Abstract
Amyloid β protein (Aβ), a pathogenic molecule associated with Alzheimer's disease, is produced by γ-secretase, which cleaves the β-carboxyl terminal fragment (βCTF) of β-amyloid precursor protein in the middle of its transmembrane domain. How the cleavage proceeds within the membrane has long been enigmatic. We hypothesized previously that βCTF is cleaved first at the membrane–cytoplasm boundary, producing two long Aβs, Aβ48 and Aβ49, which are processed further by releasing three residues at each step to produce Aβ42 and Aβ40, respectively. To test this hypothesis, we used liquid chromatography tandem mass spectrometry (LC-MS/MS) to quantify the specific tripeptides that are postulated to be released. Using CHAPSO (3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxyl-1-propanesulfonate)-reconstituted γ-secretase system, we confirmed that Aβ49 is converted to Aβ43/40 by successively releasing two or three tripeptides and that Aβ48 is converted to Aβ42/38 by successively releasing two tripeptides or these plus an additional tetrapeptide. Most unexpectedly, LC-MS/MS quantification revealed an induction period, 3–4 min, in the generation of peptides. When extrapolated, each time line for each tripeptide appears to intercept the same point on the x-axis. According to numerical simulation based on the successive reaction kinetics, the induction period exists. These results strongly suggest that Aβ is generated through the stepwise processing of βCTF by γ-secretase.
Introduction
There are three possible explanations of the intramembrane cleavage of β-carboxyl terminal fragment (βCTF) of amyloid precursor protein (APP) leading to the generation of Aβ40 and Aβ42: (1) ε-cleavage occurs first and is followed by γ-cleavage; (2) both cleavages occur at the same time; and (3) γ-cleavage occurs first and is followed by ε-cleavage. In the first case, we should find longer amyloid β protein (Aβ) species (longer than Aβ42) within the cell, whereas in the third case we should detect longer APP intracellular domain (AICD) (for example, AICD41-99 and AICD43-99, counterparts of Aβ40 and Aβ42, respectively). Despite extensive searching, we failed to find longer AICDs. In contrast, using a newly developed urea/SDS-PAGE method, we identified distinct species of longer Aβs, including Aβ48, Aβ46, Aβ45, and Aβ43 in the cell lysate (Qi-Takahara et al., 2005). We also found that treatment of cells with N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) causes intracellular accumulation of longer Aβ species together with depletion of Aβ40/42 in the culture medium. Intracellular depletion of Aβ40 accompanied the accumulation of Aβ43 at low dosage and of Aβ46 at higher dosage (Qi-Takahara et al., 2005). This accumulation profile suggests that the flow of product from Aβ46 to Aβ43 to Aβ40 is prevented downstream, which would cause differential accumulation of first Aβ43 and then Aβ46. This suggested to us that three residues along the α-helix of the transmembrane domain (TMD) of βCTF would be removed successively to generate Aβ40 (Qi-Takahara et al., 2005).
If the view that longer Aβs are processed to Aβ40/42 is correct, longer Aβ species (Aβ48–Aβ52) should generate Aβ40/42 within the cell. Using a transfection experiment, we noted an unusual rule: Aβ51 and Aβ48 preferentially generated Aβ42, whereas other longer Aβs, including Aβ49, Aβ50, and Aβ52, produced predominantly Aβ40 (Funamoto et al., 2004). Although the exact mechanism remains unknown, these results suggest that this three-residue rule is involved in the preference for the final product, Aβ40 or Aβ42. Conversely, DAPT causes accumulation of Aβ45 and Aβ48 in the CHO cells expressing mutant (mt) PS2 (N141I) and mtPS1 (M233T), respectively (Yagishita et al., 2006). Based on these results, it is reasonable to postulate that two product lines are at work: one produces Aβ40 and the other produces Aβ42. These observations led us to the view that this process mediated by γ-secretase proceeds through the stepwise (successive) cleavage of βCTF at every third residue. According to this model, Aβ40 is generated through Aβ43/46 from Aβ49, which is generated by ε-cleavage, and Aβ42 is generated through Aβ45 from Aβ48, which is generated by ε-cleavage (Qi-Takahara et al., 2005; Kakuda et al., 2006). The ε-cleaved product, Aβ49 on the γ-secretase complex, releases ITL, VIV, and IAT and is converted to Aβ40. The other ε-cleaved product, Aβ48, releases VIT and TVI and is converted to Aβ42 (tripeptide hypothesis). We tested this model by using liquid chromatography tandem mass spectrometry (LC-MS/MS) to quantify the specific tripeptides in the reaction mixture of a cell-free Aβ-generating system and a 3-[(3-cholamidopropyl)dimethylammonio]-2-hydroxyl-1-propanesulfonate (CHAPSO)-reconstituted Aβ-generating system.
Materials and Methods
Cell culture.
CHO cells inducibly expressing βCTF (C99 cells) (Qi-Takahara et al., 2005) were maintained in F-12 nutrient mixture (Invitrogen) containing 10% fetal calf serum (Invitrogen), penicillin/streptomycin, 250 μg/ml zeocin (Invitrogen), 10 μg/ml blasticidin S (Invitrogen), and 200 μg/ml G418 (Calbiochem). For βCTF expression, tetracycline (Invitrogen) was supplemented into the culture medium at a concentration of 1 μg/ml. A 4 h incubation with tetracycline was found to be suitable for the experiment; longer culture times increased the intensity of an αCTF-like band (data not shown).
Antibodies.
The monoclonal antibodies to Aβ used in this study were 82E1 (highly specific for Asp1 of human Aβ) (IBL Co. Ltd.), BA27 (raised against Aβ1-40, highly specific for Val40), BC05 (raised against Aβ35-43, specific for Aβ42), 12F4 (specific for Aβ42; Covance), and 9C4 (specific for Aβ43; Covance). A polyclonal antibody UT18 (raised against the 20 residue cytoplasmic domain of APP; a gift from Dr. T. Suzuki, Hokkaido University, Sapporo, Japan) was used to detect βCTF and AICD.
Membrane preparation.
Confluent cells were harvested and homogenized in homogenization buffer [20 mm piperazine-1,4-bis(2-ethanesulfonic acid) (PIPES), pH 7.0, 140 mm KCl, 0.25 m sucrose, and 5 mm EGTA]. Homogenized cells were centrifuged at 800 × g to remove nuclei and cell debris. The supernatant was further ultracentrifuged at 100,000 × g for 1 h. The resultant pellet containing the total membrane fraction was suspended in homogenization buffer. The membrane fraction at a protein concentration of 2.5 mg/ml in homogenization buffer containing protease inhibitor mixture [0.5 mm diisopropylfluorophosphate (DIFP), 1 μg/ml N α-p-tosyl-l-lysyl chloromethyl ketone (TLCK), 10 μg/ml antipain, 10 μg/ml leupeptin, 1 mm thiorphan, 5 mm phenanthroline, 100 μm bestatin, 10 μm amastatin, 0.1 μm arphamenine, and 5 mm EDTA] was incubated at 37°C for the time indicated, and the reaction was stopped by placing the reaction mixture on ice. After extraction of lipids twice with chloroform/methanol (2:1) and chloroform/methanol/water (1:2:0.8), the residue was extracted with 70% formic acid, and the extract was dried. The residue was dissolved in the SDS sample buffer and subjected to 16.5% SDS-PAGE, followed by Western blotting.
Preparation of C99–FLAG substrate.
A C-terminal fragment of APP (βCTF or C99) was fused at the C terminus with FLAG tag (C99–FLAG) and at the N terminus with signal peptide (MQLRNPELHLGCALALRFLALVSWDIPGARA) of human α-galactosidase A. The resultant coding fragment was inserted into pFASTBAC1 (Invitrogen). Sf9 cells were infected with recombinant baculovirus according to the instructions of the manufacturer (Kakuda et al., 2006). Infected cells (60 ml culture) were harvested after 36 h and resuspended in 0.3–0.5 ml of Tris-buffered saline (50 mm Tris HCl, pH 7.6, and 150 mm NaCl). The suspension was mixed with equal amount of 2× YI buffer [50 mm Tris HCl, pH 7.6, 150 mm NaCl, 2% NP-40, and 2× protease inhibitor cocktail (Roche Diagnostics)] and incubated on ice for 1 h. After ultracentrifugation at 245,000 × g for 20 min, the supernatant was agitated with 0.2 ml of anti-FLAG M2 agarose beads (Sigma) overnight. C99–FLAG was eluted from the beads by incubation with 0.2 ml of 100 mm glycine HCl, pH 2.7, for 10 min at room temperature, and the eluate was neutralized immediately by addition of volume of 1 m Tris HCl, pH 8.0. The concentration of residual NP-40 in the purified C99–FLAG was estimated to be 0.3–0.4%. The eluted C99–FLAG was confirmed for its purity and quantified by Coomassie Brilliant Blue staining after gel electrophoresis (Kakuda et al., 2006).
Immunoprecipitation of the γ-secretase complex.
The membrane fraction from CHO cells was adjusted to a protein concentration of 10 mg/ml with suspension buffer (50 mm PIPES, pH 7.0, 0.25 m sucrose, and 1 mm EGTA). Equal volume of 2× NK buffer (50 mm PIPES, pH 7.0, 0.25 m sucrose, 1 mm EGTA, 2% CHAPSO, 1 mm DIFP, 2 μg/ml TLCK, 20 μg/ml antipain, 20 μg/ml leupeptin, and 2 mm thiorphan) was added, and the fraction was kept on ice for 1 h. After centrifugation at 100,000 × g for 1 h, the supernatant was saved as 1% CHAPSO-solubilized membrane fraction and kept at −80°C until use. The solubilized fraction was diluted in three volumes of CHAPSO-free buffer (50 mm PIPES, pH 7.0, 0.25 m sucrose, 1 mm EGTA, 0.5 mm DIFP, 1 μg/ml TLCK, 10 μg/ml antipain, 10 μg/ml leupeptin, 1 mm thiorphan, 100 μm bestatin, 10 μm amastatin, 0.1 μm arphamenine, and 5 mm EDTA). Anti-nicastrin antibody (Sigma) and protein A-Sepharose (GE Healthcare) were added to the CHAPSO-solubilized supernatant, and the supernatant was stirred at 4°C overnight. The suspension was centrifuged, and the resultant pellet was washed sufficiently with 0.25% CHAPSO buffer (50 mm PIPES, pH 7.0, 0.25 m sucrose, 1 mm EGTA, and 0.25% CHAPSO). The γ-secretase complex bound to protein A-Sepharose was allowed to react with 250 nm C99–FLAG in 0.325% CHAPSO buffer containing 0.1% phosphatidylcholine and 1 mm thiorphan with gentle agitation at 37°C for the time indicated (Osawa et al., 2008).
