Abstract
Background
B7-H3 (CD276), an immune checkpoint molecule, regulates the tumor-immune microenvironment and controls the aggressiveness of various tumors. Although B7-H3 expression has been associated with the number of tumor-infiltrating FOXP3+ regulatory T cells, little information is available about this association in clear cell renal cell carcinoma (ccRCC).
Methods
Using 252 consecutive cases of ccRCC, we examined the association of B7-H3 expression in both the tumor cells and tumor vasculature with the number of tumor-infiltrating FOXP3+ cells and assessed whether the effects of B7-H3 expression on survival differ according to FOXP3+ cell number.
Results
High B7-H3 expression was observed in the tumor cells and tumor vasculature in 15% and 54% of ccRCC cases, respectively. High FOXP3+ cell number was positively associated with B7-H3 expression in both the tumor cells (odds ratio [OR] =2.93; P=0.0041) and tumor vasculature (OR=2.45; P=0.0007). Tumor cell B7-H3 expression was associated with increased disease-specific mortality in high FOXP3+ cell number group (hazard ratio [HR] =2.98; P=0.017), but not in low FOXP3+ group (P=0.71). Tumor vasculature B7-H3 expression was also associated with increased disease-specific mortality in high FOXP3+ cell number group (HR=4.86; P=0.0025), but not in low FOXP3+ group (P=0.48).
Conclusion
We demonstrate that B7-H3 expression in both tumor cells and the tumor vasculature is positively associated with FOXP3+ cell number. Such expression is also associated with increased mortality in high FOXP3+ cell number group, but not in low FOXP3+ cell number group. These findings suggest that B7-H3-expressing ccRCCs may exert tumor-promoting immunity by interacting with FOXP3+ regulatory T cells in the tumor microenvironment.
Keywords: immune checkpoint inhibitor, immunotherapy, prognosis, renal cancer, TIL
Introduction
Immunotherapy has emerged as a promising strategy for the treatment of various malignancies, including renal cell carcinoma (RCC).1–8 RCC is an immunosensitive cancer that is generally responsive to immune checkpoint inhibitors; however, the mechanism by which RCC exhibits susceptibility to immunotherapies is unclear.3–5,9,10 To improve outcomes for patients with RCC, additional effort should be focused on the identification of the mechanisms underlying the tumor-immune microenvironment and new molecular targets for the development of efficacious strategies against RCC.
B7-H3 plays a role in the regulation of T-cell-mediated immune responses against cancer; its expression is associated with increased mortality in patients with RCC.11–16 Recent evidence demonstrates that B7-H3 expression is positively associated with the density of tumor-infiltrating FOXP3+ regulatory T cells,17,18 which help tumor cells evade immunosurveillance.19 However, no prior studies have examined the relationship between B7-H3 expression and the numbers of FOXP3+ T cells or whether the association of B7-H3 expression with survival differs according to the density of FOXP3+ cells in cases of RCC.
In this study, using 252 consecutive cases of clear cell RCC (ccRCC), we examined the relationship between B7-H3 expression in both tumor cells and the tumor vasculature and the density of tumor-infiltrating FOXP3+ cells; we also determined whether the association between B7-H3 expression and survival differs according to FOXP3+ cell density.
Materials and methods
Study population
We enrolled 252 consecutive cases of ccRCC based on the availability of data on B7-H3 expression in both tumor cells and the tumor vasculature, the numbers of tumor-infiltrating FOXP3+ cells, and patient survival. The patients were Japanese and had undergone tumor resection between June 2005 and October 2010 at The Cancer Institute Hospital, Japanese Foundation for Cancer Research (JFCR), Tokyo, Japan. Patients were monitored until death or June 27, 2018. Pathological diagnoses were made by a urologic pathologist (K.I.), according to the 2016 WHO classification of renal cell tumors.20 All patients were pathologically staged according to the AJCC-TNM staging system, 8th edition.21 This study was approved by the institutional review board of JFCR, and written informed consent was obtained from all patients of this study. The study was conducted in accordance with the ethical standards of the Declaration of Helsinki.
