Abstract
Attention can be directed either voluntarily based on the goals of the individual or involuntarily “captured” by salient stimuli in the immediate environment. Although involuntary capture is a critical means of directing attention, the completion of many common tasks requires our ability to ignore salient, but otherwise irrelevant stimuli while restricting our attention to stimuli that are related to our goals. Here, we report neurophysiological measures of spatial attention in humans that gauge an individual's ability to resist attentional capture from salient but irrelevant information. By measuring the rapid reallocation of spatial attention immediately after the onset of distractors, we observe that the ability to override attentional capture varies substantially across individuals and is strongly predicted by the specific working memory capacity of each person. High-capacity individuals were much more capable of resisting attentional capture than low-capacity individuals, who involuntarily reallocated spatial attention when distractors were present in the display. These results provide evidence that the poor attentional abilities associated with low memory capacity may stem from an inability to override attentional capture in the initial moments after the onset of distracting information.
Introduction
Attention depends on two primary modes that compete for control over what information from the environment will be selectively enhanced or ignored. Attention can be shifted voluntarily to locations or objects to satisfy the individual's goals, and this is thought to be driven by “top-down” signals in prefrontal cortex that bias processing in posterior cortical areas (Desimone and Duncan, 1995; Miller and Cohen, 2001). In contrast, attention can be involuntarily captured by stimuli possessing highly salient properties (e.g., ambulance siren), despite being irrelevant to the individual's current goals, and this is thought to depend on “bottom-up” signals from both subcortical structures (e.g., lateral geniculate nucleus) as well as visual cortex (Yantis and Jonides, 1990; Yantis, 2000; O'Connor et al., 2002). Although recent work has begun to establish how these two modes communicate (Buschman and Miller, 2007), the means by which they compete for the control of attention are still poorly understood. Although involuntary attentional capture is an ecologically critical mechanism for detecting potentially important stimuli in the environment (Breitmeyer and Ganz, 1976), it often comes at the expense of goal-driven behavior (Posner, 1980; Berger et al., 2005). Indeed, many cognitive tasks require the ability to ignore signals from salient but irrelevant stimuli so that we may restrict attention to stimuli that are related to our goals (Kane and Engle, 2003). Thus, how effective an individual is at overriding attentional capture may be a critical factor in that individual's ability to achieve task-related goals.
Humans vary considerably in their ability to perform many cognitive tasks. One important source of this variance is an individual's working memory capacity (Cowan, 2001; Vogel and Awh, 2008). Numerous cognitive systems are thought to use working memory as an online “workspace” for representing task-relevant information. Because of its central role in cognition, individual differences in working memory capacity have been shown to be highly predictive of performance across a wide range of high-level aptitude measures, such as fluid intelligence (Kyllonen and Christal, 1990; Cowan et al., 2005). Moreover, recent work has suggested that various attentional abilities also covary with working memory capacity: high-capacity individuals show enhanced performance on attention tasks such compared with low-capacity individuals (Kane et al., 2001; Bleckley et al., 2003; Sobel et al., 2007). For example, Vogel et al. (2005) observed that low-capacity individuals were poorer at controlling which items from a display would be stored in visual working memory (WM) than high-capacity individuals. McNab and Klingberg (2008) extended these findings by observing that the control signals governing access to WM may emanate from preparatory activity in the prefrontal cortex and the basal ganglia, and that these signals are less robust in low-capacity individuals, resulting in irrelevant items unnecessarily entering the capacity-limited WM storage of the intraparietal sulcus (Todd and Marois, 2004; Xu and Chun, 2006). However, one fundamental ambiguity remains in understanding the relationship between memory capacity and attention: is poor attentional performance by low-capacity individuals the result of weak top-down control signals for selecting task-relevant items, or is it the result of an inability to override strong involuntary attentional capture signals from irrelevant items? Here, we tested between these two alternatives by measuring each subject's working memory capacity and examining its relationship with the effectiveness of their voluntary attention and their ability to resist attentional capture.
Materials and Methods
Overview
To examine how effectively individuals could override attentional capture, we recorded event-related potentials (ERPs) from healthy young adults while they performed a spatial attention task in which it was necessary to report the identity of a target presented at a cued location. On each trial, they were presented with a 100 ms bilateral display of Landolt “C”s and were asked to report the orientation of only the one item presented in the location that was indicated by a spatially informative cue at the beginning of the trial (Fig. 1A). The target was always presented along with three distractor items that were similar to the target at nearby locations, such that the four items together formed a diamond configuration in the hemifield. On half of the trials, 50 ms after the target array disappeared, a bilateral task-irrelevant probe (i.e., a filled white square) was flashed either at the location of the target for that trial or at the location of one of the distractors. On the other half of trials, no probe stimulus was presented. At the end of all trials, subjects reported the orientation of the target item by pressing one of four buttons.
Figure 1.