For the time lag (induction period) experiment, particular caution was taken to maintain the temperature of the reaction mixture at 37°C. Approximately 225 μl of 0.325% CHAPSO buffer containing 0.1% phosphatidylcholine was kept at this temperature. The protein A-Sepharose-bound γ-secretase (∼20 μl) that had been kept on ice was mixed with the preincubated CHAPSO buffer and incubated at 37°C for 5 min. The reaction was started by adding the substrate, C99–FLAG (∼5 μl) that had been kept on ice, to the preincubated mixture (∼245 μl), and the reaction mixture was agitated by inversion in a chamber kept at 37°C.
A part of the reaction mixtures was treated with TCA at the times indicated, and the remaining part was subjected to quantitative Western blotting for Aβ species using Aβ end-specific antibodies. The reaction mixtures were also separated in a two-layer separating gel system (20% acrylamide layer of 5 cm length and 10% acrylamide layer containing 8 m urea of 10 cm length) to visualize smaller Aβ species, such as Aβ37, Aβ38, and Aβ39 (see Figs. 4 A, right; 7 B, middle and bottom). Alternatively, the mixtures were loaded on another two-layer separating gel (10% acrylamide upper layer of 4 cm length and 10% acrylamide lower layer containing 8 m urea of 15 cm length) together with SeeBlue prestained standard (Invitrogen). Just before the samples ran into the 8 m urea layer, the gel plate was disassembled, and the upper separating gel containing proteins of >7 kDa and the stacking gel were removed. The lower separating gel was reassembled and again connected to a power supply for additional separation of Aβ species. This allowed us to distinguish smaller Aβ species from the truncated C99–FLAG substrate with high resolution (see Fig. 4 A, right). The blots were developed by an enhanced chemiluminescence system (GE Healthcare), and the intensity of the signals was quantified using an LAS-4000 IR multicolor luminescent image analyzer (Fujifilm). The TCA-treated reaction mixture was homogenized and kept on ice for 15 min. The suspension was centrifuged at 204,000 × g, and the supernatant was filtrated through a nitrocellulose membrane filter with 0.22 μm pore size (Millipore Corporation). The filtrate was injected into a HP1100 (Hewlett-Packard) equipped with CAPCELPAK C18 (4.6 × 250 mm; Shiseido) for concentration. The column was washed with 5% B for 20 min, and the peptides were eluted as a mixture by using a steep 5–90% B gradient for 1 min [A: 0.09% trifluoroacetic acid (TFA); B: 0.075% TFA containing 80% acetonitrile].
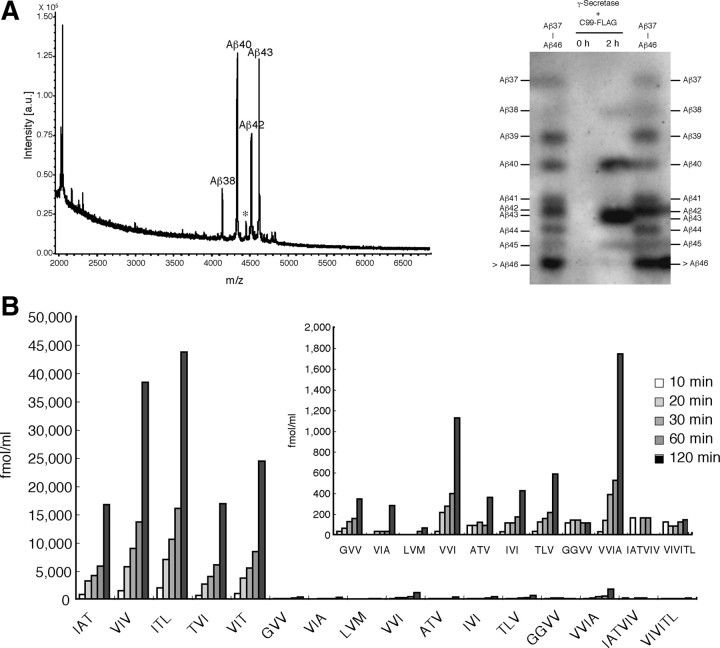
Figure 4.
The successive cleavage by the CHAPSO-reconstituted system produces finally Aβ40 and Aβ38 but not smaller Aβs. A, Left, The reaction mixture after 1 h incubation was immunoprecipitated with 82E1, and the precipitate was subjected to TOF mass spectrometry. Four distinct peaks were identified, and their m/z values corresponded to those of Aβ38, Aβ40, Aβ42, and Aβ43. A small peak indicated by * that is located between the Aβ40 and Aβ42 peaks may not be Aβ41 judging from the observed m/z. Right, 82E1 Western blotting after modified urea/SDS-PAGE, which prevented superimposition of βCTF on Aβs so that Aβs as small as Aβ37 were identified. The reaction mixture after 2 h incubation contained Aβ45 and longer species, Aβ43, Aβ42, Aβ40, and Aβ38, but not Aβ37 (lane 3), a finding that was consistent with the results of TOF mass spectrometry. Aβ42/43 were not well separated under the conditions (lanes 3, 4). Combined authentic Aβ37–Aβ49 were loaded in the leftmost and rightmost lanes. B, The reaction mixture was sampled at 10 min to 2 h and subjected to LC-MS/MS analysis. The levels of the five tripeptides increased markedly in a time-dependent manner. The levels of VVI and VVIA also increased significantly. The levels of other tripeptides, including GVV, VIA, ATV, IVI, and TLV, show very small increases (inset), and the levels of GGVV, IATVIV, and VIVITL did not change after incubation up to 2 h. The recoveries of the 12 tripeptides were the same as above, and those of the tetrapeptides and hexapeptides were assumed to be 52.8% for GGVV, 54.3% for VVIA, 46.7% for IATVIV, and 73.1% for VIVITL (see the legend to Fig. 3 and supplemental Table S2, available at www.jneurosci.org as supplemental material).
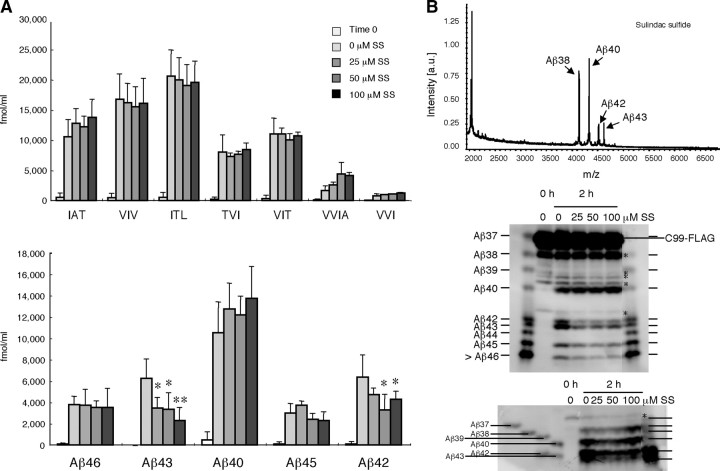
Figure 7.
The effects of sulindac sulfide on the stepwise processing. The reaction mixture containing 0, 25, 50, or 100 μm sulindac sulfide was incubated and sampled at 2 h for LC-MS/MS analysis. A, The levels of tripeptides and tetrapeptides release (top) and the calculated levels of Aβs according to the stepwise-processing model (bottom). Data are expressed as the means ± SD (n = 3). The levels of released tripeptides did not change significantly, whereas the level of VVIA tended to increase (although not significantly) with increasing concentrations of sulindac sulfide. Calculated levels of Aβ42 and Aβ43 were suppressed significantly by sulindac sulfide (*p < 0.05; **p < 0.01, ANOVA with Bonferroni's post hoc test compared with no treatment). In contrast, the levels of other Aβs, including Aβ40, Aβ45, and Aβ46, did not change significantly. The corrections for the peptides were the same as above (see the legends to Figs. 3, 4). B, Aliquots from the reaction mixture incubated with or without sulindac sulfide (100 μm) were immunoprecipitated with 82E1, and the precipitate was subjected to TOF mass spectrometry (top) and urea/SDS-PAGE [11% acrylamide gel (middle) and 20/10% discontinuous acrylamide gel (bottom)], followed by Western blotting with 82E1. TOF mass spectrometry shows that peak heights of Aβ42 and Aβ43 relative to that of Aβ40 decreased compared with those without sulindac sulfide treatment (see Fig. 4 A). The peak height of Aβ38 relative to that of Aβ40 increased substantially after the treatment (see Fig. 4 A). Western blotting (middle and bottom) showed that (1) Aβ40 stayed at a similar level, (2) Aβ42 and Aβ43 decreased markedly (middle), (3) Aβ38 increased (bottom), and (4) Aβ45 decreased somewhat (middle). Under the present conditions (middle), Aβs longer than Aβ45 were stuck at the buffer front. * indicates C-terminally truncated C99–FLAG in middle and bottom panels. Overall, the data on the effects of sulindac sulfide based on LC-MS/MS quantification of tripeptides and tetrapeptides corresponded well with those from TOF mass spectrometry and those from quantitative Aβ Western blotting.
LC-MS/MS identification and quantification of tripeptides and other oligopeptides.
