-squared test was carried out to test the hypothesis that responses were from a uniform distribution, that is, that respondents chose their response categories at random. The results show that for all factors, respondents showed significant preferences for particular categories when questioned about the modifiability of the factor.
Table 2.
Likelihood of factors to be modified (N = 103)
| Factors | Respondent ratings in % |
||||||
|---|---|---|---|---|---|---|---|
| Very Modifiable |
Not Modifiable |
Chi-squared statistic on 4 degrees of freedom; P-value | |||||
| 1 | 2 | 3 | 4 | 5 | |||
| Very Modifiable | Difficulty navigating the system when reporting | 48.5 | 42.7 | 6.8 | 1.0 | 1.0 | 114.82; <.001 |
 |
Difficulty downloading and installing the app versions of the system | 46.6 | 41.7 | 9.7 | 1.0 | 1.0 | 103.55; <.001 |
| Lack of awareness of existence of the electronic reporting system | 46.6 | 42.7 | 2.9 | 6.8 | 1.0 | 105.69; <.001 | |
| Lack of Internet access provision at the workplace | 44.7 | 37.9 | 11.7 | 4.9 | 1.0 | 81.81; <.001 | |
| Difficulty accessing the system online | 44.7 | 38.8 | 8.7 | 5.8 | 1.9 | 83.26; <.001 | |
| Extra cost of electronic reporting (Internet data costs) | 39.8 | 35.0 | 17.5 | 6.8 | 1.0 | 59.67; <.001 | |
| Lack of a culture of pharmacovigilance reporting | 36.9 | 38.8 | 16.5 | 6.8 | 1.0 | 61.22; <.001 | |
| Existence of a paper-based system as an alternative for reporting | 41.7 | 35.9 | 15.5 | 1.9 | 4.9 | 67.05; <.001 | |
| Limited access to computers at the workplace | 47.6 | 24.3 | 13.6 | 14.6 | 0.0 | 30.86; <.001 | |
| Lack of support/incentives from management to use the system for reporting | 34.0 | 38.8 | 20.4 | 2.9 | 3.9 | 56.76; <.001 | |
| Unreliable Internet coverage at the workplace | 33.0 | 38.8 | 14.6 | 11.7 | 1.9 | 48.89; <.001 | |
| Extra time involved in using the system to submit the reports | 27.2 | 45.6 | 18.4 | 2.9 | 5.8 | 62.00; <.001 | |
| Lack of an option for anonymous reporting in the system | 34.0 | 38.8 | 13.6 | 7.8 | 5.8 | 48.51; <.001 | |
| No practical benefits of using the electronic system | 28.2 | 40.8 | 19.4 | 9.7 | 1.9 | 47.92; <.001 | |
| Unreliable electricity at the workplace | 28.2 | 36.9 | 17.5 | 12.6 | 4.9 | 33.07; <.001 | |
| Least Modifiable | Dislike of computer technology | 32.0 | 32.0 | 21.4 | 8.7 | 5.8 | 31.90; <.001 |
Additional factors acting as barriers that emerged from the free-text sections of the survey included tedious transcription process from paper forms to the online system, difficulty recovering forgotten passwords on the electronic reporting portal, too much “unnecessary” information displayed on the online reporting portal making the website “too busy,” and the lack of an application version for iOS devices.
Other barriers identified by respondents were not specific to the electronic reporting system. They included lack of acknowledgment and feedback from the PPB after submission of reports, lack of dissemination of the outcomes of the reports to health workers and the public, poor coordination of pharmacovigilance activities at lower levels of care (health centers and dispensaries), poor access to pharmacovigilance reporting tools, and staffing shortages that led overwhelmed health workers to view pharmacovigilance reporting as extra “non-essential” work. Challenges associated with the devolution of Kenyan health services and the withdrawal of stavudine, an antiretroviral medicine that accounted for the largest proportion of ADR reports, from the HIV/AIDS treatment regimens in Kenya were also suspected to have an impact on overall reporting rates.54
DISCUSSION
This study achieved a high response rate of 89.5%, possibly attributable to the online questionnaire delivery method employed, which has been shown elsewhere to have a significant influence on survey response rates and average response times.55 Moreover, the growing use of smartphones and applications such as WhatsApp™ among health care professionals56,57 made it easier to reach out to respondents both individually and through their professional groups. Smartphones also allowed respondents to complete questionnaires outside of their work environments and possibly in areas with better Internet connectivity.
