Abstract
Background and objective
Asthma is one of the most common chronic diseases among women of reproductive age, and previous studies have suggested a link between female asthma and infertility. The aim of the present review is to provide an update on current knowledge of the association between female asthma and/or atopy and a reduction in fertility, ie, number of offspring, time to pregnancy (TTP) and need for fertility treatment.
Methods
Systematic review performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses-guidelines.
Results
A total of 14 studies fulfilled the predefined criteria for inclusion in the present review. Six studies investigated the association between female asthma and/or atopy and number of offspring, of which one reported a positive, two a negative, and three no association. Three studies addressed the association between asthma and TTP and found that TTP was significantly prolonged in asthmatic women compared to non-asthmatic women. Five studies investigated subfertility and the need for fertility treatments of which two studies found a higher prevalence of infertility among women prescribed anti-asthma medication. One study found no difference in the number of fertility treatments of asthmatic women compared to non-asthmatic women, whereas three studies reported that female asthma was associated with significantly more fertility treatment compared to non-asthmatic women.
Conclusion
Although the available evidence is conflicting, there is a clear trend toward an association between female asthma and a reduction in fertility, and by that a larger proportion requiring fertility treatment, even though female asthma might not negatively affect total number of offspring.
Keywords: asthma, fertility, offspring, fertility treatment, time to pregnancy
Introduction
Asthma is one of the most common chronic diseases among women of reproductive age,1 and previous studies have shown that there might be a link between asthma and a reduction in fertility.2,3
Asthma with or without concomitant atopy is an inflammatory disease and the inflammatory changes may have an effect outside the respiratory system. It has been hypothesized that the inflammation may affect the reproductive organs, and by that have an impact on various phases of the reproductive cycle, not least the receptiveness of the uterus at it may lead to inflammatory changes in the endometrium. Lower levels of vascular endothelial growth factor (VEGF) have been found in asthmatic women with unexplained infertility,4 and this might have a negative impact on the receptiveness of the endometrium.
Other inflammatory diseases, such as rheumatoid arthritis have been linked to infertility or subfertility.5,6 However, the cause of reduced fertility in rheumatoid arthritis appears to be multifactorial and related to disease activity and medical therapy, including methotrexate and systemic corticosteroid, although there is so far no clear evidence for an association between a reduction in fertility and systemic corticosteroid therapy.5 On the other hand, in inflammatory bowel diseases, no evidence has been found for physiological causes of infertility.7–11 Hence, there is no clear picture of the possible association between inflammatory diseases, in general, and reduced fertility.
The aim of the present review is to provide an update on current knowledge of fertility in women with asthma and the possible association between maternal asthma and a reduction in fertility, primarily focusing on the number of children, time to pregnancy (TTP), and fertility treatment.
Methods
This review was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses-Statement.12 A series of systematic searches were performed, using the PubMed database. The searches were last updated in January 2019.
The strategy was to assemble as much literature about female asthma and fertility as possible, to explore the association between female asthma and fertility.
The search algorithm consisted of whole words and short terms combined with Medical Subject Headings (MeSH) terms, and were carried out using the following algorithm: “Asthma AND fertility” OR “Asthma AND subfertility” OR “Asthma AND infertility” OR “Asthma AND parity” OR “Asthma AND age at pregnancy”.
