Abstract
Although nicotine is generally considered to be the main compound responsible for addictive properties of tobacco, experimental data indicate that nicotine does not exhibit all the characteristics of other substances of abuse. We recently showed that a pretreatment with mixed irreversible monoamine oxidases inhibitors (MAOIs), such as tranylcypromine, triggers a locomotor response to nicotine in mice and allows maintenance of behavioral sensitization to nicotine in rats. Moreover, we showed by microdialysis in mice that behavioral sensitization induced by compounds belonging to main groups of drugs of abuse, such as amphetamine, cocaine, morphine, or alcohol, was underlain by sensitization of noradrenergic and serotonergic neurons. Here, this neurochemical sensitization was tested after nicotine, tranylcypromine, or a mixture of both compounds. Data indicate that, whereas neither repeated nicotine nor repeated tranylcypromine alone has any effect by itself, a repeated treatment with a mixture of nicotine and tranylcypromine induces both behavioral sensitization and sensitization of noradrenergic and serotonergic neurons. The development of neurochemical and behavioral sensitizations is blocked by prazosin and SR46349B [(1Z,2E)-1-(2-fluoro-phenyl)-3-(4-hydroxyphenyl)-prop-2-en-one-O-(2-dimethylamino-ethyl)-oxime hemifumarate], two antagonists of α1b-adrenergic and 5-HT2A receptors, respectively, but not by SCH23390 [R(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride], a D1 receptor antagonist. Finally, we found that pretreatments with WAY 100635 [N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclo-hexane carboxamide trihydrochloride], a 5-HT1A receptor antagonist, can also induce a behavioral and neurochemical sensitization to repeated nicotine. Complementary experiments with 8-OHDPAT (8-hydroxy-dipropylamino-tetralin), a 5-HT1A receptor agonist, and analysis of 5-HT1A receptors expression in the dorsal raphe nucleus after a tranylcypromine injection indicate that MAOIs contained in tobacco desensitize 5-HT1A autoreceptors to trigger the strong addictive properties of tobacco.
Keywords: 5-HT2A serotonergic receptor, α1b-adrenergic receptor, nicotine, tranylcypromine, microdialysis, 5-HT1A receptor
Introduction
Tobacco is a potent reinforcing agent in humans, and nicotine is generally considered to be the major compound responsible for its addictive properties (Dani and Heinemann, 1996; Balfour et al., 1998; Di Chiara, 2000). However, animal experiments indicate some discrepancies between the effects of nicotine and those of other drugs of abuse. For example, although psychostimulants and opiates induce a substantial locomotor hyperactivity both in rats and mice, nicotine is a weak locomotor stimulant in rats and fails to induce locomotor hyperactivity in mice with the exception of C3H strain (Marks et al., 1983; Kita et al., 1988; Smolen et al., 1994; Itzhak and Martin, 1999). Moreover, repeated nicotine treatments in rats induce a behavioral sensitization that vanishes quicker than for other drugs of abuse (Ksir et al., 1985; Villégier et al., 2003).
Different studies have indicated that tobacco smokers have decreased brain levels of monoamine oxidases (Berlin et al., 1995; Fowler et al., 1996). Actually, tobacco and tobacco smoke contain monoamine oxidases inhibitors (MAOIs) (Poindexter and Carpenter, 1962; Breyer-Pfaff et al., 1996; Rommelspacher et al., 2002). A few years ago, we showed that MAOI pretreatment allows the maintenance of behavioral sensitization to nicotine in rats (Villégier et al., 2003), thus suggesting a role of MAOIs in the addictive properties of tobacco. Later, it was found that a pretreatment with tranylcypromine, a potent and irreversible MAOI, could trigger a locomotor and rewarding response to nicotine in mice (Villégier et al., 2006b) and dramatically increase nicotine self-administration in rats (Guillem et al., 2005; Villégier et al., 2006a).
More recently, we showed that repeated injections of drugs of abuse such as d-amphetamine, cocaine, morphine, or alcohol in C57BL/6 mice induce a long-lasting sensitization of noradrenergic and serotonergic neurons (Salomon et al., 2006; Lanteri et al., 2008). The reactivity of noradrenergic and serotonergic neurons was tested by measuring the cortical increase of extracellular norepinephrine (NE) and serotonin (5-HT) levels induced by an injection of d-amphetamine or para-chloroamphetamine (PCA), NE and 5-HT releasers, respectively (Salomon et al., 2006). The development of this hyperreactivity is unrelated to dopaminergic transmission (Lanteri et al., 2008) but needs the repeated stimulation of α1b-adrenergic and 5-HT2A receptors (Salomon et al., 2006; Lanteri et al., 2008). This neurochemical sensitization, which seems specific to addictive compounds, is strongly correlated with the sensitization of the behavioral response. For this reason, we proposed this neurochemical sensitization as a common physiological basis in the pathology of addiction (Lanteri et al., 2008; Tassin, 2008).
Because addictive properties of nicotine remain controversial, we tested whether a repeated treatment with nicotine could also sensitize noradrenergic and serotonergic neurons and whether an association of nicotine with tranylcypromine could trigger the same neurochemical and behavioral adaptations induced by psychostimulants, opiates, and ethanol. Furthermore, because tranylcypromine appeared necessary in the development of behavioral and neurochemical sensitizations to nicotine, we tried to understand why, in our experimental model in mice, repeated injections of nicotine alone do not induce these sensitizations. Our data suggest that nicotine induces an indirect stimulation of 5-HT1A autoreceptors that limits serotonergic transmission and that desensitization of 5-HT1A autoreceptors by MAOIs removes this limitation.
Materials and Methods
Subjects
Wild-type (WT) mice were C57BL/6J, 2–3 months of age, and male (25–35 g). They were housed four by cage and maintained on a 12 h light/dark cycle (lights on at 7:00 A.M.) with food and water available ad libitum. All animals were naive at the beginning of each experiment.
Drugs
(−)-Nicotine hydrogen tartrate, tranylcypromine hydrochloride, d-amphetamine sulfate p-chloroamphetamine hydrochloride, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclo-hexane carboxamide trihydrochloride (WAY 100635) and 8-hydroxy-dipropylamino-tetralin (8-OHDPAT) (Sigma Aldrich) were dissolved in saline. The pH values of the solutions were adjusted to 7.4 with 1N NaOH. Prazosin hydrochloride (Sigma-Aldrich) was sonicated in water and completed with saline. (1Z,2E)-1-(2-Fluoro-phenyl)-3-(4-hydroxyphenyl)-prop-2-en-one-O-(2-dimethylamino-ethyl)-oxime hemifumarate (SR46349B hemifumarate) was a generous gift from sanofi-aventis. It was dissolved with a drop of lactic acid, neutralized with 1 m NaOH, and sonicated in saline. R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390 hydrochloride) (Sigma Aldrich) was first dissolved in distilled water, which was then completed with saline. All drugs were injected intraperitoneally (0.3 ml per 100 g) except for nicotine, which was injected subcutaneously (0.15 ml per 100 g). Tetrodotoxin (TTX) (Tocris Bioscience) was perfused through the membrane dialysis at a concentration of 1 μm. Doses are expressed as salts. d-Amphetamine was given at 2 mg/kg and p-chloroamphetamine at 7 mg/kg (Itzhak et al., 2004). Doses of prazosin (1 mg/kg, i.p.) and SR46349B (1 mg/kg, i.p.) were kept identical with previous experiments (Auclair et al., 2004). Doses of nicotine (1 mg/kg, s.c.; salt instead of base) and tranylcypromine (6 mg/kg instead of 10 mg/kg) were decreased because of repeated treatments (Villégier et al., 2006a). SCH23390 was used at 0.2 mg/kg (intraperitoneally), a dose which blocks the development of behavioral and neurochemical sensitization to 2 mg/kg d-amphetamine (Salomon et al., 2006). WAY 100635 and 8-OHDPAT were injected at a dose of 1 mg/kg.
Surgery
Mice were anesthetized with sodium pentobarbital (60 mg/kg; Sanofi Santé Animale) and placed in a stereotaxic frame (Kopf Instruments). Unilateral permanent cannula (CMA/7 guide cannula; Microdialysis) was placed at the edge of the prefrontal cortex (PFC) and was secured on the skull with screw and dental cement. The coordinates for the guide cannula tip were as follows: anteroposterior, +2.6 relative to bregma; mediolateral, +0.5; and dorsoventral, 0 mm from dura (Salomon et al., 2006). After surgery, mice were placed in individual plastic cages and allowed to recover for at least 4 d.