While both the electronic pharmacovigilance reporting system and the survey questionnaire were Internet-based, the higher survey response rate could be explained by the simple mobile-optimized design of the questionnaire, the fact that it was not viewed as work carried home, and the fact that no transcription of information from a paper form to an electronic form was required, unlike the case of pharmacovigilance reporting. Some pharmacovigilance reports contain sensitive patient information that should not be taken out of hospital/clinical environments, so if there are connectivity issues in the hospital, reporting may be difficult.
Most respondents worked in hospital settings and were familiar with pharmacovigilance reporting systems, with 81.5% (n = 84) having previously submitted a report either electronically or using paper forms. This high level of familiarity agreed with the findings of a Korean study among community pharmacists.58 It contrasts, however, with the low levels of familiarity observed in other studies involving a mixture of pharmacists and other health professionals.59–62
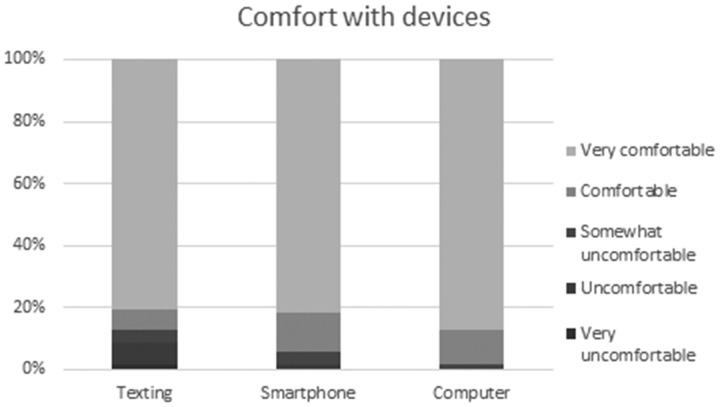
Respondents also reported a very high level of comfort with sending mobile phone text messages and using smartphones and computers (Figure 3). This suggests that discomfort with such devices was probably not a barrier to using the electronic reporting system. Desktop and smartphone application versions of the electronic reporting system existed and links to downloading them were functional, though only Windows and Linux systems for the desktop application and Android devices for the smartphone application were covered. Other platforms, such as iOS and Mac OS, were not supported. With the growing role of applications in pharmacovigilance reporting,63,64 this lack of coverage across all the major smartphone platforms used in Kenya could also have contributed to the underutilization of the electronic reporting system.
Figure 3.
Comfort with devices.
The factors acting as barriers to using the electronic reporting system cut across individual-level, organization-level, reporting system, and infrastructural barriers. The individual-level factor identified was the extra cost to the reporter associated with submitting reports to the national center via the Internet. This may include the cost of privately purchased Internet bundles where the organization does not provide workplace Internet, Internet access costs at cyber cafés, and sometimes the cost of transport from the work location to a shopping center where the Internet can be accessed. Similar barriers have been observed in a systematic review of m-Health in sub-Saharan Africa65 and in a study that explored the access of information through information and communications technology among health workers in Kenya.66 These costs can result in reporting being postponed or not done, and hence underutilization of the electronic system.