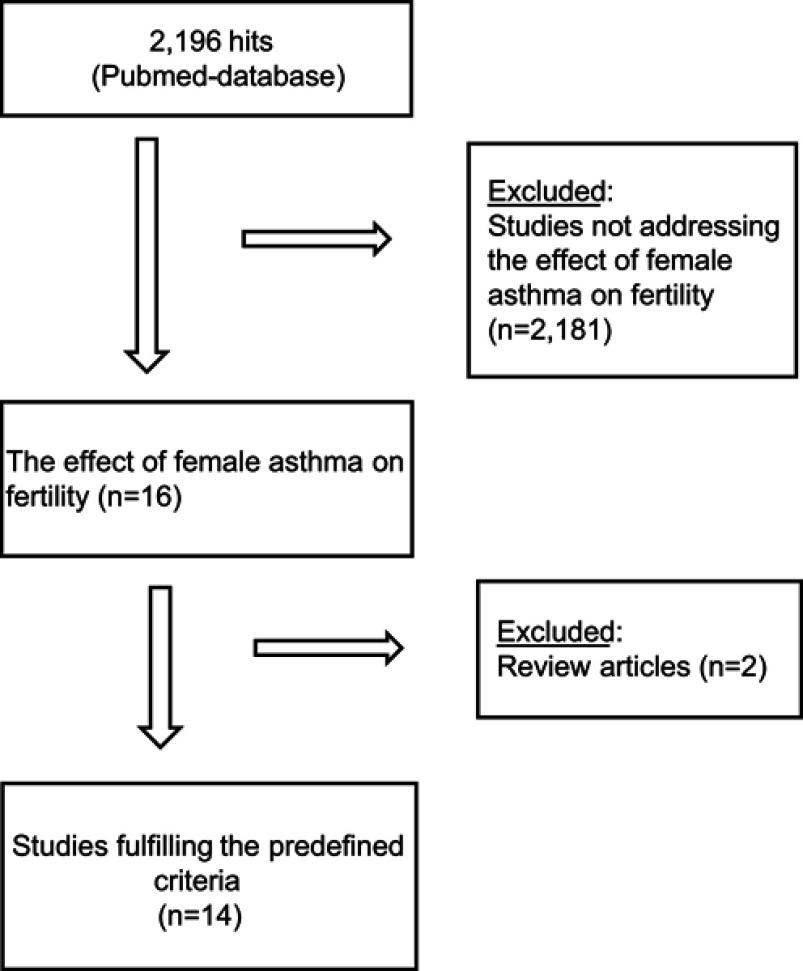
Publications were included if: 1) investigating the effect of female asthma on fertility, 2) reporting observations from original studies, 3) database or cohort studies (either prospective or retrospective in design), but excluded if: 1) reporting other reasons for impaired fertility, 2) non-original research paper, ie, reviews, and 3) non-English paper; further details are given in Figure 1.
Figure 1.
Consort diagram showing the study selection process.
Results
The search algorithm provided 2196 hits of which 14 studies fulfilled the predefined criteria and were included in the present review (Figure 1). Six studies (n=517,613) investigated the association between number of offspring and asthma, three studies (n=21,112) investigated the association between TTP and asthma, and five studies (n=1,039,893) investigated subfertility and the need of fertility treatment in asthmatic women.
Number of offspring in women with asthma and/or atopy
In 1997, based on the International Study of Asthma and Allergy in Children study, Nilsson et al,13 investigated the number of offspring among atopic and non-atopic mothers. Two groups of children were included, group 1 comprised 2338 children aged 13–14 years and group 2 comprised 1329 children 7 years of age. A questionnaire focusing on family history, allergy, smoking habits, and living conditions was completed by the parents. Maternal atopy was defined as an affirmative response to questions related to asthma, allergic rhinitis, hay fever and/or itching flexural dermatitis, using both terms of allergic rhinitis and hay fever. The study revealed that atopic mothers had more children than non-atopic mothers (2.18 children vs 1.97 children, p<0.001, in group 1 and 2.17 children vs 1.99 children, p<0.017, in group 2).
Based on data from the European Community Health Survey (ECRHS), Sunyer et al,14 assessed the association between number of offspring and atopy. ECRHS is an international survey carried out during 1991–1992 in randomly selected individuals from the general population aged 20–44 years in 16 countries (ECRHS-I), with a follow-up survey (ECRHS-II) carried out in 1999–2002. Out of 4580 women participating in ECRHS-I, a total of 2844 women (62.1%) completed the follow-up questionnaire in ECRHS-II. Atopy was defined as the presence of specific IgE toward house dust mite, cat dander, timothy grass, and/or Cladosporium herbarum, and asthma was defined based on affirmative responses to respiratory symptoms obtained from the International Union Against Tuberculosis and Lung Disease questionnaire.15,16 At baseline, the number of offspring was higher in non-atopic women than in atopic women (1.38 and 1.19, p<0.01). At the follow-up, there was no difference in the number of offspring between non-atopic and atopic women (1.79 and 1.75, p=0.35).
