Abstract
Strong unilateral contractions are accompanied by excitatory effects to the ipsilateral cortex. This activity can even result in overt contractions of muscles in the contralateral limb. We used this inadvertent, associated activity to study whether the cortical presentation of movements is organized in a directional-related or a muscle-related reference frame. We assessed the contralateral activation for the left index finger during a sustained maximal abduction of the right index finger. In the first experiment, both hands were held vertically in a symmetrical orientation, and in the second experiment the hands were in an asymmetrical orientation (left hand, palm downward; right hand, vertical). In both experiments, the direction of the contralateral associated contraction was upward, i.e., in the symmetrical hand orientation the contralateral force increased mainly in abduction direction, whereas in the asymmetrical hand orientation the contralateral force increased in the extension direction. Thus, the contralateral contractions reflected the direction of the target movement rather than simply the activity of the muscles activated on the target side. These observations provide strong evidence that motor commands are organized in an extrinsic, direction-related reference frame, as opposed to an internal muscle-related reference frame.
Introduction
Attempts at elucidating the functional organization of the motor system have taken much effort for many years. Some research groups consider the organization to reflect the activity of a target set of muscle (Todorov, 2000; Griffin et al., 2008). This view is challenged following observations indicating that the mapping is even more complicated, including high-level parameters such as movement direction (Georgopoulos et al., 1982; Kakei et al., 1999; Georgopoulos, 2000) and distinct motor actions (for review, see Graziano, 2006). Many of the conclusions are based on electrical stimulation of cortical motor areas and single neuron recordings in monkeys. In the present study, however, we used a different strategy to resolve the question whether the CNS uses the active muscle ensemble or the movement direction as the most important control variable.
It is known that during strong unilateral contractions the activity is not confined to the target muscles, but a copy of the efferent commands projects toward the contralateral hemisphere. This efferent copy can even result in overt contractions of contralateral muscles (Curschmann, 1906; Cernacek, 1961; Zijdewind and Kernell, 2001; Zijdewind et al., 2006). We refer to this inadvertent activation of the contralateral muscles as “associated activity.” Although the amount of this associated activity differs between subjects, the strength correlates strongly with the strength and duration of the target contraction (Curschmann, 1906; Todor and Lazarus, 1986; Zijdewind and Kernell, 2001; Shinohara et al., 2003). Because associated activity is inadvertent and subjects are generally unaware of this activity, it provides an excellent opportunity for studying underlying motor control strategies. Therefore, we feel that we can use this activity to investigate whether the associated activity reflects the activity of the target muscles per se or rather the direction of the intended movement.
We assessed the associated activity of the left index finger during a sustained maximal contraction with the right index finger in two experiments. In one experiment, both hands were in a vertical position (midway between supination and pronation), whereas in the second experiment, the left hand was fully pronated (palm downward), and the right hand was in vertical position. The results showed that the direction and the amount of associated activity was strongly dependent on the orientation of the contralateral hand relative to that of the target hand, such that it was mainly determined by the target movement direction rather than by the identity of the voluntarily activated target muscles.
A short account of some of the present findings has been published as an abstract (Zijdewind et al., 2008).
Materials and Methods
Subjects.
We studied 12 healthy subjects (four male and eight female; mean age, 33 ± 10 years). All subjects gave their written informed consent before participation in this study and were right handed as confirmed by the Edinburgh Handedness Inventory (Oldfield, 1971). The local ethical committee of the University Hospital Groningen approved all research procedures. All subjects participated in two experimental sessions separated by more than a week.
Experimental set-up.
We used two experimental set-ups: (1) both hands were in a vertical position, midway between supination and pronation (symmetrical orientation), and (2) the right hand was in a vertical position, but the left hand was fully pronated (palm of the hand downward; asymmetrical orientation). A graphical overview of the set-up is given in Figure 1. All subjects participated in both sessions. The order of the sessions was randomized between subjects.