Quattro Premier XE tandem quadrupole mass spectrometer accompanied by ultra performance liquid chromatography (Waters) was used to identify and quantify the tripeptides and other oligopeptides. To quantify each analyte, a combination of precursor ion product ion pair was monitored by multiple-reaction monitoring mode.
The conditions for quantification were established first for the five tripeptides ITL, VIV, IAT, VIT, and TVI, which were assumed to be liberated during incubation. Precursor and product ions to identify and quantify each tripeptide were assigned, and product ions were selected according to the signal intensity: mass-to-charge ratio (m/z) of 303.7 and 184.8 for IAT; 329.8 and 184.9 for VIV; 345.8 and 214.9 for ITL; 331.8 and 172.9 for TVI; and 331.8 and 185.0 for VIT. For Phe Leu Phe (FLF) (internal standard), the corresponding values were 425.7 and 119.1. When subjected to the column (AtlantisT3C18, 2.0 × 150 mm), all of these peptides were eluted with a 20 min linear gradient of 0–30% acetonitrile. A standard curve for each tripeptide was found to be linear from 0.4 to 100 fmol. R 2 = 0.997 for IAT, 0.996 for VIV, 0.999 for ITL, 0.998 for TVI, and 0.994 for VIT (supplemental Table S1, available at www.jneurosci.org as supplemental material).
The reaction mixture contained high concentrations of protease inhibitors (0.5 mm DIFP, 1 μg/ml TLCK, 10 μg/ml antipain, 10 μg/ml leupeptin, 1 mm thiorphan, 100 μm bestatin, 10 μm amastatin, 0.1 μm arphamenine, and 5 mm EDTA), which may affect the identification and quantification of tripeptides even by LC-MS/MS. Whereas significant proportions of each tripeptide injected alone into HPLC can be recovered in the presence of the above inhibitors, the yield decreased markedly. The reason appeared to be unusual enhancement (as much as fivefold) of the internal standard (FLF), and thus to construct the standard curve for each peptide, protease inhibitors and FLF were always added. It is impossible to assess accurate recoveries from the cell-free system because of the presence of the membrane, which brings the substrate. The measures obtained for the tripeptides released shown in Figure 2, B and C, do not account for the loss during incubation and are probably underestimations.
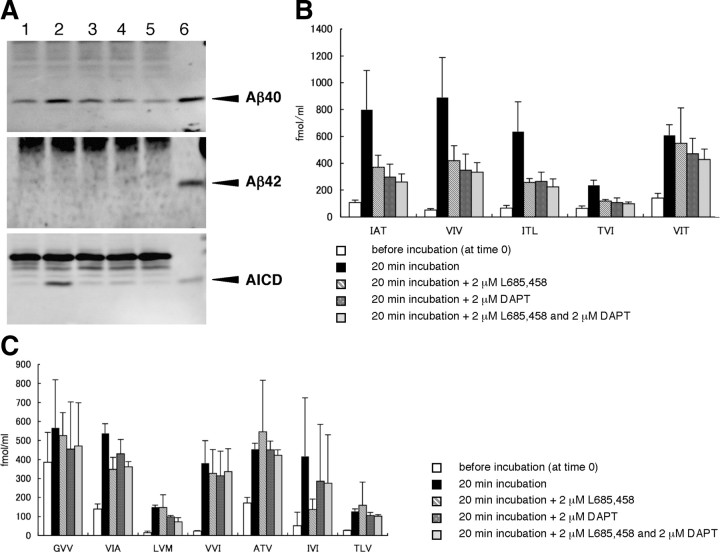
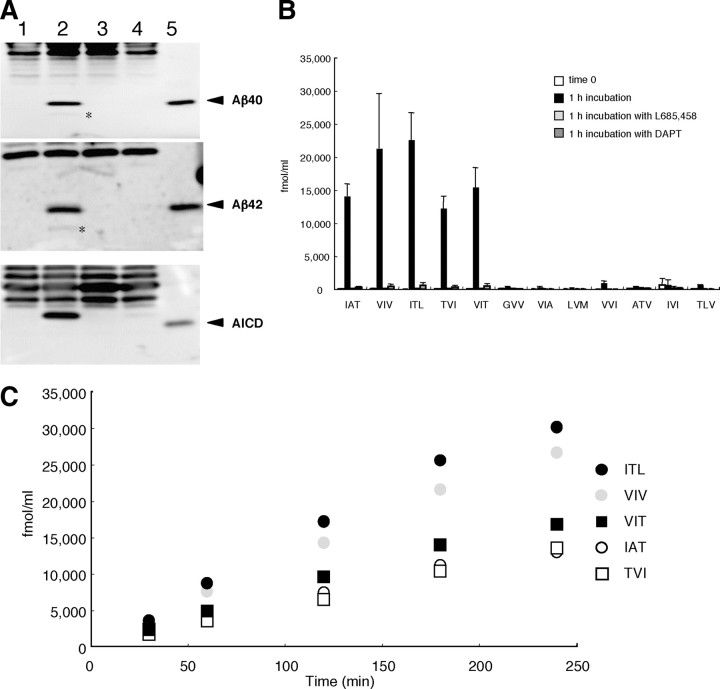
Figure 2.
The tripeptides quantified by LC-MS/MS in the reaction mixture of the cell-free Aβ-generating system. A, The cell-free reaction mixtures before and after incubation were delipidated (see Materials and Methods) and subjected to Western blotting using BA27 (specific for Aβ40), BC05 (specific for Aβ42), and UT18 (to detect AICD) (arrowheads). Small amounts of AICD and especially Aβ40 are associated with the membranes even at time 0 (lane 1), although incubation increased their levels markedly (lane 2). In contrast, in the preparation, Aβ42 was barely detectable at time 0 and even after incubation. There were no additional increases in Aβ40 and AICD in lanes 3 (in the presence of 2 μm L685,458), 4 (in the presence of 2 μm DAPT), and 5 (in the presence of both). Authentic Aβ40, Aβ42, and AICD50-99 (6.3 fmol for each) were loaded in lane 6. B, The five candidate tripeptides predicted to be released were quantified by LC-MS/MS in the cell-free system. Note the following: (1) the significant background noises at time 0; (2) remarkable increases by 20 min incubation in the amounts of tripeptides released; and (3) >50% suppression by γ-secretase inhibitors (2 μm L685,458 and 2 μm DAPT) of IAT, VIV, ITL, and TVI levels. VIT release appeared not to be suppressed significantly by the inhibitors. Data are expressed as the means ± SD (n = 3). Corrections for recoveries were not done (see Materials and Methods). C, Another seven tripeptides aligned on the TMD (from position 39 to position 50 according to Aβ numbering) of βCTF were quantified similarly by LC-MS/MS in the reaction mixture of the cell-free system. Note the following: (1) certain tripeptides gave higher signals at time 0; (2) large increases in their levels by incubation; and (3) no apparent effects on their release by γ-secretase inhibitors. Altogether, the results from the cell-free system may suggest that the tripeptide hypothesis is correct, but, to understand better the cleavage mechanism, the assay system should not be contaminated by proteases. Error bars indicate SD (n = 3). Corrections for recoveries were not done (see Materials and Methods).
In the experiments using the cell-free system, for unknown reasons, subtle differences in the elution position in LC were observed when compared with the authentic (synthetic) tripeptide. In addition, for unknown reasons, a few to several peaks for one product ion were observed, although the signal should have been double that selected (supplemental Fig. S1, available at www.jneurosci.org as supplemental material). To determine which was the peak of interest, we investigated (1) which peak intensity increased when each synthetic (authentic) peptide was added to the reaction mixture and (2) what the ratio of the intensities of the two selected product ions one precursor ion showed. Based on these two criteria, the peak of interest was identified and quantified (supplemental Fig. S1, available at www.jneurosci.org as supplemental material).
For the peptides examined here, the following precursor and product m/z values were used for identification and quantification: 274.0 and 129.0 for GVV; 330.0 and 199.0 for VVI; 302.1 and 213.1 for VIA; 290.0 and 173.0 for ATV; 344.1 and 213.1 for IVI; 332.0 and 187.1 for TLV; and 362.0 and 213.0 for LVM. The following m/z values were also selected: 331.1 and 213.9 for GGVV; 401.2 and 170.8 for VVIA; 615.2 and 185.0 for IATVIV; and 657.3 and 184.9 for VIVITL. All the synthetic peptides examined here were ready to be soluble in water and 10% TCA.
In the experiment using the CHAPSO-reconstituted system, there were almost no extra peaks in the total ion chromatogram that had unknown origins, as seen in the cell-free system, and identifying the peak was straightforward (supplemental Fig. S2, available at www.jneurosci.org as supplemental material). It is likely that the membrane fraction is a very crude source of γ-secretase and contains many proteases and membrane-associated proteins and their degraded products.
Recoveries.
For quantitative comparison between Western blotting and LC-MS/MS, recoveries of the tripeptides, tetrapeptides, and hexapeptides and Aβ40, Aβ42, and Aβ43 were measured. Eppendorf-type tubes (1.5 ml, non-stick, RNase-free microfuge tube; Ambion) were used throughout the experiment. Four hundred fifty, 2250, and 4500 fmol of each tripeptide, tetrapeptide, and hexapeptide were added to the tube containing the same reaction mixture without the substrate (250 μl), which were kept at 37°C for 0 and 1 h. The incubation was terminated by adding TCA to the mixture (the final concentration, 10%). Resultant TCA supernatants were injected to HPLC (HP1100; Hewlett-Packard) for concentration, and the eluate was dried in a speed vac centrifuge. The solubilized peptides were subjected to LC-MS/MS (Waters). Each fractionated peptide was quantified using each authentic peptide as a standard. Recoveries from non-incubated tubes were similar in the yields with 51.1–58.9% for all the tripeptides, tetrapeptides, and hexapeptides except VIVITL, which had unusually high recoveries (81.5%) (supplemental Table S2, available at www.jneurosci.org as supplemental material). Those from incubated tubes were also similar in the yields providing 46.7–54.3% for all the peptides except VIVITL, which was recovered by 73.1% (supplemental Table S2, available at www.jneurosci.org as supplemental material). After 1 h incubation, recoveries of the peptides invariably decreased, and there was no significant difference among the recoveries from 450, 2250, and 4500 fmol peptide/250 μl. These decreases may be attributable to nonspecific binding to the tube and/or hydrolytic degradation of the peptides.