In a register-based twin study using data from the Danish Twin Registry, Gade et al,17 investigated if asthma affected TTP and fertility. Asthma was defined as an affirmative answer to the question “Have you ever had asthma?”. Allergy was defined as an affirmative answer to the question “Have you ever had allergy?”. A total of 15,250 females answered the questionnaire of whom 955 reported a history of asthma. Approximately 8000 women did not respond to the questions related to fertility, with non-responders being younger than the responders (22.3 vs 32.0 years). Gade et al,17 found no difference in the number of offspring in women with and without asthma (1.86 vs 1.83; p=0.51). In contrast to previous observations, the study showed that females with asthma conceived earlier in life (23.7 vs 24.5 years; p<0.001) and were younger when giving birth for the first time (25.5 vs 26.2 years; p=0.001) compared to females without asthma.
In a population study based on a primary care database from general practices across England and Wales, Tata et al,18 investigated the fertility rates in females aged 15–44 years. Females were included from 1994 to 2004 if they had a diagnosis of allergic disease, including asthma, hay fever, and eczema (n=491,516). In total, 13%, 14%, and 12%, respectively, had a diagnosis of asthma, eczema, and hay fever. The study revealed that females with asthma had a fertility rate of 53.0 livebirths per 1000-person years vs 52.3 livebirths per 1000-person years in females without asthma (adjusted fertility rate ratio (FRR) =1.02, 95% CI: 1.00–1.04). Furthermore, the study also showed that females with asthma aged 20–29.9 years had more children (20–24.9 years FRR =57.8, 95% CI: 55.6–60.1; 25–29.9 years FRR =89.3, 95% CI: 86.4–92.3) compared to non-asthmatic females (20–24.9 years FRR =49.1, 95% CI: 48.2–50.0; 25–29.9 years FRR =79.4, 95% CI: 78.3–80.5) and the fertility rates were higher in females with eczema (59.4 vs 51.0 livebirths per 1000 person years, adjusted FRR =1.15, 95% CI: 1.13–1.17) and hay fever (57.7 vs 51.5 livebirths per 1000 person years, crude FRR =1.08, 95% CI: 1.06–1.10) compared to females without eczema and hay fever, suggesting no impairment of fertility in females with a doctor diagnosis of asthma, hay fever, or eczema.
Karmaus et al,3 investigated the association of maternal atopy and number of offspring in two different surveys based on the German part of the European Studies on Infertility and Subfecundity. In one survey, termed the pregnancy-based survey, females admitted to maternity ward in 1992 (n=1318) filled-in self-administered questionnaires. In the other survey conducted from 1991 to 1992, termed the population-based survey, women aged 25–44 (n=1531) were identified using population registers and interviewed by trained interviewers using the same questionnaire supplemented by additional questions relating to prior pregnancies. The study revealed that females with atopic manifestations as asthma, hay fever, or atopic eczema had less children compared to females without atopic manifestations in both the pregnancy-based survey (OR=0.75, 95% CI: 0.57–0.98) and the population-based survey (OR=0.79, 95% CI: 0.63–0.99). The proportion of mothers with atopic manifestations was higher in the population-based survey than the pregnancy-based survey (29.5% vs 21.0, respectively).
In three concurrent cohorts, Sunyer et al,2 studied maternal atopy and parity. They recruited mothers (n=1487) and their children to the Asthma Multicenter Infants Cohort Study in Ashford, Kent, UK (1994–1995), Menorca Island, Spain (1997–1998), and Barcelona, Spain (1996–1998). Atopy was identified by skin prick tests and information on different variables, eg, having asthma and total number of live children, was obtained by questionnaires. Asthma and hay fever, respectively, were defined as an affirmative answer to the question “Have you ever had asthma?” and “Have you ever had hay fever or allergic rhinitis?”. The studies revealed that atopic women had less children than non-atopic women (p for trend =0.002) whereas no relation was found between number of offspring and maternal asthma (p for trend =0.43).