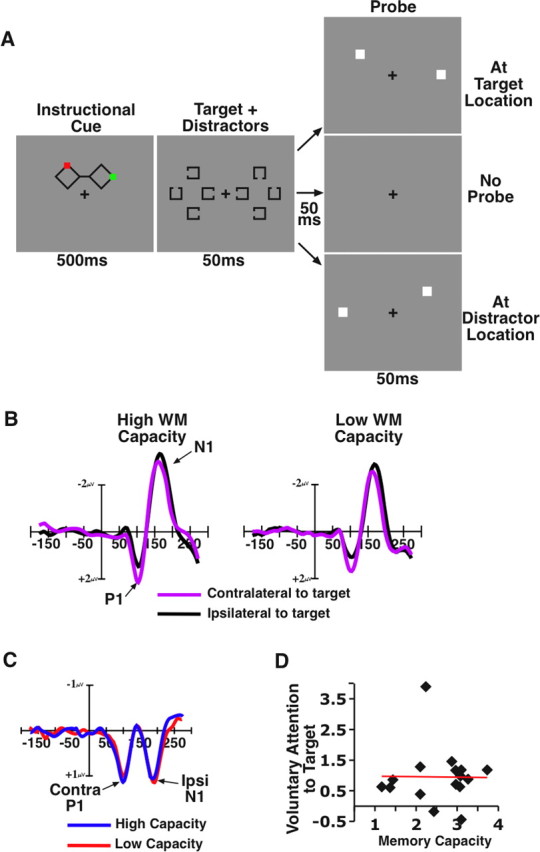
Stimuli and results from experiment 1. A, Example of the stimulus sequence and three probe conditions. The colored cue indicated the location of the target item for the trial. Half of the subjects attended red cues, and the other half attended green cues. B, Grand averaged ERP waveforms time-locked to the onset of the target array averaged across lateral occipital and temporal electrode sites and divided between the high- and low-memory-capacity groups. Note that, by convention, negative voltage is plotted upward. C, ERP difference waves (contralateral minus ipsilateral) time-locked to the target array. D, Correlation between an individual's memory capacity and the magnitude of the P1/N1 voluntary attention effect in microvolts.
Participants
All participants gave informed consent after the procedures of a protocol approved by the Human Subjects Committee at the University of Oregon. All subjects were members of the University of Oregon community and were paid $10 per hour for participation. A unique set of subjects participated in each experiment with 20 in experiment 1, 20 in experiment 2, 16 in experiment 3, and 28 in experiment 4. Subjects with eye-blink or eye-movement artifacts in excess of 25% of trials were excluded from further analysis. Four subjects in experiment 1, four subjects in experiment 2, and one subject in experiment 3 exceeded this threshold.
Measuring visual working memory capacity
For each experiment, participants first completed a behavior-only visual working memory task before starting the ERP experiment. The working memory task consisted of a change detection task with arrays of 4, 6, and 8 colored squares with a 1 s retention interval (Luck and Vogel, 1997; Vogel et al., 2001). We computed each individual's visual memory capacity with a standard formula (Pashler, 1988; Cowan, 2001). The formula is K = S(H− F), where K is the memory capacity, S is the size of the array, H is the observed hit rate, and F is the false alarm rate. Subjects were divided into high-capacity and low-capacity groups using a median split of their memory capacity estimates.
Electroencephalography recording
ERPs were recorded in each experiment using our standard recording and analysis procedures (McCollough et al., 2007), including rejection of trials contaminated by blinks or large (>1°) eye movements. We recorded from 22 standard electrode sites spanning the scalp, including international 10/20 sites F3, F4, C3, C4, P3, P4, O1, O2, PO3, PO4, T5, T6, as well as nonstandard sites occipital left (OL) and occipital right (OR) (midway between O1/2 and T5/6). The horizontal electrooculogram (EOG) was recorded from electrodes placed 1 cm to the left and right of the external canthi to measure horizontal eye movement, and the vertical EOG was recorded from an electrode beneath the right eye referenced to the left mastoid to detect blinks and vertical eye movements. Trails containing ocular artifacts, movement artifacts, or amplifier saturation were excluded from the averaged ERP waveforms. Furthermore, participants who had >20% of trial rejections in any condition were excluded from the analysis. The electroencephalography and EOG were amplified by an SA Instrumentation amplifier with a bandpass of 0.01–80 Hz (half-power cutoff, Butterworth filters) and were digitized at 250 Hz by a personal computer compatible microcomputer.
Stimuli and procedure for experiments 1 and 2
Cued target identification task
Cue and target array.
Each trial started with the onset of fixation cross (0.5° × 0.5°) centered on a gray background (red, green, blue = 125, 125, 125). Subjects were instructed to hold central fixation throughout the trial. Two hundred milliseconds later, a cue array containing two diamond-like shapes (1.6° × 1.6° for each diamond) with a green and a red dot (0.3° × 0.3°) on each was presented for 500 ms. The four corners of each diamond represented the four possible target locations in each corresponding hemifield. Half of the participants were instructed that the position of a green dot indicates where the target item will be presented in target array. The other half of the participants were instructed that the position of the red indicates the target location. After the offset of the cue (200 ms), we presented a target array composed of four boxes with a gap on a side (box size, 1° × 1°; gap size, 0.3°) for 50 ms, and participants were asked to press a button to report where the gap was on the target box. Subjects performed a total of 1200 trials (400 in each condition).
Experiment 2 used an identical procedure with two notable exceptions. First, the target array included either three similar distractors (as in experiment 1), no distractors, or four dissimilar distractors in the same hemifield with the target. The dissimilar distractors were blue circles (radius, 1°) that were presented at locations directly adjacent to the potential target positions. Second, for the no distractor and dissimilar distractor conditions, we made the size of the gap in the target item smaller (gap size, 0.15°), so that we could equate the discrimination difficulty between these conditions and the dissimilar distractor condition.