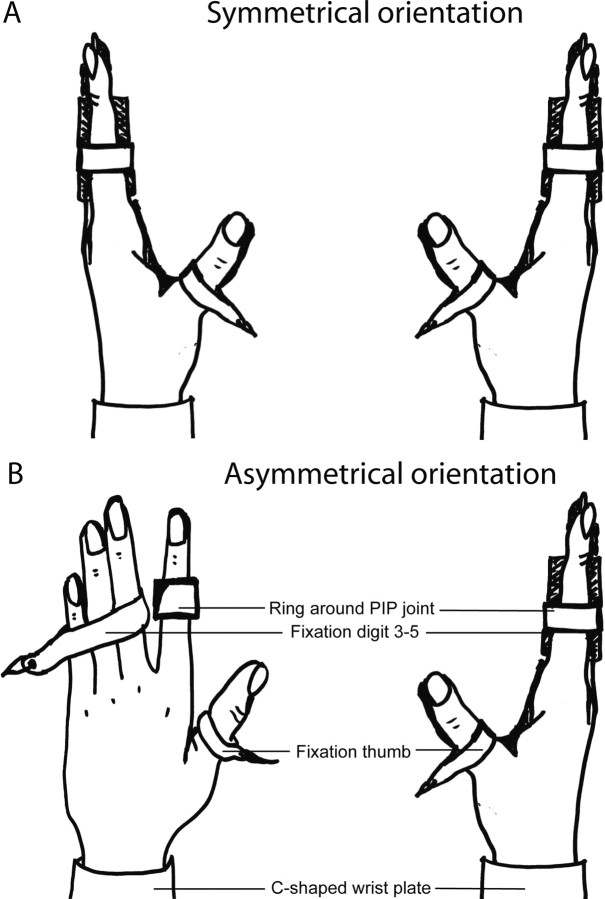
Figure 1.
Graphical overview of the symmetrical orientation (A) and the asymmetrical orientation (B) and the fixation of the hand during the task, view from above. PIP joint, Proximal interphalangeal joint.
In both experimental set-ups, subjects sat behind an experimental table with their arms on the table. The elbow angle was ∼145°. An Ω-shaped plate around the wrist stabilized the hands of the subjects in both hand positions, and digits 3–5 and thumbs of both hands were fixed such that only forces exerted by the index fingers were recorded. In the symmetrical orientation, both hands were held vertically, whereas in the asymmetrical orientation, the left hand was in a horizontal position. In both hand positions, the index fingers were held slightly abducted within a snugly fitting ring around the proximal interphalangeal joint (Zijdewind and Kernell, 1994). The ring was connected to an isometric force transducer and measured the index finger abduction force. The left force transducer was also able to measure force in flexion and extension direction.
In both positions, surface electromyography (EMG) of the first dorsal interosseus (FDI) muscles were recorded with one electrode placed over the muscle belly and a second electrode placed at the metacarpophalangeal joint of the index fingers. A band-shaped earth electrode was strapped around the wrist. Subjects received visual feedback of their target force via a screen placed in front of them and were verbally encouraged during the contractions. EMG and force signals were amplified and collected on a computer equipped with a data-acquisition interface (Cambridge Electronic Design; CED 1401; sampling frequency, 2000 and 500 Hz, for the EMG and force recordings, respectively).
Experimental protocol.
In each of the two sessions (symmetrical and asymmetrical orientation), the following measurements were performed.
We assessed the maximal voluntary abduction force of the FDI muscle in both left and right hands. Subjects performed three brief maximal abductions alternating with the left and right index finger. After these contractions subjects performed maximal contractions in flexion and extension direction of the left index finger only. Each maximal voluntary contraction (MVC) consisted of a contraction of 4 s followed by 30 s rest. The largest MVC was designated as the “control” MVC. After the MVCs, subjects performed a sustained maximal contraction in abduction direction for 2 min with the right index finger. We gave no instructions to the subjects with respect to the contralateral hand. Although the subjects had both hands connected to a force transducer and were conscious of the fact they had to generate force in both hands in turns, they were not aware of our specific interest in the activity on the “nontarget” side.
Data analysis.
EMG and force measurements were analyzed offline with a computer equipped with Spike 2 for windows (version 5.16; Cambridge Electronic Design).
For the brief MVCs, we measured the voluntary abduction force exerted by the left and right index finger, and the peak associated force exerted inadvertently by the contralateral finger in the ab/adduction plane. In addition, for the left finger only, these forces were also measured in the flexion/extension plane.