The results on the number of offspring in women with asthma and/or atopy are conflicting. Two studies found an increase in number of offspring in atopic women compared to non-atopic women,13,18 two studies found no difference,14,17 one study found a decrease in number of offspring in women with asthma or atopic symptoms,3 and lastly one study found that atopic women had less children than healthy women though no relation between asthma and number of offspring was found.2 Only one of the six studies had a doctor diagnosis of asthma or allergy from a database,18 whereas the remaining studies2,3,13,14,17 relied on questionnaires, of which at least three used affirmative answers to questions like “Have you ever had asthma” or “Have you ever had allergy”.2,13,17 Two of the studies identified atopy by the presence of specific IgE or positive skin prick tests,2,14 and one study used trained interviewers.3 The discrepancy in results is therefore likely to be caused by differences in methodology, as registered diagnosis and laboratory test are likely to be more reliable compared to questionnaire. The difference in observations in the two surveys by Sunyer et al,14 could be due to the fact that data were missing for more than one-third of the women participating in the first survey and only 62% of the women participated in the follow-up survey. Furthermore, atopic women were younger at baseline than the non-atopic women. Additionally, Gade et al,17 also had a high proportion of non-responders that potentially can bias the results. The non-responders were younger than the responders, and the study found that women with asthma conceived earlier than non-asthmatic women. It could, therefore, be argued that the high number of non-responders was due to a lower age of the women. Presently, it is, therefore, difficult to draw valid conclusions with regard to the impact of female asthma and/or atopy on total number of offspring.
Time to pregnancy
In the register-based twin study (described above), Gade et al,17 also investigated TTP and found TTP to be significantly prolonged in asthmatic females compared to non-asthmatic females (27.0% vs 21.6%, p=0.009). In asthmatic females >30 years of age, TTP was even more prolonged (32.2% vs 24.9%, p=0.04). Furthermore, asthmatic females not prescribed asthma therapy had a significantly increased risk of prolonged TTP compared to non-asthmatic females (30.5% vs 21.6%, p=0.0004), and the same was found for asthmatic females prescribed inhaled corticosteroids (ICS) compared to non-asthmatics (33.0% vs 21.6%, p=0.003). However, asthmatics receiving any kind of asthma treatment tended to have shorter TTP than untreated asthmatics though this did not reach statistical significance (23.8% vs 30.5%, p=0.134). Furthermore, the study revealed that females with allergy had the same TTP as the general population (21.2% vs 21.6%; OR 0.91, 95% CI 0.76–1.10) and females with asthma and allergy had a slightly, not statistically significantly, prolonged TTP (26.5% vs 21.6%; OR 1.28, 95% CI 0.96–1.72).
In a clinical observational study, Gade et al,19 investigated women (n=245) with unexplained infertility who were undergoing fertility treatments from 2011 to 2013. At a screening visit, the women were interviewed and objectively assessed for asthma and allergy including lung function test, methacholine and/or mannitol challenge test, measurement of exhaled nitric oxide fraction, skin prick test, and blood samples. Of the 245 women, 81 were found to have current asthma. The study revealed that asthmatic women had a significantly prolonged TTP compared to non-asthmatics (55.6 months vs 32.3 months, p<0.001), also after adjusting for age, body mass index (BMI), former smoking, age at menarche, previous pregnancies/births, semen quality and motility, and number of treatment cycles received in fertility treatment (p=0.003). Females >35 years of age were less likely to conceive compared to females <35 years of age (25.9 vs 61.4%, p<0.001). There was no significant difference in TTP in women with treated asthma vs women with untreated asthma (HR 1.13, 95% CI 0.60–2.15, p=0.706).
