Abstract
Dopamine (DA) transmission plays a critical role in the processing of emotionally salient information and in associative learning and memory processes. Within the mammalian brain, neurons within the medial prefrontal cortex (mPFC) are involved critically in the encoding, expression, and extinction of emotionally salient learned information. Within the mPFC, dopaminergic transmission is involved importantly in controlling attentional and motivational processes, particularly within the context of emotionally salient sensory information. Considerable evidence suggests differential roles for DA D1-like versus D2-like receptors, including the D4 receptor subtype, in the regulation of neuronal activity and emotional processing within the mPFC. Using an olfactory fear-conditioning assay in rats, we compared the roles of DA D1 versus D4 receptor activation during the encoding and recall phases of emotional learning and memory. We report that specific activation of DA D4 receptors within the mPFC strongly potentiates the salience of normally nonsalient emotional associative fear memories and blocks the encoding of suprathreshold conditioned fear associations. However, D4 receptor activation has no effect on the recall of previously learned emotionally salient conditioned memories. In contrast, intra-mPFC D1 receptor activation failed to increase the emotional salience of subthreshold fear stimuli but completely blocked the expression of previously learned emotionally relevant information, demonstrating that DA D4 versus D1 subtype receptor transmission within the mPFC plays distinct functional roles in the processing of emotionally salient versus nonsalient associative information and differentially modulates the encoding versus recall phases of emotional memory within the mPFC.
Introduction
The ability to assign appropriate emotional significance to incoming sensory information is essential for normal cognitive function and adaptive behavior. These psychological processes require prefrontal cortical mechanisms that allow accurate filtering of salient versus nonsalient sensory information. Disturbances in these processes may lead to the formation of inappropriately reinforced associative memories, maladaptive behaviors, and delusional ideation, all of which are typically present in schizophrenia-related psychoses. Within the medial prefrontal cortex (mPFC), cognitive, emotional, and motivational processes are strongly modulated by dopamine (DA) transmission and, in particular, through signaling via the DA D1 and the D4 receptor subtypes (Seamans et al., 1998; Pezze et al., 2003; Laviolette et al., 2005; Onn et al., 2006).
Dopaminergic modulation of mPFC neuronal networks is known to control the encoding of working memory and the temporal sequencing of behavioral output (Goldman-Rakic et al., 1989; Goldman-Rakic, 1995; Seamans et al., 1998). Functionally, DA transmission within the mPFC influences neuronal network activity in a biphasic manner, with D1 receptor activation increasing the intrinsic excitability of inhibitory GABAergic interneurons and D2-like receptor activation decreasing the activity of these interneurons, presumably leading to decreased probability of tonic inhibitory GABA release within the mPFC (Seamans et al., 2001). The D4 subtype is expressed highly in mPFC relative to other brain regions and plays a critical role in modulating neuronal activity in both prelimbic and infralimbic mPFC regions (Ceci et al., 1999; Onn et al., 2005). Additional support for a modulatory role of DA D4 receptor transmission is suggested by findings that DA-mediated amplification of mPFC neuronal activity is preferentially mediated through dopaminergic actions on the D4 receptor subtype (Ceci et al., 1999). Given the critical role of the mPFC in the encoding and expression of emotionally salient information and its ability to amplify dopaminergic effects within mPFC neuronal networks, one possibility is that DA D4 receptor activation within the mPFC may amplify the emotional salience of sensory information and the subsequent encoding of this associative information.
A subpopulation of neurons within the mPFC is capable of encoding and expressing emotional conditioned associations via inputs from the basolateral nucleus of the amygdala (BLA) (Laviolette et al., 2005; Laviolette and Grace, 2006a,b). We have reported previously that D4 receptor blockade, either systemically or directly within the mPFC, can prevent the acquisition (but not expression) of emotionally salient associative fear memories, as demonstrated behaviorally and at the level of the single neuron (Laviolette et al., 2005). However, it is presently not known how activation of DA D4 versus D1 receptor transmission within the mPFC may be involved in modulating the acquisition and/or expression (recall) of emotionally salient information or how these receptors may modulate salient versus nonsalient sensory stimuli in the context of emotional memory encoding. Accordingly, we conducted a series of behavioral pharmacological studies comparing the potential roles of DA D4 versus D1 receptor transmission during the acquisition or expression phases of emotional learning and memory processing, by comparing emotionally salient versus nonsalient associative conditioning stimuli during an olfactory fear-conditioning procedure.
Materials and Methods
Animals and surgery.
Male Sprague Dawley rats (300–350 g) were obtained from Charles River Laboratories. All procedures were performed in accordance with the Canadian Council on Animal Care and were approved by the Council on Animal Care at the University of Western Ontario. Rats were anesthetized with a ketamine (80 mg/ml)–xylazine (6 mg/kg) mixture administered intraperitoneally and placed in a stereotaxic device. All stereotaxic coordinates were based on the atlas of Paxinos and Watson (1996). Two stainless steel guide cannulae (22 gauge) were implanted into the mPFC using the following stereotaxic coordinates (15° angle; in millimeters from bregma): anteroposterior (AP), +2.9; lateral (LAT), ±1.9; ventral (V), −3.0 from the dural surface. For bilateral intra-BLA placements, the following stereotaxic coordinates were used (0° angle): AP, −3.0; LAT, ±5.0; V, −8.0 from the dural surface (see Fig. 4). Jeweler's screws and dental acrylic were used to secure the cannulae.
Figure 4.
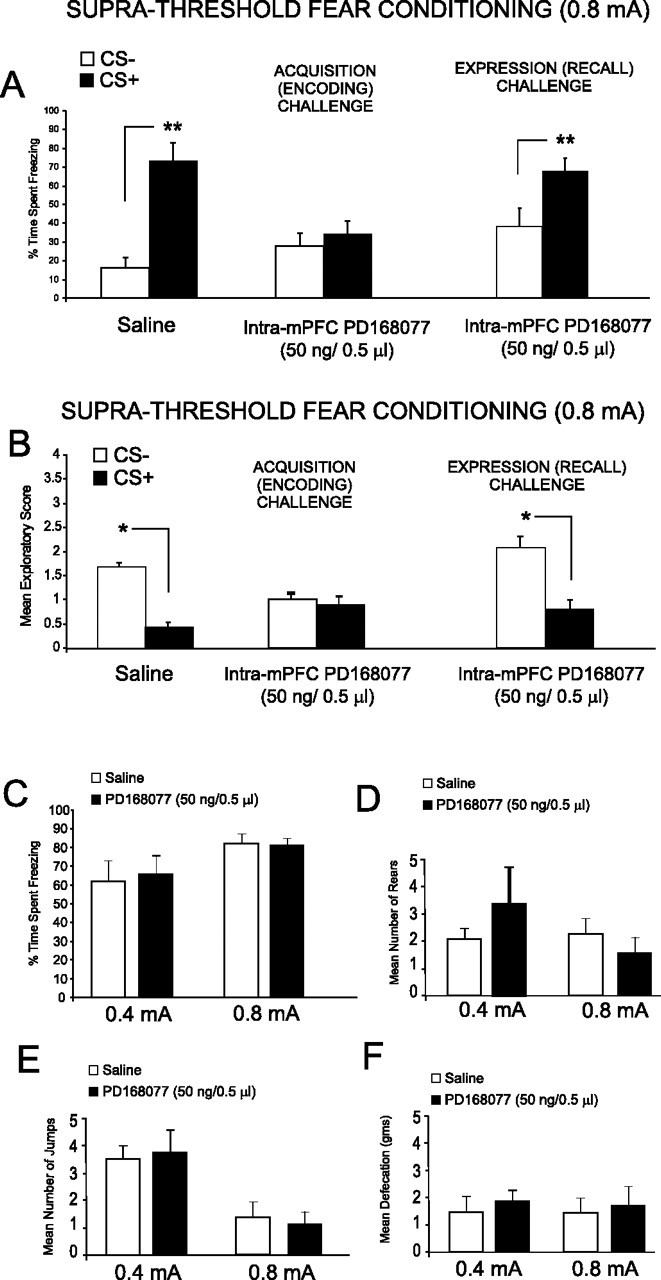
Intra-mPFC D4 agonist effects on suprathreshold olfactory fear conditioning and footshock sensitivity analyses. A, Saline control rats showed significant freezing behavior to CS+ versus CS− presentations after conditioning with suprathreshold footshock (0.8 mA) levels. In contrast, preconditioning microinfusion of intra-mPFC PD 168077 (50 ng/0.5 μl) blocked the acquisition of suprathreshold olfactory fear conditioning as demonstrated by no significant difference in the percentage of time spent freezing to the CS+ relative to the CS− at testing. However, intra-mPFC PD 168077 (50 ng/0.5 μl) microinfusions immediately before testing did not block the expression (recall) of suprathreshold olfactory fear conditioning. B, Analysis of exploratory activity after CS+ or CS− presentation revealed that whereas saline control rats showed significant conditioned suppression of exploratory behavior in response to CS+ versus CS− presentations, this effect was blocked in rats receiving intra-mPFC PD 168077 (50 ng/0.5 μl) immediately before conditioning. In contrast, intra-mPFC PD 168077 (50 ng/0.5 μl) administered immediately before testing did not block the expression (recall) of suprathreshold olfactory fear conditioning observed as a significant decrease in exploratory behavior in response to CS+ presentations. C, Footshock sensitivity testing (see Materials and Methods) revealed no significant differences between control groups receiving a subthreshold footshock (0.4 mA) versus intra-mPFC saline control (n = 5) or intra-mPFC PD 168077 (50 ng/μl; n = 5) or between control groups receiving a suprathreshold level of footshock (0.8 mA) versus intra-mPFC saline control (n = 6) or intra-mPFC PD 168077 (50 ng/μl, n = 6) in the percentage of time spent freezing in response to footshock presentations. D, Similarly, no significant differences were observed in the mean number of rears between groups in response to either level of footshock. E, No significant differences were observed between the mean numbers of jumps between groups in response to either level of footshock. F, No significant differences were observed in the mean amount of defecation between groups in response to either level of footshock.