Immunohistochemical analyses
Using formalin-fixed, paraffin-embedded tissue specimens that had been collected for the pathological diagnoses of ccRCC, we constructed tissue microarrays (TMAs), as previously described.22 In brief, we punched sections from donor paraffin blocks using a 2-mm-diameter coring needle and transferred the tissue to recipient paraffin blocks using a tissue microarrayer (KIN-1, Azumaya, Tokyo, Japan). For each case, a urologic pathologist (K.I.) selected one 2-mm-diameter area, showing the tumor’s most representative histology.22
B7-H3 immunostaining was performed, as previously described.23 Sections with a thickness of 4 μm from the TMAs were immunostained for B7-H3 with an anti-B7-H3 mouse monoclonal antibody (clone: BD/5A11; Daiichi Sankyo Co., Ltd., Tokyo, Japan; diluted 1:400) using the Leica Bond III automated system (Leica Biosystems Melbourne Pvt., Ltd., Melbourne, Australia). The sections were incubated at pH 9 for 10 min at 100 °C. For both the positive and negative controls, we used a B7 subfamily cell array (provided by Daiichi Sankyo Co., Ltd.), which was composed of CHO-K1 cells overexpressing B7 subfamily members, including B7-H1 (also known as PD-L1), B7-H2, B7-H3, B7-H4, B7-1, or B7-2, as well as mock-transfected control cells, as previously described.23,24 Using this cell array, we verified the specificity and sensitivity of the anti-B7-H3 antibody (clone: BD/5A11).23,24 B7-H3 expression in tumor cells was measured, as described previously.23,24 The intensity of B7-H3 expression in tumor cell membranes was defined as absent–weak or moderate–strong. The percentages of tumor cells at both B7-H3 intensities were determined. Specimens were categorized into two groups based on the staining intensity and percentage of positive cells: low B7-H3 expression in tumor cells (moderate–strong <50%) and high B7-H3 expression (moderate–strong ≥50%). For B7-H3 expression in the tumor vasculature, absent–focal and moderate–diffuse expression levels were categorized as low and high, respectively (Figure 1A–C).
Figure 1.
(A–C) Immunohistochemical staining for B7-H3 in tumor cells and the tumor vasculature of clear cell renal cell carcinoma. (A) Representative tumor with low B7-H3 expression in tumor cells and the tumor vasculature. (B) Representative tumor with high B7-H3 expression in tumor cells. (C) Representative tumor with high B7-H3 expression in the tumor vasculature. (D and E) Immunohistochemical staining for FOXP3 in tumor-infiltrating cells in clear cell renal cell carcinoma. (D) Representative tumor with a low FOXP3+ cell density. (E) Representative tumor with a high FOXP3+ cell density. Scale bar, 100 µm.
FOXP3 immunostaining was also conducted using 4-μm-thick sections from the TMAs. The sections were immunostained for FOXP3 using an anti-FOXP3 mouse monoclonal antibody (clone: 236A/E7; Abcam, Cambridge, UK; diluted 1:100) using the Leica Bond III automated system. These sections were incubated at pH 9 for 20 min at 100 °C. Tumor-infiltrating FOXP3+ cells were counted using a 40× objective in five randomly selected fields, and the results were averaged. We selected the median value of the counts as the cutoff value to define high and low densities of FOXP3+ cells (Figure 1D and E).
Immunostaining results were interpreted by a urologic pathologist (K.I.) in a blinded manner. All samples were blindly examined by a second pathologist (G.A.). There was good concordance between the two observers, as verified by a kappa coefficient of 0.72 [95% confidence interval (CI) =0.61–0.83; P<0.0001] for B7-H3 expression in tumor cells, 0.79 (95% CI=0.72–0.87; P<0.0001) for B7-H3 expression in the tumor vasculature, and 0.73 (95% CI=0.65–0.81; P<0.0001) for tumor-infiltrating FOXP3+ cell density.
Statistical analyses
Statistical analyses were conducted using JMP 12 software (SAS Institute Inc., Cary, NC, USA) and Excel 2016 software (Microsoft, Redmond, WA, USA). All tests were two sided, and P-values less than 0.05 were considered statistically significant. The kappa coefficient was calculated to assess the level of agreement between the two pathologists in the immunohistochemical analyses. To investigate the associations of B7-H3 expression levels with clinicopathological characteristics, the chi-square or Fisher’s exact test was appropriately performed. The relationships between the clinicopathological features and B7-H3 expression levels were also assessed by univariable and multivariable logistic regression models. For the multivariable analysis, we initially included age (<65 years vs ≥65 years), sex (male vs female), tumor size (<40 mm vs ≥40 mm), Fuhrman grade (1–2 vs 3–4), pathological stage (I–II vs III–IV), and FOXP3+ cell density (low vs high). For survival analyses, survival duration was defined as the interval between the date of tumor resection and the date of death or last follow-up. The Kaplan–Meier method and log-rank test were used for survival analysis. For the analysis of disease-specific mortality, deaths as a result of other causes were censored. To adjust for confounding variables, we used Cox proportional hazards regression models, and calculated hazard ratios (HRs) for mortality. The multivariable models initially included the same set of variables as in multivariable logistic regression analysis.
Results
B7-H3 expression in RCC
Among 252 cases of ccRCC, high tumor cell expression of B7-H3 was observed in 37 cases (15%), whereas high expression levels of B7-H3 in the tumor vasculature were observed in 137 cases (54%; Figure 1). Table 1 shows the clinicopathological characteristics of ccRCC, according to B7-H3 expression levels in both tumor cells and the tumor vasculature. B7-H3 expression in tumor cells was positively associated with the density of tumor-infiltrating FOXP3+ cells (P=0.0069). Similarly, B7-H3 expression in the tumor vasculature was positively associated with the density of tumor-infiltrating FOXP3+ cells (P=0.0015). High B7-H3 expression in the vasculature was also associated with large tumor size (P=0.013), advanced pathological stage (P=0.015), old age (P=0.029), and high Fuhrman grade (P=0.032).