Force was expressed as absolute force (N) in the ab/adduction plane and the flexion/extension plane. Abduction and extension were arbitrarily assigned a positive sign, adduction and flexion a negative sign. To assess the relative level of the associated force, we also expressed force as a percentage of the individuals' MVC. Because MVC values differed in flexion and extension direction, we determined mean group values for the flexion and extension direction separately (if a subject produced associated activity in extension direction, the force in flexion direction was designated to zero and vice versa).
For the EMG of the target FDI, we calculated the root mean square (RMS) for a period of 100 ms around the voluntary force peak.
During the sustained contraction, we calculated mean abduction force and RMS EMG of the FDI for 10 s epochs in both right and left index finger. Furthermore, in the left index finger, the mean force in extension/flexion direction for each 10 s epoch was calculated. As during the brief contractions, abduction and extension were arbitrarily assigned a positive sign, adduction and flexion a negative sign. Additionally, the mean force was expressed as a percentage of the MVC in the same direction.
For the brief MVCs, voluntary force was analyzed using a 2 (orientation) × 2 (hand) repeated-measures ANOVA. For the left index finger, effects of hand orientation on the direction of the associated activity was analyzed using a 2 (hand orientation) × 2 (direction of associated force) repeated-measures ANOVA. If this interaction showed significant effects, post hoc analyses were performed for each force direction separately with planned contrasts.
During the sustained 2 min contraction, voluntary force and RMS EMG as well as associated RMS EMG values were analyzed with a 2 (hand orientation) × 12 (time) repeated-measures ANOVA. To detect effects of hand orientation on the direction of the associated force, we used a 2 (hand orientation) × 2 (direction of associated activity) × 12 (time). If an interaction was found between orientations, force direction and time, post hoc analyses were performed for each force direction separately. Differences were tested with a 2 (hand orientation) × 12 (time) repeated-measures ANOVA. We present the group data as mean ± SDs in the text and mean ± SEMs in the figures. Statistical significance was set at p < 0.05.
Results
Brief MVCs
Voluntary contractions
Maximal abduction force generated in the symmetrical orientation was 40.22 ± 10.02 N for the right index finger and 38.89 ± 11.76 N for the left index finger. These values were not significantly different from the forces in the asymmetrical orientation: 42.14 ± 9.21 N for the right index finger and 41.75 ± 8.35 N for the left index finger (F(1,11) = 1.533, p > 0.2). In the left index finger, the maximal force in extension direction was smaller in the vertical hand position compared with the fully pronated position (14.55 ± 4.89 N vs 25.55 ± 16.57 N, respectively; p = 0.018). In nine subjects, we also recorded maximal flexion force of the left index finger in both positions. The contractions in flexion direction did not show a difference between hand positions: in the vertical position, subjects attained 39.66 ± 21.61 N and in the horizontal position 33.83 ± 13.07 N. The large SDs in the extension and flexion forces were attributable to one subject who was substantially stronger than the other subjects; exclusion of this subject did not affect the statistical analysis; therefore, this subject was retained in the data pool.
During the brief abductions with the left index finger, the abduction was often accompanied by index finger flexion (35.34 ± 12.99 %MVC, n = 8). Furthermore, in three subjects, index finger flexion changed to extension during the contraction (average extension force: 6.95 ± 9.90 %MVC).
Associated contractions during brief MVCs
Most subjects showed associated contractions in the left index finger in both hand orientations. However, the preferred direction of the associated contraction was different between hand orientations (effect of orientation, F(1,11) = 9.691; p = 0.01).
In the symmetrical hand orientation, the associated force was stronger in abduction direction (2.71 ± 4.16 N; 6.06 ± 9.27 %MVC) compared with flexion (3.32 ± 5.09 %MVC) and extension direction (1.38 ± 1.57 %MVC); absolute force in the flexion/extension plane equaled −1.07 ± 2.34 N (resultant force in flexion direction).