Olfactory fear conditioning.
Rats were taken from their home cages, received sham microinfusions into the mPFC, and were habituated for 30 min in a ventilated conditioning chamber with an electric grid floor inside a sound-attenuated room. Olfactory fear conditioning took place in one of two distinct environments, counterbalanced within groups: “shock” environment A was a 30 × 30 inch Plexiglas box with black stripes on a white background and a metallic grid shock floor, whereas shock environment B was a 30 × 30 inch Plexiglas box with black dots on a white background with a grid shock floor. Testing 24 h later took place in one of two alternate environments, in which animals had not previously received electric shock, counterbalanced within groups: test environment A had walls with black dots and a gray Plexiglas floor, whereas test environment B had walls with black and white stripes and a gray Plexiglas floor. On day 1 (habituation phase), animals were habituated to a random combination of shock environment A or B and test environment A or B in a counterbalanced order for 30 min in each environment. On day 2 (conditioning phase), animals were returned to the conditioning room and placed in the previously assigned shock environment. During the conditioning phase, one of the odors (almond or peppermint) was presented to the animal for 19 s, and a footshock was then delivered (0.4 or 0.8 mA) for 1 s [positive conditioned stimulus (CS+)]. The two different levels of footshock (0.4 and 0.8 mA) correspond to subthreshold and suprathreshold levels of emotional stimuli, as reported previously (Laviolette and Grace, 2006a). One hundred twenty seconds later, the alternate odor was presented for 20 s [negative conditioned stimulus (CS−)] in the absence of footshock. This cycle was repeated five times. On the following day (test phase), rats were returned to the test room and placed in the previously assigned test environment. Before odor presentation, the rat was allowed to explore the environment for 1 min, during which time baseline levels of freezing and exploratory behavior were observed. Odors (CS+ or CS−) were then presented for 5 min each to the animal in a counterbalanced order, and the amount of time freezing was recorded. Freezing was defined as complete immobility with the exception of respiratory-related movement. We also analyzed exploratory behavior in response to presentations of CS+ or CS− odors, as described previously (Rosenkranz and Grace, 2003). Exploratory behavior was scored as follows, with a score assigned for every minute of each of the 5 min during the CS+ or CS− odor presentations: 0, no locomotion; 1, ambulation across one side of the testing chamber; 2, ambulation across two sides; 3, exploration of the full perimeter of the testing chamber; 4, exploration of the center and entire perimeter of the test chamber. To analyze DA receptor modulation on acquisition of emotionally salient information, microinfusions were given directly before the conditioning procedure. To examine the effects of DA receptor manipulation on the expression phase of learned emotional memory drug, microinfusions were given directly before testing (24 h after fear conditioning). Experimental procedures for examining acquisition (encoding) versus expression (recall) of associative fear conditioning are summarized schematically in Figure 1C.
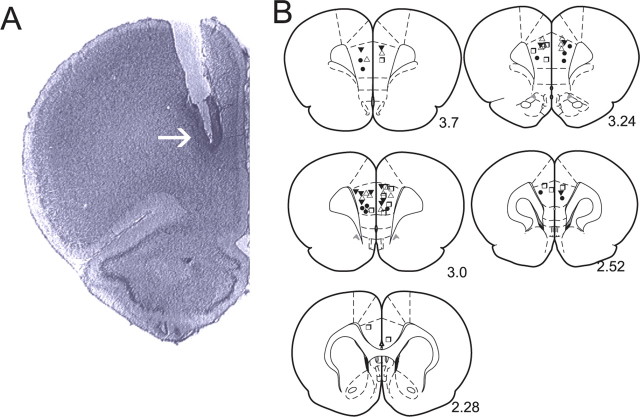
Figure 1.
Histological analysis of intra-mPFC microinjection sites. A, Microphotograph of a representative injector placement within the mPFC. B, Schematic illustration showing representative bilateral placements of microinjection cannulae. For illustrative clarity, only a subset of experimental groups is presented. Symbols represent separate experimental groups: ▵, PD 168077, 50 ng/0.5 μl versus acquisition of subthreshold stimuli; □, SKF 38393, 1000 ng/0.5 μl versus acquisition of subthreshold stimuli; ▾, PD 168077, 50 ng/0.5 μl versus acquisition of suprathreshold stimuli; •, SKF 38393, 1000 ng/0.5 μl versus expression of suprathreshold stimuli.
Drug administration.
For mPFC and BLA microinfusions, stainless steel guide cannulae (22 gauge) were implanted bilaterally into the mPFC or BLA, and drugs were administered through a 28 gauge microinfusion injector (Plastics One). All drugs were dissolved in physiological saline, with pH adjusted to 7.4. Bilateral intra-mPFC microinjection of saline vehicle, the highly selective D4 agonist PD 168077 (dose range, 2.5–50 ng/0.5 μl; Tocris), the D1-like receptor agonist SKF 38393 (dose range, 100–1000 ng/0.5 μl; Sigma), the D2-like agonist quinpirole (dose range, 100–1000 ng/0.5 μl; Sigma), or the D4 antagonist L-741,741 (1000 ng; Tocris) and the full D1 receptor agonist SKF 81297 (dose range, 1–100 ng/0.5 μl; Tocris) were microinfused immediately before the olfactory conditioning procedure (acquisition phase challenge) or immediately before the testing phase (recall challenge). For studies examining the role of BLA inputs, bilateral intra-BLA microinjections of either saline vehicle or muscimol (500 ng/0.5 μl) were performed immediately before intra-mPFC PD 168077 (50 mg/0.5 μl) microinjections.
Footshock sensitivity analysis.
To determine whether our intra-mPFC pharmacological manipulations induced alterations in sensitivity to footshock, we rigorously monitored behavioral responses to these manipulations before and after drug administration. For intra-mPFC experiments, we performed separate control experiments, wherein animals received either bilateral intra-mPFC saline (control) or the D4 agonist PD 186077 (50 ng/0.5 μl) and were placed in a clear Plexiglas environment with a footshock grid floor. Based on previously published methodology (Laviolette and Grace, 2006), we presented either a behaviorally subthreshold footshock level (0.4 mA, 1 s) or a behaviorally suprathreshold footshock level (0.8 mA) over five times and measured sensitivity to footshock over four separate variables: (1) percentage of time spent freezing in 20 s postfootshock intervals; (2) number of jumps in response to footshock; (3) amount of defecation (in pieces) during footshock session; and (4) number of times the animal reared during the sensitivity trial. All of these behavioral indices of footshock sensitivity have been reported as reliable indicators of fear reactivity to the presentation of a footshock stimulus (Antoniadis and McDonald, 1999).
Histology.
After completion of the experiment, the animals were perfused, and brains were removed and stored in formalin with 25% sucrose solution for at least 24 h. Brains were then sectioned into 40 μm coronal slices, mounted, and stained using cresyl violet to allow for histological analysis of the site of injection. The majority of mPFC placements were localized within the boundaries of the prelimbic cortical area (PLC).
Data analysis.
Data were analyzed with one-, two-, or three-way ANOVA where appropriate or Student's t tests. Post hoc analyses were performed with Newman–Keuls tests.
Results
Intra-mPFC histological analysis
Histological analysis revealed microinfusion injector cannlae placements to be bilaterally localized within the anatomical boundaries of the mPFC region, as determined by the atlas of Paxinos and Watson (1996). In Figure 1A, we present a microphotograph showing a typical, representative injector placement within the mPFC. In Figure 1B, we present a schematic illustration showing representative intra-mPFC bilateral cannulae placements along the rostrocaudal axis of the mPFC.