Microdialysis experiments
Acute treatment.
The day of the experiment, the microdialysis probe was inserted in the PFC (CMA/7; membrane length, 2 mm, and diameter, 0.24 mm; cutoff, 6000 Da; Microdialysis). Artificial CSF (in mm: 147 NaCl, 3.5 KCl, 1 CaCl2, 1.2 MgCl2, 1 NaH2PO4, 25 NaHCO3, pH 7.6) was perfused with a CMA100 microinjection pump through the probe at a rate of 1 μl/min via FEP catheter (internal diameter, 0.12 mm) connected to a fluid swivel. Adequate steady state of monoamines levels in perfusate samples was reached 140 min after probe insertion, and samples were collected in 300 μl vials placed into a refrigerated computer-controlled fraction collector (CMA/170). Samples (20 μl every 20 min) were collected during 100 min, to determine basal extracellular values. After d-amphetamine or PCA injections, samples were collected for 200 min. After tranylcypromine injection, samples were collected for 240 min. In the last series of experiments, 100 min after tranylcypromine injection, either 8-OHDPAT (0.5 mg/kg) was injected systemically, or TTX (1 μm) was perfused locally through the probe. Samples were analyzed for NE or 5-HT levels the day of the experiment.
Repeated treatment.
The experimental conditions are shown in Table 1. Briefly, mice received four consecutive daily injections of saline or tranylcypromine or nicotine or tranylcypromine plus nicotine or WAY 100635 or WAY 100635 plus nicotine and waited 4 d before the dialysis experiment. To test the effect of the pretreatment on the development of neurochemical sensitization to tranylcypromine plus nicotine, mice received every single day a pretreatment (saline or prazosin plus SR46349B or SCH23390) 30 min before the injection of tranylcypromine. When animals were pretreated with prazosin and SR46349B, two subsequent injections of prazosin were administered at 60 and 150 min after tranylcypromine injection, because of its short half-life (100 min) (Auclair et al., 2004). Every day after drug injection, mice were immediately placed for 2 h in the cylindrical compartment used to perform microdialysis. Control mice were injected on 4 consecutive days with 0.9% saline (0.1 ml/injection) or SR46349B plus prazosin or SCH23390, and their response to d-amphetamine or PCA was measured 4 d later.
Table 1.
Mice pretreatments before challenges by d-amphetamine or para-chloro-amphetamine
| Day 1 | Day 2 | Day 3 | Day 4 | Days 5–8 | Day 9 |
|---|---|---|---|---|---|
| Saline | Saline | Saline | Saline | 4 d Withdrawal | d-Amph |
| Id | Id | Id | Id | Id | PCA |
| Tranyl | Tranyl | Tranyl | Tranyl | Id | d-Amph |
| Id | Id | Id | Id | Id | PCA |
| Nicotine | Nicotine | Nicotine | Nicotine | Id | d-Amph |
| Id | Id | Id | Id | Id | PCA |
| Tranyl + nico | Tranyl + nico | Tranyl + nico | Tranyl + nico | Id | d-Amph |
| Id | Id | Id | Id | Id | PCA |
| SR/Pz + tranyl + nico | SR/Pz + tranyl + nico | SR/Pz + tranyl + nico | SR/Pz + tranyl + nico | Id | d-Amph |
| Id | Id | Id | Id | Id | PCA |
| SCH + tranyl + nico | SCH + tranyl + nico | SCH + tranyl + nico | SCH + tranyl + nico | Id | d-Amph |
| Id | Id | Id | Id | Id | PCA |
| Way + nico | Way + nico | Way + nico | Way + nico | Id | d-Amph |
In order to test neurochemical and behavioral sensitizations, mice received once a day during 4 d different pretreatments before a 4 d withdrawal. Way, WAY 100635; SCH, SCH23390; d-Amph, d-amphetamine; nico, nicotine; tranyl, tranylcypromine; Id, idem; Pz, prazosin; SR, SR46349B.
Biochemistry
Dialysate samples were completed to 30 μl with the adapted mobile phase and placed into a refrigerated automatic injector (Triathlon; Spark Holland). Twenty-five microliters of the sample were injected every 30 min through a rheodyne valve in the mobile phase circuit. High-performance liquid chromatography was performed with a reverse-phase column (80 × 4.6 mm; 3 μm particle size; HR-80; ESA). Mobile phase (for NE analysis: 0.1 m NaH2PO4, 0.1 mm EDTA, 3.8 mm octane sulfonic acid, 0.25 mm triethylamine, 10% methanol, pH 2.9; and for 5-HT analysis: 0.1 m NaH2PO4, 0.1 mm EDTA, 1.5 mm octane sulfonic acid, 0.25 mm triethylamine, 15% methanol, 5% acetonitrile, pH 2.9) was delivered at 0.7 ml/min by an ESA-580 pump. Electrochemical detection was performed with an ESA coulometric detector (Coulochem II 5100A, with a 5014B analytical cell; Eurosep). The conditioning electrode was set at −0.175 mV, and the detecting electrode was set at +0.175 mV, allowing a good signal-to-noise ratio. External standards were regularly injected to determine the stability of the sensitivity (0.2 pg for NE and 0.3 pg for 5-HT).
Locomotor activity
Acute treatment.
Mice were introduced in a circular corridor (4.5 cm width, 17 cm external diameter) crossed by four infrared beams (1.5 cm above the base) placed at every 90° (Imetronic). The locomotor activity was counted when animals interrupted two successive beams and thus had traveled one-quarter of the circular corridor. Spontaneous activity was recorded for 120 min (habituation to the experimental procedure), and then mice were injected intraperitoneally with d-amphetamine or p-chloroamphetamine or tranylcypromine or nicotine or tranylcypromine plus nicotine or WAY 100635 or WAY 100635 plus nicotine or 8-OHDPAT or 8-OHDPAT plus nicotine, and locomotor responses were recorded for an additional 200 min period. Injection of nicotine occurred 100 min after injection of tranylcypromine (Villégier et al., 2006a), 30 min after injection of WAY 100635, and 60, 100, or 120 min after injection of 8-OHDPAT.
Repeated treatment.
The experimental conditions are shown in Table 1. Briefly, mice received four consecutive daily injections of saline or tranylcypromine or nicotine or tranylcypromine plus nicotine, or WAY 100635 or WAY 100635 plus nicotine, and their locomotor activity was recorded after a d-amphetamine, p-chloroamphetamine, or WAY 100635 plus nicotine injection after a 4 d withdrawal with the same protocol as for an acute treatment. To test the effect of the pretreatment on the development of behavioral sensitization to tranylcypromine plus nicotine, mice received every single day a pretreatment (saline or prazosin plus SR46349B or SCH23390) 30 min before the injection of tranylcypromine. When animals were pretreated with prazosin plus SR46349B, two subsequent injections of prazosin were administered at 60 and 150 min after tranylcypromine injection because of its short half-life (100 min). Finally, locomotor responses to d-amphetamine or PCA were tested 4 d after the last drug injection. Control mice were injected on 4 consecutive days with saline or SR46349B plus prazosin or SCH23390, and their locomotor response to d-amphetamine or PCA was recorded 4 d later.
Histology
At the end of the experiment, brains were put into a formaldehyde solution and cut on a microtome in serial coronal slices according to the atlas of Paxinos and Franklin (1997). Histological examination of cannula tip placement was subsequently made on 100 μm safranine-stained coronal sections (Fig. 1).
Figure 1.
Schematic illustration of the localization of the membrane dialysis. The drawing is from the atlas by Paxinos and Franklin (1997). The number corresponds to the anteriority of the slice from the bregma.
Immunodetection of 5-HT1A receptors
Preparation of membrane protein enriched fraction.
Mice were injected with saline or tranylcypromine (6 mg/kg, i.p.) in their home cage. One-hundred minutes later, they were killed by decapitation, and their brains were rapidly removed from the calvarium and immediately frozen in isopentane (−30°C), before being stored at −80°C. Then, serial coronal sections (500 μm) were obtained using a cryostat (MICROM HM560). One hundred milligrams of prefrontal cortex (corresponding to four mice in each group) were collected and 50 mg of dorsal raphe nucleus (DRN) (corresponding to eight mice in each group) were punched from the slices according to the atlas of Paxinos and Franklin (1997). These experiments were performed at −20°C.
