Abstract
Extracellular acetylcholine (ACh) levels in the dorsal hippocampus increases during learning or exploration, exhibiting a sex-specific 24 h release profile. To examine the activational effect of gonadal steroid hormones on the sex-specific ACh levels and its correlation with spontaneous locomotor activity, we observed these parameters simultaneously for 24 h. Gonadectomy severely attenuated the ACh levels, whereas the testosterone replacement in gonadectomized males or 17β-estradiol replacement in gonadectomized females successfully restored the levels. 17β-Estradiol-priming in gonadectomized males could not restore the ACh levels, and testosterone replacement in gonadectomized females failed to raise ACh levels to those seen in testosterone-primed gonadectomized males, revealing a sex-specific activational effect. Spontaneous locomotor activity was not changed in males by gonadectomy or the replacement of gonadal steroids, but 17β-estradiol enhanced the activity in gonadectomized females. Gonadectomy severely reduced the correlation between ACh release and activity levels, but the testosterone replacement in gonadectomized males or 17β-estradiol replacement in gonadectomized females successfully restored it. To further analyze the sex-specific effect of gonadal steroids, we examined the organizational effect of gonadal steroids on the ACh release in female rats. Neonatal testosterone or 17β-estradiol treatment not only increased the ACh levels but also altered them to resemble male-specific ACh release properties without affecting levels of spontaneous locomotor activity. We conclude that the activational effects of gonadal steroids maintaining the ACh levels and the high correlation with spontaneous locomotor activity are sex-specific, and that the organizational effects of gonadal steroids suggest estrogen receptor-mediated masculinization of the septo-hippocampal cholinergic system.
Introduction
Cholinergic activation of the hippocampus is necessary for both spatial and contextual memory consolidation (Gale et al., 2001; Wallenstein and Vago 2001; Herrera-Morales et al., 2007). Extracellular acetylcholine (ACh) levels in the hippocampus increase during exploration or learning (Ragozzino et al., 1996; Stancampiano et al., 1999; Giovannini et al., 2001), exhibiting a clear diurnal rhythm (Mitsushima et al., 1998). The episodic activation of septo-hippocampal cholinergic system is important for the generation of theta oscillations (Lee et al., 1994; Buzsáki 2002) that modulate the induction of long-term potentiation (LTP) in the hippocampal CA1 neurons (Hyman et al., 2003). Moreover, the released ACh not only enhances synaptic plasticity via the muscarinic M1/M2 receptors (Seeger et al., 2004; Shinoe et al., 2005), but is also responsible for neurogenesis in the dentate gyrus (Mohapel et al., 2005; Kotani et al., 2006).
Spatial memory in rats requires the dorsal hippocampus (Moser et al., 1995; O'Keefe and Burgess 1996), which exhibits sex-specific extracellular ACh levels (Masuda et al., 2005). Male rats have significantly higher ACh levels in the dorsal hippocampus than females, which is consistent with superior spatial ability in males (Jones et al., 2003; Jonasson 2005). We previously hypothesized that sex-specific ACh levels could be attributed to the organizational and/or activational effects of gonadal steroids (Mitsushima et al., 2008b). Although neuroanatomical, neurochemical, and behavioral evidence have suggested an activational effect of gonadal steroids on the septo-hippocampal cholinergic neurons for decades (Luine et al., 1986; Gibbs and Pfaff 1992; Daniel et al., 1997; Mufson et al., 1999; Kritzer et al., 2001; Markowska and Savonenko 2002; Miettinen et al., 2002; Nakamura et al., 2002), conclusive evidence has been lacking in freely behaving rats. Further, the organizational effect of gonadal steroids on ACh levels is completely unknown.
Acetylcholine levels episodically change with spontaneous movement (Day et al., 1991; Mitsushima et al., 1996) that stimulates electrical activity of cholinergic neurons in the basal forebrain (Buzsáki et al., 1988). Moreover, voluntary running enhances neurogenesis, spatial learning, and synaptic plasticity in mice (van Praag et al., 1999). In contrast, a restriction of exploratory behavior reduces ACh levels as well as the spatial learning (Mitsushima et al., 1998, 2001). Therefore, it is necessary to rule out the possibility that the effects of gonadal steroids reflect the change in spontaneous locomotor activity. By simultaneous monitoring of ACh levels and spontaneous locomotor activity, we revealed a sex-specific activational effect of gonadal steroids on the 24 h ACh release profile in freely behaving rats. Moreover, the sex-specific activational effect was affected by neonatal estradiol injection, suggesting sexual differentiation of septo-hippocampal cholinergic system.
Materials and Methods
Subjects
Male and female Wistar-Imamichi rats were obtained from Animal Reproduction Research at 7–8 weeks of age. Same sex groups of 2–3 rats were housed in plastic cages (length 31 cm, width 47 cm, height 20 cm) at a constant temperature of 23 ± 1°C under a constant cycle of light and dark (lights on: 5:00 A.M. to 7:00 P.M.). Food and water were available ad libitum in all experimental periods. All animal housing and surgical procedures were in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Animal Research Center, Yokohama City University Graduate School of Medicine.
Experimental design
Experiment I.
To examine the activational effect of gonadal steroids on sex-specific extracellular ACh levels, each sex was divided into 4–5 groups. Male groups were (1) gonadally intact male rats (Males), (2) orchidectomized rats (ORX), (3) orchidectomized testosterone-primed rats (ORX+T), and (4) orchidectomized 17β-estradiol-primed rats (ORX+E); female groups were (1) gonadally intact diestrous female rats (Diestrous Females), (2) gonadally intact female rats from proestrus to estrus (Proestrous Females) (3) ovariectomized rats (OVX), (4) ovariectomized testosterone-primed rats (OVX+T), and (5) ovariectomized 17β-estradiol-primed rats (OVX+E). Gonadectomy was performed 14.8 ± 0.6 d before the in vivo experiment, and a testosterone or 17β-estradiol capsule was subcutaneously implanted on the day of the gonadectomy. To make a 17β-estradiol capsule, we packed a 1:4 mixture of 17β-estradiol (Sigma) and cholesterol crystals in a piece of SILASTIC tubing (15 mm length per 250 g body weight, i.d. 2.0 mm, o.d. 3.0 mm; Dow Corning). To make a testosterone capsule, we packed testosterone crystals (Sigma) in a piece of SILASTIC tubing (30 mm length per 250 g body weight, i.d. 2.0 mm, o.d. 3.0 mm; Dow Corning). Using the testosterone capsule, we maintained serum testosterone levels at the levels in intact males. Using the 17β-estradiol capsule, we maintained serum 17β-estradiol levels at the levels in proestrous female rats (Mitsushima et al., 2008b). We previously confirmed the efficacy of the hormone treatments at the behavioral level: OVX+E females showed high lordosis quotient (∼90%) and persistent vaginal cornification (N = 10). In contrast, ORX+T males displayed vigorous mounting and anogenital sniffing toward the receptive females (N = 10).
