Abstract
The vitamin A metabolite, retinoic acid (RA), is well known for its roles in neural development and regeneration. We have previously shown that RA can induce positive growth cone turning in regenerating neurons in vitro. In this study, we address the subcellular mechanisms underlying this chemo-attractive response, using identified central neurons from the adult mollusc, Lymnaea stagnalis. We show that the RA-induced positive growth cone turning was maintained in the presence of the transcriptional inhibitor, actinomycin D. We also physically transected the neurites from the cell body and showed that isolated growth cones retain the capacity to turn toward a gradient of RA. Moreover, this attractive turning is dependent on de novo local protein synthesis and Ca2+ influx. Most of RA's actions during neurite outgrowth and regeneration require gene transcription, although these data show for the first time in any species, that the chemotropic action of RA in guiding neurite outgrowth, involves a novel, nongenomic mechanism.
Introduction
Retinoic acid (RA), the active metabolite of vitamin A, plays diverse roles during development and regeneration, including patterning of the nervous system and neurite outgrowth (Clagett-Dame et al., 2006; Maden, 2007). In particular, RA potentiates outgrowth from embryonic spinal cord, dorsal root ganglia, and cerebellum in vitro (Wuarin et al., 1990; Yamamoto et al., 1996; Corcoran et al., 2000) as well as from adult newt spinal cord (Dmetrichuk et al., 2005). In addition to potentiating outgrowth and regeneration, RA appears to exert tropic effects to guide neurite outgrowth from dissociated chick neural tube cells (Maden et al., 1998) and newt spinal cord explants in vitro (Dmetrichuk et al., 2005).
RA's primary mode of action involves signaling through nuclear receptors, the RA receptors (RARs) and the retinoid X receptors (RXRs). When bound, these receptors dimerize, acting as transcription factors. Gene products under the control of RA include those involved in neurite outgrowth, such as neuron navigator 2 (Muley et al., 2008), NEDD9 (Knutson and Clagett-Dame, 2008), neurotrophins and even the retinoid receptors themselves (Mey and McCaffery, 2004). However, RA has also been proposed to exert nongenomic actions, either via activation of extra-nuclear retinoid receptors (Chen and Napoli, 2008) or by direct interaction with other signaling molecules (Ochoa et al., 2003).
It was initially thought that RA signaling was a vertebrate innovation. Recent evidence, however, suggests a more primitive origin, as RA also plays a role in other bilaterian animals (Campo-Paysaa et al., 2008). We also demonstrated a conserved role for RA in the induction of neurite outgrowth from adult molluscan neurons in culture (Dmetrichuk et al., 2006) and have demonstrated the presence of RA in the molluscan CNS (Dmetrichuk et al., 2008). Moreover, we have cloned the RA synthesizing enzyme, retinal dehydrogenase, from the mollusc Lymnaea stagnalis (C. J. Carter, G. E. Spencer, unpublished data; GenBank Accession No. FJ539101), as well as an RXR receptor with 80% amino acid homology to the vertebrate RXRα (C. Carter, R. Carlone, J. Dmetrichuk, G. Spencer, unpublished data; GenBank Accession No. AY846875). Using cultured neurons from Lymnaea stagnalis, we previously showed for the first time in any species that individual growth cones are attracted to and turn toward a source of exogenously applied RA (Dmetrichuk et al., 2006, 2008). In this study, we have now investigated the subcellular mechanisms underlying the RA-induced chemo-attraction and provide unique evidence that growth cone turning toward RA is transcriptionally independent and involves a novel mechanism requiring local protein synthesis and Ca2+ influx.
Materials and Methods
Cell culture.
Lymnaea stagnalis, bred in our laboratory, were kept in open air tanks containing artificial, aerated pond water (5 g/L; Instant Ocean Sea Salt). Animals used for cell culture ranged in size from 20 to 25 mm. All cell culture procedures were performed in an identical manner to those described previously (Dmetrichuk et al., 2006, 2008). Briefly, individually identifiable neurons were removed from dissected CNSs and were plated on poly-l-lysine-coated culture dishes containing brain conditioned medium (CM) and RA to promote neurite outgrowth (Dmetrichuk et al., 2006, 2008). CM was prepared by incubating 10 CNSs in 6 ml of defined medium (DM) for 3 d, allowing (unknown) trophic factors to be released into the DM. CM was used to promote neurite outgrowth, as DM alone does not contain any growth factors. Pedal A (PeA) ciliary motoneurons and Visceral F (VF) neurons were used in this study, and all cell cultures were maintained overnight in a dark environment at 22°C.
Growth cone turning assays.
After 18 to 24 h in culture, individual neuronal cell bodies had extended regenerated neurites. Before each growth cone turning assay, individual growth cones were monitored to ensure active outgrowth that was uniform in speed (μm/min) and direction. Growth cones exhibiting a variable growth trajectory were not used. All-trans RA (10−5 m in the pipette) was applied to the individual growth cones using a pressure pipette (Eppendorf-Femtojet; 4–8 μm) placed ∼50 to 150 μm from the growth cone. Pressures between 5 and 12 hPa were used to apply RA, while holding pressures of 1–2 hPa were used during rest periods to prevent backflow of bath solution. The concentration of RA at the growth cone was likely 100–1000× less than that contained within the pipette (Lohof et al., 1992). Control experiments using the vehicle solution for RA [0.1% ethanol (EtOH) in DM in pipette] were performed in the exact same manner. Control experiments using DM alone were not performed, as it has been previously shown not to produce growth cone turning (Dmetrichuk et al., 2006). Isolated neurites were mechanically separated from the cell body using a sharp glass electrode (see Fig. 2Ai–ii). After transection, processes were permitted to recover and were monitored to ensure continued growth and stability. Growth cone turning in both intact and isolated neurites was then evaluated in the presence of a series of pharmacological agents and growth cone behavior was monitored for at least 1 h.