The prefrontal cortex and DRN punched samples were homogenized using a 1 ml Potter-Elvehjem PTFE pestle and glass tube homogenizer (Sigma-Aldrich), (1 g/10 V), in ice-cold classic Tris-HCl buffer (10 mm, pH 8; Sigma-Aldrich) containing 0.25 m sucrose and Protease Inhibitor Mixture tablet (Roche Diagnostics). These homogenates were centrifuged at 22,000 × g for 10 min at 4°C. The supernatants were carefully decanted, and then centrifuged at 50,000 × g for 1 h at 4°C. The supernatants resulting of this second centrifugation were removed, and the remaining pellets containing membrane proteins were finally resuspended in 50 μl of homogenized buffer described above. The preparation of this membrane enriched fraction was adapted from a protocol previously described for myometrial cell membranes (Legrand et al., 1987; Mhaouty-Kodja et al., 2001).
Western blot analysis.
The protein concentration of all extracted samples was measured using Bio-Rad Protein Assay (Bio-Rad Laboratories) and bovine serum albumin (BSA) was used as a protein standard. Protein samples (40 μg) were boiled 5 min at 95°C in Laemmli buffer containing glycerol and β-mercaptoethanol (Laemmli, 1970). Lysates were subjected to SDS-PAGE and transferred on HybondC EC nitrocellulose membrane (GE Healthcare). Membranes were then blocked 1 h at room temperature in buffer containing 50 mm Tris, 150 mm NaCl, 0.1% Tween 20, and 2% BSA, and incubated overnight at 4°C in a 1:500e dilution of rabbit polyclonal antibody against 5-HT1A receptor (el Mestikawy et al., 1990) or in a 1:1500e dilution of rabbit polyclonal antibody against 5-HT transporter (5-HTT) (Qian et al., 1995). Secondary antibody, goat anti-rabbit IgG conjugated to HRP (7074 and 7072; Cell Signaling), was used in 1:3000 dilution in TBS-Tween (0.1%) solution. Membranes were incubated 1 h at room temperature, and visualization of immunoreactivity was performed by chemiluminescence using ECL kit (Pierce EC Western Blotting Substrate kit; Thermo Scientific), and detection of chemiluminescence was performed using Kodak developer and fixer, and Kodak Hyperfilm ECL (GE Healthcare). Beta Actine (A544J; Sigma-Aldrich) was immunoprobed as index of cellular protein level. Immunostaining quantification was performed by using the ChemiGenius2 analyzer image and software (Ozyme). 5-HT1A knock-out (KO) mice (Heisler et al., 1998) were used as control of antibody specificity. The 5-HTT was used as a specific marker of serotonergic cells and plasma membrane proteins.
Statistics
Statistical analysis was performed using GraphPad Prism 3.0 software. Data from microdialysis and locomotor activity experiments were described as a function of time. Data from microdialysis were expressed as a percentage of the respective mean basal value. The extracellular monoamines levels and the locomotor activity obtained after different treatments were compared and analyzed with a two-way ANOVA (repeated measures). In this test, there are two factors that affect the dependent variables: effect of time (effect A) and effect of drug (effect B). Each factor has two or more levels within it, and the degrees of freedom (df) for each factor is 1 less than the number of levels. It is assumed (1) that main effect A has a levels [and A has (a − 1) df], (2) that main effect B has b levels [and B has (b − 1) df], and (3) that n is the sample size of each treatment. In these conditions, when there is a significant interaction between the two factors, we compare FAB to a F with (a − 1) and (a − 1)(b − 1) degrees of freedom. When there is no significant interaction, we compare FAB to a F with (a − 1) and a(n − 1) degrees of freedom, the residual. Differences in 5-HT1A receptors expression were determined using an unpaired t test. Significant differences were set at p < 0.05.
Results
Repeated nicotine alone or tranylcypromine alone leads neither to noradrenergic and serotonergic neuron sensitization nor to behavioral sensitization
As previously observed (Salomon et al., 2006), repeated treatment with saline does not alter the NE and 5-HT release in the prefrontal cortex, when compared with acutely treated animals, after a d-amphetamine and PCA injection, respectively (F(1,24) = 2.848, p < 0.1, and F(1,24) = 1.696, p < 0.2, respectively). Similarly, repeated treatments with nicotine do not induce increases in extracellular NE or 5-HT levels higher than those induced in animals pretreated with saline (F(1,24) = 2.816, p < 0.1, and F(1,24) = 1.149, p < 0.2, for NE and 5-HT levels induced by d-amphetamine and PCA, respectively) (Fig. 2A,C). Furthermore, repeated treatments with tranylcypromine induce a slight but significant decrease in extracellular NE and 5-HT levels (F(1,5) = 5.224, p < 0.05, and F(1,5) = 7.356, p < 0.01, for NE and 5-HT levels induced by d-amphetamine and PCA, respectively).
Figure 2.
Repeated nicotine requires tranylcypromine to sensitize noradrenergic and serotonergic neurons and obtain behavioral sensitization. Nicotine (1 mg/kg), tranylcypromine (6 mg/kg), and nicotine plus tranylcypromine were used for repeated treatments. Four days after the last injection, d-amphetamine (2 mg/kg) or PCA (7 mg/kg) was administered and cortical extracellular NE (A) or 5-HT (C) levels, respectively or locomotor responses (B, D, respectively) were analyzed. Each group contained at least five animals for microdialysis experiments and eight animals for locomotor activity determination. A, Cortical extracellular NE levels are expressed as a percentage of the respective mean basal value. There was no significant difference in basal cortical extracellular NE levels in the different conditions (mean basal NE values, 0.67 pg NE/20 min ± 0.08). B, Locomotor response to d-amphetamine (2 mg/kg) is measured in the same experimental conditions as in A. C, Cortical extracellular 5-HT levels are expressed as a percentage of the respective mean basal value. There was no significant difference in basal cortical extracellular 5-HT levels in the different conditions (mean basal 5-HT values, 0.81 pg 5-HT/20 min ± 0.06). D, Locomotor response to PCA (7 mg/kg) was measured in the same experimental conditions as in C. Error bars indicate SEM. nico, Nicotine; tranyl, tranylcypromine.
Finally, repeated treatments with nicotine or tranylcypromine do not induce behavioral sensitization to d-amphetamine or PCA treatments [(F(1,147) = 1.59, p < 0.8, and F(1,147) = 1.86, p < 0.1, after d-amphetamine for repeated nicotine or tranylcypromine, respectively, when compared with repeated saline) and (F(1,147) = 2.975, p < 0.08, and F(1,147) = 0.6404, p < 0.4, after PCA for repeated nicotine or tranylcypromine, respectively, when compared with repeated saline)] (Fig. 2B,D).
Repeated treatments with a nicotine and tranylcypromine mixture lead to noradrenergic and serotonergic neuron sensitization and behavioral sensitization
When animals received repeated treatments with both nicotine and tranylcypromine, we found an important increase in extracellular NE and 5-HT levels (F(1,5) = 294.4, p < 0.0001, and F(1,5) = 93.76, p < 0.0001, for NE and 5-HT levels induced by d-amphetamine and PCA, respectively) (Fig. 2A,C). Similarly, after repeated treatments with nicotine plus tranylcypromine, we found a significant behavioral sensitization to d-amphetamine and PCA (F(1,20) = 354.7, p < 0.0001, and F(1,20) = 224.1, p < 0.0001, for repeated tranylcypromine plus nicotine when compared with repeated saline, after d-amphetamine or PCA, respectively) (Fig. 2B,D).
Pretreatments with prazosin and SR46349B, but not with SCH23390, before nicotine and tranylcypromine mixture injection, protect noradrenergic and serotonergic neurons from sensitization and block the development of behavioral sensitization
To verify that, as in the case of other drugs of abuse, increased reactivity of noradrenergic and serotonergic neurons induced by repeated treatments with nicotine and tranylcypromine is related to the stimulation of α1b-adrenergic and 5-HT2A receptors, prazosin (1 mg/kg) and SR46349B (1 mg/kg) were injected 30 min before tranylcypromine plus nicotine mixture. Pretreatment with these two receptor antagonists blocked the NE and 5-HT neuron sensitization induced by repeated tranylcypromine plus nicotine mixture. It was found that extracellular NE and 5-HT levels induced by d-amphetamine and PCA were significantly different between animals pretreated with tranylcypromine and nicotine and those pretreated with prazosin and SR46349B in addition to tranylcypromine and nicotine (F(1,5) = 368.4, p < 0.0001, and F(1,5) = 157.7, p < 0.0001, for NE and 5-HT extracellular levels, respectively) (Fig. 3A,C). Indeed, after pretreatment with these two receptor antagonists, extracellular NE and 5-HT levels induced by d-amphetamine and PCA were not different from those of animals repeatedly treated with saline (F(1,24) = 3.765, p < 0.06, and F(1,24) = 3.663, p < 0.06, for NE and 5-HT levels, respectively).