Key organization-level barriers that were identified included failure to provide Internet at the workplace, lack of support from management to use the electronic system, and lack of a pharmacovigilance reporting culture in the organizations. Health organizations that embrace the culture of pharmacovigilance reporting may make more effort to provide Internet access or support for electronic submission of reports via either Internet cafés or personal mobile data. Failing to provide Internet at the workplace, however, may not be within the control of management, especially in small government health facilities in rural areas, which often lack the financial capacity and influence of top management.67,68 Also important, especially in a devolved health care system such as in Kenya, is the political will to provide and sustain Internet services in health facilities to facilitate important services such as electronic pharmacovigilance reporting.69
Barriers related to the reporting system included challenges associated with the coexistence of an electronic system with a paper-based system. As in many other electronic system launches, the launch of the electronic pharmacovigilance system in Kenya in 2013 was dubbed “going paperless,”51 and this could have unintentionally painted the paper-based system as cumbersome and ineffective, leading to a reduction in the printing and distribution of paper-based reporting tools. It may also have led to a reluctance by county pharmacists to ask for paper forms from the national pharmacovigilance office, because they felt they were supposed to use the new electronic reporting system. This problem was highlighted in the free-text section of the questionnaire by some respondents, who complained of difficulties in accessing the reporting tools (both paper and electronic). A hybrid phase in which paper and electronic systems coexist is important not only to provide backup in case the electronic system fails, but also to use at sites where the electronic system cannot yet be accessed.
Extra time required to submit reports using the electronic system was also highlighted as a barrier. This may have been a result of the usability challenges associated with the electronic reporting tool, which can lead to underuse.70 Comments from respondents confirmed the existence of usability challenges, including unnecessary information being displayed on the website, unnecessary information required during reporting, difficulties in resetting forgotten passwords, and the poor website navigation system. These usability challenges meant that it possibly took longer to report electronically, which further contributed to underutilization of the system. The importance of usability design and testing prior to implementation of electronic systems in health care settings continues to be emphasized.45,70
Another possible reason for the extra time that respondents mentioned was the requirement to transfer information from paper reports – usually filled at lower-level health facilities and brought to the central hospital – into the electronic system before submission to the national pharmacovigilance center. Comments from respondents revealed frustrations with the lengthy transcription process, with the suggestion that the PPB also accept scanned paper forms, shown to be feasible and time-saving elsewhere.71
On infrastructural barriers, poor Internet coverage was identified as an impediment to the optimal use of the electronic system for report submission. The problem of unreliable/unavailable Internet and the potential that this could interfere with electronic systems in health care has been observed in previous studies.72–74 Smartphone and desktop application versions of the electronic reporting system can, however, be used to capture reports in offline mode, then submit them when there is sufficient Internet coverage. For areas with no Internet coverage, the paper system may be the solution, or a text message reporting system could be developed.
A factor local to Kenya that had a significant impact on pharmacovigilance reporting was the devolution of health services from the national government to county governments. This transition led to a disruption of administrative functions at the county level, low staff morale, resignations of key health personnel, and confusion arising from politicization of the health function.75 These affected pharmacovigilance reporting, as hospital financial flows were disrupted and low morale meant that reporting was no longer a priority. Staff shortages following resignations further worsened the situation.
Factors that were least likely to act as barriers included a dislike of computer technology, the perception that the electronic reporting system had no practical benefits, and unreliable electricity at the workplace. The first factor could be explained by the high level of comfort with electronic devices among the respondents, as shown in Figure 3. Comfort with technology among intended users of an electronic system is important in ensuring uptake, and the lack of it can result in system rejection or underuse.38,76 Despite unreliable electricity not being viewed as a major barrier by respondents, it can be a serious impediment to implementation of electronic systems, particularly those running on desktop personal computers and local area networks.77,78 Mobile devices using mobile Internet are less affected due to long-lasting batteries, and falling costs of solar power should reduce this barrier going forward.
All the factors were considered modifiable, possibly indicating respondents’ confidence with their respective management. This should, however, be viewed with caution because of the possibility of social desirability bias and acquiescence bias in responses due to the manner in which the questionnaires were sent to the respondents and the use of Likert scale questions in the survey, respectively. However, it is likely that the anonymous nature of the questionnaire mitigated against social desirability bias, especially as this was explicitly communicated to the respondents prior to their participation. For all factors, the agreement that they were modifiable was statistically significant.