In the Screening for Pregnancy endpoints study, Grzeskowiak et al,20 recruited healthy nulliparous women (n=5617) and investigated the impact of asthma and asthma medication on fecundability and TTP. The study was a multicenter multinational prospective cohort study carried out in Auckland (New Zealand), Adelaide (Australia), Cork (Ireland), Manchester and London (United Kingdom) and the women were recruited between November 2004 and February 2011. Asthma was defined by the question “Have you been diagnosed with asthma by a doctor?” Of the 5617 included women, 1106 reported doctor-diagnosed asthma and these women were divided into subgroups classified as former asthmatics (doctor diagnosed, but no symptoms and no asthma medication within the last 12 months) and current asthmatics (doctor diagnosed with symptoms and use of asthma reliever or controller medication within the last 12 months), with the latter group further sub-divided based on type of asthma medication into intermittent asthma (use of reliever medication, ie, short-acting β2-agonist, only) and persistent asthma (use ofICS with or without long-acting β-agonists. TTP was self-reported and defined as the duration of pre-conception sex (in months) without the use of contraceptives and subfertility was defined as TTP >12 months. Grzeskowiak et al, reported that the group of current asthmatics using only reliever therapy (n=421) had a fecundability odds ratio (FORs) of 0.85 (95% CI 0.75–0.96) compared to non-asthmatics, the group of current asthmatics using controller medication (n=235) had a FORs of 0.98 (95% CI 0.84–1.15) and former asthmatics (n=450) had a FORs of 1.00 (95% CI 0.89–1.13) compared to non-asthmatics. Their findings revealed a reduction in fecundity, or prolonged TTP, (FORs <1) in asthmatic women only using rescue medication for asthma compared to non-asthmatic women. On the other hand, no significant difference in fecundity was found between women with former asthma or current asthmatic women on controller medication compared to non-asthmatic women. However, the women with both former and current asthma on controller medication were younger, had a higher BMI, were more likely to smoke, be of Caucasian ethnicity and have a lower socioeconomic status. Furthermore, the self-reported diagnosis of asthma and TTP may be considered as a weakness, whereas objective data on asthma medication, although not reported in detail, is likely to strengthen the identification of women with asthma.
In both studies, Gade et al,17,19 found prolonged TTP in asthmatic women compared to non-asthmatic women. In contrast to Grzeskowiak et al,20 the studies by Gade et al,17,19 did not observe any significant differences in TTP in women prescribed asthma medication. Unfortunately, the register-based twin study17 had a high proportion of non-responders, whereas in the observational study,19 the extensive examination of the participants is a clear strength of the study. However, as a single center study, the included women might not be fully representative according to factors such as socioeconomic class and lifestyle. The currently available studies suggest that women with asthma have prolonged TTP, which is probably not caused by potential adverse effects of asthma medication.
Subfertility and need for fertility treatment
In a case–control study, Grodstein et al,21 investigated the association between the use of pharmaceuticals and infertility in the United States and Canada. The study focused on women with ovulatory dysfunction as a cause of infertility (n=597) compared to women admitted for delivery of a livebirth at hospitals (n=3833). Trained nurses interviewed the women and collected information on medical history, use of contraceptives, reproductive history and personal habits. The study revealed that women who had used asthma medication for more than 6 months had an increased risk of infertility (RR 1.7, 95% CI 0.7–3.5). The risk was 2.5 (95% CI 1.0–5.9) for women prescribed asthma medication before the age of 21 in contrast to 0.5 (95% CI 0.0–2.8) for women prescribed asthma medication at an older age. Furthermore, the study showed that the risk of ovulatory infertility was significantly increased in women using beta-agonists (RR 3.2, 95%CI 0.8–11) but only slightly increased in women treated with theophylline (RR 1.2, 95% CI 0.3–3.2).
In the clinical study (described above) of women with unexplained infertility by Gade et al,19 asthmatic women were less likely to conceive than non-asthmatic women, especially in women >35 years of age compared to women <35 years (25.9% vs 61.4%, p<0.001). The pregnancy rates for non-asthmatic women >35 years of age was 56.7% and for women <35 years 80.6%. Furthermore, there was no difference in number of fertility treatments in the asthmatic group and the non-asthmatic group (p=0.862).