Table 1.
Clinicopathological characteristics of clear cell renal cell carcinoma according to B7-H3 expression in tumor cells and the tumor vasculature
| Variables | N of samples (%) | B7-H3 expression in tumor cells | B7-H3 expression in tumor vasculature | |||||
|---|---|---|---|---|---|---|---|---|
| Low N=215 (85%) |
High N=37 (15%) |
P-values | Low N=115 (46%) |
High N=137 (54%) |
P-values | |||
| Age | 0.92 | 0.029 | ||||||
| <65 years | 148 (59%) | 126 (59%) | 22 (59%) | 76 (66%) | 72 (53%) | |||
| ≥65 years | 104 (41%) | 89 (41%) | 15 (41%) | 39 (34%) | 65 (47%) | |||
| Sex | 0.82 | 0.50 | ||||||
| Male | 187 (74%) | 159 (74%) | 28 (76%) | 83 (72%) | 104 (76%) | |||
| Female | 65 (26%) | 56 (26%) | 9 (25%) | 32 (28%) | 33 (24%) | |||
| Tumor size | 0.40 | 0.013 | ||||||
| <40 mm | 132 (52%) | 115 (53%) | 17 (46%) | 70 (61%) | 62 (45%) | |||
| ≥40 mm | 120 (48%) | 100 (47%) | 20 (54%) | 45 (39%) | 75 (55%) | |||
| Fuhrman grade | 0.059 | 0.032 | ||||||
| 1–2 | 195 (77%) | 171 (80%) | 24 (65%) | 96 (83%) | 99 (72%) | |||
| 3–4 | 57 (23%) | 44 (20%) | 13 (35%) | 19 (17%) | 38 (28%) | |||
| Pathological stage | 0.45 | 0.015 | ||||||
| I–II | 221 (88%) | 190 (88%) | 31 (84%) | 107 (93%) | 114 (83%) | |||
| III–IV | 31 (12%) | 25 (12%) | 6 (16%) | 8 (7.0%) | 23 (17%) | |||
| Performance status | 0.65 | 0.76 | ||||||
| 0 | 234 (96%) | 200 (96%) | 34 (94%) | 108 (96%) | 126 (95%) | |||
| ≥1 | 10 (4.1%) | 8 (3.9%) | 2 (5.6%) | 4 (3.6%) | 6 (4.6%) | |||
| FOXP3+ cell density | 0.0069 | 0.0015 | ||||||
| Low | 126 (50%) | 115 (53%) | 11 (30%) | 70 (61%) | 56 (41%) | |||
| High | 126 (50%) | 100 (47%) | 26 (70%) | 45 (39%) | 81 (59%) | |||
Logistic regression analysis to assess relationships with B7-H3 expression
To adjust for confounding variables, we conducted logistic regression analysis and assessed relationships with B7-H3 expression in tumor cells and the tumor vasculature (Table 2). In an independent manner, FOXP3+ cell density was positively associated with B7-H3 expression in both tumor cells [odds ratio (OR) =2.93, 95% CI=1.40–6.56; P=0.0041] and the tumor vasculature (OR=2.45, 95% CI=1.46–4.17; P=0.0007; Table 2).
Table 2.
The associations of B7-H3 expression with clinicopathological features in clear cell renal cell carcinoma
| B7-H3 expression | |||||
|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis* | ||||
| OR (95% CI) | P-values | OR (95% CI) | P-values | ||
| Factors to predict high B7-H3 expression in tumor cells | |||||
| FOXP3+ cell density | high (vs low) | 2.72 (1.31–5.99) | 0.0069 | 2.93 (1.40–6.56) | 0.0041 |
| Fuhrman grade | 3–4 (vs 1–2) | 2.11 (0.97–4.42) | 0.059 | 2.36 (1.07–5.09) | 0.034 |
| Tumor size (mm) | ≥40 (vs <40) | 1.35 (0.67–2.75) | 0.40 | ||
| Pathological stage | III–IV (vs I–II) | 1.47 (0.51–3.68) | 0.45 | ||
| Sex | male (vs female) | 1.10 (0.50–2.59) | 0.82 | ||
| Age (years) | <65 (vs ≥65) | 1.04 (0.51–2.14) | 0.92 | ||
| Factors to predict high B7-H3 expression in tumor vasculature | |||||
| FOXP3+ cell density | high (vs low) | 2.25 (1.36–3.75) | 0.0015 | 2.45 (1.46–4.17) | 0.0007 |
| Tumor size (mm) | ≥40 (vs <40) | 1.88 (1.14–3.13) | 0.013 | 2.01 (1.19–3.41) | 0.0084 |
| Age (years) | ≥65 (vs <65) | 1.76 (1.06–2.95) | 0.029 | 1.81 (1.07–3.10) | 0.027 |
| Pathological stage | III–IV (vs I–II) | 2.70 (1.20–6.67) | 0.015 | ||
| Fuhrman grade | 3–4 (vs 1–2) | 1.94 (1.06–3.66) | 0.032 | ||
| Sex | male (vs female) | 1.22 (0.69–2.14) | 0.50 | ||
Notes: *The multivariable model initially included age (<65 years vs ≥65 years), sex (male vs female), tumor size (<40 mm vs ≥40 mm), Fuhrman grade (1–2 vs 3–4), pathological stage (I–II vs III–IV), and FOXP3+ cell density (low vs high). A backward stepwise elimination with a threshold P-value of 0.05 was used to select variables in the final model.