Based on the weighting of the distribution of responses, factors related to the reporting system were generally deemed more modifiable, while those perceived to be less modifiable included unreliability of the electricity supply and dislike of computer technology (Table 2). Interestingly, the factors considered least modifiable were also the ones considered by the respondents to be least likely to act as barriers. This was a positive finding that may need to be verified in a separate study, since this study was based on perceived rather than actual barriers to using the electronic system.
This study shows that it is challenging to successfully scale up important e-Health projects in low-income settings, but that users can have clear and consistent views about what the barriers to scaling up are and how to address them. It further shows than in an engaged group of health professionals such as pharmacists, high response rates can be achieved on surveys even in low-income settings.
This study had some limitations. There was sampling bias, as the surveyed participants were drawn from one region of the country and were public sector pharmacists only. A more general sample of staff in a national-level study would have been more powerful. The review done for the study was nonsystematic and was limited to English-language articles only. It was also performed in databases that may be biased toward studies from developed countries, and because of this, the results leading to the survey may have omitted some questions relevant to the study setting. The distribution of questionnaires to participants directly from their supervisors also may have introduced some social desirability bias, especially on aspects of the survey touching on the managers. Completion of the survey using mobile devices by some participants could also have affected the data quality, due to the relatively smaller screen size, which is thought to affect the length and quality of free-text answers.79,80 Another potential limitation of the study is its timing 3 years into the implementation; this should give a good idea of long-term performance, but an earlier study could have shed more light on the implementation process. The final limitation is that the study examined perceived rather than actual factors that acted as barriers to electronic pharmacovigilance reporting. While the perceptions of users were important, it is equally important to establish the actual barriers to using the electronic reporting system.
CONCLUSION
The main barriers to using the electronic pharmacovigilance reporting system were access to the Internet, the system’s design and usability problems, and challenges related to the hybrid system of reporting. All these factors were perceived by users of the system to be modifiable. Formative evaluation of the performance of such systems is necessary to allow for early detection of problems and for improvements and learning. With large numbers of health facilities in Kenya using electronic health record systems to support care and reporting, these systems could also make important contributions to the generation and submission of AE reports, potentially speeding the process and reducing workload and errors. We would encourage the PPB to address the problems identified in the current electronic pharmacovigilance system and institute follow-up studies to assess reporting rates and user experiences.
FUNDING
OOA was supported by a Commonwealth Scholarship for Master's Study in the UK. HF was a Marie Skłodowska-Curie Fellow, funded by the European Union's Horizon 2020 research and innovation program under grant agreement No. 661289, “Global eHealth.”
COMPETING INTERESTS
None.
ACKNOWLEDGMENTS
We would like to thank the Kenya Pharmacy and Poisons Board and the pharmacists who participated in the survey.
