Abstract
Effects of antipsychotic drugs have widely been considered to be mediated by blockade of postsynaptic dopamine D2 receptors. Effects of antipsychotics on presynaptic functions of dopaminergic neurotransmission might also be related to therapeutic effects of antipsychotics. To investigate the effects of antipsychotics on presynaptic functions of dopaminergic neurotransmission in relation with occupancy of dopamine D2 receptors, changes in dopamine synthesis capacity by antipsychotics and occupancy of dopamine D2 receptors were measured by positron emission tomography (PET) in healthy men. PET studies using [11C]raclopride and l-[β-11C]DOPA were performed under resting condition and oral administration of single dose of the antipsychotic drug risperidone on separate days. Although occupancy of dopamine D2 receptors corresponding dose of risperidone was observed, the changes in dopamine synthesis capacity by the administration of risperidone were not significant, nor was the relation between the occupancy of dopamine D2 receptors and these changes. A significant negative correlation was observed between the baseline dopamine synthesis capacity and the changes in dopamine synthesis capacity by risperidone, indicating that this antipsychotic can be assumed to stabilize the dopamine synthesis capacity. The therapeutic effects of risperidone in schizophrenia might be related to such stabilizing effects on dopaminergic neurotransmission responsivity.
Introduction
Effects of antipsychotic drugs have widely been considered to be mediated by blockade of postsynaptic dopamine D2 receptors (Carlsson and Lindqvist, 1963; Creese et al., 1976; Seeman et al., 1976). This hypothesis has been supported by positron emission tomography (PET) studies to determine the occupancy of dopamine D2 receptors in schizophrenia patients treated with first-generation antipsychotics, e.g., haloperidol (Farde et al., 1988; Baron et al., 1989) and second-generation antipsychotics, e.g., risperidone (Nyberg et al., 1993).
Effects of antipsychotics on presynaptic functions of dopaminergic neurotransmission might also be related to the therapeutic effects of antipsychotics. It has been reported that antipsychotic drugs, chlorpromazine and haloperidol, increased dopamine metabolites in mouse brain tissue (Carlsson and Lindqvist, 1963; O'Keeffe et al., 1970), and also that risperidone and clozapine increased dopamine release in rat brain (Hertel et al., 1996). Increases and decreases in the activity of aromatic l-amino acid decarboxylase (AADC) by antagonists and agonists of dopamine D2 receptors were also observed in rat brain tissue, respectively (Zhu et al., 1992, 1993). The regional activity of AADC in brain indicating the dopamine synthesis capacity can be estimated using radiolabeled l-DOPA (Gjedde et al., 1991). Significant increases and decreases in dopamine synthesis capacities by antagonists and agonists of dopamine D2 receptors were observed in animal studies using [3H]DOPA, l-[β-11C]DOPA, or 6-[18F]fluoro-l- DOPA, respectively (Cumming et al., 1997; Torstenson et al., 1998; Danielsen et al., 2001). These findings indicate that pharmacological effects on dopaminergic autoreceptors might cause changes in the presynaptic dopamine synthesis capacity (Carlsson and Lindqvist, 1963).
Effects of antipsychotics on the dopamine synthesis capacity in brain have been investigated in human subjects. A significant increase in dopamine synthesis capacity after acute administration of antipsychotic drug haloperidol was observed using PET with 6-[18F]fluoro-l-DOPA in healthy human subjects (Vernaleken et al., 2006). On the other hand, a significant decrease in dopamine synthesis capacity after chronic administration of haloperidol was observed using 6-[18F]fluoro-l-DOPA in patients with schizophrenia (Gründer et al., 2003). A significant increase in the plasma concentration of homovanillic acid after acute administration of antipsychotics, haloperidol or fluphenazine, was observed in patients with schizophrenia, indicating an increase in dopamine turnover (Davila et al., 1988; Pickar et al., 1988). During chronic administration, a significant decrease in the plasma concentration of homovanillic acid was also observed (Davila et al., 1988; Pickar et al., 1988). However, the effects of antipsychotics on the dopamine synthesis capacity have not been investigated in relation to the occupancy of dopamine D2 receptors in human subjects.