Abbreviations: CI, confidence interval; OR, odds ratio.
B7-H3 expression and RCC mortality
Among the 252 patients with ccRCC, there were 58 deaths, including 41 disease-specific deaths, during a median follow-up period of 99 months.
We initially assessed the association of B7-H3 expression in tumor cells with survival. In the Kaplan–Meier analysis, high B7-H3 expression was associated with increased disease-specific death (log-rank P=0.012; Figure 2A) and overall mortality (log-rank P=0.050; Figure 2B). In a Cox regression univariable analysis, high B7-H3 expression was associated with increased disease-specific death (HR=2.37, 95% CI=1.13–4.62; P=0.024; Table 3). In a multivariate analysis, B7-H3 expression was not independently associated with mortality (P≥0.28).
Figure 2.
Kaplan–Meier curves for disease-specific survival (A) and overall survival (B) in patients with clear cell renal cell carcinoma according to B7-H3 expression in tumor cells. Kaplan–Meier curves for disease-specific survival (C) and overall survival (D) in patients with clear cell renal cell carcinoma according to B7-H3 expression in the tumor vasculature.
Table 3.
B7-H3 expression and patient mortality* in clear cell renal cell carcinoma, stratified by density of tumor-infiltrating FOXP3+ cells
| Disease-specific mortality | Overall mortality | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis** | Univariable analysis | Multivariable analysis** | ||||||
| HR (95% CI) | P-values | HR (95% CI) | P-values | HR (95% CI) | P-values | HR (95% CI) | P-values | ||
| All patients | |||||||||
| B7-H3 expression in tumor cells: low (N=215) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |||||
| B7-H3 expression in tumor cells: high (N=37) | 2.37 (1.13–4.62) | 0.024 | 1.67 (0.78–3.30) | 0.18 | 1.84 (0.95–3.32) | 0.068 | 1.44 (0.74–2.62) | 0.28 | |
| FOXP3+ cell density: low | |||||||||
| B7-H3 expression in tumor cells: low (N=115) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |||||
| B7-H3 expression in tumor cells: high (N=11) | 1.33 (0.21–4.72) | 0.71 | 1.66 (0.25–6.70) | 0.55 | 1.35 (0.32–3.90) | 0.64 | 1.67 (0.38–5.14) | 0.45 | |
| FOXP3+ cell density: high | |||||||||
| B7-H3 expression in tumor cells: low (N=100) | 1 (referent) | 1 (referent) | 1 (reference) | 1 (reference) | |||||
| B7-H3 expression in tumor cells: high (N=26) | 2.98 (1.23–6.91) | 0.017 | 1.51 (0.58–3.74) | 0.39 | 2.06 (0.93–4.28) | 0.074 | 1.30 (0.56–2.83) | 0.53 | |
| All patients | |||||||||
| B7-H3 expression in tumor vasculature: low (N=115) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |||||
| B7-H3 expression in tumor vasculature: high (N=137) | 2.48 (1.27–5.19) | 0.0069 | 1.45 (0.73–3.12) | 0.30 | 2.41 (1.38–4.42) | 0.0017 | 1.86 (1.05–3.45) | 0.035 | |
| FOXP3+ cell density: low | |||||||||
| B7-H3 expression in tumor vasculature: low (N=70) | 1 (referent) | 1 (referent) | 1 (referent) | 1 (referent) | |||||
| B7-H3 expression in tumor vasculature: high (N=56) | 1.42 (0.54–3.79) | 0.48 | 1.21 (0.45–3.28) | 0.71 | 1.64 (0.74–3.71) | 0.22 | 1.46 (0.65–3.31) | 0.36 | |
| FOXP3+ cell density: high | |||||||||
| B7-H3 expression in tumor vasculature: low (N=45) | 1 (referent) | 1 (referent) | 1 (reference) | 1 (reference) | |||||
| B7-H3 expression in tumor vasculature: high (N=81) | 4.86 (1.65–20.7) | 0.0025 | 1.93 (0.57–8.90) | 0.31 | 3.85 (1.61–11.4) | 0.0016 | 3.13 (1.28–9.38) | 0.010 | |
Notes: *Cox proportional hazards regression models were used to calculate the HR and 95% CI. **The multivariable model initially included age (<65 years vs ≥65 years), sex (male vs female), tumor size (<40 mm vs ≥40 mm), Fuhrman grade (1–2 vs 3–4), pathological stage (I–II vs III–IV), and FOXP3+ cell density (low vs high). A backward stepwise elimination with a threshold P-value of 0.05 was used to select variables in the final model.