In a register study based on the Swedish Medical Birth Register from 1995 to 2004, Källén et al,22 investigated the use of anti-asthmatic drugs in the first trimester of pregnancy and during pregnancy. The study included women that reported the use of anti-asthmatic drugs early in pregnancy (n=24,368), women prescribed anti-asthmatic drugs later in pregnancy (n=7778), with all women (n=860,215) used as controls. Information regarding subfertility was obtained from the first prenatal visit to the midwife. The study revealed that women using anti-asthmatic drugs had experienced a period of subfertility and the association was stronger for women receiving drugs later during pregnancy than for women who used drugs early in pregnancy (OR 1.45, 95% CI 1.34–1.57 vs OR 1.19, 95% CI 1.14–1.25), however, the pattern of drug use for asthma was not reported in detail.
In a retrospective population-based study, Sheiner et al,23 investigated singleton pregnancies in mothers with and without asthma based on deliveries in southern Israel during the years 1988–2002. The diagnosis of asthma was based on the patient’s chart and patients with less than three visits in the prenatal care were excluded. A total of 139,168 singleton deliveries were included during the period and 1.4% were deliveries of asthmatic mothers. The study revealed that asthmatic mothers had significantly more fertility treatments compared to non-asthmatic mothers (3.3% vs 1.8%, p<0.001). The study also found that the maternal age was significantly higher in asthmatic mothers compared to the non-asthmatic mothers (29.1±5.7 vs 28.4±5.8, p<0.001).
A very recent case–control study by Vejen Hansen et al,24 explored the association between asthma and need for fertility treatment among women with life births. The sample comprised women with asthma (n=932) and non-asthmatic matched controls (n=2757), of whom 12% (n=114) and 8% (n=212), respectively, had fertility treatment (OR 1.67, 95% CI 1.32–2.13; p<0.001). Furthermore, this association between female asthma and need for fertility treatment remained significant after adjusting for age, BMI, and smoking habits; and subdividing the cohort according to age showed that in women ≥35 years, 25% (n=63) and 13% (n=82) among case and controls, respectively, had fertility treatment (OR 2.12, 95% CI 1.47 to 3.07; p<0.001). Of note is that 64% of the enrolled women with asthma were prescribed ICS either as monotherapy or in combination with a long-acting beta2-agonist (22% of the sample), suggesting that the majority of the sample had mild to moderate asthma.
The current evidence seems somewhat contradicting, as Sheiner et al,23 found that asthmatic women had more fertility treatments than non-asthmatic women, whereas Gade et al,19 found no evidence of the need of more fertility treatments. However, compared to previous observations, that asthma affects up to 10% of the women of childbearing age,1 the proportion of asthmatic women in the study by Sheiner et al,23 is markedly lower. It could be hypothesized that there might be women with undiagnosed asthma included in the study which might bias the results. However, the study by Gade et al,19 recruited a selected group of women with unexplained infertility, and the observation of more need for fertility treatment among women with asthma by Sheiner et al,23 is strongly supported by the observations in the study by Vejen Hansen et al,24 Furthermore, in the study by Källén et al,22 the results are based on the women’s perceived experience of subfertility without any given definitions of subfertility. Overall, the current evidence points toward a higher need for fertility treatment among women with asthma, possibly irrespective of severity of the disease.
Discussion
The present review revealed that two out of six studies looking at number of offspring reported that women with asthma or atopic symptoms had less children than healthy women.2,3 Three studies found no difference in number of offspring14,17,18 and one study found that atopic women had more children than non-atopic women.13 In the investigation of TTP in asthmatic women, all three studies included found prolonged TTP17,19,20 but found conflicting results regarding the effect of asthma medication on TTP as two studies found no significant difference in the prolonged TTP in treated asthmatic women compared to untreated asthmatic women,17,19 and one study20 found no significant difference in TTP in asthmatic women prescribed controller medication compared to non-asthmatic women. In the need of fertility treatments, the included studies19,23,24 reported different outcomes. One study19 found no difference in the number of fertility treatments in asthmatic and non-asthmatic women, whereas the two other studies reported that asthmatic women had significantly more fertility treatments.23,24 One study reported that women using asthma medication reported having experienced a period of subfertility.21
Reviewing the available evidence reveals inconsistencies in findings. First of all, there is a variation in the definition of atopy and asthma. Some studies use affirmative answers to questions related to previous diagnoses and/or symptoms,3,13,17,20,21 while other base the diagnosis on the women’s medical records or objective tests18,19,22–24 or a combination of tests and questions.2,14 Second of all, there is a difference in how specific a disease is defined, some studies address women with atopic manifestations,13 others asthma17,20–24 and some look at both asthma and atopic manifestations.2,3,14,17–19 Further studies of more well-characterized cohorts are, therefore, needed in order to obtain more accurate information on the association between asthma and/or atopic disease and fertility.