Figure 2.
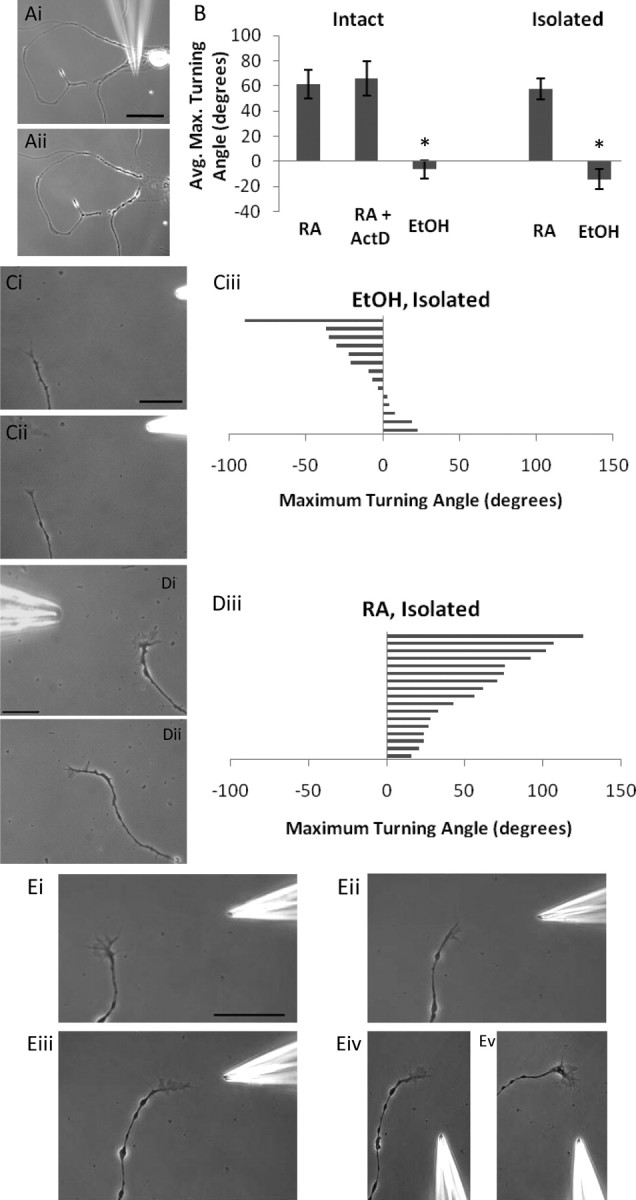
Isolated neurites retain the capacity to turn toward RA. Ai, ii, The process of mechanical isolation of the neurites from the cell body, using a sharp glass pipette. Scale bar, 60 μm. B, Summary graph of mean growth cone turning angles to both RA and EtOH application in intact and isolated growth cones; *p < 0.001 compared with RA. C, Isolated neurites do not turn toward a gradient of EtOH. i, 0 min; ii, 23 min. Scale bar, 30 μm. iii, Histogram depicts maximum turning angle of isolated growth cones to EtOH and each bar represents one growth cone. Positive values represent a turn toward the pipette and negative values a turn away from the pipette. D, Isolated PeA growth cones turn toward a gradient of RA. i, 0 min; ii, 39 min. Scale bar, 30 μm. iii, Histogram depicts maximum turning angle of isolated growth cones to RA. E, Representative example of an isolated VF growth cone turning toward RA. i, 0 min; ii, 45 min; iii, 75 min. iv, v, Changing the angle of RA application by moving the pipette induced a second change in direction of the VF growth cone (Ev, 195 min). Scale bar, 100 μm.
Chemicals.
All chemicals were obtained from Sigma-Aldrich. All-trans RA was prepared in absolute EtOH and diluted in DM to a final concentration of 10−5 m (in 0.1% EtOH). Actinomycin D (5 × 10−5 m) was used as an inhibitor of transcription, while anisomycin (4.5 × 10−5 m) was used as a protein synthesis inhibitor (Hamakawa et al., 1999). The PKCα inhibitor Gö6976 (10−5 m) and Ca2+ channel blocker, cadmium (10−5 m), were also used. Actinomycin D and Gö6976 were initially dissolved in DMSO, while anisomycin and cadmium were dissolved in water. Dilutions to the final concentrations were performed using DM. For experiments with inhibitors dissolved in DMSO, control experiments with 0.01% DMSO in the bath were also performed.
Analysis.
The rate of neurite outgrowth was first monitored for ∼1 h in the presence of each inhibitor to ensure sustained outgrowth. The average rate of outgrowth for neurites in CM alone was 0.702 ± 0.459 μm/min, and this was not significantly altered by any of the inhibitors. Positive controls for actinomycin D were performed by incubation of cells in CM with 50 μm actinomycin D and measuring the maximum neurite length for each cell at 48 h in either DMSO (n = 20) or in actinomycin D (n = 13).
All images were captured and analyzed using Northern Eclipse software (Empix imaging). Unless stated otherwise, all statistical analyses were performed using a one-way ANOVA with a Tukey–Kramer post hoc test (SigmaStat Software). All data are expressed as mean ± SEM.