Figure 3.
Development of neurochemical and behavioral sensitizations induced by repeated tranylcypromine plus nicotine is blocked by prazosin and SR46349B but not by SCH23390. Experimental conditions were identical with those of Figure 2 for determinations of extracellular NE and 5-HT levels as well as for monitoring locomotor activity (A–D). Each group consisted of at least five and eight animals for microdialysis and locomotor activity, respectively. It was verified that data obtained with repeated SR46349B plus prazosin or repeated SCH233890 were not significantly different from that of repeated saline. Amph, d-Amphetamine; SR, SR46349B; Pz, prazosin; SCH, SCH23390. Error bars indicate SEM.
At the opposite, injections of a D1 receptor antagonist, SCH23390, at 0.2 mg/kg, a dose that blocks behavioral and neurochemical sensitizations to d-amphetamine (Salomon et al., 2006), 30 min before injections of the tranylcypromine plus nicotine mixture did not modify the increased responses in extracellular NE and 5-HT levels induced by d-amphetamine and PCA in animals pretreated with saline before the tranylcypromine plus nicotine mixture (F(1,24) = 0.0028, p < 0.9, and F(1,24) = 0.5170, p < 0.4, when compared with saline-pretreated animals for NE and 5-HT levels after d-amphetamine and PCA, respectively) (Fig. 3A,C).
Effects obtained for behavioral sensitizations to d-amphetamine and PCA in presence of these three receptors ligands are identical with those obtained for neurochemical sensitizations. Indeed, pretreatments with prazosin and SR46349B block the development of the behavioral sensitizations to d-amphetamine and PCA (F(1,147) = 3.61, p < 0.06, and F(1,147) = 2.765, p < 0.09, for repeated tranylcypromine plus nicotine when compared with repeated saline after d-amphetamine or PCA, respectively), whereas SCH23390 has no effect on the development of behavioral sensitization to d-amphetamine and PCA induced by tranylcypromine plus nicotine (F(1,147) = 3.885, p < 0.06, and F(1,147) = 3.691, p < 0.06, for d-amphetamine and PCA, respectively, when compared with animals pretreated with saline instead of SCH23390) (Fig. 3B,D).
Finally, behavioral experiments were performed in presence of either prazosin (1 mg/kg) or SR46349B (1 mg/kg) to determine the respective roles of noradrenergic and serotonergic transmissions, respectively, in the development of behavioral sensitizations to repeated tranylcypromine plus nicotine. We found that each receptor antagonist (i.e., prazosin or SR46349B) was able to block almost completely the behavioral sensitizations induced by tranylcypromine plus nicotine as revealed by d-amphetamine or PCA administrations (data not shown).
Effects of the acute injection of the mixture tranylcypromine plus nicotine on locomotor response and cortical extracellular NE and 5-HT levels
We showed previously (Villégier et al., 2006a,b) that, whereas C57BL/6 mice do not exhibit a locomotor response to nicotine (1 mg/kg; base), nicotine-induced locomotor hyperactivity could be obtained when tranylcypromine (10 mg/kg) was injected 100 min before nicotine. Here, we confirm these data when a lower dose of tranylcypromine (6 mg/kg) is injected 100 min before a lower dose of nicotine (1 mg/kg; salt) (F(1,48) = 461.4, p < 0.0001, when tranylcypromine plus nicotine is compared with tranylcypromine plus saline) (Fig. 4A).
Figure 4.

Locomotor activity and cortical extracellular NE and 5-HT levels in response to nicotine in presence or absence of tranylcypromine. Animals received tranylcypromine (6 mg/kg) or saline (first arrow) and, 100 min later, an injection of nicotine (1 mg/kg) or saline (second arrow), and locomotor activity (A) and extracellular NE (B) and 5-HT (C) levels were monitored. Each group consisted of at least five and eight animals for microdialysis and locomotor activity, respectively. Mean basal cortical extracellular NE and 5-HT levels were similar to those of Figure 2. Error bars indicate SEM. nico, Nicotine; tranyl, tranylcypromine.
Because the sensitizing effects of the mixture tranylcypromine plus nicotine are blocked by α1-adrenergic and 5-HT2A receptor antagonists, we analyzed the time course of cortical extracellular NE and 5-HT levels in the same conditions as those of the development of the locomotor response. As shown on Figure 4B, cortical extracellular NE levels increase dramatically 20 min after tranylcypromine (6 mg/kg) and then decrease regularly to attain initial values 100 min after the injection (F(1,12) = 121.2, p < 0.0001, when compared with saline injection). Nicotine, however, has no effect on extracellular NE levels in presence or absence of tranylcypromine (F(1,52) = 2.344, p < 0.1, and F(1,52) = 3.578, p < 0.06, when compared with respective controls, with saline or with tranylcypromine) (Fig. 4B).
Figure 4C shows that, unlike extracellular NE levels, extracellular 5-HT levels increase regularly after tranylcypromine (6 mg/kg) and increase quicker when nicotine is injected instead of saline 100 min after tranylcypromine (F(1,12) = 240.8, p < 0.0001, and F(1,12) = 39.08, p < 0.0001, when compared with saline and nicotine, respectively). Finally, it was verified that nicotine alone had no effect on cortical extracellular 5-HT levels (F(1,52) = 3.242; p < 0.07) (Fig. 4C). It can be noted that these data obtained on cortical extracellular 5-HT levels are similar to those obtained when extracellular 5-HT levels were monitored in the nucleus accumbens after tranylcypromine and nicotine (Villégier et al., 2006a).
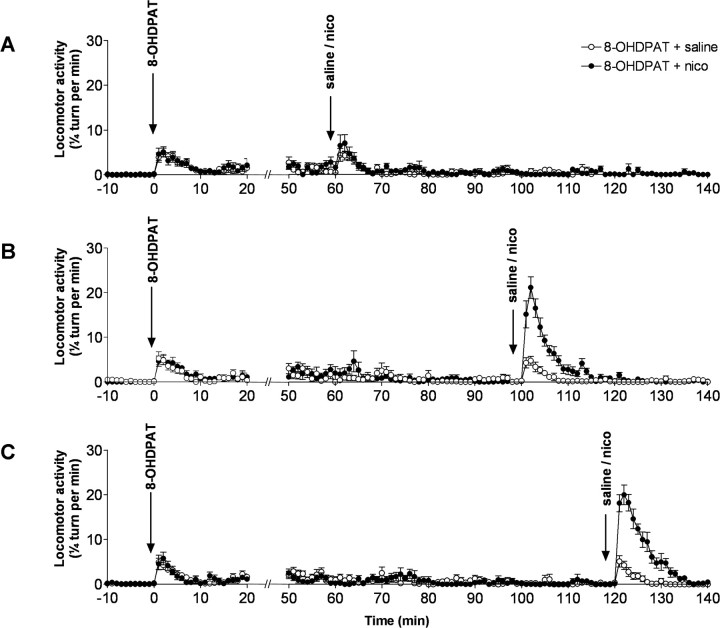
Effects of 8-OHDPAT, a 5-HT1A receptor agonist, on locomotor response to nicotine
Our data indicate that a modification of 5-HT transmission by nicotine is associated with a locomotor response to nicotine. Engberg et al. (2000) showed that nicotine could indirectly inhibit serotonergic neurons through the stimulation of 5-HT1A receptors. We therefore made the hypothesis that tranylcypromine, because of its effects on extracellular 5-HT levels, was desensitizing 5-HT1A receptors, thus allowing nicotine to trigger a locomotor response. This was tested by pretreating the animals with 8-OHDPAT (1 mg/kg), a 5-HT1A receptor agonist, at different times before nicotine injection (1 mg/kg). Figure 5 shows that, whereas 8-OHDPAT has no effect on nicotine-induced locomotor response 60 min after the agonist injection (F(1,987) = 3.09, p < 0.07, when compared with saline), nicotine induces a significant locomotor response 100 min (F(1,140) = 573.3; p < 0.0001) as well as 120 min (F(1,140) = 850.8; p < 0.0001) after 8-OHDPAT injection (Fig. 5A–C).