REFERENCES
- 1. World Health Organization. Pharmacovigilance. www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmvigi/en/. Accessed November 8, 2016. [Google Scholar]
- 2. Kim JH, Scialli AR. Thalidomide: the tragedy of birth defects and the effective treatment of disease. Toxicol Sci. 2011;1221:1–6. [DOI] [PubMed] [Google Scholar]
- 3. Ridings JE. The thalidomide disaster, lessons from the past. Methods Mol Biol. 2013;947:575–86. [DOI] [PubMed] [Google Scholar]
- 4. Casadevall N, Edwards IR, Felix T et al. , Pharmacovigilance and biosimilars: considerations, needs and challenges. Expert Opin Biol Ther. 2013;13(7):1039–47. [DOI] [PubMed] [Google Scholar]
- 5. Lucio SD, Stevenson JG, Hoffman JM. Biosimilars: implications for health-system pharmacists. Am J Health-Syst Pharm. 2013;7022:2004–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sewak NPS, Whitcher C, Neophytou I. The introduction of biosimilar monoclonal antibodies into developed markets: what are payers concerned about? Value Health. 2013;163:A229. [Google Scholar]
- 7. Vermeer NS, Spierings I, Mantel-Teeuwisse AK et al. , Traceability of biologicals: present challenges in pharmacovigilance. Expert Opin Drug Saf. 2015;141:63–72. [DOI] [PubMed] [Google Scholar]
- 8. Emmanouilides CE, Karampola MI, Beredima M. Biosimilars: hope and concern. J Oncol Pharm Pract. 2015;224:618–24. [DOI] [PubMed] [Google Scholar]
- 9. Bellis JR, Kirkham JJ, Thiesen S et al. , Adverse drug reactions and off-label and unlicensed medicines in children: a nested case? Control study of inpatients in a pediatric hospital. BMC Med. 2013;11:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eguale T, Buckeridge DL, Verma A et al. , Association of off-label drug use and adverse drug events in an adult population. JAMA Intern Med. 2016;1761:55–63, 69. [DOI] [PubMed] [Google Scholar]
- 11. Pellegrino P, Carnovale C, Cattaneo D et al. , Pharmacovigilance knowledge in family paediatricians. A survey study in Italy. Health Policy. 2013;113(1–2):216–20. [DOI] [PubMed] [Google Scholar]
- 12. Star K, Edwards IR. Pharmacovigilance for children’s sake. Drug Saf. 2014;372:91–98. [DOI] [PubMed] [Google Scholar]
- 13. Naik P. The Future of Pharmacovigilance. J Pharmacovigil 2015;3:159. [Google Scholar]
- 14. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol. 2014;4:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bhagavathula AS, Elnour AA, Shehab A. Pharmacovigilance on sexual enhancing herbal supplements. Saudi Pharm J. 2016;241:115–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singh H, Dulhani N, Kumar B, Singh P, Tewari P, Nayak K. A pharmacovigilance study in medicine department of tertiary care hospital in Chhattisgarh (Jagdalpur), India. J Young Pharm. 2010;21:95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lange C, Abubakar I, Alffenaar J-WC et al. , Management of patients with multidrug-resistant/extensively drug-resistant tuberculosis in Europe: a TBNET consensus statement. Eur Respir J. 2014;441:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Almuzaini T, Choonara I, Sammons H. Substandard and counterfeit medicines: a systematic review of the literature. BMJ Open. 2013;38:e002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Amundstuen Reppe L, Spigset O, Schjøtt J. Drug information services today: current role and future perspectives in rational drug therapy. Clin Ther. 2016;382:414–21. [DOI] [PubMed] [Google Scholar]
- 20. Montastruc J-L, Bondon-Guitton E, Abadie D et al. , Pharmacovigilance, risks and adverse effects of self-medication. Thérapie. 2016;712:257–62. [DOI] [PubMed] [Google Scholar]
- 21. Wicks P, Chiauzzi E. “Trust but verify” – five approaches to ensure safe medical apps. BMC Med. 2015;13:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Parvizi N, Woods K. Regulation of medicines and medical devices: contrasts and similarities. Clin Med. 2014;141:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uppsala Monitoring Centre. www.who-umc.org. Accessed May 18, 2016.