Results
Pedal A motoneuron growth cones turned toward a gradient of RA
We have previously shown that identified VF neuronal growth cones turn toward an exogenous source of both all-trans and 9-cis-RA (Dmetrichuk et al., 2006, 2008). In this study, we have used both identified PeA and VF neurons. The first aim was, therefore, to demonstrate that RA acted as a chemo-attractant to PeA neurites in a similar manner as with the VF neurites. PeA neurons were cultured in CM for a period of 18–24 h, allowing sufficient time for neurite outgrowth. Application of RA (10−5 m) or the control vehicle EtOH (0.1%) was performed in a pulsatile manner using the pipette assay technique. First of all, we demonstrated that application of the EtOH vehicle alone did not produce a positive turning response in the PeA growth cones (−6.4 ± 7.2°; n = 10) (Fig. 1Ai–iii). In contrast, we found that the growth cones of PeA neurons turned toward the gradient of RA (61.5 ± 11.6°; n = 10) (Fig. 1Bi–iii). There was no significant difference between the turning response in the PeA growth cones and that of the VF growth cones shown previously (66.9 ± 12.3°; n = 7; p > 0.05, t test) (Dmetrichuk et al., 2006). In contrast, there was a significant difference (p < 0.001) between the turning angles produced by RA and EtOH in the PeA growth cones.
Figure 1.
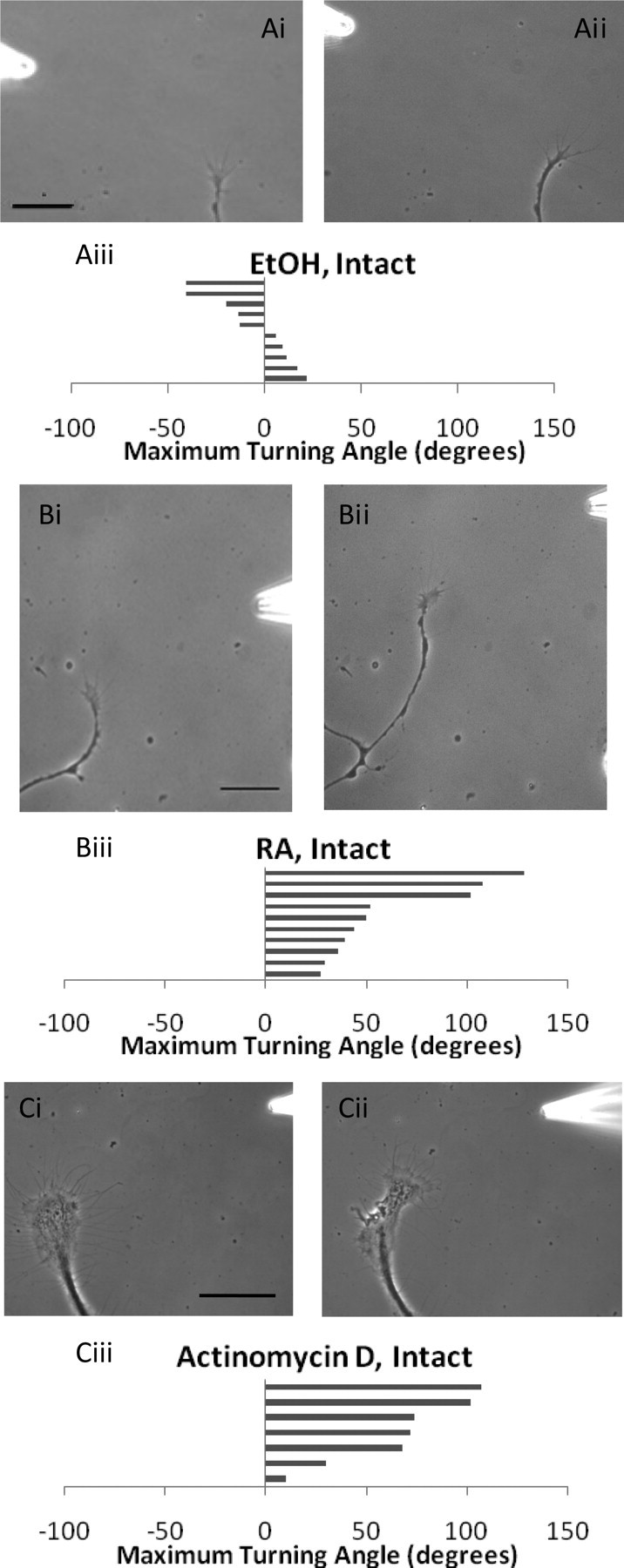
RA-induced chemoattraction of PeA growth cones is independent of transcription. A, Individual PeA growth cones do not turn toward a gradient of EtOH. i, 0 min; ii, 45 min. Scale bar, 30 μm. iii, Histogram depicts maximum turning angle to EtOH, and each bar represents one growth cone. Positive values represent a turn toward the pipette and negative values a turn away from the pipette. B, Individual PeA growth cones turn toward an exogenously applied gradient of RA. i, 0 min; ii, 67 min. Scale bar, 30 μm. iii, Histogram depicts maximum turning angle to RA. C, Growth cone turning toward RA was maintained in the presence of the transcription inhibitor, actinomycin D. i, 0 min; ii, 60 min. Scale bar, 100 μm. iii, Histogram depicts maximum turning angle toward RA in the presence of actinomycin D.
The RA-induced growth cone turning was independent of transcription and occurred in isolated neurites
Most known effects of RA in the developing or regenerating nervous system occur as a result of RA binding to nuclear receptors (RAR/RXR) to activate gene transcription. However, the RA-induced growth cone turning often occurred within minutes of RA application, strongly suggesting that the response may be independent of gene transcription. To determine whether gene transcription plays a role in the RA-induced growth cone turning, a transcription inhibitor, actinomycin D, was used. We chose to use 50 μm actinomycin D, as this concentration is effective in cultured Aplysia neurons (Lyles et al., 2006), and it is known that a lower concentration of 0.8 μm is also effective in cultured Lymnaea neurons (Hamakawa et al., 1999). As a positive control, we demonstrated that inhibition of transcription by culturing PeA neurons in 50 μm actinomycin D resulted in significant inhibition of neurite outgrowth (Lovell and Moroz, 2006), compared with DMSO controls (paired t test, p < 0.0001; data not shown).