Figure 5.
Effects of 8-OHDPAT, a 5-HT1A receptor agonist, on locomotor activity induced by nicotine. Three groups of eight animals received an injection of 8-OHDPAT (1 mg/kg) and 60 min (A), 100 min (B), or 120 min (C) later, an injection of nicotine (1 mg/kg) or of saline, and locomotor activity was monitored. Error bars indicate SEM. nico, Nicotine.
Effects of WAY 100635, a 5-HT1A receptor antagonist, on locomotor response to nicotine
To verify that the effect of 8-OHDPAT on locomotor response to nicotine was attributable to the desensitization/inactivation of 5-HT1A receptors, analysis of locomotor response to nicotine was performed in presence of WAY 100635, a 5-HT1A receptor antagonist. Figure 6 shows that, 30 min after the injection of WAY100635, nicotine induces a significant locomotor response (F(1,60) = 867.5; p < 0.0001), whereas no significant effect is observed when nicotine or WAY 100635 is injected alone (F(1,427) = 3.341, p < 0.1, and F(1,427) = 0.3173, p < 0.5, when compared with saline, for nicotine and WAY 100635, respectively) (Fig. 6A).
Figure 6.
Effects of WAY 100635, a 5-HT1A receptor antagonist, on locomotor activity induced by nicotine after acute or repeated treatments. A, C, Acute, Animals received an injection of WAY 100635 (1 mg/kg) or saline and, 30 min later, an injection of nicotine (1 mg/kg) or saline, and locomotor activity was monitored. B, D, Pretreated, Animals received 4 consecutive days, once a day, either repeated saline plus saline, saline plus nicotine, WAY 100635 plus saline, or WAY plus nicotine. Then, 4 d later, all animals received an injection of WAY 100635 and, 30 min later, an injection of nicotine, and locomotor activity was measured. C, Histograms of acute locomotor responses after different conditions (white rectangles). ***p < 0.0001 from corresponding saline, WAY 100635, or nicotine controls. D, Histograms of locomotor responses to WAY 100635 plus nicotine after different repeated pretreatments. ***p < 0.0001 from acute WAY 100635 plus nicotine and repeated saline plus saline, WAY 100635 plus saline, or saline plus nicotine. Each group contained at least eight animals. sal, Saline; nico, nicotine; WAY, WAY 100635. Error bars indicate SEM.
Interestingly, when WAY 100635 and nicotine were injected once a day on 4 consecutive days and WAY 100635 and nicotine were injected after a 4 d withdrawal, a significant increase in locomotor response to nicotine is obtained (F(1,60) = 812.2, p < 0.0001, when compared with acute locomotor response induced by WAY 100635 plus nicotine) (Fig. 6B–D). This behavioral sensitization is not observed when animals are pretreated once a day on 4 consecutive days with WAY 100635 or nicotine alone (F(1,427) = 0.3173, p < 0.5, and F(1,427) = 0.09002, p < 0.7643, for WAY 100635 and nicotine, respectively) (Fig. 6B,D).
Does behavioral sensitization obtained with WAY 100635 plus nicotine correlate with sensitization of noradrenergic and serotonergic neurons?
To test whether behavioral sensitization to nicotine obtained with repeated injections of WAY 100635 plus nicotine was parallel to neurochemical sensitization, cortical extracellular NE and 5-HT levels were monitored after d-amphetamine and PCA injections. Figure 7 shows that animals repeatedly treated with WAY 100635 and nicotine exhibit a higher increase of extracellular NE and 5-HT levels than corresponding controls (F(1,5) = 53.01, p < 0.0001, and F(1,5) = 38.33, p < 0.0001, for NE and 5-HT levels, respectively), whereas extracellular NE and 5-HT levels are not different from controls when animals are repeatedly treated with WAY 100635 alone (F(1,24) = 0.5650, p < 0.4, and F(1,24) = 0.2904, p < 0.5, for NE and 5-HT levels, respectively) (Fig. 7A,C). Similarly, extracellular NE and 5-HT levels are not different from controls when animals are repeatedly treated with nicotine alone (Fig. 2A,C).
Figure 7.
Effects of repeated WAY 100635 plus nicotine on sensitization of noradrenergic and serotonergic neurons and behavioral sensitization. Animals were repeatedly treated once a day during 4 d by WAY 100635 (1 mg/kg) and nicotine (1 mg/kg), and WAY 100635 and saline. Four days after the last injection, d-amphetamine (2 mg/kg) or PCA (7 mg/kg) was administered and cortical extracellular NE (A) or 5-HT (C) levels, respectively, or locomotor responses (B, D, respectively) were analyzed. Data of repeated nicotine, presented in Figure 2, have been omitted for sake of clarity. Each group contained at least five animals for microdialysis experiments and eight animals for locomotor activity determination. Mean basal extracellular NE and 5-HT levels were similar to those of Figure 2. Amph, d-Amphetamine; nico, nicotine; WAY, WAY 100635. Error bars indicate SEM.
An increase in locomotor responses to d-amphetamine and PCA is also observed in animals repeatedly treated with WAY 100635 plus nicotine (F(1,20) = 295.7, p < 0.0001, and F(1,20) = 394.7, p < 0.0001, when compared with saline pretreated animals for d-amphetamine and PCA, respectively), whereas this effect is not observed in animals repeatedly treated with WAY 100635 alone (F(1,147) = 3.529, p < 0.06, and F(1,147) = 0.3204, p < 0.5, for d-amphetamine and PCA, respectively) (Fig. 7B,D). Similarly, there is no effect on locomotor response when animals are repeatedly treated with nicotine alone (Fig. 2B,D).
Analysis of the effects of tranylcypromine on 5-HT1A receptors
To test early effects of tranylcypromine on 5-HT1A receptor function, we analyzed by microdialysis the ability of 8-OHDPAT to decrease 5-HT extracellular levels 100 min after a tranylcypromine injection. As expected, 8-OHDPAT (0.5 mg/kg) induces a potent decrease in 5-HT extracellular levels (F(1,7) = 628.5, p < 0.0001, when compared with saline) (Fig. 8A). In contrast, 100 min after a tranylcypromine injection, 8-OHDPAT (0.5 mg/kg) fails to significantly alter 5-HT extracellular levels (F(1,32) = 0.8414, p < 0.3, when compared with saline). Same data were obtained with 1 mg/kg 8-OHDPAT (data not shown).
Figure 8.

Short-term effects of tranylcypromine on 5-HT1A receptors. A, Cortical 5-HT extracellular levels were measured in mice after injection of either saline or tranylcypromine (first arrow) and, 100 min later, saline or 8-OHDPAT (second arrow). In another series of experiments, animals received, 100 min after a tranylcypromine injection, a perfusion of TTX (1 μm) (dotted lines). N = 5 animals per group. B, Mice were injected with either saline or tranylcypromine and PFC or DRNs were punched 100 min later. Experiments were also performed with cerebral tissues from animals deprived of 5-HT1A receptors (KO). Levels of 5-HTT and 5-HT1A receptors were determined in the presence of respective antibodies using β-actin as a reference. Experiments have been performed three times with N = 5 mice for prefrontal cortex and N = 8 mice for raphe nuclei. C, Histograms of relative expression of 5-HT1A receptors when compared with β-actin. ***p < 0.001 when compared with respective controls. aExperiments with KO mice have been performed twice. sal, Saline; tranyl or tran, tranylcypromine. Error bars indicate SEM.
However, because tranylcypromine may increase 5-HT extracellular levels through an impulse-independent process that would be insensitive to 5-HT1A receptors stimulation, the effect of a local perfusion of TTX was tested on the increased tranylcypromine-induced 5-HT extracellular levels. Data indicate that, when 1 μm TTX is perfused through the dialysis probe 100 min after a tranylcypromine injection, it induces a dramatic reduction of 5-HT extracellular cortical levels (F(1,7) = 1.137, p < 0.0001, when compared with saline) (Fig. 8A).