- 24. Ampadu HH, Hoekman J, de Bruin ML et al. , Adverse drug reaction reporting in Africa and a comparison of individual case safety report characteristics between Africa and the rest of the world: analyses of spontaneous reports in VigiBase®. Drug Saf. 2016;39:335–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lu Z. Information technology in pharmacovigilance: benefits, challenges, and future directions from industry perspectives. Drug Healthc Patient Saf. 2009;1:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh AR, Tiwari R. A review on pharmacovigilance a powerful weapon for tracking safety and efficacy of drugs. ResearchGate. 2011;46:867–71. [Google Scholar]
- 27. Menniti M, Menniti A, Patanè M et al. , Informatics applied to pharmacovigilance: future perspectives. J Pharmacol Pharmacother. 2013;4(Suppl. 1):S43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ribeiro-Vaz I, Silva A-M, Costa Santos C, Cruz-Correia R. How to promote adverse drug reaction reports using information systems – a systematic review and meta-analysis. BMC Med Inform Decis Mak. 2016;16:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tozzi AE, Gesualdo F, D’Ambrosio A, Pandolfi E, Agricola E, Lopalco P. Can digital tools be used for improving immunization programs? Front Public Health. 2016;4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ortega A, Aguinagalde A, Lacasa C, Aquerreta I, Fernández-Benítez M, Fernández LM. Efficacy of an adverse drug reaction electronic reporting system integrated into a hospital information system. Ann Pharmacother. 2008;4210:1491–96. [DOI] [PubMed] [Google Scholar]
- 31. Haber P, Iskander J, Walton K, Campbell SR, Kohl KS. Internet-based reporting to the vaccine adverse event reporting system: a more timely and complete way for providers to support vaccine safety. Pediatrics. 2011;127(Suppl 1):S39–44. [DOI] [PubMed] [Google Scholar]
- 32. Abadie D, Chebane L, Bert M, Durrieu G, Montastruc J-L. Online reporting of adverse drug reactions: a study from a French regional pharmacovigilance center. Thérapie. 2014;695:395–400. [DOI] [PubMed] [Google Scholar]
- 33. Ribeiro-Vaz I, Santos C, da Costa-Pereira A, Cruz-Correia R. Promoting spontaneous adverse drug reaction reporting in hospitals using a hyperlink to the online reporting form: an ecological study in Portugal. Drug Saf. 2012;355:387–94. [DOI] [PubMed] [Google Scholar]
- 34. Bates DW, Evans RS, Murff H, Stetson PD, Pizziferri L, Hripcsak G. Detecting adverse events using information technology. J Am Med Inform Assoc. 2003;102:115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Murff HJ, Patel VL, Hripcsak G, Bates DW. Detecting adverse events for patient safety research: a review of current methodologies. J Biomed Inform. 2003;36(1–2):131–43. [DOI] [PubMed] [Google Scholar]
- 36. Ussai S, Spartà MC. PIH1 – Adverse drug events: how information technology will meet the challenges of pharmacovigilance. Value Health. 2014;177:A750. [DOI] [PubMed] [Google Scholar]
- 37. Bahk CY, Goshgarian M, Donahue K et al. , Increasing patient engagement in pharmacovigilance through online community outreach and mobile reporting applications: an analysis of adverse event reporting for the Essure device in the US. Pharm Med. 2015;29(6):331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson K, Atkinson KM, Westeinde J et al. , An evaluation of the feasibility and usability of a proof of concept mobile app for adverse event reporting post influenza vaccination. Hum Vaccines Immunother 2016;127:1738–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu X, Chen H. A research framework for pharmacovigilance in health social media: identification and evaluation of patient adverse drug event reports. J Biomed Inform. 2015;58:268–79. [DOI] [PubMed] [Google Scholar]