To test the involvement of gene transcription in growth cone turning, we incubated the cultured PeA neurons in 50 μm actinomycin D and showed that the transcriptional inhibitor did not inhibit the normal growth cone turning response to exogenously applied RA (63.4 ± 13.4°; n = 7; p > 0.05) (Fig. 1Ci–iii). This led us to hypothesize that a local rather than a genomic mechanism may be involved.
To confirm our above finding and to assess whether a local mechanism was involved in the RA-mediated chemo-attraction, we took advantage of the ability of molluscan neurites to survive in the absence of the cell body and to continue to grow in their isolated state for up to 48 h (van Kesteren et al., 2006). In this study, neurites were physically transected from the cell body (Fig. 2Ai,ii) and then allowed to recover from injury. Once it was determined that the transected neurites remained viable and continued to grow, they were exposed to a gradient of RA (10−5 m) or EtOH vehicle (as control). As with the intact neurites, application of the EtOH vehicle to isolated neurites did not produce positive growth cone turning (−14.1 ± 7.7°; n = 14) (Fig. 2Ci–iii). We next showed, for the first time, that the RA-induced chemo-attractive turning also occurred in transected, isolated neurites (57.8 ± 8.4°; n = 17) (Fig. 2Di–iii, Ei–iii). Furthermore, we also demonstrated that moving the RA-containing pipette after the first isolated growth cone turn induced a second change in direction (Fig. 2Eiv,v). A significant difference was found between the EtOH and RA conditions (p < 0.001). Together, the above data demonstrate that the RA-induced growth cone turning occurs in isolated neurites in the absence of the cell body and thus in the absence of gene transcription (summarized in Fig. 2B).
Local protein synthesis was required for RA-induced growth cone turning
The above findings indicate that RA is not acting through nuclear-located receptors to produce growth cone turning in cultured neurons. Our next aim was to determine whether the local growth cone turning response to RA required protein synthesis. The dependency of some growth cone turning responses on local protein synthesis has been well established (Lin and Holt, 2007), but it is not required for responses to all guidance cues (Roche et al., 2009). To determine the role of protein synthesis, we used bath application of the protein synthesis inhibitor anisomycin (4.5 × 10−5 m). We first monitored outgrowth for 1 h before the turning assays and demonstrated that the inhibitor alone had no effect on the rate of outgrowth (0.73 ± 0.299 μm/min; p > 0.05, when compared with CM alone). Experiments with anisomycin were first performed in intact neurons, and we found that incubation with anisomycin blocked the normal turning response to exogenously applied RA (6.8 ± 5.8°; n = 11; p < 0.05) (Fig. 3Ai–iii). We next used isolated neurites to determine whether de novo protein synthesis was localized to the neurite and/or growth cone. As with the intact neurites, isolated neurites in the presence of anisomycin failed to turn toward the RA gradient (1.2 ± 12.0°; n = 11; p < 0.05) (Fig. 3Bi–iii), which strongly suggests that the RA-induced growth cone turning requires de novo local protein synthesis (Fig. 3C).
Figure 3.
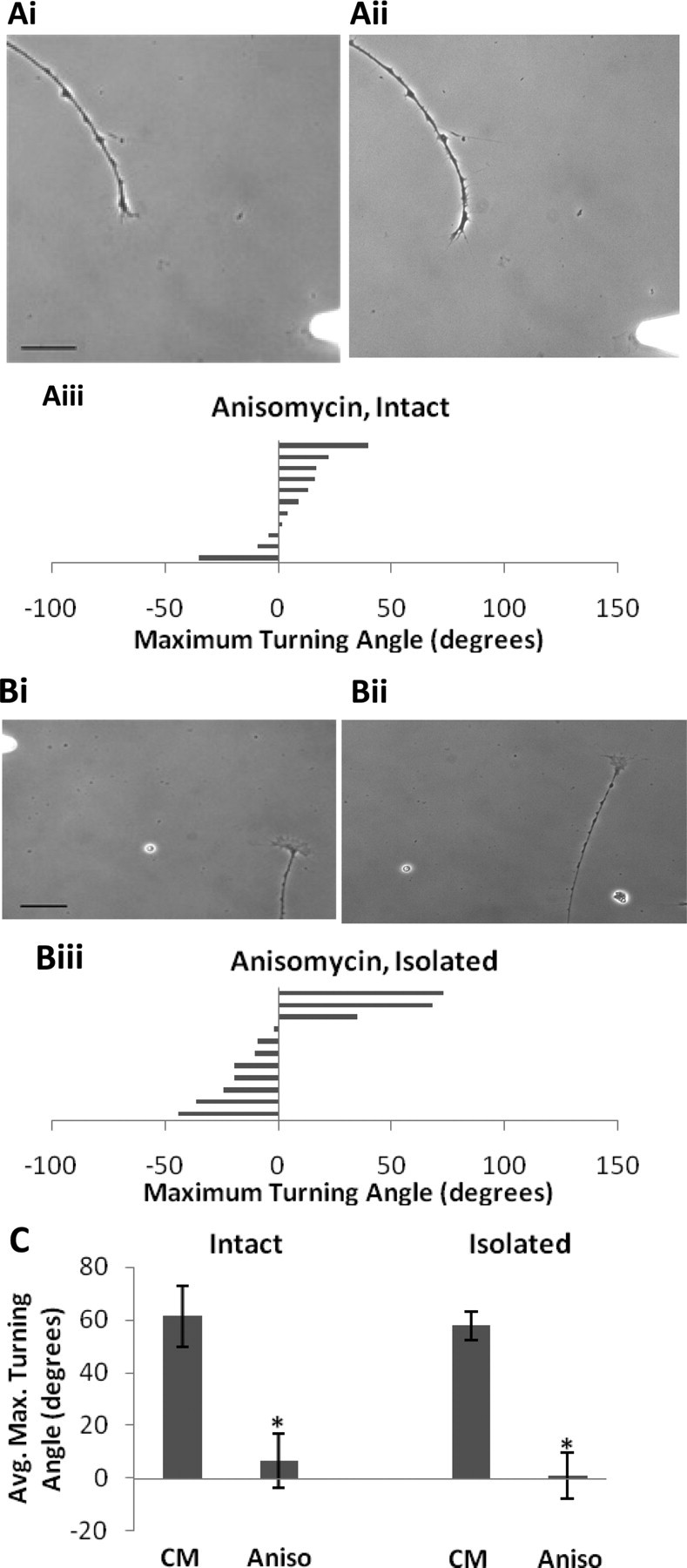