Abbreviations: CI, confidence interval; HR, hazard ratio.
We next assessed the association of B7-H3 expression in the tumor vasculature with survival. In the Kaplan–Meier analysis, high B7-H3 expression in the tumor vasculature was associated with increased disease-specific death (log-rank P=0.0081; Figure 2C) and overall mortality (log-rank P=0.0021; Figure 2D). In the Cox regression univariable analysis, high B7-H3 expression in the tumor vasculature was associated with increased disease-specific death (HR=2.48, 95% CI=1.27–5.19; P=0.0069) and overall mortality (HR =2.41, 95% CI=1.38–4.42; P=0.0017; Table 3). In the multivariable analysis, high B7-H3 expression in the tumor vasculature was independently associated with increased overall mortality (HR=1.86, 95% CI=1.05–3.45; P=0.035).
B7-H3 expression, FOXP3+ cell density, and RCC mortality
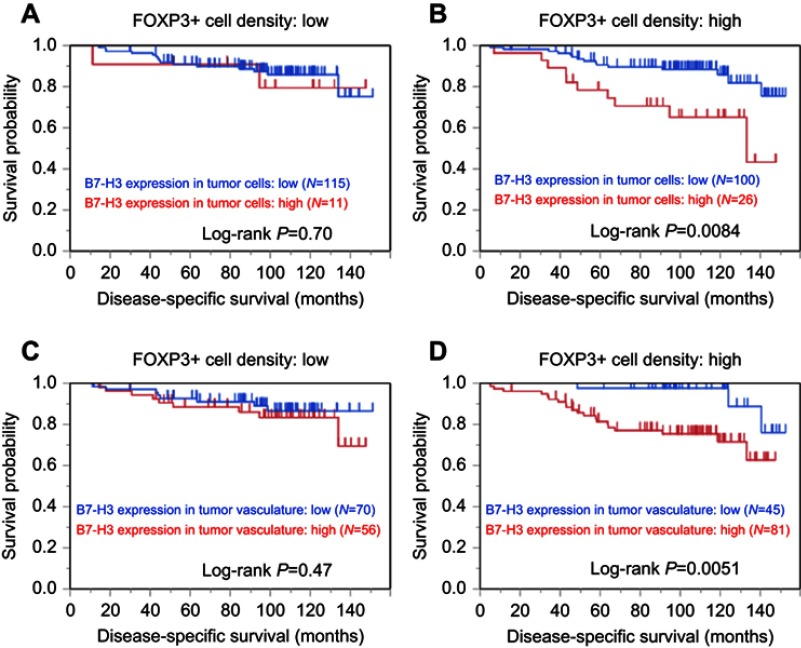
We examined the association between B7-H3 expression and mortality according to the density of tumor-infiltrating FOXP3+ cells (Figure 3 and Table 3). High B7-H3 expression in tumor cells was associated with increased disease-specific mortality in the high FOXP3+ cell density group (log-rank P=0.0084; Figure 3B) but not in the low cell density group (log-rank P=0.70; Figure 3A). High B7-H3 expression in the tumor vasculature was also associated with increased disease-specific death in the high FOXP3+ cell density group (log-rank P=0.0051; Figure 3D) but not in the low cell density group (log-rank P=0.47; Figure 3C). In a Cox regression univariable analysis, high B7-H3 expression in tumor cells was associated with increased disease-specific mortality in the high FOXP3+ cell density group (HR=2.98, 95% CI=1.23–6.91; P=0.017) but not in the low cell density group (P=0.71; Table 3). High B7-H3 expression in the tumor vasculature was also associated with increased disease-specific mortality in the high FOXP3+ cell density group (HR=4.86, 95% CI=1.65−20.7; P=0.0025) but not in the low cell density group (P=0.48; Table 3). High B7-H3 expression in the tumor vasculature was independently associated with increased overall mortality in the high FOXP3+ cell density group (HR =3.13, 95% CI=1.28−9.38; P=0.010) but not in the low cell density group (P=0.36).
Figure 3.
Kaplan–Meier curves for disease-specific survival in patients with clear cell renal cell carcinoma according to B7-H3 expression in tumor cells (A and B) and the tumor vasculature (C and D), stratified by the density of tumor-infiltrating FOXP3+ cells. (A and C) Low FOXP3+ cell density group. (B and D) High FOXP3+ cell density group.
Discussion
The expression of B7-H3, a regulator of T-cell-mediated immune responses, appears to control the aggressiveness of various malignancies, including RCC.11–16,25–27 Recent evidence suggests a positive association between tumor B7-H3 expression and the density of tumor-infiltrating FOXP3+ regulatory T cells.17,18 However, no prior studies have examined the association of B7-H3 expression with tumor-infiltrating FOXP3+ cells or whether the association between B7-H3 expression and patient survival differs according to tumor-infiltrating FOXP3+ cell density in RCC. We herein demonstrate a positive association of B7-H3 expression in both tumor cells and the tumor vasculature with the density of tumor-infiltrating FOXP3+ cells. Furthermore, high B7-H3 expression was associated with increased mortality in the high FOXP3+ cell density group but not in the low cell density group. Our results suggest the potential interplay between B7-H3 expression and FOXP3+ regulatory T cells in the tumor-immune microenvironment of RCC.