In the asymmetrical hand orientation, the associated forces were stronger in abduction (2.05 ± 3.25 N; 4.64 ± 7.17 %MVC) and extension direction (4.21 ± 6.30 %MVC) than in flexion direction (1.14 ± 2.56 %MVC); absolute force in the flexion/extension plane was 0.70 ± 2.00 N (resultant force in extension direction).
Sustained contraction
Voluntary contractions
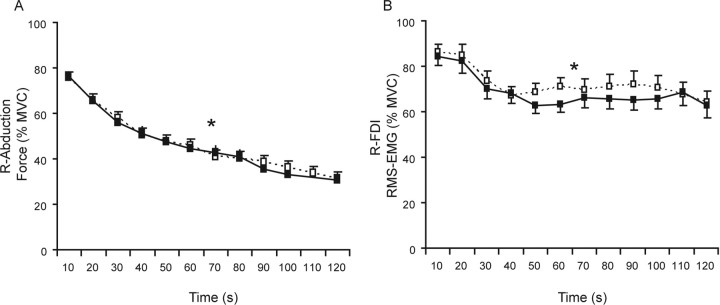
As expected, in both the symmetrical and asymmetrical orientation the force of the target finger during the sustained maximal contraction declined over time (effect of time, F(11,121) = 148.27; p < 0.001) (Fig. 2A). No difference was found in the degree of fatigue of the target muscle across the two tested orientations (mean final force, 12.50 ± 3.74 N; 30.98 ± 8.67 %MVC; no orientation effect, F(1,11) = 0.169; p > 0.6). The RMS EMG of the FDI of the target hand also declined over time (F(11,121) = 12.642, p < 0.001), without a difference between hand orientations (mean final RMS EMG, 63.44 ± 17.42 %MVC; F(1,11) = 0.627; p > 0.4) (Fig. 2B).
Figure 2.
Mean index finger abduction force (A) and RMS EMG of the first dorsal interosseus (B) of the target hand over 10 s epochs during a sustained maximal abduction with the right index finger (%MVC). The two lines represent the two experiments: black symbols (uninterrupted line) refer to the experiment with the hands in a symmetrical orientation (both hands vertical, midway between pronation and supination); white symbols (dashed line) refer to the experiment with the hands in an asymmetrical orientation (left hand fully pronated, right hand vertical). Asterisk denotes significant effect for time.
Associated activity during the sustained contraction
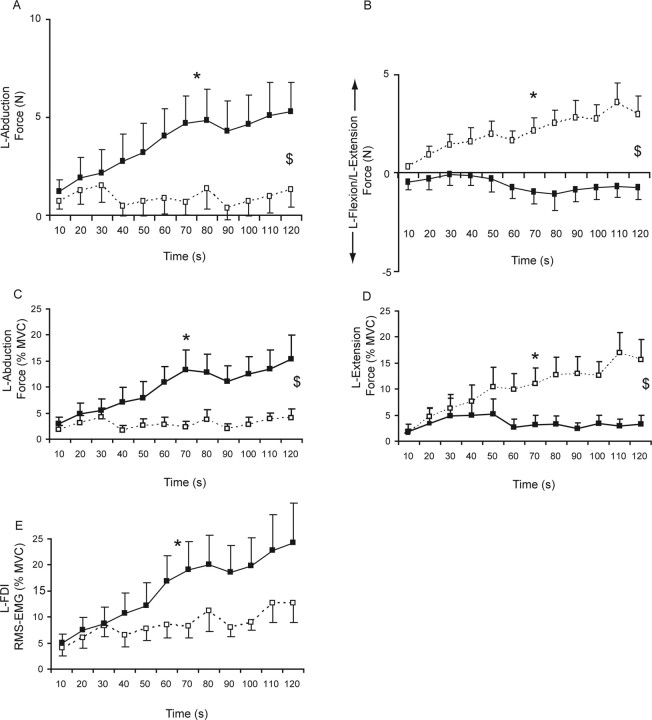
During the fatigue task, associated index finger force was seen in both the symmetrical and asymmetrical orientation. However, the main direction of the associated activity differed significantly (interaction effect between orientation, direction and time: F(11,121) = 3.965, p < 0.001) (Fig. 3).
Figure 3.