RA-mediated growth cone turning requires protein synthesis. A, Intact neurites in the presence of anisomycin (4.5 × 10−5 m) failed to turn toward a gradient of RA. i, 0 min; ii, 33 min. Scale bar, 30 μm. iii, Histogram depicts maximum turning angle of intact growth cones to RA in the presence of the translational inhibitor, anisomycin, and each bar represents one growth cone. Positive values represent a turn toward the pipette and negative values a turn away from the pipette. B, Neurites isolated from their cell body also fail to turn toward the RA gradient when in the presence of anisomycin. i, 0 min; ii, 103 min. Scale bar, 30 μm. iii, Histogram depicts the maximal turning angle of isolated growth cones toward RA in the presence of the translational inhibitor, anisomycin. C, Summary graph showing the mean turning angles toward RA of intact and isolated growth cones in the presence of anisomycin. *p < 0.05 compared with CM.
The RA-induced turning response was Ca2+-dependent
The above data demonstrated that RA induced the growth cone turning via a nongenomic mechanism requiring local protein synthesis. Nongenomic effects of RA have previously been reported, one example being the direct binding of RA to PKCα (Ochoa et al., 2003). PKCα has been shown to mediate nongenomic actions of other steroid hormones via modulation of Ca2+ influx (Capiati et al., 2001), and Ca2+ is well known to be an important signaling molecule in many growth cone signaling pathways (Gomez and Zheng, 2006). Based on these previous reports, we next tested the possible involvement of PKCα and Ca2+ influx in the RA-induced growth cone turning. Experiments with bath application of the vehicle DMSO showed that the average turning angle toward RA (33.6 ± 7.5°; n = 11) was substantially less than previously found in CM only. This suggested that the control vehicle DMSO may have perturbed the turning response to RA. In the presence of the PKCα inhibitor Gö6976 (10−5 m), the growth cone turning in response to RA (24.1 ± 6.2°; n = 10) was not significantly different (p > 0.05) from that in the presence of the vehicle DMSO, suggesting that PKC activation is not involved (Fig. 4Ai–iii). However, the turning angle in the PKC inhibitor was significantly less than the turning angle in CM alone (p < 0.05) (Fig. 4C), raising the possibility that disruption of the turning response by the DMSO vehicle may have masked any further inhibition by the PKC antagonist.
Figure 4.
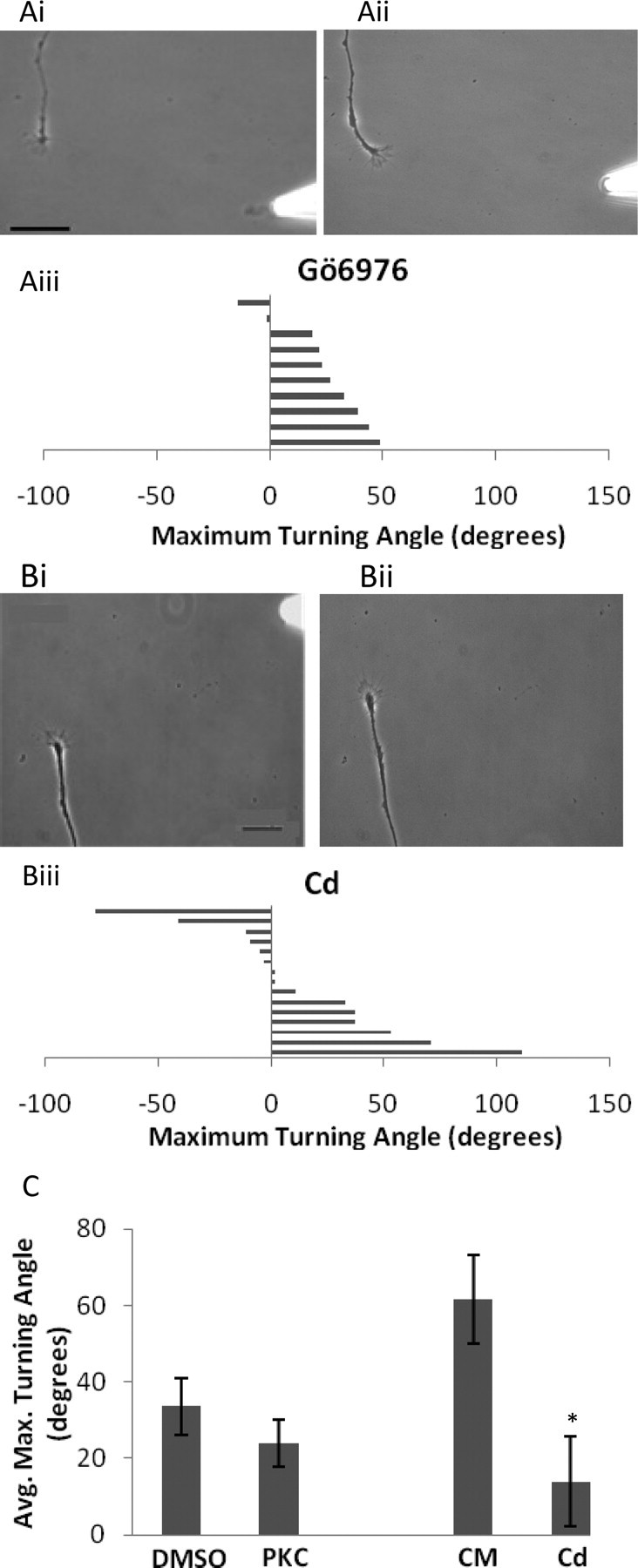
Blocking Ca2+ influx prevents RA-induced chemo-attraction. Ai, ii, Bath-applied PKC inhibitor, Gö6976 (10−5 m), had no significant effect on the turning response to RA, compared with DMSO controls. i, 0 min; ii, 50 min. Scale bar, 30 μm. iii, Histogram displays the maximal turning angle of each growth cone in the presence of Gö6976, and each bar represents one growth cone. Positive values represent a turn toward the pipette and negative values a turn away from the pipette. B, Bath applied cadmium (10−5 m) disrupted the normal chemo-attraction induced by RA. i, 0 min; ii, 154 min. Scale bar, 30 μm. iii, Histogram displays the maximal turning angle of each growth cone in the presence of cadmium (Cd). C, Summary graph showing the effects of the PKC inhibitor (PKC) and Ca2+ channel blocker (Cd) on RA-mediated growth cone turning; *p < 0.05 compared with CM.