Intra-mPFC DA D4 receptor activation potentiates associative learning to emotionally nonsalient conditioning stimuli
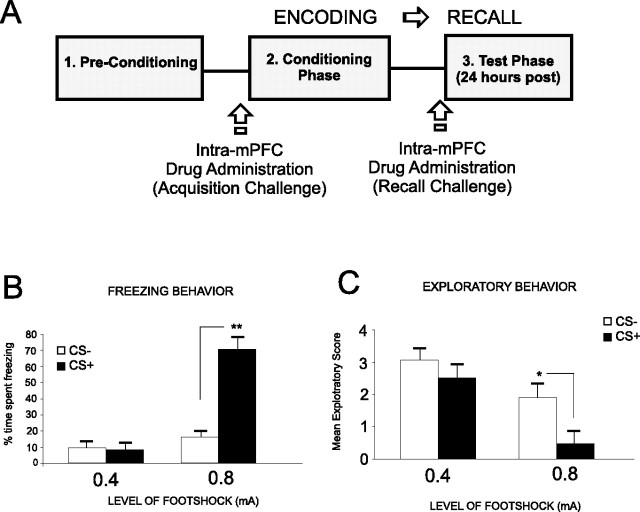
For this and all subsequent behavioral experiments, we present a schematic illustration in Figure 2A, showing our fear-conditioning procedure in terms of conditioning timelines and methods for challenging either the acquisition (encoding phase) or the expression (recall phase) of emotional associative learning. To establish salient versus nonsalient levels of footshock for use in our fear-conditioning procedures, we selected two levels of footshock (0.4 and 0.8 mA) for use in our olfactory fear-conditioning procedures, based on a previously published report (Laviolette and Grace, 2006). Preliminary studies determined that whereas the lower level of footshock (0.4 mA; n = 5) (Fig. 2B) produced no significant associative memory encoding (animals demonstrated no conditioned freezing behavior in response to CS+ relative to CS− presentations; t(4) = 0.15; p > 0.05), a higher level of footshock (0.8 mA; n = 6) (Fig. 2B) produced strong associative fear conditioning, demonstrated by significantly greater levels of conditioned freezing in response to CS+ relative to CS− presentations (t(5) = 5.84; p < 0.01). Similarly, whereas the lower level of footshock (0.4 mA; n = 5) produced no associative suppression of exploratory behavior in response to CS+ versus CS− presentations (Fig. 2C) (t(4) = 1.78; p > 0.05), a higher level of footshock (0.8 mA; n = 6) (Fig. 2B) produced conditioned suppression of exploratory behavior specifically in response to CS+ relative to CS− presentations (Fig. 2C) (t(5) = 2.2; p < 0.05). Thus, similar to previous reports (Laviolette et al., 2005; Laviolette and Grace, 2006a), these levels of footshock were selected for subsequent experiments to represent “salient” versus “nonsalient” emotional conditioning stimuli. Using our associative olfactory fear-conditioning procedure (see Materials and Methods) (Laviolette et al., 2005; Laviolette and Grace, 2006a) with an emotionally nonsalient (0.4 mA) level of footshock (which produces no associative fear conditioning in control animals) (Laviolette and Grace, 2006a), we next performed bilateral intra-mPFC microinfusions of either saline or the specific DA D4 agonist PD 168077 (2.5, 25, or 50 ng/0.5 μl) immediately before fear conditioning, to determine the effects of D4 receptor activation during the acquisition phase (encoding) of this associative information. ANOVA revealed a significant interaction between group and treatment (F(3,53) = 11.2; p < 0.001) on times spent freezing to either CS+ or CS− cue presentations at testing. Post hoc analysis illustrated a dose-dependent potentiation in associative fear-conditioning expression at the two higher doses of PD 168077, 25 ng (n = 7; p < 0.05) and 50 ng (n = 8; p < 0.01), demonstrated by a significantly greater time spent freezing to the CS+ compared with CS− (Fig. 3A). In contrast, post hoc analysis revealed no significant difference between freezing time to the CS+ compared with CS− for the saline (n = 5; p > 0.05) control group or animals receiving the lowest dose of PD 168077 (2.5 ng/0.5 μl; n = 7; p > 0.05). To demonstrate the receptor specificity of our D4 agonist effect, we performed a separate control experiment (n = 7) wherein rats received coinfusion of the highest effective dose of PD 168077 (50 ng/0.5 μl) with a competitive antagonist of the D4 receptor, L-741,741 (1000 ng/0.5 μl), before subthreshold olfactory fear conditioning. Statistical analysis revealed that D4 antagonist coadministration blocked the potentiating effect of PD 168077 because rats showed no significant difference between percentage of time spent freezing (t(6) = 1.22; p > 0.05) in response to the CS+ relative to the CS− (Fig. 3A). Analysis of spontaneous exploratory behavior during presentations of either CS+ or CS− (Fig. 3B) revealed a significant interaction between group and treatment (F(3,63) = 2.57; p < 0.01) with post hoc analysis revealing that rats treated with the highest dose PD 169077 (50 ng/0.5 μl; n = 8) or 25 ng/0.5 μl (n = 6) displayed significantly decreased exploratory behavior in response to the CS+ relative to the CS− presentations (p < 0.01 and p < 0.05, respectively). In addition, post hoc analysis revealed there was no significant difference in exploratory behavior observed in response to the CS+ relative to the CS− with the lower doses of PD 168077, 2.5 ng/0.05 μl (n = 7; p > 0.05) and 25 ng/0.5 μl (n = 7; p > 0.05), as well as the saline control group (n = 5; p > 0.05) (Fig. 3B). In addition, in the pharmacological control group receiving coadministration of PD 168077 (50 ng/0.5 μl) with the D4 antagonist L-741,741 (1000 ng/0.5 μl) demonstrated no significant difference between exploratory behavior in response to the CS+ and CS− presentations (t(6) = 1.05; p > 0.05) (Fig. 3B). To further characterize the specific role of the DA D4 subtype relative to other D2-like receptors within the mPFC in our observed emotional associative learning potentiation effects, we ran an additional control experiment using a nonspecific agonist of D2-like receptors, quinpirole, with relative Ki values of 4.8, ∼24, ∼30, and ∼1900 nm at the D2, D3, D4, and D1 DA receptor subtypes, respectively (Seeman and Van Tol, 1994; Levant et al., 1996), for comparison with the effects of the specific D4 receptor agonist PD 168077 (Fig. 3A). We performed bilateral intra-mPFC microinfusions of quinpirole, immediately before conditioning, at two different concentrations: a lower concentration (100 ng/0.5 μl), which should display greater selectivity for the D2 receptor subtype, and a higher concentration (1000 ng/0.5 μl), which would not provide specificity at the D2 receptor but, rather, likely interact with all D2-like subtypes, including the D4 receptors. ANOVA revealed a significant effect of treatment on time spent freezing (Fig. 3C) (F(2, 33) = 5.6; p < 0.01). Post hoc analysis revealed no significant difference in the time spent freezing to the CS+ relative to the CS− in rats receiving a lower, D2 receptor-specific dose of quinpirole (n = 6; p > 0.05). In contrast, post hoc analysis revealed that intra-mPFC microinfusions of quinpirole at the higher (nonspecific) dose (1000 ng/0.5 μl) resulted in a potentiation of subthreshold fear stimuli as demonstrated by a significant increase in the time spent freezing to the CS+ relative to the CS− (n = 6; p < 0.01) (Fig. 3C). Whereas it is not possible to directly compare in vitro binding kinetics with that which would be observed in vivo, these results demonstrate the DA activation within the mPFC specifically potentiates emotional associative learning via actions on a highly sensitive D4 receptor population (showing emotional learning potentiation at doses as low as 25 ng/0.5 ml (Fig. 3A) rather than via nonspecific effects on all D2-like receptor subtypes within the mPFC. These behavioral results are consistent with anatomical and electrophysiological evidence showing higher relative concentrations of the D4 subtype and greater neuronal sensitivity within the mPFC to DA D4 receptor activation, relative to other DA receptor subtypes (Mrzljak et al., 1996; Ceci et al., 1999; Wedzony et al., 2000; Onn et al., 2005).
Figure 2.
Experimental protocol summary and footshock sensitivity level assay. A, Schematic representation showing experimental associative fear conditioning assay and timeline for examining acquisition (encoding) versus expression (recall) phases of associative olfactory fear memory. B, C, Behavioral sensitivity assay demonstrating that although subthreshold (0.4 mA) footshock stimuli produce neither conditioned freezing behavior (B) nor conditioned suppression of exploratory behavior (C), presentation of a suprathreshold (0.8 mA) footshock level produces significant levels of freezing (B) and suppression of exploratory behavior (C). Error bars represent SEM for this and all subsequent figures. For this and all subsequent figures, *p < 0.05; **p < 0.01.