To test whether this lack of effect of 8-OHDPAT is attributable to a reduction of 5-HT1A receptor density on serotonergic cells, we analyzed 5-HT1A receptors expression by Western blot on a plasma membrane enriched fraction from prefrontal cortex (heteroreceptors) and DRN (autoreceptors) 100 min after a tranylcypromine injection. Figure 8B shows that the 5-HT1A protein migrates as immunoreactive bands of ∼50 and ∼63 kDa. This finding is in agreement with that previously described in rat brain (el Mestikawy et al. 1990). As expected, the 5-HT1A immunostaining strongly decreases in the 5-HT1A knock-out mice tissues, confirming the specificity of 5-HT1A antibody. Our data show that tranylcypromine specifically decreases the 5-HT1A immunoreactive bands in DRN (p < 0.0001), but not in prefrontal cortex, whereas the total amount of membrane protein loaded, as shown with the 5-HTT staining, stays constant in all conditions (Fig. 8C). This relative diminution of 5-HT1A protein levels in the membrane enriched fraction suggests that the 5-HT1A autoreceptors have been internalized.
Discussion
The main finding of our study is that nicotine needs the association with an irreversible and nonselective MAOI to induce the same neurochemical modifications as those observed with compounds belonging to the main groups of drugs of abuse (i.e., amphetamine, cocaine, morphine, or alcohol) (Salomon et al., 2006; Lanteri et al., 2008). Moreover, although repeated injections of nicotine alone do not enhance the hyperlocomotor effects of amphetamine or PCA, tranylcypromine pretreatment allows nicotine to induce a robust and persistent cross-sensitization to these two drugs. Our data also show that, as with all drugs of abuse tested up to now, the neurochemical and behavioral effects of nicotine revealed by MAO inhibition are prevented by coadministration of α1b-adrenergic and 5-HT2A receptor antagonists but not by a D1 receptor antagonist. This confirms that repeated stimulation of α1b-adrenergic and 5-HT2A receptors, but not of D1 receptors, is a critical step in the development of long-lasting effects of drugs of abuse (Auclair et al., 2004; Salomon et al., 2006; Lanteri et al., 2008).
First, we may wonder why nicotine does not induce by itself sensitization of noradrenergic and serotonergic neurons nor behavioral sensitization?
The mechanism by which drugs of abuse are supposed to increase locomotor activity has been linked to their ability to stimulate mesolimbic dopaminergic transmission (Kelly et al., 1975; Kelly and Iversen, 1976). Nicotine is known to stimulate dopamine (DA) release in the nucleus accumbens via the stimulation of nicotinic receptors located in the ventral tegmental area (Di Chiara and Imperato, 1988; Vezina et al., 1992; Nisell et al., 1994; Pontieri et al., 1996). Therefore, it has been proposed that MAO inhibition contributes synergistically with nicotine to enhance its reinforcing effects by maintaining an increased DA concentration in the nucleus accumbens (Lewis et al., 2007). However, we previously showed that GBR12783 [1-[2-(diphenylmethoxy)ethyl]-4-(3-phenyl-2-(propenyl)-piperazine], which specifically blocks DA uptake, does not trigger locomotor response to nicotine in C57BL/6 mice (Villégier et al., 2006b). This strongly suggests that DA release is not a limiting factor in the development of a locomotor response to nicotine. Interestingly, we found that only a compound that increases 5-HT release could elicit a locomotor response to nicotine in these mice (Villégier et al., 2006b).
Functional interactions between ascending monoaminergic systems have been well characterized and are thought to play a key role in addictive processes. Indeed, noradrenergic and serotonergic neurons control behavioral response and subcortical DA release induced by psychostimulants or opiates through the stimulation of α1b-adrenergic and 5-HT2A receptors (Blanc et al., 1994; Darracq et al., 1998; Auclair et al., 2002, 2004; Drouin et al., 2002).
In rats, nicotine has been shown to increase noradrenergic neurons activity (Egan and North, 1986; Engberg, 1989) and to stimulate NE release both in vitro (Clarke and Reuben, 1996) and in vivo (Mitchell, 1993). Here, in mice, we failed to detect an increase in cortical NE levels in response to nicotine in absence or presence of tranylcypromine. Although it may be attributable to species differences, this suggests that microdialysis may, under certain experimental conditions, be not sensitive enough to detect short-lasting releases of neurotransmitters (Brazell et al., 1991).
Depending on experimental conditions, nicotine can either increase or decrease serotonergic neurons firing (Mihailescu et al., 1998; Engberg et al., 2000). Indeed, activation of presynaptic nicotinic receptors in DRN depolarizes most of serotonergic cells through the release of NE and the stimulation of α1-adrenergic receptors (Li et al., 1998). However, the pharmacological blockade by prazosin of this response unmasks a hyperpolarization mediated by 5-HT acting on somatodendritic 5-HT1A autoreceptors (Li et al., 1998). Nicotine-induced activation of serotonergic neurons would be, in absence of MAOI, quickly blocked by indirect activation of an inhibitory retrocontrol, thus explaining why nicotine fails to increase locomotor activity in mice. Incidentally, it has been shown that 5-HT1A receptor agonists can reduce locomotor hyperactivity induced by cocaine (Carey et al., 2004), confirming the critical role played by 5-HT in the behavioral output.
Our data indicate that a 5-HT1A receptor agonist reveals a locomotor response to nicotine if nicotine is injected 100 min after the agonist administration. Paradoxically, a 5-HT1A receptor antagonist mimics this effect and, moreover, when repeatedly injected with nicotine, induces behavioral sensitization and sensitization of noradrenergic and serotonergic neurons. This indicates that a locomotor response to nicotine can occur if 5-HT1A receptors are inactivated after either their desensitization or their blockade. Interestingly, 100 min are needed to observe the effects of tranylcypromine on nicotine-induced locomotor response (Villégier et al., 2006a,b), a delay similar to that necessary for 8-OHDPAT to induce a locomotor response to nicotine (Fig. 5B).
Finally, our data show that 8-OHDPAT, at a dose shown to inhibit neuronal firing, fails to decrease 5-HT extracellular levels 100 min after a tranylcypromine injection. This lack of effect of 8-OHDPAT is correlated to a decreased membrane expression of 5-HT1A receptor in the DRN, which is not observed in the prefrontal cortex. This latter point may be attributable to a different coupling to G-protein in the raphe nucleus or to a lower tranylcypromine-induced increase in 5-HT extracellular levels in the prefrontal cortex than in the DRN. In any case, this result is consistent with previous findings showing an internalization of 5-HT1A autoreceptors but not heteroreceptors 60 min after an acute injection of 8-OHDPAT or fluoxetine (Riad et al., 2001; 2004). Together, these findings indicate that tranylcypromine is able to trigger, as soon as 100 min after its injection, a strong desensitization of 5-HT1A autoreceptors.
Following that line, it can be proposed that tranylcypromine effects on nicotine-induced locomotor activity, as well as on behavioral and neurochemical sensitizations, are attributable to the desensitization of 5-HT1A autoreceptors. The absence of behavioral response to nicotine in absence of MAOIs may therefore be attributable to the blockade of DRN neurons electrical activity (Engberg et al., 2000; Li et al., 1998) induced by the indirect agonist effect of nicotine on 5-HT1A receptors. MAOIs, because they enhance serotonergic tone, desensitize 5-HT1A autoreceptors and allow the stimulating effects of nicotine on serotonergic cells.
The second challenge of this study is to understand how repeated injections of nicotine and tranylcypromine trigger a neurochemical and behavioral sensitization. Whereas tobacco addiction is generally thought to be mediated by mesolimbic dopaminergic system (Balfour et al., 1998; Di Chiara, 2000), our findings indicate that the development of the sensitization of noradrenergic and serotonergic neurons by nicotine is not related to its ability to activate D1 transmission. Similarly, we previously showed that DA transmission is not involved in the development of noradrenergic and serotonergic sensitization after psychostimulants, opiates, or alcohol (Lanteri et al., 2008). Together, these data lead us to conclude that, although there is no doubt that DA transmission is involved in the expression of behavioral sensitization induced by drugs of abuse (Nestler, 1992; Vezina, 1996; Pierce and Kalivas, 1997), DA transmission is not involved in the development of neurochemical and behavioral sensitizations, whatever the drug of abuse.