A, B, Associated force in ab/adduction (A) and flexion/extension direction (B) of the left index finger during a sustained maximal abduction with the right index finger. Data are averaged over 10 s epochs. The two lines represent the two experiments: black symbols (uninterrupted line) refer to the experiment with the hands in a symmetrical orientation (both hands vertical, midway between pronation and supination); white symbols (dashed line) refer to the experiment with the hands in an asymmetrical orientation (left hand fully pronated, right hand vertical). C, Shows the mean associated force (%MVC) of the left hand in abduction direction during the symmetrical and asymmetrical orientation. D shows the mean associated force in extension direction (%MVC); for calculation of the mean values, the force in flexion direction is designated as 0. In contrast to C and D, A and B show the associated force calculated as the mean of the absolute forces (abduction and extension force was arbitrarily designated as positive values and adduction and flexion as negative values). E shows the RMS EMG values obtained in the left first dorsal interosseus. Asterisk denotes significant effect for time, and $ denotes significant interaction effect between hand orientation and time (p < 0.05). The error bars represent SEM.
In the symmetrical hand orientation, the associated force in the contralateral hand increased over time in abduction direction to 5.30 ± 5.29 N (15.26 ± 16.07 %MVC; F(11,121) = 4.497, p < 0.001) (Fig. 3A,C). No significant increase in associated force in extension (final force, 3.27 ± 5.71 %MVC) or flexion (final force, 6.22 ± 15.03 %MVC) direction was seen (Fig. 3B,D) (final absolute force, −0.74 ± 2.07 N; F(11,121) = 1.022, p > 0.4). The associated force in flexion/extension direction was variable between subjects; in the latter part of the contraction, five subjects showed associated force in flexion direction, and three subjects showed associated force in extension direction. In four subjects, the force in flexion/extension direction was close to zero (< 2 %MVC).
In the asymmetrical hand orientation, no increase in associated force over time was seen in abduction (mean: 1.79 ± 2.78 N, 2.84 ± 0.89 %MVC; effect time: F(11,121) = 0.5, p > 0.5) (Fig. 3A,C) or flexion direction (0.22 ± 0.13 %MVC). Yet, in this orientation, the force in the extension direction increased progressively to 15.54 ± 13.41 %MVC (absolute force, 3.01 ± 3.17 N; F(11,121) = 5.390; p < 0.001) (Fig. 3B,D).
The RMS EMG of the contralateral FDI showed a tendency toward a significant effect of task (F(1,11) = 4.290, p = 0.063) (Fig. 3E). During the sustained contraction in the symmetrical hand orientation, a larger and more consistent increase in EMG was seen (to 24.24 ± 26.14 %MVC; effect time, F(11,121) = 4.879; p < 0.001) compared with the RMS EMG increase in the asymmetrical orientation (to 12.55 ± 12.55 %MVC; effect time, F(11,121) = 1.777; p = 0.065).
Discussion
Our data clearly provide strong evidence that the coding of motor signals is organized in an external frame of reference (i.e., direction of force in space), rather than in an intrinsic reference frame (i.e., muscle specific). Here, we show that the direction of inadvertent contralateral-associated activity, measured during a sustained maximal voluntary contraction of the target hand, reflected the spatial direction of the generated target force. Our study is of interest because the task that subjects purposely performed (right index finger abduction) was similar in the two conditions, yet the (inadvertent) output of the left hand differed depending on its orientation. In review papers concerning contralateral activation (Hoy et al., 2004; Carson, 2005; Cincotta and Ziemann, 2008), associated activity is often implicitly connected with the homologous muscle. Our observations suggest that the direction of contralateral associated activity is organized according to movement direction rather than in relation to active muscle identities. This observation demonstrates that the organization of associated activity is more complicated than previously thought. If we assume that the copy of the command signal that is projected to the nontarget hemisphere is similar in the two setups, our data demonstrates that the hand orientation actually modulates the output of the command signal.