- 40. Sarker A, Ginn R, Nikfarjam A et al. , Utilizing social media data for pharmacovigilance: a review. J Biomed Inform. 2015;54:202–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baron S, Goutard F, Nguon K, Tarantola A. Use of a text message-based pharmacovigilance tool in Cambodia: pilot study. J Med Internet. Res 2013;154:e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Leeb A, Regan AK, Peters IJ, Leeb C, Leeb G, Effler PV. Using automated text messages to monitor adverse events following immunisation in general practice. Med J Aust 2014;2007:416–18. www.mja.com.au/journal/2014/200/7/using-automated-text-messages-monitor-adverse-events-following-immunisation. Accessed June 22, 2016. [DOI] [PubMed] [Google Scholar]
- 43. Vergeire-Dalmacion G, Castillo-Carandang NT, Juban NR, Amarillo ML, Tagle MP, Baja ES. Texting-based reporting of adverse drug reactions to ensure patient safety: a feasibility study. JMIR Public Health Surveill. 2015;12:e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maigetter K, Pollock AM, Kadam A, Ward K, Weiss MG. Pharmacovigilance in India, Uganda and South Africa with reference to WHO’s minimum requirements. Int J Health Policy Manag. 2015;45:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Olsson S, Pal SN, Dodoo A. Pharmacovigilance in resource-limited countries. Expert Rev Clin Pharmacol. 2015;84:449–60. [DOI] [PubMed] [Google Scholar]
- 46. Swamy S, Mourya M, Kadhe G, Mane A, Sawant S. Safety reporting through a comprehensive and pragmatic pharmacovigilance process for India and emerging markets: an industry perspective. Expert Opin Drug Saf. 2015;149:1409–20. [DOI] [PubMed] [Google Scholar]
- 47. Mitchell I, Schuster A, Smith K, Pronovost P, Wu A. Patient safety incident reporting: a qualitative study of thoughts and perceptions of experts 15 years after “To Err is Human.” BMJ Qual Saf. 2016;252:92–99. [DOI] [PubMed] [Google Scholar]
- 48. Kumar P, Paton C, Kirigia D. I’ve got 99 problems but a phone ain’t one: electronic and mobile health in low and middle income countries. Arch Dis Child. 2016;10110:974–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kilbridge PM, Classen DC. The informatics opportunities at the intersection of patient safety and clinical informatics. J Am Med Inform Assoc. 2008;154:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gonzalez-Gonzalez C, Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Strategies to improve adverse drug reaction reporting: a critical and systematic review. Drug Saf. 2013;365:317–28. [DOI] [PubMed] [Google Scholar]
- 51. Pharmacovigilance Reporting Goes Digital in Kenya. Management Sciences for Health. www.msh.org/news-events/stories/pharmacovigilance-reporting-goes-digital-in-kenya. Accessed November 8, 2016.
- 52. BOS Online Survey Tool. www.onlinesurveys.ac.uk/. Accessed November 16, 2016.
- 53. van Grootheest AC, de Jong-van den Berg LTW. The role of hospital and community pharmacists in pharmacovigilance. Res Soc Adm. Pharm 2005;11:126–33. [DOI] [PubMed] [Google Scholar]
- 54. PPB Publications. Pharmacy and Poisons Board. http://pharmacyboardkenya.org/?page_id=442. Accessed November 8, 2016. [Google Scholar]
- 55. Hardigan PC, Popovici I, Carvajal MJ. Response rate, response time, and economic costs of survey research: a randomized trial of practicing pharmacists. Res Soc Adm Pharm. 2016;121:141–48. [DOI] [PubMed] [Google Scholar]
- 56. Kamel Boulos MN, Giustini DM, Wheeler S. Instagram and WhatsApp in health and healthcare: an overview. Future Internet. 2016;83:37. [Google Scholar]
- 57. Nardo B, Cannistrà M, Diaco V et al. , Optimizing patient surgical management using WhatsApp application in the Italian healthcare system. Telemed J E-Health. 2016;229:718–25. [DOI] [PubMed] [Google Scholar]
- 58. Yu YM, Lee E, Koo BS et al. , Predictive factors of spontaneous reporting of adverse drug reactions among community pharmacists. PLoS ONE. 2016;115:e0155517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kiguba R, Karamagi C, Waako P, Ndagije HB, Bird SM. Recognition and reporting of suspected adverse drug reactions by surveyed healthcare professionals in Uganda: key determinants. BMJ Open. 2014;411:e005869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Suyagh M, Farah D, Abu Farha R. Pharmacist’s knowledge, practice and attitudes toward pharmacovigilance and adverse drug reactions reporting process. Saudi Pharm J. 2015;232:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elkalmi RM, Hassali MA, Ibrahim MIM, Liau SY, Awaisu A. A qualitative study exploring barriers and facilitators for reporting of adverse drug reactions (ADRs) among community pharmacists in Malaysia. J Pharm Health Serv Res. 2011;22:71–78. [Google Scholar]