Probe array.
After the offset of target array (50 ms), on two-thirds of trials, task irrelevant bilateral probes (white squares, 0.9° × 0.9°) were presented for 50 ms at either the target location or one of the distractor locations with equal probability. No probe was presented on the other one-third of trials to obtain an ERP response elicited exclusively by the target array.
ERP analyses
Voluntary attention (time locking to target array).
Only the probe-absent trials were analyzed to establish a pure measure of the ERP components evoked by the target array without subsequent overlap from the evoked activity to the probe. The amplitude of P1/N1 attentional modulation was measured as the difference in mean amplitude between contralateral activity and ipsilateral activity from 75 to 175 ms after the onset of target array. We computed contralateral waveforms by averaging the activity recorded at right hemisphere electrode sites when subjects were cued to attend the left hemifield with the activity recorded from left hemisphere electrode sites when they were cued to attend the right hemifield. P1/N1 attention effects were measured by averaging across four posterior pairs of lateral electrode sites (PO3, PO4, OL, OR, T5, and T6). Mean amplitudes were compared across conditions by repeated-measures ANOVA.
Resistance to attentional capture (time locking to probe array).
To eliminate the overlapping activity evoked by the target array from that evoked by the probe itself, we computed a difference wave between probe-present trials and probe-absent trials. The logic of this approach is that overlapping activity from the target array would be equivalent for both probe-present and probe-absent trials and thus would be eliminated in the subtraction leaving only the evoked response to the probe. The amplitude of P1/N1 attentional modulation to the probe was measured as the difference in mean amplitude between contralateral activity and ipsilateral activity from 75 to 175 ms after the onset of probe array. The measurements of P1/N1 were obtained by averaging across four posterior pairs of lateral electrode sites (PO3, PO4, OL, OR, T5, and T6).
Stimuli and procedure for experiment 3
Working memory filtering task
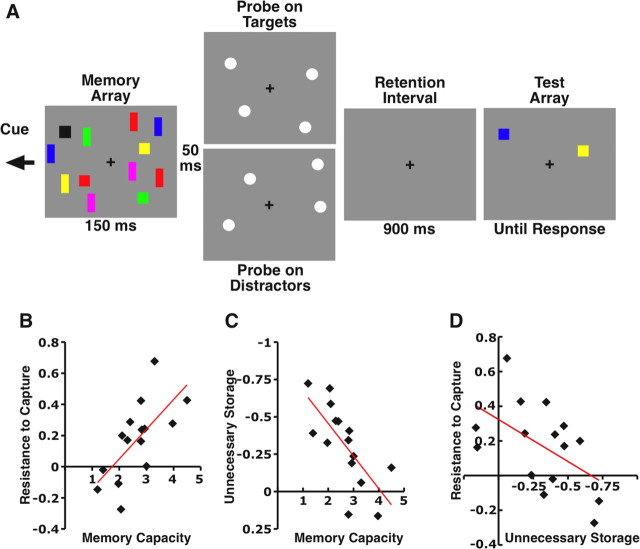
At the beginning of each trial, an arrow cue is presented above the fixation cross for 200 ms to indicate which side of the screen (left/right) to perform the task while ignoring the other hemifield. After the offset of cue array (500 ms), the memory array was presented for 150 ms. In the distractors-absent conditions, the memory array contained either two squares (square size, 1° × 1°) (T2D0) or six squares (T6D0) in each hemifield. In the distractors-present condition, the memory array consisted of two squares and four very square-like rectangles (width to height ratio, 2/3:3/2) (T2D4). After 900 ms of blank screen (retention interval), a test array was presented with one colored square in each hemifield. The subjects were asked to make an unspeeded response as to whether the colored square was either the same or different to its original value in the memory array. On two-thirds of distractor-present trials, two task-irrelevant probes were presented 50 ms after the offset of memory array in each hemifield. On half of these trials, the probes were presented at target locations, and on the other half of the trials, the probes were presented at distractor locations. Each participant completed 200 trials for each distractor-absent condition (T2D0 and T6D0) and 300 trials for each distractor-present condition (T2D4 no probe, T2D4 target probe, and T2D4 distractor probe).
ERP analyses
Contralateral delay activity.
The contralateral delay activity (CDA) was measured as the difference in mean amplitude between ipsilateral and contralateral waveforms recorded at posterior parietal, lateral occipital, and posterior temporal electrode sites (PO3, PO4, T5, T6, OL, and OR) from 300–900 ms after the onset of the memory array. The amplitude of the CDA was measured on probe-absent trials only to avoid any ERP overlap from the probe onset. The unnecessary storage effect was measured as the mean difference in CDA amplitude between the two target distractor present trials (T2D4) and the two target distractor absent trials (T2D0).
Resistance to attentional capture—P1/N1 attentional modulation.
Attentional responses to the probe in experiment 3 were measured using the same procedure as that in the first two experiments.