During the brief maximal index finger abductions with the hands in a symmetrical orientation, the mean amount of associated activity in the FDI and abduction force was comparable with earlier studies using index finger abduction (∼5 %MVC) (Zijdewind and Kernell, 2001; Shinohara et al., 2003; Post et al., 2008). The small amount of associated activity during the brief contractions and at the start of the sustained contraction (0–30 s) in combination with the large variability between subjects reduces the chances of finding statistical significant differences. Therefore, it is unclear whether the small difference between associated force in abduction and extension direction represents a difference in organization between brief and sustained contractions or is just caused by lack of statistical power. During the sustained contraction, the differences in associated activity across hand positions became very apparent. It was already known that the amount of associated activity increases during a sustained contraction (Zijdewind and Kernell, 2001), and this observation has recently been confirmed by fMRI data showing an increase in ipsilateral activation of the precentral and postcentral gyrus during a sustained maximal contraction (Post et al., 2009). In our opinion, this observation demonstrates that the increase in associated activity during sustained contractions is attributable to an increased central drive toward the target muscles accompanied by increased activity to the nontarget hemisphere resulting in increased associated activity. This implies that the small difference in direction sensitivity during the brief contractions is very likely attributable to lack of statistical power and not to a difference in command strategy between brief and sustained contractions.
Several reviews (Hoy et al., 2004; Carson, 2005; Cincotta and Ziemann, 2008) discuss the possible neuronal pathways involved in associated activity. In short, the models attribute associated activity to activation coming through ipsilateral pathways, contralateral pathways, or an interaction between the ipsilateral and contralateral areas [Zijdewind et al. (2006), their Fig. 1]. It seems unlikely that associated activity via ipsilateral projections would be strongly affected by the position of the ipsilateral, nontarget hand. Therefore, we feel that the present data clearly indicate that the associated activity comes through the cortex contralateral to the muscle showing associated activity (see also Zijdewind et al., 2006).
It is generally thought that the amount of associated activity is not only affected by excitatory but also by inhibitory effects on the ipsilateral motor cortex (Leocani et al., 2000; Arányi and Rösler, 2002). These inhibitory interactions are important in suppressing the tendency to move in symmetry. Therefore, it is interesting that our results underline data obtained in a study of Duque et al. (2005). They studied the effects of hand position on inhibition in the corticospinal tract to the contralateral FDI. Just before index finger abduction with the hands in a symmetrical orientation—in their study both hands were fully pronated—the MEP was depressed compared with the asymmetrical orientation (left hand fully pronated, right hand midway between pronation and supination). Their data suggest that the distribution of inhibition of the ipsilateral cortex is also related to the direction of the movement of the opposite hand rather than reflecting the active muscles in the opposite hand.
Summarizing, our data extends previous data mainly obtained in monkeys that demonstrate that neuronal activity in motor areas is not simply related to a single muscle representation but reflects many features, such as direction of the movement (Georgopoulos et al., 1982, 1986; Kakei et al., 1999), motor actions (Graziano, 2006), and force production (Evarts, 1968). In our set-up, we studied inadvertent movements of the opposite index finger, and our results underline the experiments of Kakei et al. (1999), suggesting that the coding of the voluntary movement is strongly determined by an external reference frame as opposed to a muscle-related (internal) reference frame. Furthermore, as we assume that the associated activity is the resultant of a copy of the efference command toward the nontarget hemisphere, our data propose a future study to determine whether direction sensitivity as coded in the efference copy is a property of the motor cortex or that it is the result of a (direction-sensitive) modulation of the corticospinal output, e.g., by proprioceptive feedback or visual input.
Footnotes
We thank Koen Vaartjes for the technical support during the experiments and Dr. D. Kernell for useful comments on an earlier version of this manuscript.