Experiment II.
To examine the organizational effect of gonadal steroids on the ACh levels, an in vivo microdialysis study was performed in neonatally steroid-treated female rats. On the day of birth and 24 h later, testosterone propionate (100 μg/50 μl, Sigma), 17β-estradiol benzoate (100 μg/50 μl, Sigma), 5α-dihydrotestosterone (100 μg/50 μl, Fluka Chemie), or sesame oil (50 μl) was subcutaneously injected into the back skin of pups as previously described (Mong et al., 1999; Amateau et al., 2004). After maturation (8 weeks old), the rats were ovariectomized and a testosterone capsule was subcutaneously implanted. We performed in vivo microdialysis 16.2 ± 0.4 d after the procedure. To confirm that the neonatal steroid treatment was effective in inducing sexual differentiation, we observed behavioral responses to a sexually receptive female for 10 min after the dialysate sampling.
Surgery
Under sodium pentobarbital anesthesia (30–50 mg/kg, i.p.), a stainless-steel guide cannula (outer diameter, 0.51 mm) was implanted stereotaxically into the right side of the dorsal hippocampus. The coordinates were 4.0–4.3 mm anterior from the ear-bar, 3.0 mm lateral to the midline, and 2.1–2.2 mm below the surface of the brain according to the brain atlas of Paxinos and Watson (1997). The coordinates were adjusted based on sex and body weight. After cannula implantation, a stylet was inserted into the guide until the microdialysis was performed. Although rats were reared and housed in group cages, each rat was individually housed in a cylindrical plastic cage (diameter = 35 cm, height = 45 cm) for 10.5 ± 1.4 d. During this period, vaginal smears were taken from the female rats to confirm expression of the normal estrous cycle. Male rats were handled for a short time daily.
In vivo microdialysis
The experiment was performed in an electromagnetic- and sound-shielded room (Mitsushima et al., 2006; Jitsuki et al., 2009) (length 1.2 m, width 2.2 m, height 2.3 m). The stylet was replaced with a microdialysis probe the day before the experiment (outer diameter = 0.31 mm, AI-8-1; Eicom Co.). A two-channel fluid swivel device (SSU-20; Eicom Co.) was connected to the inlet and outlet of the probe. During the experiment, an artificial CSF solution (containing, in mm: 147 NaCl, 4 KCl, 1.2 CaCl2, 0.9 MgCl2) was infused through the dialysis probe with 1.0-mm-long semipermeable membrane at a rate of 1.2 μl/min using a microdialysis pump (CMA/102; Carnegie Medicin). The rats were housed individually in their cage, and the dialysis was performed under unanesthetized, freely moving conditions (Mitsushima et al., 2006, 2008b). After the overnight stabilization period, dialysates were automatically collected in an autoinjector (24 μl) (EAS-20; Eicom Co.) every 20 min for 24 h and the same volume of ethylhomocholine solution (100 nm) was mixed in as the internal standard. This mixture was injected directly into a HPLC column every 20 min (Takase et al., 2007; Mitsushima et al., 1998). In intact female rats, we collected dialysates on diestrous days (i.e., from diestrous 1 to diestrous 2) or the days from proestrus to estrus.
Biochemical analysis of ACh
To examine in vivo ACh levels without artificial chemicals, no eserine was used in the present study (Takase et al., 2007). ACh was quantified by a combination of HPLC column, enzyme reaction, and electrochemical detection (HTEC-500; Eicom Co.). A solution consisting of 0.1 mm Na2HPO4, pH 8.5, containing 200 mg/L sodium 1-decanesulfonate (Aldrich Chemical Company) was delivered as the HPLC mobile phase at a rate of 150 μl/min. After sample separation in a styrene polymer column (AC-GEL; Eicom Co.), ACh was converted to hydrogen peroxide by a postcolumn enzyme reactor (AC-ENZYMPAK; Eicom Co.) containing immobilized acetylcholinesterase and choline oxidase. The hydrogen peroxide was detected with an electrochemical detector with a least detectable amount of 5–10 fmol/sample.
To calculate the recovery rate of each dialysis probe, in vitro experiments were also performed. The amount of ACh collected every 20 min was divided by the in vitro recovery rate to estimate the extracellular ACh levels. The in vitro recovery rate was determined for individual probes and applied to the results from individual rats (mean ± SEM; 13.4 ± 0.5%).
Measurement of spontaneous locomotor activity
During dialysate collection, the rats were individually housed in cylindrical plastic cages (diameter 35 cm, height 45 cm) placed on dielectric constant sensors with counters (ACTMONITOR II; Dia Medical System Co.). The spontaneous locomotor activity was evaluated by changes in the dielectric constant and recorded in a personal computer every 20 min for 24 h (VAIO PCG-Z1/P, Sony) using an interface unit (DAS-008; Neuroscience Inc.) (Mitsushima et al., 1996, 1997). Activity counts were individually normalized by body weight using a previously described formula (Takase et al., 2007).
Histology
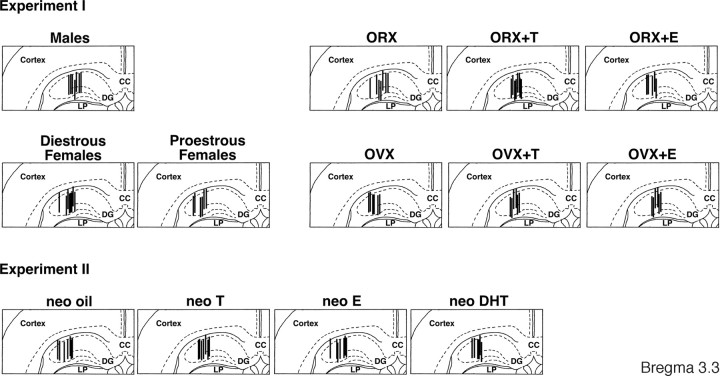
After the sampling, the animals were deeply anesthetized and perfused with a 10% formalin solution. Frozen coronal sections (50 μm thick) were sequentially cut from the forebrain using a microtome (MA-101; Yamato Koki Co.). The location of the dialysis probe was microscopically verified in the frozen sections (Fig. 1).