Stimuli and procedure for experiment 4 (spatial blink test)
This task was a modified version of the original spatial blink task introduced in Folk et al. (2002). Participants observed a rapid serial stream (RSVP) of colored letters presented at fixation, and their goal was to identify a single letter drawn in a target color within the stream. Target color was red for half of participants and green for the other half of subjects, counterbalanced. In each RSVP stream, each letter was presented for 45 ms and followed by 45 ms of blank screen. All the letters except for Q, R, P, I, and O were used to create a stream with 15 letters without repetition. For red target participants, all distractor letters were randomly assigned to four possible isoluminant distractor colors (blue, yellow, magenta, and green), and for green target participants, red was used as one of the distractor colors. On half of the trials, four “#” signs were presented simultaneously around the fixation cross (figure X) at either lag 0, 1, 2, 3, 4, or 5 before the target letter (flanker condition). For half of these flanker-present trials, one of the flanking “#” signs was assigned with one of the distractor colors (irrelevant color flanker); for the other half of trials, it was assigned a target color (relevant color flanker). The remaining three “#” signs were gray. Overall, each participant completed 960 RSVP trials.
Results
To measure spatial attention, we examined the early visually evoked ERP waveforms (i.e., P1 and N1) that reflect sensory processing in extrastriate cortical areas beginning within the first 75 ms of stimulus onset. These ERP components are modulated by spatial attention, with larger amplitudes observed for stimuli that appear in attended locations compared with reduced amplitudes for stimuli presented in unattended locations (Mangun et al., 1993; Hillyard et al., 1998). In lateralized displays, such as those used here, the P1 attention effect is typically observed as a larger positive voltage at electrodes over the hemisphere that is contralateral to the attended side of the display; whereas the N1 attention effect is often observed as a larger negative voltage at electrodes over the hemisphere that is ipsilateral to the attended sided of the display (Heinze et al., 1990). Thus, these ERP components provide a sensitive measure of where spatial attention is directed at a particular moment during a task.
Measuring voluntary and involuntary attention
We measured both voluntary and involuntary control mechanisms by examining the spatial attention during two separate moments of task performance. By examining the P1/N1 attention effect to the target array, we could measure how effectively an individual could interpret the cue, voluntarily orient attention toward the impending target location, and modulate the perceptual response to the display. In contrast, the P1/N1 response to the task-irrelevant probe that followed the target array provides a measure of whether the individual could override attentional capture by the distractors. For example, imagine an individual that could completely resist attentional capture from the distractors surrounding the target by locking attention exclusively to the target position. When the task-irrelevant probe appears at the target location, there should be a large P1/N1 response, because that location is still attended; however, when the probe is presented at distractor position, a negligible P1/N1 response is expected, because attention is focused solely on the target location. Thus, perfect override of attentional capture would produce a steep gradient in the P1/N1 responses between probes presented at target and distractor locations. In contrast, consider an individual who was incapable of resisting attentional capture by the distractors surrounding the target and involuntarily reoriented attention to include one or more of the distractor locations. When the probe is presented at the target location, the P1/N1 response may be reduced, because attention has been withdrawn from the target location; however, probes presented at the distractor location may show an increased P1/N1 response, because one or more of these locations is now attended. Thus, a perfect inability to override capture would produce a small difference in the P1/N1 responses to probes presented at target and distractor locations. Consequently, by measuring the difference in the response to probes at target and distractor locations, we can gauge an individual's effectiveness at overriding involuntary attentional capture.
Both groups showed large and significant early attention effects to the target array (Fig. 1B). However, the magnitude of this effect was not different between the groups [F <1, nonsignificant (n.s.)]. Thus, we observe no relationship between how well one can voluntarily orient attention in this task and his or her memory capacity (r = 0.11, n.s.). However, by examining the response to the probe, such a relationship emerges (Fig. 2). High-capacity individuals showed a large attention effect for probes at target locations (p < 0.001) and a small, nonsignificant effect for probes at distractor positions (p > 0.25), consistent with an ability to hold attention exclusively at the target position. In contrast, the low-capacity individuals showed a considerably smaller attention effect for probes at target positions (p < 0.01), and the magnitude of this effect was not reliably greater than the attention effect observed for probes at distractor positions (p > 0.30): consistent with an inability to maintain focus on the target location and a reorienting of attention to include distractor positions. We calculated the difference in amplitude between probe responses at target locations and those at distractor locations and plotted this value for each subject as a function of his or her working memory capacity (Fig. 2C). We observed a highly significant positive correlation between these two factors (r = 0.73, p < 0.001), with low-capacity individuals showing poor resistance to attentional capture and high-capacity individuals showing strong resistance to capture.
Figure 2.
Attention responses to the probe in experiment 1. A, ERP difference waves (contralateral minus ipsilateral) time-locked to the onset of the probe divided across high- and low-memory-capacity groups. B, Mean amplitude (in microvolts) of the P1/N1 attention effect to the probe. Error bars reflect 95% confidence intervals. C, Correlation between memory capacity and susceptibility to attentional capture. Attentional capture is measured as the amplitude difference (in microvolts) between the P1/N1 responses to probes at target and distractor locations. Note that smaller values indicate poorer resistance to capture.
Experiment 2: capture by any distracting items or only similar distractors?