Recently, we have validated quantitative analyses in l-[β-11C]DOPA PET studies (Ito et al., 2006, 2007). In the present study, to elucidate changes in dopamine synthesis capacity by antipsychotics in relation to the occupancy of dopamine D2 receptors, dopamine D2 receptor bindings and dopamine synthesis capacities at resting condition and after oral administration of a single dose of the antipsychotic drug risperidone were measured in the same human subjects by PET with [11C]raclopride and l-[β-11C]DOPA, respectively.
Materials and Methods
Subjects.
The study was approved by the Ethics and Radiation Safety Committees of the National Institute of Radiological Sciences, Chiba, Japan. Twelve healthy men (21–29 years of age, 24.3 ± 2.9 years [mean ± SD]) were recruited and written informed consent was obtained. The subjects were free of somatic, neurological or psychiatric disorders on the basis of their medical history and magnetic resonance (MR) imaging of the brain. They had no history of current or previous drug abuse according to interview.
PET procedures.
All PET studies were performed with a Siemens ECAT Exact HR+ system, which provides 63 sections with an axial field of view of 15.5 cm (Brix et al., 1997). The intrinsic spatial resolution was 4.3 mm in-plane and 4.2 mm full-width at half maximum (FWHM) axially. With a Hanning filter (cutoff frequency: 0.4 cycle/pixel), the reconstructed in-plane resolution was 7.5 mm FWHM. Data were acquired in three-dimensional mode. Scatter was corrected (Watson et al., 1996). A 10 min transmission scan using a 68Ge-68Ga line source was performed for correction of attenuation. A head fixation device with thermoplastic attachments for individual fit minimized head movement during the PET measurements.
PET studies were performed under resting condition (baseline study) and oral administration of risperidone (drug challenge study) on separate days. The interval between the 2 studies was 7 d in 10 subjects, and 14 d in 2 subjects. In each study, both PET scans with [11C]raclopride and l-[β-11C]DOPA were performed sequentially. After intravenous rapid bolus injection of [11C]raclopride, dynamic PET scanning was performed for 60 min. After 1 h from the end of the [11C]raclopride PET measurement, dynamic PET scanning was performed for 89 min after intravenous rapid bolus injection of l-[b-11C]DOPA. The frame sequence consisted of twelve 20 s frames, sixteen 1 min frames, and ten 4 min frames for [11C]raclopride, and seven 1 min frames, five 2 min frames, four 3 min frames, and twelve 5 min frames for l-[β-11C]DOPA. The radioactivity injected was 220–230 MBq and 342–395 MBq in the baseline studies, and 205–274 MBq and 344–388 MBq in the drug challenge studies for [11C]raclopride and l-[β-11C]DOPA, respectively. The specific radioactivity was 168–517 GBq/μmol and 26–88 GBq/μmol in the baseline studies, and 162–535 GBq/μmol and 39–90 GBq/μmol in the drug challenge studies for [11C]raclopride and l-[β-11C]DOPA, respectively. A venous blood sample was taken at the beginning of l-[β-11C]DOPA PET scanning for measurement of natural neutral amino acid (NAA) concentration in plasma. NAA concentration was measured by HPLC (L-8500 amino acid analyzer system, Hitachi Corp.). The amino acids are phenylalanine, tryptophan, leucine, methionine, isoleucine, tyrosine, histidine, valine and threonine, which are transported via the same carrier at the blood–brain barrier as l-DOPA (Sugaya et al., 2001). A weighted sum of the NAAs, which was the l-DOPA corresponding concentration of the nine NAAs for the carrier system, was calculated according to our previous work (Ito et al., 2006).
In the drug challenge studies, risperidone at 0.5–2.0 mg was orally administrated 2 h before the start of PET scanning with [11C]raclopride. The dose of risperidone was 0.5 mg in 3 subjects, 1.0 mg in 3 subjects, 1.5 mg in 3 subjects, and 2.0 mg in 3 subjects. To estimate the plasma concentration of risperidone and its active metabolite (9-hydroxy-risperidone), venous blood sampling was performed at the start and end of each PET scan. The plasma concentrations of risperidone and 9-hydroxy-risperidone were determined by validated liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) method. Since risperidone and 9-hydroxy-risperidone have similar binding profiles to neuroreceptors (Leysen et al., 1994), the sum of their plasma concentrations was used as the plasma concentration of the antipsychotic drug in the present study.