Figure 1.
Location of the microdialysis probes within the dorsal hippocampus. Vertical lines represent the 1.0 mm length of the dialysis membrane. CC, Corpus callosum; DG, dentate gyrus; LP, lateral posterior nucleus. The number indicates the distance posterior to the bregma.
Statistics
In experiment I, extracellular ACh levels or spontaneous locomotor activity were analyzed by three-way ANOVA with repeated measures; between group factors were sex and steroid, and within group factor was time points. Correlation coefficient or slope was analyzed by two-way factorial ANOVA, where the variables were sex and steroid. These were followed by post hoc ANOVAs with the Fisher protected least significant difference test. Simple linear regression was used to evaluate the relationship between ACh levels and spontaneous locomotor activity. Pearson's correlation coefficient (Mitsushima et al., 1996) and slope of the best fit line (Takase et al., 2009) were calculated for each individual rat. To perform the ANOVAs, proestrous and diestrous female groups were combined as cycling females.
In experiment II, ACh levels or spontaneous locomotor activity were analyzed by two-way ANOVA with repeated measures; between group factor was neonatal steroid treatment, and within group factor was time points. This was followed by post hoc ANOVAs with the Fisher protected least significant difference test. The effect of neonatal gonadal steroids on correlation coefficient, slope, or sexual behavior was analyzed by one-way factorial ANOVA followed by the post hoc test. p < 0.05 was considered statistically significant.
Results
Extracellular ACh levels
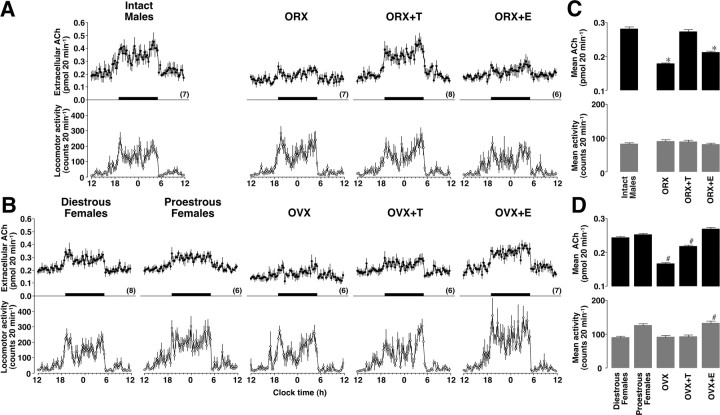
All groups exhibited an episodic ACh release profile in the dorsal hippocampus throughout the day, confirming the presence of a significant diurnal rhythm. In male groups (Fig. 2A), the orchidectomy clearly reduced extracellular ACh levels, which were successfully restored by testosterone replacement. However, 17β-estradiol replacement could not restore ACh levels in ORX males. In female groups (Fig. 2B), the extracellular ACh levels were clearly reduced by ovariectomy, and 17β-estradiol replacement successfully restored them. Testosterone replacement slightly, but significantly, increased the levels. Three-way ANOVA found the main effects of steroid (F(3,3763) = 9.159, p < 0.01) and time (F(71,3763) = 56.026, p < 0.01). Significant interactions were also observed between sex and steroid (F(3,3763) = 2.977, p < 0.04), between steroid and time (F(213,3763) = 1.780, p < 0.01), and among sex, steroid, and time (F(213,3763) = 2.098, p < 0.01). Intact male rats had significantly greater ACh levels than cycling female rats (p < 0.01). Moreover, ORX+T males had significantly greater ACh levels than OVX+T females (p < 0.01), whereas ORX+E males had significantly smaller ACh levels than OVX+E females (p < 0.01). The results of the statistical analysis are summarized in Figure 2, C and D.
Figure 2.
A–D, Activational effects of gonadal steroids on the extracellular ACh levels and spontaneous locomotor activity. In male groups (A), ORX reduced the ACh levels without changing the spontaneous locomotor activity. Replacement of testosterone (+T) but not 17β-estradiol (+E) restored the ACh levels. In female groups (B), OVX reduced the ACh levels. Testosterone replacement in OVX females failed to raise ACh levels to those seen in testosterone-primed ORX males. Replacement of 17β-estradiol not only maintained ACh levels, but also enhanced spontaneous locomotor activity. Horizontal black bars indicate the dark phase. Overall levels of ACh and spontaneous locomotor activity were statistically summarized for male (C) and female (D) groups. *p < 0.01 compared with gonadally intact males. #p < 0.01 compared with cycling females (diestrous and proestrous groups were combined for statistical analysis). The number of rats in each group is shown in parentheses. Data are expressed as the mean ± SEM.
Spontaneous locomotor activity
All groups exhibited an episodic locomotor activity profile throughout the day, showing a clear diurnal rhythm. In male groups (Fig. 2A), orchidectomy and hormone replacement after orchidectomy did not alter observed locomotor activity. In contrast, female groups (Fig. 2B) exhibited some differences in response to ovariectomy and hormone replacement. Although proestrous and OVX+E females had relatively higher locomotor activity than diestrous females, the levels in OVX or OVX+T females were similar to the levels in diestrous females. Three-way ANOVA found the main effects of sex (F(1,3763) = 7.318, p < 0.01) and time (F(71,3763) = 46.084, p < 0.01). Significant interaction was also observed among sex, steroid and time (F(213,3763) = 1.252, p < 0.01). OVX+E females had significantly greater locomotor activity than cycling, OVX, or OVX+T females (p < 0.01). The results of the statistical analysis are summarized in Figure 2, C and D.