- 62. Khan TM. Community pharmacists’ knowledge and perceptions about adverse drug reactions and barriers towards their reporting in Eastern region, Alahsa, Saudi Arabia. Ther Adv Drug Saf. 2013;42:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heger M. Regulators move toward adverse event reporting via mobile apps. Nat Med. 2015;212:104–04. [DOI] [PubMed] [Google Scholar]
- 64. Kuchya S, Kalaiselvan V, Kaur I, Singh GN. Mobile application: an approach to enhance easy adverse drug reactions reporting in India. Health Technol. 2016;62:157–58. [Google Scholar]
- 65. Betjeman TJ, Soghoian SE, Foran MP, Betjeman TJ, Soghoian SE, Foran MP. mHealth in Sub-Saharan Africa. Int J Telemed Appl. 2013;2013:e482324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Muinga N, Sen B, Todd J, Ayieko P, English M. Access to and value of information to support good practice for staff in Kenyan hospitals. Glob Health Action 2015;8:26559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pirmohamed M, Atuah KN, Dodoo ANO, Winstanley P. Pharmacovigilance in developing countries. BMJ. 2007;3357618:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Isah AO, Pal SN, Olsson S, Dodoo A, Bencheikh RS. Specific features of medicines safety and pharmacovigilance in Africa. Ther Adv Drug Saf. 2012;31:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Graham JE, Borda-Rodriguez A, Huzair F, Zinck E. Capacity for a global vaccine safety system: the perspective of national regulatory authorities. Vaccine. 2012;3033:4953–59. [DOI] [PubMed] [Google Scholar]
- 70. Wiele P, Rantanen E. Usability of incident reporting systems: preliminary results of a case study. Proc Int Symp Hum Factors Ergon Healthc. 2015;41:168–73. [Google Scholar]
- 71. Kabanywanyi AM, Mulure N, Migoha C et al. , Experience of safety monitoring in the context of a prospective observational study of artemether-lumefantrine in rural Tanzania: lessons learned for pharmacovigilance reporting. Malar J. 2010;91:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Shiferaw F, Zolfo M. The role of information communication technology (ICT) towards universal health coverage: the first steps of a telemedicine project in Ethiopia. Glob Health Action. 2012;5:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mostert-Phipps N, Pottas D, Korpela M. A South African perspective on factors that impact on the adoption and meaningful use of health information technologies. South Afr Fam Pract. 2013;556:545–54. [Google Scholar]
- 74. Oluoch T, de Keizer NF. Evaluation of health IT in low-income countries. Stud Health Technol Inform. 2016;222:324–35. [PubMed] [Google Scholar]
- 75. Challenges Facing Devolution in Kenya: A Comparative Study. www.academia.edu/9385903/Challenges_Facing_Devolution_in_Kenya_A_Comparative_Study. Accessed November 8, 2016. [Google Scholar]
- 76. Garrett P, Brown CA, Hart-Hester S et al. , Identifying barriers to the adoption of new technology in rural hospitals: a case report. Perspect Health Inf Manag. 2006;3 www.ncbi.nlm.nih.gov/pmc/articles/PMC2047308/. Accessed June 3, 2016. [PMC free article] [PubMed] [Google Scholar]
- 77. Luna D, Almerares A, Mayan JC, González Bernaldo de Quirós F, Otero C. Health informatics in developing countries: going beyond pilot practices to sustainable implementations: a review of the current challenges. Healthc Inform Res. 2014;201:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Latourette MT, Siebert JE, Barto RJ et al. , Magnetic resonance imaging research in sub-Saharan Africa: challenges and satellite-based networking implementation. J Digit Imaging. 2011;244:729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mavletova A. Data quality in PC and mobile web surveys. Soc Sci Comput Rev. 2013;316:725–43. [Google Scholar]
- 80. Lambert AD, Miller AL. Living with smartphones: does completion device affect survey responses? Res High Educ. 2014;562:166–77. [Google Scholar]