The results of experiment 1 suggest that although voluntary attention ability does not vary with memory capacity, the ability to override attentional capture signals from distractors does strongly correlate with memory capacity. However, it is unclear whether low-capacity subjects involuntarily reorient spatial attention when any distracting items are present or if it is restricted only to distractors that are similar to the target (Folk et al., 1992; Theeuwes, 1994)? Therefore, in experiment 2, we manipulated the similarity of the distractors to the target by presenting either no distractors, similar distractors (as in experiment 1), or highly dissimilar distractors (i.e., blue circles). If low-capacity individuals reorient spatial attention for any distracting material regardless of its similarity to the target, we would expect equivalent capture effects for the dissimilar and similar distractor conditions. However, if the low-capacity subject's difficulties in overriding attentional capture occur only when the distractors share visual features with the target, we should observe the relationship with memory capacity only in the similar distractor condition. Figure 3 shows the relationship between memory capacity and the distractor capture attention effect to the probe across the three conditions. When the distractors were similar to the target, we replicated the strong correlation with memory capacity that was observed in experiment 1 (r = 0.64, p < 0.001). However, this relationship was not observed for trials with either no distractors (r = 0.15, n.s.) or dissimilar distractors (r = 0.14, n.s.), which indicates that this relationship is restricted to capture triggered by the presence distractors that are highly similar to the target.
Figure 3.
Stimulus displays and results from experiment 2. A, Top, Example of a similar distractors trial. Bottom, Correlation between memory capacity and susceptibility to attentional capture with similar distractors. B, Top, Example of a no distractors trial. Bottom, Correlation between memory capacity and susceptibility to capture with no distractors. C, Top, Example of a dissimilar distractors trial. Bottom, Correlation between memory capacity and susceptibility to capture with dissimilar distractors.
Behavioral performance for experiments 1 and 2
Working memory task
The mean working memory capacity estimates were 2.6 (SD, 0.78) and 2.9 (SD, 0.80), for experiment 1 and experiment 2, respectively. Across the two experiments, working memory capacity estimates ranged from 1.2 to 4.2.
Cued target task
In experiment 1, the mean accuracy was 94% (SD, 0.05) for no probe condition, 90% (SD, 0.07) for the target probe condition, and 94% (SD, 0.05) for the distractor probe condition. A repeated-measure ANOVA showed a significant difference in accuracy between the target probe condition and the other two conditions (F(1,14) = 19.68, p < 0.01). There was no significant difference between the distractor probe and no probe condition (F(1,14) = 0.11, p > 0.7). The result indicates that a probe at the target location made the task slightly harder than a probe at a distractor location or no probe. However, individual differences in working memory capacity were not predictive of performance in any condition (p values > 0.25).
In experiment 2, when there were no distractors present, mean accuracy was 91% (SD, 0.07) for no probe condition, 89% (SD, 0.08) for the target probe condition, and 89% (SD, 0.08) for the distractor-position probe condition. Repeated-measure ANOVA showed a small but significant difference in accuracy between no probe condition and probe conditions (F(1,13) = 11.43, p < 0.01) but no significant difference between the two probe conditions (F(1,13) = 3.29, p > 0.05). Similar to experiment 1, individual working memory capacity was not predictive of performance in any condition (p values > 0.05).
In the similar distractor conditions, the mean accuracy was 84% (SD, 0.11) for no probe condition, 68% (SD, 0.14) for the target probe condition, and 82% (SD, 0.13) for the distractor probe condition. A repeated-measure ANOVA showed a significant difference in accuracy between no probe condition and two probe conditions (F(1,15) = 54.96, p < 0.01). There was also a significant difference between the two probe conditions (F(1,15) = 32.38, p < 0.01) with lower accuracy for the target probe trials. Similar to previous experiments, individual working memory capacity was not predictive of performance in any condition (p values > 0.05).
In dissimilar distractor conditions, the mean accuracy revealed a similar pattern as the similar distractor conditions [no probe condition = 83% (SD, 0.08), target probe condition = 71% (SD, 0.10), distractor probe condition = 81% (SD, 0.09)]. A repeated-measure ANOVA showed a significant difference in accuracy between no probe conditions and probe conditions (F(1,15) = 66.49, p < 0.01). There was also a significant difference between distractor probe and target probe conditions (F(1,15) = 36.79, p < 0.01). Similar to previous experiments, individual working memory capacity was not predictive of performance in any condition (p values > 0.05).
One interesting result from experiment 2 was that high-capacity individuals showed a larger cost in performance, attributable to the presence of a probe at the target location (r = 0.49, p < 0.05). This suggests that high-capacity individuals were more susceptible to the masking effect by the probe. Such relationship between the probe-induced masking effect and working memory capacity was only observed in the similar distractor condition, which supports the idea that high-capacity individuals had tighter focus of attention on the target location and thus incurred more cost because of the probe flash.
Experiment 3: capture as an early mechanism for unnecessary storage in WM?