Correlation between the ACh and locomotor activity
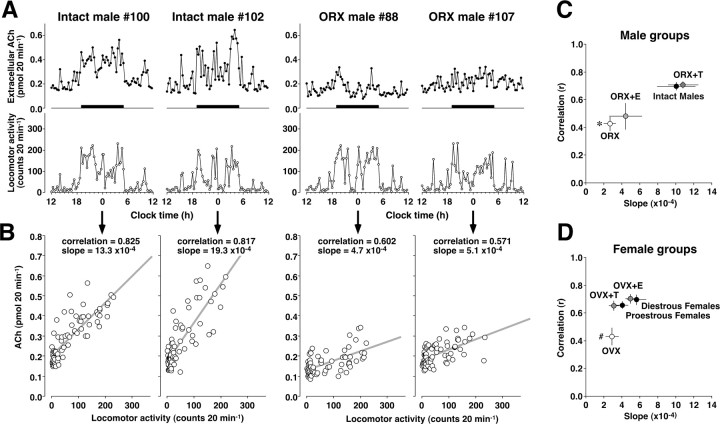
Both sexes exhibited a positive correlation between the ACh levels and the spontaneous locomotor activity. Representative cases of 2 gonadally intact and 2 ORX males are shown in Figure 3A. Although the ACh levels in intact males (#100 and #102) were highly correlated with the spontaneous locomotor activity, the ACh levels in ORX males (#88 and #107) were not always high when they are moving. The individual results of simple linear regression analysis are shown in Figure 3B. Intact males had a higher correlation coefficient and a steeper slope of the fit line than ORX males. All correlation and slope data were summarized in Figure 3, C and D. For the correlation coefficient, two-way ANOVA showed that the main effect of steroid (F(3,53) = 16.328, p < 0.01) and the interaction between sex and steroid (F(3,53) = 3.501, p < 0.02) were significant. For the slope of the fit line, the main effects of sex (F(1,53) = 13.220, p < 0.01) and steroid (F(3,53) = 7.137, p < 0.01), as well as the interaction between sex and steroid (F(3,53) = 6.568, p < 0.01) were significant. In the male groups, reduced correlation and slope in ORX males (p < 0.01) were successfully restored in ORX+T males (Fig. 3C). Similarly, in the female groups, a reduced correlation in OVX females (p < 0.01) was successfully restored by treatment with either 17β-estradiol or testosterone, although the slope was relatively small and without difference (Fig. 3D). Moreover, data from intact males had a significantly steeper slope than cycling females (p < 0.01), showing a sex-specific ACh release property.
Figure 3.
A, Correlation between ACh levels and spontaneous locomotor activity in 2 gonadally intact and 2 ORX male rats. B, Simple linear regression analysis showed that the data from intact males (#100 and #102) had a steep slope of the fit line, whereas the data from ORX males (#88 and #107) had a gentle slope. C, Orchidectomy reduced both correlation and slope, with testosterone replacement (+T) restoring the property in male groups. D, OVX reduced the correlation, with steroid replacement either testosterone or 17β-estradiol (+E) restoring the property in female groups. *p < 0.01 compared with males (both correlation and slope). #p < 0.01 compared with cycling females (correlation only). Moreover, intact males had significantly steeper slope than cycling females (p < 0.01). Data in C and D represent the means ± SEM.
Neonatal gonadal steroid treatments
To examine the organizational effect of gonadal steroids on 24 h ACh release, we subcutaneously injected oil, testosterone, 17β-estradiol, or 5α-dihydrotestosterone into neonatal female rats (Experiment II, see Materials and Methods). At 8 weeks of age, either dihydrotestosterone- or oil-treated rats showed normal 4 d estrous cycles, whereas 17β-estradiol-treated rats showed clear constant estrus. The vagina did not open in all testosterone-treated rats. Then, the four groups of rats were bilaterally ovariectomized, and a testosterone capsule was implanted.
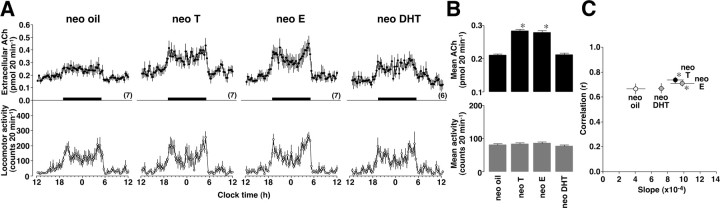
The ACh levels exhibited a clear diurnal rhythm in all 4 groups. Although neonatal testosterone or estradiol treatment significantly increased the ACh levels, the effect was not clear in neonatal dihydrotestosterone treatment (Fig. 4A). Two-way ANOVA found the main effect of time (F(71,1633) = 26.201, p < 0.01) and the interaction between treatment and time (F(213,3763) = 1.252, p < 0.01). The results of the statistical analysis are summarized in Figure 4B.
Figure 4.
Organizational effects of gonadal steroids on ACh levels in female rats. At postnatal days 0 and 1, female pups were given oil (neo oil), testosterone (neo T), 17β-estradiol (neo E), or 5α-dihydrotestosterone (neo DHT). After maturation, the rats were bilaterally ovariectomized and given testosterone replacement. A, Neo oil or neo DHT rats exhibited relatively low ACh levels, whereas neo T or neo E rats enhanced the ACh levels compared with male rats. B, Overall levels of ACh and spontaneous locomotor activity were statistically summarized for neonatally steroid-treated female rats. Neo T or neo E treatment significantly enhanced the mean ACh levels in female rats without changing the spontaneous locomotor activity. C, In simple linear regression analysis, neo T or neo E data had significantly steeper slope than neo oil data. *p < 0.01 compared with neo oil. The number of rats in each group is shown in parentheses. Data are expressed as the mean ± SEM.
Locomotor activity exhibited a clear diurnal rhythm in all 4 groups, but the effect of neonatal treatment was not clear (Fig. 4A). Two-way ANOVA found the main effect of time (F(71,1633) = 20.406, p < 0.01), but the main effect of treatment or the interaction was not significant (Fig. 4B).
The results of regression analysis are shown in Figure 4C. Although there was no difference in the correlation coefficient (F(3,23) = 1.405, p > 0.05), data from neonatally testosterone (neo T)- or 17β-estradiol-treated (neo E) rats had a steeper slope of the fit line than oil-treated rats (neo oil), revealing male-specific ACh release (F(3,23) = 5.230, p < 0.01). In addition, neonatal dihydrotestosterone treatment may masculinize the ACh release property (neo DHT, p = 0.06 vs neo oil). Moreover, the neonatal testosterone or estradiol treatment enhanced male-like copulatory behavior in the rats (Table 1, F(3,23) = 10.139, p < 0.01).
Table 1.
Mounting behaviors in neonatally steroid-treated rats
| Group | Number of animals | Number of mounts per 10 min |
|---|---|---|
| neo oil | 7 | 8.3 ± 1.4 |
| neo T | 7 | 23.0 ± 3.3* |
| neo E | 7 | 23.9 ± 2.5* |
| neo DHT | 6 | 10.3 ± 2.8 |
*Significantly different from neo oil (p < 0.01).