Furthermore, there also seem to be inconsistencies in the observations with regard to atopic and non-atopic asthma, as the studies have reported that atopic asthma does not affect fertility, whereas non-atopic asthma tends to have a negative effect on fertility. At present, the underlying mechanisms for these observations are unknown, but it may be caused by differences in nature of inflammation, and the observations, therefore, clearly calls for further studies addressing the possible association between type of inflammation, including systemic inflammation, possible inflammatory changes in the uterus together with the effect of levels of VEGF, in women with atopic and non-atopic asthma and fertility.
In conclusion, although the studies have produced conflicting results, the presently available studies clearly suggest an association between female asthma and a reduction in fertility, not least in women toward the end of the reproductive age and perhaps also non-atopic asthma, although the evidence so far does not clearly answer the question of whether or not female asthma and/atopy has an impact on total number of offspring. The underlying mechanisms are largely unknown and further investigation needs to be done on how female asthma affects the reproductive organs and fertility, also in relation to the level of asthma therapy. Furthermore, studies on interventions aiming at reducing probably both airway and systemic inflammation, including in the uterus, are clearly needed in order to improve fertility in women with asthma.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Virchow JC. Asthma and pregnancy. Semin Respir Crit Care Med. 2012;33(6):630–644. doi: 10.1055/s-0032-1326961 [DOI] [PubMed] [Google Scholar]
- 2.Sunyer J, Anto JM, Harris J, et al. Maternal atopy and parity. Clin Exp Allergy. 2001;31(9):1352–1355. [DOI] [PubMed] [Google Scholar]
- 3.Karmaus W, Eneli I. Maternal atopy and the number of offspring: is there an association? Pediatr Allergy Immunol. 2003;14(6):470–474. [DOI] [PubMed] [Google Scholar]
- 4.Gade EJ, Thomsen SF, Lindenberg S, Macklon NS, Backer V. Lower values of VEGF in endometrial secretion are a possible cause of subfertility in non-atopic asthmatic patients. J Asthma. 2015;52(4):336–342. doi: 10.3109/02770903.2014.966915 [DOI] [PubMed] [Google Scholar]
- 5.Ince-Askan H, Dolhain RJ. Pregnancy and rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2015;29(4–5):580–596. doi: 10.1016/j.berh.2015.07.001 [DOI] [PubMed] [Google Scholar]
- 6.Provost M, Eaton JL, Clowse ME. Fertility and infertility in rheumatoid arthritis. Curr Opin Rheumatol. 2014;26(3):308–314. doi: 10.1097/BOR.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 7.Ban L, Tata LJ, Humes DJ, Fiaschi L, Card T. Decreased fertility rates in 9639 women diagnosed with inflammatory bowel disease: a United Kingdom population-based cohort study. Aliment Pharmacol Ther. 2015;42(7):855–866. doi: 10.1111/apt.13354 [DOI] [PubMed] [Google Scholar]
- 8.Hashash JG, Kane S. Pregnancy and inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2015;11(2):96–102. [PMC free article] [PubMed] [Google Scholar]
- 9.McConnell RA, Mahadevan U. Pregnancy and the patient with inflammatory bowel disease: fertility, treatment, delivery, and complications. Gastroenterol Clin North Am. 2016;45(2):285–301. doi: 10.1016/j.gtc.2016.02.006 [DOI] [PubMed] [Google Scholar]