Figure 3.
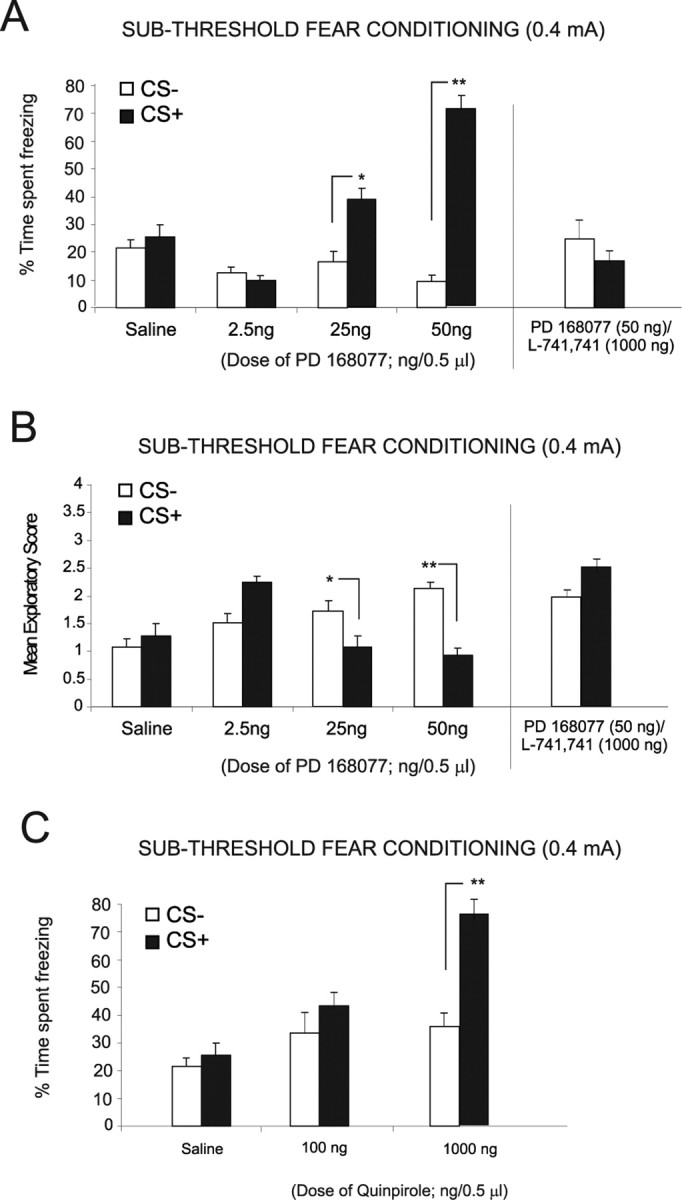
Bilateral intra-mPFC DA D4 and D2 receptor manipulations versus subthreshold levels of fear-conditioning stimuli. A, Bilateral intra-mPFC microinfusions of the DA D4 receptor agonist PD 168077 (2.5, 25, and 50 ng/0.5 μl) dose-dependently potentiated the acquisition of subthreshold (0.4 mA footshock) olfactory fear conditioning. Both saline controls and rats receiving a lower dose of PD 168077 (2.5 ng/0.5 μl) display no significant difference in time freezing to the CS+ relative to the CS− presentations. In contrast, rats receiving higher doses of PD 168077 (25 ng and 50 ng/0.5 μl) display significantly greater levels of freezing specifically in response to presentation of the CS+ relative to CS−. In a separate control experiment, this D4 receptor-induced potentiation was blocked when the D4 receptor antagonist L-741,741 (1000 ng/0.5 μl) was coadministered with PD 168077 (50 ng/0.5 μl), as rats display no significant difference in conditioned freezing behavior in response to the CS+ relative to CS− presentations. B, Measurement of exploratory behavior in response to the CS+ and CS− odors revealed saline control rats and rats receiving the lower two doses of PD 168077 (2.5 and 25 ng/0.5 μl) showed no significant difference in exploratory scores. However, with the 50 ng/0.5 μl PD 168077 dose, there was a significant decrease in spontaneous exploratory behavior after CS+ relative to CS− presentation. Similarly, intra-mPFC coadministration with the D4 receptor antagonist L-741,741 (1000 ng/0.5 μl) with PD 168077 (50 ng/0.5 μl) blocked conditioned suppression of exploratory behavior, demonstrated by no significant difference between exploratory behavior in response to CS+ and CS− presentations. C, Bilateral intra-mPFC microinfusions of the D2-like receptor agonist quinpirole failed to potentiate subthreshold fear-conditioning acquisition at a lower dose of 100 ng/0.5 μl, demonstrated by no significant freezing response to CS+ versus CS− presentations. However, bilateral intra-mPFC microinfusions of quinpirole at a higher (nonspecific) dose (1000 ng/0.5 μl) resulted in a potentiation of subthreshold fear stimuli as demonstrated by a significant increase in the time spent freezing to the CS+ relative to the CS−.
Intra-mPFC DA D4 receptor activation blocks the encoding but not the expression of associative learning to emotionally salient conditioning stimuli
We next examined the possible effects of mPFC DA D4 activation on the encoding of suprathreshold fear-conditioning stimuli, using a higher, emotionally salient dose of footshock (0.8 mA). We first examined the acquisition phase of emotional learning by administering an effective dose of the D4 agonist (50 ng/0.5 μl) immediately before conditioning. A control group (n = 7) receiving intra-mPFC saline before fear conditioning to the suprathreshold footshock level showed significant levels of conditioned fear at testing, demonstrated by a significant increase both in freezing behavior (n = 7; t(6) = 4.29; p < 0.01) (Fig. 4A) and a decrease in exploratory behavior (n = 7; t(6) = 3.45; p < 0.05) (Fig. 4B) in response to the CS+ presentation relative to the CS−. In contrast, rats receiving bilateral intra-mPFC microinfusions of the effective dose of PD 168077 (n = 8; 50 ng/0.5 μl) demonstrated a complete block in the acquisition of emotionally salient fear stimuli as demonstrated by no significant difference in response to CS+ versus CS− presentations in either freezing behavior (t(7) = 1.14; p > 0.05) (Fig. 4A) or exploratory behavior (t(7) = 1.15; p > 0.05) (Fig. 4B). Thus, activation of mPFC D4 receptors blocked the encoding of emotional associative learning to a suprathreshold, emotionally salient footshock stimulus. We next examined the expression phase of emotional learning by performing bilateral intra-mPFC microinfusions of the D4 agonist (n = 8; 50 ng/0.5 μl) immediately before the testing (recall phase; 24 h after conditioning). Analysis revealed intra-mPFC PD 168077 (50 ng/0.5 μl) did not block the expression of previously learned emotionally salient information as demonstrated by a significant increase in the percentage of time spent freezing to the CS+ relative to the CS− (t(7) = 3.03; p < 0.05) (Fig. 4A), as well as a significant decrease in the exploratory behavior observed in response to the CS+ relative to the CS− presentations (t(7) = 3.46; p < 0.05) (Fig. 4B). Thus, activation of intra-mPFC DA D4 receptors specifically blocks the encoding of emotionally salient associative learning, while having no effect on the expression (recall) of this information.