Discussion
In experiment I, we found an activational effect of gonadal steroids on the extracellular ACh levels in the dorsal hippocampus of behaving rats; the levels were severely reduced after gonadectomy and testosterone replacement in ORX males or 17β-estradiol replacement in OVX females successfully restored them. Moreover, testosterone replacement in OVX females failed to increase ACh to the levels seen in ORX+T males. In experiment II, neonatal androgenization not only increased ACh levels but also altered ACh release to resemble that of males without affecting spontaneous activity levels. This is the first report showing an organizational effect of gonadal steroids on sex-specific ACh release profiles in behaving rats.
The activational effects of gonadal steroids on cholinergic neurons are consistent with previous neuroanatomical and neurochemical findings. For example, orchidectomy decreases the density of cholinergic fibers in the dorsal hippocampus, whereas testosterone replacement in ORX male rats maintains fiber density (Nakamura et al., 2002). Also, 17β-estradiol increases the induction of choline acetyltransferase in the basal forebrain in OVX female rats (Luine et al., 1986; McEwen and Alves, 1999). A previous in vitro study demonstrated that 17β-estradiol treatment increases both high affinity choline uptake and ACh synthesis in basal forebrain neurons (Pongrac et al., 2004). Furthermore, we recently reported an activational effect of gonadal steroids on the maintenance of stress-induced ACh release in the dorsal hippocampus in immobilized rats (Mitsushima et al., 2008b). Despite all of this evidence suggesting the activational effect of gonadal steroids on ACh release in the dorsal hippocampus, conclusive evidence such as dynamic ACh changes under physiological conditions has not been presented in behaving animals. Because spontaneous movement increases extracellular ACh levels (Day et al., 1991; Mitsushima et al., 1996), we simultaneously analyzed ACh levels and spontaneous locomotor activity to determine the precise effect of gonadal steroids. In the present study, we found that gonadectomy impaired ACh levels without affecting spontaneous locomotor activity levels. Moreover, the activational effect on ACh levels was clear especially during active period, although it is not clear during resting period (Mitsushima et al., 2008b). Our results provide the first evidence that the 24 h extracellular ACh levels in the dorsal hippocampus are dependent on the presence of gonadal steroids.
Acetylcholine levels were still sex-specific under the comparable gonadal steroids levels. For example, testosterone replacement in OVX females failed to increase ACh levels to those seen in ORX+T males. Moreover, 17β-estradiol replacement was unable to restore ACh levels in ORX males. These results may be consistent with previous neuroanatomical reports at the spine synapse level. First, estradiol benzoate consistently increases the NMDA receptor binding and spine density in the CA1 area of OVX females, although the treatment fails to increase the parameters in ORX males (Romeo et al., 2005; Parducz et al., 2006). Second, androgens are important in maintaining normal spine synapse numbers on CA1 pyramidal neurons, whereas estrogen is important only in females (MacLusky et al., 2006). These findings suggest that the activational effects of gonadal steroids are sex-specific and essential for maintaining hippocampal functions. Based on the reports, together with the present data from experiment I, we hypothesized that the action of sex-specific steroids is the result of neonatal sexual differentiation rather than the activational effect of gonadal steroids in adult rats.
In experiment II, we examined the organizational effects of testosterone under the comparable gonadal steroids levels. Because ACh levels exhibited distinct sex difference in gonadectomized testosterone-primed condition (see ORX+T vs OVX+T), we chose the steroid condition to evaluate the organizational effects. Without gonadal steroid-priming, gonadectomized rats did not show a clear sex difference. In gonadectomized 17β-estradiol-primed condition, it would be difficult to interpret the sex-specific ACh levels, because 17β-estradiol increases spontaneous locomotor activity in OVX females. We found that neonatal testosterone injection not only increased the ACh levels but also altered the ACh release property to resemble the male-specific profile without changing spontaneous activity levels. These results provide the first evidence that neonatal testosterone exposure sexually differentiates septo-hippocampal cholinergic neurons.
We further analyzed the organizational effect of testosterone using 17β-estradiol and 5α-dihydrotestosterone. 17β-Estradiol is the aromatized product of testosterone, acting as an estrogen receptor agonist. In contrast, 5α-dihydrotestosterone is the 5α-reduced metabolite of testosterone, acting as an androgen receptor agonist. Because testosterone can be converted to 17β-estradiol or 5α-dihydrotestosterone in neonatal rat brain (Zwain and Yen, 1999), neonatal testosterone can activate both estrogen and androgen receptors (McEwen, 1981). Concerning other sex-specific functions, estrogen receptor is known to mediate the activation of male copulatory behavior and the disruption of estrous cyclicity (McEwen, 1981; Herath et al., 2001), whereas androgen receptors mediate the masculinization of social play (Meaney et al., 1983). In experiment II, not only testosterone but also 17β-estradiol treatment in neonatal female pups masculinized ACh release property in adults, suggesting estrogen receptor-mediated masculinization of septo-hippocampal cholinergic systems. Consistently, testosterone or estradiol treatment in neonatal female pups improves their adult spatial performance, whereas neonatal gonadectomy in male pups causes decrements in the performance (Williams and Meck 1991). In contrast, neonatal 5α-dihydrotestosterone treatment may partly masculinized the ACh release property, but failed to increase the overall ACh levels. Although 5α-dihydrotestosterone has been classically considered a prototypical androgen receptor agonist, a metabolite of 5α-dihydrotestosterone, 5α-androstane-3β,17β-diol (3β-diol), has higher affinity for estrogen receptor β (Lund et al., 2006). Therefore, 5α-dihydrotestosterone and its metabolites (3β-diol) may stimulate both androgen receptor and estrogen receptor β, whereas 17β-estradiol stimulates estrogen receptor α and β. Considering the action of gonadal steroids and their metabolites, estrogen receptor α may mediate the organizational effect on septo-hippocampal cholinergic system and spatial learning performance.
Acetylcholine in the dorsal hippocampus has physiological relevance. Behavioral studies have demonstrated that the level of extracellular ACh increases during learning (Ragozzino et al., 1996; Stancampiano et al., 1999) and is positively correlated with memory performance (Gold, 2003). Endogenous ACh in the hippocampus plays an important role in both spatial and contextual memory consolidation. Bilateral injections of scopolamine into the dorsal hippocampus impair both spatial learning (Herrera-Morales et al., 2007) and contextual encoding in male rats (Wallenstein and Vago 2001). At the cellular level, both pyramidal and nonpyramidal neurons in the hippocampal CA1 area receive direct cholinergic afferents mediated by the muscarinic receptors (Cole and Nicoll 1983; Widmer et al., 2006).