It is unclear whether this involuntary reorienting toward distractors by low-capacity subjects at early processing stages incurs significant consequences at later stages of processing or whether these distractors are briefly attended but then quickly disregarded before they are processed further. We examined this question in experiment 3 by asking subjects to perform a visual WM task in which they must selectively remember the colors of only the squares in a display that also contained colored rectangles (Fig. 4A). Similar to the previous experiments, we flashed task-irrelevant probes either at the target or the distractor locations within the attended hemifield shortly after the memory array so that we could examine the early attentional capture effect. Additionally, we examined the CDA during the memory retention period, which has been shown to be sensitive to the number of items that are currently being maintained in visual working memory (Vogel and Machizawa, 2004; McCollough et al., 2007). We used the CDA as a measure of whether or not the irrelevant items (i.e., the rectangles) were unnecessarily maintained in visual working memory by comparing CDA amplitude on trials containing two targets and no distractors with trials containing two targets and four distractors. Efficient exclusion of the distractors from memory would result in equivalent CDA amplitudes between the conditions, whereas an inability to exclude the distractors would result in larger CDA amplitudes for the distractor present conditions than the target only conditions. Here, we examined whether an individual's ability to resist attentional capture during the initial moments of processing (i.e., P1/N1) predicts whether that individual ultimately stores the irrelevant items in memory. As before, we again observed a strong correlation between the early attentional capture effect and memory capacity (r = 0.67, p < 0.001) (Fig. 4B). Moreover, we replicated our previous finding of a strong correlation between unnecessary storage of distractors and memory capacity (r = 0.71, p < 0.001) (Fig. 4C). Importantly, we found a strong correlation between an individual's P1/N1 capture effect and the CDA irrelevant memory storage effect (r = 0.55, p < 0.01) (Fig. 4D), with individuals who were more prone to attentional capture showing larger unnecessary storage effects in memory. Although we cannot conclude causality, these results indicate how well an individual can override attentional capture within the first 100 ms of processing predicts whether or not irrelevant items are unnecessarily stored in working memory.
Figure 4.
Stimuli and results from experiment 3. A, Example of a selective working memory trial with distractors present for the left hemifield. Subjects must remember the colors of only the squares across the retention period. B, Correlation between memory capacity and susceptibility to attentional capture for distractor present trials. C, Correlation between memory capacity and the unnecessary storage effect during the retention period. Unnecessary storage was measured as the difference in mean amplitude (in microvolts) of the CDA component between the distractors present and distractors absent trials at the lateral occipital and posterior parietal electrode sites. Note that more negative values indicate greater amounts of unnecessary storage in memory. D, Correlation between susceptibility to attentional capture during encoding and unnecessary storage of distractors during the retention period.
Behavioral performance for experiment 3
Working memory task
The mean working memory capacity estimate was 2.64 (SD, 0.88). The range in the estimate was 1.3–4.5.
Filtering task
The mean accuracy for T2D0 condition was 89.3% (SD, 7.2%). The mean accuracy for T2D4 condition was 65.1% (SD, 10.1%). Filtering cost was calculated as the decline in accuracy from T2D0 condition to T2D4 condition (mean, 24.2%; SD, 8.3%).
There was a significant negative correlation between individuals' working memory capacity and the filtering cost (r = 0.63, p = 0.01). This shows that lower working memory capacity individuals suffered more by the presence of distractors than high-capacity subjects.
Moreover, the behavioral filtering cost showed a strong relationship with ERP measures of unnecessary storage as well as involuntary attentional capture. First, it showed a negative correlation with the increase in CDA amplitude because of distractors (r = 0.77, p < 0.01). This nicely demonstrates that the behavioral measure of filtering cost is reflecting the consequence of storing irrelevant objects as measured by the CDA. Furthermore, behavioral filtering cost negatively correlated with the difference between P1/N1 attentional responses to target probes and distractor probes (r = −0.71, p < 0.05). This is in line with the ERP finding that the involuntary attentional capture by similar distractors led to inefficient filtering.
Experiment 4: do these relationships generalize to other capture paradigms?
In the final experiment, we sought to determine whether the results of the first three experiments generalize to other standard behavioral measures of attentional capture. In particular, we used a “spatial blink” task (Folk et al., 2002) in which subjects must report the identity of a single target letter drawn in red which is embedded in a rapid serial stream of colored letters (Fig. 5A). On a portion of trials shortly before the target appeared, task-irrelevant “#” signs flanked the target stream as a means of temporarily capturing attention away from stream, resulting in reduced accuracy for reporting the target (Fig. 5B). These flanker trials contained either a single red “#” (contingent color flanker, i.e., the same as the target) or a single blue “#” (irrelevant color flanker). Consequently, this task provides three measures of attentional control for each subject. By examining performance on no-flanker trials, we can assess how effectively each subject could voluntarily orient attention to the target stream and select the target. Despite the very challenging task (mean accuracy was 78%), high- and low-memory-capacity individuals performed equivalently (F <1). Furthermore, on irrelevant color flanker trials, target accuracy was reduced significantly, but again, no relationship to memory capacity was observed (r = −0.14, n.s.). However, on contingent color flanker trials, target accuracy was again reduced, but here the magnitude of the capture effect was strongly correlated with memory capacity (Fig. 5C) (r = 0.52, p < 0.01), with low-capacity individuals showing the largest reduction in performance. This pattern of results converges nicely with those in the first three experiments. Specifically, although voluntary attention appears to be unaffected, low-memory-capacity individuals are substantially impaired at resisting attentional capture from distractors that share features with the current target.
Figure 5.
Stimuli and results from experiment 4. A, Example of the spatial blink task for relevant and irrelevant colored flankers. B, Target accuracy for all subjects for each of the three conditions. Please note that “lag” is the temporal distance between the flankers and the target. Error bars reflect the SEM. C, Correlation between memory capacity and magnitude of contingent capture (r = 0.52, p < 0.01).
Behavioral performance for experiment 4
Working memory task
Individual working memory capacity estimate (mean, 2.9; SD, 0.8) ranged from 1.4 to 4.5.