Appropriate amounts of Aβ40, Aβ42, and Aβ43 that were solubilized in DMSO and kept at −80°C were added to the tubes containing the same reaction mixture without the substrate. The tubes containing final concentrations, 4, 8, or 16 pmol/ml for each Aβ, were kept at 37°C for 0 and 1 h. Aliquots were taken from the incubated mixtures and subjected to quantitative Western blotting using specific monoclonal antibodies. Incubation did not affect the yields of the three Aβs, but the yields differed a little from each another: the yield of Aβ40 was the highest at 85.6%, that of Aβ43 was 84.6%, and that of Aβ42 was 80.7% (supplemental Table S2, available at www.jneurosci.org as supplemental material).
Quantitative Western blotting.
Aliquots from the reaction mixture were dissolved in the SDS sample buffer and subjected to 16.5% SDS-PAGE, followed by Western blotting using the end-specific Aβ antibodies BA27, 12F4 (Covance), 9C4 (Covance), and UT18. Aβ40, Aβ42, Aβ43, and AICD on the blot were quantified by an LAS-4000 IR multi color luminescent image analyzer (Fujifilm) with defined amounts of each authentic Aβ (Peptide Institute) and AICD-50-99-FLAG (Kakuda et al., 2006) as the standards. The protein amounts of the authentic standards were determined by amino acid analysis (Peptide Institute).
Other methods and reagents.
Tripeptides and other oligopeptides were custom made by the Peptide Institute. Their quantities were determined by amino acid analysis (Peptide Institute).
The γ-secretase inhibitors L685,458 [1S-benzyl-4R-(1S-carbamoyl-2-phenylethylcarbamoyl-1S-3-methylbutylcarbamoyl)-2R-hydroxy-5-phenylpentyl] carbamic acid tert-butyl ester] and DAPT were purchased from Calbiochem. Both were solubilized with DMSO and kept −80°C until use. Protein concentrations were determined using the bicinchoninic acid protein assay (Pierce Chemical).
Numerical simulation.
Simulations were performed by numerical integration using fourth-order Runge–Kutta method. The reactions simulated were those shown in supplemental Appendix (Eqs. A1–A5) (available at www.jneurosci.org as supplemental material), which are described in the differential Equations B1–B16 in supplemental Appendix (available at www.jneurosci.org as supplemental material), in which we assume that only [E] and [C99] have the non-zero initial concentrations.
The parameters were determined using genetic algorithm (GA) to fit the time lines of tripeptide generated, by minimizing the sum of squared differences between all data points in Figure 8 A and the numerical calculations. The population size for GA is typically 100, and each individual has a virtual chromosome encoding 16 floating-point real values representing all of the undetermined model system parameters shown in supplemental Table 4S (available at www.jneurosci.org as supplemental material). The number of typical iteration steps is 20,000, and point mutations and crossover mutations of the virtual chromosomes are allowed to occur during optimization. A set of parameters is provided in supplemental Table 4S (available at www.jneurosci.org as supplemental material).
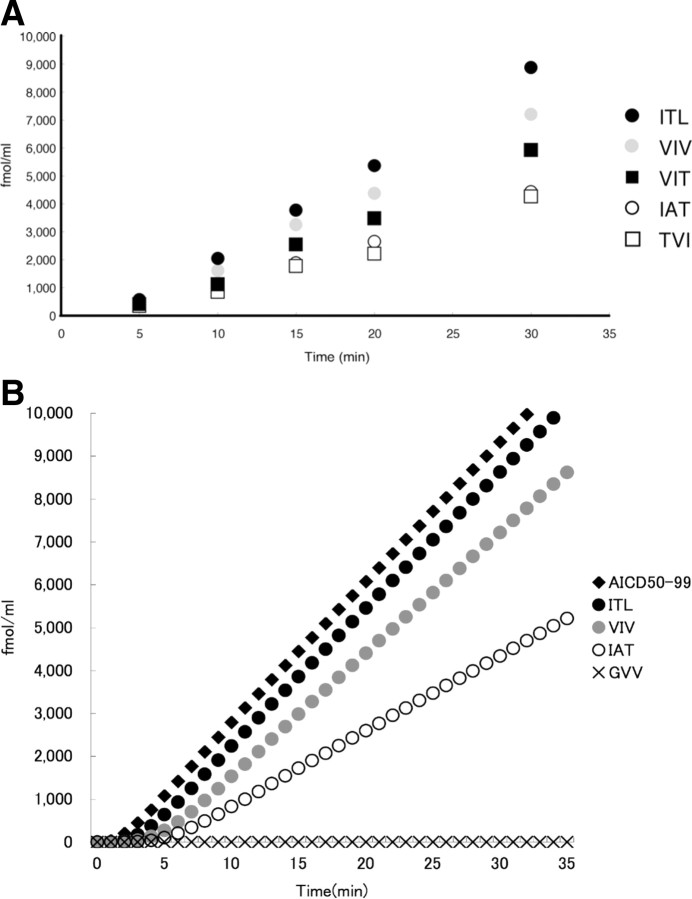
Figure 8.
A, The very early phase of the tripeptide-release reaction. The reaction mixture was sampled at 5–30 min and subjected to LC-MS/MS analysis for tripeptide quantification. Approximately 245 μl of 0.25% CHAPSO buffer containing 0.1% phosphatidylcholine was preincubated at 37°C. The CHAPSO buffer that was kept at 37°C was added to the γ-secretase-bound protein A-Sepharose (∼20 μl) in an Eppendorf tube that had been kept on ice, and the mixture was incubated at 37°C for 5 min. The reaction was started by addition of the substrate, C99–FLAG (∼5 μl) that had been kept on ice, and the reaction mixture continued with agitation in a chamber kept at 37°C. Extrapolation of each time line for each tripeptide appears to intercept the same point on the x-axis, indicating that the reactions start after a 3–4 min induction period. The corrections for the peptides were the same as above (see the legends to Figs. 3, 4). Note that the relationships ITL > VIV > IAT and VIT > TVI were maintained up to 10 min. Black circles, ITL; gray circles, VIV; white circles, IAT; black squares, VIT; white squares, TVI. B, Tripeptide-release kinetics simulated based on the stepwise-processing model. A set of parameters were optimized using genetic algorithm to fit the experimental data in Figure 8 A (supplemental Table S4, available at www.jneurosci.org as supplemental material). The simulation shows that there are induction periods for the generation of AICD50-99 as well as for that of tripeptides. After induction periods, 3–4 min for tripeptides and ∼2 min for AICD49-99, the reactions proceeded in a linear manner up to 6 h (data not shown).
Results
Distinct tripeptides appear to be released from membrane preparations, and their release is suppressed by specific γ-secretase inhibitors
We sought to identify by LC-MS/MS the particular tripeptides postulated to be released from the TMD of βCTF during Aβ generation (Qi-Takahara et al., 2005) (Fig. 1 A). The cell-free Aβ-generating system, in which the membrane fraction was prepared from CHO cells expressing βCTF (C99), was examined at first because we consider it to be similar to the living cell conditions. The tripeptide hypothesis claims (1) that intramembrane cleavage proceeds from ε-cleavage to γ-cleavage by releasing tripeptides along the α-helix of the TMD of βCTF (one helical turn is equivalent to 3.6 residues), and (2) that two product lines result: the first starts from Aβ49 (Leu49) through Aβ46 and Aβ43 to Aβ40 and the second starts from Aβ48 (Thr48) through Aβ45 to Aβ42 (Fig. 1 A). According to this hypothesis, the ε-cleavage primarily determines the preference for the final γ-cleavage products. Under normal conditions, ε-cleavage occurs mostly at the carboxyl side of Leu49 and would occur occasionally at the carboxyl side of Thr48, thus resulting in predominantly Aβ40 with a smaller proportion (10–20%) of Aβ42 in the cell-free Aβ-generating system (Sato et al., 2003).
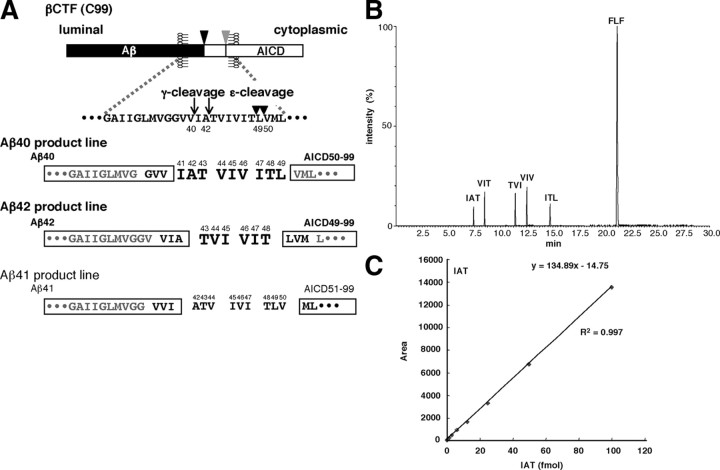
Figure 1.