All MR imaging studies were performed with a 1.5-tesla MR scanner (Philips Medical Systems, Best, The Netherlands). Three-dimensional volumetric acquisition of a T1-weighted gradient echo sequence produced a gapless series of thin transverse sections (echo time, 9.2 ms; repetition time, 21 ms; flip angle: 30°; field of view: 256 mm; acquisition matrix: 256 × 256; slice thickness: 1 mm).
Regions of interest.
All MR images were coregistered to the PET images with the statistical parametric mapping (SPM2) system (Friston et al., 1990). Regions of interest (ROIs) were drawn on coregistered MR images and transferred to the PET images. ROIs were defined for the cerebellar cortex, putamen, caudate head, and occipital cortex. Each ROI was drawn in three adjacent sections and data were pooled to obtain the average radioactivity concentration for the whole volume of interest. To obtain regional time-activity curves, regional radioactivity was calculated for each frame, corrected for decay, and plotted versus time. In-house software was used to draw ROIs.
Calculation of occupancy of dopamine D2 receptors.
For both PET studies with [11C]raclopride, the binding potential (BPND) was calculated by the reference tissue model method (Lammertsma et al., 1996; Lammertsma and Hume, 1996). With this method, the time-activity curve in the brain region is described by that in the reference region with no specific binding, assuming that both regions have the same level of nondisplaceable radioligand binding:
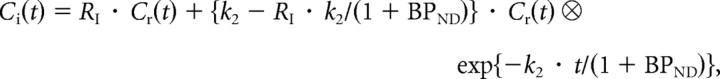 |
where Ci is the radioactivity concentration in a brain region; Cr is the radioactivity concentration in the reference region; RI is the ratio of K1/K1′ (K1, influx rate constant for the brain region; K1′, influx rate constant for the reference region), k2 is the efflux rate constant for the brain region, and ⊗ denotes the convolution integral. In this analysis, three parameters (BPND, RI, and k2) were estimated by nonlinear least-squares curve fitting. The cerebellum was used as a reference region. The occupancy of dopamine D2 receptors by risperidone was calculated as follows:
where BPND(baseline) is the BPND value in the baseline study, and BPND(drug) is the BPND value in the drug challenge study.
Calculation of dopamine synthesis capacity.
The uptake rate constant for l-[β-11C]DOPA indicating the dopamine synthesis capacity was estimated by graphical analysis (Patlak and Blasberg, 1985; Gjedde, 1988; Hartvig et al., 1991), which allows for the calculation of the uptake rate constant ki using time-activity data in a reference brain region with no irreversible binding. The ki values can be estimated by using simple linear least-squares fitting as follows:
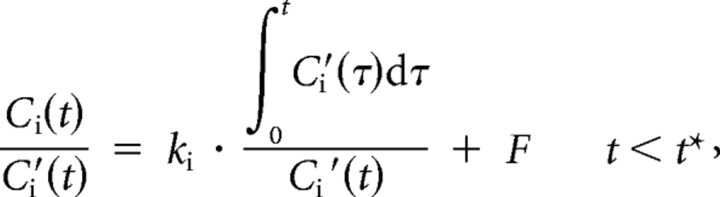 |
where Ci and Ci′ are the total radioactivity concentrations in a brain region with and without irreversible binding, respectively, and t* is the equilibrium time of the compartment for unchanged radiotracer in brain tissue. Plotting Ci(t)/Ci′(t) versus Ci′(τ)dτ/Ci′(t), after time t* yields a straight line with the slope ki and intercept F. In the present study, the occipital cortex was used as a reference region with no irreversible binding, because this region is known to have the lowest dopamine concentration (Brown et al., 1979) and lowest aromatic l-amino acid decarboxylase activity (Lloyd and Hornykiewicz, 1972). The range of equilibrium time t* of 29–89 min was used (Ito et al., 2006, 2007). The percentage change in ki by oral administration of risperidone was calculated as follows:
where ki(baseline) is the ki value in the baseline study, and ki(drug) is the ki value in the drug challenge study.
Results
The ranges of occupancy of dopamine D2 receptors in the striatum for each dose of risperidone measured by PET with [11C]raclopride are given in Table 1. The occupancies of dopamine D2 receptors ranged from 39% to 75% in the putamen and from 33% to 79% in the caudate. The sums of the plasma concentrations of risperidone and 9-hydroxy-risperidone during [11C]raclopride and l-[β-11C]DOPA PET studies, averaged between the start and end of each scanning, ranged from 3.8 to 23.1 ng/ml (12.2 ± 6.6 ng/ml, mean ± SD) and from 2.6 to 19.5 ng/ml (10.5 ± 5.8 ng/ml), respectively.