Spatial blink task
At the group level, we successfully replicated the primary result of Folk et al. (2002). We observed that target identification accuracy is significantly lower when flankers appeared shortly before the target onset. First of all, when there was no flanker presented along with the RSVP stream, mean accuracy was 78% (SD, 11%). When one of the flankers had an irrelevant color, accuracy was significantly lower (p = 0.01) at Lag 2 [180 ms stimulus onset asynchrony (SOA)]. When one of the flankers shared a target color, the drop was significant across Lag 0 (0 ms SOA) through Lag 5 (450 ms SOA) (p values <0.03). This suggests attentional capture was larger and lasted longer when a flanker shared color value with the target.
Discussion
The ability to override the involuntary capture of attention by salient but irrelevant information is an important determinant for the successful completion of many goal-directed behaviors. By using a novel neural measure of attentional capture, our results reveal that there is systematic variability across human individuals in the ability to override the capture signal from distractors and lock spatial attention on target locations. More importantly, these results help to resolve the question of why low-capacity individuals often have poor performance on various types of attention tasks. That is, our observation that the voluntary P1/N1 attention effects to the target array were large, reliable, and entirely uncorrelated with memory capacity indicates that the low-capacity individuals were unimpaired in their ability to consistently follow the goal-based instructions of the task and could voluntarily orient their spatial attention just as effectively as their high-capacity counterparts. Instead, these results suggest that the poor attentional ability associated with low memory capacity is a consequence of a deficit in adequately overriding involuntary attentional capture signals from distracting information. Poor override of attentional capture led to a rapid and involuntary reorienting of spatial attention toward the locations of the distractors which likely resulted in their unnecessary storage in WM. Thus, a surprising secondary conclusion from these results is that the poor attentional ability of low-capacity subjects appears to stem from the allocation of attention within the initial moments after the onset of distracting information, a time course that is much earlier in processing than has been previously assumed on the basis of various late-stage tasks such as Stroop (Kane and Engle, 2003), anti-saccade (Unsworth et al., 2004), and partial report (Vogel et al., 2005).
We observed the relationship between memory capacity and attentional capture only in situations when the identity of the distractors was highly similar to the target, which is inconsistent with a purely “stimulus-driven” (Theeuwes, 1994) form of attentional capture. That is, because information regarding the individual's goals (e.g., target identity) influenced whether the distractors triggered a reorienting of attention, these results are instead consistent with a “contingent” form of attentional capture in which only distracting items that partially overlap with the subject's current “control settings” capture attention (Folk et al., 1992, 1994). Such a mechanism appears to rest at the critical interaction point of voluntary and involuntary modes of attention (Yantis, 2000). By this view, the ability to quickly override attentional capture from items that partially but not completely match the goals of the individual may be a way to effectively coordinate both modes of attentional control to facilitate such complex yet disparate goal-directed behaviors as controlling working memory (Vogel et al., 2005), tracking moving objects (Drew and Vogel, 2008), and abstract reasoning (Halford et al., 2007).
Footnotes
This study was supported by a grant from the National Science Foundation to E.K.V.
References
- Berger et al., 2005.Berger A, Henik A, Rafal R. Competition between endogenous and exogenous orienting of visual attention. J Exp Psychol Gen. 2005;134:207–221. doi: 10.1037/0096-3445.134.2.207. [DOI] [PubMed] [Google Scholar]
- Bleckley et al., 2003.Bleckley MK, Durso FT, Crutchfield JM, Engle RW, Khanna MM. Individual differences in working memory capacity predict visual attention allocation. Psychon Bull Rev. 2003;10:884–889. doi: 10.3758/bf03196548. [DOI] [PubMed] [Google Scholar]
- Breitmeyer and Ganz, 1976.Breitmeyer BG, Ganz L. Implications of sustained and transient channels for theories of visual-pattern masking, saccadic suppression, and information-processing. Psychol Rev. 1976;83:1–36. [PubMed] [Google Scholar]
- Buschman and Miller, 2007.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- Cowan, 2001.Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–185. doi: 10.1017/s0140525x01003922. [DOI] [PubMed] [Google Scholar]
- Cowan et al., 2005.Cowan N, Elliott EM, Scott Saults J, Morey CC, Mattox S, Hismjatullina A, Conway AR. On the capacity of attention: its estimation and its role in working memory and cognitive aptitudes. Cogn Psychol. 2005;51:42–100. doi: 10.1016/j.cogpsych.2004.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone and Duncan, 1995.Desimone R, Duncan J. Neural mechanisms of selective visual-attention. Annu Rev Neurosci. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Drew and Vogel, 2008.Drew T, Vogel EK. Neural measures of individual differences in selecting and tracking multiple moving objects. J Neurosci. 2008;28:4183–4191. doi: 10.1523/JNEUROSCI.0556-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk et al., 1992.Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Folk et al., 1994.Folk CL, Remington RW, Wright JH. The structure of attention control—contingent attentional capture by apparent motion, abrupt onset, and color. J Exp Psychol Hum Percept Perform. 1994;20:317–329. doi: 10.1037//0096-1523.20.2.317. [DOI] [PubMed] [Google Scholar]