Illustration of γ-secretase-mediated successive tripeptide release from βCTF (the tripeptide hypothesis). A, ε-Cleavage at the membrane–cytoplasmic boundary of βCTF (C99) generates Aβ49 and Aβ48 and their counterparts AICD50-99 and AICD49-99 (boxed), respectively. γ-Secretase then successively cleaves in a direction from the ε-cleavage (arrowheads) to γ-cleavage (arrows) sites Aβ49 and Aβ48 by releasing tripeptides at every α-helical turn, finally producing Aβ40 and Aβ42, respectively. Thus, the processing of Aβ49 accompanies the release of ITL, VIV, and IAT in this order, whereas that of Aβ48 accompanies the release of VIT, followed by TVI. Because only two species of AICD50-99 and AICD49-99 have been detected (Sato et al., 2003), the Aβ40 product line starting from Aβ49 and the Aβ42 product line starting from Aβ48 should be predominant. The Aβ41 product line beginning from Aβ50 should be negligible. B, Chromatogram of the five tripeptides (indicated by larger bold letters in A) that are predicted from the tripeptide hypothesis. The authentic tripeptides (12.5 fmol for each) and FLF (100 fmol), an internal control, were subjected to LC-MS/MS (Waters). Each of the five tripeptides and FLF eluted at a distinct position. C, Various concentrations of IAT together with all protease inhibitors that are added to the reaction mixture and FLF were subjected to LC-MS/MS analysis (see Materials and Methods). The resulting peak areas of the product ion were measured and plotted against the IAT concentration, which showed excellent linearity between 0.4 and 100 fmol of IAT (for other peptides, see supplemental Table S1, available at www.jneurosci.org as supplemental material).
Before the LC-MS/MS quantification of the tripeptides, several conditions were set. The mass chromatogram of the five candidate tripeptides and FLF, an internal standard, is shown in Figure 1 B. Each 12.5 fmol of synthetic IAT, VIV, ITL, TVI, and VIT and 100 fmol of FLF were injected. Each peptide eluted at a distinct time in the LC-MS/MS [ultra performance liquid chromatography accompanied by Quattro Premier XE (Waters)]. A linear standard curve was obtained between 0.4 and 100 fmol for each tripeptide examined (Fig. 1 C) (supplemental Table S1, available at www.jneurosci.org as supplemental material). The enzymatic reaction was terminated with TCA (final concentration, 10%), and the acid supernatant was subjected to HPLC (HP1100; Hewlett-Packard) for concentration. The concentrates were subjected to LC-MS/MS, and each peptide was quantified using synthetic tripeptides as standards.
When the membrane preparation is incubated at 37°C, Aβ and AICD are generated and their production is blocked by specific γ-secretase inhibitors (Sato et al., 2003) (Fig. 2 A). The production rates for both molecules were similar (Fig. 2 A) and constant up to 10 min and then declined (Sato et al., 2003). As shown in Figure 2 B, the amounts of the five tripeptides released increased markedly after 20 min incubation. To confirm that these increases were mediated by γ-secretase, two inhibitors, L685,458 (transition-state analog) or DAPT (non-transition-state analog), were added. Either inhibitor suppressed the production of IAT, VIV, ITL, and TVI >50% but little that of VIT (Fig. 2 B). Thus, the cell-free Aβ-generating system appeared to generate the candidate tripeptides (Fig. 2 B) as well as Aβ and AICD. To examine whether only these five tripeptides are released in the cell-free system, another seven randomly selected tripeptides aligned on the TMD sequence of βCTF (GVV, VIA, LVM, VVI, ATV, IVI, and TLV) (Fig. 1 A) were quantified similarly. Among these, ATV, IVI, and TLV together represent a possible Aβ41-producing process (Fig. 1 A). Although the production of these seven tripeptides increased substantially after incubation, the amount produced was less than that of the five postulated tripeptides and was not suppressed significantly by L685,458 or DAPT (Fig. 2 C). This suggests that these seven tripeptides are produced primarily by other protease(s), probably those associated with the membrane, and not by γ-secretase.
The CHAPSO-reconstituted γ-secretase system generates exclusively the candidate tripeptides, and their release is suppressed completely by specific γ-secretase inhibitors
The above results from the cell-free system suggest that the generation of the five candidate tripeptides is mediated by γ-secretase, an observation that may support the tripeptide hypothesis. However, significant ambiguity exists, possibly because of contamination of the γ-secretase system by active proteases. We were surprised by the findings that large amounts of tripeptides were generated, probably from membrane-associated proteins other than βCTF, and that this was insensitive to γ-secretase inhibitors. Thus, we chose the CHAPSO-reconstituted γ-secretase system with minor modification developed previously in our laboratory (Kakuda et al., 2006; Osawa et al., 2008). This comprises immunoprecipitated CHAPSO-solubilized γ-secretase and βCTF (C99–FLAG) purified from infected Sf9 cells. Incubating the CHAPSO-containing reaction mixture increased the levels of Aβ49, Aβ48, Aβ45, Aβ43, Aβ42, and Aβ40 linearly up to ∼2 h, as judged by Aβ Western blotting (Kakuda et al., 2006) (Fig. 3 A). Probably because of the lipidated substrate, the specific activity of γ-secretase in the reconstituted system was 20 times higher than that reported (Kakuda et al., 2006). The CHAPSO-reconstituted system is characterized by production of abundant Aβ40, Aβ42, and Aβ43 and small or trace amounts of Aβ45, Aβ48, and Aβ49 (Kakuda et al., 2006). In our hands, Aβ46 was undetectable by Western blotting (Kakuda et al., 2006). As expected, L685,458 uniformly suppressed the production of all Aβ species. Unexpectedly, DAPT did not cause buildups of Aβ43 and Aβ46 but uniformly suppressed the production of all Aβ species, as did L685,458 (Kakuda et al., 2006).
Figure 3.
LC-MS/MS quantification of the tripeptides released in the CHAPSO-reconstituted γ-secretase system. A, The reaction mixture was sampled at time 0 (leftmost lane) and 1 h (the next three lanes) and subjected to SDS-PAGE followed by Western blotting using BA27 (top), BC05 (middle), and UT18 (bottom) (arrowheads). Lane 1, Before incubation (at time 0); lane 2, 1 h incubation; lane 3, 1 h incubation in the presence of 2 μm L685,458; lane 4, 1 h incubation in the presence of 2 μm DAPT; lane 5, authentic Aβ40 (top), Aβ42 (middle), and AICD50-99 (bottom) with 18.8 fmol for each. Barely discernible bands (*) below the Aβ40 or Aβ42 band presumably represent p3 (Aβ17-40 and Aβ17-42; top and middle). AICD in lane 2 (bottom) migrates slower than AICD in lane 5 because of the added FLAG sequence in the former. B, LC-MS/MS quantification of the 12 tripeptides released in the CHAPSO-reconstituted γ-secretase system by 1 h incubation. Note the following: (1) the levels of released tripeptides in the reconstituted system were approximately fivefold higher than in the cell-free system; (2) background noises were very low at time 0 (column 1 for each tripeptide); (3) release of all of the predicted tripeptides was increased markedly by incubation (column 2); (4) release of the tripeptides was blocked completely by 1 μm L685,458 (column 3) or 1 μm DAPT (column 4); and (5) compared with these five tripeptides, the levels of the other seven tripeptides were almost negligible ( to the values of the 5 tripeptides). Data are expressed as the means ± SD (n = 3). The recoveries of the tripeptides were assumed to be 49.4% for IAT, 50.4% for VIV, 47.8% for IAT, 48.3% for TVI, 48.4% for VIT, 48.2% for GVV, 48.2% for VIA, 46.8% for LVM, 49.2% for VVI, 50.2% for ATV, 53.2% for IVI, and 48.7% for TLV (see Materials and Methods) (supplemental Table S2, available at www.jneurosci.org as supplemental material). C, Aliquots were taken from the reaction mixture at the times indicated and subjected to LC-MS/MS analysis. The release reactions proceeded linearly up to ∼1 h and then declined gradually. The relationships ITL > VIV > IAT and VIT > TVI were maintained throughout. Corrections for the recoveries were the same as above. Black circles, ITL; gray circles, VIV; white circles, IAT; black squares, VIT; white squares, TVI.
γ-Secretase complex immunoprecipitated by anti-nicastrin antibody from the CHAPSO-solubilized membrane fraction was allowed to react with 250 nm C99–FLAG in 0.325% CHAPSO buffer containing 0.1% phosphatidylcholine at 37°C (Osawa et al., 2008). The amounts of the five predicted peptides increased greatly during the incubation, but, in sharp contrast, those of the other seven tripeptides no longer increased to such extents in this system (Fig. 3 B). The production of the predicted five tripeptides was suppressed almost completely by 1 μm L685,458 or 1 μm DAPT (Fig. 3 B). This strongly suggests that the five predicted tripeptides are produced specifically by γ-secretase. These results substantiate the tripeptide hypothesis that particular tripeptides are released exclusively and further support our view that Aβ generation involves two product lines.
Time course followed by LC-MS/MS indicates that the processing proceeds in a direction from ε- to γ-cleavage
Because the profile of tripeptides released was consistent with the tripeptide hypothesis (Qi-Takahara et al., 2005), the release kinetics in the CHAPSO-solubilized system was followed by LC-MS/MS. As shown in Figure 3 C, the levels of all five tripeptides elevated linearly up to ∼1 h and then declined gradually. This suggests that other proteases did not contaminate the reaction mixture to a significant level. This pattern corresponded well with the time course of Aβ produced shown by Western blotting (Kakuda et al., 2006). Importantly, the levels of tripeptides were invariably ITL > VIV > IAT and VIT > TVI from 30 min to ∼4 h. These relationships were maintained strictly up to as early as 10 min (see below).
The tripeptide hypothesis claims that particular tripeptides are released successively to produce Aβ49, Aβ46, Aβ43, and Aβ40, and Aβ48, Aβ45, and Aβ42 in this order (Qi-Takahara et al., 2005). A critical requirement for this model is that the amounts of tripeptides released earlier are definitely larger than the amounts released later. Thus, for the tripeptide hypothesis to be valid, the relationship ITL > VIV > IAT and VIT > TVI should be observed at any time during the reaction. Notably, this relationship itself indicates strongly that the direction of reaction is from ε- to γ-cleavage.