We next aimed to investigate a potential role for Ca2+ influx in the RA-mediated growth cone turning response. There are no specific Ca2+ channel blockers that are effective in Lymnaea neurons, so to this end, we used the general Ca2+ channel blocker, cadmium, which has previously been shown to be effective in Lymnaea neurons (Feng et al., 2000; Dmetrichuk et al., 2008). Again, we monitored neurite outgrowth for 1 h after the addition of cadmium to the bath (10−5 m, final concentration) and found that it had no adverse effect on the rate of neurite outgrowth (0.682 ± 0.369 μm/min; p > 0.05). When exposed to the RA gradient in the presence of cadmium, the growth cone turning was significantly reduced (14 ± 11.8°; n = 15; p < 0.05) (Fig. 4Bi–iii). These data strongly suggest that Ca2+ influx may be an important requirement for RA-induced growth cone turning (Fig. 4C).
Discussion
In this study, we have shown that PeA motoneuron growth cones turned toward an exogenous gradient of RA and for the first time in any species, have addressed the subcellular mechanisms that underlie this turning response. The most significant finding of this study is that the RA-induced growth cone turning was independent of transcription and occurred in isolated neurites. We show that neurites physically transected from their cell body retained the ability to turn toward a gradient of RA, with a turning angle that did not differ from intact neurites. Although growth cone turning toward RA occurred in the absence of the nucleus, we did show that the response was dependent on local protein synthesis, as turning was abolished with the protein synthesis inhibitor anisomycin. Other growth cone responses dependent on local protein synthesis include attractive responses to netrin-1 (Campbell and Holt, 2001) and BDNF (Yao et al., 2006). Furthermore, the response of growth cones to Sema3A involves local translation of RhoA (Wu et al., 2005), while responses to slit2 result in the local synthesis of cofilin-1 (Piper et al., 2006). However, not all responses to guidance cues depend on local protein synthesis (Roche et al., 2009), and such differences may depend on organism, neuronal age, and/or other cues normally present in the cell's microenvironment.
Our novel results strongly imply that a local mechanism, operating at the level of the growth cone, is responsible for the RA-induced turning behavior. Classically, the actions of RA have been mediated by the nuclear receptors RARs and RXRs, acting via gene transcription. There is now, however, precedent in the literature for nongenomic actions of RA. Some of these actions likely occur as a result of RA binding to extra-nuclear receptors and involve inhibition of transmitter release (Liao et al., 2004), activation of ERK (Cañón et al., 2004), and increased dendritic growth and translation of GluR1 (Chen and Napoli, 2008). There is also evidence in the literature for retinoid receptors localized to axonal (Calderon and Kim, 2007) and dendritic (Chen and Napoli, 2008; Poon and Chen 2008) compartments, as well as association with dendritic RNA granules (Chen et al., 2008). Carter et al. (2008) have recently shown the presence of retinoid X receptors in the growth cones of cultured Lymnaea PeA neurons, but whether or not these receptors are involved in the RA-mediated local growth cone turning response is yet to be determined.