Footshock sensitivity analysis
Although no existing evidence supports a modulatory role for mPFC DA D4 receptor transmission in the processing of nociceptive information, an alternative explanation for our observed conditioning effects on potentiation of subthreshold (0.4 mA) emotional fear conditioning or blockade of the acquisition of suprathreshold (0.8 mA) footshock presentations may be a D4 receptor-mediated increase or decrease in peripheral nociceptive sensitivity to footshock presentations. To rule out this possibility, we analyzed behavioral sensitivity to our low footshock level (0.4 mA), comparing rats receiving microinfusions of either saline (n = 5) or the effective dose of the D4 agonist PD 168077 (n = 5; 50 ng/0.5 μl). In addition, we analyzed behavioral sensitivity to our higher, suprathreshold level of footshock (0.8 mA) comparing rats receiving microinfusions of either saline (n = 6) or the effective dose of the D4 agonist PD 168077 (n = 6; 50 ng/0.5 μl). We compared physiological and behavioral reactions to footshock presentations as described previously (see Materials and Methods) (Laviolette and Grace, 2006a). For control groups receiving the subthreshold footshock presentations (0.4 mA), comparing saline control versus intra-mPFC PD 168077 pretreated groups revealed no significant differences in footshock-induced freezing behavior (t(4) = 0.28; p > 0.5) (Fig. 4C). Furthermore, no significant differences were observed in the number of rears between groups in response to footshock (t(4) = 1.29; p > 0.05) (Fig. 4D), in the quantity of footshock-related jumping between groups (t(4) = 0.20; p > 0.5) (Fig. 4E), and in the amount of defecation between groups during the footshock presentation session (t(4) = 0.23; p > 0.05) (Fig. 4F). For control groups receiving the suprathreshold footshock presentations (0.8 mA), comparing saline control versus intra-mPFC PD 168077 pretreated groups revealed no significant differences in footshock-induced freezing behavior (t(5) = 0.88; p > 0.5) (Fig. 4C). Furthermore, no significant differences were observed in the number of rears between groups in response to footshock (t(5) = 0.92; p > 0.05) (Fig. 4D), in the quantity of footshock-related jumping between groups (t(5) = 0.23; p > 0.5) (Fig. 4E), and in the amount of defecation between groups during the footshock presentation session (t(5) = 0.18; p > 0.05) (Fig. 4F). These behavioral and physiological indices are well established correlates of footshock-related sensitivity (Antoniadis and McDonald, 1999; Laviolette and Grace, 2006a) and demonstrate that activation of DA D4 receptors with the highest effective dose of intra-mPFC PD 168077 (50 ng/0.5 μl) does not produce any appreciable alterations in physiological/peripheral sensitivity to subthreshold (0.4 mA) or suprathreshold (0.8 mA) footshock presentations. In addition, because the present studies used a context-independent form of associative emotional memory conditioning, our observed effects are not likely related to any effects of DA D1 or D4 modulation of spatial memory processing, as emotional associative memory encoding was linked specifically to olfactory cues, independent of spatial/environmental context, further underscoring the importance of DA D1 and D4 transmission during the encoding, modulation, and expression of associative learning and memory, specifically in the context of emotionally salient experience.
D4 receptor-mediated potentiation of emotional learning in the mPFC is dependent on the BLA
We have reported previously that a subpopulation of neurons within the mPFC that receive a direct, functional input from the BLA, encode and express emotional associative learning (Laviolette et al., 2005; Laviolette and Grace, 2006a). Indeed, pharmacological inactivation of the BLA before single neuron associative conditioning in the mPFC blocks the encoding of this information (Laviolette et al., 2005; Laviolette and Grace, 2006a). To examine the potential role of the BLA in our observed potentiation of emotional learning via D4 receptor activation within the mPFC, we examined the effects of pharmacological inactivation of the BLA before administration of intra-mPFC PD68077 (50 ng/0.5 μl) using a subthreshold level of footshock as the conditioning cue (0.4 mA). Bilateral microinfusions of the GABAA receptor agonist muscimol (n = 7; 500 ng/0.5 μl) or saline (n = 7) were performed in the BLA, immediately before intra-mPFC D4 agonist microinfusions. Histological analysis confirmed the placement of intra-amygdala cannulae within the anatomical boundaries of the BLA (Fig. 5A–C). Analysis revealed that intra-BLA muscimol pretreatment (500 ng/0.5 μl) significantly blocked the effects of intra-mPFC microinfusions of PD 168077 (50 ng/0.5 μl) as demonstrated by no significant difference in the percentages of time spent freezing (n = 7; t(6) = 0.31; p > 0.05) (Fig. 5D) or exploratory behavior (n = 7; t(6) = 0.31; p > 0.05) (Fig. 5E) in response to the CS+ versus CS− presentations, compared with saline control groups. Thus, the ability of mPFC D4 receptor activation to potentiate subthreshold emotional associative learning is dependent on the BLA, suggesting an important role for BLA>mPFC inputs in the processing of emotional learning and memory within the mPFC, as reported previously (Laviolette et al., 2005; Laviolette and Grace, 2006a).
Figure 5.
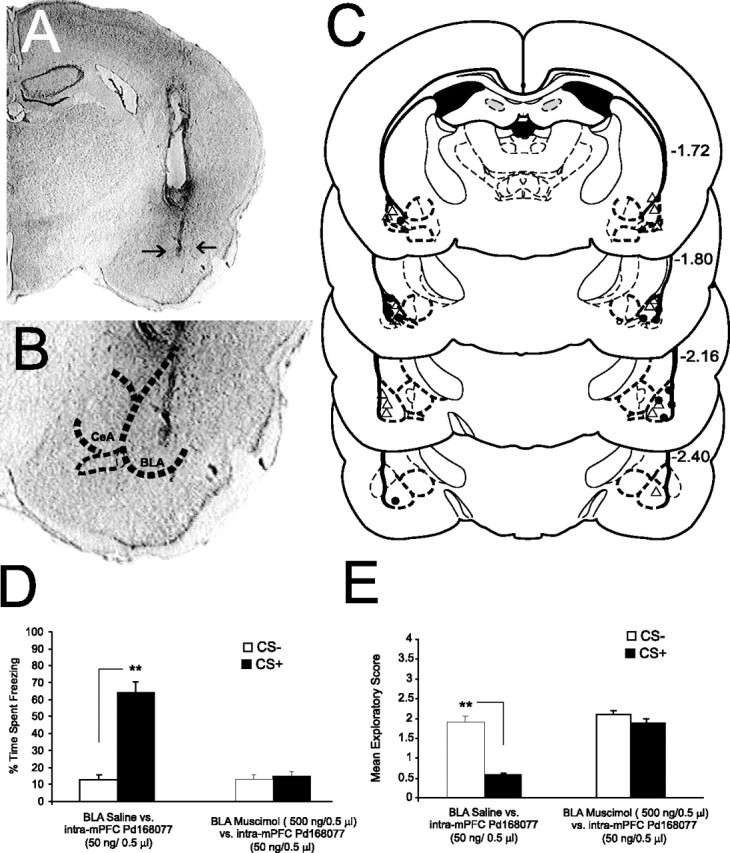
Histological analysis of intra-BLA cannulae placements and effects of BLA inactivation on D4-mediated emotional associative learning potentiation. A, A microphotograph of a coronal section of the BLA showing a representative microinjection site (arrows). B, Microphotograph of the BLA at higher magnification with a superimposed outline of the anatomical boundaries of the BLA relative to the adjacent central nucleus of the amygdala (CeA). C, Representative schematic diagram showing representative intra-BLA microinjection sites: ▵, BLA saline control placements; •, intra-BLA muscimol (500 ng/0.5 μl) injector locations. D, Intra-BLA saline administration before intra-mPFC PD 168077 (50 ng/0.5 μl) had no significant effect on the ability of mPFC D4 receptor activation to potentiate subthreshold (0.4 mA) olfactory fear-conditioning freezing behavior, as rats displayed significant levels of freezing after CS+ presentations. Intra-BLA muscimol administration (500 ng/0.5 μl) before intra-mPFC PD 168077 (50 ng/0.5 μl) significantly blocked the ability of mPFC D4 receptor activation to potentiate subthreshold olfactory fear conditioning, as rats displayed no differences in freezing behavior to CS+ versus CS− presentations. E, Similar to the effects observed with freezing behavior, intra-BLA saline did not affect the ability of intra-mPFC D4 activation to potentiate emotional associative learning, as rats showed significantly greater conditioned suppression of exploratory behavior in response to CS+ relative to CS− presentations. In contrast, this associative response was blocked in rats receiving intra-BLA muscimol before intra-mPFC PD 168077 (50 ng/0.5 μl).