The final products of the CHAPSO-reconstituted γ-secretase system are Aβ40 and Aβ38, the latter of which accompanies the release of tetrapeptide
In the cell culture system, smaller Aβs, including Aβ39, Aβ38, Aβ37, Aβ35, and Aβ34, are found in the medium, and their production seems to be mediated by γ-secretase because their production is suppressed with a specific γ-secretase inhibitor (Beher et al., 2002). However, one should be cautious about the interpretation because the data do not exclude the possibility that the culture medium contains carboxyl peptidases with robust activity toward Aβs of conventional lengths (for example, Aβ38, Aβ40, or Aβ42) and thus generates smaller Aβs (smaller than Aβ38). We examined to what extent the CHAPSO-reconstituted system processes βCTF and whether the smaller Aβs as observed often in the culture media are produced. After incubation, the reaction mixture was immunoprecipitated with 82E1 (specific for the N terminus of human Aβ), and the precipitate was subjected to time of flight (TOF) mass spectrometry. Four distinct peaks were observed, and their observed m/z values corresponded to those of Aβ38, Aβ40, Aβ42, and Aβ43 (Fig. 4 A, left). Longer Aβs, including Aβ45, Aβ48, and Aβ49 that exist in the reaction mixture (Kakuda et al., 2006), were undetectable by TOF mass spectrometry, presumably because they existed in only small amounts in the mixture and because their ionization efficiencies are very low. Notably, Aβs smaller than Aβ38 were undetectable by TOF mass spectrometry. In this case, ionization efficiencies should not be low, and thus it is more likely that Aβs smaller than Aβ38 would be absent or at negligible levels in the reaction mixture. To confirm this result, we used a modified urea/SDS-PAGE protocol that makes it possible to identify Aβs as small as Aβ37. We detected Aβ38, Aβ40, Aβ42, and Aβ43 (and longer Aβ species) but not Aβ37 or Aβ39 in the reaction mixture (Fig. 4 A, right), an observation that is consistent with the TOF mass spectrometry result. We conclude that the CHAPSO-reconstituted system proceeds to the production of Aβ40 and Aβ38 but no further.
This raised an important question: is Aβ38 produced from Aβ42 by releasing tetrapeptide, VVIA, instead of a tripeptide (Qi-Takahara et al., 2005)? If so, this would contradict the tripeptide hypothesis. We examined again in depth whether the reaction mixture contains the peptides including GVV, VVI, VIA, and VVIA, which would explain the generation of small Aβs. Removing GVV from Aβ40 should generate Aβ37, removing VVI from Aβ41 should generate Aβ38, and removing VIA from Aβ42 should generate Aβ39. Among these five peptides, only VVIA was detected consistently in the reaction mixture, although its level was relatively low (Fig. 4 B). Thus, the presence of VVIA indicates conversion of Aβ42 to Aβ38 by releasing tetrapeptide, which contradicts the tripeptide rule. Tetrapeptide release is apparently inherent in the Aβ42 processing because the amount of GGVV that may have been released to produce Aβ36 from Aβ40 was unaltered (Fig. 4 B, inset). Occasionally, VVI was released to a significant level, indicating a very low level of Aβ41 generated in the reaction mixture. This means that Aβ38 generation also requires a very small contribution from Aβ41. The failure to detect incubation-dependent changes in the levels of two hexapeptides, IATVIV and VIVITL, is noteworthy. This indicates clearly that cleavage of hexapeptide by skipping one helical turn, which still follows the α-helical model, never occurred (Fig. 5) (Yagishita et al., 2008).
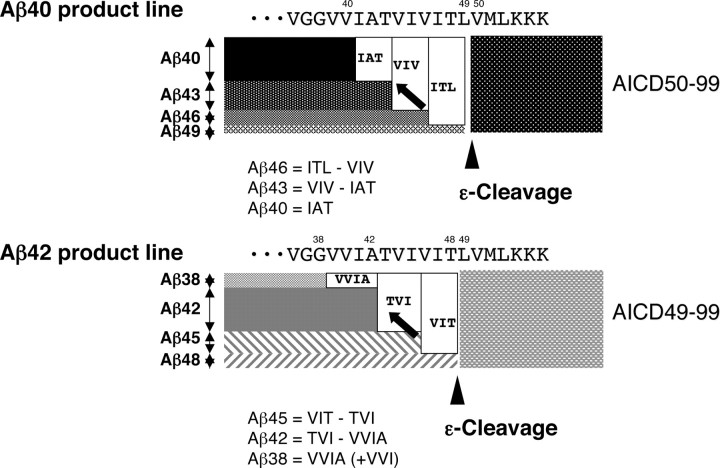
Figure 5.
Illustration of the stepwise processing of βCTF. Aβ40 product line: Aβ49 generated by ε-cleavage (arrowhead) is processed successively probably on the γ-secretase complex. Aβ49 is converted to Aβ46 by releasing ITL, and Aβ46 is converted to Aβ43 by releasing VIV. Finally, Aβ43 is converted to Aβ40 by releasing IAT (arrow). The characteristic of this model is that the difference in the amounts of tripeptide released determines the amount of Aβ produced. Accordingly, Aβ46 = ITL − VIV, Aβ43 = VIV − IAT, and Aβ40 = IAT. Aβ42 product line: Aβ48 generated by ε-cleavage (arrowhead) is converted to Aβ45, and Aβ45 in turn is converted to Aβ42. Aβ42 is converted finally to Aβ38 (arrow). Similarly, Aβ45 = VIT − TVI, Aβ42 = TVI − VVIA, and Aβ38 = VVIA (see Results). The final step in the Aβ42 product line is removal of VVIA, instead of VIA.
Closer inspection of Figure 4 B shows that very minute amounts ( to of the candidate tripeptides) of GVV, VIA, ATV, IVI, and TLV were released and that the amount increased in a time-dependent manner, which was detectable by LC-MS/MS (Fig. 4 B, inset). Among the above peptides, ATV, IVI, and TLV were involved in the Aβ41-producing process (Fig. 1 A). It is likely that, although very minor, Aβ41 product line starting from ε-cleavage at the carboxyl side of Val-50 also appeared in the CHAPSO-reconstituted system.
Comparison between Aβ levels determined by Western blotting and those assessed by released tripeptide and tetrapeptide levels according to the stepwise-processing model
According to the stepwise-processing model that was developed from the tripeptide hypothesis (Fig. 5), Aβ46 = ITL − VIV; Aβ43 = VIV − IAT; and Aβ40 = IAT (when additional cleavage does not occur); and Aβ45 = VIT − TVI; Aβ42 = TVI − VVIA; and Aβ38 = VVIA (+ VVI) (when additional cleavage does not occur). The Aβ quantities inferred from LC-MS/MS according to the model were found to be in parallel with those by Western blotting. In the CHAPSO-reconstituted system, Aβ40, Aβ42, and Aβ43 were the most abundant species identified by Western blotting (Fig. 6 A). This was also the case with LC-MS/MS quantification (Figs. 5, 6 B). Whereas Aβ46 was undetectable by Western blotting (Kakuda et al., 2006), LC-MS/MS was able to measure the lowest amounts of possible Aβ46 level (Fig. 3 C).
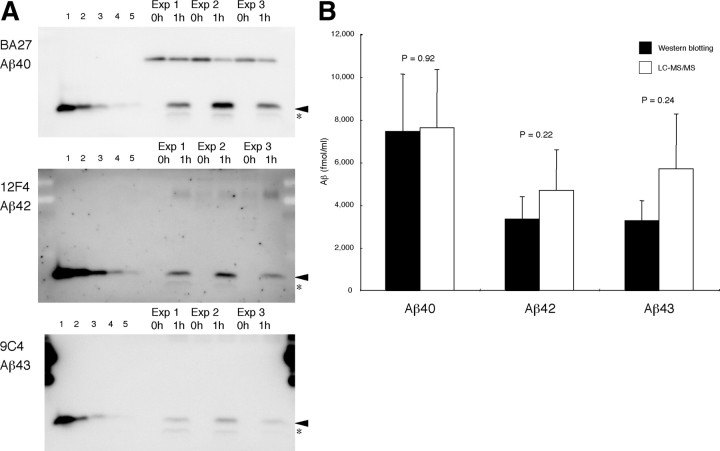
Figure 6.
Quantitative comparisons between Western blotting and LC-MS/MS. Three experiments were performed using the same lot of substrate (250 nm C99–FLAG) and the same CHAPSO-solubilized supernatant for immunoprecipitation. One-half of the aliquots taken from the reaction mixture was subjected to conventional PAGE, and the other half was treated with TCA and subjected to LC-MS/MS to quantify the tripeptides. A, The left five lanes in each panel were for the standards: 2.5, 5, 12.5, 25, and 50 fmol of each authentic Aβ was loaded in lanes 1–5 and subjected to quantitative Western blotting with BA27 for Aβ40 (top), with 12F4 (middle) for Aβ42, and with 9C4 (bottom) for Aβ43. The samples before and after 1 h incubation were loaded in the right six lanes. The reaction mixture generated significant levels of Aβ40, Aβ42, or Aβ43 after 1 h incubation (arrowheads). Barely discernible bands indicated by * may represent p3 (Aβ17-40 and Aβ17-42). B, Quantification of generated Aβ40, Aβ42, and Aβ43 by Western blotting (black column) and by LC-MS/MS based on the stepwise-processing model (white column). Data are expressed as the means ± SD (n = 3). The recoveries for Aβs were assumed to be 85.6% for Aβ40, 80.7% for Aβ42, and 84.6% for Aβ43 (supplemental Table S3, available at www.jneurosci.org as supplemental material). No significant difference (p = 0.92 for Aβ40, 0.22 for Aβ42, and 0.24 for Aβ43, t test) between Western blotting and LC-MS/MS data.