- 10.Tavernier N, Fumery M, Peyrin-Biroulet L, Colombel JF, Gower-Rousseau C. Systematic review: fertility in non-surgically treated inflammatory bowel disease. Aliment Pharmacol Ther. 2013;38(8):847–853. doi: 10.1111/apt.12478 [DOI] [PubMed] [Google Scholar]
- 11.van der Woude CJ, Ardizzone S, Bengtson MB, et al. The second European evidenced-based consensus on reproduction and pregnancy in inflammatory bowel disease. J Crohns Colitis. 2015;9(2):107–124. doi: 10.1093/ecco-jcc/jju006 [DOI] [PubMed] [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 13.Nilsson L, Kjellman NLM, Löfman O, Björkstén B. Parity among atopic and non‐atopic mothers. Pediatr Allergy Immunol. 1997;8(3):134–136. [DOI] [PubMed] [Google Scholar]
- 14.Sunyer J, Anto JM, Plana E, et al. Maternal atopy and changes in parity. Clin Exp Allergy. 2005;35(8):1028–1032. doi: 10.1111/j.1365-2222.2005.02300.x [DOI] [PubMed] [Google Scholar]
- 15.Burney P, Chinn S. Developing a new questionnaire for measuring the prevalence and distribution of asthma. CHEST. 1987;91(6):79S–83S. doi: 10.1378/chest.91.6_Supplement.79S [DOI] [PubMed] [Google Scholar]
- 16.Burney P, Luczynska C, Chinn S, Jarvis D. The European community respiratory health survey. Eur Respir J. 1994;7(5):954–960. [DOI] [PubMed] [Google Scholar]
- 17.Gade EJ, Thomsen SF, Lindenberg S, Kyvik KO, Lieberoth S, Backer V. Asthma affects time to pregnancy and fertility: a register-based twin study. Eur Respir J. 2014;43(4):1077–1085. doi: 10.1183/09031936.00148713 [DOI] [PubMed] [Google Scholar]
- 18.Tata LJ, Hubbard RB, McKeever TM, et al. Fertility rates in women with asthma, eczema, and hay fever: a general population-based cohort study. Am J Epidemiol. 2007;165(9):1023–1030. doi: 10.1093/aje/kwk092 [DOI] [PubMed] [Google Scholar]
- 19.Gade EJ, Thomsen SF, Lindenberg S, Backer V. Fertility outcomes in asthma: a clinical study of 245 women with unexplained infertility. Eur Respir J. 2016;47(4):1144–1151. doi: 10.1183/13993003.01389-2015 [DOI] [PubMed] [Google Scholar]
- 20.Grzeskowiak LE, Smithers LG, Grieger JA, et al. Asthma treatment impacts time to pregnancy: evidence from the international SCOPE study. Eur Respir J. 2018;51:2. doi: 10.1183/13993003.02035-2017 [DOI] [PubMed] [Google Scholar]
- 21.Grodstein F, Goldman MB, Ryan L, Cramer DW. Self-reported use of pharmaceuticals and primary ovulatory infertility. Epidemiology. 1993;4(2):151–156. [DOI] [PubMed] [Google Scholar]
- 22.Kallen B, Otterblad Olausson P. Use of anti-asthmatic drugs during pregnancy. 1. Maternal characteristics, pregnancy and delivery complications. Eur J Clin Pharmacol. 2007;63(4):363–373. doi: 10.1007/s00228-006-0257-1 [DOI] [PubMed] [Google Scholar]
- 23.Sheiner E, Mazor M, Levy A, Wiznitzer A, Bashiri A. Pregnancy outcome of asthmatic patients: a population-based study. J Matern Fetal Neonatal Med. 2005;18(4):237–240. doi: 10.1080/14767050500260616 [DOI] [PubMed] [Google Scholar]
- 24.Hansen AV, Ali Z, Malchau SS, Blafoss J, Pinborg A, Ulrik CS. Fertility treatment among women with asthma – a case-control study of 3689 women with live births. Eur Respir J. 2019;53(2). [DOI] [PubMed] [Google Scholar]