Because prazosin and SR46349B prevent sensitization of noradrenergic and serotonergic neurons induced by nicotine when associated with tranylcypromine, this may indicate that, as it was shown for others drugs of abuse, the addictive properties of nicotine are linked to its ability to increase extracellular NE and 5-HT levels. However, repeated injections of tranylcypromine alone, which, as shown here, release both NE and 5-HT, do not lead to sensitization of noradrenergic and serotonergic neurons nor to behavioral sensitization. It may be recalled that, similarly, repeated injections of venlafaxine or clorimipramine, two nonaddictive compounds that block both NE and 5-HT reuptake activities (Millan et al., 2001), do not increase reactivity of noradrenergic and serotonergic neurons (Lanteri et al. 2008). One hypothesis could be that development of this neurochemical sensitization needs repeated intense and phasic stimulations of α1b-adrenergic and 5-HT2A receptors, whereas antidepressants induce only slow and moderate increases of noradrenergic and serotonergic transmissions (Millan et al., 2001), as well as shown here for tranylcypromine and 5-HT release. Because each antagonist could block behavioral sensitization to nicotine plus tranylcypromine, it may be assumed that activation of one system by nicotine needs the activation of the other, as shown by Li et al. (1998) with regard to the activation of 5-HT cells by NE.
The question of the mechanism of sensitization of noradrenergic and serotonergic neurons remains, however, unanswered. Anatomical and functional studies between noradrenergic and serotonergic systems indicate that the firing rate of serotonergic neurons in raphe nuclei is under the excitatory control of α1b-adrenergic receptors (Baraban and Aghajanian, 1980; Bortolozzi and Artigas, 2003). Conversely, serotonergic neurons hyperpolarize noradrenergic cells in locus ceruleus through the tonic stimulation of 5-HT2A receptors expressed by GABAergic interneurons (Gorea and Adrien, 1988; Szabo and Blier, 2001). In addition, both systems innervate the prefrontal cortex, an area that controls behavioral responses through glutamatergic afferents onto the ventral tegmental area (Kalivas and Duffy, 1998) and the nucleus accumbens (Pierce et al., 1996).
We propose that, when 5-HT1A receptors are desensitized or blocked, nicotine releases NE and 5-HT and stimulates α1b-adrenergic and 5-HT2A receptors. Repeating nicotine consumption would repeatedly stimulate these receptors and impair the mutual regulation between noradrenergic and serotonergic systems, thus leading to an increased reactivity of both groups of neurons. Increased responses of noradrenergic and serotonergic neurons to sensory stimulations may therefore represent a common mechanism underlying long-lasting effects of all drugs of abuse, including tobacco (Lanteri et al., 2008; Tassin, 2008).
In humans, nicotine replacement therapies are the most widely used form of pharmacological intervention, but have proven to be remarkably unsuccessful (Silagy et al., 2004; Medioni et al., 2005). Interestingly, most tobacco smokers relapse after a few week withdrawal (i.e., when inhibition of monoamine oxidases activity by tobacco and tobacco smoke is likely to have disappeared). MAOIs, or any compound able to desensitize 5-HT1A autoreceptors, may provide a more complete scheme of the addictive properties of tobacco in experimental models of reward.
Footnotes
This work was supported by Institut National de la Santé et de la Recherche Médicale and Université Pierre et Marie Curie, and C.L. and L.S. have received a fellowship from the Mission Interministérielle de Lutte Contre la Drogue et la Toxicomanie and Ministère de la Recherche et de la Technologie, respectively. We thank Drs. Joëlle Adrien, Edith Doucet, Laurence Lanfumey, and Michel Hamon for the 5-HT1A and 5-HTT antibodies, for their advice in the determination of 5-HT1A receptor desensitization, and for the gift of mice depleted in 5-HT1A receptors. We thank Drs. Véronique Béréziat, Sakina Mhaouty-Kodja, and Sébastien Parnaudeau for their precious advice on protein membrane fractioning and Western blotting. We thank Patricia Cougnot and Robert Poulhe for skillful technical assistance.
References
- Auclair A, Cotecchia S, Glowinski J, Tassin JP. d-Amphetamine fails to increase extracellular dopamine levels in mice lacking α1b-adrenergic receptors: relationship between functional and nonfunctional dopamine release. J Neurosci. 2002;22:9150–9154. doi: 10.1523/JNEUROSCI.22-21-09150.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004;20:3073–3084. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Balfour DJ, Benwell ME, Birrell CE, Kelly RJ, Al-Aloul M. Sensitization of the mesoaccumbens dopamine response to nicotine. Pharmacol Biochem Behav. 1998;59:1021–1030. doi: 10.1016/s0091-3057(97)00537-6. [DOI] [PubMed] [Google Scholar]
- Baraban JM, Aghajanian GK. Suppression of firing activity of 5-HT neurons in the dorsal raphe by alpha-adrenoceptor antagonists. Neuropharmacology. 1980;19:355–363. doi: 10.1016/0028-3908(80)90187-2. [DOI] [PubMed] [Google Scholar]
- Berlin I, Said S, Spreux-Varoquaux O, Olivares R, Launay JM, Puech AJ. Monoamine oxidase A and B activities in heavy smokers. Biol Psychiatry. 1995;38:756–761. doi: 10.1016/0006-3223(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Blanc G, Trovero F, Vezina P, Hervé D, Godeheu AM, Glowinski J, Tassin JP. Blockade of prefronto-cortical alpha 1-adrenergic receptors prevents locomotor hyperactivity induced by subcortical d-amphetamine injection. Eur J Neurosci. 1994;6:293–298. doi: 10.1111/j.1460-9568.1994.tb00272.x. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Artigas F. Control of 5-hydroxytryptamine release in the dorsal raphe nucleus by the noradrenergic system in rat brain. Role of alpha1-adrenoceptors. Neuropsychopharmacology. 2003;28:421–434. doi: 10.1038/sj.npp.1300061. [DOI] [PubMed] [Google Scholar]
- Brazell MP, Mitchell SN, Gray JA. Effect of acute administration of nicotine on in vivo release of noradrenaline in the hippocampus of freely moving rats: a dose-response and antagonist study. Neuropharmacology. 1991;30:823–833. doi: 10.1016/0028-3908(91)90116-s. [DOI] [PubMed] [Google Scholar]
- Breyer-Pfaff U, Wiatr G, Stevens I, Gaertner HJ, Mundle G, Mann K. Elevated norharman plasma levels in alcoholic patients and controls resulting from tobacco smoking. Life Sci. 1996;58:1425–1432. doi: 10.1016/0024-3205(96)00112-9. [DOI] [PubMed] [Google Scholar]
- Carey RJ, Depalma G, Damianopoulos E, Müller CP, Huston JP. The 5-HT1A receptor and behavioral stimulation in the rat: effects of 8-OHDPAT on spontaneous and cocaine-induced behavior. Psychopharmacology (Berl) 2004;177:46–54. doi: 10.1007/s00213-004-1917-4. [DOI] [PubMed] [Google Scholar]
- Clarke PB, Reuben M. Release of [3H]-noradrenaline from rat hippocampal synaptosomes by nicotine: mediation by different nicotinic receptor subtypes from striatal [3H]-dopamine release. Br J Pharmacol. 1996;117:595–606. doi: 10.1111/j.1476-5381.1996.tb15232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani JA, Heinemann S. Molecular and cellular aspects of nicotine abuse. Neuron. 1996;16:905–908. doi: 10.1016/s0896-6273(00)80112-9. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin JP. Importance of the noradrenaline-dopamine coupling in the locomotor activating effects of d-amphetamine. J Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. α1b-adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan TM, North RA. Actions of acetylcholine and nicotine on rat locus coeruleus neurons in vitro. Neuroscience. 1986;19:565–571. doi: 10.1016/0306-4522(86)90281-2. [DOI] [PubMed] [Google Scholar]
- el Mestikawy S, Riad M, Laporte AM, Vergé D, Daval G, Gozlan H, Hamon M. Production of specific anti-rat 5-HT1A receptor antibodies in rabbits injected with a synthetic peptide. Neurosci Lett. 1990;118:189–192. doi: 10.1016/0304-3940(90)90623-h. [DOI] [PubMed] [Google Scholar]
- Engberg G. Nicotine-induced excitation of locus coeruleus neurons is mediated via release of excitatory amino acids. Life Sci. 1989;44:1535–1540. doi: 10.1016/0024-3205(89)90446-3. [DOI] [PubMed] [Google Scholar]
- Engberg G, Erhardt S, Sharp T, Hajós M. Nicotine inhibits firing activity of dorsal raphe 5-HT neurones in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:41–45. doi: 10.1007/s002100000252. [DOI] [PubMed] [Google Scholar]