Other studies have shown that nongenomic effects of RA may occur in the absence of retinoid receptors. For example, RA can rapidly activate CREB (via PKC, ERK, and p90 ribosomal S6 kinase) in human bronchial cells in a manner that is independent of retinoid receptors (Aggarwal et al., 2006). RA has also been proposed to directly modulate PKC activity, and studies suggest the existence of an RA-binding site on the PKC molecule (Radominska-Pandya et al., 2000; Ochoa et al., 2003). However, whether RA binding to PKC activates or inactivates the molecule seems unclear and may depend on cell-type and/or concentration (Aggarwal et al., 2006). In this study, our results with the PKC inhibitor were also somewhat unclear, since the turning response in the presence of the inhibitor was significantly reduced compared with CM alone but was not significantly different to that in the DMSO vehicle control. It should be noted that the turning angle in the vehicle DMSO was substantially (albeit nonsignificantly) reduced compared with CM alone, which might suggest that DMSO, even at a low concentration, disrupts the growth cone's ability to experience the normal chemo-attraction to RA. DMSO is known to have effects on the integrity and stability of cell membranes (Yu and Quinn, 1998) and may affect the growth cones ability to sense guidance cues, masking a possible reduction in response due to the PKC antagonist. Thus, we cannot conclusively rule out the involvement of PKC at this time.
We also showed using cadmium that Ca2+ influx plays an important role in the turning response. This result is not surprising, as Ca2+ influx has been known to contribute to many different growth cone responses (Gomez and Zheng, 2006). Interestingly, Liou et al. (2005) showed that the effects of RA on transmitter release involved changes in intracellular Ca2+, although the effect was not inhibited by cadmium, but instead involved release of Ca2+ from intracellular stores. Previous work in our lab showed that cadmium reduced RA-mediated outgrowth over an extended incubation of 72 h (Dmetrichuk et al., 2008). However, in this study, short incubation times in cadmium did not reduce the rate of outgrowth, and thus it is unlikely that cadmium perturbed the turning response by slowing neurite outgrowth. Although it remains to be established what downstream targets are activated by the establishment of this internal Ca2+ gradient, our data show that Ca2+ influx plays a role in the RA-mediated growth cone turning response.
In summary, using physically isolated neurites, we have shown for the first time that the chemo-attractive effects of RA (at least in Lymnaea) involve a novel, localized mechanism that is independent of nuclear-localized receptors and gene transcription. Furthermore, we demonstrated that the RA-induced growth cone turning requires de novo local protein synthesis and Ca2+ influx.
Footnotes
This work was supported by a Discovery Grant to G.E.S. from The Natural Sciences and Engineering Research Council (NSERC), Canada. N.R.F. and J.M.D. were supported by scholarships from NSERC (Canada). We are grateful to G. Tattersall for help with the final version of this manuscript.
References
- Aggarwal S, Kim SW, Cheon K, Tabassam FH, Yoon JH, Koo JS. Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol Biol Cell. 2006;17:566–575. doi: 10.1091/mbc.E05-06-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon F, Kim HY. Role of RXR in neurite outgrowth induced by docosahexaenoic acid. Prostaglandins Leukot Essent Fatty Acids. 2007;77:227–232. doi: 10.1016/j.plefa.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Campo-Paysaa F, Marlétaz F, Laudet V, Schubert M. Retinoic acid signaling in development: tissue-specific functions and evolutionary origins. Genesis. 2008;46:640–656. doi: 10.1002/dvg.20444. [DOI] [PubMed] [Google Scholar]
- Cañón E, Cosgaya JM, Scsucova S, Aranda A. Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol Biol Cell. 2004;15:5583–5592. doi: 10.1091/mbc.E04-05-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capiati DA, Vazquez G, Boland RL. Protein kinase C α modulates the Ca2+ influx phase of the Ca2+ response to 1α,25-dihydroxy-vitamin-D3 in skeletal muscle cells. Horm Metab Res. 2001;33:201–206. doi: 10.1055/s-2001-14950. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Dmetrichuk JM, Carlone R, Spencer GE. Immunohistochemical detection of a conserved retinoid X receptor (RXR) in the molluscan CNS: a putative role in neuronal regeneration. Soc Neurosci Abstr. 2008;34:725–1. [Google Scholar]
- Chen N, Napoli JL. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARα. FASEB J. 2008;22:236–245. doi: 10.1096/fj.07-8739com. [DOI] [PubMed] [Google Scholar]
- Chen N, Onisko B, Napoli JL. The nuclear transcription factor RARα associates with neuronal RNA granules and suppresses translation. J Biol Chem. 2008;283:20841–20847. doi: 10.1074/jbc.M802314200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clagett-Dame M, McNeill EM, Muley PD. Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J Neurobiol. 2006;66:739–756. doi: 10.1002/neu.20241. [DOI] [PubMed] [Google Scholar]