RCC is an immunosensitive tumor as shown by its spontaneous regression, high levels of tumor-infiltrating inflammatory cells, and susceptibility to immunotherapies.3–5,9,10 As with melanoma and non-small cell lung cancer (NSCLC),6–8 RCC is generally susceptible to immune checkpoint inhibitors.3–5,9,10 Such susceptibility in melanoma and NSCLC is thought to be attributed to the high mutational burden of these malignancies.28 On the other hand, the mutational load of RCC is not as high as that observed in other immunotherapy-susceptible tumor types;28 therefore, the reasons for its responsiveness to immune checkpoint blockades are unclear. Interactive associations between the immune checkpoint pathway and host immunity must be elucidated to develop an efficacious immunotherapeutic strategy for RCC.
B7-H3 is a member of the B7 family of immunoregulatory proteins, which includes PD-L1 (also known as B7-H1), and is thought to control tumor aggressiveness in various types of cancer, including RCC.11–16,25–27 B7-H3 has been linked to tumor angiogenesis,29,30 and its expression is associated with increased mortality in patients with RCC not only in the tumor cells but also in the tumor vasculature.14–16 Furthermore, B7-H3 has been known to be overexpressed during pathological, but not physiological, angiogenesis. While physiological functions of B7-H3 remain elusive, B7-H3 is highly expressed in the tumor vessels of renal, lung, colon, breast, endometrial, and ovarian cancers, but not in the blood vessels of their corresponding normal tissues.14,15,29,31 In RCC, anti-B7-H3 therapy may simultaneously target both the tumor and stromal components, resulting in enhanced therapeutic efficacy.31
B7-H3 plays a critical role in the suppression of T-cell-mediated antitumor immune responses, although conflicting evidence exists.11–13,32 B7-H3 inhibits the proliferation of both CD4+ and CD8+ T cells and reduces the production of effector cytokines (eg, IL-2 and interferon-gamma) by suppressing NFAT, NFKB, and AP-1 transcriptional activities.33,34 In B7-H3-deficient mice, Th1 (T helper 1)-mediated hypersensitivity and onset of experimental autoimmune encephalomyelitis (EAE) are induced, and treatment with a blocking anti-B7-H3 antibody worsens EAE.33,34 Moreover, administration of an anti-B7-H3 antibody during the induction phase enhances the severity of Th2 (T helper 2)-mediated experimental allergic conjunctivitis.35 Dendritic cell (DC)-associated B7-H3 induced by regulatory T cells impairs T cell stimulatory function.36 On the other hand, FOXP3 is a master regulator of the development and function of regulatory T cells and is regarded, to date, as the most specific and reliable marker of regulatory T cells.37–39 FOXP3+ regulatory T cells impede antitumor immunity;19 therefore, the targeting FOXP3+ regulatory T cells can be a potential strategy for the treatment of malignancies with high numbers of tumor-infiltrating FOXP3+ regulatory T cells.40,41 Studies have demonstrated that B7-H3 expression is positively associated with the density of tumor-infiltrating FOXP3+ regulatory T cells,17,18 which help tumor cells evade immunosurveillance.19 In NSCLC, Jin et al have reported a positive correlation between B7-H3 expression and FOXP3+ cell density as well as a differential association between B7-H3 expression and survival according to FOXP3+ cell density.17 Consistent with their findings, we demonstrated a positive relationship between B7-H3 expression and the density of tumor-infiltrating FOXP3+ cells as well as pronounced effects of B7-H3 expression on survival in RCC patients with a high FOXP3+ cell density. B7-H3 blockade, in combination with the targeting of FOXP3+ cells, might be an effective strategy for managing B7-H3-expressing RCC.
Limitations of this study deserve comments. First, the observational nature of the study makes it challenging to determine the causal association between B7-H3 expression and tumor-infiltrating FOXP3+ cells. However, mechanistic studies have shown that B7-H3 can regulate T-cell-mediated antitumor immunity.11–13, Second, we used TMAs to assess B7-H3 expression; therefore, intratumor heterogeneity can affect our results, although we used relatively large rods (2 mm in diameter). Third, there are no standardized methods to immunohistochemically evaluate B7-H3 expression status and the density of tumor-infiltrating FOXP3+ cells, which may alter our results. However, two pathologists conducted blinded and independent assessments of B7-H3 expression status and FOXP3+ cell density with good interobserver agreement. Fourth, we recognize the inherent limitations in assessing only the tumor cells, tumor vasculature, and FOXP3+ cells because the tumor-immune microenvironment comprises neoplastic cells, immune cells, fibroblasts, endothelial cells, extracellular matrix, proteases, and cytokines. Therefore, comprehensive studies are required to dissect the complex crosstalk between these components. Fifth, data regarding treatment effect on advanced-stage tumors were not available in the current study. Sixth, our database was created retrospectively. Seventh, the total number of patients (N=252) was not large enough; therefore, statistical power was limited, particularly for subgroup analyses. Finally, this study lacks generalizability because we evaluated Japanese patients at a single cancer hospital. Thus, further investigations with larger numbers of patients of other ethnicities are required to confirm our findings.