Interestingly, both in vivo learning (Whitlock et al., 2006) and in vitro bath application of carbachol, a cholinergic agonist, induce LTP in the hippocampal CA1 region without artificial tetanus stimulus (Auerbach and Segal 1996). Furthermore, gene disruption of muscarinic M2 receptors not only impairs spatial learning but also blocks the carbachol-induced LTP in the Schaffer collateral pathway to the hippocampal CA1 region (Seeger et al., 2004), suggesting the involvement of muscarinic M2 receptors. Although the molecular mechanism of carbachol-induced LTP was unknown, Shi et al. (2001) demonstrated that synaptic delivery of AMPA receptors is a mechanism of electrically induced LTP. Moreover, in vitro patch clamp recording demonstrated that focal application of ACh induces long-lasting enhancement of Schaffer collateral EPSCs at the CA1 pyramidal neurons (Fernández de Sevilla et al., 2008). Based on these findings, we hypothesized that the ACh released in the hippocampus enhances glutamatergic transmission in CA1 pyramidal neurons, playing a principal role in hippocampal learning. Finally, by combining Herpes virus-mediated in vivo gene delivery with in vitro patch-clamp recordings (Takahashi et al., 2003), we previously revealed that learning-induced ACh release mediates synaptic delivery of AMPA receptors in CA1 pyramidal neurons (Mitsushima et al., 2008a).
In the present study, we found reduced correlation between ACh levels and locomotor activity levels in gonadectomized rats, suggesting that hippocampal function in rats may not always be activated at a subthreshold level of gonadal steroids. High positive correlation between ACh levels and activity levels depends on the presence of gonadal steroids. It is therefore possible, that the learning impairment in gonadectomized rats (Gibbs and Pfaff 1992; Kritzer et al., 2001; Markowska and Savonenko 2002; Luine et al., 2003) may be attributable to insufficient ACh levels in the hippocampus when it is required for memory. These results suggest that circulating gonadal steroids strengthen the coupling between spontaneous behaviors and ACh levels, which in turn, may activate the learning function in the hippocampus at the appropriate time.
In humans, circulating levels of gonadal steroids decline with age. Moreover, a reduction in ACh synthesis is known as a common feature of Alzheimer's disease (Coyle et al., 1983), afflicting >18 million people worldwide (Ferri et al., 2005; Mount and Downtown 2006). The disease is the most common form of dementia (Cummings 2004) and is frequently accompanied by insomnia, poor concentration, or day/night confusion (McCurry et al., 2004; Starkstein et al., 2005). Centrally active acetylcholinesterase inhibitor (donepezil) is effective in not only mild, but also moderate to severe cases (Petersen et al., 2005; Winblad et al., 2006), proving the importance of endogenous ACh in humans. In addition, women are twice as likely to develop the disease (Swaab and Hofman 1995), and estradiol seems to play a protective role (Zandi et al., 2002; Norbury et al., 2007). A recent study using single photon emission tomography showed that estrogen replacement therapy in healthy women increases muscarinic M1/M4 receptor binding in the hippocampus (Norbury et al., 2007). Conversely in men, testosterone but not estradiol seems to play a protective role (Moffat et al., 2004; Rosario et al., 2004) and testosterone supplementation clearly improved hippocampus-dependent learning deficits in men with Alzheimer's disease (Cherrier et al., 2005). These results suggest a sex-specific activational effect of gonadal steroids on the cholinergic system in humans. Thus, there are many similarities between the rat model and the human studies, supporting the idea that the gonadal steroids replacement or an increase in their bioavailability is necessary when there is a subthreshold level of the hormone. Based on the neonatal sexual differentiation of the septo-hippocampal cholinergic system, we may have to search for sex-specific clinical strategies for Alzheimer's disease.
Footnotes
This work was supported by Grant-in-Aid 18590219 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to D.M.).
References
- Amateau SK, Alt JJ, Stamps CL, McCarthy MM. Brain estradiol content in newborn rats: sex differences, regional heterogeneity, and possible de novo synthesis by the female telencephalon. Endocrinology. 2004;145:2906–2917. doi: 10.1210/en.2003-1363. [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Physiol. 1996;492:479–493. doi: 10.1113/jphysiol.1996.sp021323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Buzsáki G, Bickford RG, Ponomareff G, Thal LJ, Mandel R, Gage FH. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988;8:4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- Cole AE, Nicoll RA. Acetylcholine mediates a slow synaptic potential in hippocampal pyramidal cells. Science. 1983;221:1299–1301. doi: 10.1126/science.6612345. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Alzheimer's disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- Daniel JM, Fader AJ, Spencer AL, Dohanich GP. Estrogen enhances performance of female rats during acquisition of a radial arm maze. Horm Behav. 1997;32:217–225. doi: 10.1006/hbeh.1997.1433. [DOI] [PubMed] [Google Scholar]
- Day J, Damsma G, Fibiger HC. Cholinergic activity in the rat hippocampus, cortex and striatum correlates with locomotor activity: an in vivo microdialysis study. Pharmacol Biochem Behav. 1991;38:723–729. doi: 10.1016/0091-3057(91)90233-r. [DOI] [PubMed] [Google Scholar]
- Fernández de Sevilla D, Núñez A, Borde M, Malinow R, Buño W. Cholinergic-mediated IP3-receptor activation induces long-lasting synaptic enhancement in CA1 pyramidal neurons. J Neurosci. 2008;28:1469–1478. doi: 10.1523/JNEUROSCI.2723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale GD, Anagnostaras SG, Fanselow MS. Cholinergic modulation of Pavlovian fear conditioning: effects of intrahippocampal scopolamine infusion. Hippocampus. 2001;11:371–376. doi: 10.1002/hipo.1051. [DOI] [PubMed] [Google Scholar]
- Gibbs RB, Pfaff DW. Effects of estrogen and fimbria/fornix transection on p75NGFR and ChAT expression in the medial septum and diagonal band of Broca. Exp Neurol. 1992;116:23–39. doi: 10.1016/0014-4886(92)90173-n. [DOI] [PubMed] [Google Scholar]