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- Gorea E, Adrien J. Serotonergic regulation of noradrenergic coerulean neurons: electrophysiological evidence for the involvement of 5-HT2 receptors. Eur J Pharmacol. 1988;154:285–291. doi: 10.1016/0014-2999(88)90203-8. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, Stinus L. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J Neurosci. 2005;25:8593–8600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Chu HM, Brennan TJ, Danao JA, Bajwa P, Parsons LH, Tecott LH. Elevated anxiety and antidepressant-like responses in serotonin 5-HT1A receptor mutant mice. Proc Natl Acad Sci U S A. 1998;95:15049–15054. doi: 10.1073/pnas.95.25.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain Res. 1999;818:204–211. doi: 10.1016/s0006-8993(98)01260-8. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Achat-Mendes CN, Ali SF, Anderson KL. Long-lasting behavioral sensitization to psychostimulants following p-chloroamphetamine-induced neurotoxicity in mice. Neuropharmacology. 2004;46:74–84. doi: 10.1016/s0028-3908(03)00316-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. Repeated cocaine administration alters extracellular glutamate in the ventral tegmental area. J Neurochem. 1998;70:1497–1502. doi: 10.1046/j.1471-4159.1998.70041497.x. [DOI] [PubMed] [Google Scholar]
- Kita T, Nakashima T, Shirase M, Asahina M, Kurogochi Y. Effects of nicotine on ambulatory activity in mice. Jpn J Pharmacol. 1988;46:141–146. doi: 10.1254/jjp.46.141. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Iversen SD. Selective 6OHDA-induced destruction of mesolimbic dopamine neurons: abolition of psychostimulant-induced locomotor activity in rats. Eur J Pharmacol. 1976;40:45–56. doi: 10.1016/0014-2999(76)90352-6. [DOI] [PubMed] [Google Scholar]
- Kelly PH, Seviour PW, Iversen SD. Amphetamine and apomorphine responses in the rat following 6-OHDA lesions of the nucleus accumbens septi and corpus striatum. Brain Res. 1975;94:507–522. doi: 10.1016/0006-8993(75)90233-4. [DOI] [PubMed] [Google Scholar]
- Ksir C, Hakan R, Hall DP, Jr, Kellar KJ. Exposure to nicotine enhances the behavioral stimulant effect of nicotine and increases binding of [3H]acetylcholine to nicotinic receptors. Neuropharmacology. 1985;24:527–531. doi: 10.1016/0028-3908(85)90058-9. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non dopaminergic mechanism. Neuropsychopharmacology. 2008;33:1724–1734. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- Legrand C, Maltier JP, Benghan-Eyene Y. Rat myometrial adrenergic receptors in late pregnancy. Biol Reprod. 1987;37:641–650. doi: 10.1095/biolreprod37.3.641. [DOI] [PubMed] [Google Scholar]
- Lewis A, Miller JH, Lea RA. Monoamine oxidase and tobacco dependence. Neurotoxicology. 2007;28:182–195. doi: 10.1016/j.neuro.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Li X, Rainnie DG, McCarley RW, Greene RW. Presynaptic nicotinic receptors facilitate monoaminergic transmission. J Neurosci. 1998;18:1904–1912. doi: 10.1523/JNEUROSCI.18-05-01904.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Burch JB, Collins AC. Genetics of nicotine response in four inbred strains of mice. J Pharmacol Exp Ther. 1983;226:291–302. [PubMed] [Google Scholar]
- Medioni J, Berlin I, Mallet A. Increased risk of relapse after stopping nicotine replacement therapies: a mathematical modelling approach. Addiction. 2005;100:247–254. doi: 10.1111/j.1360-0443.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- Mhaouty-Kodja S, Houdeau E, Cohen-Tannoudji J, Legrand C. Catecholamines are not linked to myometrial phospholipase C and uterine contraction in late pregnant and parturient mouse. J Physiol. 2001;536:123–131. doi: 10.1111/j.1469-7793.2001.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihailescu S, Palomero-Rivero M, Meade-Huerta P, Maza-Flores A, Drucker-Colín R. Effects of nicotine and mecamylamine on rat dorsal raphe neurons. Eur J Pharmacol. 1998;360:31–36. doi: 10.1016/s0014-2999(98)00658-x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Lejeune F, Newman-Tancredi A, Rivet JM, Auclair A, Peglion JL. S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J Pharmacol Exp Ther. 2001;298:565–580. [PubMed] [Google Scholar]
- Mitchell SN. Role of the locus coeruleus in the noradrenergic response to a systemic administration of nicotine. Neuropharmacology. 1993;32:937–949. doi: 10.1016/0028-3908(93)90058-b. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular mechanisms of drug addiction. J Neurosci. 1992;12:2439–2450. doi: 10.1523/JNEUROSCI.12-07-02439.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Infusion of nicotine in the ventral tegmental area or the nucleus accumbens of the rat differentially affects accumbal dopamine release. Pharmacol Toxicol. 1994;75:348–352. doi: 10.1111/j.1600-0773.1994.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Ed 2. New York: Academic; 1997. [Google Scholar]
- Pierce RC, Kalivas PW. Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci. 1997;17:3254–3261. doi: 10.1523/JNEUROSCI.17-09-03254.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. J Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poindexter E, Carpenter R. The isolation of harmane and norharmane from tobacco and cigarette smoke. Phytochemistry. 1962;1:215–221. [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Qian Y, Melikian HE, Rye DB, Levey AI, Blakely RD. Identification and characterization of antidepressant-sensitive serotonin transporter proteins using site-specific antibodies. J Neurosci. 1995;15:1261–1274. doi: 10.1523/JNEUROSCI.15-02-01261.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Watkins KC, Doucet E, Hamon M, Descarries L. Agonist-induced internalization of serotonin-1a receptors in the dorsal raphe nucleus (autoreceptors) but not hippocampus (heteroreceptors) J Neurosci. 2001;21:8378–8386. doi: 10.1523/JNEUROSCI.21-21-08378.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riad M, Zimmer L, Rbah L, Watkins KC, Hamon M, Descarries L. Acute treatment with the antidepressant fluoxetine internalizes 5-HT1A autoreceptors and reduces the in vivo binding of the PET radioligand [18F]MPPF in the nucleus raphe dorsalis of rat. J Neurosci. 2004;24:5420–5426. doi: 10.1523/JNEUROSCI.0950-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rommelspacher H, Meier-Henco M, Smolka M, Kloft C. The levels of norharman are high enough after smoking to affect monoamine oxidase B in platelets. Eur J Pharmacol. 2002;441:115–125. doi: 10.1016/s0014-2999(02)01452-8. [DOI] [PubMed] [Google Scholar]
- Salomon L, Lanteri C, Glowinski J, Tassin JP. Behavioral sensitization to amphetamine results from an uncoupling between noradrenergic and serotonergic neurons. Proc Natl Acad Sci U S A. 2006;103:7476–7481. doi: 10.1073/pnas.0600839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;2004 doi: 10.1002/14651858.CD000146.pub2. CD000146. [DOI] [PubMed] [Google Scholar]
- Smolen A, Marks MJ, DeFries JC, Henderson ND. Individual differences in sensitivity to nicotine in mice: response to six generations of selective breeding. Pharmacol Biochem Behav. 1994;49:531–540. doi: 10.1016/0091-3057(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Res. 2001;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- Tassin JP. Uncoupling between noradrenergic and serotonergic neurons as a molecular basis of stable changes in behavior induced by repeated drugs of abuse. Biochem Pharmacol. 2008;75:85–97. doi: 10.1016/j.bcp.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P, Blanc G, Glowinski J, Tassin JP. Nicotine and morphine differentially activate brain dopamine in prefrontocortical and subcortical terminal fields: effects of acute and repeated injections. J Pharmacol Exp Ther. 1992;261:484–490. [PubMed] [Google Scholar]
- Villégier AS, Blanc G, Glowinski J, Tassin JP. Transient behavioral sensitization to nicotine becomes long-lasting with monoamine oxidases inhibitors. Pharmacol Biochem Behav. 2003;76:267–274. doi: 10.1016/s0091-3057(03)00223-5. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP. Monoamine oxidases inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology. 2006a;31:1704–1713. doi: 10.1038/sj.npp.1300987. [DOI] [PubMed] [Google Scholar]
- Villégier AS, Salomon L, Blanc G, Godeheu G, Glowinski J, Tassin JP. Irreversible blockade of monoamine oxidases reveals the critical role of 5-HT transmission in locomotor response induced by nicotine in mice. Eur J Neurosci. 2006b;24:1359–1365. doi: 10.1111/j.1460-9568.2006.05011.x. [DOI] [PubMed] [Google Scholar]