References
- Arányi Z, Rösler KM. Effort-induced mirror movements. A study of transcallosal inhibition in humans. Exp Brain Res. 2002;145:76–82. doi: 10.1007/s00221-002-1101-1. [DOI] [PubMed] [Google Scholar]
- Carson RG. Neural pathways mediating bilateral interactions between the upper limbs. Brain Res Brain Res Rev. 2005;49:641–662. doi: 10.1016/j.brainresrev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Cernacek J. Contralateral motor irradiation–cerebral dominance. Its changes in hemiparesis. Arch Neurol. 1961;4:165–172. doi: 10.1001/archneur.1961.00450080047005. [DOI] [PubMed] [Google Scholar]
- Cincotta M, Ziemann U. Neurophysiology of unimanual motor control and mirror movements. Clin Neurophysiol. 2008;119:744–762. doi: 10.1016/j.clinph.2007.11.047. [DOI] [PubMed] [Google Scholar]
- Curschmann H. Beitrage zur physiologie und pathologie der kontralateralen mitbewegungen. Deutsche Zeitschrift for nervenheilkunde. 1906;31:1–52. [Google Scholar]
- Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG. Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex. 2005;15:588–593. doi: 10.1093/cercor/bhh160. [DOI] [PubMed] [Google Scholar]
- Evarts EV. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968;31:14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP. Neural aspects of cognitive motor control. Curr Opin Neurobiol. 2000;10:238–241. doi: 10.1016/s0959-4388(00)00072-6. [DOI] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Schwartz AB, Kettner RE. Neuronal population coding of movement direction. Science. 1986;233:1416–1419. doi: 10.1126/science.3749885. [DOI] [PubMed] [Google Scholar]
- Graziano M. The organization of behavioral repertoire in motor cortex. Annu Rev Neurosci. 2006;29:105–134. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hudson HM, Belhaj-Saïf A, McKiernan BJ, Cheney PD. Do corticomotoneuronal cells predict target muscle EMG activity? J Neurophysiol. 2008;99:1169–1986. doi: 10.1152/jn.00906.2007. [DOI] [PubMed] [Google Scholar]
- Hoy KE, Fitzgerald PB, Bradshaw JL, Armatas CA, Georgiou-Karistianis N. Investigating the cortical origins of motor overflow. Brain Res Brain Res Rev. 2004;46:315–327. doi: 10.1016/j.brainresrev.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Kakei S, Hoffman DS, Strick PL. Muscle and movement representations in the primary motor cortex. Science. 1999;285:2136–2139. doi: 10.1126/science.285.5436.2136. [DOI] [PubMed] [Google Scholar]
- Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M. Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain. 2000;123:1161–1173. doi: 10.1093/brain/123.6.1161. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Post M, Bayrak S, Kernell D, Zijdewind I. Contralateral muscle activity and fatigue in the human first dorsal interosseous muscle. J Appl Physiol. 2008;105:70–82. doi: 10.1152/japplphysiol.01298.2007. [DOI] [PubMed] [Google Scholar]
- Post M, Steens A, Renken R, Maurits NM, Zijdewind I. Voluntary activation and cortical activity during a sustained maximal contraction: an fMRI study. Hum Brain Mapp. 2009;30:1014–1027. doi: 10.1002/hbm.20562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara M, Keenan KG, Enoka RM. Contralateral activity in a homologous hand muscle during voluntary contractions is greater in old adults. J Appl Physiol. 2003;94:966–974. doi: 10.1152/japplphysiol.00836.2002. [DOI] [PubMed] [Google Scholar]
- Todor JI, Lazarus JA. Exertion level and the intensity of associated movements. Dev Med Child Neurol. 1986;28:205–212. doi: 10.1111/j.1469-8749.1986.tb03856.x. [DOI] [PubMed] [Google Scholar]
- Todorov E. Direct cortical control of muscle activation in voluntary arm movements: a model. Nat Neurosci. 2000;3:391–398. doi: 10.1038/73964. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Index finger position and force of the human first dorsal interosseus and its ulnar nerve antagonist. J Appl Physiol. 1994;77:987–997. doi: 10.1152/jappl.1994.77.2.987. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Kernell D. Bilateral interactions during contractions of intrinsic hand muscles. J Neurophysiol. 2001;85:1907–1913. doi: 10.1152/jn.2001.85.5.1907. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Butler JE, Gandevia SC, Taylor JL. The origin of activity in the biceps brachii muscle during voluntary contractions of the contralateral elbow flexor muscles. Exp Brain Res. 2006;175:526–535. doi: 10.1007/s00221-006-0570-z. [DOI] [PubMed] [Google Scholar]
- Zijdewind I, Post M, Mulder P, Bakels R. Contralateral activity during a unilateral contraction mirrors the direction of movements rather than the active muscle assemblies. Soc Neurosci Abstr. 2008;34 277.24/LL16. [Google Scholar]