Conclusion
The current study demonstrates a positive association between B7-H3 expression and the number of tumor-infiltrating FOXP3+ cells as well as the pronounced effects of B7-H3 expression on survival in RCC patients with a high FOXP3+ cell density. These results suggest that the B7-H3 signaling pathway plays a critical role in immunosuppression via interactions with FOXP3+ regulatory T cells in the RCC microenvironment. On the basis of our findings, the targeting of B7-H3 and FOXP3+ regulatory T cells may serve as a promising strategy for the treatment of B7-H3-expressing RCC.
Acknowledgment
The authors thank Mr. Motoyoshi Iwakoshi, Ms. Miyuki Kogure, Ms. Tomoyo Kakita, and Ms. Naoko Takahashi for their technical assistance, and Ms. Yuki Takano and Ms. Chikako Yoshida for their secretarial expertise. The authors thank Daiichi Sankyo Co., Ltd. for providing us with anti-B7-H3 antibody (clone: BD/5A11) and B7-H3 subfamily cell array. This study was supported in part by Suzuki Foundation for Urological Medicine.
Abbreviations
ccRCC, clear cell renal cell carcinoma; CI, confidence interval; EAE, experimental autoimmune encephalomyelitis; HR, hazard ratio; JFCR, Japanese Foundation for Cancer Research; NSCLC, non-small cell lung cancer; OR, odds ratio; RCC, renal cell carcinoma; Th1, T helper 1; Th2, T helper 2; TMA, tissue microarray.
Disclosure
Kentaro Inamura received a research grant from Konica Minolta, Inc. Takeshi Yuasa received remuneration for a lecture from Astellas Pharma, Sanofi Japan, Pfizer Japan, Novartis Pharma Japan, Ono Pharma, Bristol-Myers Squibb Japan, and Daiichi Sankyo Co., Ltd. Yuichi Ishikawa received research grants from Daiichi Sankyo Co., Ltd. and Chugai Pharmaceutical Co., Ltd. and is a consultant for Fujirebio Inc. The authors report no other conflicts of interest in this work.
References
- 1.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geissler K, Fornara P, Lautenschlager C, Holzhausen HJ, Seliger B, Riemann D. Immune signature of tumor infiltrating immune cells in renal cancer. Oncoimmunology. 2015;4(1):e985082. doi: 10.4161/2162402X.2014.985082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 Study. J Clin Oncol. 2017;35(34):3851–3858. doi: 10.1200/jco.2016.72.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tie Y, Ma X, Zhu C, et al. Safety and efficacy of nivolumab in the treatment of cancers: A meta-analysis of 27 prospective clinical trials. Int J Cancer. 2017;140(4):948–958. doi: 10.1002/ijc.30501 [DOI] [PubMed] [Google Scholar]
- 7.von Pawel J, Bordoni R, Satouchi M, et al. Long-term survival in patients with advanced non-small-cell lung cancer treated with atezolizumab versus docetaxel: results from the randomised phase III OAK study. Eur J Cancer. 2019;107:124–132. doi: 10.1016/j.ejca.2018.11.020 [DOI] [PubMed] [Google Scholar]
- 8.Namikawa K, Kiyohara Y, Takenouchi T, et al. Efficacy and safety of nivolumab in combination with ipilimumab in Japanese patients with advanced melanoma: an open-label, single-arm, multicentre phase II study. Eur J Cancer. 2018;105:114–126. doi: 10.1016/j.ejca.2018.09.025 [DOI] [PubMed] [Google Scholar]
- 9.Chevrier S, Levine JH, Zanotelli VRT, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169(4):736–749 e718. doi: 10.1016/j.cell.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017;3:17009. doi: 10.1038/nrdp.2017.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapoval AI, Ni J, Lau JS, et al. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339 [DOI] [PubMed] [Google Scholar]
- 12.Ceeraz S, Nowak EC, Noelle RJ. B7 family checkpoint regulators in immune regulation and disease. Trends Immunol. 2013;34(11):556–563. doi: 10.1016/j.it.2013.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L, Kang FB, Shan BE. B7-H3-mediated tumor immunology: friend or foe? Int J Cancer. 2014;134(12):2764–2771. doi: 10.1002/ijc.28474 [DOI] [PubMed] [Google Scholar]
- 14.Crispen PL, Sheinin Y, Roth TJ, et al. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. doi: 10.1158/1078-0432.CCR-08-0536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin X, Zhang H, Ye D, Dai B, Zhu Y, Shi G. B7-H3 is a new cancer-specific endothelial marker in clear cell renal cell carcinoma. Onco Targets Ther. 2013;6:1667–1673. doi: 10.2147/OTT.S53565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Ji J, Zhang G, et al. Expression and significance of B7-H3 and Tie-2 in the tumor vasculature of clear cell renal carcinoma. Onco Targets Ther. 2017;10:5417–5424. doi: 10.2147/OTT.S147041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin Y, Zhang P, Li J, et al. B7-H3 in combination with regulatory T cell is associated with tumor progression in primary human non-small cell lung cancer. Int J Clin Exp Pathol. 2015;8(11):13987–13995. [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Zhou L, Chang X, Pang X, Zhang H, Zhang S. B7-H3, B7-H4, Foxp3 and IL-2 expression in cervical cancer: associations with patient outcome and clinical significance. Oncol Rep. 2016;35(4):2183–2190. doi: 10.3892/or.2016.4607 [DOI] [PubMed] [Google Scholar]