Effects of intra-mPFC DA D1 receptor activation on the encoding of nonsalient versus salient emotional stimuli during associative fear conditioning
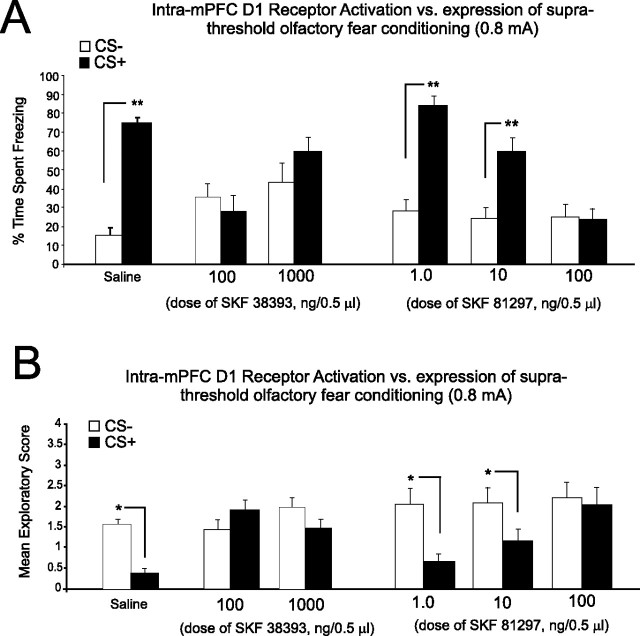
We next examined the potential role of mPFC DA D1 receptor transmission during the encoding of emotionally nonsalient sensory information, by performing bilateral microinfusions of the DA D1-like agonists (SKF 38393; 100 and 1000 ng/0.5 μl) or SKF 81297 (100 ng/0.5 μl) before conditioning rats with our subthreshold level of footshock (0.4 mA). Initial pilot studies revealed that doses of intra-mPFC SKF 81297 higher than 100 ng/0.5 μl produced seizures and/or aversive reactions in some animals; hence, 100 ng was the highest dose of this compound used for this and all subsequent experiments. For experiments using SKF 38393, ANOVA revealed no significant effect of treatment or group (F(2,33) = 0.68; p > 0.05) on the acquisition of subthreshold fear conditioning as demonstrated by no significant difference in the time spent freezing (n = 6, 100 ng/0.5 μl; n = 6, 1000 ng/0.5 μl; p > 0.05) (Fig. 6A) or exploratory behavior (F(2,33) = 0.97; p > 0.05; n = 6, 100 ng/0.5 μl; n = 6, 1000 ng/0.5 μl; p > 0.05) (Fig. 6B) in response to the CS+ compared with CS− presentations. Given that intra-mPFC D1 receptor activation had no observable effect on fear conditioning to subthreshold (emotionally nonsalient) levels of footshock, we next investigated the possible role of D1 receptor activation on the acquisition of suprathreshold (emotionally salient levels of footshock; 0.8 mA). Rats received either bilateral microinfusions of intra-mPFC SKF 38393 (n = 8; 1000 ng/0.5 μl) or saline, immediately before olfactory fear conditioning with the suprathreshold footshock stimulus. ANOVA revealed a significant main effect of treatment on the percentage of time spent freezing in response to CS+ versus CS− presentations (F(1,25) = 83.2; p < 0.0001) with post hoc analysis revealing that both SKF 38393 and saline pretreated groups displayed significantly greater amounts of freezing to the CS+, relative to CS− (Fig. 6C) (p < 0.01). Analysis of spontaneous exploratory behavior revealed a significant group by treatment interaction on mean exploratory behavior scores after CS+ versus CS− presentations (F(1,25) = 3.8; p < 0.01), with post hoc analysis revealing that both SKF 38393 and saline pretreated groups displayed significantly attenuated exploratory behavior in response to CS+ versus CS− presentations (Fig. 6D) (p < 0.01).
Figure 6.
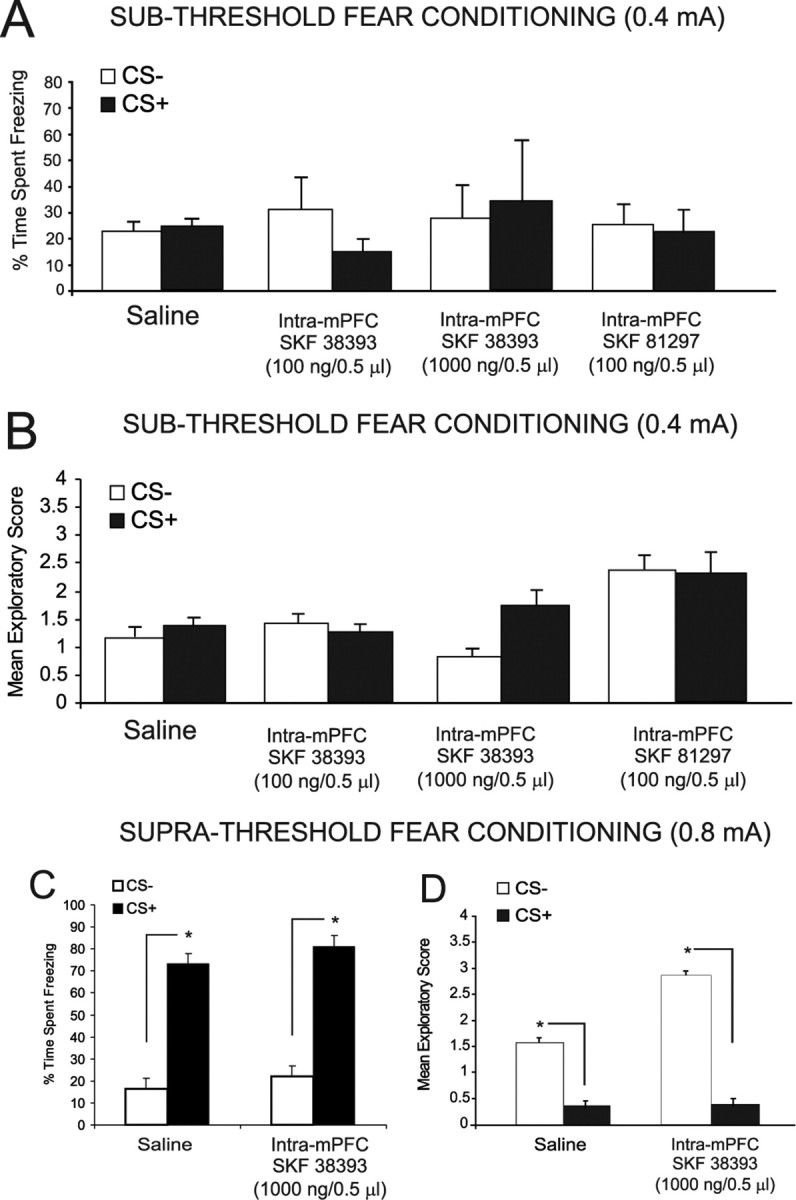
Behavioral effects of bilateral intra-mPFC DA D1 receptor activation on the acquisition of salient or nonsalient associative fear conditioning memory. A, Intra-mPFC DA D1 agonist SKF 38393 (100 and 1000 ng/0.5 μl; n = 7 and n = 8, respectively) had no effect on the percentage of time spent freezing to CS+ versus CS− presentations after olfactory fear conditioning with subthreshold footshock levels (0.4 mA). Bilateral intra-mPFC administration of another D1 receptor agonist, SKF 81297 (100 ng/0.5 μl), similarly had no effect on conditioning to a subthreshold level of footshock. B, Intra-mPFC SKF 38393 (100 and 1000 ng/0.5 μl) had no effect on mean exploratory scores after CS+ versus CS− presentations after olfactory fear conditioning with subthreshold footshock stimuli. Bilateral intra-mPFC administration of the D1 receptor agonist SKF 81297 (100 ng/0.5 μl) similarly had no effect on exploratory scores after CS+ or CS− presentations when conditioned with this subthreshold footshock level. C, Intra-mPFC saline control and intra-mPFC SKF 38393 (1000 ng/0.5 μl) groups displayed a significant increase in freezing in response to CS+ versus CS− presentations after fear conditioning with suprathreshold footshock levels (0.8 mA). D, In addition, intra-mPFC saline control and intra-mPFC SKF 38393 (1000 ng/0.5 μl) groups displayed a significant decrease in exploratory behavior in response to CS+ versus CS− presentations after fear conditioning with suprathreshold levels of footshock.