- Folk et al., 2002.Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Percept Psychophys. 2002;64:741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Halford et al., 2007.Halford GS, Cowan N, Andrews G. Separating cognitive capacity from knowledge: a new hypothesis. Trends Cogn Sci. 2007;11:236–242. doi: 10.1016/j.tics.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze et al., 1990.Heinze HJ, Luck SJ, Mangun GR, Hillyard SA. Visual event-related potentials index focused attention within bilateral stimulus arrays. I. Evidence for early selection. Electroencephalogr Clin Neurophysiol. 1990;75:511–527. doi: 10.1016/0013-4694(90)90138-a. [DOI] [PubMed] [Google Scholar]
- Hillyard et al., 1998.Hillyard SA, Vogel EK, Luck SJ. Sensory gain control (amplification) as a mechanism of selective attention: electrophysiological and neuroimaging evidence. Philos Trans R Soc Lond B Biol Sci. 1998;353:1257–1270. doi: 10.1098/rstb.1998.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane and Engle, 2003.Kane MJ, Engle RW. Working-memory capacity and the control of attention: the contributions of goal neglect, response competition, and task set to Stroop interference. J Exp Psychol Gen. 2003;132:47–70. doi: 10.1037/0096-3445.132.1.47. [DOI] [PubMed] [Google Scholar]
- Kane et al., 2001.Kane MJ, Bleckley MK, Conway AR, Engle RW. A controlled-attention view of working-memory capacity. J Exp Psychol Gen. 2001;130:169–183. doi: 10.1037//0096-3445.130.2.169. [DOI] [PubMed] [Google Scholar]
- Kyllonen and Christal, 1990.Kyllonen PC, Christal RE. Reasoning ability is (little more than) working-memory capacity. Intelligence. 1990;14:389–433. [Google Scholar]
- Luck and Vogel, 1997.Luck SJ, Vogel EK. The capacity of visual working memory for features and conjunctions. Nature. 1997;390:279–281. doi: 10.1038/36846. [DOI] [PubMed] [Google Scholar]
- Mangun et al., 1993.Mangun GR, Hillyard SA, Luck SJ. Electrocortical substrates of visual selective attention. In: Meyer D, Kornblum S, editors. Attention and performance XIV. Cambridge, MA: MIT; 1993. pp. 219–243. [Google Scholar]
- McCollough et al., 2007.McCollough AW, Machizawa MG, Vogel EK. Electrophysiological measures of maintaining representations in visual working memory. Cortex. 2007;43:77–94. doi: 10.1016/s0010-9452(08)70447-7. [DOI] [PubMed] [Google Scholar]
- McNab and Klingberg, 2008.McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nat Neurosci. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Miller and Cohen, 2001.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- O'Connor et al., 2002.O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5:1203–1209. doi: 10.1038/nn957. [DOI] [PubMed] [Google Scholar]
- Pashler, 1988.Pashler H. Familiarity and visual change detection. Percept Psychophys. 1988;44:369–378. doi: 10.3758/bf03210419. [DOI] [PubMed] [Google Scholar]
- Posner, 1980.Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Sobel et al., 2007.Sobel KV, Gerrie MP, Poole BJ, Kane MJ. Individual differences in working memory capacity and visual search: the roles of top-down and bottom-up processing. Psychon Bull Rev. 2007;14:840–845. doi: 10.3758/bf03194109. [DOI] [PubMed] [Google Scholar]
- Theeuwes, 1994.Theeuwes J. Stimulus-driven capture and attentional set - selective search for color and visual abrupt onsets. J Exp Psychol Hum Percept Perform. 1994;20:799–806. doi: 10.1037//0096-1523.20.4.799. [DOI] [PubMed] [Google Scholar]
- Todd and Marois, 2004.Todd JJ, Marois R. Capacity limit of visual short-term memory in human posterior parietal cortex. Nature. 2004;428:751–754. doi: 10.1038/nature02466. [DOI] [PubMed] [Google Scholar]
- Unsworth et al., 2004.Unsworth N, Schrock JC, Engle RW. Working memory capacity and the antisaccade task: individual differences in voluntary saccade control. J Exp Psychol Learn Mem Cogn. 2004;30:1302–1321. doi: 10.1037/0278-7393.30.6.1302. [DOI] [PubMed] [Google Scholar]
- Vogel and Awh, 2008.Vogel EK, Awh E. How to exploit diversity for scientific gain: using individual differences to constrain cognitive theory. Curr Dir Psychol Sci. 2008;17:171–176. [Google Scholar]
- Vogel and Machizawa, 2004.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel et al., 2001.Vogel EK, Woodman GF, Luck SJ. Storage of features, conjunctions, and objects in visual working memory. J Exp Psychol Hum Percept Perform. 2001;27:92–114. doi: 10.1037//0096-1523.27.1.92. [DOI] [PubMed] [Google Scholar]
- Vogel et al., 2005.Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Xu and Chun, 2006.Xu Y, Chun MM. Dissociable neural mechanisms supporting visual short-term memory for objects. Nature. 2006;440:91–95. doi: 10.1038/nature04262. [DOI] [PubMed] [Google Scholar]
- Yantis, 2000.Yantis S, editor. Cambridge, MA: MIT; 2000. Goal-directed and stimulus-driven determinants of attentional control. [Google Scholar]
- Yantis and Jonides, 1990.Yantis S, Jonides J. Abrupt visual onsets and selective attention—voluntary versus automatic allocation. J Exp Psychol Hum Percept Perform. 1990;16:121–134. doi: 10.1037//0096-1523.16.1.121. [DOI] [PubMed] [Google Scholar]