Table 1.
Dose of risperidone and ranges of occupancy of dopamine D2 receptors
| Dose of risperidone (mg) | Occupancy (%) |
|
|---|---|---|
| Putamen | Caudate | |
| 0.5 | 39–46% | 33–44% |
| 1.0 | 48–52% | 48–60% |
| 1.5 | 61–69% | 63–71% |
| 2.0 | 71–75% | 75–79% |
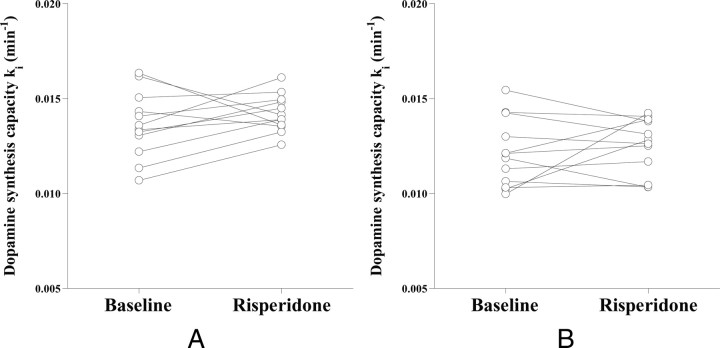
The uptake rate constant ki of l-[β-11C]DOPA in the striatum indicating the dopamine synthesis capacity for baseline and drug challenge study results are shown in Figure 1. The ki values were 0.0136 ± 0.0017 min−1 and 0.0142 ± 0.0010 min−1 (mean ± SD) in the putamen and 0.0121 ± 0.0018 min−1 and 0.0125 ± 0.0015 min−1 in the caudate for baseline and drug challenge studies, respectively. No significant differences in ki were observed between the two studies. Weighted sums of the natural neutral amino acids (NAAs) in plasma were 1251 ± 198 nmol/ml for the baseline studies and 1207 ± 199 nmol/ml (mean ± SD) for the drug challenge studies. No significant differences in values were observed between the two studies.
Figure 1.
The uptake rate constant ki indicating the dopamine synthesis capacity for the baseline study and drug challenge study with risperidone in the putamen (A) and caudate (B).
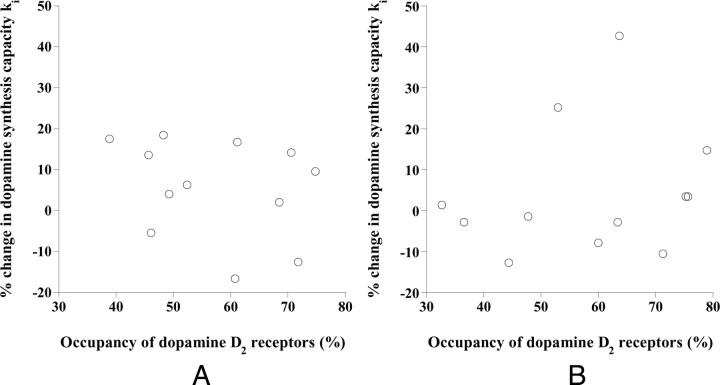
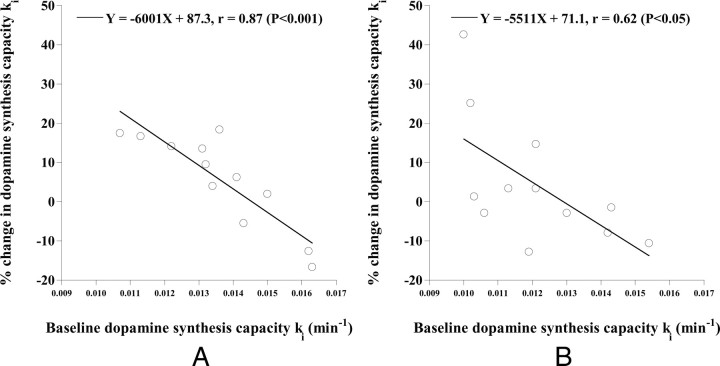
The relations between the occupancy of dopamine D2 receptors and the percentage change in ki by drug challenge are shown in Figure 2. No significant correlations were observed. The relations between ki in the baseline study and percentage change in ki by the drug challenge are shown in Figure 3. Significant negative correlations were observed (putamen: p < 0.001, caudate: p < 0.05).