Effects of intra-mPFC DA D1 receptor activation on the expression (recall) of emotionally salient associative fear conditioning
Given that intra-mPFC D1 receptor activation produced no observable effects on the encoding of emotional associative learning using a nonsalient or salient footshock stimuli (Fig. 6), we next examined the potential role of mPFC D1 receptor transmission on the expression (recall) phase of emotional associative learning, using a suprathreshold, emotionally salient level of footshock (see Materials and Methods) as the conditioning stimulus. We administered either intra-mPFC SKF 38393 (100 and 1000 ng/0.5 μl) or the full D1 receptor agonist SKF 81297 (1–100 ng/0.5 μl) directly before the testing phase of olfactory fear conditioning (24 h after conditioning). For experiments using SKF 38393, ANOVA revealed an interaction between treatment and group (F(3, 50) = 5.81; p < 0.01). Post hoc analysis revealed that rats pretreated with either saline (n = 8) or a lower dose of SKF 38393 (10 ng/0.5 μl) demonstrated significant fear conditioning at testing, with greater amounts of time spent freezing to CS+ relative to CS− presentations (p < 0.01). In contrast, higher doses of intra-mPFC SKF 38393 produced a complete block of associative emotional memory recall, demonstrated by no significant difference in times spent freezing to CS+ versus CS− presentations (100 ng/0.5 μl, n = 7; 1000 ng/0.5 μl, n = 8; p > 0.05) (Fig. 7A). Analysis of exploratory behavior revealed a significant interaction between group and treatment (F(3,57) = 3.71; p < 0.01) with post hoc analysis revealing that groups treated with saline (n = 8) or a lower dose of SKF 38393 (n = 8; 10 ng/0.5 μl) displayed significant conditioned suppression of exploratory behavior in response to CS+ versus CS− presentations (p < 0.05), whereas this effect was blocked in groups receiving the higher doses of intra-mPFC SKF 38393 (100 ng/0.5 μl, n = 7; 1000 ng/0.5 μl, n = 8; p > 0.05) (Fig. 7B) in response to CS+ versus CS− presentations. For experiments using SKF 81297 (1–100 ng/0.5 μl), ANOVA revealed an interaction between treatment and group (F(3, 59) = 6.4; p < 0.001). Post hoc analysis revealed that rats pretreated with either saline (n = 6) or the lower doses of SKF 81297 (1 or 10 ng/0.5 μl; n = 8 for both groups) demonstrated significant fear conditioning at testing, with greater amounts of time spent freezing to CS+ relative to CS− presentations (p < 0.01). In contrast, higher doses of intra-mPFC SKF 81297 (100 ng/0.5 μl) produced a complete block of associative emotional memory recall, demonstrated by no significant difference in times spent freezing to CS+ versus CS− presentations (n = 8; p > 0.05) (Fig. 7A). For groups receiving intra-mPFC SKF 38393, analysis of exploratory behavior revealed a significant interaction between group and treatment (F(3,57) = 3.71; p < 0.01) with post hoc analysis revealing that groups treated with saline (n = 8) or a lower dose of SKF 38393 (n = 8; 10 ng/0.5 μl) displayed significant conditioned suppression of exploratory behavior in response to CS+ versus CS− presentations (p < 0.05), whereas this effect was blocked in groups receiving the higher doses of intra-mPFC SKF 38393 (100 ng/0.5 μl, n = 7; 1000 ng/0.5 μl, n = 8; p > 0.05) (Fig. 7B) in response to CS+ versus CS− presentations. For groups receiving intra-mPFC SKF 81297, analysis of exploratory behavior revealed a significant main effect of treatment (F(3,59) = 19.5; p < 0.0001) with post hoc analysis revealing that groups treated with saline (n = 6) or the lower doses of SKF 81297 (1 or 10 ng/0.5 μl; n = 8) displayed significant conditioned suppression of exploratory behavior in response to CS+ versus CS− presentations (p < 0.05), whereas this effect was blocked in groups receiving the higher doses of intra-mPFC SKF 81297 (100 ng/0.5 μl; n = 8) (Fig. 7B) in response to CS+ versus CS− presentations. Thus, in contrast to our observations with intra-mPFC DA D4 receptor modulation, pharmacological activation of mPFC D1 receptors produced no effect on the encoding of salient or nonsalient fear-conditioning stimuli during the acquisition phase of associative learning (Fig. 6) but dose-dependently blocks the expression (recall) of previously encoded emotional associative memories (Fig. 7).
Figure 7.
Behavioral effects of bilateral intra-mPFC DA D1 receptor activation on the expression (recall) of suprathreshold associative fear-conditioning memory. A, Rats receiving intra-mPFC saline displayed normal expression (recall) of suprathreshold (0.8 mA footshock) fear conditioning when administered saline immediately before testing. In contrast, administration of a D1 agonist (SKF 38393; 100–1000 ng/0.5 μl) immediately before testing completely blocked the expression (recall) of previously acquired emotional learning, with rats displaying no significant difference in freezing behavior in response to CS+ relative to CS− presentations. Similarly, bilateral intra-mPFC administration of the full D1 receptor agonist SKF 81297 (1–100 ng/0.5 μl) produced a dose-dependent attenuation of emotional memory recall at the highest dose of 100 ng, but not at subthreshold doses (1–10 ng/0.5 μl). B, Similar effects were observed in conditioned suppression of exploratory behavior scores after CS+ versus CS− presentations, with intra-mPFC D1 receptor activation blocking conditioned suppression of exploratory behavior after pretesting microinfusions of SKF 38393 (100–1000 ng/0.5 μl), relative to saline controls. Bilateral intra-mPFC administration of another D1 receptor agonist, SKF 81297 (1–100 ng/0.5 μl), also produced a dose-dependent attenuation of emotional memory recall reflected in conditioned suppression of exploratory behavior, at the highest dose of 100 ng but not at subthreshold doses of 1–10 ng/0.5 μl.
Discussion
Evidence from behavioral, electrophysiological, and neuroanatomical studies suggests a complex role for DA transmission within the mPFC during the processing of emotional and cognitive information. Studies examining executive processes such as set-shifting and cognitive flexibility suggest that optimal levels of D1 or D2-like receptor activity are essential for normal cognition and memory function. For example, supranormal stimulation of either D1 or D4 receptors within the mPFC impairs spatial working memory and/or performance on set-shifting tasks in rodents (Zahrt et al., 1997; Floresco et al., 2006). Anomalies in D4 receptor transmission have been reported also in schizophrenia (Seeman et al., 1993; Stefanis et al., 1998). Nevertheless, little is known about the relative roles of mPFC DA D1 versus D4 receptor transmission specifically in the context of emotional learning and memory processing.
DA D4 receptors modulate the encoding of salient and nonsalient emotional information in the mPFC
Given the ability of D4 receptor activation to amplify DA-mediated neuronal activity within the mPFC (Ceci et al., 1999), we hypothesized that activation of mPFC D4 receptors may potentiate the emotional salience of normally subthreshold sensory stimuli, leading to heightened emotional memory encoding. Our results demonstrate that activation of mPFC D4 receptors potentiates the emotional salience of subthreshold fear-conditioning stimuli during acquisition but not expression (recall) phases of associative learning. However, once associative memories are encoded, D4 manipulations no longer appear capable of modulating these memories, consistent with findings in single mPFC neurons (Laviolette et al., 2005). Whereas future studies are required to examine the precise neuronal basis of this effect, recent in vivo electrophysiological evidence demonstrates that D4 receptor agonists can decrease feedforward inhibition on mPFC neurons receiving input from the BLA (Floresco and Tse, 2007), suggesting the possibility that our observed potentiation of emotional associative memory may be attributable to increasing associative information input from the BLA to the mPFC, in effect “priming” neurons in the mPFC to receive emotionally salient associative information from the BLA. We have reported previously that blockade of the D4 receptor within the mPFC can block the encoding of emotionally salient olfactory associative learning (Laviolette et al., 2005). This may suggest that the encoding of emotionally salient memories requires an optimal level of DA D4 receptor stimulation as either overstimulation or blockade of intra-mPFC D4 receptor transmission can inhibit the encoding of this information.
The observed emotional memory potentiating effects of D4 receptor activation are intriguing given previous evidence linking D4 receptor activation with bidirectional modulation of α-Ca2+/calmodulin-dependent protein kinase II (α-CaMKII), a molecule involved in synaptic plasticity, learning, and memory (Malenka and Nicoll, 1999; Frankland et al., 2001). Gu and Yan (2004) have reported that in in vitro slice preparations of rodent mPFC, D4 receptor activation strongly increases α-CaMKII activity during low baseline levels of neuronal activity. In contrast, during high levels of neuronal activity, D4 agonist application causes a marked decrease in α-CaMKII levels, demonstrating that D4 receptor signaling within the mPFC bidirectionally modulates α-CaMKII activity as a function of mPFC neuronal activity levels. Thus, one possibility is that our observed potentiation of normally nonsalient emotional stimuli (Fig. 3) may be related to the ability of D4 receptor activation to increase endogenous levels of α-CaMKII during periods of low mPFC neuronal activity (as may be predicted during exposure to subthreshold footshock). Conversely, the ability of mPFC D4 receptor activation to block emotional memory formation to suprathreshold, emotionally salient footshock stimuli (Fig. 4 A,B) may be related to D4 receptor-mediated attenuation of endogenous α-CaMKII levels, specifically during periods of high neuronal activity within the mPFC (as would be predicted after exposure to higher footshock levels) (Gu and Yan, 2004; Gu et al., 2006).
The present results demonstrate a critical role for prefrontal cortical DA D4 receptor signaling during the encoding phase of emotional associative memory and suggest that perturbations in this system may result in inappropriate associative encoding of sensory stimuli via D4 receptor-mediated modulation of emotional salience variables. Given that similar psychopathological symptoms are present in syndromes such as schizophrenia, autism, and posttraumatic stress disorder, the present results may implicate abnormalities in prefrontal cortical DA D4 transmission in the emotional processing and memory disturbances observed in such disorders.