- Corcoran J, Shroot B, Pizzey J, Maden M. The role of retinoic acid receptors in neurite outgrowth from different populations of embryonic mouse dorsal root ganglia. J Cell Sci. 2000;113:2567–2574. doi: 10.1242/jcs.113.14.2567. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk JM, Spencer GE, Carlone RL. Retinoic-acid dependent attraction of adult spinal cord axons towards regenerating newt limb blastemas in vitro. Dev Biol. 2005;281:112–120. doi: 10.1016/j.ydbio.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk JM, Carlone RL, Spencer GE. Retinoic acid induces neurite outgrowth and growth cone turning in invertebrate neurons. Dev Biol. 2006;294:39–49. doi: 10.1016/j.ydbio.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Dmetrichuk JM, Carlone RL, Jones TR, Vesprini ND, Spencer GE. Detection of endogenous retinoids in the molluscan CNS and characterization of the trophic and tropic actions of 9-cis retinoic acid on isolated neurons. J Neurosci. 2008;28:13014–13024. doi: 10.1523/JNEUROSCI.3192-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng ZP, Hasan SU, Lukowiak K, Syed NI. Target cell contact suppresses neurite outgrowth from soma-soma paired Lymnaea neurons. J Neurobiol. 2000;42:357–369. doi: 10.1002/(sici)1097-4695(20000215)42:3<357::aid-neu7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Gomez TM, Zheng JQ. The molecular basis for calcium-dependent axon pathfinding. Nat Rev Neurosci. 2006;7:115–125. doi: 10.1038/nrn1844. [DOI] [PubMed] [Google Scholar]
- Hamakawa T, Woodin MA, Bjorgum MC, Painter SD, Takasaki M, Lukowiak K, Nagle GT, Syed NI. Excitatory synaptogenesis between identified Lymnaea neurons requires extrinsic trophic factors and is mediated by receptor tyrosine kinases. J Neurosci. 1999;19:9306–9312. doi: 10.1523/JNEUROSCI.19-21-09306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson DC, Clagett-Dame M. atRA regulation of NEDD9, a gene involved in neurite outgrowth and cell adhesion. Arch Biochem Biophys. 2008;477:163–174. doi: 10.1016/j.abb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Liao YP, Ho SY, Liou JC. Non-genomic regulation of transmitter release by retinoic acid at developing motoneurons in Xenopus cell culture. J Cell Sci. 2004;117:2917–2924. doi: 10.1242/jcs.01153. [DOI] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Local translation and directional steering in axons. EMBO J. 2007;26:3729–3736. doi: 10.1038/sj.emboj.7601808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou JC, Ho SY, Shen MR, Liao YP, Chiu WT, Kang KH. A rapid, nongenomic pathway facilitates the synaptic transmission induced by retinoic acid at the developing synapse. J Cell Sci. 2005;118:4721–4730. doi: 10.1242/jcs.02603. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell P, Moroz LL. The largest growth cones in the animal kingdom: an illustrated guide to the dynamics of Aplysia neuronal growth in cell culture. Integr Comp Biol. 2006;46:847–870. doi: 10.1093/icb/icl042. [DOI] [PubMed] [Google Scholar]
- Lyles V, Zhao Y, Martin KC. Synapse formation and mRNA localization in cultured Aplysia neurons. Neuron. 2006;49:349–356. doi: 10.1016/j.neuron.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci. 2007;8:755–765. doi: 10.1038/nrn2212. [DOI] [PubMed] [Google Scholar]
- Maden M, Keen G, Jones GE. Retinoic acid as a chemotactic molecule in neuronal development. Int J Dev Neurosci. 1998;16:317–322. doi: 10.1016/s0736-5748(98)00046-x. [DOI] [PubMed] [Google Scholar]
- Mey J, McCaffery P. Retinoic acid signaling in the nervous system of adult vertebrates. Neuroscientist. 2004;10:409–421. doi: 10.1177/1073858404263520. [DOI] [PubMed] [Google Scholar]
- Muley PD, McNeill EM, Marzinke MA, Knobel KM, Barr MM, Clagett-Dame M. The atRA–responsive gene neuron navigator 2 functions in neurite outgrowth and axonal elongation. Dev Neurobiol. 2008;68:1441–1453. doi: 10.1002/dneu.20670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa WF, Torrecillas A, Fita I, Verdaguer N, Corbalan-Garcia S, Gomez-Fernandez JC. Retinoic acid binds to the C2-domain of protein kinase C (alpha) Biochem. 2003;42:8774–8779. doi: 10.1021/bi034713g. [DOI] [PubMed] [Google Scholar]
- Piper M, Anderson R, Dwivedy A, Weinl C, van Horck F, Leung KM, Cogill E, Holt C. Signaling mechanisms underlying slit2-induced collapse of Xenopus retinal growth cones. Neuron. 2006;49:215–228. doi: 10.1016/j.neuron.2005.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon MM, Chen L. Retinoic acid-gated sequence-specific translational control by RARα. Proc Natl Acad Sci U S A. 2008;105:20303–20308. doi: 10.1073/pnas.0807740105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radominska-Pandya A, Chen G, Czernik PJ, Little JM, Samokyszyn VM, Carter CA, Nowak G. Direct interaction of all-trans-retinoic acid with protein kinase C (PKC). Implications for PKC signaling and cancer therapy. J Biol Chem. 2000;275:22324–22330. doi: 10.1074/jbc.M907722199. [DOI] [PubMed] [Google Scholar]
- Roche FK, Marsick BM, Letourneau PC. Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J Neurosci. 2009;29:638–652. doi: 10.1523/JNEUROSCI.3845-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kesteren RE, Carter C, Dissel HM, van Minnen J, Gouwenberg Y, Syed NI, Spencer GE, Smit AB. Local synthesis of actin-binding protein β-thymosin regulates neurite outgrowth. J Neurosci. 2006;26:152–157. doi: 10.1523/JNEUROSCI.4164-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020–1024. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuarin L, Sidell N, de Vellis J. Retinoids increase perinatal spinal cord neuronal survival and astroglial differentiation. Int J Dev Neurosci. 1990;8:317–326. doi: 10.1016/0736-5748(90)90038-4. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, McCaffery P, Dräger UC. Influence of the choroid plexus on cerebellar development: analysis of retinoic acid synthesis. Brain Res Dev Brain Res. 1996;93:182–190. doi: 10.1016/0165-3806(96)00038-7. [DOI] [PubMed] [Google Scholar]
- Yao J, Sasaki Y, Wen Z, Bassell GJ, Zheng JQ. An essential role for beta-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nat Neurosci. 2006;9:1265–1273. doi: 10.1038/nn1773. [DOI] [PubMed] [Google Scholar]
- Yu ZW, Quinn PJ. The modulation of membrane structure and stability by dimethyl sulphoxide. Mol Membr Biol. 1998;15:59–68. doi: 10.3109/09687689809027519. [DOI] [PubMed] [Google Scholar]