Figure 6, A and B, shows three experiments for the quantitative comparison between Western blotting and LC-MS/MS data. Appropriate amounts of aliquots taken from the reaction mixture after 1 h were subjected to Western blotting (Fig. 6 A) and LC-MS/MS (Fig. 6 B). Instead of urea/SDS-PAGE, conventional SDS-PAGE was used because the former yields broad bands with unclear boundaries, which make it difficult to quantify accurately. To quantify each Aβ species using SDS-PAGE and Western blotting, we used end-specific monoclonal antibodies, BA27, 12F4, and 9C4, that can distinguish between Aβ40, Aβ42, and Aβ43, which have the same electrophoretic mobility (Fig. 6 A). Aβ42 was also quantified with BC05. Although BC05 cross-reacts with Aβ43 (Iwatsubo et al., 1994), the reactivity is ⅕ to less than that with Aβ42 in Western blotting. BC05 and 12F4 gave very similar results (data not shown). As shown in Figure 6 B, the results of the two methods corresponded roughly to each other within at most a twofold difference. Some issues remain to be addressed. Recovery values used here were derived on some assumptions (see Materials and Methods). As described above, the levels of Aβs estimated from the levels of tripeptide and tetrapeptide quantified by LC-MS/MS are based on the assumption that there is no leaky pathway other than the two product lines. In view of the completely different methodologies and assumptions for quantification, the results of these methods should appear consistent. Thus, the above results suggest that the stepwise-processing model is accurate and that the action of γ-secretase or the effect of its inhibitors can be considered based on this model.
The above results suggest that the tetrapeptide-release step from Aβ42 to Aβ38 would be a specific target of Aβ42-lowering nonsteroidal anti-inflammatory drugs (NSAIDs) (Weggen et al., 2001; Eriksen et al., 2003) and other Aβ42-enhancing compounds (Kukar et al., 2005). If elucidated, the cleavage mechanism at this step would lead to the development of efficient and selective Aβ modulator. We examined whether sulindac sulfide affects the release of peptides in the CHAPSO-reconstituted system using LC-MS/MS (Fig. 7 A) and urea/SDS-PAGE followed by Western blotting (Fig. 7 B). Addition of sulindac sulfide caused a nonsignificant increase in the amount of VVIA in the reaction mixture (Fig. 7 A, top), whereas the amount of VVI stayed at the same. The putative levels of Aβ40, Aβ46, and Aβ45 did not change significantly, but the levels of Aβ43 and Aβ42 decreased significantly (Fig. 7 A, bottom). TOF mass spectrometry and urea/SDS-PAGE show a characteristic “NSAID effect” pattern in the CHAPSO-reconstituted system (Fig. 7 B): a decrease in Aβ42 level and an increase in Aβ38 level (for control, see Fig. 4 A). A decrease in Aβ43 level has not been reported thus far, possibly because Aβ43 is retained in the cell and is not secreted into the medium, which most commonly serves as the sample for TOF mass spectrometry and Western blotting. Thus, it is possible that, without analysis of the lysates, one could have overlooked changes in the Aβ43 levels caused by sulindac sulfide. It is possible that sulindac sulfide enhances the final steps of both product lines (release of IAT and VVIA) and thereby lowers the levels of Aβ43 and Aβ42, respectively.
LC-MS/MS reveals the presence of an induction period in the generation of tripeptides from βCTF, which may be explained by the stepwise-processing model
Because the profile of tripeptides released was consistent with the tripeptide hypothesis (Qi-Takahara et al., 2005), the kinetics of the CHAPSO-solubilized system was traced back by LC-MS/MS up to as early as 5 min (Fig. 8 A). We thought that accurate quantification of very low amounts of tripeptides, especially in the early phase of the reaction, would provide insight into the cleavage mechanism. We emphasize that the levels of tripeptides were invariably ITL > VIV > IAT and VIT > TVI even at earlier times, as early as 10 min. Most unexpectedly, LC-MS/MS quantification revealed an induction period, 3–4 min, in the generation of peptides. When extrapolated, each time line for each tripeptide, appears to intercept almost the same point on the x-axis (Fig. 8 A). After this induction period, the reaction proceeds at a constant rate for each tripeptide.
We are careful about performing this kind of experiment because the γ-secretase fraction and substrate were kept on ice and then mixed with preincubated CHAPSO buffer. The temperature of the reaction mixture may not reach 37°C quickly and thus could be responsible for the induction period when the reaction proceeded very slowly. Despite repeated, very carefully performed, experiments (see Materials and Methods), we still observed the induction period. Thus, the lag cannot be explained by delayed elevation of the mixture temperature to 37°C. We concluded that the induction period is real in the CHAPSO-reconstituted γ-secretase system and sought to test whether numerical simulation based on the stepwise-processing model can explain this phenomenon. This is because the differential equations (supplemental Appendix, available at www.jneurosci.org as supplemental material) derived from the model do not appear to have analytical solutions.
Here we consider only the Aβ40 product line. When the first-order kinetics is postulated, Equations B1–B16 in supplemental Appendix (available at www.jneurosci.org as supplemental material) can be derived from the stepwise-processing model (Eqs. A1–A5 in supplemental Appendix, available at www.jneurosci.org as supplemental material). These reactions were simulated on the assumption that, in the initial conditions, only [E] and [C99] have the non-zero initial concentrations. The parameters (supplemental Table S4, available at www.jneurosci.org as supplemental material) were fitted to the time lines of tripeptides (Fig. 8 A). The simulations showed the following: (1) in the system from as low as 1 fm to as high as 1 μm C99 (data not shown), there existed a similar induction period, 3–4 min, for each tripeptide and also a shorter induction period for AICD (Fig. 8 B), and then the reactions proceeded linearly up to 6 h (data not shown; see Fig. 3 C); and (2) at 250 nm C99 (experimental condition), it takes 10–15 min for the system to reach the steady state (supplemental Fig. S3, available at www.jneurosci.org as supplemental material). Consistent with these simulation results, we found that an induction period similar to those of tripeptides existed also for AICD, although AICD49-99 and AICD50-99 were not separately measured in the CHAPSO-reconstituted system using quantitative Western blotting (data not shown). Furthermore, in the experiment using 1 μm C99, the generation of AICD and tripeptides exhibited induction periods that were very similar to those in the case of 250 nm C99 (data not shown). The simulation showed that the tripeptide generated early has a shorter induction period than one generated late (Fig. 8 B). This may not have been resolved experimentally.
Altogether, the successive reaction kinetics is a reasonable explanation for the induction period in the generation of tripeptides. This suggests strongly that the stepwise-processing model is correct.
Discussion
Our stepwise-processing model for γ-secretase cleavage is based on three assumptions: (1) ε-cleavage occurs first and produces Aβ49 and Aβ48; (2) the cleavage proceeds from ε-site to γ-site, and every three-residue and an additional tetrapeptide are removed successively from the TMD of Aβ49/48; and (3) two predominant product lines are involved: Aβ49 is processed to Aβ43/40, and Aβ48 is processed to Aβ42/38 (Qi-Takahara et al., 2005). The model implies that, after ε-cleavage, the longer Aβs to be processed are kept bound to the γ-secretase complex until the final processing to Aβ40/38; that is, the k − (k c−, k 49−, k 46−, or k 43−) value for each longer Aβ is very small (supplemental Appendix, available at www.jneurosci.org as supplemental material). In other words, long Aβs, including Aβ43, Aβ45, Aβ48, and Aβ49, detach only rarely from the enzyme complex during the processing (represented by k −) because they accumulate gradually in a linear manner in the reaction mixture (Kakuda et al., 2006). Regarding the first point, extensive searching has failed to find such longer AICDs. Although in our present study we did not investigate the temporal profile for the generation of AICD, the presence of an induction period for the generation of tripeptides is consistent with the view that ε-cleavage precedes γ-cleavage. On the second point, LC-MS/MS showed unambiguously that these postulated tripeptides and tetrapeptides are released into the reaction mixture. Tetrapeptide release is apparently inherent to the Aβ42 processing, because GGVV, which may have been released to produce Aβ36, was undetectable. Relating to the third point, our previous results suggest strongly that the Aβ40-producing process is distinct from the Aβ42-producing process (Funamoto et al., 2004; Qi-Takahara et al., 2005). The latter process was less sensitive to DFK-167, a difluoro ketone peptidomimetic, than was the former. In addition, accumulating Aβ45 in the rafts prepared from N141I mtPS2-transfected cells is not processed to Aβ42, suggesting again that both product lines differ functionally from each other (Yagishita et al., 2008).
The accuracy of LC-MS/MS quantification has also revealed an unexpected cleavage process, which contradicts the three-residue rule, although it must be a very minor (almost negligible) process. Occasionally, VVI was detected at significant levels, suggesting that a small amount of Aβ41 existed, and thus a small proportion of Aβ38 was derived from Aβ41 in the reaction mixture (Figs. 4, 7). We noted that more VVI was released than was ATV, IVI, or TLV in the Aβ41 product line. Following the stepwise-processing model, the relatively high level of VVI released would be explained by a contribution from an additional product line(s). For example, the release of VIT in the Aβ42 product line, followed by the release of tetrapeptide ATVI, would lead to Aβ41. In any case, the production of Aβ38 would be contributed not only from Aβ42 but also from Aβ41 by releasing VVI (see below). Thus, quantification of VVIA alone may underestimate the level of Aβ38. Such unusual cleavage that does not follow the tripeptide (or sometimes tetrapeptide) rule may tend to occur in the CHAPSO-reconstituted system that allows free collision between the enzyme and substrate. Conversely, membrane integrity would enhance these minor processes and production of smaller Aβs that are barely detectable in the reconstituted system. In relation to this unusual cleavage, we note that intracellular generation of Aβ49 or Aβ48 induced by transfection leads to production of small but significant amounts of Aβ40 or Aβ42, respectively. This suggests that the two product lines are not completely independent and may have some junctions. It should be also noted that the present theoretical model does not take into account minor and unusual cleavage(s) or interdependence between the product lines.
The stepwise-processing model has the inherent characteristic that the difference in the levels of tripeptide released is equal to the amount of Aβ produced (Fig. 5). Only the levels of the final products, Aβ40 and Aβ38, are equal to the released amounts of tripeptides and tetrapeptides, IAT and VVIA (but see above), respectively. Because the levels of other Aβs represent the difference of tripeptide (and tetrapeptide) released, it is possible that, even if the trimming activity of γ-secretase increases markedly, the level of Aβ of interest is low. Conversely, the level of Aβ of interest may increase despite suppressed activity of γ-secretase. Accordingly, the levels of Aβs do not reflect the cleavage activity at each step. One noteworthy example is the effect of sulindac sulfide on the γ-secretase assay system (Fig. 7), i.e., the cleaving activities of γ-secretase do not significantly alter. Nonetheless, the levels of produced Aβ43 and Aβ42 decreased, whereas the tetrapeptide VVIA tended to increase. Because the levels of Aβ40 stayed constant, only the level of Aβ42 appeared to decrease, leading to the prevailing view that sulindac sulfide is selective for the Aβ42 product line. However, our results (Fig. 7) suggest that this may not be correct. It is possible that sulindac sulfide affects the final processes of Aβ generation, by enhancing of cleaving of IAT and VVIA, which decreased the levels of Aβ43 and Aβ42.