Figure 2.
The relations between the occupancy of dopamine D2 receptors and the percentage change in ki by drug challenge with risperidone in the putamen (A) and caudate (B).
Figure 3.
The relations between ki in the baseline study and the percentage changes in ki by drug challenge with risperidone in the putamen (A) and caudate (B).
Discussion
Effects of antipsychotics on presynaptic dopamine synthesis might be caused by pharmacological activity on dopaminergic autoreceptors (Carlsson and Lindqvist, 1963). Although occupancy of dopamine D2 receptors corresponding to the dose of risperidone was observed, no significant changes in the dopamine synthesis capacity ki by administration of risperidone were observed in the present study. Furthermore, there were no significant correlations between the occupancy of dopamine D2 receptors and changes in dopamine synthesis capacity ki by risperidone. No significant changes in the dopamine synthesis capacity after acute administration of risperidone in healthy human subjects were also reported using 6-[18F]-l-m-tyrosine (Mamo et al., 2004). On the other hand, a significant increase in the dopamine synthesis capacity measured using 6-[18F]fluoro-l-DOPA and a significant increase in the plasma concentration of homovanillic acid have been observed after acute administration of antipsychotics, haloperidol or fluphenazine, in healthy human subjects (Vernaleken et al., 2006) and patients with schizophrenia (Davila et al., 1988; Pickar et al., 1988), respectively. The discrepancy between the present and previous results might have resulted from the use of different antipsychotics. However, in rat brain, it has been reported that risperidone and clozapine also increased dopamine release (Hertel et al., 1996). Another reason for this discrepancy might due to differences in the radiotracers used. However, in animal studies with [3H]DOPA, l-[β-11C]DOPA, or 6-[18F]fluoro-l- DOPA, significant increases and decreases in dopamine synthesis capacities by antagonists and agonists of dopamine D2 receptors, respectively, have been observed (Cumming et al., 1997; Torstenson et al., 1998; Danielsen et al., 2001).
In the present study, significant negative correlations were observed between the baseline dopamine synthesis capacity ki and the percentage changes in the dopamine synthesis capacity by risperidone. This indicates that the increase and decrease in dopamine synthesis capacity by administration of risperidone are observed in subjects with low and high baseline dopamine synthesis capacity, respectively, and the degrees of increase and decrease in dopamine synthesis capacity are greater as the baseline dopamine synthesis capacities are smaller and larger, respectively. Negative correlations between baseline cerebral 6-[18F] fluoro-l-DOPA utilization and change in 6-[18F]fluoro-l-DOPA storage capacity by haloperidol challenge have also been observed in healthy human subjects (Vernaleken et al., 2008), corresponding to our present results. In addition, the coefficients of variation of dopamine synthesis capacity ki were smaller in studies with the administration of risperidone than in baseline studies. Thus, the antipsychotic drug risperidone can be assumed to stabilize the dopamine synthesis capacity. The concept of phasic and tonic dopamine release with relation to the modulation of dopaminergic neurotransmission has been proposed, and abnormal responsivity in both phasic and tonic dopamine release in schizophrenia has been considered (Grace, 1991). The therapeutic effects of risperidone might be related to stabilizing effects on such dopaminergic responsivity. In addition, it has been reported that an antipsychotic drug, clozapine, normalized dopamine turnover in the primate phencyclidine model, indicating that the effects of clozapine in schizophrenia might be related to the restoration of dopamine tone (Elsworth et al., 2008). In this study, only an acute intervention was performed on healthy subjects, and therefore, the chronic effects of antipsychotics on patients with schizophrenia should be investigated in future.
It has been reported that the working memory and learning functions were correlated with the baseline dopamine synthesis capacity (Cools et al., 2008, 2009). Further studies to investigate the effects of antipsychotics on such higher brain functions in relation with changes in dopamine synthesis capacity should be considered (Vernaleken et al., 2008).