Previous studies suggest a functional link between the BLA and mPFC both in terms of modulating DA-mediated mPFC neuronal network activity and in regulating associative encoding within mPFC neurons (Laviolette et al., 2005, 2006a; Floresco et al., 2007). We found that BLA inactivation prevented mPFC D4 agonist-mediated potentiation of memory for nonsalient fear stimuli, suggesting that mPFC D4 modulation of emotional learning depends on functional amygdala input. Although the present studies do not differentiate between ascending versus descending pathways within the BLA>mPFC circuit, given that mPFC neurons that respond to BLA inputs are involved in emotional memory encoding in mPFC neurons, BLA inactivation may prevent the flow of first-order emotional salience information from the amygdala to the mPFC, thereby blocking any potentiating effects of D4 receptor activation within the mPFC, as suggested by previous studies (Laviolette et al., 2005; Laviolette and Grace, 2006a).
DA D1 receptors modulate the expression (recall), but not the encoding, of emotionally salient associative information in mPFC
At the behavioral systems level, systemic or intra-mPFC activation of D1 receptors impairs working memory performance (Zahrt et al., 1997) and delayed response performance (Cai and Arnsten, 1990; Arnsten et al., 1994). Although few studies have examined directly the role of mPFC D1 receptor transmission during the encoding and/or recall of emotionally salient associative memories, current theories of mPFC DA function have suggested that D1 receptor transmission may be crucial for the preservation and recall of memories over temporal delay periods (Durstewitz and Seamans, 2008). For example, Seamans et al. (1998) reported that blockade of mPFC D1 receptors blocked the ability of rodents to use previously acquired spatial memory during a delayed performance radial arm task, while having no effect on nondelayed memory recall. These studies suggest that executive memory functions within mPFC neuronal networks may require an optimal level of DA D1 signaling, specifically during periods of memory recall, rather than during associative memory encoding. Such a role for mPFC D1 signaling is consistent with our findings that whereas D1 receptor activation had no influence on the acquisition (encoding phase) of emotional memory, activation of D1 receptors potently blocked the ability to recall emotional associative memory during the expression (test phase) of this learning. Our results suggest that D1 receptor-mediated inhibition of mPFC pyramidal neuron activity may be a mechanism whereby recall of emotionally salient information can be suppressed through dopaminergic modulation of mPFC neuronal associative memory networks.
In terms of DA D1 receptor-mediated control of mPFC neuronal network activity, behavioral and electrophysiological evidence implicates DA D1 receptor transmission in the modulation of inhibitory, GABAergic interneurons. Specifically, activation of mPFC D1 receptors is reported to increase feedforward GABAergic inhibition on mPFC pyramidal neurons (Seamans et al., 2001; Seamans and Yang, 2004). However, because single neurons within the mPFC are capable of actively encoding emotional associative learning during the acquisition phase (Laviolette et al., 2005; Laviolette and Grace, 2006a), one prediction would be that activation of mPFC D1 receptors, which is known to increase feedforward inhibition on mPFC pyramidal neurons (Seamans et al., 2001; Gorelova et al., 2002), also would block associative memory encoding during the acquisition phase of learning. Instead, we observed no blockade of emotional associative learning acquisition after intra-mPFC D1 receptor activation (Fig. 6), further demonstrating a functional dissociation in mPFC DA D1 function during the acquisition (encoding) versus recall phases of emotional learning and memory.
Conclusions
Our results demonstrate distinct functional roles for DA D1 versus D4 receptor subtypes during the encoding versus recall phases of emotional associative memory within the mPFC and also demonstrate a functional role for DA D4 transmission in the modulation of the emotional salience value of sensory stimuli during associative learning and memory processing. Although the present experiments focused primarily on the prelimbic (PLC) division of the mPFC, during the acquisition and expression (recall) phases of emotional associative memory, considerable evidence points to a functional dissociation between the prelimbic and infralimbic divisions of the mPFC during the acquisition versus extinction of emotional memory (Quirk and Mueller, 2008; Santini et al., 2008), such that neurons within the PLC appear critical for emotional memory acquisition whereas infralimbic neurons appear critical for extinction-related learning. The present results, although not directly examining the role of the infralimbic cortex in DA-related emotional memory acquisition and recall, also demonstrate an important role for the PLC in the encoding phase of emotional associative memory. Future studies are required to examine the potential roles of DA D1 versus D4 receptor transmission during the extinction phase of emotional memory processing. A clearer understanding of the roles of DA D1 versus D4 receptor transmission within the mPFC during the processing of emotionally salient associative learning and memory may help elucidate how abnormalities in mPFC DA transmission may lead to disturbed emotional processing and learning present in disorders such as schizophrenia and autism.
Footnotes
This work was supported by the Ontario Mental Health Foundation and the Natural Sciences and Engineering Research Council.
References
- Antoniadis EA, McDonald RJ. Discriminative fear conditioning to context expressed by multiple measures of fear in the rat. Behav Brain Res. 1999;101:1–13. doi: 10.1016/s0166-4328(98)00056-4. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology. 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Cai JX, Arnsten AFT. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1990;282:1–7. [PubMed] [Google Scholar]
- Ceci A, Brambilla A, Duranti P, Grauert M, Grippa N, Borsini F. Effect of antipsychotic and selective dopaminergic antagonists on dopamine-induced facilitatory activity in prelimbic cortical pyramidal neurons: an in vitro study. Neuroscience. 1999;93:107–115. doi: 10.1016/s0306-4522(99)00123-2. [DOI] [PubMed] [Google Scholar]
- Durstewitz D, Seamans JK. The dual-state theory of prefrontal cortex dopamine function with relevance to catechol-O-methytransferase genotypes and schizophrenia. Biol Psychiatry. 2008;64:739–749. doi: 10.1016/j.biopsych.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT. Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci. 2007;27:2045–2057. doi: 10.1523/JNEUROSCI.5474-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- Frankland PW, O'Brien C, Ohon C, Kirkwood A, Silva AJ. Alpha-CamKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranthi C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang C. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Gu Z, Yan Z. Bidirectional regulation of Ca2+/calmodulin-dependent protein kinase II activity by dopamine D4 receptors in prefrontal cortex. Mol Pharmacol. 2004;66:948–955. doi: 10.1124/mol.104.001404. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jiang Q, Yuen EY, Yan Z. Activation of dopamine D4 receptors induces synaptic translocation of CaMKII in cultured prefrontal cortical neurons. Mol Pharmacol. 2006;69:813–822. doi: 10.1124/mol.105.018853. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. Cannabinoids potentiate emotional learning plasticity in neurons of the medial prefrontal cortex through basolateral amygdala inputs. J Neurosci. 2006a;26:6458–6468. doi: 10.1523/JNEUROSCI.0707-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in neural emotional learning circuits: implications for schizophrenia and addiction. Cell Mol Life Sci. 2006b;63:1597–1613. doi: 10.1007/s00018-006-6027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdale input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levant B, Moehlekamp JD, Morgan KA, Leonard NL, Cheng CC. Modulation of [3H] quinpirole binding in brain by monoamine oxidase inhibitors: evidence for a potential novel binding site. J Pharmacol Exp Ther. 1996;278:145–153. [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation: a decade of progress? Science. 1999;285:325–334. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS. Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature. 1996;381:245–248. doi: 10.1038/381245a0. [DOI] [PubMed] [Google Scholar]
- Onn SP, Wang XB, Grace AA. Dopamine D1 and D4 receptor subtypes differentially modulate recurrent excitatory synapses in prefrontal cortical pyramidal neurons. Neuropsychopharmacology. 2006;31:318–338. doi: 10.1038/sj.npp.1300829. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 2. San Diego: Academic; 1996. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Bast T, Feldon J. Significance of dopamine transmission in the rat medial prefrontal cortex for conditioned fear. Cereb Cortex. 2003;13:371–380. doi: 10.1093/cercor/13.4.371. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J Neurosci. 2003;23:11054–11064. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–57. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal–prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Gorelova N, Durstewitz D, Yang CR. Bidirectional dopamine modulation of GABAergic inhabition in prefrontal cortical pyramidal neurons. J Neurosci. 2001;21:3628–3638. doi: 10.1523/JNEUROSCI.21-10-03628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P, Van Tol HH. Dopamine receptor pharmacology. Trends Pharmacol Sci. 1994;15:264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors are elevated in schizophrenia. Nature. 1993;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Bresnick JN, Kerwin RW, Schofield WN, McAllister G. Elevation of D4 dopamine receptor mRNA in postmortem schizophrenic brain. Brain Res Mol Brain Res. 1998;53:112–119. doi: 10.1016/s0169-328x(97)00285-4. [DOI] [PubMed] [Google Scholar]
- Wedzony K, Chocyk A, Mackowiak M, Fijal K, Czyrak A. Cortical localization of dopamine D4 receptors in the rat brain-immunocytochemical study. J Physiol Pharmacol. 2000;51:205–221. [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AFT. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]