This model explains why it was difficult for us to find possible intermediates within the lysates because these intermediates are characterized by their very low levels in the cell. If an intermediate is a good substrate for γ-secretase, it would be cleaved promptly, generating another intermediate and leaving nothing behind. Thus, only intermediates relatively resistant to γ-secretase would be easy to detect in the cell. Why we often detect Aβ46 in the cell would be most likely attributable to its relative resistance to VIV-cleaving activity of γ-secretase within the membrane. In contrast, in the CHAPSO-reconstituted system, Aβ46 was very susceptible to this cleaving activity, and only a very low level of Aβ46 was produced, which was undetectable by Western blotting.
We do not know why the CHAPSO-reconstituted system processes βCTF up to Aβ40/38 but no further to smaller species, neither do we know how the substrate is translocated on the catalytic site of the γ-secretase complex during the successive cleavage process. Apparently, this step is energy independent, because the cleavage does not require ATP or GTP. Interestingly, an exogenously added Aβ46, Aβ48, or Aβ49 is never processed to smaller species by the CHAPSO-reconstituted γ-secretase (data not shown). Presumably, a distinct cleavage-sensitive conformation would be required for long Aβs to bind to the γ-secretase complex. This may relate to the previous observation that longer Aβ species generated by transfection are easily processed, and smaller Aβ species are more difficult to process (Funamoto et al., 2004).
We do not have a proper explanation about why γ-secretase cleaves out VVIA to produce Aβ38 but never VIA to produce Aβ39 in this reconstituted system. Other cleavage sites definitely follow the tripeptide rule and fit well within the α-helical model proposed originally by Lichtenthaler et al. (2002). When one looks at the sequence of TMD of βCTF, bulky hydrophobic residues except Ala42 are filled between the ε- and γ-cleavage sites up to the GG portion in which the smallest residue appears serially. The α-helical surface of the TMD may be shallow grooved in this portion, which may provide a stronger signal for binding to the catalytic sites of γ-secretase. In other words, the γ-secretase may prefer to cleave near the less hindered G–V amide bond rather than the more hindered V–V amide bond.
Although the present CHAPSO-reconstituted system demonstrates the framework of γ-secretase cleavage, stepwise processing, it lacks some features observed in cell-free system or cultured cells. First, remarkable accumulation of Aβ43/46 in cultured cells treated by DAPT cannot be seen in the CHAPSO-reconstituted system. Presumably, differential sensitivity of γ-secretase/Aβ complex to effective concentrations of DAPT in the cell could explain the phenomenon. The γ-secretase/Aβ43 complex is more sensitive to DAPT than is the γ-seretase/Aβ46 complex. Alternatively, the catalytic sites in the membrane of γ-seretase/Aβ43 would be located in the outer portion of the membrane and would thus be more sensitive to DAPT, whereas the γ-secretase/Aβ46 complex would be located in the inner portion of the membrane and be less sensitive to DAPT. The CHAPSO-reconstituted system has no membrane barrier, resulting in loss of differential sensitivities. Second, more Aβ40 than Aβ42 is usually produced in the cells or cell-free system (Fig. 2). In contrast, the CHAPSO-reconstituted system generates similar levels of Aβ40, Aβ42, and Aβ43 (Kakuda et al., 2006). The two product lines are predominant and substantially independent; this comes from abundance of Aβ48 relative to Aβ49 in ε-cleaved products in the CHAPSO-reconstituted system. This suggests that the cleavage at the carboxyl side of Thr48 would be enhanced in the CHAPSO-reconstituted system compared with the cell-free system. It is possible that membrane integrity or some regulatory protein factors suppress the generation of Aβ48 and thus the generation of Aβ42. LC-MS/MS analysis of the raft system (Yagishita et al., 2006) may provide insight into the characteristics of γ-secretase embedded in the membrane.
Although the CHAPSO-reconstituted system has some inherent problems, we believe that this stepwise-processing model provides the framework for investigating the cleavage by γ-secretase. The characteristics revealed here should be considered when studying the structure–function relationship of γ-secretase complex (Lazarov et al., 2006; Osenkowski et al., 2009).
Footnotes
This work was supported in part by a Grant-in-Aid for Scientific Research on Priority Areas, Research on Pathomechanisms of Brain Disorders (Y.I.), by a Grant-in-Aid for Scientific Research for the Encouragement of Young Scientists (A) (S.F.) from the Ministry of Education, Culture, Sports, Science, and Technology, and by the grant program “Collaborative Development of Innovative Seeds” (S.F.) from the Japan Science and Technology Agency. We also thank Information-Technology Promotion Agency, Japan and Exploratory IT Human Resources Project (MITOH Program) for kind support (Y.N.). We thank Takeda Chemical Industries for BA27 and BC05, Dr. Toshiharu Suzuki (Hokkaido University) for UT18, and Naoki Sawano for technical assistance.
References
- Beher D, Wrigley JD, Owens AP, Shearman MS. Generation of C-terminally truncated amyloid-β peptides is dependent on γ-secretase activity. J Neurochem. 2002;82:563–575. doi: 10.1046/j.1471-4159.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- Eriksen JL, Sagi SA, Smith TE, Weggen S, Das P, McLendon DC, Ozols VV, Jessing KW, Zavitz KH, Koo EH, Golde TE. NSAIDs and enantiomers of flurbiprofen target γ-secretase and lower Aβ 42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funamoto S, Morishima-Kawashima M, Tanimura Y, Hirotani N, Saido TC, Ihara Y. Truncated carboxyl-terminal fragments of β-amyloid precursor protein are processed to amyloid β-proteins 40 and 42. Biochemistry. 2004;43:13532–13540. doi: 10.1021/bi049399k. [DOI] [PubMed] [Google Scholar]
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of Aβ 42(43) and Aβ 40 in senile plaques with end-specific Aβ monoclonals: evidence that an initially deposited species is Aβ 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- Kakuda N, Funamoto S, Yagishita S, Takami M, Osawa S, Dohmae N, Ihara Y. Equimolar production of amyloid β-protein and amyloid precursor protein intracellular domain from β-carboxyl-terminal fragment by γ-secretase. J Biol Chem. 2006;281:14776–14786. doi: 10.1074/jbc.M513453200. [DOI] [PubMed] [Google Scholar]
- Kukar T, Murphy MP, Eriksen JL, Sagi SA, Weggen S, Smith TE, Ladd T, Khan MA, Kache R, Beard J, Dodson M, Merit S, Ozols VV, Anastasiadis PZ, Das P, Fauq A, Koo EH, Golde TE. Diverse compounds mimic Alzheimer disease-causing mutations by augmenting Aβ42 production. Nat Med. 2005;11:545–550. doi: 10.1038/nm1235. [DOI] [PubMed] [Google Scholar]
- Lazarov VK, Fraering PC, Ye W, Wolfe MS, Selkoe DJ, Li H. Electron microscopic structure of purified, active γ-secretase reveals an aqueous intramembrane chamber and two pores. Proc Natl Acad Sci U S A. 2006;103:6889–6894. doi: 10.1073/pnas.0602321103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler SF, Beher D, Grimm HS, Wang R, Shearman MS, Masters CL, Beyreuther K. The intramembrane cleavage site of the amyloid precursor protein depends on the length of its transmembrane domain. Proc Natl Acad Sci U S A. 2002;99:1365–1370. doi: 10.1073/pnas.032395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osawa S, Funamoto S, Nobuhara M, Wada-Kakuda S, Shimojo M, Yagishita S, Ihara Y. Phosphoinositides suppress γ-secretase in both the detergent-soluble and -insoluble states. J Biol Chem. 2008;283:19283–19292. doi: 10.1074/jbc.M705954200. [DOI] [PubMed] [Google Scholar]
- Osenkowski P, Li H, Ye W, Li D, Aeschbach L, Fraering PC, Wolfe MS, Selkoe DJ, Li H. Cryoelectron microscopy structure of purified γ-secretase at 12 A resolution. J Mol Biol. 2009;385:642–652. doi: 10.1016/j.jmb.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y. Longer forms of amyloid β-protein: implications for the mechanism of intramembrane cleavage by γ-secretase. J Neurosci. 2005;25:436–445. doi: 10.1523/JNEUROSCI.1575-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Dohmae N, Qi Y, Kakuda N, Misonou H, Mitsumori R, Maruyama H, Koo EH, Haass C, Takio K, Morishima-Kawashima M, Ishiura S, Ihara Y. Potential link between amyloid β-protein 42 and C-terminal fragment γ 49-99 of β-amyloid precursor protein. J Biol Chem. 2003;278:24294–24301. doi: 10.1074/jbc.M211161200. [DOI] [PubMed] [Google Scholar]
- Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- Yagishita S, Morishima-Kawashima M, Tanimura Y, Ishiura S, Ihara Y. DAPT-induced intracellular accumulations of longer amyloid β-proteins: further implications for the mechanism of intramembrane cleavage by γ-secretase. Biochemistry. 2006;45:3952–3960. doi: 10.1021/bi0521846. [DOI] [PubMed] [Google Scholar]
- Yagishita S, Morishima-Kawashima M, Ishiura S, Ihara Y. Aβ46 is processed to Aβ40 and Aβ43, but not to Aβ42, in the low density membrane domains. J Biol Chem. 2008;283:733–738. doi: 10.1074/jbc.M707103200. [DOI] [PubMed] [Google Scholar]