Serotonin 5-HT2A receptor antagonists have been reported to modulate endogenous dopamine release (Pehek et al., 2001), and to reduce extrapyramidal side effects (Balsara et al., 1979; Korsgaard et al., 1985; Hicks, 1990). Risperidone is an antagonist for dopamine D2 receptors and serotonin 5-HT2A receptors with high affinity (Leysen et al., 1994), and it has been reported to modulate endogenous dopamine release. These findings indicate that changes in the dopamine synthesis capacity by administration of risperidone might be due to not only pharmacological effects on dopaminergic autoreceptors, but also on serotonin 5-HT2A receptors. Thus, the stabilizing effects of risperidone on the dopamine synthesis level might also be related to its antagonism toward serotonin 5-HT2A receptors. To elucidate this, further studies based on the same design using a selective antagonist for dopamine D2 receptors, such as sulpiride, should be considered. In addition, a new antipsychotic drug aripiprazole that is a partial agonist to dopamine D2 receptors has recently been used for treatment of schizophrenia (Mamo et al., 2007). Further studies to investigate the effects of aripiprazole on dopamine synthesis capacity should also be considered.
In conclusion, dopamine D2 receptor bindings and dopamine synthesis capacities at resting condition and after oral administration of a single dose of the antipsychotic drug risperidone were measured in the same human subjects. Although occupancy of dopamine D2 receptors corresponding to the dose of risperidone was observed, no significant changes in dopamine synthesis capacity by administration of risperidone were observed. It was also noted that there was no significant correlation between occupancy of dopamine D2 receptors and changes in dopamine synthesis capacity by risperidone. On the other hand, a significant negative correlation was observed between the baseline dopamine synthesis capacity and the changes in dopamine synthesis capacity by risperidone. This indicates that the antipsychotic drug risperidone can be considered to stabilize the dopamine synthesis capacity. This suggests that the therapeutic effects of risperidone in schizophrenia might be related to the stabilizing effects on dopaminergic neurotransmission responsivity.
Footnotes
This study was supported in part by a Grant-in-Aid for Molecular Imaging Program from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japanese government, and a Grant-in-Aid for Scientific Research (C) (No. 21591587) from the Japan Society for the Promotion of Science. We thank Katsuyuki Tanimoto, Takahiro Shiraishi, and Toshio Miyamoto for their assistance in performing the PET experiments at the National Institute of Radiological Sciences. We also thank Yoshiko Fukushima and Masako Fukushima of the National Institute of Radiological Sciences for their help as clinical research coordinators.
References
- Balsara JJ, Jadhav JH, Chandorkar AG. Effect of drugs influencing central serotonergic mechanisms on haloperidol-induced catalepsy. Psychopharmacology (Berl) 1979;62:67–69. doi: 10.1007/BF00426037. [DOI] [PubMed] [Google Scholar]
- Baron JC, Martinot JL, Cambon H, Boulenger JP, Poirier MF, Caillard V, Blin J, Huret JD, Loc'h C, Maziere B. Striatal dopamine receptor occupancy during and following withdrawal from neuroleptic treatment: correlative evaluation by positron emission tomography and plasma prolactin levels. Psychopharmacology (Berl) 1989;99:463–472. doi: 10.1007/BF00589893. [DOI] [PubMed] [Google Scholar]
- Brix G, Zaers J, Adam LE, Bellemann ME, Ostertag H, Trojan H, Haberkorn U, Doll J, Oberdorfer F, Lorenz WJ. Performance evaluation of a whole-body PET scanner using the NEMA protocol. J Nucl Med. 1997;38:1614–1623. [PubMed] [Google Scholar]