- 19.Nomura T, Sakaguchi S. Naturally arising CD25+CD4+ regulatory T cells in tumor immunity. Curr Top Microbiol Immunol. 2005;293:287–302. [DOI] [PubMed] [Google Scholar]
- 20.Moch H, Humphrey PA, Ulbright TM, Reuter VE. WHO Classification of Tumours of the Urinary System and Male Genital Organs. 4th ed. Lyon: IARC Press; 2016. [DOI] [PubMed] [Google Scholar]
- 21.Amin MB, Edge SB, Greene FL, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 22.Inamura K, Yokouchi Y, Sakakibara R, et al. Relationship of tumor PD-L1 expression with EGFR wild-type status and poor prognosis in lung adenocarcinoma. Jpn J Clin Oncol. 2016;46(10):935–941. doi: 10.1093/jjco/hyw087 [DOI] [PubMed] [Google Scholar]
- 23.Inamura K, Yokouchi Y, Kobayashi M, et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer. 2017;103:44–51. doi: 10.1016/j.lungcan.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 24.Inamura K, Takazawa Y, Inoue Y, et al. Tumor B7-H3 (CD276) expression and survival in pancreatic cancer. J Clin Med. 2018;7(7):E172. doi: 10.3390/jcm7070172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang C, Zhang Z, Li F, et al. Large-scale analysis reveals the specific clinical and immune features of B7-H3 in glioma. Oncoimmunology. 2018;7(11):e1461304. doi: 10.1080/2162402x.2018.1461304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han S, Shi X, Liu L, et al. Roles of B7-H3 in cervical cancer and its prognostic value. J Cancer. 2018;9(15):2612–2624. doi: 10.7150/jca.24959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Wang Z, Zhang C, et al. Genetic and clinical characterization of B7-H3 (CD276) expression and epigenetic regulation in diffuse brain glioma. Cancer Sci. 2018;109(9):2697–2705. doi: 10.1111/cas.13744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seaman S, Stevens J, Yang MY, Logsdon D, Graff-Cherry C, St Croix B. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell. 2007;11(6):539–554. doi: 10.1016/j.ccr.2007.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mesri M, Birse C, Heidbrink J, et al. Identification and characterization of angiogenesis targets through proteomic profiling of endothelial cells in human cancer tissues. PLoS One. 2013;8(11):e78885. doi: 10.1371/journal.pone.0078885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seaman S, Zhu Z, Saha S, et al. Eradication of tumors through simultaneous ablation of CD276/B7-H3-positive tumor cells and tumor vasculature. Cancer Cell. 2017;31(4):501–515.e508. doi: 10.1016/j.ccell.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci U S A. 2008;105(30):10495–10500. doi: 10.1073/pnas.0802423105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967 [DOI] [PubMed] [Google Scholar]
- 34.Prasad DV, Nguyen T, Li Z, et al. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500 [DOI] [PubMed] [Google Scholar]
- 35.Fukushima A, Sumi T, Fukuda K, et al. B7-H3 regulates the development of experimental allergic conjunctivitis in mice. Immunol Lett. 2007;113(1):52–57. doi: 10.1016/j.imlet.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 36.Mahnke K, Ring S, Johnson TS, et al. Induction of immunosuppressive functions of dendritic cells in vivo by CD4+CD25+ regulatory T cells: role of B7-H3 expression and antigen presentation. Eur J Immunol. 2007;37(8):2117–2126. doi: 10.1002/eji.200636841 [DOI] [PubMed] [Google Scholar]
- 37.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 38.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 39.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909 [DOI] [PubMed] [Google Scholar]
- 40.Asaka S, Yen TT, Wang TL, Shih IM, Gaillard S. T cell-inflamed phenotype and increased Foxp3 expression in infiltrating T-cells of mismatch-repair deficient endometrial cancers. Mod Pathol. 2018:in press. doi: 10.1038/s41379-018-0172-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shitara K, Nishikawa H. Regulatory T cells: a potential target in cancer immunotherapy. Ann N Y Acad Sci. 2018;1417(1):104–115. doi: 10.1111/nyas.13625 [DOI] [PubMed] [Google Scholar]