- Giovannini MG, Rakovska A, Benton RS, Pazzagli M, Bianchi L, Pepeu G. Effects of novelty and habituation on acetylcholine, GABA, and glutamate release from the frontal cortex and hippocampus of freely moving rats. Neuroscience. 2001;106:43–53. doi: 10.1016/s0306-4522(01)00266-4. [DOI] [PubMed] [Google Scholar]
- Gold PE. Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem. 2003;80:194–210. doi: 10.1016/j.nlm.2003.07.003. [DOI] [PubMed] [Google Scholar]
- Herath CB, Watanabe G, Katsuda S, Yoshida M, Suzuki AK, Taya K. Exposure of neonatal female rats to p-tert-octylphenol disrupts afternoon surges of luteinizing hormone, follicle-stimulating hormone and prolactin secretion, and interferes with sexual receptive behavior in adulthood. Biol Reprod. 2001;64:1216–1224. doi: 10.1095/biolreprod64.4.1216. [DOI] [PubMed] [Google Scholar]
- Herrera-Morales W, Mar I, Serrano B, Bermúdez-Rattoni F. Activation of hippocampal postsynaptic muscarinic receptors is involved in long-term spatial memory formation. Eur J Neurosci. 2007;25:1581–1588. doi: 10.1111/j.1460-9568.2007.05391.x. [DOI] [PubMed] [Google Scholar]
- Hyman JM, Wyble BP, Goyal V, Rossi CA, Hasselmo ME. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J Neurosci. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitsuki S, Kimura F, Funabashi T, Takahashi T, Mitsushima D. Sex-specific 24-h profile of extracellular serotonin levels in the medial prefrontal cortex. Brain Res. 2009;1260:30–37. doi: 10.1016/j.brainres.2008.12.084. [DOI] [PubMed] [Google Scholar]
- Jonasson Z. Meta-analysis of sex differences in rodent models of learning and memory: a review of behavioral and biological data. Neurosci Behav Rev. 2005;28:811–825. doi: 10.1016/j.neubiorev.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Jones CM, Braithwaite VA, Healy SD. The evolution of sex differences in spatial ability. Behav Neurosci. 2003;117:403–411. doi: 10.1037/0735-7044.117.3.403. [DOI] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–514. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Kritzer MF, McLaughlin PJ, Smirlis T, Robinson JK. Gonadectomy impairs T-maze acquisition in adult male rats. Horm Behav. 2001;39:167–174. doi: 10.1006/hbeh.2001.1645. [DOI] [PubMed] [Google Scholar]
- Lee MG, Chrobak JJ, Sik A, Wiley RG, Buzsáki G. Hippocampal theta activity following selective lesion of the septal cholinergic system. Neuroscience. 1994;62:1033–1047. doi: 10.1016/0306-4522(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Luine VN, Renner KJ, McEwen BS. Sex-dependent differences in estrogen regulation of choline acetyltransferase are altered by neonatal treatments. Endocrinology. 1986;119:874–878. doi: 10.1210/endo-119-2-874. [DOI] [PubMed] [Google Scholar]
- Luine VN, Jacome LF, Maclusky NJ. Rapid enhancement of visual and place memory by estrogens in rats. Endocrinology. 2003;144:2836–2844. doi: 10.1210/en.2003-0004. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5α-dihydrotestosterone and its metabolite 5α-androstan-3β,17β-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor β-expressing neurons in the hypothalamus. J Neurosci. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLusky NJ, Hajszan T, Prange-Kiel J, Leranth C. Androgen modulation of hippocampal synaptic plasticity. Neuroscience. 2006;138:957–965. doi: 10.1016/j.neuroscience.2005.12.054. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Savonenko AV. Effectiveness of estrogen replacement in restoration of cognitive function after long-term estrogen withdrawal in aging rats. J Neurosci. 2002;22:10985–10995. doi: 10.1523/JNEUROSCI.22-24-10985.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda J, Mitsushima D, Funabashi T, Kimura F. Sex and housing conditions affect the 24-h acetylcholine release profile in the hippocampus in rats. Neuroscience. 2005;132:537–542. doi: 10.1016/j.neuroscience.2005.01.010. [DOI] [PubMed] [Google Scholar]
- McCurry SM, Logsdon RG, Vitiello MV, Teri L. Treatment of sleep and nighttime disturbances in Alzheimer's disease: a behavior management approach. Sleep Med. 2004;5:373–377. doi: 10.1016/j.sleep.2003.11.003. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Neural gonadal steroid actions. Science. 1981;211:1303–1311. doi: 10.1126/science.6259728. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20:279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J, Poulin P, McEwen BS. Sexual differentiation of social play in rat pups is mediated by the neonatal androgen-receptor system. Neuroendocrinology. 1983;37:85–90. doi: 10.1159/000123524. [DOI] [PubMed] [Google Scholar]
- Miettinen RA, Kalesnykas G, Koivisto EH. Estimation of the total number of cholinergic neurons containing estrogen receptor-α in the rat basal forebrain. J Histochem Cytochem. 2002;50:891–902. doi: 10.1177/002215540205000703. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Mizuno T, Kimura F. Age-related changes in diurnal acetylcholine release in the prefrontal cortex of male rats as measured by microdialysis. Neuroscience. 1996;72:429–434. doi: 10.1016/0306-4522(95)00572-2. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Jinnai K, Kimura F. Possible role of the γ-aminobutyric acid-A receptor system in the timing of the proestrous luteinizing hormone surge in rats. Endocrinology. 1997;138:1944–1948. doi: 10.1210/endo.138.5.5097. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamanoi C, Kimura F. Restriction of environmental space attenuates locomotor activity and hippocampal acetylcholine release in male rats. Brain Res. 1998;805:207–212. doi: 10.1016/s0006-8993(98)00735-5. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Funabashi T, Shinohara K, Kimura F. Impairment of maze learning in rats by restricting environmental space. Neurosci Lett. 2001;297:73–76. doi: 10.1016/s0304-3940(00)01670-0. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Yamada K, Takase K, Funabashi T, Kimura F. Sex differences in the basolateral amygdala: the extracellular levels of serotonin and dopamine, and their responses to restraint stress in rats. Eur J Neurosci. 2006;24:3245–3254. doi: 10.1111/j.1460-9568.2006.05214.x. [DOI] [PubMed] [Google Scholar]
- Mitsushima D, Ishihara K, Kamiya Y, Malinow R, Takahashi T. Acetylcholine release during learning mediates synaptic delivery of AMPA receptors into Schaffer collateral synapses in the dorsal hippocampus. The 31st Annual Meeting of the Japan Neuroscience Society Abstr O3–C04.2008a. [Google Scholar]