- Brown RM, Crane AM, Goldman PS. Regional distribution of monoamines in the cerebral cortex and subcortical structures of the rhesus monkey: concentrations and in vivo synthesis rates. Brain Res. 1979;168:133–150. doi: 10.1016/0006-8993(79)90132-x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3-methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Cools R, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D'Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science. 1976;192:481–483. doi: 10.1126/science.3854. [DOI] [PubMed] [Google Scholar]
- Cumming P, Ase A, Laliberté C, Kuwabara H, Gjedde A. In vivo regulation of DOPA decarboxylase by dopamine receptors in rat brain. J Cereb Blood Flow Metab. 1997;17:1254–1260. doi: 10.1097/00004647-199711000-00014. [DOI] [PubMed] [Google Scholar]
- Danielsen EH, Smith D, Hermansen F, Gjedde A, Cumming P. Acute neuroleptic stimulates DOPA decarboxylase in porcine brain in vivo. Synapse. 2001;41:172–175. doi: 10.1002/syn.1071. [DOI] [PubMed] [Google Scholar]
- Davila R, Manero E, Zumarraga M, Andia I, Schweitzer JW, Friedhoff AJ. Plasma homovanillic acid as a predictor of response to neuroleptics. Arch Gen Psychiatry. 1988;45:564–567. doi: 10.1001/archpsyc.1988.01800300060007. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Jentsch JD, Morrow BA, Redmond DE, Jr, Roth RH. Clozapine normalizes prefrontal cortex dopamine transmission in monkeys subchronically exposed to phencyclidine. Neuropsychopharmacology. 2008;33:491–496. doi: 10.1038/sj.npp.1301448. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Halldin C, Sedvall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatry. 1988;45:71–76. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Dolan RJ, Lammertsma AA, Frackowiak RS. The relationship between global and local changes in PET scans. J Cereb Blood Flow Metab. 1990;10:458–466. doi: 10.1038/jcbfm.1990.88. [DOI] [PubMed] [Google Scholar]
- Gjedde A. Exchange diffusion of large neutral amino acids between blood and brain. In: Rakic L, Begley DJ, Davson H, Zlokovic BV, editors. Peptide and amino acid transport mechanisms in the cerebral nervous system. New York: Stockton; 1988. pp. 209–217. [Google Scholar]
- Gjedde A, Reith J, Dyve S, Leger G, Guttman M, Diksic M, Evans A, Kuwabara H. Dopa decarboxylase activity of the living human brain. Proc Natl Acad Sci U S A. 1991;88:2721–2725. doi: 10.1073/pnas.88.7.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Gründer G, Vernaleken I, Müller MJ, Davids E, Heydari N, Buchholz HG, Bartenstein P, Munk OL, Stoeter P, Wong DF, Gjedde A, Cumming P. Subchronic haloperidol downregulates dopamine synthesis capacity in the brain of schizophrenic patients in vivo. Neuropsychopharmacology. 2003;28:787–794. doi: 10.1038/sj.npp.1300103. [DOI] [PubMed] [Google Scholar]
- Hartvig P, Agren H, Reibring L, Tedroff J, Bjurling P, Kihlberg T, Långström B. Brain kinetics of l-[β-11C]dopa in humans studied by positron emission tomography. J Neural Transm Gen Sect. 1991;86:25–41. doi: 10.1007/BF01250373. [DOI] [PubMed] [Google Scholar]
- Hertel P, Nomikos GG, Iurlo M, Svensson TH. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl) 1996;124:74–86. doi: 10.1007/BF02245607. [DOI] [PubMed] [Google Scholar]
- Hicks PB. The effect of serotonergic agents on haloperidol-induced catalepsy. Life Sci. 1990;47:1609–1615. doi: 10.1016/0024-3205(90)90365-x. [DOI] [PubMed] [Google Scholar]
- Ito H, Ota M, Ikoma Y, Seki C, Yasuno F, Takano A, Maeda J, Nakao R, Suzuki K, Suhara T. Quantitative analysis of dopamine synthesis in human brain using positron emission tomography with l-[β-11C]DOPA. Nucl Med Commun. 2006;27:723–731. doi: 10.1097/01.mnm.0000230069.08576.6d. [DOI] [PubMed] [Google Scholar]
- Ito H, Shidahara M, Takano H, Takahashi H, Nozaki S, Suhara T. Mapping of central dopamine synthesis in man using positron emission tomography with l-[β-11C]DOPA. Ann Nucl Med. 2007;21:355–360. doi: 10.1007/s12149-007-0033-z. [DOI] [PubMed] [Google Scholar]
- Korsgaard S, Gerlach J, Christensson E. Behavioral aspects of serotonin-dopamine interaction in the monkey. Eur J Pharmacol. 1985;118:245–252. doi: 10.1016/0014-2999(85)90135-9. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- Leysen JE, Janssen PM, Megens AA, Schotte A. Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry. 1994;55(Suppl):5–12. [PubMed] [Google Scholar]
- Lloyd KG, Hornykiewicz O. Occurrence and distribution of aromatic l-amino acid (L-DOPA) decarboxylase in the human brain. J Neurochem. 1972;19:1549–1559. doi: 10.1111/j.1471-4159.1972.tb05099.x. [DOI] [PubMed] [Google Scholar]
- Mamo D, Remington G, Nobrega J, Hussey D, Chirakal R, Wilson AA, Baker G, Houle S, Kapur S. Effect of acute antipsychotic administration on dopamine synthesis in rodents and human subjects using 6-[18F]-l-m-tyrosine. Synapse. 2004;52:153–162. doi: 10.1002/syn.20016. [DOI] [PubMed] [Google Scholar]
- Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D2, 5-HT2, and 5-HT1A receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164:1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Farde L, Eriksson L, Halldin C, Eriksson B. 5-HT2 and D2 dopamine receptor occupancy in the living human brain. A PET study with risperidone. Psychopharmacology (Berl) 1993;110:265–272. doi: 10.1007/BF02251280. [DOI] [PubMed] [Google Scholar]
- O'Keeffe R, Sharman DF, Vogt M. Effect of drugs used in psychoses on cerebral dopamine metabolism. Br J Pharmacol. 1970;38:287–304. doi: 10.1111/j.1476-5381.1970.tb08517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Pehek EA, McFarlane HG, Maguschak K, Price B, Pluto CP. M100,907, a selective 5-HT2A antagonist, attenuates dopamine release in the rat medial prefrontal cortex. Brain Res. 2001;888:51–59. doi: 10.1016/s0006-8993(00)03004-3. [DOI] [PubMed] [Google Scholar]
- Pickar D, Breier A, Kelsoe J. Plasma homovanillic acid as an index of central dopaminergic activity: studies in schizophrenic patients. Ann N Y Acad Sci. 1988;537:339–346. doi: 10.1111/j.1749-6632.1988.tb42118.x. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Sugaya Y, Sasaki Y, Goshima Y, Kitahama K, Kusakabe T, Miyamae T, Kato T, Misu Y. Autoradiographic studies using l-[14C]DOPA and l-DOPA reveal regional Na+-dependent uptake of the neurotransmitter candidate l-DOPA in the CNS. Neuroscience. 2001;104:1–14. doi: 10.1016/s0306-4522(01)00008-2. [DOI] [PubMed] [Google Scholar]
- Torstenson R, Hartvig P, Långström B, Bastami S, Antoni G, Tedroff J. Effect of apomorphine infusion on dopamine synthesis rate relates to dopaminergic tone. Neuropharmacology. 1998;37:989–995. doi: 10.1016/s0028-3908(98)00085-9. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Kumakura Y, Cumming P, Buchholz HG, Siessmeier T, Stoeter P, Müller MJ, Bartenstein P, Gründer G. Modulation of [18F]fluorodopa (FDOPA) kinetics in the brain of healthy volunteers after acute haloperidol challenge. Neuroimage. 2006;30:1332–1339. doi: 10.1016/j.neuroimage.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Vernaleken I, Kumakura Y, Buchholz HG, Siessmeier T, Hilgers RD, Bartenstein P, Cumming P, Gründer G. Baseline [18F]-FDOPA kinetics are predictive of haloperidol-induced changes in dopamine turnover and cognitive performance: a positron emission tomography study in healthy subjects. Neuroimage. 2008;40:1222–1231. doi: 10.1016/j.neuroimage.2007.12.045. [DOI] [PubMed] [Google Scholar]
- Watson CC, Newport D, Casey ME. A single scatter simulation technique for scatter correction in 3D PET. In: Grangeat P, Amans JL, editors. Three-dimensional image reconstruction in radiology and nuclear medicine. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 255–268. [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. Regulation of aromatic l-amino acid decarboxylase by dopamine receptors in the rat brain. J Neurochem. 1992;58:636–641. doi: 10.1111/j.1471-4159.1992.tb09765.x. [DOI] [PubMed] [Google Scholar]
- Zhu MY, Juorio AV, Paterson IA, Boulton AA. Regulation of striatal aromatic l-amino acid decarboxylase: effects of blockade or activation of dopamine receptors. Eur J Pharmacol. 1993;238:157–164. doi: 10.1016/0014-2999(93)90843-7. [DOI] [PubMed] [Google Scholar]