- Mitsushima D, Takase K, Funabashi T, Kimura F. Gonadal steroid hormones maintain the stress-induced acetylcholine release in the hippocampus: simultaneous measurements of the extracellular acetylcholine and serum corticosterone levels in the same subjects. Endocrinology. 2008b;149:802–811. doi: 10.1210/en.2007-0827. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Kawas C, Blackman MR, Harman SM, Resnick SM. Free testosterone and risk for Alzheimer disease in older men. Neurology. 2004;62:188–193. doi: 10.1212/wnl.62.2.188. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939–946. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Mong JA, Glaser E, McCarthy MM. Gonadal steroids promote glial differentiation and alter neuronal morphology in the developing hypothalamus in a regionally specific manner. J Neurosci. 1999;19:1464–1472. doi: 10.1523/JNEUROSCI.19-04-01464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser MB, Moser EI, Forrest E, Andersen P, Morris RGM. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci U S A. 1995;92:9697–9701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount C, Downtown C. Alzheimer disease: progress or profit? Nat Med. 2006;12:780–784. doi: 10.1038/nm0706-780. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Cai WJ, Jaffar S, Chen E, Stebbins G, Sendera T, Kordower JH. Estrogen receptor immunoreactivity within subregions of the rat forebrain: neuronal distribution and association with perikarya containing choline acetyltransferase. Brain Res. 1999;849:253–274. doi: 10.1016/s0006-8993(99)01960-5. [DOI] [PubMed] [Google Scholar]
- Nakamura N, Fujita H, Kawata M. Effects of gonadectomy on immunoreactivity for choline acetyltransferase in the cortex, hippocampus, and basal forebrain of adult male rats. Neuroscience. 2002;109:473–485. doi: 10.1016/s0306-4522(01)00513-9. [DOI] [PubMed] [Google Scholar]
- Norbury R, Travis MJ, Erlandsson K, Waddington W, Ell PJ, Murphy DGM. Estrogen therapy and brain muscarinic receptor density in healthy females: a SPET study. Horm Behav. 2007;51:249–257. doi: 10.1016/j.yhbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N. Geometric determinations of the place fields of the hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- Parducz A, Hajszan T, Maclusky NJ, Hoyk Z, Csakvari E, Kurunczi A, Prange-Kiel J, Leranth C. Synaptic remodeling induced by gonadal hormones: neuronal plasticity as a mediator of neuroendocrine and behavioral responses to steroids. Neuroscience. 2006;138:977–985. doi: 10.1016/j.neuroscience.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. 3rd ed. London: Academic; 1997. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, Galasko D, Jin S, Kaye J, Levey A, Pfeiffer E, Sano M, van Dyck CH, Thal LJ. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Pongrac JL, Gibbs RB, Defranco DB. Estrogen-mediated regulation of cholinergic expression in basal forebrain neurons requires extracellular signal-regulated kinase activity. Neuroscience. 2004;124:809–816. doi: 10.1016/j.neuroscience.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci U S A. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-α. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- Rosario ER, Chang L, Stanczyk FZ, Pike CJ. Age-related testosterone deplation and the development of Alzheimer disease. JAMA. 2004;292:1431–1432. doi: 10.1001/jama.292.12.1431-b. [DOI] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci. 2004;24:10117–10127. doi: 10.1523/JNEUROSCI.3581-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci. 2005;25:11194–11200. doi: 10.1523/JNEUROSCI.2338-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stancampiano R, Cocco S, Cugusi C, Sarais L, Fadda F. Serotonin and acetylcholine release response in the rat hippocampus during a spatial memory task. Neuroscience. 1999;89:1135–1143. doi: 10.1016/s0306-4522(98)00397-2. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG. The construct of minor and major depression in Alzheimer's disease. Am J Psychiatry. 2005;162:2086–2093. doi: 10.1176/appi.ajp.162.11.2086. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Hofman MA. Sexual differentiation of the human hypothalamus in relation to gender and sexual orientation. Trend Neurosci. 1995;18:264–270. [PubMed] [Google Scholar]
- Takahashi T, Svoboda K, Malinow R. Experience strengthening transmission by driving AMPA receptors into synapses. Science. 2003;299:1585–1588. doi: 10.1126/science.1079886. [DOI] [PubMed] [Google Scholar]
- Takase K, Mitsushima D, Funabashi T, Kimura F. Sex difference in the 24-h acetylcholine release profile in the premotor/supplementary motor area of behaving rats. Brain Res. 2007;1154:105–115. doi: 10.1016/j.brainres.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Takase K, Kimura F, Yagami T, Mitsushima D. Sex-specific 24-h acetylcholine release profile in the medial prefrontal cortex: simultaneous measurement of spontaneous locomotor activity in behaving rats. Neuroscience. 2009;159:7–15. doi: 10.1016/j.neuroscience.2008.12.039. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc Natl Acad Sci U S A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallenstein GV, Vago DR. Intrahippocampal scopolamine impairs both acquisition and consolidation of contextual fear conditioning. Neurobiol Learn Mem. 2001;75:245–252. doi: 10.1006/nlme.2001.4005. [DOI] [PubMed] [Google Scholar]
- Whitlock JR, Heynen AJ, Shuler MG, Bear MF. Learning induces long-term potentiation in the hippocampus. Science. 2006;313:1093–1097. doi: 10.1126/science.1128134. [DOI] [PubMed] [Google Scholar]
- Widmer H, Ferrigan L, Davies CH, Cobb SR. Evoked slow muscarinic acetylcholinergic synaptic potentials in rat hippocampal interneurons. Hippocampus. 2006;16:617–628. doi: 10.1002/hipo.20191. [DOI] [PubMed] [Google Scholar]
- Williams CL, Meck WH. The organizational effects of gonadal steroids on sexually dimorphic spatial ability. Psychoneuroendocrinology. 1991;16:155–176. doi: 10.1016/0306-4530(91)90076-6. [DOI] [PubMed] [Google Scholar]
- Winblad B, Kilander L, Eriksson S, Minthon L, Båtsman S, Wetterholm AL, Jansson-Blixt C, Haglund A. Donepezil in patients with severe Alzheimer's disease: double-blind, parallel-group, placebo-controlled study. Lancet. 2006;367:1057–1065. doi: 10.1016/S0140-6736(06)68350-5. [DOI] [PubMed] [Google Scholar]
- Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JCS. Hormone replacement therapy and incidence of Alzheimer disease in older women. JAMA. 2002;288:2123–2129. doi: 10.1001/jama.288.17.2123. [DOI] [PubMed] [Google Scholar]
- Zwain IH, Yen SSC. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]